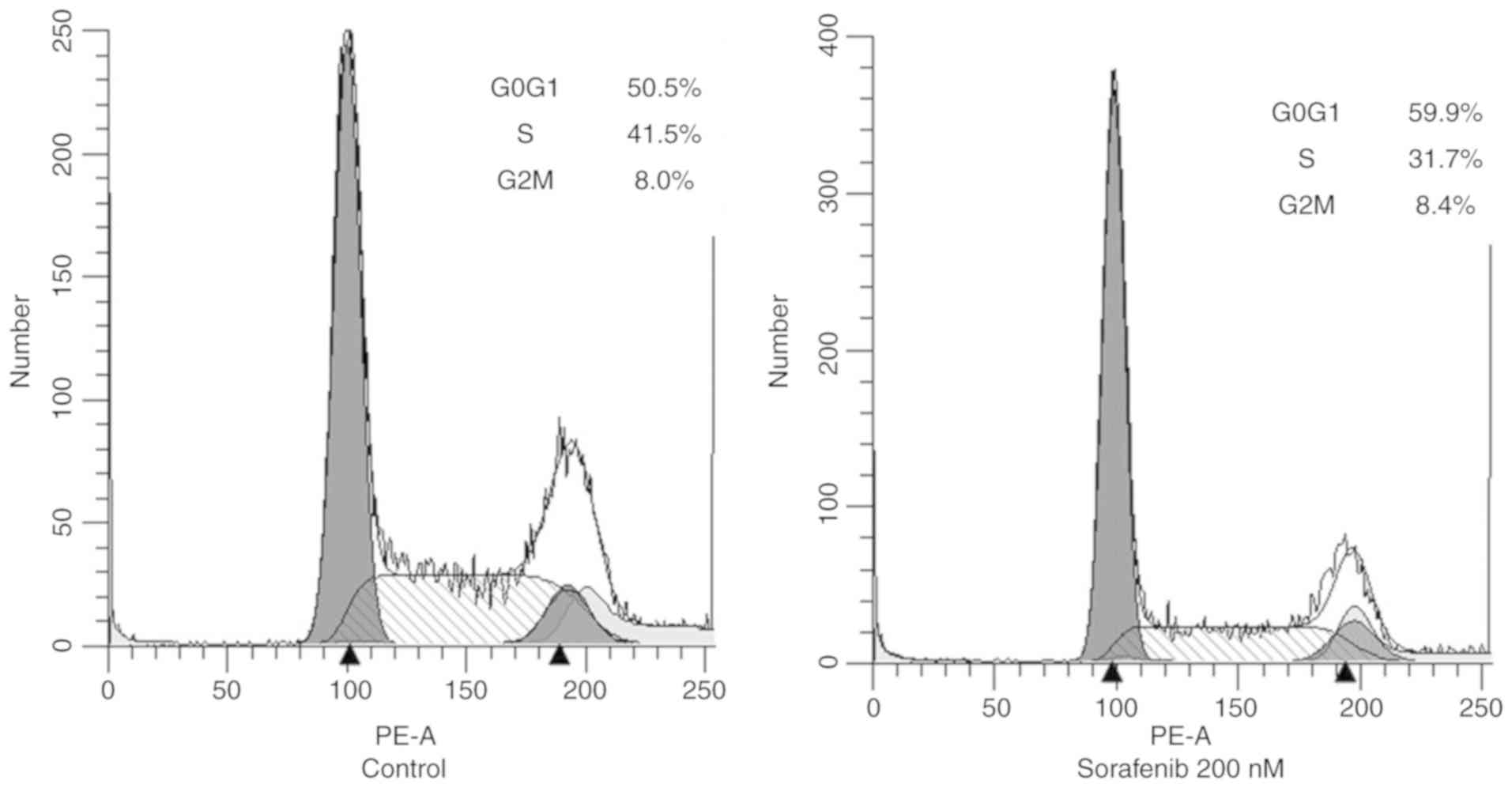
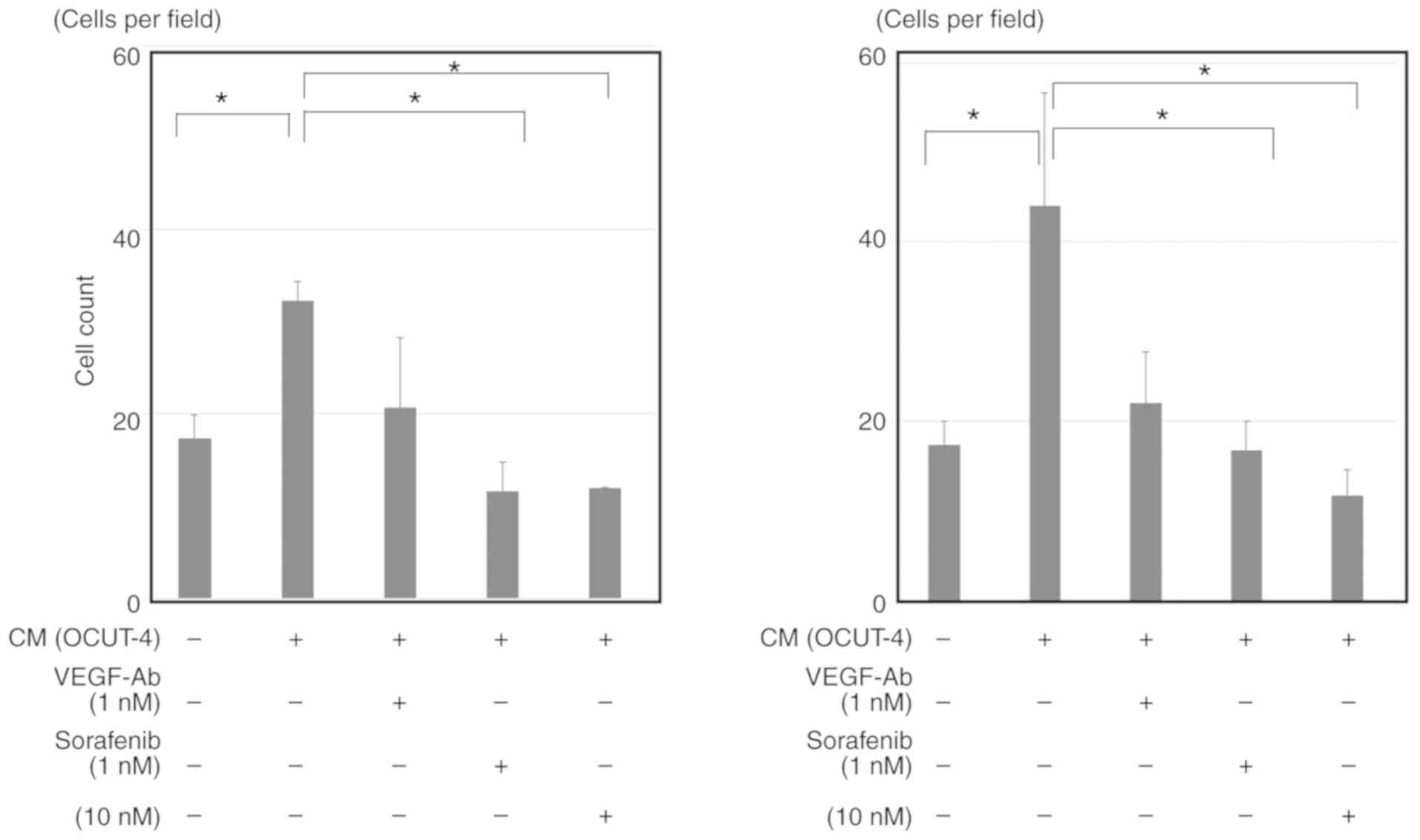
|
1
|
Sugitani I, Miyauchi A, Sugino K, Okamoto
T, Yoshida A and Suzuki S: Prognostic factors and treatment
outcomes for anaplastic thyroid carcinoma: ATC Research Consortium
of Japan cohort study of 677 patients. World J Surg. 36:1247–1254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haymart MR, Banerjee M, Yin H, Worden F
and Griggs JJ: Marginal treatment benefit in anaplastic thyroid
cancer. Cancer. 119:3133–3139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung SH, Onoda N, Ishikawa T, Ogisawa K,
Takenaka C, Yano Y, Hato F and Hirakawa K: Peroxisome
proliferator-activated receptor gamma activation induces cell cycle
arrest via the p53-independent pathway in human anaplastic thyroid
cancer cells. Jpn J Cancer Res. 93:1358–1365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nobuhara Y, Onoda N, Yamashita Y, Yamasaki
M, Ogisawa K, Takashima T, Ishikawa T and Hirakawa K: Efficacy of
epidermal growth factor receptor-targeted molecular therapy in
anaplastic thyroid cancer cell lines. Br J Cancer. 92:1110–1116.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurata K, Onoda N, Noda S, Kashiwagi S,
Asano Y, Hirakawa K and Ohira M: Growth arrest by activated BRAF
and MEK inhibition in human anaplastic thyroid cancer cells. Int J
Oncol. 49:2303–2308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onoda N, Nakamura M, Aomatsu N, Noda S,
Kashiwagi S, Kurata K, Uchino S and Hirakawa K: Significant
cytostatic effect of everolimus on a gefitinib-resistant anaplastic
thyroid cancer cell line harboring PI3KCA gene mutation. Mol Clin
Oncol. 3:522–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasaian K, Wiseman SM, Walker BA, Schein
JE, Zhao Y, Hirst M, Moore RA, Mungall AJ, Marra MA and Jones SJ:
