Introduction
Lung cancer is a major cause of cancer-related
mortality in China (1,2). The overall 5-year survival rate for
patients with non-small cell lung cancer (NSCLC) is ~17.1%
(2). In the past years, extensive
research has been performed to obtain a better understanding of the
underlying molecular biology of lung cancer (3–5).
Recent studies have shed light on the role of cancer stem cells
(CSCs) in lung cancer (6–8).
CSCs are a subpopulation of cells within a tumor
that possess self-renewal and tumor-initiating capacities (9). Cluster of ddifferentiation 24 (CD24)
is a small membrane glycoprotein that has emerged as a major
determinant of stemness in various cancer types (10,11).
Although CD24 has been used extensively in combination with other
putative markers to isolate CSCs (12–14),
the lack of their universal expression limits their usage to lung
cancer (15,16). However, CD24 has been suggested as
a biomarker for carcinoma progression in lung cancer (17,18).
The aim of the present study was to assess the tumor
promotion roles of CD24 in the subtypes of lung cancer. Firstly,
the significance of CD24 mRNA in human lung cancer was evaluated
using the Oncomine database. Secondly, CD24high and
CD24low cells were isolated from Lewis lung carcinoma
(LLC) cells and the tumorigenic ability of these cells in
vitro and in vivo was identified. Furthermore, a focus
was placed on the roles of nicotine in CD24 expression, and the
associated molecular signaling pathways were investigated. The
findings of this study may be useful in improving the clinical
effectiveness for a better prognosis in patients with lung
adenocarcinoma.
Materials and methods
Oncomine database analysis
CD24 mRNA levels in NSCLC tissues were
compared with their matched normal tissues using the Oncomine
database (http://www.oncomine.org). The threshold
used to obtain the most significant probes of the queried gene for
each microarray data included a 2-fold difference in expression
between cancer and normal tissues, with a P-value of
<1×10−4.
Kaplan-Meier plotter analysis
The prognostic value of CD24 mRNA in lung
cancer was analyzed using Kaplan-Meier (KM)-Plotter (http://kmplot.com/analysis/). Overall survival of the
patients with high and low levels of CD24 mRNA was shown by
the log-rank test.
Cell culture
LLC cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and grown in RPMI-1640
medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine
serum and antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin). Cells were maintained in a humidified cell incubator
with 5% CO2 at 37°C.
Fluorescence-activated cell sorting
(FACS)
Sorting of the side population (SP) cells from LLC
cells was performed as described previously (19). SP cells were washed twice with PBS
and suspended in 100 µl assay buffer [PBS, 0.5% bovine serum
albumin (BSA), 2 mM EDTA (pH 7.2)] with 10 µl phycoerythrin
(PE)-conjugated anti-CD24 antibody (1:50; catalog no. 555428; BD
Biosciences, Franklin Lakes, NJ, USA) and 20 µl fluorescein
isothiocyanate-conjugated anti-CD133/2 (clone 293C3) antibody
[1:20; catalog no. 130-104-322; Miltenyi Biotec Technology and
Trading (Shanghai) Co., Ltd. Shanghai, China]. The cells were then
incubated in the dark at 4°C for 30 min, washed twice with 1 ml
assay buffer and centrifuged at 300 × g for 10 min. The cell pellet
was subjected to FACS using a BD Aria II sorter (BD
Biosciences).
Reverse transcription-polymerase chain
reaction (PCR)
Total RNA was isolated from cells using an RNeasy
Mini kit (Beijing Bomed Gene Technology Co., Ltd., Beijing, China).
cDNA was reverse transcribed from 1 µg total RNA using a
Takara Reverse Transcription kit (Takara Biotechnology, Co., Ltd.,
Dalian, China), and then amplified using the following primers:
CD24 sense, 5′-ACTCAGGCCAGGAAACGTCTCT-3′ and antisense,
5′-AACAGCCAATTCGAGGTG GAC-3′; ATP-binding cassette subfamily G
member 2 (Junior blood group) (ABCG2) sense,
5′-AGCTGCAAGGAAAGATC CAA-3′ and antisense
5′-TCCAGACACACCACGGATAA-3′; and GAPDH sense,
5′-AGAAGGCTGGGGCTCATTTG-3′ and antisense,
5′-AGGGGCCATCCACAGTCTTC-3′. The PCR products were electrophoresed
on a 1.5% agarose gel, and visualized by ethidium bromide staining
under an ultraviolet imaging system (UVP, LLC, Phoenix, AZ, USA).
