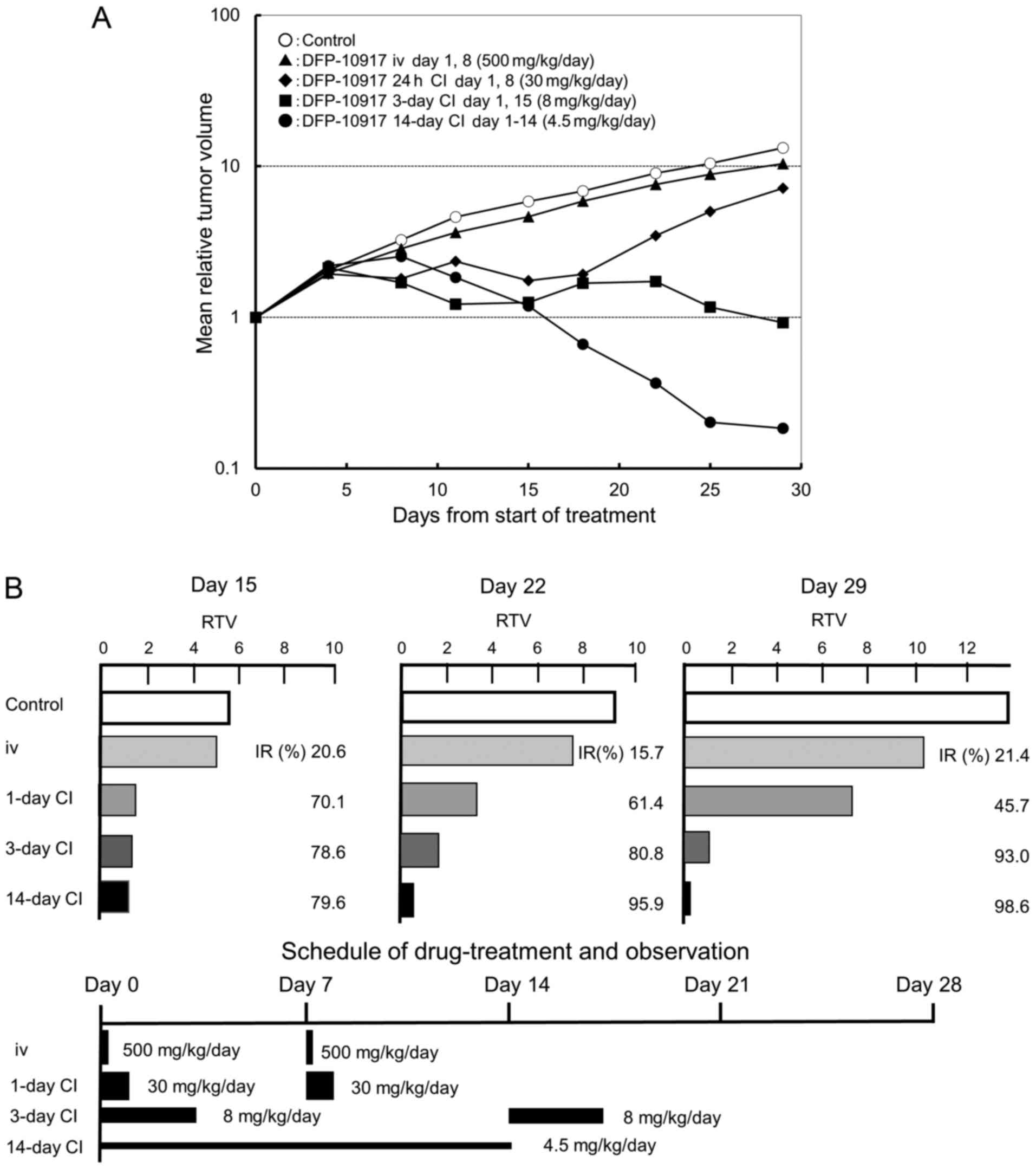
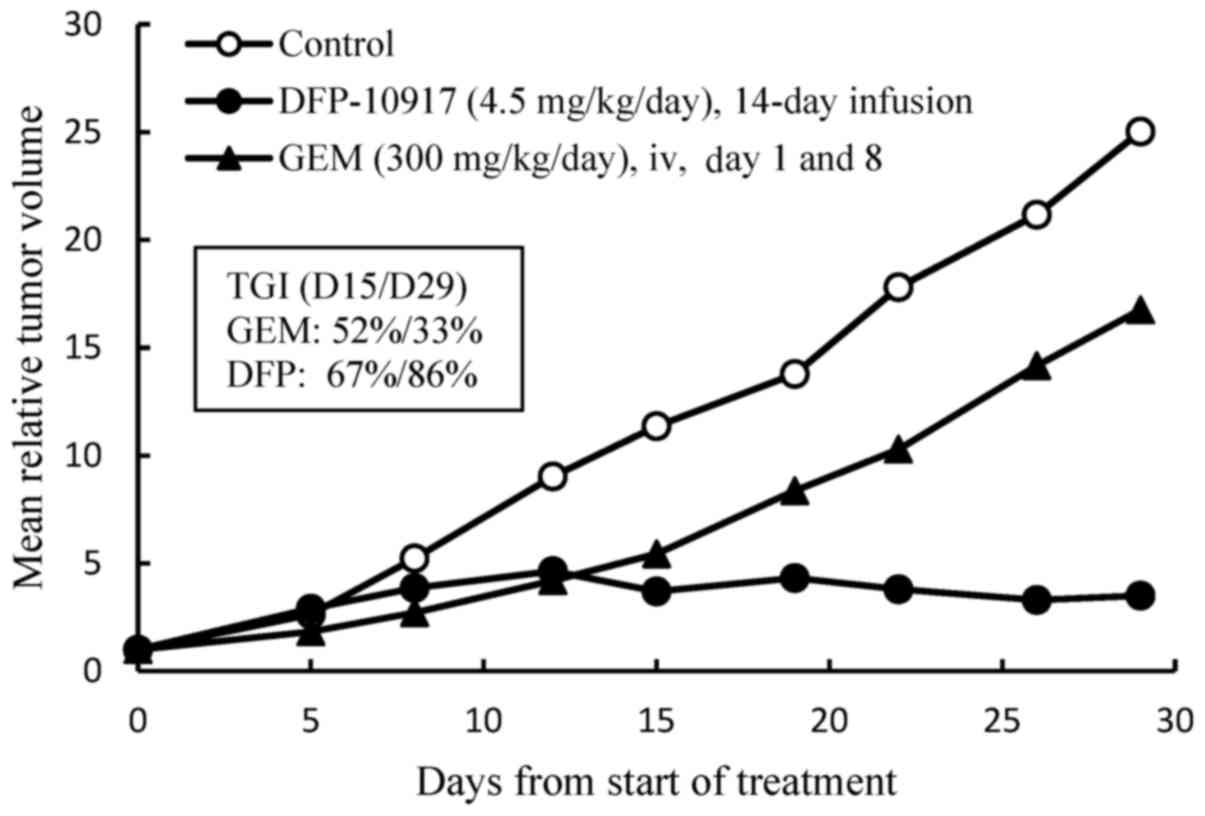
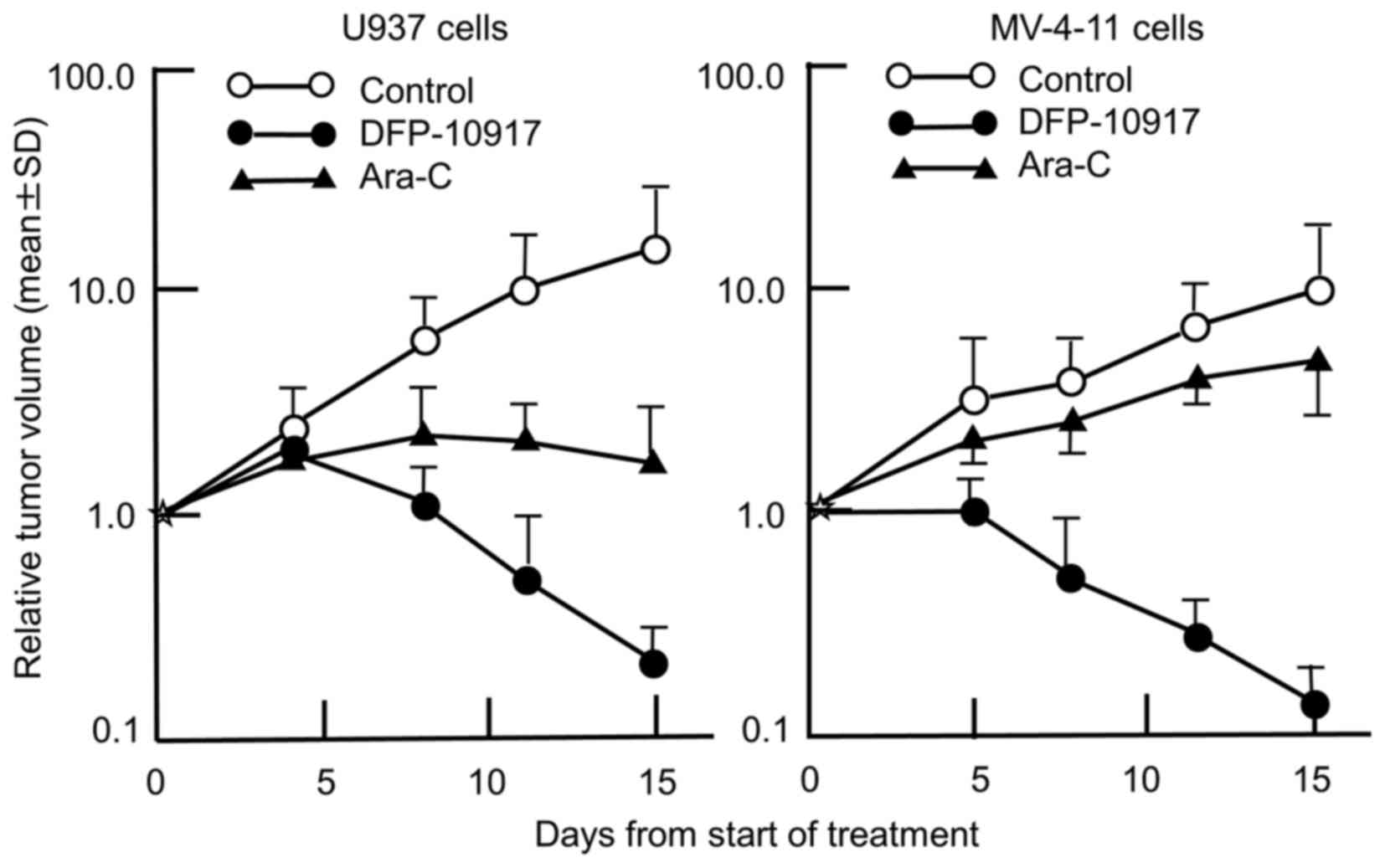
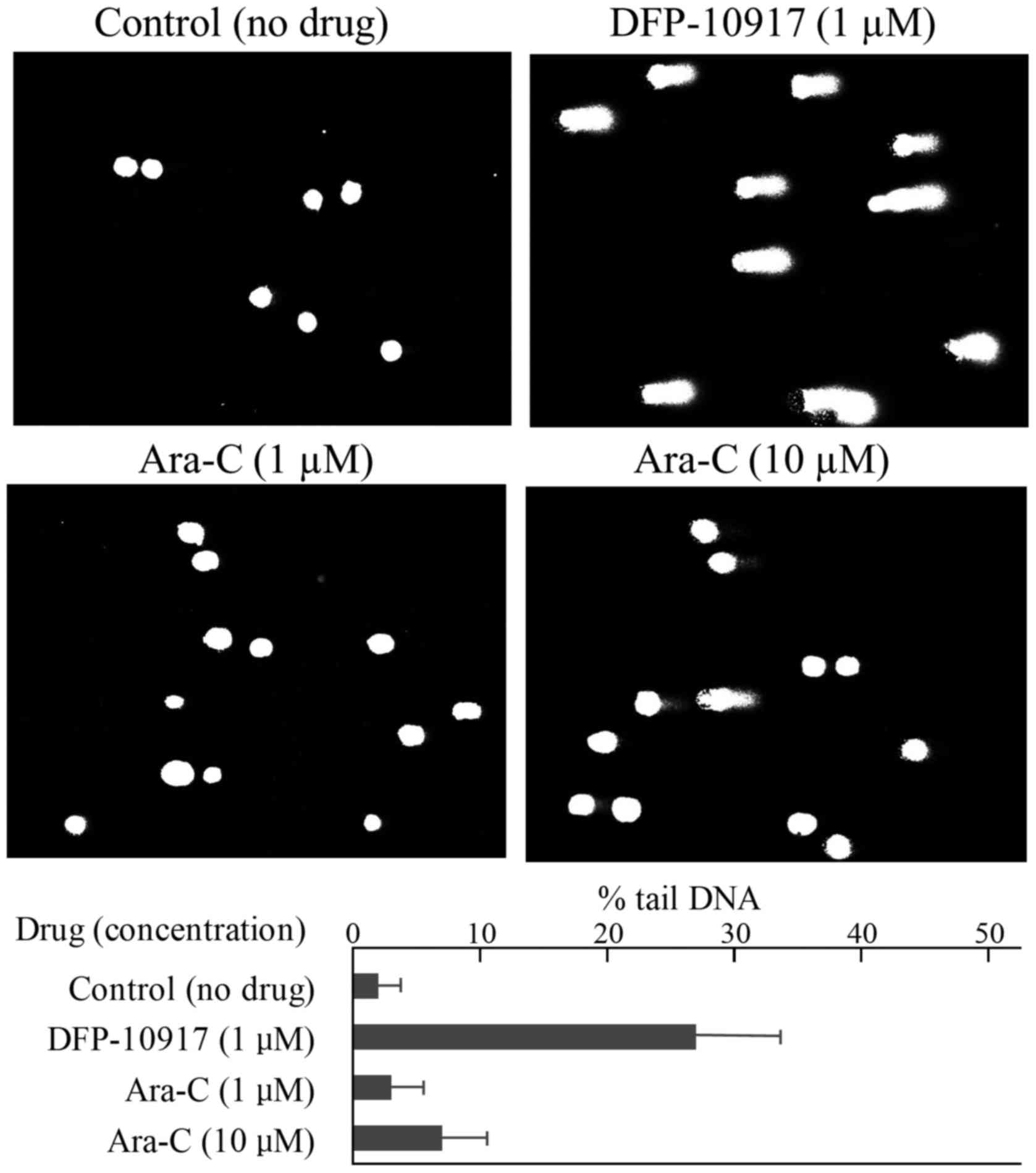
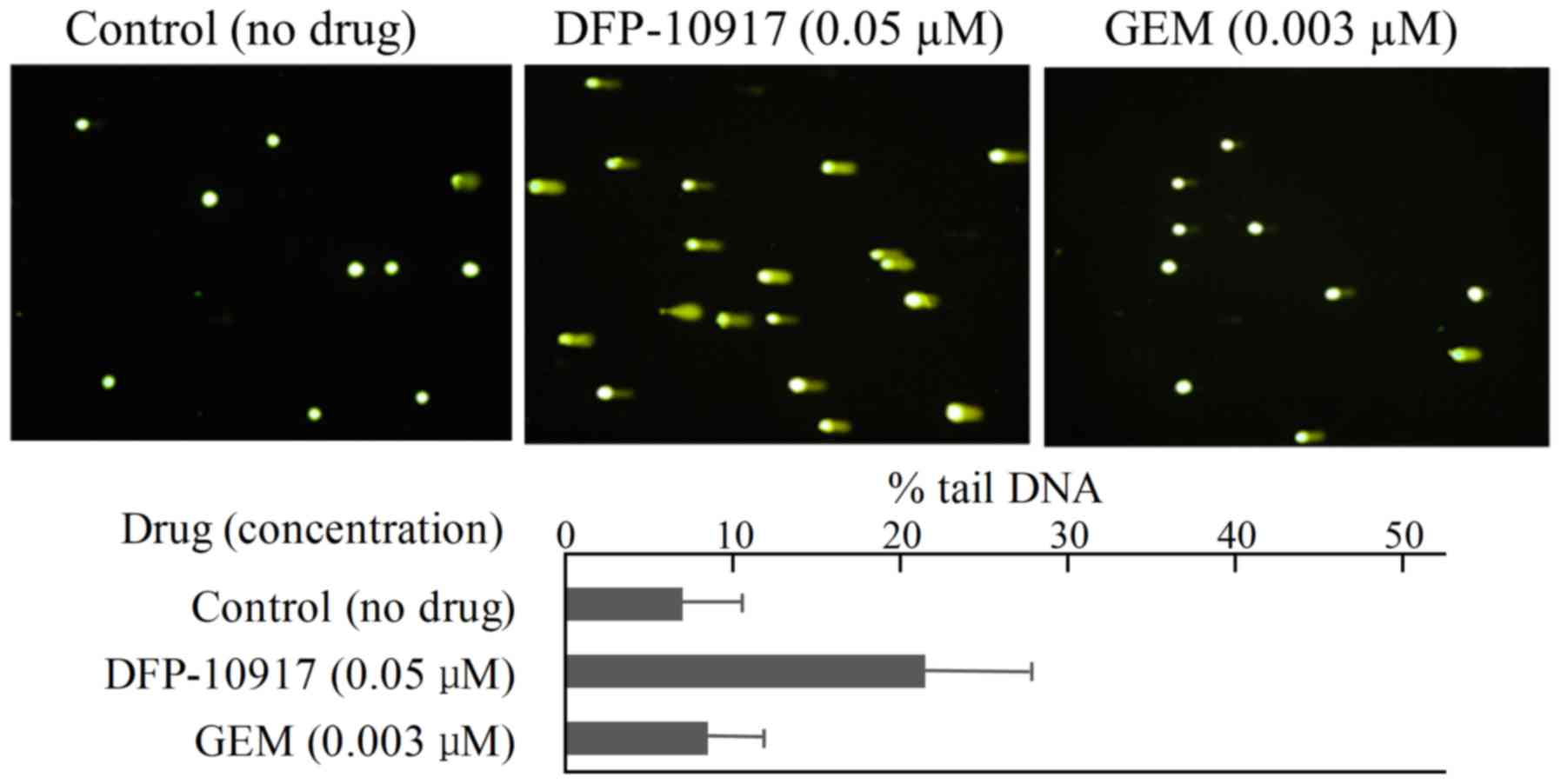
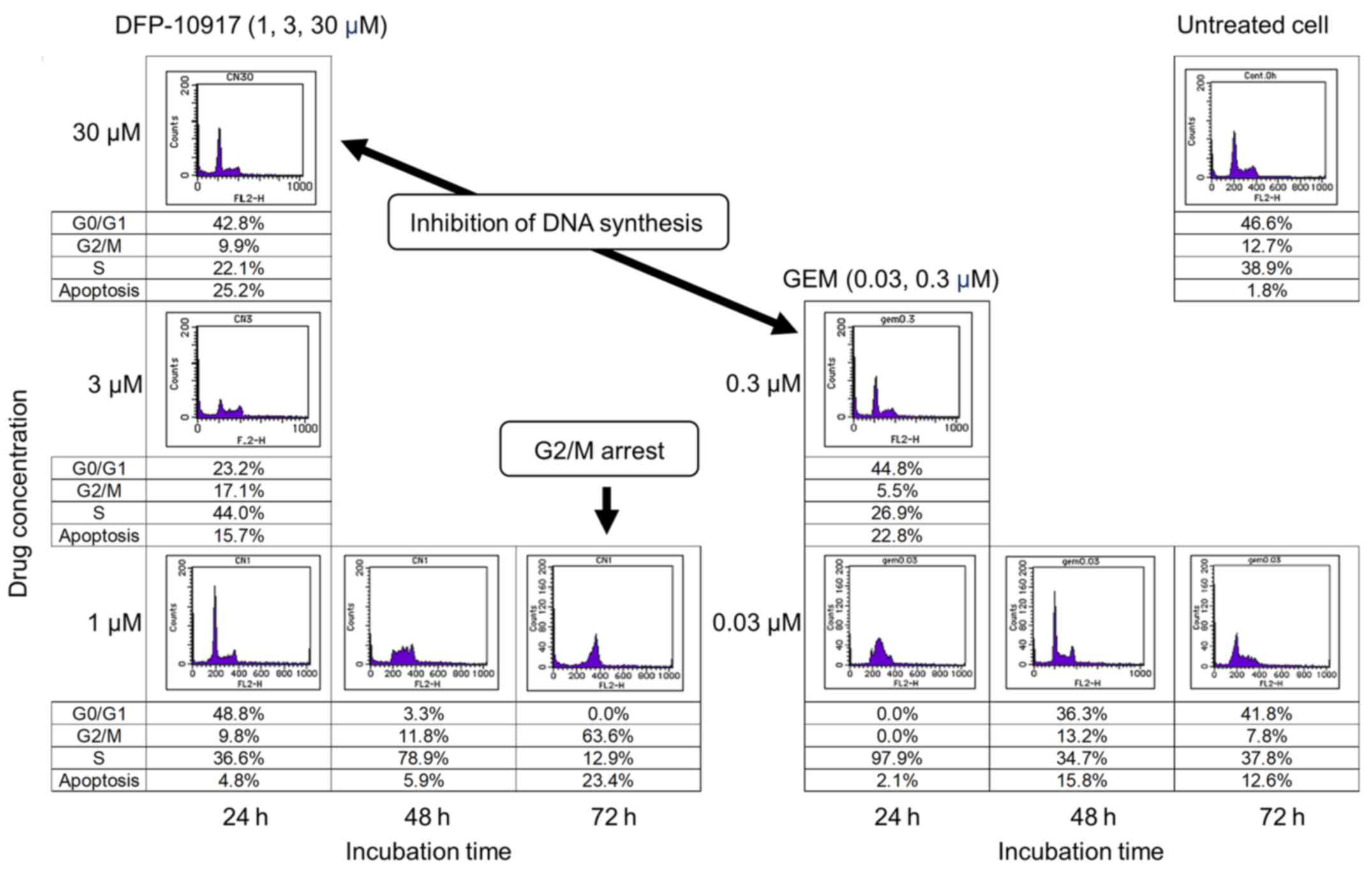
|
1
|
Noble S and Goa KL: Gemcitabine A review
of its pharmacology and clinical potential in non-small cell lung
cancer and pancreatic cancer. Drugs. 54:447–472. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barton-Burke M: Gemcitabine: A
