According to Globocan, breast cancer (BC) is the
most common cancer in women, with an estimated 1.67 million new
cancer cases being diagnosed in 2012 (1). Globocan estimates that BC is the 5th
most common cause of mortality among the overall cancer mortalities
(522,000 mortalities) and is considered the principal cause of
cancer-associated mortality in women in less developed regions
(324,000 mortalities). The recent decline in mortality rates of BC
in developed regions has been associated with more effective
screening programmes, improved chemotherapeutic options and
targeted therapy (2-4).
For a number of years, pathologists have recognized
the biological heterogeneity among BCs. BCs are diversified into a
few biologically distinct subtypes with specific pathological
features and different clinical behaviours (5-7).
Initially, breast tumours were classified according to histological
type, grade and the expression of steroid receptors (8,9).
However, pioneering gene expression profile studies demonstrated
that the morphological heterogeneity of BC is also reflected at the
gene expression level (10-13).
These studies allowed for the classification of BCs into five
intrinsic molecular subtypes: Luminal A and B; normal breast-like;
with overexpression of erb-b2 receptor tyrosine kinase 2/human
epidermal growth factor receptor 2 (HER2); and basal-like
carcinomas (10-13). However, gene expression
profiling-based techniques are not very common in daily clinical
practice due to their relatively high cost. Routine
histopathological subclassification of BC is primarily accomplished
by immunohistochemical (IHC) detection of oestrogen receptor (ER),
progesterone receptor (PR), HER2 and marker of proliferation Ki-67
(Ki-67) (14). Different
expression patterns of these biomarkers provide a simple clinical
classification system for BCs and allow for the determination of
therapeutic approaches. According to The St. Gallen surrogate
classification, BCs may be classified by IHC into four subtypes:
Luminal A (ER+, PR+, HER2− and low
Ki-67 index); luminal B (ER+, PR+ and
HER2+ or high Ki-67 index); HER2-positive
(ER−, PR−, HER2+); and triple
negative breast cancer (TNBC; ER−, PR−,
HER2−) (15). TNBC is
one of the most challenging subtypes among BC due to the lack of
targeted therapies and its highly aggressive clinical behaviour
(16-18). TNBCs may also be clustered into at
least two distinct molecular classes according to their different
behaviours: The basal phenotype and non-basal-like TNBCs (5). This distinction within TNBCs is
important from a clinical perspective due to the different
responses to chemotherapeutic treatment of each subtype (19). The basal-like subtype originates in
the myoepithelial/basal cells that line the outer layer of mammary
ducts, and is characterized by a high proliferation rate,
aggressive pathological features and an extremely poor clinical
outcome (20-22). Although the identification of
basal-like cancer is optimally performed by gene expression
profiling, attempts have been made to establish a reliable IHC
panel for its identification. According to Nielsen et al
(23), basal-like BCs are
characterized as ER, PR and HER2-negative tumours with
overexpression of basal cytokeratins 5/6 or epidermal growth factor
receptor (EGFR). This IHC panel, termed 'Core Basal', has been
validated and widely applied for basal-like subtype identification
(23,24). However, to depict the heterogeneity
of BCs, the evidence from a number of studies demonstrates that
certain basal-like by gene expression tumours weakly express
steroid receptors, which results in the misclassification of the
majority of them as luminal tumours by IHC (25-30).
According to the current standards, the luminal subtype is reported
when <1% of the tumour cells express ER by IHC. Tumours weakly
expressing ER comprise a relatively rare subgroup characterized by
positive nuclear staining of 1-10% cells (29,31),
with an incidence ranging between 1 and 6.7% (32,33).
A number of studies demonstrated that approximately one-half of
weakly ER-positive cases exhibit different gene expression patterns
compared with luminal tumours, overlapping with the basal-like gene
profile and predicting poor prognosis (29,31).
Currently, patients in this subgroup are scheduled to receive
endocrine therapy, which in that case may not only be ineffective,
but may also expose them to unnecessary side effects, thus limiting
their opportunities to benefit from chemotherapy-based treatments.
To date, a single panel of IHC markers for the specific
identification of basal-like tumours remains to be validated.
However, considering recent findings, nestin, a marker of stem
cells, appears to be a sensitive IHC marker for basal-like subtype
identification, regardless of ER status (34,35).
Nestin (a neural stem cell protein) is a member of
the class VI family of intermediate filament (IF) proteins,
originally identified as a marker of neural progenitors and
subsequently observed to be expressed in a wide range of other cell
types (36-38). Nestin was initially identified as a
protein expressed in the developing central nervous system (CNS) in
neuroepithelial stem cells that give rise to neurons and glia
(39,40). The expression of nestin in adults
is uncommon; it is restricted to very specific cell types and may
be induced only during particular processes i.e., development and
regeneration (41-44). In tissues, nestin-positive cells
are limited to defined locations where they may function as
quiescent reserve cells, which are capable of proliferation,
differentiation and migration when reactivated (37). During cell differentiation, nestin
expression is subsequently downregulated and replaced by
tissue-specific IF proteins (45).
Except for the expression in the CNS, nestin has additionally been
identified in stem and progenitor cells of the muscles (46-48),
teeth (49), testes (50), pancreas (51), intestines (52), bone marrow (53), hair follicles (54), endothelium (55,56)
and other tissues. Nestin expression in tumour cells was initially
reported in neuroectodermal tumours of the CNS (57,58).
Further studies on nestin expression demonstrated that it is also
present in epithelial cancer, including breast (59), prostate (60), pancreatic (61), lung (62), ovarian (63) and other cancer types. Additionally,
in a number of tumours the expression of nestin is considered a
factor indicating poor prognosis (64-67).
Furthermore, nestin expression in tumours is not limited to cancer
cells, and is also present in newly-forming tumour vessels and
cancer stem cells (CSCs) (56,68).
The present review discusses the role of nestin as a
significant agent involved in the mechanisms underlying BC
progression. Being involved in proliferation, angiogenesis and
self-renewal, nestin may be hypothesized to be an important factor
in BC pathogenesis.
The human nestin protein consists of 1,621 amino
acids with a predicted molecular weight of 177.4 kDa. Nestin was
classified as new type (type VI) of IF as it did not fall clearly
into any of the previously described types (69). As in all IFs, nestin has a highly
conserved α-helical core domain of 300-330 amino acids flanked by
N- and C-terminal domains (36).
However, nestin has two intrinsic features that determine its two
unique properties. A short N-terminal head domain prevents nestin
self-assembly and, as a result, nestin co-polymerizes forming
heterodimers with other IFs, most favourably with vimentin
(70,71). Additionally, nestin has an
unconventionally long C-tail domain that protrudes from the
filament structure, being accessible to post-translational
modifications and protein interactions (36).
Although the exact mechanisms of action of nestin in
BC development remain unknown, there have been certain studies
elucidating its role in the cell cycle, proliferation and survival.
Accumulating data suggests that IF protein phosphorylation markedly
alters the structure/dynamics of IFs in cells (72,73).
Nestin has multiple phosphorylation sites and has been demonstrated
to be phosphorylated by kinases involved in cell cycle regulation,
for example cyclin-dependent kinase 1 [additionally termed cell
division control protein 2 homolog (cdc2)] and cyclin-dependent
kinase 5 (Cdk5) (74,75). In rat cells, cdc2 kinase is
involved in the phosphorylation of nestin in the highly conserved
rod domain, regulating its intracellular organization during
mitosis (4). Recently, it was
reported that nestin phosphorylation directly affects the
proliferation of cancer cells (76). Nestin also modulates Cdk5 activity,
a kinase that has recently been demonstrated to be involved in
tumorigenesis, with functions ranging from cell proliferation to
invasion and angiogenesis (74,77-79).
In BC, the overexpression of the Cdk5/p35 complex is associated
with markers of poor prognosis, TNBC, Her2+ expression
and high grade of malignancy (79). In normal cells, nestin serves as a
potent survival determinant acting via cytoplasmic sequestration of
the Cdk5/p35 complex, which protects cells from oxidative
stress-induced cell death (80).
