Introduction
Lung cancer is a common malignant tumor type that
has become a major public health concern globally. In a survey of
85.5 million people in China in 2015, the number of lung cancer
cases was 7.333 million and the death toll was 6.102 million
(1). Considerable progress in lung
cancer treatment strategies, including surgery, radiation therapy
and chemotherapy, has recently been achieved (2). However, lung cancer is characterized
by high invasiveness, increased metastasis and drug resistance;
hence, the survival rate of patients with this disease is poor
(3). Thus, novel methods to treat
lung cancer are urgently needed.
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
~22 nucleotides long (4). miRNAs
have recently become a popular focus of cancer research due to
their ability to act as oncogenes or tumor suppressor genes. As
regulators of gene expression, miRNAs repress protein translation
or promote mRNA degradation by binding to the 3′-untranslated
region (3′-UTR) of their target mRNAs (5,6).
Selinexor may upregulate the expression of miR-145 by inhibiting
exportin 1, which controls the proliferation and invasiveness of
pancreatic cancer cells (7). This
finding demonstrates that miRNAs may act as tumor suppressors.
However, in gastric cancer cells, miR-181a-5p directly reduces the
expression of protein-tyrosine phosphatase MEG2, which functions as
a tumor suppressor gene, suggesting that this miRNA acts as an
oncogene (8).
miR-33a is located in the sterol regulatory
element-binding protein 2 gene of human chromosome 22 (9). miR-33a is the previous name of
miR-33a-5p, which, together with miR-33a-3p, derives from the same
pre-miRNA hairpin. miR-33a regulates the lipid balance effect by
decreasing ATP binding cassette subfamily A member 1 and ATP
binding cassette subfamily G member 1-mediated cholesterol efflux
(10). In addition, as a tumor
suppressor gene, miR-33a inhibits the proliferation and metastasis
of breast cancer cells by suppressing a disintegrin and
metalloproteinase domain 9 and ROS proto-oncogene 1 (4). Similarly, miR-33a may negatively
regulate twist family bHLH transcription factor 1expression and
inhibit lung cancer cellular metastasis in the SPC-A-1 and
NCL-H1299 cell lines (11).
The anticancer effect of miRNAs has recently become
a hot topic for research. Combining chemotherapeutic drugs with
miRNAs has resulted in synergistic anticancer effects. In
hepatocellular carcinoma (HCC) cells, miR-122 increases sensitivity
to adriamycin and vincristine (12). Similarly, miR-145 improves
sensitivity to paclitaxel (13).
Celastrol, as an active compound, is extracted from Tripterygium
wilfordii. Celastrol is an effective treatment for multiple
diseases, including inflammation, neuropathic pain and
atherosclerosis. Multiple studies have demonstrated that celastrol
can modulate multiple signaling pathways involved in tumorigenesis,
including tumor protein p53, androgen receptor/Ets transcription
factor/nuclear factor-κB and caspase (14–16).
Furthermore, celastrol has been reported to exhibit potential
therapeutic efficacy against various types of cancer, including
HCC, prostate and breast cancer (17–19).
However, the exact anticancer mechanism of celastrol has not been
fully elucidated. Based on the above studies, it was hypothesized
that combining celastrol and miRNAs may be more effective in cancer
treatment than either treatment alone. Therefore, the aim of the
present study was to explore the mechanisms through which celastrol
and miR-33a-5p may treat lung cancer. The results of the present
study could provide the basis for a novel therapeutic approach for
lung cancer.
Materials and methods
Lung adenocarcinoma tissues
Specimens of lung adenocarcinoma and paracarcinoma
normal tissues were collected from 14 patients (7 males and 7
females, aged 40–59 years, 4 patients in stage IB and 10 patients
in stage IIB) who were pathologically diagnosed with lung
adenocarcinoma at YantaiShan Hospital (Yantai, China) between March
5 and August 31, 2015. All patients were diagnosed for the first
time and had not received chemotherapy. Fresh tissues from the
patients were prepared for RNA analysis immediately following
surgery. All experiments were performed in accordance with the
relevant guidelines and approved by the Medical Ethics Committee of
Binzhou Medical University (Yantai, China). Prior to study
inclusion, written informed consent was obtained from all
patients.
Determination of miR-33a-5p expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Specimens of lung adenocarcinoma and paracarcinoma
tissues from 14 patients, and xenograft tumors from nude mice, were
collected and ground into powder in liquid nitrogen; the cultured
cells did not need to be homogenized. miRNAs from lung
adenocarcinoma cells or tissues were isolated using a miRNA kit
(Takara Bio, Inc., Otsu, Japan). After measuring the concentration
of miRNAs, poly(A) was added using poly(A) polymerase (Ambion;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Then,
PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio, Inc.) was
used to perform the RT reaction with primer
[5′-AACATGTACAGTCCATGGATGd(T)30N(A, G, C or T)-3′]. The SYBR Premix
Ex Taq kit (Takara Bio, Inc.) was used to perform qPCR with the
7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR conditions were as follows: initial
denaturation at 95°C for 30 sec; 40 cycles of 95°C for 10 sec, 60°C
annealing for 20 sec and extension at 72°C for 20 sec. Then,
fluorescence was detected at 585 nm. The primers used to amplify
miR-33a-5p were 5′-GTGCATTGTAGTTGCATT-3′ (forward) and
5′-AACATGTACAGTCCATGGATG-3′ (reverse). The primers for 5S rRNA were
5′-GCCATACCACCCTGAACG-3′ (forward) and 5′-AACATGTACAGTCCATGGATG-3′
(reverse). The human 5S rRNA gene served as the control. The
results were calculated using the 2−ΔΔCq value (20).
miRNA synthesis and vector
construction
miR-33a-5p mimics and negative control (nc)
oligonucleotides were chemically synthesized. The sequences of the
miR-33a-5p mimics were as follows: GUGCAUUGUAGUUGCAUUGCA (sense)
and UGCAAUGCAACUACAAUGCACUU (antisense). The sequences of the nc
oligonucleotides were as follows: CAGUACUUUUGUGUAGUACAA (sense) and
GUACUACACAAAAGUACUGUU (antisense). These sequences were inserted
into the pGCMV/EGFP/miR/blasticidin vector during the construction
of the miRNA overexpression vector. This part of the experiment was
performed by GenePharma Biotech Co., Ltd. (Shanghai, China).
Cell culture and transfection
Lung adenocarcinoma (LTEP-a-2 and A549) cells and
human bronchial epithelial (HBE) cells, which are all adherent
cells, were maintained in 1640 medium supplemented with 10% fetal
bovine serum (FBS) (both from Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin, and 100 μg/ml streptomycin at
37°C with 5% CO2 under saturation humidity. The
synthesized miRNA mimics were transfected when cells had reached
50–60% confluence. A total of 1 μg miRNA mimics was mixed
with 3 μl Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), in accordance with the manufacturer's
instructions. Cells were treated with 50 μM miRNA mimics.
Blasticidin (Solarbio Science and Technology Co., Ltd., Beijing,
China) was used to select antibiotic-resistant cells and to detect
cells that expressed miR-33a-5p stably. Cells with stable
miR-33a-5p expression were used for the xenograft experiments in
mice. In addition, 0.75 μM celastrol (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and 25 μM miRNA was used to detect
the effects of combined treatment. Celastrol was added when
changing the calf serum medium 6 h after transfection.
MTT assay to measure cell
proliferation
Logarithmic-phase cells (1×104) in each
well of 96-well plates were treated with miRNA or celastrol for 48
h. At 4 h before the end of incubation, 10 μl MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to each well. Then, 100
μl DMSO (Sigma-Aldrich; Merck KGaA) was added, and the plate
was shaken until MTT was dissolved. The optical density (OD) was
measured at 490 nm using an auto-microplate reader (Thermo Fisher
Scientific, Inc.) to compare the proliferation of each group of
cells.
Flow cytometry to assess cellular
apoptosis
Cells were treated with miRNA or celastrol for 48 h,
and the culture medium was discarded. The cells were digested from
the bottom of the culture flask with 0.25% trypsin enzyme without
EDTA, then centrifuged at 100 × g for 5 min. PBS was used to wash
the cells. The cellular apoptosis ratio was detected using Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
(BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA). Annexin
V-FITC (5 μl) was added to the collected cells. After
complete mixing, 5 μl PI was added. Finally,
1×104 cells were analyzed using a flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA). The data analysis was
performed using the CytExpert 1.2.11.0 software (Beckman Coulter,
Inc.).