The RT-PCR conditions were as follows: 10 min at 95°C for
dena-turation, followed by 35 cycles of 20 sec at 95°C, 40 sec at
56°C and 30 sec at 72°C, and a final extension step of 5 min at
72°C.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 30
min, followed by washing twice in PBS at room temperature (RT) for
5 min. Non-specific binding sites were blocked with 3% BSA in PBS
for 1 h at RT. PE-conjugated anti-CD24 antibody (as
aforementioned), diluted in 3% BSA/PBS, was applied overnight at
4°C. For every coverslip, the cells were observed and images were
captured in 5 random fields using an Olympus CX71 fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Colony-formation assay
Cells were plated at a density of 2×105
cells/well in 24-well plates under serum-free, colony-specific
conditions. Fresh aliquots of epidermal growth factor and basic
fibroblast growth factor were added every day. Subsequent to
culturing the cells for 3 weeks, colonies were visible under a
light microscope (Olympus CX31; Olympus).
Transwell assay
Cell suspension (200 µl; 1×105
cells/ml, RPMI-1640 medium with 1% FBS) was placed into the upper
chamber of a Transwell (8-µM pore size polycarbonate
membrane; Cell Biolabs, San Diego, CA, USA). In the lower chamber,
RPMI-1640 medium with 10% FBS was added. Subsequent to culture for
24 h, the cells that had migrated through and adhered to the lower
surface of the membrane were fixed with paraformaldehyde for 15 min
and stained with 0.1% crystal violet for 10 min at RT. Next, five
fields of view were randomly selected for the counting of cells
under a light microscope (Olympus CX31; Olympus).
Nicotine treatment and Ras inhibitor
(salirasib)
According to the method used in the study by Chu
et al (20),
CD24low and CD24high cells were split every 3
days with medium supplemented with 0.5 µM nicotine to keep
the drug at a constant concentration. For the controls,
CD24low and CD24high cells treated with
nicotine were incubated with salirasib (100 µM; 162520-00-5;
Tocris Bioscience, Bristol, UK) for 24 h at 37°C.
In vivo assays
Liaoning Medical University Ethics Committee
(Jinzhou, Liaoning, China) approved the research protocols
performed in this study. NOD SCID mice (25–40 g; 4 to 6-weeks-old;
male; NOD.CB17-Prkdcscid/NcrCrl; Charles River Laboratories, Inc.,
Wilmington, MA, USA) received standard laboratory food and water
ad libitum and were maintained in micro-isolator cages with
filtered air and handled under sterile conditions under a laminar
flow hood. A subcutaneous injection of cells [1×107
cells in 200 µl PBS, including main population (MP), SP,
CD24high, CD24low,
CD133highCD24high or
CD133highCD24low; 30 mice in each treatment
group] was administered into the flank of each mouse. Tumors were
measured using calipers, and tumor volumes were calculated (tumor
volume = length × width2 × 0.52) (21). Once the tumor diameters had reached
3–5 mm, the mice were used in the following studies. According to
the methods used in the study by Cavarra et al (22), male mice injected with
CD24high or CD24low cells were exposed to the
smoke of four cigarettes/day at 10 a.m. and 4 p.m. for 3 months
(1.2 mg of nicotine; Honghe filter cigarettes; Honghe Cigarette
Factory, Yunnan, China), 1 month prior to cell inoculation and 2
months after cell inoculation), in specially designed cages. The
mice were examined at 0, 10, 20, 30, 40, 50 and 60 days, and tumor
growth was evaluated by measuring the length and width of the tumor
mass. Subsequently, the animals were euthanized with pentobarbital
sodium via the tail vein (100 mg/kg). The survival state of the
immunodeficient mice was observed day and night, and euthanasia was
available to use at the first sign of any mental or dietary
problems.
Immunohistochemical staining
Endogenous peroxidase activity was blocked in
4-µm tumor sections with 3% hydrogen peroxide for 30 min at
RT. Antigen retrieval was performed in citrate buffer (10 mM; pH
6.0) for 30 min at 95°C in a pressure cooker. Primary antibodies
were incubated with sections at 1:500 overnight at 4°C (Table I). Sections were then incubated
with horseradish peroxidase-labeled polymer-conjugated goat
anti-mouse/goat anti-rabbit secondary antibody (1:100; catalog no.