The genomic and transcriptomic landscape of anaplastic thyroid
cancer: Implications for therapy. BMC Cancer. 15:9842015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi H, Ye X, Long B, Ye T, Zhang L, Yan F,
Yang Y and Li L: Inhibition of the AKT/mTOR pathway augments the
anticancer effects of sorafenib in thyroid cancer. Cancer Biother
Radiopharm. 32:176–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Savvides P, Nagaiah G, Lavertu P, Fu P,
Wright JJ, Chapman R, Wasman J, Dowlati A and Remick SC: Phase II
trial of sorafenib in patients with advanced anaplastic carcinoma
of the thyroid. Thyroid. 23:600–604. 2013. View Article : Google Scholar :
|
|
14
|
Ito Y, Onoda N, Ito KI, Sugitani I,
Takahashi S, Yamaguchi I, Kabu K and Tsukada K: Sorafenib in
japanese patients with locally advanced or metastatic medullary
thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid.
27:1142–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for anaplastic thyroid cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugitani I, Onoda N, Ito KI and Suzuki S:
Management of anaplastic thyroid carcinoma: The fruits from the ATC
research consortium of Japan. J Nippon Med Sch. 85:18–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brose MS, Worden FP, Newbold KL, Guo M and
Hurria A: Effect of age on the efficacy and safety of lenvatinib in
radio-iodine-refractory differentiated thyroid cancer in the phase
III SELECT trial. J Clin Oncol. 35:2692–2699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaman A, Wu W and Bivona TG: Targeting
oncogenic BRAF: Past, present, and future. Cancers (Basel).
11:E11972019. View Article : Google Scholar
|
|
19
|
Sanz-Garcia E, Argiles G, Elez E and
Tabernero J: BRAF mutant colorectal cancer: Prognosis, treatment,
and new perspectives. Ann Oncol. 28:2648–2657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ascierto PA, Kirkwood JM, Grob JJ, Simeone