Transwell cell migration assays
Cells were treated with miRNAs or celastrol for 24 h
and collected. Then, the cells were seeded into the upper chamber
(105 cells/well in 400 μl 1640 medium, FBS-free)
of Transwells (Corning Inc., Corning, NY, USA). The lower chamber
was filled with 600 μl 1640 medium supplemented with 20%
calf serum (Gibco; Thermo Fisher Scientific, Inc.). After 24 h, the
liquid in the upper chamber was removed with wet swabs, and the
upper surface was carefully washed with methanol to fix the cells.
Then, the cells that had traversed the membrane were stained with
0.1% crystal violet and counted under an inverted light microscope
(Leica Microsystems GmbH, Wetzlar, Germany). The migration
capability of the cells in vitro was assessed according to
the number of transmembrane cells. Average trans-membrane cells
number were determined in five ×200 fields.
Western blot analysis
Cells were lysed with lysis buffer (21), and all proteins were collected. The
xenograft tumors were collected, ground into powder in liquid
nitrogen, then lysed with lysis buffer to collect all proteins.
Then, 40 μg protein was loaded into individual lanes and
separated via SDS-PAGE. Subsequently, the proteins were transferred
onto polyvinylidene fluoride membranes (Sigma-Aldrich; Merck KGaA),
which were blocked with 7% nonfat milk in TBST for 2 h. After
washing with TBST, the membranes were incubated with rabbit
anti-human mTOR (1:800; cat. no. BS3611; Bioworld Technology,
Nanjing, China), rabbit anti-human phosphorylated (p)-p70 ribosomal
protein S6 kinase (p70S6K; 1:500; cat. no. BS4439), rabbit
anti-human p-eukaryotic translation initiation factor 4E binding
protein 1 (4EBP1; 1:500; cat. no. BS4746), and rabbit anti-human
GAPDH (1:6,000; cat. no. AP0063) (all from Bioworld Technology) in
TBST at 4°C overnight. Horseradish peroxidase-labeled goat
anti-rabbit Immunoglobulin G (1:6,000; cat. no. ZB-2301; Beijing
ZhongShan Golden Bridge Technology Co., Ltd., Beijing, China) was
added, and the samples were incubated for 2 h at room temperature.
Finally, images of the membranes were captured using a
chemiluminescent imager (Tanon Science and Technology Co. Ltd.,
Shanghai, China). The densities of the bands were analyzed using
Gel Image System 4.2 software (Tanon Science and Technology Co.
Ltd.).
Luciferase assays
mTOR-3′-UTR double-stranded DNA (203 bp) containing
an incomplete matched area of miR-33a-5p was synthesized and
inserted into the dual-luciferase reporter pmirGLO vector (Promega
Corp., Madison, WI, USA) by SacI/XhoI dual-enzyme
digestion (Takara Bio, Inc.). Thus, GP-miRGLO-mTOR-WT was
constructed. In addition, the nucleotide sequence was altered to
construct the GP-miRGLO-mTOR-MUT vector as the control. A549 and
LTEP-a-2 cells were transfected with miR-33a-5p and the
dual-luciferase reporter pmirGLO vector, which simultaneously
expressed the Firefly and Renilla luciferases. Cells were
collected after 48 h incubation, and 100 μl 1X passive lysis
buffer was added to each well. Then, 20 μl sample and 100
μl Luciferase Assay Reagent II were added to each well of
96-well white flat bottom plates. The activity of Firefly
lucif-erase in each well was detected using a luminescent detection
system (Tecan Group, Ltd., Mannedorf, Switzerland) read as M1.
Then, 100 μl 1X Stop & Glo reagent was added to each
well. The activity of Renilla luciferase was detected using
the luminescent detection system, read as M2. The ratio of M1/M2
was calculated, and the relative luciferase activity of each group
was evaluated.
Xenografts in mice
Cells were transfected with miR-33a-5p
overexpression vector and selected with blasticidin. A total of 16
BALB/c-nu/nu 5-6 week-old male mice with an average weight of 18-20
g (Charles River, Beijing, China) were randomly divided into 4
groups of 4 mice in each. They were kept in a laminar airflow
cabinet under specific pathogen-free conditions with a controlled
temperature (23±2°C), humidity (40–70%) with free access to food
and water. The cells were transplanted subcutaneously into the
right or left flanks of these mice. Tumors appeared after ~3 days.
The tumor volume was measured daily with calipers, with the
following formula: Tumor volume (mm3) = A x
B2/2, where 'A' and 'B' are the maximum and minimum
tumor diameters, respectively. When the tumor volume of the control
group increased to ~150 mm3, half of the mice from the
control and the miRNA-overexpressing groups were randomly selected.
Celastrol was injected into these selected mice at 2 mg/kg/day, 5
days/week. The other half of the groups was intraperitoneally
injected with saline as a control. Following drug treatments for 6
weeks, all of the mice were sacrificed for tumor isolation by
cervical vertebra dislocation. The tumors were then collected and
weighed. Subsequently, all of the tumors were used for RNA
extraction and total protein detection. All animal experiments were
approved by the Committee on the Ethics of Animal Experiments of
Binzhou Medical University and conducted based on the National
Institutes of Health (Bethesda, MD, USA) Guide for the Care and Use
of Laboratory Animals.
Statistical analysis
SPSS v22.0 software (IBM Corp., Armonk, NY, USA) was
utilized for statistical analysis. Student's t-test and one-way
analysis of variance followed by a Tukey's test were used to
compare variables among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
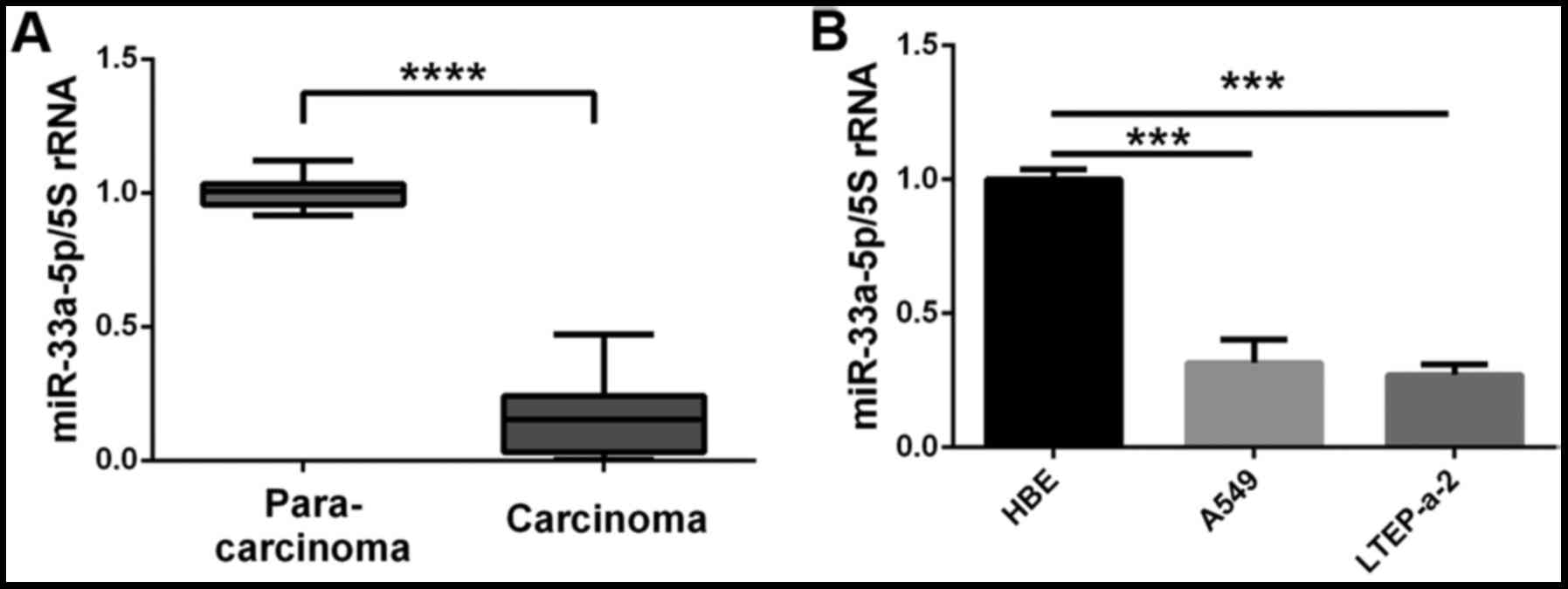
Expression of miR-33a-5p is low in lung
cancer tissues and cells
The expression of miR-33a-5p was measured in lung
tumors and the corresponding adjacent tissues to examine a
potential role of miR-33a-5p on the development of lung
adenocarcinoma. The results demonstrated that the expression levels
of miR-33a-5p were significantly lower in lung cancer tissues
compared with the corresponding adjacent tissues (Fig. 1A). When examining cell lines, the
expression levels of miR-33a-5p were lower in the lung
adenocarcinoma cell lines LTEP-a-2 and A549 compared with the
normal HBE cells (Fig. 1B). Thus,
downregulation of miR-33a-5p expression is likely to be involved in
the development of lung adenocarcinoma.