In the nucleus, Cdk5 forms a complex with p25, which is generated
as a product of the proteolytic cleavage of p35. Nestin, acting as
a protein scaffold, stabilizes p35 and prevents the translocation
of Cdk5/p25 to the nucleus, which is a required step for the
induction of cell death (80).
Nestin was also reported to drive protein hedgehog (Hh)-dependent
tumorigenesis by binding to transcriptional activator Gli3, a zinc
finger transcription factor that negatively regulates Hh signalling
(81). The Hh pathway has been
recognized as one of the key signalling pathways involved in the
pathogenesis of TNBC (82). Lines
of evidence suggest that nestin is directly involved in the
mechanisms underlying tumour growth and progression. However,
further studies are required to clarify its oncogenic role in
BC.
The first evidence of nestin expression in human BC
tissues was demonstrated by Li et al (59). In that study, nestin expression was
detected in the regenerative compartment of the normal human
mammary gland in addition to BC tumour cells. The study
demonstrated that in mammary ducts and lobules, nestin was
expressed in the subluminal compartment in two morphologically
distinct cell types: In columnar basal cells expressing tumour
protein p63 and normal cytokeratin-14; and in filamentous
myoepithelial cells expressing desmin (59). Additionally, the study demonstrated
that during murine pregnancy, nestin coordinately colocalized with
p63 in columnar basal cells and was involved in the regenerative
cycle of the mammary gland. On the other hand, nestin expression in
myoepithelial cells appeared to be stable throughout the course of
pregnancy. Nestin-expressing cells have also been isolated from
human breast milk, which suggests that it may be a novel source of
putative stem cells (83-85). A further study indicated that these
nestin-expressing cells were able to differentiate into neural stem
cells and neurons (86). The role
of nestin in the process of self-renewal in BC has been confirmed
by additional studies (87-89).
Although gene expression profiling is the gold
standard facilitating accurate classification of basal-like
tumours, its application is expensive and impractical in everyday
practice. Thus, surrogate IHC panels are the first-choice strategy
in basal-like subtype identification, even with their lower
sensitivity and specificity. In order to identify the best
individual IHC marker, Won et al (93) tested 46 biomarkers associated with
the basal-like subtype, against a gene expression profile gold
standard. On the basis of the highest odds ratio, nestin was the
best positive biomarker identifying the basal-like intrinsic
subtype among all of the investigated biomarkers. Furthermore, a
combined IHC panel composed of nestin positivity and/or inositol
polyphosphate-4-phosphatase negativity
(nestin+/INPP4B−) had a better sensitivity
and specificity compared with any other proposed IHC panel for
basal-like subtype identification (93). In their further study, application
of the nestin+/INPP4B− panel enabled the
classification of the majority of weakly ER-positive cases as
basal-like, in accordance with the results obtained by the gold
standard assay (35). To validate
these findings, the researchers evaluated the prognostic value of
nestin as a single positive biomarker of basal-like cancer in a
large cohort of 3,641 early-stage invasive BC cases (34). Currently, all established IHC
panels for basal-like cancer identification require ER negativity
(23,102,103), therefore any new IHC marker that
may be interpreted independently of ER may be a valuable diagnostic
tool. Therefore, nestin, as an abundantly and stably expressed
cytoskeletal protein, may be helpful for classifying clinically
problematic cases with weak expression of ER. In a cohort
comprising 2,323 ER+ cases as defined by IHC,
nestin+ expression was noted in 5% of ER+
tumours (120 patients) and was an independent poor prognostic
factor for patients within this subgroup (34). The same trend for nestin was
observed in ER+ patients receiving endocrine therapy
only; however, due to the small number of cases, the results were
not statistically significant. To support the findings that nestin
expression may reliably identify basal-like tumours within the
ER+ subgroup, Asleh et al (34) analysed 672 nestin+ cases
against a gene expression profiling assay. As in their previous
study (35), nestin expression was
strongly associated with the intrinsic basal-like subtype,
established by the gold standard assay. These results contributed
to the improved understanding of the issue of nestin+
ER+ cases reported by Parry et al (90), which according to the standards
were classified as luminal by IHC. In the study by Asleh et
al (34), nestin expression
was observed in 371 (10%) out of all the investigated cases and was
associated with high-risk clinicopathological factors (younger age,
higher grade and high proliferation index). In contrast to Parry
et al (90) and Liu et
al (91), the analysis of
long-term survival data demonstrated that nestin positivity was an
independent poor prognostic factor in BC. However, the previous
studies examining the prognostic value of nestin have certain
limitations which may clarify such an inconsistency in the results.
For instance, in the studies by Liu et al (91) and Parry et al (90), the number of nestin+
cases included in the analyses was relatively low, comprising 24
and 20 cases respectively. Additionally, the study by Asleh et
al (34) analysed early-stage
BC cases of which 42% of the patients had not received any systemic
treatment, whereas all the patients studied by Parry et al
(90) received anthracycline-based
adjuvant chemotherapy, which may have influenced the survival
rates. Recent data from a second large cohort by Kruger et
al (96) also support the
finding that nestin is associated with aggressive tumour
characteristics (size, grade, proliferation, p53 expression and
blood vessel invasion) and the basal-like BC phenotype. Notably, in
contrast to Liu et al (91), Kruger et al noted nestin
expression more frequently in patients with negative lymph node
status. In fact, it had been proposed in previous studies that
basal-like BCs preferentially spread haematogenously, giving rise
to metastatic deposits in the brain and lungs (104). Similar to Asleh et al
(34), the researchers confirmed
the predictive value of nestin as an independent prognostic factor
associated with poor prognosis (96). Additionally, the study by Kruger
et al (96) demonstrated
that nestin protein, in addition to nestin mRNA expression, was
associated with DNA repair associated (BRCA1) germline mutations,
particularly among young women. Nestin appeared to be a stronger
predictor of BRCA1 germline mutation compared with any other
investigated core marker [including cytokeratin 5 (CK-5), EGFR,
P-cadherin and intrinsic basal-like subtype]. Moreover, nestin mRNA
expression levels and signature scores varied among TNBC subgroups,
as described by Lehmann et al (105), being highest in the basal-like 1,
mesenchymal and mesenchymal stem-like subgroups (96). Recently, the potential of treatment
of BRCA-associated cancer with poly-(ADP ribose) polymerase
inhibitors (PARPi) has been recognised, with a number of molecules
being under clinical trials (106). As a strong predictor of BRCA1
mutation, nestin may be a valuable tool for identifying patients
eligible for PARPi targeted therapy, although the validity of its
predictive value requires further study.
An additional study of 2,930 cases investigated
whether the protein expression pattern in primary tumours may
influence the first site of distant metastases (107). It was revealed that the
expression of nestin, prominin-1 (CD133) or CK-5 in the primary
tumour were significantly associated with brain metastases as the
first site of distant recurrence. Since nestin and CD133 are
proteins expressed by neural stem cells, that previous study
appeared to be in accordance with Paget's 'seed and soil' theory,
postulating that metastasis depends on the interaction between
cancer cells and the specific organ microenvironment (108). Hypothetically, BC cells
expressing neural stem cells markers may also share the same
features and may thus be particularly well adapted to the brain
microenvironment to initiate brain metastases. However, the
biological mechanisms associated with these proteins and BC brain
metastases remain unknown. According to prognostic prediction, the
1-year survival rate of patients with BC brain metastases is
<20% (109). Meisen et
al (110) proposed
nestin-targeted therapy for breast cancer metastases in two murine
models. Targeting BC brain metastases with an oncolytic virus
programmed to kill nestin-expressing cells, and to deliver the
extracellular domain of brain angiogenesis inhibitor 1,
significantly enhanced the survival of mice with established
metastatic BC brain tumours. Meisen et al (110) also investigated the cytotoxic
effect of the same nestin-targeting virus on four human BC cell
lines corresponding to the different biological types of BC.
According to Neve et al (111), BC cell lines may be clustered
into three major subsets (luminal, basal A and basal B), which
reflect the molecular subtypes of primary tumours. Using the
publicly accessible 'Neve breast cancer dataset', Meisen et
al (110) compared nestin
gene expression in 50 BC cell lines included in the analysis.