A0208/A0216; Beyotime Institute of Biotechnology, Beijing, China)
for 60 min at RT, followed by incubation with a streptavidin
horseradish peroxidase complex (Beyotime Institute of
Biotechnology) for 60 min at RT. Bound antibody was visualized with
3,3′-diaminobenzidine tetrahydrochloride (Beyotime Institute of
Biotechnology). Sections were also counterstained with hematoxylin
for 30 sec at RT (Beyotime Institute of Biotechnology). The results
were visible under a light microscope (Olympus CX31; Olympus).
 | Table IAntibodies used in the western
blotting and immunohistochemistry analyses. |
Table I
Antibodies used in the western
blotting and immunohistochemistry analyses.
| Protein | Producer | Catalog no. | Dilution |
|---|
| HSP90 | Cell
Signaling
Technology, Inc.a | 4874 | 1:100 |
| CD24 | Santa
Cruz
Biotechnology, Inc.b | sc-7034 | 1:200 |
| p-RAF | Santa
Cruz
Biotechnology, Inc.b | sc-16806 | 1:200 |
| p-RAS | Santa
Cruz
Biotechnology, Inc.b | sc-521 | 1:200 |
| E-cadherin | Santa Cruz
sc-8426
Biotechnology, Inc.b | 1:100 | |
| β-actin | Santa
Cruz
Biotechnology, Inc.b | sc-47778 | 1:1,000 |
Western blot analysis
Protein was extracted in lysis buffer (P0013B;
Beyotime Institute of Biotechnology) for 30 min on ice. The extract
was centrifuged at 4,000 × g for 5 min at 4°C to remove debris.
Total protein concentration was determined using the bicinchoninic
acid protein assay kit (P0010; Beyotime Institute of
Biotechnology). Extracted proteins (30 µg) were separated by
10% SDS-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes. Membranes were blocked in 5%
BSA/Tris-buffered saline plus Tween-20 at RT for 1 h and then
incubated with primary antibodies at 4°C overnight (Table I). The reaction was followed by
probing with peroxidase-coupled secondary antibodies at 1:1,000
dilution (catalog no. A0216; goat anti-mouse; and catalog no.
A0208; goat anti-rabbit; Beyotime Institute of Biotechnology), and
binding results were visualized by enhanced chemiluminescence kit
(Amersham; GE Healthcare, Chicago, IL, USA).
Ingenuity Pathway Analysis
The Ingenuity Pathway Analysis software
(Ingenuity® Systems; www.ingenuity.com) was used to build networks and
identify pathways of CD24 based on data mining.
Statistical analysis
Each experiment was performed in triplicate. Data
were analyzed using GraphPad Prism 5 software (GraphPad Software,
San Diego, CA, USA). Statistical analysis was performed using
one-way analysis of variance and Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Associations between CD24 status and
clinical parameters of lung cancer patients
Oncomine analysis of cancer versus normal tissue
showed that the CD24 mRNA level was higher in lung
adenocarcinoma, large cell lung carcinoma, squamous cell lung
carcinoma and small cell lung carcinoma (Fig. 1). No difference in CD24 mRNA
was found between male and female lung cancer patients (data not
shown). Notably, the patients aged 40 to 49 years old exhibited a
higher CD24 mRNA level compared with that of patients of other age
groups (data not shown). Another notable result was that smoking
decreased CD24 expression in lung cancer patients (Fig. 2).