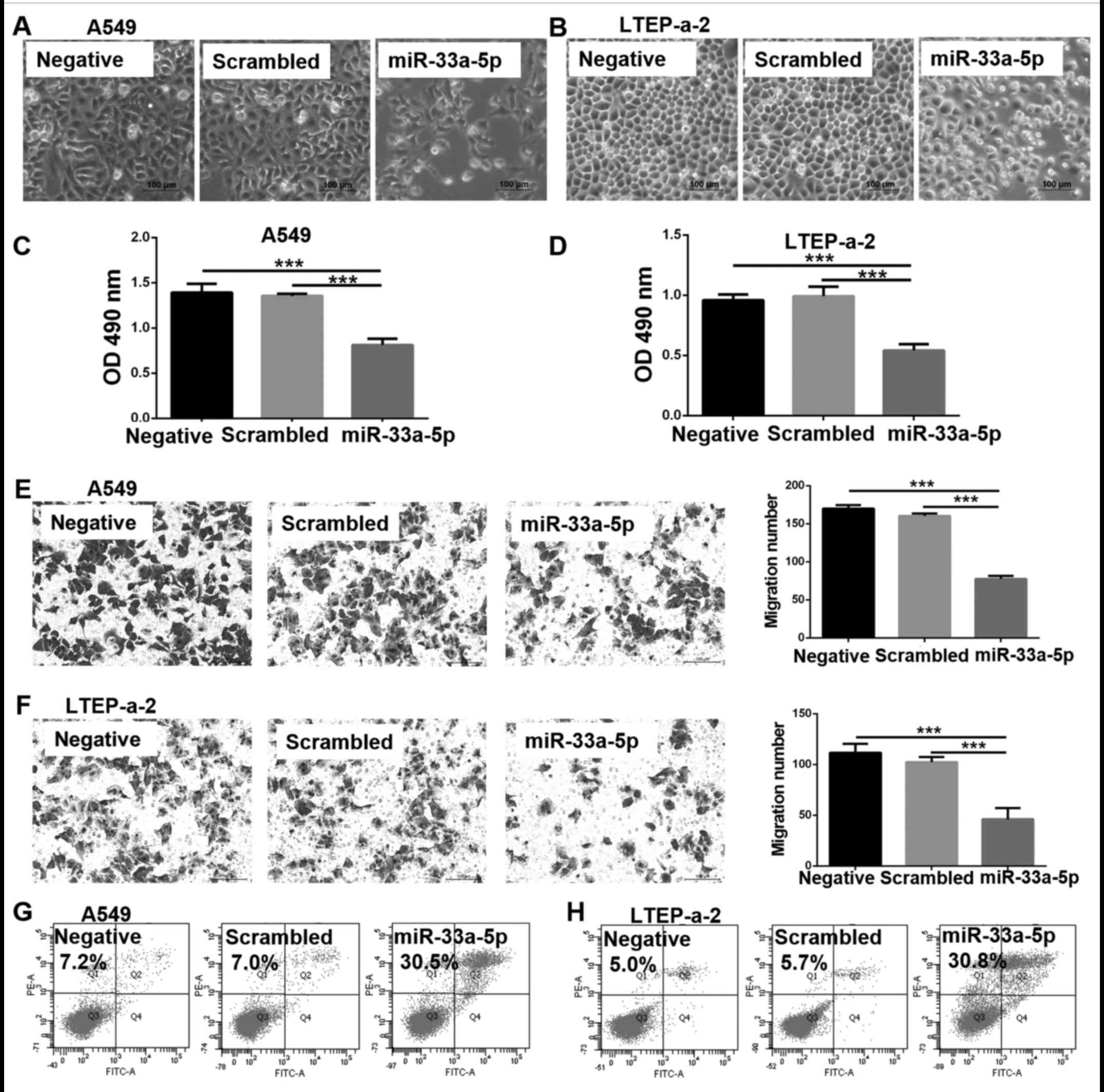
miR-33a-5p inhibits the proliferation of
lung adenocarcinoma cells
Considering the decrease in miR-33a-5p expression in
lung adenocarcinoma tissues and cells, the effect of miR-33a-5p on
the proliferation of lung adenocarcinoma cells was examined in
vitro. At 48 h following transfection of miR-33a-5p mimics into
A549 and LTEP-a-2 cells, a lower number of cells was observed in
the miR-33a-5p overexpression group compared with the scrambled
control group, in both the cell lines tested (Fig. 2A and B). In addition, MTT assay was
used to measure cell viability. The OD value of the overexpression
group was decreased compared with the scrambled control group
(Fig. 2C and D), which indicated
that cell proliferation in the overexpression group was inhibited.
The results of the Transwell cell migration assays demonstrated
that the migration ability of A549 and LTEP-a-2 cells was
significantly inhibited following miR-33a-5p overexpression
(Fig. 2E and F). Furthermore,
miR-33a-5p overexpression enhanced apoptosis; the % of apoptotic
cells was over four times higher in the miR-33a-5p overexpression
group compared with the scrambled control group for both the A549
(Fig. 2G) and LTEP-a-2 (Fig. 2H) cells.