Although nestin mRNA was detected in all investigated cell lines,
increased expression levels were observed in the basal A cluster,
which matched the Perou basal-like signature of primary tumours
(10,11). The in vitro cell viability
assay on the cell lines representing the luminal (MCF7 and SKBR3),
Basal A (MDA-MB-468) and Basal B (MDA-MB-231) clusters indicated
that the same oncolytic virus exerted its cytotoxic effect on all
the investigated cell lines (110). Predictably, the most significant
cytotoxicity was observed in the MDA-MB-436 cells expressing the
highest levels of nestin.
CSCs are defined as a small subpopulation of
undifferentiated tumour cells that are characterized by their
capacity for self-renewal and ability to differentiate into
multiple tumour cell types (68,112,113). The ability of CSCs to reproduce a
continuously growing tumour, described as 'tumorigenicity', is
responsible for therapeutic resistance and metastasis (68,114). The first study demonstrating the
involvement of nestin in the biology of CSCs analysed nestin
expression in CSCs isolated from different types of human CNS
tumours (115,116). Further studies have confirmed the
utility of nestin as a potent marker of tumour cells exhibiting a
stem-like phenotype. In BCs, cells with a stem-like phenotype may
be identified based on the expression patterns of cell surface
markers, and are described as CD44 antigen (CD44)+ and
signal transducer CD24 (CD24)−/low (117). Further studies demonstrated that
a high number of CD44+/CD24−/low cells may be
particularly observed in TN tumours and cell lines, being a
predictor of poor prognosis and aggressive behaviour (118,119). Liu et al (120) demonstrated that these
CD44+/CD24−/low cells, which highly expressed
nestin and another relevant stem cell marker [POU domain, class 5,
transcription factor 1 (Oct-4)], had a greater ability to form
mammospheres in vitro (119). Furthermore, it was demonstrated
that nestin and Oct-4 co-expression was significantly associated
with younger age, higher histological grade, lymph node metastasis
and TN phenotype. Furthermore, multivariate analysis of survival
indicated that the co-expression of these two markers was
associated with shorter survival independent of age, lymph node
metastasis and TN phenotype. In IBC, nestin expression was detected
in cells forming tumour emboli within lymphatic vessels (100). These nestin+ cells
formed spheroids expressing other CSC markers (including
CD44+/CD24−/low, aldehyde dehydrogenase
cytosolic 1 and CD133) and stemness-associated transcription
factors [Oct-4, homeobox protein NANOG (Nanog) and transcription
factor (SOX-2)]. Furthermore, it was identified that
nestin+ spheroids were resistant to radiotherapy and
chemotherapy and strongly contributed to distant metastasis
(121). The study by Apostolou
et al (89) confirmed the
co-expression of nestin and other stemness markers (including
Oct3/4, Nanog and SOX-2) in circulating tumour cells (CTCs)
isolated from clinical patients. Notably, their results indicated a
marked association between the expression of these markers in CTCs
and the stage of the disease. The mechanism underlying the
involvement of nestin in the biology of CSCs was described by Zhao
et al (88). In that study,
the researchers investigated the role of nestin in primary CSCs
isolated from BC tumours. First, they studied the behaviour of
naturally occurring nestinhigh and nestinlow
populations of CSCs. Subsequently, the authors examined the
behaviour of primary CSCs with genetically modified nestin
expression by overexpressing or silencing the NES gene.
Cells with nestinhigh and nestin-overexpressing cells
had potent tumorigenicity, displayed by rapid mammosphere formation
in vitro and the induction of solid tumours in vivo.
Silencing of nestin expression induced cell cycle arrest at the
G2/M phase, promoted apoptosis and reduced the expression of
epithelial-mesenchymal transition (EMT) markers (including
N-cadherin, vimentin and α-smooth muscle actin). EMT is a dynamic
process that appears to facilitate tumour metastasis by switching
the cell phenotype from epithelial to mesenchymal (122,123). Furthermore, nestin silencing
significantly upregulated the expression of proteins involved in
the inhibition of the Wnt/β-catenin pathway, which is crucial for
the regulation of the proliferation and tumorigenicity of stem
cells (124-127). In addition, the inhibition of the
Wnt/β-catenin pathway in nestinhigh CSCs significantly
limited their tumorigenicity.
To date, a number of natural compounds have been
investigated to target breast CSCs. The inhibitory effect of Huaier
aqueous extract and nitidine chloride (a natural polyphenolic
compound isolated from the root of Zanthoxylum nitidum) on
CSCs has been tested in BC cell lines (128,129). The two compounds significantly
reduced the number of CD44+/CD24−/low cells
and decreased the expression of nestin and other pluripotency
markers (including Nanog and Oct-4). It was suggested that the
inhibition of nestin, Nanog and Oct-4 occurred via the inhibition
of the Hh pathway (128,129). The Hh signalling pathway is
associated with normal mammary gland development and its
activation, either by mutation or aberrant expression of pathway
components, leads to BC development (130). A recent study reported that in
TNBC, nestin expression may be regulated by the SOX-10
transcription factor, which directly binds to the nestin promoter
and enhances its expression (131). SOX-10 is specifically expressed
in mammary cells exhibiting the highest levels of stem/progenitor
activity, and in breast tumours with stem-like behaviour (132). SOX10 knockdown
significantly inhibits nestin expression and decreases the
stem-like features of TNBC, in terms of the
CD44+/CD24−/low cell ratio and tumour
sphere-forming abilities (131).
Importantly, the effect of SOX10 knockdown on the properties
of CSCs is partly counteracted by enforcing nestin expression.
Angiogenesis is the process of new blood vessel
formation, which involves numerous mechanisms mediating the growth
and modification of a capillary network (133,134). The process was first described in
breast tumours by Folkman (135)
and became the basis for further research, demonstrating that
sustained angiogenesis is an integral part of the progression of
the majority of cancer types (135-137). Studies on nestin expression in
human tumours have demonstrated that nestin is expressed in tumour
cells and also in tumour vessels (55,138). These observations led to further
research examining the role of nestin in the process of
angiogenesis in breast and other cancer types.
The first immunohistochemical study on the
nestin-expressing microvasculature in BC clinical samples was
conducted by Kruger et al (139). The study aimed to evaluate
angiogenesis via the assessment of immature (nestin+)
and proliferating (Ki-67+) endothelial cells in breast
tumour tissue. The study demonstrated that nestin and Ki-67
co-expression in tumour vessels, in addition to nestin+
microvessel density (nestin+MVD), were significantly
associated with the basal-like phenotype and a negative ER and PR
status. The study also assessed the prognostic value of the
vascular proliferation index (VPI), which was calculated as the
ratio between the number of nestin+Ki-67+
microvessels and the total number of nestin+
microvessels expressed as a percentage. It was demonstrated that
the VPI was increased in invasive ductal carcinoma (IDC) compared
with invasive lobular carcinoma (ILC). Furthermore, an increased
VPI was significantly associated with a shorter overall survival in
univariate and multivariate analyses. However, the prognostic value
of nestin+MVD was not confirmed in the study (139). The prognostic value of
nestin+MVD was demonstrated in our recent study
(56). It was observed that the
number of nestin-expressing microvessels was noticeably increased
in invasive tumours compared with pre-invasive lesions.
Additionally, a high value of nestin+MVD was associated
with disease progression, stage, lymph node metastasis, high tumour
grade and the TN phenotype. Our study demonstrated that a high
value of nestin+MVD was an independent factor for poor
prognosis in BC. Notably, it was also reported that the number of
nestin-expressing vessels was significantly correlated with
immature CD34+, although not with mature platelet
endothelial cell adhesion molecule (CD31)+ vessels
(56). To confirm these results,
nestin expression was analysed in endothelial cell lines isolated
from various types of human vessels. Different patterns of nestin
expression were noted in the human endothelial cells according to
their maturity. It was observed that particularly high nestin
expression occurred in an early endothelial progenitor cell line,
originating from human umbilical cord blood (140), compared with endothelial cells
from dermal microvessels and the umbilical vein (56). Taken together, these results
suggested that nestin expression may reflect the progenitor nature
of vessels, and that it is primarily limited to undifferentiated
and newly forming vessels.