pharmacologic and clinical overview. Cancer Nurs. 22:176–183. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toschi L, Finocchiaro G, Bartolini S,
Gioia V and Cappuzzo F: Role of gemcitabine in cancer therapy.
Future Oncol. 1:7–17. 2005. View Article : Google Scholar
|
|
4
|
Cole N and Gibson BE: High-dose cytosine
arabinoside in the treatment of acute myeloid leukaemia. Blood Rev.
11:39–45. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kern W and Estey EH: High-dose cytosine
arabinoside in the treatment of acute myeloid leukemia: Review of
three randomized trials. Cancer. 107:116–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reese ND and Schiller GJ: High-dose
cytarabine (HD araC) in the treatment of leukemias: A review. Curr
Hematol Malig Rep. 8:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Gong X, Sun M, Zhao X, Gong B, Wei
H, Mi Y and Wang J: High-dose cytarabine in acute myeloid leukemia
treatment: A systematic review and meta-analysis. PLoS One.
9:e1101532014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Somasekaram A, Jarmuz A, How A, Scott J
and Navaratnam N: Intracellular localization of human cytidine
deaminase. Identification of a functional nuclear localization
signal. J Biol Chem. 274:28405–28412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azuma A, Nakajima Y, Nishizono N, Minakawa
N, Suzuki M, Hanaoka K, Kobayashi T, Tanaka M, Sasaki T and Matsuda
A: Nucleosides and nucleotides. 122
2′-C-cyano-2′-deoxy-1-β-D-arabinofuranosylcytosine and its
derivatives A new class of nucleoside with a broad antitumor
spectrum. J Med Chem. 36:4183–4189. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azuma A, Hanaoka K, Kurihara A, Kobayashi
T, Miyauchi S, Kamo N, Tanaka M, Sasaki T and Matsuda A:
Nucleosides and nucleotides. 141. Chemical stability of a new
antitumor nucleoside,
2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine in
alkaline medium: Formation of
2′-C-cyano-2′-deoxy-1-β-D-ribo-pentofuranosylcytosine and its
antitumor activity. J Med Chem. 38:3391–3397. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka M, Matsuda A, Terao T and Sasaki T:
Antitumor activity of a novel nucleoside,
2′-C-cyano-2′-deoxy-1-β-D-arabinofuranosylcytosine (CNDAC) against
murine and human tumors. Cancer Lett. 64:67–74. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuma A, Huang P, Matsuda A and Plunkett
W: Cellular pharmacokinetics and pharmacodynamics of the
deoxycytidine analog
2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine (CNDAC).
Biochem Pharmacol. 61:1497–1507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayakawa Y, Kawai R, Otsuki K, Kataoka M
and Matsuda A: Evidence supporting the activity of
2′-C-cyano-2′-deoxy-1-β-D-arabino-pentafuranosylcytosine as a
terminator in enzymatic DNA-chain elongation. Bioorg Med Chem Lett.