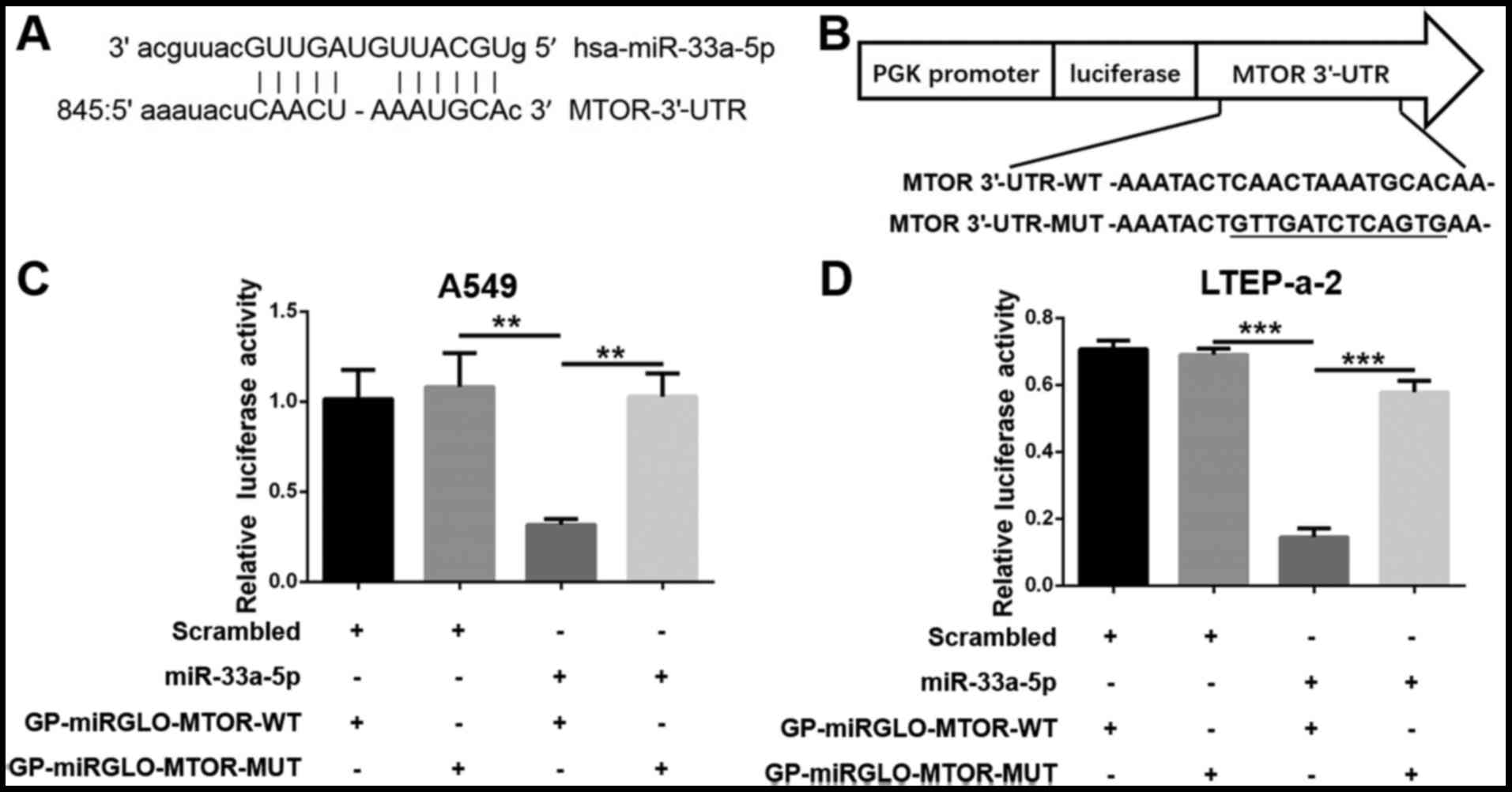
mTOR is a direct target of
miR-33a-5p
A miRNA could inhibit the proliferation of lung
adenocarcinoma cells by modulating the expression of its target
genes. Therefore, predicted target genes were examined for
miR-33a-5p by bioinformatics analysis (www.microrna.org/microrna/getMirnaForm.do and
www.targetscan.org/index.html) and
matching sites were identified on the 3′-UTR of the mTOR gene
(Fig. 3A). According to the
mechanism of miRNA function, miR-33a-5p likely affects the
expression of mTOR by binding to the mTOR-3′-UTR. Wild-type (WT)
mTOR-3′-UTR was cloned downstream of the Firefly luciferase gene to
construct the GP-miRGLO-mTOR-WT dual-luciferase vector. In
addition, the predicted binding sites (852-863 bp) were replaced
with the complementary sequences to construct the mutant (MUT)
GP-miRGLO-mTOR-MUT vector (Fig.
3B). The cells were transfected with the GP-miRGLO-mTOR-WT or
GP-miRGLO-mTOR-MUT vector, together with miR-33a-5p mimics or
scrambled control. Luciferase activity was detected after 48 h
incubation. The results demonstrated that the relative luciferase
activity significantly decreased in the cells transfected with the
GP-miRGLO-mTOR-WT vector and miR-33a-5p mimics (Fig. 3C and D). No notable difference was
observed between cells transfected with GP-miRGLO-mTOR-MUT vector
and miR-33a-5p mimics (Fig. 3C and
D).
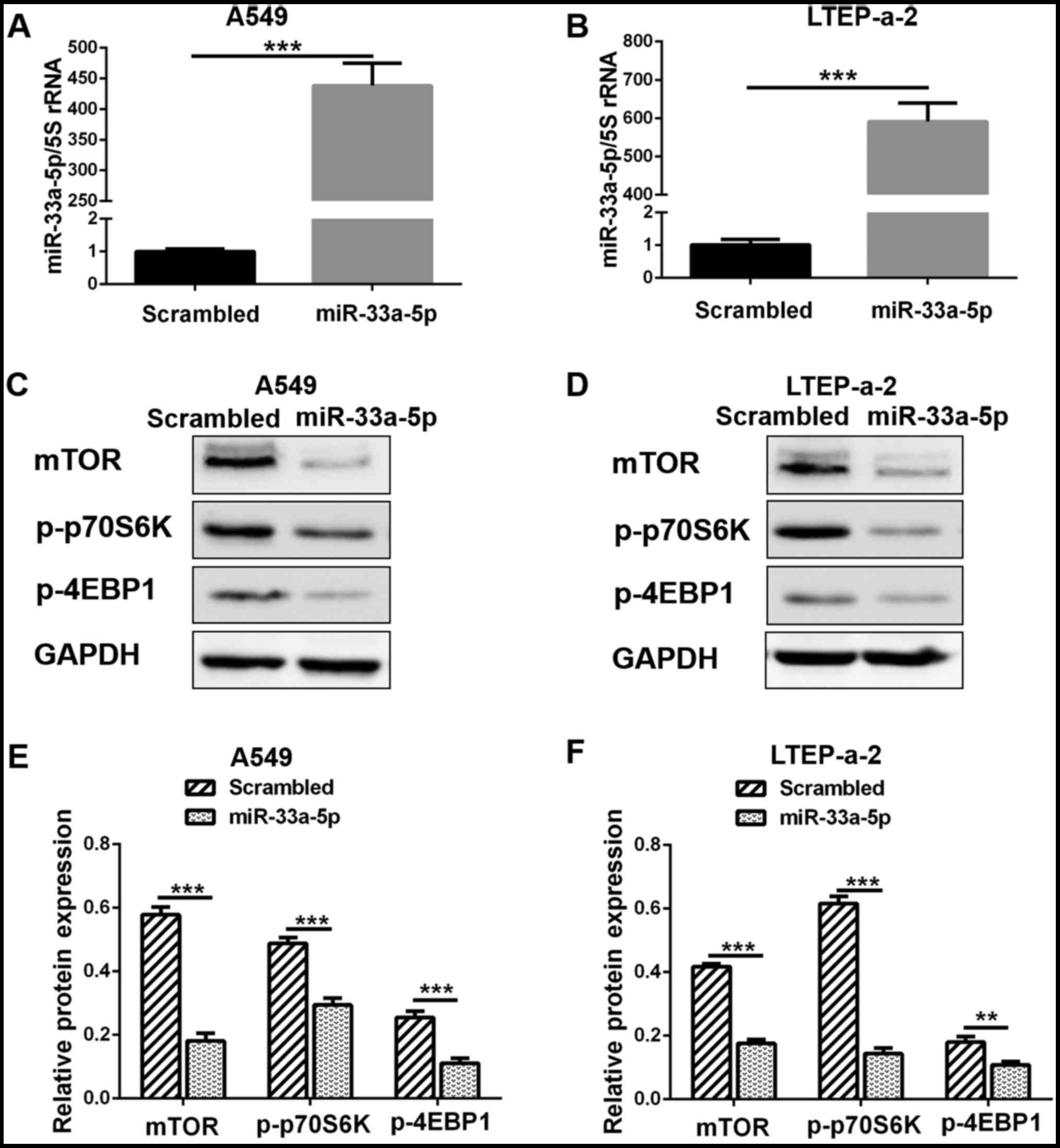
miR-33a-5p negatively regulates mTOR and
downstream effector expression in lung adenocarcinoma cells
The effect of miR-33a-5p transfection on mTOR
expression in lung adenocarcinoma cells was determined. RT-qPCR
analysis confirmed that the expression levels of miR-33a-5p
significantly increased in the A549 and LTEP-a-2 cells following
mimics transfection (Fig. 4A and
B). The results from the western blot analysis of the
transfected cells indicated that the protein expression levels of
mTOR were significantly inhibited following miR-33a-5p
overexpression (Fig. 4C–F).
Furthermore, the protein expression levels for the downstream
effectors of mTOR, p-p70S6K and p-4EBP1, were significantly
decreased following miR-33a-5p overexpression (Fig. 4C–F). These results indicated that
miR-33a-5p inhibited the proliferation of lung adenocarcinoma cells
via the mTOR signaling pathway.