In another study, it was demonstrated that nestin
expression in BC cells was positively correlated with the area and
number of vessels expressing different endothelial antigens,
including nestin, CD31, CD34 and transcription factor SOX-18
(97). Another recent study also
confirmed that nestin expression in tumour cells was associated
with higher VPI and blood vessel, although not lymphatic vessel,
invasion (96). Furthermore, a
positive correlation was observed between the area and number of
nestin-expressing vessels and vessels expressing the transcription
factor SOX-18, which is an important regulator of vascular
development contributing to the differentiation of mesenchymal stem
cells into endothelial cells (144-146). Notably, it was demonstrated that
nestin expression in tumour cells correlated with the area and the
number of nestin+ vessels, which may support hypotheses
on vascular mimicry and/or the trans-differentiation of CSCs into
endothelial cells (147,148). In BC, numerous studies have
reported the presence of dysfunctional and disorganized vessels
with a defective endothelium, in addition to the presence of
vascular-like channels formed of tumour cells (149-151). Bussolati et al (87) demonstrated that nestin-expressing
breast tumour CSCs were able to differentiate into epithelial cells
and also into endothelial cells, in vitro and in
vivo. Culturing nestin+ CSCs in the presence of
serum promoted their differentiation towards the epithelial
lineage. On the other hand, culturing nestin+ CSCs in
the presence of VEGF resulted in their differentiation into
endothelial cells. Differentiated endothelial cells were able to
express endothelial markers and organize into capillary-like
structures in Matrigel. Furthermore, implantation of
nestin+ CSCs in severe combined immunodeficient (SCID)
mice led to tumour development in which some of the intratumoural
vessels were of human origin, suggesting the in vivo
endothelial differentiation of CSCs. Notably, the study also
demonstrated that endothelial-differentiated cells derived from
nestin+ CSCs were able to form an in vivo human
vessel network and eventually an epithelial tumour when implanted
in Matrigel in SCID mice. This may suggest that a
non-differentiated population of tumorigenic nestin+
CSCs is maintained among endothelial-differentiated breast CSCs
(87).
Undoubtedly, nestin is a valuable predictive marker
which has the potential to be implemented in clinical practice, in
order to achieve improved and more accurate diagnosis of BC. Since
the use of nestin-targeted treatment in the mouse model of
BC-related brain metastasis has resulted in a notable inhibition of
disease progression, further research on possible applications in
clinical patients is worthy of attention (110). A number of studies on different
types of neoplasms have confirmed that inhibition of nestin results
in the decreased proliferation, migration and invasion of tumour
cells (61,88,152-154). Notably, nestin expression is
primarily restricted to a few types of normal cells, while it is
abundantly expressed by tumour cells, newly-formed vessels and
CSCs. Therefore, the therapy targeting nestin may be a potent and
multidirectional therapeutic strategy with limited side effects.
Improved understanding of the mechanisms through which nestin
facilitates BC may aid the development of a powerful novel
therapeutic agent.
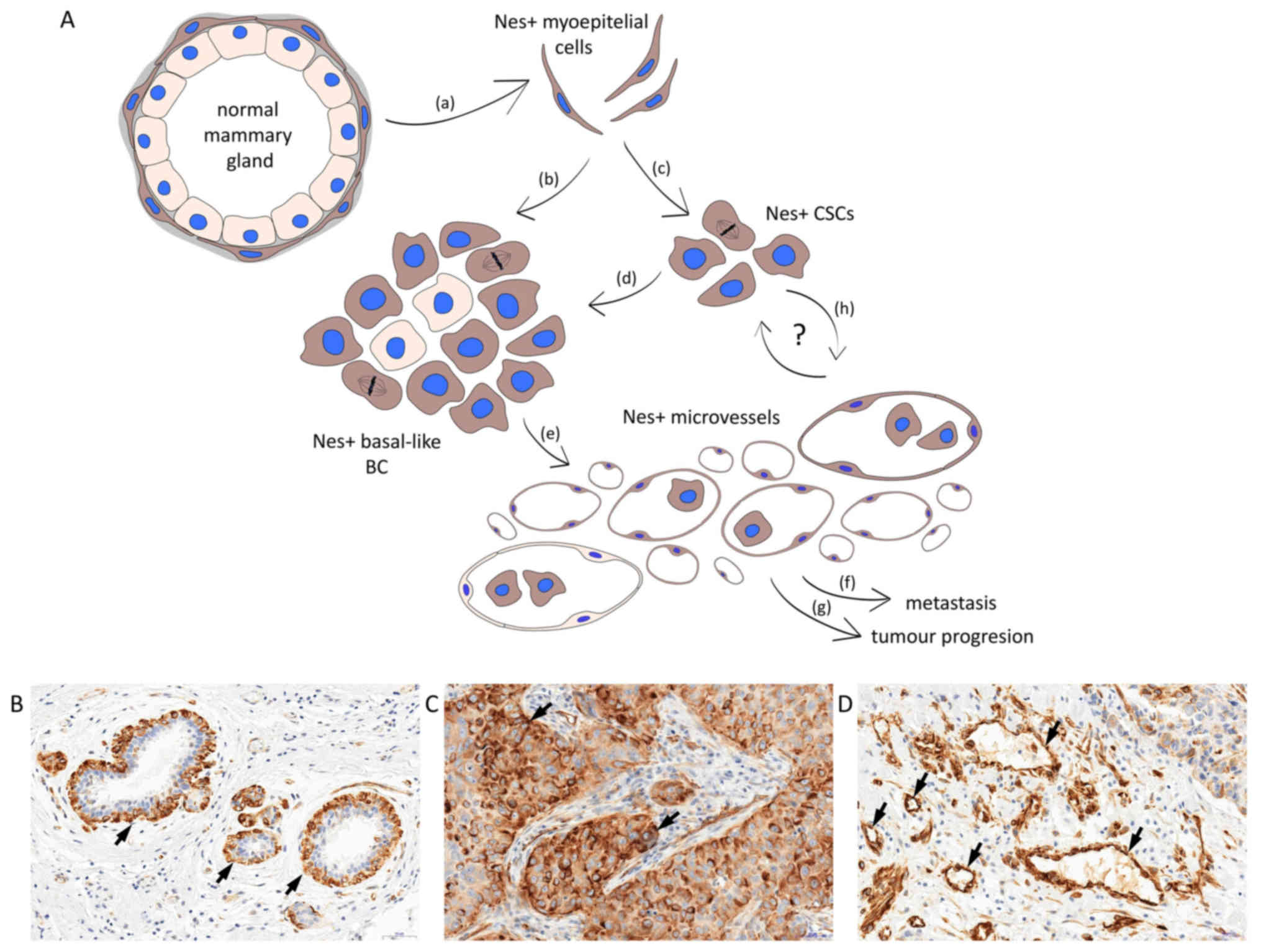
The emerging evidence suggests that nestin is
undeniably an important factor involved in BC progression (Fig. 1A). In the breast, nestin expression
is detected in the basal/myoepithelial layer of normal mammary
glands (Fig. 1B) in basal-like BC
(Fig. 1C) and BC-associated
vessels (Fig. 1D). As a specific
marker of basal-like tumours and a marker of poor prognosis, it has
the potential to become a biomarker used in daily clinical
practice. Numerous studies have confirmed the role of nestin in
important processes in tumour progression, including self-renewal
and angiogenesis; however, the molecular mechanisms underlying
these phenomena require further clarification. In conclusion, in
BC, nestin may serve as a promising prognostic factor and a
potential therapeutic target for tumour suppression and
angiogenesis inhibition.
Not applicable.
The present study was supported by Wroclaw Medical
University (grant no. Pbmn 192).
Not applicable.
AN made substantial contributions to the conception
of the article and created the major draft of the manuscript; PD
contributed to the conception of the article and revised the
manuscript critically for important intellectual content.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, McCarron P and Parkin DM: The
changing global patterns of female breast cancer incidence and
mortality. Breast Cancer Res. 6:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Bray F, Ferlay J,
Lortet-Tieulent J, Anderson BO and Jemal A: International variation
in female breast cancer incidence and mortality rates. Cancer
Epidemiol Biomarkers Prev. 24:1495–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ekwueme DU, Guy GP Jr, Rim SH, White A,
Hall IJ, Fairley TL and Dean HD: Health and economic impact of
breast cancer mortality in young women, 1970–2008. Am J Prev Med.