8:2559–2562. 1998. View Article : Google Scholar
|
|
14
|
Azuma A, Huang P, Matsuda A and Plunkett
W: 2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine: A
novel anticancer nucleoside analog that causes both DNA strand
breaks and G(2) arrest. Mol Pharmacol. 59:725–731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Guo Y, Li Y, Jiang Y, Chubb S,
Azuma A, Huang P, Matsuda A, Hittelman W and Plunkett W: Molecular
basis for G2 arrest induced by
2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine and
consequences of checkpoint abrogation. Cancer Res. 65:6874–6881.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y and Liu X: M<atsuda A and
Plunkett W: Repair of
2′-C-cyano-2′-deoxy-1-β-D-arabino-pantofuranosylcytosine-induced
DNA single-starand breaks by transcription-coupled nucleotide
excision repair. Cancer Res. 68:3881–2889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Wang Y, Benaissa S, Matsuda A,
Kantarjian H, Estrov Z and Plunkett W: Homologous recombination as
a resistance mechanism to replication-induced double-strand breaks
caused by the antileukemia agent CNDAC. Blood. 116:1737–1746. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang P, Chubb S, Hertel LW, Grindey GB
and Plunkett W: Action of 2′,2′-difluorodeoxycytidine on DNA
synthesis. Cancer Res. 51:6110–6117. 1991.PubMed/NCBI
|
|
19
|
Jiang HY, Hickey RJ, Abdel-Aziz W and
Malkas LH: Effects of gemcitabine and araC on in vitro DNA
synthesis mediated by the human breast cell DNA synthesome. Cancer
Chemother Pharmacol. 45:320–328. 2000. View Article : Google Scholar
|
|
20
|
Miura S and Izuta S: DNA polymerases as
targets of anticancer nucleosides. Curr Drug Targets. 5:191–195.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao W, McNutt MA and Zhu WG: The comet
assay: A sensitive method for detecting DNA damage in individual
cells. Methods. 48:46–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Food and Drug Administration HHS: HHS
International conference on harmonisation; guidance on S9
nonclincal evaluation for anticancer pharmaceuticals; availability.
Fed Regist. 75:10487–10488. 2010.
|
|
23
|
Cook N, Hansen AR, Siu LL and Abdul Razak
AR: Early phase clinical trials to identify optimal dosing and
safety. Mol Oncol. 9:997–1007. 2015. View Article : Google Scholar :
|
|
24
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sandler AB, Nemunaitis J, Denham C, von
Pawel J, Cormier Y, Gatzemeier U, Mattson K, Manegold C, Palmer MC,
Gregor A, et al: Phase III trial of gemcitabine plus cisplatin
versus cisplatin alone in patients with locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 18:122–130.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spratlin J, Sangha R, Glubrecht D, Dabbagh
L, Young JD, Dumontet C, Cass C, Lai R and Mackey JR: The absence
of human equilibrative nucleoside transporter 1 is associated with
reduced survival in patients with gemcitabine-treated pancreas
adenocarcinoma. Clin Cancer Res. 10:6956–6961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho CC, Kuo SH, Huang PH, Huang HY, Yang
CH, Yang PC and Ho CC1: Caveolin-1 expression is significantly
associated with drug resistance and poor prognosis in advanced
non-small cell lung cancer patients treated with gemcitabine-based
chemotherapy. Lung Cancer. 59:105–110. 2008. View Article : Google Scholar
|
|
28
|
Veerman G, Ruiz van Haperen VW, Vermorken
JB, Noordhuis P, Braakhuis BJ, Pinedo HM and Peters GJ: Antitumor
activity of prolonged as compared with bolus administration of
2′,2′-difluorodeoxycytidine in vivo against murine colon tumors.
Cancer Chemother Pharmacol. 38:335–342. 1996. View Article : Google Scholar
|
|
29
|
Kirstein MN, Wieman KM, Williams BW,
Fisher JE, Marker PH, Le CT, Yee D and Kratzke RA: Short versus
continuous gemcitabine treatment of non-small cell lung cancer in
an in vitro cell culture bioreactor system. Lung Cancer.