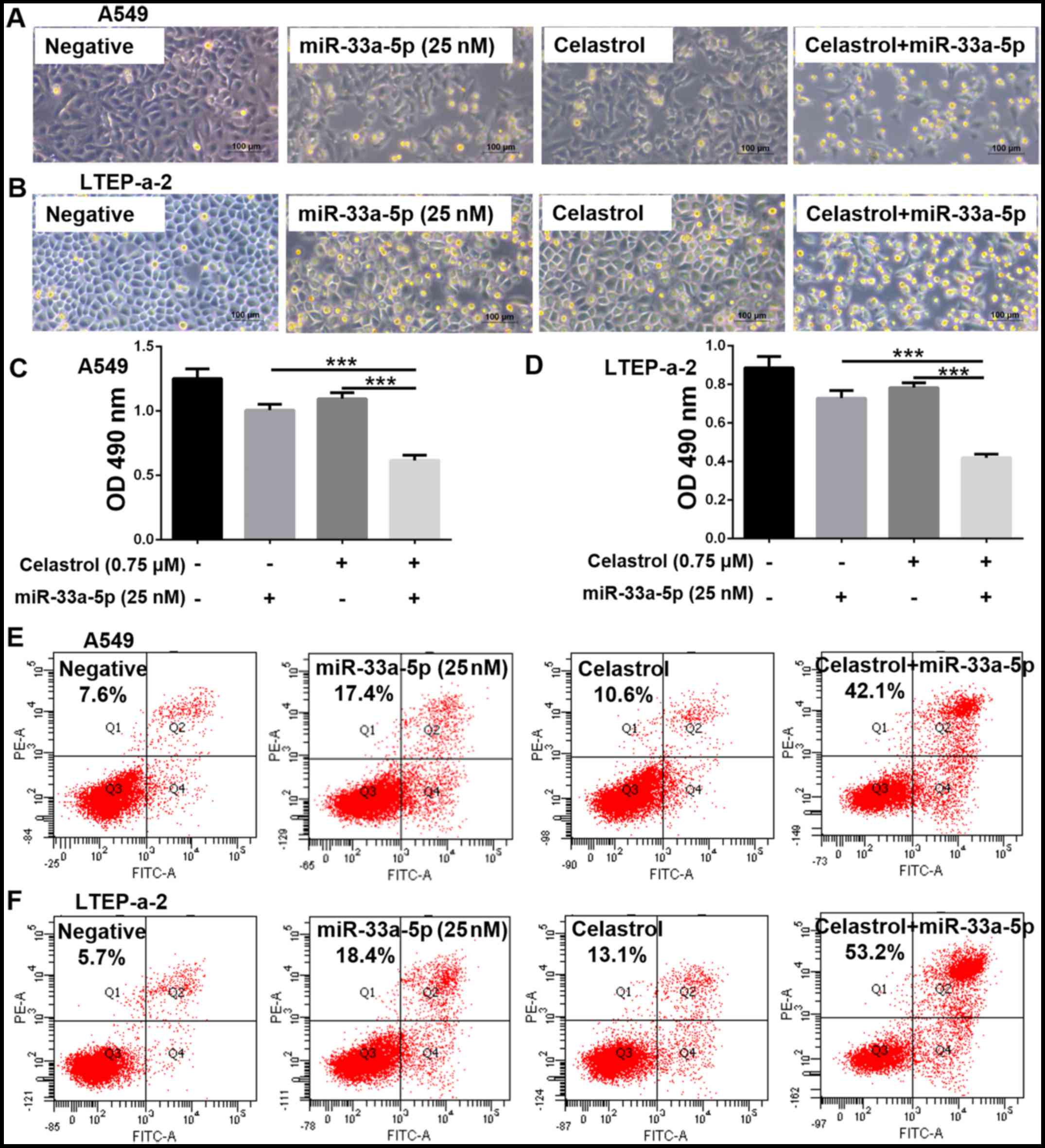
miR-33a-5p enhances cellular sensitivity
to celastrol
Celastrol treatment was combined with miR-33a-5p
overexpression and microscopic observation of the cells revealed
that the number of visible living cells was significantly reduced
in the combination treatment group compared with the negative
control group, and the single-use 0.75 μM celastrol or 25 nM
miR-33a-5p groups (Fig. 5A and B).
In the MTT assay, the OD of the combination treatment group was
lower compared with the other groups, which indicated a reduction
in cell viability (Fig. 5C and D).
Therefore, the combination treatment inhibited cell proliferation
more efficiently than either treatment alone. The % of apoptotic
cells in the A549 and LTEP-a-2 cells was ~50% in the combination
treatment group, but <20% in the single treatment groups
(Fig. 5E and F).
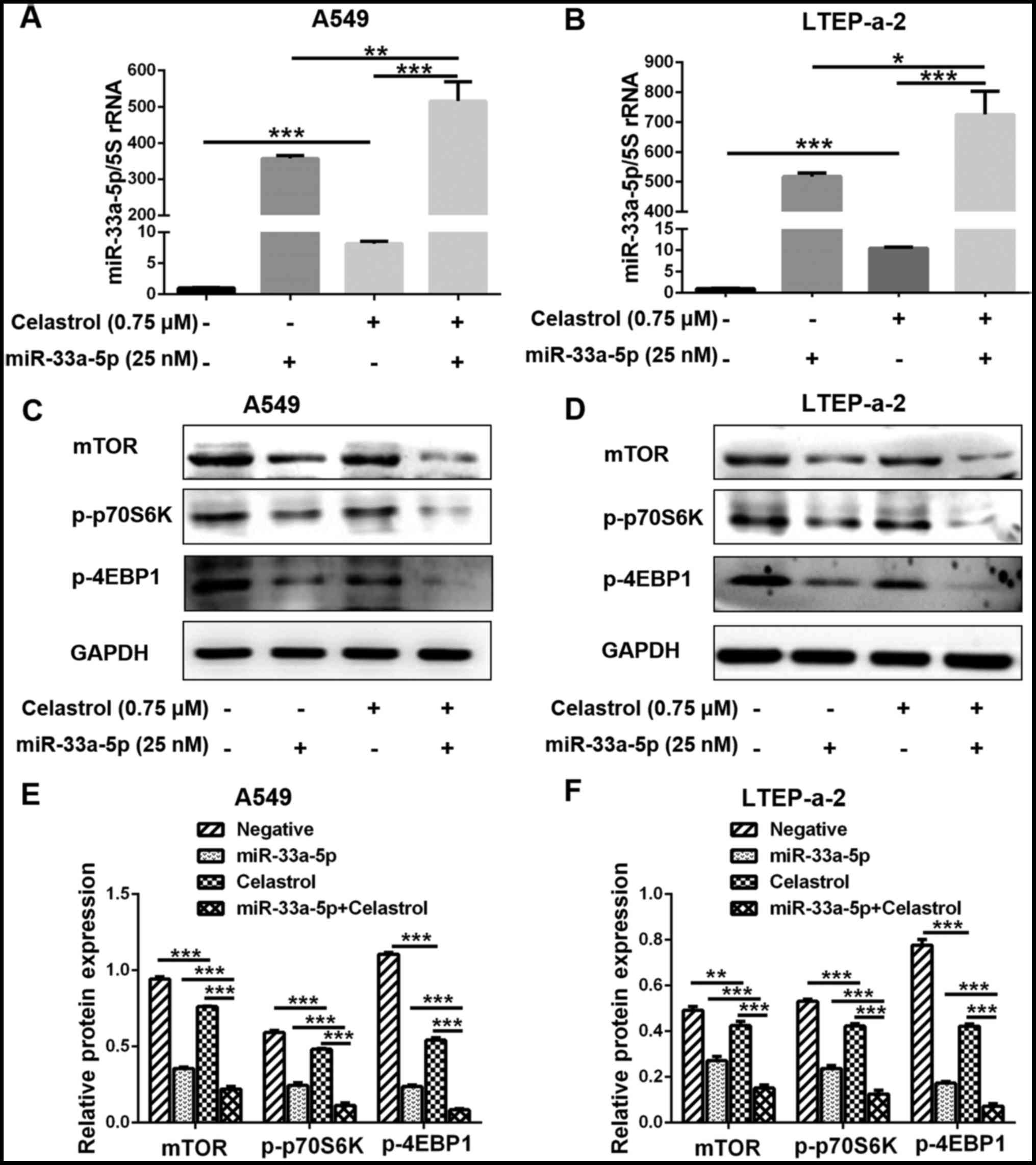
Combination of celastrol and miR-33a-5p
increases the expression of miR-33a-5p to inhibit the mTOR
signaling pathway
The expression levels of miR-33a-5p were examined by
RT-qPCR, in order to explore the mechanism by which miR-33a-5p
enhances the sensitivity of lung adenocarcinoma cells to celastrol.