46:71–79. 2014. View Article : Google Scholar
|
|
5
|
Dai X, Xiang L, Li T and Bai Z: Cancer
Hallmarks, Biomarkers and breast cancer molecular subtypes. J
Cancer. 7:1281–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zardavas D, Irrthum A, Swanton C and
Piccart M: Clinical management of breast cancer heterogeneity. Nat
Rev Clin Oncol. 12:381–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koren S and Bentires-Alj M: Breast tumor
heterogeneity: Source of fitness, Hurdle for Therapy. Mol Cell.
60:537–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacroix M, Toillon RA and Leclercq G:
Stable 'portrait' of breast tumors during progression: Data from
biology, pathology and genetics. Endocr Relat Cancer. 11:497–522.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simpson PT, Reis-Filho JS, Gale T and
Lakhani SR: Molecular evolution of breast cancer. J Pathol.
205:248–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner NC and Reis-Filho JS: Basal-like
breast cancer and the BRCA1 phenotype. Oncogene. 25:5846–5853.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang Y, Zhang Q, Wang X, Yang X, Wang X,
Huang Z, Jiao Y and Wang J: Quantitative phosphoproteomics reveals
genistein as a modulator of cell cycle and DNA damage response
pathways in triple-negative breast cancer cells. Int J Oncol.
48:1016–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gnant M, Harbeck N and Thomssen C: St.
Gallen 2011: Summary of the Consensus Discussion. Breast Care
(Basel). 6:136–141. 2011. View Article : Google Scholar
|
|
16
|
Raman V, Fuentes Lorenzo JL, Stashenko EE,
Levy M, Levy MM and Camarillo IG: Lippia origanoides extract
induces cell cycle arrest and apoptosis and suppresses NF-κB
signaling in triple-negative breast cancer cells. Int J Oncol.
51:1801–1808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee A and Djamgoz MBA: Triple negative
breast cancer: Emerging therapeutic modalities and novel
combination therapies. Cancer Treat Rev. 62:110–122. 2018.
View Article : Google Scholar
|
|
18
|
Yao H, He G, Yan S, Chen C, Song L, Rosol
TJ and Deng X: Triple-negative breast cancer: Is there a treatment
on the horizon? Oncotarget. 8:1913–1924. 2017.
|
|
19
|
Prat A, Pineda E, Adamo B, Galván P,
Fernández A, Gaba L, Díez M, Viladot M, Arance A and Muñoz M:
Clinical implications of the intrinsic molecular subtypes of breast
cancer. Breast. 24(Suppl 2): S26–S35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fulford LG, Easton DF, Reis-Filho JS,
Sofronis A, Gillett CE, Lakhani SR and Hanby A: Specific
morphological features predictive for the basal phenotype in grade
3 invasive ductal carcinoma of breast. Histopathology. 49:22–34.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gudjonsson T, Adriance MC, Sternlicht MD,
Petersen OW and Bissell MJ: Myoepithelial cells: Their origin and
function in breast morphogenesis and neoplasia. J Mammary Gland
Biol Neoplasia. 10:261–272. 2005. View Article : Google Scholar
|
|
22
|
Badowska-Kozakiewicz AM and Budzik MP:
Immunohisto-chemical characteristics of basal-like breast cancer.
Contemp Oncol (Pozn). 20:436–443. 2016.
|
|
23
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheang MC, Voduc D, Bajdik C, Leung S,
McKinney S, Chia SK, Perou CM and Nielsen TO: Basal-like breast
cancer defined by five biomarkers has superior prognostic value
than triple-negative phenotype. Clin Cancer Res. 14:1368–1376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prat A, Adamo B, Cheang MC, Anders CK,
Carey LA and Perou CM: Molecular characterization of basal-like and
non-basal-like triple-negative breast cancer. Oncologist.
18:123–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lachapelle J and Foulkes W:
Triple-negative and basal-like breast cancer: Implications for
oncologists. Curr Oncol. 18:161–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bertucci F, Finetti P, Viens P and
Birnbaum D: Difference in therapeutic response between basal and
nonbasal triple-negative breast cancers. Oncologist. 18:1060–1061.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar
|
|
29
|
Sheffield BS, Kos Z, Asleh-Aburaya K, Wang
XQ, Leung S, Gao D, Won J, Chow C, Rachamadugu R, Stijleman I, et
al: Molecular subtype profiling of invasive breast cancers weakly
positive for estrogen receptor. Breast Cancer Res Treat.
155:483–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prabhu JS, Korlimarla A, Desai K,
Alexander A, Raghavan R, Anupama C, Dendukuri N, Manjunath S,
Correa M, Raman N, et al: A majority of low (1-10%) ER positive
breast cancers behave like hormone receptor negative tumors. J
Cancer. 5:156–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwamoto T, Booser D, Valero V, Murray JL,
Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y, et al: Estrogen
receptor (ER) mRNA and ER-related gene expression in breast cancers
that are 1% to 10% ER-positive by immunohistochemistry. J Clin
Oncol. 30:729–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nadji M, Gomez-Fernandez C, Ganjei-Azar P
and Morales AR: Immunohistochemistry of estrogen and progesterone
receptors reconsidered: Experience with 5,993 breast cancers. Am J
Clin Pathol. 123:21–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khoshnoud MR, Löfdahl B, Fohlin H,
Fornander T, Stål O, Skoog L, Bergh J and Nordenskjöld B:
Immunohistochemistry compared to cytosol assays for determination
of estrogen receptor and prediction of the long-term effect of
adjuvant tamoxifen. Breast Cancer Res Treat. 126:421–430. 2011.
View Article : Google Scholar
|
|
34
|
Asleh K, Won JR, Gao D, Voduc KD and
Nielsen TO: Nestin expression in breast cancer: Association with
prognosis and subtype on 3641 cases with long-term follow-up.
Breast Cancer Res Treat. 168:107–115. 2018. View Article : Google Scholar
|
|
35
|
Asleh-Aburaya K, Sheffield BS, Kos Z, Won
JR, Wang XQ, Gao D, Wolber R, Gilks CB, Bernard PS, Chia SK, et al:
Basal biomarkers nestin and INPP4b identify intrinsic subtypes
accurately in breast cancers that are weakly positive for oestrogen
receptor. Histopathology. 70:185–194. 2017. View Article : Google Scholar
|
|
36
|
Michalczyk K and Ziman M: Nestin structure
and predicted function in cellular cytoskeletal organisation.
Histol Histopathol. 20:665–671. 2005.PubMed/NCBI
|
|
37
|
Wiese C, Rolletschek A, Kania G, Blyszczuk
P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR and Wobus AM:
Nestin expression - a property of multi-lineage progenitor cells?
Cell Mol Life Sci. 61:2510–2522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mokrý J and Nĕmecek S: Immunohistochemical
detection of intermediate filament nestin. Acta Medica (Hradec
Kralove). 41:73–80. 1998.
|
|
39
|
Cattaneo E and McKay R: Proliferation and
differentiation of neuronal stem cells regulated by nerve growth
factor. Nature. 347:762–765. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krum JM and Rosenstein JM: Transient
coexpression of nestin, GFAP, and vascular endothelial growth
factor in mature reactive astroglia following neural grafting or
brain wounds. Exp Neurol. 160:348–360. 1999. View Article : Google Scholar
|
|
42
|
Vaittinen S, Lukka R, Sahlgren C, Hurme T,
Rantanen J, Lendahl U, Eriksson JE and Kalimo H: The expression of
intermediate filament protein nestin as related to vimentin and
desmin in regenerating skeletal muscle. J Neuropathol Exp Neurol.
60:588–597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lindqvist J, Torvaldson E, Gullmets J,
Karvonen H, Nagy A, Taimen P and Eriksson JE: Nestin contributes to
skeletal muscle homeostasis and regeneration. J Cell Sci.