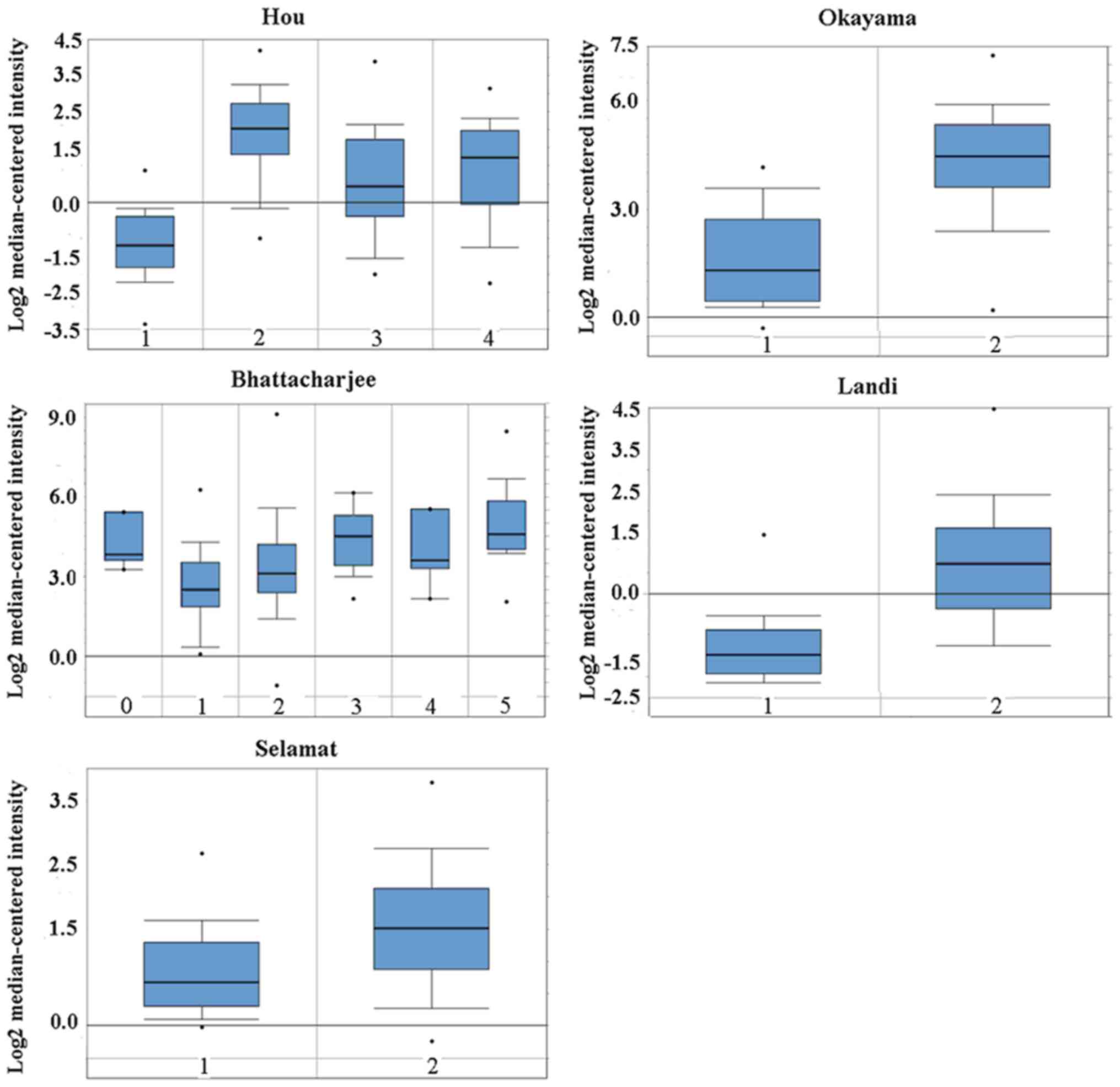 | Figure 1Cluster of ddifferentiation 24 mRNA
was evaluated in subtypes of lung cancer using Oncomine analysis.
Hou: 1, lung (n=65); 2, large cell lung carcinoma (n=19); 3, lung
adenocarcinoma (n=45); and 4, squamous cell lung carcinoma (n=27).
Bhattacharjee: 0, no value (n=7); 1, lung (n=17); 2, lung
adenocarcinoma (n=132); 3, lung carcinoid tumor (n=20); 4, small
cell lung carcinoma (n=6); and 5, squamous cell lung carcinoma
(n=21). Okayama: 0, lung (n=20); and 1, lung adenocarcinoma
(n=226). Selamat: 1, lung (n=58); and 2, lung adenocarcinoma
(n=58). Landi: 1, lung (n=49); and 2, lung adenocarcinoma (n=58).
The data are presented as the mean ± standard deviation. The dots
represent abnormal values/outliers. |
In addition, the prognostic values of CD24
mRNA were analyzed in subtypes of lung cancer by KM-plotter.
CD24 mRNA can be used as a prognostic marker for patients
with lung adenocarcinoma (P=0.0029) (Fig. 3). No influence of CD24 mRNA
on squamous cell lung carcinoma was found (P=0.65) (Fig. 3). A high level of CD24 mRNA
was shown to improve the survival rate of male lung cancer patients
(P=0.0066) (Fig. 3). A high level
of CD24 mRNA could also improve the prognosis of never
smokers (P=0.00036), while no influence of CD24 mRNA on
former smokers and current smokers was found (P=0.06) (Fig. 3).