Although miR-33a-5p expression was increased in the celastrol alone
group, it was considerably higher in the celastrol and miR-33a-5p
combination treatment group (Fig. 6A
and B). Furthermore, results from western blot analysis
demonstrated that the expression of mTOR was markedly decreased in
the combination treatment group as the expression of miR-33a-5p
increased (Fig. 6D–F). Similarly,
p-p70S6K and p-4EBP1 expression was also further decreased in the
combination treatment group (Fig.
6D–F). These results suggested that mTOR was involved in the
mechanism by which miR-33a-5p enhanced the sensitivity of lung
adenocarcinoma cells to celastrol. The results were similar in A549
(Fig. 6C and E) and LTEP-a-2
(Fig. 6D and F) cells.
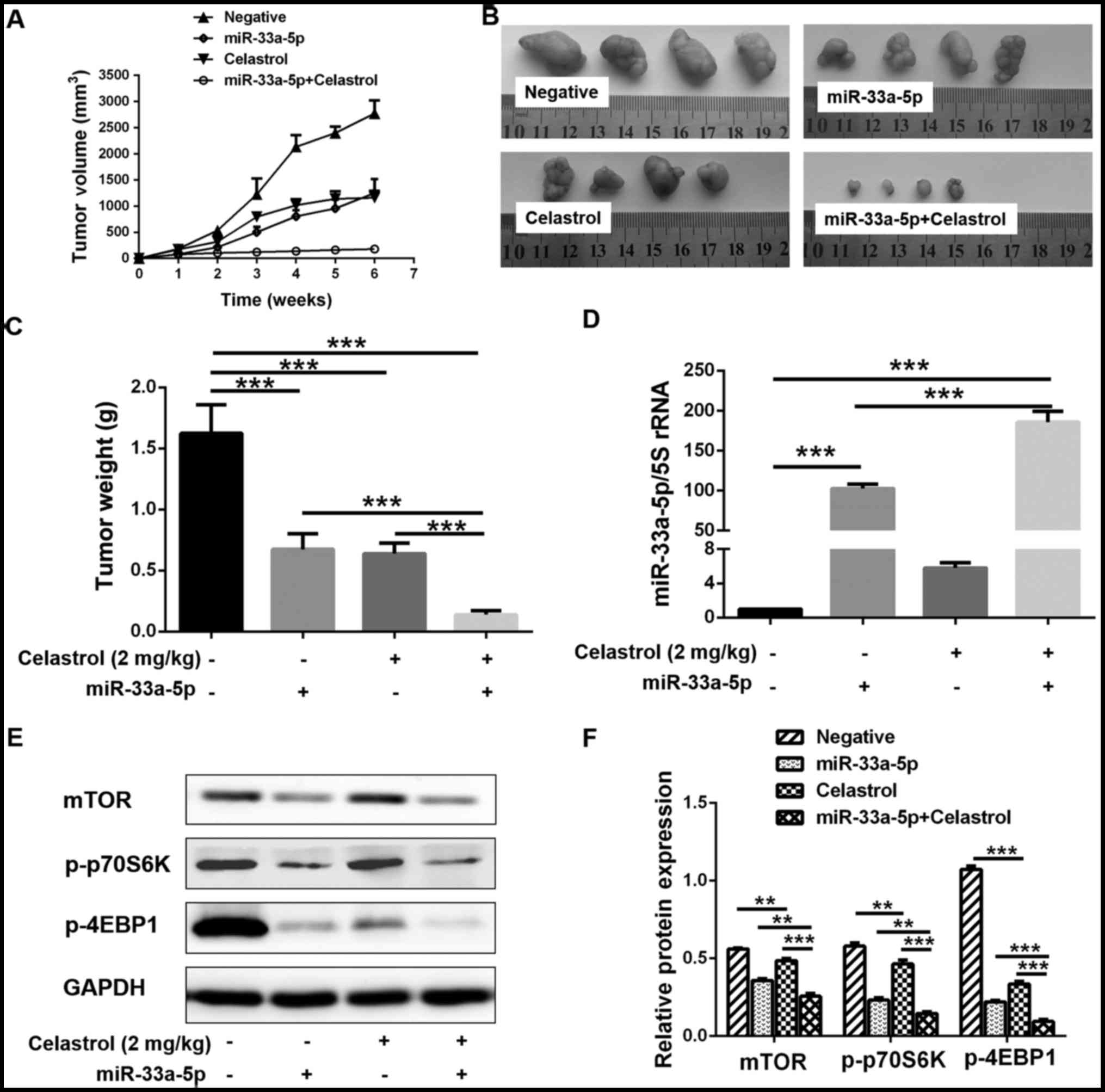
Combination of celastrol and miR-33a-5p
inhibits LTEP-a-2 cell growth in vivo
Parental untreated LTEP-a-2 cells (negative) or
LTEP-a-2 cells stably transfected with miR-33a-5p overexpression
vector (miR-33a-5p) were injected into the flanks of nude mice.
Mice were then administered with celastrol or saline treatment to
verify that the combination of celastrol and miR-33a-5p could
inhibit LTEP-a-2 cell growth in vivo. The growth curve of
the xenograft tumors revealed that the tumor volume increased with
prolonged incubation time in the negative control group. The tumor
growth rate was considerably lower in the celastrol treatment alone
group or the miR-33a-5p overexpression alone group compared with
the negative control group. In the combination treatment group, the
tumor growth was almost completely arrested after 2 weeks (Fig. 7A). The xenograft tumors were
extracted at the end of the experiment and the volumes and weight
measurements of the xenograft tumors varied among the different
groups (Fig. 7B and C). The
xenograft tumors in the combination treatment group were the
smallest (Fig. 7B and C). RT-qPCR
analysis of the xenograft tumors indicated that the cells stably
transfected with the miR-33a-5p overexpression vector maintained a
higher expression level of miR-33a-5p compared with the parental
cells (Fig. 7D). Celastrol
treatment further enhanced the expression of miR-33a-5p (Fig. 7D), which was consistent with the
in vitro experiments. Finally, similarly to the in
vitro experiments, higher expression levels of miR-33a-5p
resulting from the combination treatment significantly inhibited
the expression of mTOR and its downstream effectors in the
xenograft tumors (Fig. 7E and
F).
Discussion
As small noncoding RNA molecules, miRNAs have vital
roles in various cellular processes during cancer development, such
as proliferation, apoptosis, invasion and migration (22,23).
miRNAs may function as tumor oncogenes or suppressors (24). As an oncogene, miR-374a
overexpression significantly promotes HCC cell viability compared
with control. Further investigation indicated that miR-374a
enhances HCC proliferation by targeting mitogen-inducible gene 6
(25). Conversely, researchers
have discovered that miRNAs may act as tumor suppressors by
inhibiting proliferation and promoting apoptosis. miR-133b
expression is significantly lower in colorectal cancer (CRC)
samples or cell lines compared with normal controls. In addition,
transfection with miR-133b can markedly hinder CRC cell
proliferation and invasion in vitro and in vivo
(26). The expression of
miR-140-5p is reduced five-fold in breast cancer tissues compared
with adjacent normal tissues, and is associated with advanced
clinical procedure and poor prognosis (27). In the present study, miR-33a-5p
expression was demonstrated to be decreased in lung adenocarcinoma
tissues compared with normal adjacent tissues. Similarly,
miR-33a-5p expression was reduced in lung adenocarcinoma cell lines
compared with normal HBE cells. These results indicated miR-33a-5p
may act as a tumor suppressor gene.