130:2833–2842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
About I, Laurent-Maquin D, Lendahl U and
Mitsiadis TA: Nestin expression in embryonic and adult human teeth
under normal and pathological conditions. Am J Pathol. 157:287–295.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin RC, Matesic DF, Marvin M, McKay RD and
Brüstle O: Re-expression of the intermediate filament nestin in
reactive astrocytes. Neurobiol Dis. 2:79–85. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sejersen T and Lendahl U: Transient
expression of the intermediate filament nestin during skeletal
muscle development. J Cell Sci. 106:1291–1300. 1993.PubMed/NCBI
|
|
47
|
Kachinsky AM, Dominov JA and Miller JB:
Myogenesis and the intermediate filament protein, nestin. Dev Biol.
165:216–228. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kachinsky AM, Dominov JA and Miller JB:
Intermediate filaments in cardiac myogenesis: Nestin in the
developing mouse heart. J Histochem Cytochem. 43:843–847. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Terling C, Rass A, Mitsiadis TA, Fried K,
Lendahl U and Wroblewski J: Expression of the intermediate filament
nestin during rodent tooth development. Int J Dev Biol. 39:947–956.
1995.PubMed/NCBI
|
|
50
|
Fröjdman K, Pelliniemi LJ, Lendahl U,
Virtanen I and Eriksson JE: The intermediate filament protein
nestin occurs transiently in differentiating testis of rat and
mouse. Differentiation. 61:243–249. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zulewski H, Abraham EJ, Gerlach MJ, Daniel
PB, Moritz W, Müller B, Vallejo M, Thomas MK and Habener JF:
Multipotential nestin-positive stem cells isolated from adult
pancreatic islets differentiate ex vivo into pancreatic endocrine,
exocrine, and hepatic phenotypes. Diabetes. 50:521–533. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vanderwinden JM, Gillard K, De Laet MH,
Messam CA and Schiffmann SN: Distribution of the intermediate
filament nestin in the muscularis propria of the human
gastrointestinal tract. Cell Tissue Res. 309:261–268. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vogel W, Grünebach F, Messam CA, Kanz L,
Brugger W and Bühring HJ: Heterogeneity among human bone
marrow-derived mesenchymal stem cells and neural progenitor cells.
Haematologica. 88:126–133. 2003.PubMed/NCBI
|
|
54
|
Amoh Y, Yang M, Li L, Reynoso J, Bouvet M,
Moossa AR, Katsuoka K and Hoffman RM: Nestin-linked green
fluorescent protein transgenic nude mouse for imaging human tumor
angiogenesis. Cancer Res. 65:5352–5357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mokrý J, Cízková D, Filip S, Ehrmann J,
Osterreicher J, Kolár Z and English D: Nestin expression by newly
formed human blood vessels. Stem Cells Dev. 13:658–664. 2004.
View Article : Google Scholar
|
|
56
|
Nowak A, Grzegrzolka J, Paprocka M,
Piotrowska A, Rys J, Matkowski R and Dziegiel P: Nestin-positive
microvessel density is an independent prognostic factor in breast
cancer. Int J Oncol. 51:668–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tohyama T, Lee VM, Rorke LB, Marvin M,
McKay RD and Trojanowski JQ: Nestin expression in embryonic human
neuroepithelium and in human neuroepithelial tumor cells. Lab
Invest. 66:303–313. 1992.PubMed/NCBI
|
|
58
|
Dahlstrand J, Collins VP and Lendahl U:
Expression of the class VI intermediate filament nestin in human
central nervous system tumors. Cancer Res. 52:5334–5341.
1992.PubMed/NCBI
|
|
59
|
Li H, Cherukuri P, Li N, Cowling V,
Spinella M, Cole M, Godwin AK, Wells W and DiRenzo J: Nestin is
expressed in the basal/myoepithelial layer of the mammary gland and
is a selective marker of basal epithelial breast tumors. Cancer
Res. 67:501–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kleeberger W, Bova GS, Nielsen ME, Herawi
M, Chuang AY, Epstein JI and Berman DM: Roles for the stem cell
associated intermediate filament Nestin in prostate cancer
migration and metastasis. Cancer Res. 67:9199–9206. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sterlacci W, Savic S, Fiegl M, Obermann E
and Tzankov A: Putative stem cell markers in non-small-cell lung
cancer: A clinicopathologic characterization. J Thorac Oncol.
9:41–49. 2014. View Article : Google Scholar
|
|
63
|
Qin Q, Sun Y, Fei M, Zhang J, Jia Y, Gu M,
Xia R, Chen S and Deng A: Expression of putative stem marker nestin
and CD133 in advanced serous ovarian cancer. Neoplasma. 59:310–315.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ishiwata T, Matsuda Y and Naito Z: Nestin
in gastrointestinal and other cancers: Effects on cells and tumor
angiogenesis. World J Gastroenterol. 17:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Piras F, Perra MT, Murtas D, Minerba L,
Floris C, Maxia C, Demurtas P, Ugalde J, Ribatti D and Sirigu P:
The stem cell marker nestin predicts poor prognosis in human
melanoma. Oncol Rep. 23:17–24. 2010.
|
|
66
|
Zhong B, Wang T, Lun X, Zhang J, Zheng S,
Yang W, Li W, Xiang AP and Chen Z: Contribution of nestin positive
esophageal squamous cancer cells on malignant proliferation,
apoptosis, and poor prognosis. Cancer Cell Int. 14:572014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li S, Lai Y, Fan J, Shen C and Che G:
Clinicopathological and prognostic significance of Nestin
expression in patients with non-small cell lung cancer: A
systematic review and meta-analysis. Clin Exp Med. 17:161–174.
2017. View Article : Google Scholar
|
|
68
|
Neradil J and Veselska R: Nestin as a
marker of cancer stem cells. Cancer Sci. 106:803–811. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Guérette D, Khan PA, Savard PE and Vincent
M: Molecular evolution of type VI intermediate filament proteins.
BMC Evol Biol. 7:1642007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chou YH, Khuon S, Herrmann H and Goldman
RD: Nestin promotes the phosphorylation-dependent disassembly of
vimentin intermediate filaments during mitosis. Mol Biol Cell.
14:1468–1478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sjöberg G, Jiang WQ, Ringertz NR, Lendahl
U and Sejersen T: Colocalization of nestin and vimentin/desmin in
skeletal muscle cells demonstrated by three-dimensional
fluorescence digital imaging microscopy. Exp Cell Res. 214:447–458.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Holle AW, Kalafat M, Ramos AS, Seufferlein
T, Kemkemer R and Spatz JP: Intermediate filament reorganization
dynamically influences cancer cell alignment and migration. Sci
Rep. 7:451522017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Makihara H, Inaba H, Enomoto A, Tanaka H,
Tomono Y, Ushida K, Goto M, Kurita K, Nishida Y, Kasahara K, et al:
Desmin phosphorylation by Cdk1 is required for efficient separation
of desmin intermediate filaments in mitosis and detected in murine
embryonic/newborn muscle and human rhabdomyosarcoma tissues.
Biochem Biophys Res Commun. 478:1323–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sahlgren CM, Mikhailov A, Vaittinen S,
Pallari HM, Kalimo H, Pant HC and Eriksson JE: Cdk5 regulates the
organization of Nestin and its association with p35. Mol Cell Biol.
23:5090–5106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sahlgren CM, Mikhailov A, Hellman J, Chou
YH, Lendahl U, Goldman RD and Eriksson JE: Mitotic reorganization
of the intermediate filament protein nestin involves
phosphorylation by cdc2 kinase. J Biol Chem. 276:16456–16463. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Matsuda Y, Ishiwata T, Yoshimura H,
Yamahatsu K, Minamoto T and Arai T: Nestin phosphorylation at
threonines 315 and 1299 correlates with proliferation and
metastasis of human pancreatic cancer. Cancer Sci. 108:354–361.