CD133highCD24low
and CD133highCD24high fractions in LLC
cells
In our previous study, SP cells were isolated from
LLC cells using Hoechst 33342 efflux analysis (19). ABCG2 protein is a surface marker of
SP cells (19). In the present
study, SP cells were further isolated by use of two CSC-specific
markers, CD133 and CD24 (Fig. 4A).
In addition, it was found that CD24 mRNA and protein expression was
significantly higher in the CD133highCD24high
cells compared with that in the
CD133highCD24low cells (Fig. 4B and C). ABCG2 mRNA and protein was
higher in CD133highCD24high cells and
CD133highCD24low cells than that in MP cells.
Immunofluorescence results showed that CD24 was localized in the
membrane of CD133highCD24high and
CD133highCD24low cells (Fig. 4D).
Tumorigenic ability of
CD133highCD24low and
CD133highCD24high LLC cells in vitro and in
vivo
Colony formation assays were performed to detect the
proliferation of MP, SP, CD133highCD24high
and CD133highCD24low cells. Sphere clusters
were clearly observed in SP,
CD133highCD24high and
CD133highCD24low cells (P<0.05), with no
difference among these three cell types (Fig. 5A). Migration of MP, SP,
CD133highCD24high and
CD133highCD24low cells was detected using
Transwell assay. It was found that more SP,
CD133highCD24high and
CD133highCD24low cells migrated to the lower
membrane compared with MP cells (P<0.05) (Fig. 5B). No difference in mobility was
found among SP, CD133highCD24high and
CD133highCD24low cells (Fig. 5B). Furthermore, it was found that
SP, CD133highCD24high and
CD133highCD24low cells had similar abilities
to transfer the tumors into immunocompromised mice (Fig. 6A and B). The diameters of the
largest single subcutaneous tumors observed in the SP,
CD133highCD24high and
CD133highCD24low groups were 4.4±0.3, 4.6±0.3
and 4.7±0.2 mm. These results indicated that CD24 expression did
not increase the tumor-forming ability of the LLC cells.
Potential mechanism of nicotine-inhibited
CD24 expression
Based on the results of Ingenuity Pathways Analysis
software (version 6.3; Ingenuity Systems, Redwood, CA, USA), the
differentially expressed proteins in CD24-expressing cells were
determined (data not shown). In addition, immunohistochemistry
(IHC) results showed that the level of RAS was markedly higher in
the cancer tissues of
CD133highCD24low-injected mice compared with
that in the MP group, while almost no RAS expression was found in
the CD133highCD24high group (Fig. 6C). The results also revealed that
CD24 expression was associated with HSP90 expression (Fig. 6C). Low E-cadherin expression was
found in all MP, SP, CD133highCD24high and
CD133highCD24low groups (Fig. 6C).
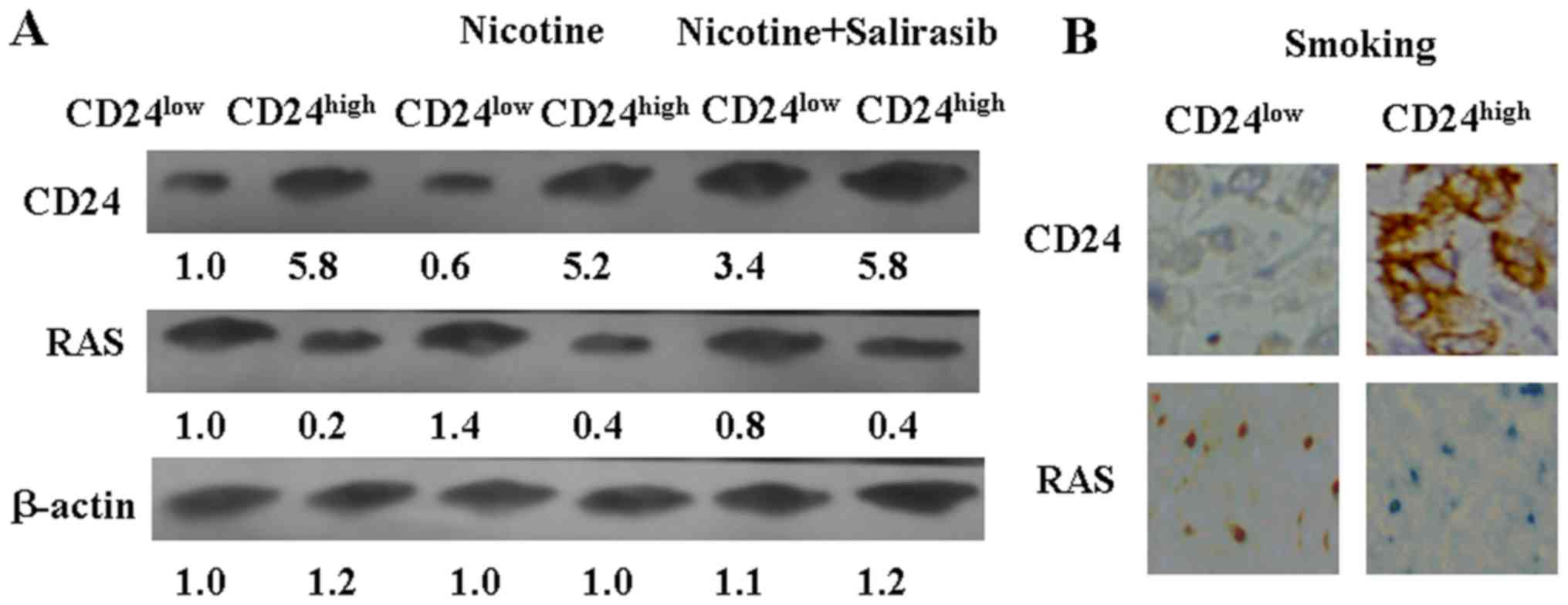
Furthermore, it was found that nicotine treatment