Previous studies have reported that miR-33a-5p has a
critical role in tumor growth and proliferation. Wang et al
(28) determined that miR-33a acts
as a cell proliferation suppressor in gastric cancer by targeting
CDK6, cyclin D1 and Pim-1 proto-oncogene serine/threonine protein
kinase. However, other researchers have reported different findings
on the function of miR-33a-5p in tumors. miR-33a promotes the
proliferation and inhibits the apoptosis of liver cancer cells by
targeting peroxisome proliferator-activated receptor-α (29). In order to explore the function of
miR-33a-5p in lung adenocarcinoma, lung adenocarcinoma cells were
transfected with miR-33a-5p mimics. The results demonstrated that
upregulating the expression of miR-33a-5p could markedly inhibit
the growth of the lung adenocarcinoma cell lines.
It is widely believed that miRNAs can regulate
protein expression by binding to the 3′-UTR of mRNA (8,30,31).
mTOR is a serine/threonine kinase that is involved in the growth
and proliferation of cancer cells (32). Previous studies have demonstrated
that mTOR can promote the activation of p70S6K and the
phosphorylation of 4E-BP1, which is closely related to tumor growth
and proliferation (33,34). In the present study, miRNA target
prediction software was employed and a luciferase reporter assay
was conducted to further analyze the relationship between
miR-33a-5p and mTOR. The miRNA target prediction software indicated
that multiple miR-33a-5p binding sites are located in the mTOR
3′-UTR. miR-33a-5p was demonstrated to directly target mTOR, as
evidenced by the luciferase reporter assay results. In addition,
overexpression of miR-33a-5p significantly decreased the protein
expression levels of mTOR, thereby weakening the phosphorylation of
p70S6K and 4EBP1. Other studies have demonstrated that many other
proteins can also be targeted by miR-33a-5p. As a tumor suppressor,
miR-33a can downregulate PIM1 by directly targeting its 3′-UTR in
prostate cancer (35). Similarly,
in melanoma cells, miR-33a, whose target is CDK16, is an important
negative regulator of cell proliferation (36). In addition, as a bone metastasis
suppressor in lung cancer, miR-33a targets parathyroid
hormone-related protein (37).
As a chemotherapeutic drug, celastrol not only
inhibits cancer cell proliferation, but also regulates miRNA
expression. In prostate cancer cells, celastrol downregulates
miR-17-92a, which can lead to autophagy induction (38). Similarly, celastrol inhibits HCC
cell migration and invasion by regulating miR-224 expression
(39). In the current study,
miR-33a-5p was demonstrated to be upregulated in lung
adenocarcinoma cells following celastrol treatment. Therefore, we
focused on the effect of the combined celastrol and miR-33a-5p
treatment on lung adenocarcinoma cells. There is considerable
evidence supporting the benefits of combining chemotherapeutic
drugs and miRNAs. miR-223 improved the sensitivity of gallbladder
cancer cells to docetaxel by downregulating stathmin 1 (40). Similarly, miR-101 enhances the
cytotoxic effects of fluorouracil and cisplatin by inhibiting the
proliferation of colon cancer cells (41). In the present study, compared with
the miR-33a-5p or celastrol alone groups, the group administered
with combined miR-33a-5p and celastrol treatments exhibited higher
proliferation inhibition and apoptosis rates, as determined by MTT
and flow cytometry analyses, respectively. Western blot analysis
demonstrated that the combination treatment group also had low
expression levels of mTOR and its downstream effectors. Similar
results were observed in the xenografted animal model. The present
data suggest that combined treatment with celastrol and miR-33-5p
exerted a more notable effect on lung adenocarcinoma compared with
either treatment alone. However, isobologram analysis was not
performed in the present study, as the IC50 of the
miRNA-mediated inhibitory effect on cell growth is difficult to
calculate; similarly, other previous studies examining the
combination of miRNA and chemical in the treatment of cancer also
did not perform isobologram analysis (40,42).
In conclusion, miR-33a-5p overexpression inhibited
the proliferation of lung adenocarcinoma cells and enhanced the
anticancer effects of celastrol. In addition, miR-33a-5p improved
the sensitivity of lung adenocarcinoma cells to celastrol by
targeting the mTOR signaling pathway. Therefore, a combination of
miR-33a-5p and celastrol treatment may be a promising therapeutic
strategy for patients with lung adenocarcinoma.
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
mTOR
|
mechanistic target of rapamycin
|
|
UTR
|
untranslated region
|
|
p70S6K
|
p70 ribosomal protein S6 kinase
|
|
4EBP1
|
eukaryotic translation initiation
factor 4E binding protein 1
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
DMSO
|
dimethyl sulfoxide
|
|
OD
|
optical density
|
|
HCC
|
hepatocellular carcinoma
|
|
CRC
|
colorectal cancer
|
|
CDK
|
cyclin-dependent kinase
|
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the National
Natural Science Foundation (grant nos. 31371321, 81572735 and
81772281), the Shandong Science and Technology Committee (grant
nos. ZR2014HQ079, ZR2014HL056 and ZR2013HL003), and the Foundation
of Shandong Educational Committee (grant nos. J17KA121 and
J13LE11).
[2] Availability
of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
[3] Author's
contributions
YJL, WC and SYX conceived and designed the
experiments. YJL, YXS, RMH, PW, LJZ, XM, YM, PYW and NX performed
the experiments. YJL, PYW, SYX and WC analyzed the data. YJL, YXS,
SYX and WC wrote the manuscript. All authors have read and approved
the final manuscript.
[4] Ethics
approval and consent to participate
All experiments with human specimens were performed
in accordance with the relevant guidelines and approved by the
Medical Ethics Committee of Binzhou Medical University (Yantai,
China). Prior to study inclusion, written informed consent was
obtained from all patients. All animal experiments were approved by
the Committee on the Ethics of Animal Experiments of Binzhou
Medical University and conducted based on the National Institutes
of Health (Bethesda, MD, USA) Guide for the Care and Use of
Laboratory Animals.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: Non-small cell lung cancer, version 1. J Natl
Compr Canc Netw. 12:1738–1761. 2015. View Article : Google Scholar
|
|
3
|
Tan CS, Gilligan D and Pacey S: Treatment
approaches for EGFR-inhibitor-resistant patients with
non-small-cell lung cancer. Lancet Oncol. 16:e447–e459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang C, Zhang Y, Ding W, Lin Y, Huang Z
and Luo Q: MiR-33a suppresses breast cancer cell proliferation and
metastasis by targeting ADAM9 and ROS1. Protein Cell. 6:881–889.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pu M, Li C, Qi X, Chen J, Wang Y, Gao L,
Miao L and Ren J: MiR-1254 suppresses HO-1 expression through seed
region-dependent silencing and non-seed interaction with TFAP2A
transcript to attenuate NSCLC growth. PLoS Genet. 13:e10068962017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo X, Zhu Y and Hong X, Zhang M, Qiu X,
Wang Z, Qi Z and Hong X: miR-181d and c-myc-mediated inhibition of
CRY2 and FBXL3 reprograms metabolism in colorectal cancer. Cell
Death Dis. 8:e29582017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azmi AS, Li Y, Muqbil I, Aboukameel A,
Senapedis W, Baloglu E, Landesman Y, Shacham S, Kauffman MG, Philip
PA, et al: Exportin 1 (XPO1) inhibition leads to restoration of
tumor suppressor miR-145 and consequent suppression of pancreatic
cancer cell proliferation and migration. Oncotarget. 8:82144–82155.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Sun F, Hong Y, Liu Y, Fen M, Yin K,
Ge X, Wang F, Chen X and Guan W: MEG2 is regulated by miR-181a-5p
and functions as a tumour suppressor gene to suppress the
proliferation and migration of gastric cancer cells. Mol Cancer.
16:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Najafi-Shoushtari SH, Kristo F, Li Y,
Shioda T, Cohen DE, Gerszten RE and Näär AM: MicroRNA-33 and the
SREBP host genes cooperate to control cholesterol homeostasis.