58:196–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajdev L, Goldberg G, Hopkins U and
Sparano JA: A phase I trial of gemcitabine administered as a 96-h
continuous intravenous infusion in patients with advanced carcinoma
and lymphoma. Med Oncol. 23:369–376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tempero M, Plunkett W, Ruiz Van, Haperen
V, Hainsworth J, Hochster H, Lenzi R and Abbruzzese J: Randomized
phase II comparison of dose-intense gemcitabine: Thirty-minute
infusion and fixed dose rate infusion in patients with pancreatic
adenocarcinoma. J Clin Oncol. 21:3402–3408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cappuzzo F, Novello S, De Marinis F,
Selvaggi G, Scagliotti GV, Barbieri F, Maur M, Papi M, Pasquini E,
Bartolini S, et al: A randomized phase II trial evaluating standard
(50 mg/min) versus low (10 mg/min) infusion duration of gemcitabine
as first-line treatment in advanced non-small-cell lung cancer
patients who are not eligible for platinum-based chemotherapy. Lung
Cancer. 52:319–325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ho DH, Brown NS, Benvenuto J, McCredie KB,
Buckels D and Freireich EJ: Pharmacologic studies of continuous
infusion of arabinosylcytosine by liquid infusion system. Clin
Pharmacol Ther. 22:371–374. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kreis W, Chaudhri F, Chan K, Allen S,
Budman DR, Schulman P, Weiselberg L, Freeman J, Deere M and
Vinciguerra V: Pharmacokinetics of low-dose
1-beta-D-arabinofuranosylcytosine given by continuous intravenous
infusion over twenty-one days. Cancer Res. 45:6498–6501.
1985.PubMed/NCBI
|
|
35
|
Spriggs DR, Robbins G, Takvorian T and
Kufe DW: Continuous infusion of high-dose
1-beta-D-arabinofuranosylcytosine: A phase I and pharmacological
study. Cancer Res. 45:3932–3936. 1985.PubMed/NCBI
|
|
36
|
Donehower RC, Karp JE and Burke PJ:
Pharmacology and toxicity of high-dose cytarabine by 72-hour
continuous infusion. Cancer Treat Rep. 70:1059–1065.
1986.PubMed/NCBI
|
|
37
|
Spriggs DR, Sokal JE, Griffin J and Kufe
DW: Low-dose ara-C administered by continuous subcutaneous
infusion: A pharmacologic evaluation. Cancer Drug Deliv. 3:211–216.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bolwell BJ, Cassileth PA and Gale RP:
Low-dose cytosine arabinoside in myelodysplasia and acute
myelogenous leukemia: A review. Leukemia. 1:575–579.
1987.PubMed/NCBI
|
|
39
|
Stentoft J: The toxicity of cytarabine.
Drug Saf. 5:7–27. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stone RM, Spriggs DR, Dhawan RK, Arthur
KA, Mayer RJ and Kufe DW: A phase I study of intermittent
continuous infusion high-dose cytosine arabinoside for acute
leukemia. Leukemia. 4:843–847. 1990.PubMed/NCBI
|
|
41
|
Schiller G, Gajewski J, Nimer S, Territo
M, Ho W, Lee M and Champlin R: A randomized study of intermediate
versus conventional-dose cytarabine as intensive induction for
acute myelogenous leukaemia. Br J Haematol. 81:170–177. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fleming RA, Capizzi RL, Rosner GL, Oliver
LK, Smith SJ, Schiffer CA, Silver RT, Peterson BA, Weiss RB, Omura
GA, et al: Clinical pharmacology of cytarabine in patients with
acute myeloid leukemia: A cancer and leukemia group B study. Cancer
Chemother Pharmacol. 36:425–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bishop JF, Matthews JP, Young GA, Szer J,
Gillett A, Joshua D, Bradstock K, Enno A, Wolf MM, Fox R, et al: A
randomized study of high-dose cytarabine in induction in acute
myeloid leukemia. Blood. 87:1710–1717. 1996.PubMed/NCBI
|
|
44
|
Löwenberg B, Pabst T, Vellenga E, van
Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Biemond BJ,
Gratwohl A, et al Dutch-Belgian Cooperative Trial Group for
Hemato-Oncology (HOVON) and Swiss Group for Clinical Cancer
Research (SAKK) Collaborative Group: Cytarabine dose for acute
myeloid leukemia. N Engl J Med. 364:1027–1036. 2011. View Article : Google Scholar : PubMed/NCBI
|