inhibited CD24 expression in vitro (Fig. 7A). The level of RAS was moderately
increased in CD24low and CD24high LLC cells
after nicotine treatment (Fig.
7A). To test the hypothesis that CD24 expression partly
depended on downregulation of RAS in LLC cells, salirasib was used
as a RAS blocker. Downregulation of RAS using salirasib could
induce CD24 expression in CD24low LLC cells (Fig. 7A). Altogether, these results
suggest that nicotine could inhibit CD24 expression in LLC cells
via activating RAS.
CD24high and CD24low
cell-injected mice were subjected to cigarette smoke, as
aforementioned. The tumor volumes of these two groups exhibited no
significant changes from the beginning to the end of the experiment
(Fig. 6B). IHC results also showed
that RAS and CD24 expression were not changed in the mice subjected
to smoke (Fig. 7B).
Discussion
In the present study, the prognostic value of
CD24 mRNA in NSCLC was analyzed using the Oncomine database.
A number of previous studies have discussed the roles of CD24 in
lung cancer (17,23). In the study by Karimi-Busheri et
al (17), upregulation of CD24
was observed in >75% of NSCLC patients. Kristiansen et al
(23) reported a higher incidence
of CD24 expression in NSCLC tissues. Consistent with these previous
results, the present study also found higher CD24 mRNA
expression in lung cancer tissues compared with that in matched
normal tissues. Kristiansen et al (23) found that CD24 expression is an
independent predictor of a shortened survival time in NSCLC
patients. Lee et al (24)
demonstrated a significant association between CD24 expression and
shorter NSCLC patient survival times. However, in the present
study, it was found that CD24 mRNA was associated with a
longer survival time in the patients with lung adenocarcinoma. The
main reason for this difference may be that dynamic changes of CD24
protein throughout the development of cancer (25). CD24 is a heavily glycosylated
protein that also demonstrates increased additional structural
flexibility in its mature form (25).
Previous studies showed that CD24 could not be used
as a CSC marker for human lung adenocarcinoma (16,26).
Roudi et al (16) found
that CD24 could not be considered a potential marker for isolating
CSCs in the human lung adenocarcinoma A549 cell line. Xu et
al (15) also found that
CD24− A549 cells possess partial CSC properties, but
actually are not CSCs. In the present study, another lung
adenocarcinoma cell line, LLC, was used to analyze the roles of
CD24 in the stemness of lung adenocarcinoma. The in vitro
and in vivo experiments demonstrated that
CD24high LLC cells showed no significant differences in
terms of metastasis and tumorgenicity compared with
CD24low cells. These results indicated that CD24 could
not be used to isolate CSCs from lung adenocarcinoma cells.