Science. 328:1566–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao M, Lei H, Liu Q, Chen Y, Zhao L, Li Q,
Luo S, Zuo Z, He Q, Huang W, et al: Effects of miR-33a-5P on
ABCA1/G1-mediated cholesterol efflux under inflammatory stress in
THP-1 macrophages. PLoS One. 9:e1097222014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Yang J, Li J, Shen X, Le Y, Zhou
C, Wang S, Zhang S, Xu D and Gong Z: MircoRNA-33a inhibits
epithelial-to-mesenchymal transition and metastasis and could be a
prognostic marker in non-small cell lung cancer. Sci Rep.
5:136772015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Xia F, Ma L, Shan J, Shen J, Yang Z,
Liu J, Cui Y, Bian X, Bie P, et al: MicroRNA-122 sensitizes HCC
cancer cells to adri-amycin and vincristinethrough modulating
expression of MDR and inducing cell cycle arrest. Cancer Lett.
310:160–169. 2011.PubMed/NCBI
|
|
13
|
Zhu X, Li Y, Xie C, Yin X, Liu Y, Cao Y,
Fang Y, Lin X, Xu Y, Xu W, et al: miR-145 sensitizes ovarian cancer
cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer.
135:1286–1296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang R, Gu X, Dai W, Ye J, Lu F, Chai Y,
Fan G, Gonzalez FJ, Duan G and Qi Y: A lipidomics investigation
into the intervention of celastrol in experimental colitis. Mol
Biosyst. 12:1436–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Li Y, Ren J, Zhu C, Fu J, Lin D
and Qiu Y: Celastrol attenuates inflammatory and neuropathic pain
mediated by cannabinoid receptor type 2. Int J Mol Sci.
15:13637–13648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y,
Meng G, Xie L, Wang J, Xiao Y, et al: Celastrol prevents
atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS
One. 8:e654772013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang W, He W, Li PP, Song SS, Yuan PF, Lu
JT and Wei W: Protective effects of celastrol on
diethylnitrosamine-induced hepatocellular carcinoma in rats and its
mechanisms. Eur J Pharmacol. 784:173–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao L, Zhou Z, Cai Y, Castro P, Dakhov O,
Shi P, Bai Y, Ji H, Shen W and Wang J: Celastrol suppresses tumor
cell growth through targeting an AR-ERG-NF-κB pathway in
TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS One.
8:e583912013. View Article : Google Scholar
|
|
19
|
Yang HS, Kim JY, Lee JH, Lee BW, Park KH,
Shim KH, Lee MK and Seo KI: Celastrol isolated from Tripterygium
regelii induces apoptosis through both caspase-dependent and
-independent pathways in human breast cancer cells. Food Chem
Toxicol. 49:527–532. 2011. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) methods. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Zhang X, Chen S and Wang Y: Honokiol
up-regulates prostacyclin synthease protein expression and inhibits
endothelial cell apoptosis. Eur J Pharmacol. 554:1–7. 2007.
View Article : Google Scholar
|
|
22
|
Labak CM, Wang PY, Arora R, Guda MR,
Asuthkar S, Tsung AJ and Velpula KK: Glucose transport: Meeting the
metabolic demands of cancer, and applications in glioblastoma
treatment. Am J Cancer Res. 6:1599–1608. 2016.PubMed/NCBI
|
|
23
|
Jiang R, Zhang C, Liu G, Gu R and Wu H:
MicroRNA-101 inhibits proliferation, migration and invasion in
osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 7:88–97.
2017.PubMed/NCBI
|
|
24
|
Baranwal and Alahari SK: miRNA control of
tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.
|
|
25
|
Li H, Chen H, Wang H, Dong Y, Yin M, Zhang
L and Wei J: MicroRNA-374a promotes hepatocellular carcinoma cell
proliferation by targeting mitogen-inducible gene-6 (MIG-6). Oncol
Res. Jul 21–2017.Epub ahead of print. View Article : Google Scholar
|
|
26
|
Wang X, Bu J, Liu X, Wang W, Mai W, Lv B,
Zou J, Mo X, Li X, Wang J, et al: miR-133b suppresses metastasis by
targeting HOXA9 in human colorectal cancer. Oncotarget.
8:63935–63948. 2017.PubMed/NCBI
|
|
27
|
Lu Y, Qin T, Li J, Wang L, Zhang Q, Jiang
Z and Mao J: MicroRNA-140-5p inhibits invasion and angiogenesis
through targeting VEGF-A in breast cancer. Cancer Gene Ther.
24:386–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Zhou X, Shan B, Han J, Wang F, Fan
X, Lv Y, Chang L and Liu W: Downregulation of microRNA-33a promotes
cyclin-dependent kinase 6, cyclin D1 and PIM1 expression and
gastric cancer cell proliferation. Mol Med Rep. 12:6491–6500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang W, Zhang L, Xian Y and Yu Z:
MicroRNA-33a promotes cell proliferation and inhibits apoptosis by
targeting PPARα in human hepatocellular carcinoma. Exp Ther Med.
13:2507–2514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng
L, Ding L, Zhang Y, Zhang L, Li N, et al: Exosomes derived from
gemcitabine-resistant cells transfer malignant phenotypic traits
via delivery of miRNA-222-3p. Mol Cancer. 16:1322017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li JA, Wang YD, Wang K, Wang ZL, Jia DY,
Yang BY and Xiong CB: Downregulation of miR-214-3p may contribute
to pathogenesis of ulcerative colitis via targeting STAT6. BioMed
Res Int. 2017:85249722017.PubMed/NCBI
|
|
32
|
Perl A: mTOR activation is a biomarker and
a central pathway to autoimmune disorders, cancer, obesity, and
aging. Ann NY Acad Sci. 1346:33–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lekmine F, Uddin S, Sassano A, Parmar S,
Brachmann SM, Majchrzak B, Sonenberg N, Hay N, Fish EN and
Platanias LC: Activation of the p70 S6 kinase and phosphorylation
of the 4E-BP1 repressor of mRNA translation by type I interferons.
J Biol Chem. 278:27772–27780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mi C, Ma J, Wang KS, Zuo HX, Wang Z, Li
MY, Piao LX, Xu GH, Li X, Quan ZS, et al: Imperatorin suppresses
proliferation and angiogenesis of human colon cancer cell by
targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J
Ethnopharmacol. 203:27–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karatas OF, Wang J, Shao L, Ozen M, Zhang
Y, Creighton CJ and Ittmann M: miR-33a is a tumor suppressor
microRNA that is decreased in prostate cancer. Oncotarget.
8:60243–60256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian F, Wei H, Tian H, Qiu Y and Xu J:
miR-33a is downregulated in melanoma cells and modulates cell
proliferation by targeting PCTAIRE1. Oncol Lett. 11:2741–2746.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuo PL, Liao SH, Hung JY, Huang MS and Hsu
YL: MicroRNA-33a functions as a bone metastasis suppressor in lung
cancer by targeting parathyroid hormone related protein. Biochim
Biophys Acta. 1830:3756–3766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo J, Mei Y, Li K, Huang X and Yang H:
Downregulation of miR-17-92a cluster promotes autophagy induction
in response to celastrol treatment in prostate cancer cells.
Biochem Biophys Res Commun. 478:804–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Li Y, Liu D, Sun H and Liu J:
miR-224 is critical for celastrol-induced inhibition of migration
and invasion of hepatocellular carcinoma cells. Cell Physiol
Biochem. 32:448–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu W, Hu Y, Ma Q, Zhou L, Jiang L, Li Z,
Zhao S, Xu Y, Shi W, Li S, et al: miR-223 increases gallbladder
cancer cell sensitivity to docetaxel by downregulating STMN1.
Oncotarget. 7:62364–62376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen LG, Xia YJ and Cui Y: Upregulation of
miR-101 enhances the cytotoxic effect of anticancer drugs through
inhibition of colon cancer cell proliferation. Oncol Rep.
38:100–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Asukai K, Kawamoto K, Eguchi H, Konno M,
Asai A, Iwagami Y, Yamada D, Asaoka T, Noda T, Wada H, et al:
Micro-RNA-130a-3p regulates gemcitabine resistance via PPARG in
cholangiocarcinoma. Ann Surg Oncol. 24:2344–2352. 2017. View Article : Google Scholar : PubMed/NCBI
|