2017. View Article : Google Scholar :
|
|
77
|
Pozo K and Bibb JA: The Emerging Role of
Cdk5 in Cancer. Trends Cancer. 2:606–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chiker S, Pennaneach V, Loew D, Dingli F,
Biard D, Cordelières FP, Gemble S, Vacher S, Bieche I, Hall J, et
al: Cdk5 promotes DNA replication stress checkpoint activation
through RPA-32 phosphorylation, and impacts on metastasis free
survival in breast cancer patients. Cell Cycle. 14:3066–3078. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liang Q, Li L, Zhang J, Lei Y, Wang L, Liu
DX, Feng J, Hou P, Yao R, Zhang Y, et al: CDK5 is essential for
TGF-β1-induced epithelial-mesenchymal transition and breast cancer
progression. Sci Rep. 3:29322013. View Article : Google Scholar
|
|
80
|
Sahlgren CM, Pallari HM, He T, Chou YH,
Goldman RD and Eriksson JE: A nestin scaffold links Cdk5/p35
signaling to oxidant-induced cell death. EMBO J. 25:4808–4819.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Choi SS, Syn WK, Karaca GF, Omenetti A,
Moylan CA, Witek RP, Agboola KM, Jung Y, Michelotti GA and Diehl
AM: Leptin promotes the myofibroblastic phenotype in hepatic
stellate cells by activating the hedgehog pathway. J Biol Chem.
285:36551–36560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Habib JG and O'Shaughnessy JA: The
hedgehog pathway in triple-negative breast cancer. Cancer Med.
5:2989–3006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fan Y, Chong YS, Choolani MA, Cregan MD
and Chan JK: Unravelling the mystery of stem/progenitor cells in
human breast milk. PLoS One. 5:e144212010. View Article : Google Scholar
|
|
84
|
Patki S, Kadam S, Chandra V and Bhonde R:
Human breast milk is a rich source of multipotent mesenchymal stem
cells. Hum Cell. 23:35–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cregan MD, Fan Y, Appelbee A, Brown ML,
Klopcic B, Koppen J, Mitoulas LR, Piper KM, Choolani MA, Chong YS,
et al: Identification of nestin-positive putative mammary stem
cells in human breastmilk. Cell Tissue Res. 329:129–136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hosseini SM, Talaei-Khozani T, Sani M and
Owrangi B: Differentiation of human breast-milk stem cells to
neural stem cells and neurons. Neurol Res Int. 2014:8078962014.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bussolati B, Grange C, Sapino A and
Camussi G: Endothelial cell differentiation of human breast tumour
stem/progenitor cells. J Cell Mol Med. 13:309–319. 2009. View Article : Google Scholar
|
|
88
|
Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M
and Liu C: Nestin positively regulates the Wnt/β-catenin pathway
and the proliferation, survival and invasiveness of breast cancer
stem cells. Breast Cancer Res. 16:4082014. View Article : Google Scholar
|
|
89
|
Apostolou P, Toloudi M, Chatziioannou M,
Ioannou E and Papasotiriou I: Cancer stem cells stemness
transcription factors expression correlates with breast cancer
disease stage. Curr Stem Cell Res Ther. 7:415–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Parry S, Savage K, Marchiò C and
Reis-Filho JS: Nestin is expressed in basal-like and triple
negative breast cancers. J Clin Pathol. 61:1045–1050. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin
F, Xu H and Lu P: Clinical implications for nestin protein
expression in breast cancer. Cancer Sci. 101:815–819. 2010.
View Article : Google Scholar
|
|
92
|
Piras F, Ionta MT, Lai S, Perra MT, Atzori
F, Minerba L, Pusceddu V, Maxia C, Murtas D, Demurtas P, et al:
Nestin expression associates with poor prognosis and triple
negative phenotype in locally advanced (T4) breast cancer. Eur J
Histochem. 55:e392011. View Article : Google Scholar
|
|
93
|
Won JR, Gao D, Chow C, Cheng J, Lau SY,
Ellis MJ, Perou CM, Bernard PS and Nielsen TO: A survey of
immunohistochemical biomarkers for basal-like breast cancer against
a gene expression profile gold standard. Mod Pathol. 26:1438–1450.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tampaki EC, Tampakis A, Nonni A,
Kontzoglou K, Patsouris E and Kouraklis G: Nestin and cluster of
differentiation 146 expression in breast cancer: Predicting early
recurrence by targeting metastasis? Tumour Biol.
39:1010428317691181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gao N, Xu H, Liu C, Xu H, Chen G, Wang X,
Li Y and Wang Y: Nestin: Predicting specific survival factors for
breast cancer. Tumour Biol. 35:1751–1755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Krüger K, Wik E, Knutsvik G, Nalwoga H,
Klingen TA, Arnes JB, Chen Y, Mannelqvist M, Dimitrakopoulou K,
Stefansson IM, et al: Expression of Nestin associates with BRCA1
mutations, a basal-like phenotype and aggressive breast cancer. Sci
Rep. 7:10892017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nowak A, Grzegrzółka J, Kmiecik A,
Piotrowska A, Matkowski R and Dzięgiel P: Role of nestin expression
in angiogenesis and breast cancer progression. Int J Oncol.
52:527–535. 2018.PubMed/NCBI
|
|
98
|
Huang A, Cao S and Tang L: The tumor
microenvironment and inflammatory breast cancer. J Cancer.
8:1884–1891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
van Uden DJ, van Laarhoven HW, Westenberg
AH, de Wilt JH and Blanken-Peeters CF: Inflammatory breast cancer:
An overview. Crit Rev Oncol Hematol. 93:116–126. 2015. View Article : Google Scholar
|
|
100
|
Xiao Y, Ye Y, Yearsley K, Jones S and
Barsky SH: The lymphovascular embolus of inflammatory breast cancer
expresses a stem cell-like phenotype. Am J Pathol. 173:561–574.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rögelsperger O, Ekmekcioglu C, Jäger W,
Klimpfinger M, Königsberg R, Krenbek D, Sellner F and Thalhammer T:
Coexpression of the melatonin receptor 1 and nestin in human breast
cancer specimens. J Pineal Res. 46:422–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Laakso M, Loman N, Borg A and Isola J:
Cytokeratin 5/14-positive breast cancer: True basal phenotype
confined to BRCA1 tumors. Mod Pathol. 18:1321–1328. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Badve S, Dabbs DJ, Schnitt SJ, Baehner FL,
Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR,
et al: Basal-like and triple-negative breast cancers: A critical
review with an emphasis on the implications for pathologists and
oncologists. Mod Pathol. 24:157–167. 2011. View Article : Google Scholar
|
|
104
|
Foulkes WD, Metcalfe K, Hanna W, Lynch HT,
Ghadirian P, Tung N, Olopade O, Weber B, McLennan J, Olivotto IA,
et al: Disruption of the expected positive correlation between
breast tumor size and lymph node status in BRCA1-related breast
carcinoma. Cancer. 98:1569–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zimmer AS, Gillard M, Lipkowitz S and Lee
JM: Update on PARP inhibitors in breast cancer. Curr Treat Options
Oncol. 19:212018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sihto H, Lundin J, Lundin M, Lehtimäki T,
Ristimäki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T,
Isola J, et al: Breast cancer biological subtypes and protein
expression predict for the preferential distant metastasis sites: A
nationwide cohort study. Breast Cancer Res. 13:R872011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
de Groot AE, Roy S, Brown JS, Pienta KJ
and Amend SR: Revisiting Seed and Soil: Examining the primary tumor
and cancer cell foraging in metastasis. Mol Cancer Res. 15:361–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Custódio-Santos T, Videira M and Brito MA:
Brain metastasization of breast cancer. Biochim Biophys Acta.
1868:132–147. 2017.PubMed/NCBI
|
|
110
|
Meisen WH, Dubin S, Sizemore ST,
Mathsyaraja H, Thies K, Lehman NL, Boyer P, Jaime-Ramirez AC, Elder
JB, Powell K, et al: Changes in BAI1 and nestin expression are
prognostic indicators for survival and metastases in breast cancer
and provide opportunities for dual targeted therapies. Mol Cancer
Ther. 14:307–314. 2015. View Article : Google Scholar :
|
|
111
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sin WC and Lim CL: Breast cancer stem
cells-from origins to targeted therapy. Stem Cell Investig.