The most important result of the present study is
that CD24 expression is critically dependent on the smoking status
of lung cancer patients. It was found that nicotine could inhibit
CD24 expression in LLC cells by upregulation of RAS. Nicotine is
believed to promote the tumorigenesis of lung cancer cells
(15). An increase in Ras
activity/expression is frequently found in numerous cancer types
(27,28). More and more evidence indicates
that nicotine is able to activate Ras upon its interaction with
nicotine acetylcholine receptors (15). In agreement with previous studies,
the present study also confirmed that nicotine could induce RAS
expression in LLC cells. In previous studies, the activation of RAS
was able to downregulate CD24 expression at the mRNA and protein
levels (29,30). The present study also demonstrated
that the expression of oncogenic Ras directly downregulated the
expression of CD24 at the protein level. Furthermore, inhibition of
RAS could partially restore CD24 expression in LLC cells. These
results provided an integrated insight regarding the mechanism of
nicotine-inhibited CD24 expression in LLC cells. Nicotine is well
known to be an addictive component of cigarettes. Notably, no
effects of smoking on CD24 expression were found in vivo.
The main reason for this is that the level of nicotine may have
been too low to influence established xenograft tumor models.
In summary, the principal findings of the present
study were that: i) CD24 could be used as a prognostic marker in
lung adenocarcinoma; ii) in vitro and in vivo
experiments did not find a significant influence of CD24 on
tumorgenicity of LLC cells; and iii) nicotine inhibited CD24
expression in LLC cells by upregulation of RAS. However, the
downstream proteins of RAS should be analyzed in further
studies.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Scientific Foundation of China (grant no. 81502558), the President
Fund of Liaoning Medical University (grant no. XZJJ20140102) and
the Biological Anthropology Innovation Team Project of Jinzhou
Medical University (grant no. JYLJ201702).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
PX and FR were responsible for the study design,
original article drafting and editing, data acquisition and data
analysis. DHL, MA and BLB performed the experiments. DHL and PX
were responsible for data analysis. DHL, MA and BLB were
responsible for data acquisition. DHL, MA, BLB and PX were
responsible for data interpretation and methodology. PX and FR were
responsible for supervision of the whole study and funding
acquisition. PX revised the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethical Committee of Jinzhou Medical University
approved this investigation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naylor EC, Desani JK and Chung PK:
Targeted Therapy and Immunotherapy for Lung Cancer. Surg Oncol Clin
N Am. 25:601–609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng M: Classification and pathology of
lung cancer. Surg Oncol Clin N Am. 25:447–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of Lung Cancer. Surg Oncol Clin N Am. 25:439–445.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shan GP, Zhang P, Li P, Du FL and Yang YW:
Numb gene enhances radiation sensitivity of nonsmall cell lung
cancer stem sells. Cancer Biother Radiopharm. 31:180–188. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao C, Shen Y, Jin F, Miao Y and Qiu X:
Cancer stem cells in small cell lung cancer cell line H446: Higher
dependency on oxidative phosphorylation and mitochondrial
substrate-level phosphorylation than non-stem cancer cells. PLoS
One. 11:e01545762016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang KJ, Yang MH, Zheng JC, Li B and Nie
W: Arsenic trioxide inhibits cancer stem-like cells via
downregulation of Gli1 in lung cancer. Am J Transl Res.
8:1133–1143. 2016.
|
|
9
|
Fabregat I, Malfettone A and Soukupova J:
New insights into the crossroads between EMT and stemness in the
context of cancer. J Clin Med. 5:E372016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheridan C, Kishimoto H, Fuchs RK,
Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S and
Nakshatri H: CD44+/CD24− breast cancer cells
exhibit enhanced invasive properties: An early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar
|
|
11
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: An enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Wang YJ, Bian L, Fang ZH, Zhang QY
and Cheng JX: CD44+/CD24+ cervical cancer
cells resist radiotherapy and exhibit properties of cancer stem
cells. Eur Rev Med Pharmacol Sci. 20:1745–1754. 2016.PubMed/NCBI
|
|
13
|
Todoroki K, Ogasawara S, Akiba J, Nakayama
M, Naito Y, Seki N, Kusukawa J and Yano H:
CD44v3+/CD24− cells possess cancer stem
cell-like properties in human oral squamous cell carcinoma. Int J
Oncol. 48:99–109. 2016. View Article : Google Scholar
|
|
14
|
Salaria S, Means A, Revetta F, Idrees K,
Liu E and Shi C: Expression of CD24, a stem cell marker, in
pancreatic and small intestinal neuroendocrine tumors. Am J Clin
Pathol. 144:642–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Mu J, Xiao J, Wu X, Li M, Liu T and
Liu X: CD24 negative lung cancer cells, possessing partial cancer
stem cell properties, cannot be considered as cancer stem cells. Am
J Cancer Res. 6:51–60. 2015.