4:962017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Albini A, Bruno A, Gallo C, Pajardi G,
Noonan DM and Dallaglio K: Cancer stem cells and the tumor
microenvironment: Interplay in tumor heterogeneity. Connect Tissue
Res. 56:414–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shima H, Yamada A, Ishikawa T and Endo I:
Are breast cancer stem cells the key to resolving clinical issues
in breast cancer therapy? Gland Surg. 6:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
116
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang H, Wang L, Song Y, Wang S, Huang X,
Xuan Q, Kang X and Zhang Q: CD44+/CD24−
phenotype predicts a poor prognosis in triple-negative breast
cancer. Oncol Lett. 14:5890–5898. 2017.PubMed/NCBI
|
|
119
|
Ma F, Li H, Wang H, Shi X, Fan Y, Ding X,
Lin C, Zhan Q, Qian H and Xu B: Enriched CD44(+)/CD24(−) population
drives the aggressive phenotypes presented in triple-negative
breast cancer (TNBC). Cancer Lett. 353:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Liu C, Cao X, Zhang Y, Xu H, Zhang R, Wu
Y, Lu P and Jin F: Co-expression of Oct-4 and Nestin in human
breast cancers. Mol Biol Rep. 39:5875–5881. 2012. View Article : Google Scholar
|
|
121
|
Xiao Y, Ye Y, Zou X, Jones S, Yearsley K,
Shetuni B, Tellez J and Barsky SH: The lymphovascular embolus of
inflammatory breast cancer exhibits a Notch 3 addiction. Oncogene.
30:287–300. 2011. View Article : Google Scholar
|
|
122
|
Chang R, Zhang P and You J:
Post-translational modifications of EMT transcriptional factors in
cancer metastasis. Open Life Sci. 11:237–243. 2016.
|
|
123
|
Grzegrzolka J, Biala M, Wojtyra P,
Kobierzycki C, Olbromski M, Gomulkiewicz A, Piotrowska A, Rys J,
Podhorska-Okolow M and Dziegiel P: Expression of EMT markers SLUG
and TWIST in breast cancer. Anticancer Res. 35:3961–3968.
2015.PubMed/NCBI
|
|
124
|
Liang Q, Li W, Zhao Z and Fu Q:
Advancement of Wnt signal pathway and the target of breast cancer.
Open Life Sci. 11:98–104. 2016.
|
|
125
|
Luo G, Huang D, Tao R and Chen J: The role
of E-cadherin - 160C/A polymorphism in breast cancer. Open Life
Sci. 11:110–115. 2016.
|
|
126
|
Odiba A, Ottah V, Anunobi O, Edeke AA,
Ukegbu CY, Chukwunonyelum I, Onosakponome I and Joshua PE: Research
progress in oncology. Highlighting and exploiting the roles of
several strategic proteins in understanding cancer biology. Open
Life Sci. 11:331–347. 2016.
|
|
127
|
de Sousa E Melo F and Vermeulen L: Wnt
Signaling in cancer stem cell biology. Cancers (Basel).
8:82016.
|
|
128
|
Sun M, Zhang N, Wang X, Li Y, Qi W, Zhang
H, Li Z and Yang Q: Hedgehog pathway is involved in nitidine
chloride induced inhibition of epithelial-mesenchymal transition
and cancer stem cells-like properties in breast cancer cells. Cell
Biosci. 6:442016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang X, Zhang N, Huo Q, Sun M, Dong L,
Zhang Y, Xu G and Yang Q: Huaier aqueous extract inhibits stem-like
characteristics of MCF7 breast cancer cells via inactivation of
hedgehog pathway. Tumour Biol. 35:10805–10813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hatsell S and Frost AR: Hedgehog signaling
in mammary gland development and breast cancer. J Mammary Gland
Biol Neoplasia. 12:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Feng W, Liu S, Zhu R, Li B, Zhu Z, Yang J
and Song C: SOX10 induced Nestin expression regulates cancer stem
cell properties of TNBC cells. Biochem Biophys Res Commun.
485:522–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dravis C, Spike BT, Harrell JC, Johns C,
Trejo CL, Southard-Smith EM, Perou CM and Wahl GM: Sox10 regulates
stem/progenitor and mesenchymal cell states in mammary epithelial
cells. Cell Reports. 12:2035–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Krupkova O Jr, Loja T, Zambo I and
Veselska R: Nestin expression in human tumors and tumor cell lines.
Neoplasma. 57:291–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Krüger K, Stefansson IM, Collett K, Arnes
JB, Aas T and Akslen LA: Microvessel proliferation by co-expression
of endothelial nestin and Ki-67 is associated with a basal-like
phenotype and aggressive features in breast cancer. Breast.
22:282–288. 2013. View Article : Google Scholar
|
|
140
|
Paprocka M, Krawczenko A, Dus D, Kantor A,
Carreau A, Grillon C and Kieda C: CD133 positive progenitor
endothelial cell lines from human cord blood. Cytometry A.
79:594–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Alliot F, Rutin J, Leenen PJ and Pessac B:
Pericytes and periendothelial cells of brain parenchyma vessels
co-express aminopeptidase N, aminopeptidase A, and nestin. J
Neurosci Res. 58:367–378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Nakagawa S, Miki Y, Miyashita M, Hata S,
Takahashi Y, Rai Y, Sagara Y, Ohi Y, Hirakawa H, Tamaki K, et al:
Tumor micro-environment in invasive lobular carcinoma: Possible
therapeutic targets. Breast Cancer Res Treat. 155:65–75. 2016.
View Article : Google Scholar
|
|
143
|
Morikawa S, Baluk P, Kaidoh T, Haskell A,
Jain RK and McDonald DM: Abnormalities in pericytes on blood
vessels and endothelial sprouts in tumors. Am J Pathol.
160:985–1000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ikhapoh IA, Pelham CJ and Agrawal DK:
Sry-type HMG box 18 contributes to the differentiation of bone
marrow-derived mesenchymal stem cells to endothelial cells.
Differentiation. 89:87–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Downes M and Koopman P: SOX18 and the
transcriptional regulation of blood vessel development. Trends
Cardiovasc Med. 11:318–324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pula B, Olbromski M, Wojnar A,
Gomulkiewicz A, Witkiewicz W, Ugorski M, Dziegiel P and
Podhorska-Okolow M: Impact of SOX18 expression in cancer cells and
vessels on the outcome of invasive ductal breast carcinoma. Cell
Oncol (Dordr). 36:469–483. 2013. View Article : Google Scholar
|
|
147
|
Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T,
Gu Q, Yao Z, Dong XY, Zhao N and Liu N: CD133+ cells
with cancer stem cell characteristics associates with vasculogenic
mimicry in triple-negative breast cancer. Oncogene. 32:544–553.
2013. View Article : Google Scholar
|
|
148
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Folberg R, Hendrix MJ and Maniotis AJ:
Vasculogenic mimicry and tumor angiogenesis. Am J Pathol.
156:361–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Baluk P, Hashizume H and McDonald DM:
Cellular abnormalities of blood vessels as targets in cancer. Curr
Opin Genet Dev. 15:102–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Shirakawa K, Kobayashi H, Heike Y,
Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M
and Wakasugi H: Hemodynamics in vasculogenic mimicry and
angiogenesis of inflammatory breast cancer xenograft. Cancer Res.
62:560–566. 2002.PubMed/NCBI
|
|
152
|
Narita K, Matsuda Y, Seike M, Naito Z,
Gemma A and Ishiwata T: Nestin regulates proliferation, migration,
invasion and stemness of lung adenocarcinoma. Int J Oncol.
44:1118–1130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Akiyama M, Matsuda Y, Ishiwata T, Naito Z
and Kawana S: Inhibition of the stem cell marker nestin reduces
tumor growth and invasion of malignant melanoma. J Invest Dermatol.
133:1384–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Matsuda Y, Ishiwata T, Yoshimura H,
Yamashita S, Ushijima T and Arai T: Systemic administration of
small interfering RNA targeting human nestin inhibits pancreatic
cancer cell proliferation and metastasis. Pancreas. 45:93–100.
2016. View Article : Google Scholar
|