|
|
16
|
Roudi R, Madjd Z, Ebrahimi M, Samani FS
and Samadikuchaksaraei A: CD44 and CD24 cannot act as cancer stem
cell markers in human lung adenocarcinoma cell line A549. Cell Mol
Biol Lett. 19:23–36. 2014. View Article : Google Scholar
|
|
17
|
Karimi-Busheri F, Rasouli-Nia A,
Zadorozhny V and Fakhrai H: CD24+/CD38− as
new prognostic marker for non-small cell lung cancer. Multidiscip
Respir Med. 8:652013. View Article : Google Scholar
|
|
18
|
Majores M, Schindler A, Fuchs A, Stein J,
Heukamp L, Altevogt P and Kristiansen G: Membranous CD24 expression
as detected by the monoclonal antibody SWA11 is a prognostic marker
in non-small cell lung cancer patients. BMC Clin Pathol. 15:192015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia P, Gou WF, Zhao S and Zheng HC:
Crizotinib may be used in Lewis lung carcinoma: A novel use for
crizotinib. Oncol Rep. 30:139–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu M, Guo J and Chen CY: Long-term
exposure to nicotine, via ras pathway, induces cyclin D1 to
stimulate G1 cell cycle transition. J Biol Chem. 280:6369–6379.
2005. View Article : Google Scholar
|
|
21
|
Alessandri G, Filippeschi S, Sinibaldi P,
Mornet F, Passera P, Spreafico F, Cappa PM and Gullino PM:
Influence of gangliosides on primary and metastatic neoplastic
growth in human and murine cells. Cancer Res. 47:4243–4247.
1987.PubMed/NCBI
|
|
22
|
Cavarra E, Bartalesi B, Lucattelli M,
Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA and
Lungarella G: Effects of cigarette smoke in mice with different
levels of alpha(1)-proteinase inhibitor and sensitivity to
oxidants. Am J Respir Crit Care Med. 164:886–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kristiansen G, Schlüns K, Yongwei Y,
Denkert C, Dietel M and Petersen I: CD24 is an independent
prognostic marker of survival in nonsmall cell lung cancer
patients. Br J Cancer. 88:231–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ, Choe G, Jheon S, Sung SW, Lee CT
and Chung JH: CD24, a novel cancer biomarker, predicting
disease-free survival of non-small cell lung carcinomas: A
retrospective study of prognostic factor analysis from the
viewpoint of forthcoming (seventh) new TNM classification. J Thorac
Oncol. 5:649–657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ayre DC, Pallegar NK, Fairbridge NA,
Canuti M, Lang AS and Christian SL: Analysis of the structure,
evolution, and expression of CD24, an important regulator of cell
fate. Gene. 590:324–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hermann PC, Sancho P, Cañamero M,
Martinelli P, Madriles F, Michl P, Gress T, de Pascual R, Gandia L,
Guerra C, et al: Nicotine promotes initiation and progression of
KRAS-induced pancreatic cancer via Gata6-dependent
dedifferentiation of acinar cells in mice. Gastroenterology.
147:1119–33.e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Veluchamy JP, Spanholtz J, Tordoir M,
Thijssen VL, Heideman DA, Verheul HM, de Gruijl TD and van der
Vliet HJ: Combination of NK cells and cetuximab to enhance
anti-tumor responses in RAS mutant metastatic colorectal cancer.
PLoS One. 11:e01578302016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hrustanovic G and Bivona TG: RAS-MAPK
signaling influences the efficacy of ALK-targeting agents in lung
cancer. Mol Cell Oncol. 3:e10910612015. View Article : Google Scholar
|
|
29
|
Pallegar NK, Ayre DC and Christian SL:
Repression of CD24 surface protein expression by oncogenic Ras is
relieved by inhibition of Raf but not MEK or PI3K. Front Cell Dev
Biol. 3:472015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|