E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM and
Mozzillo N: The role of BRAF V600 mutation in melanoma. J Transl
Med. 10:852012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schreuer M, Jansen Y, Planken S, Chevolet
I, Seremet T, Kruse V and Neyns B: Combination of dabrafenib plus
trametinib for BRAF and MEK inhibitor pretreated patients with
advanced BRAFV600-mutant melanoma: An open-label, single arm,
dual-centre, phase 2 clinical trial. Lancet Oncol. 18:464–472.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Planchard D, Besse B, Groen HJM, Souquet
PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, et
al: Dabrafenib plus trametinib in patients with previously treated
BRAF(V600E)-mutant metastatic non-small cell lung cancer: An
open-label, multicentre phase 2 trial. Lancet Oncol. 17:984–993.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang HM, Huang YW, Huang JS, Wang CH, Kok
VC, Hung CM, Chen HM and Tzen CY: Analastic carcinoma of the
thyroid arising more often from follicular carcinoma than papillary
carcinoma. Ann Surg Oncol. 14:3011–3018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onoda N, Nakamura M, Aomatsu N, Noda S,
Kashiwagi S and Hirakawa K: Establishment, characterization and
comparison of seven authentic anaplastic thyroid cancer cell lines
retaining clinical features of the original tumors. World J Surg.
38:688–695. 2014. View Article : Google Scholar
|
|
26
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Costache MI, Ioana M, Iordache S, Ene D,
Costache CA and Săftoiu A: VEGF expression in pancreatic cancer and
other malignancies: A review of the literature. Rom J Intern Med.
53:199–208. 2015.PubMed/NCBI
|
|
28
|
Kim S, Yazici YD, Calzada G, Wang ZY,
Younes MN, Jasser SA, El-Naggar AK and Myers JN: Sorafenib inhibits
the angiogenesis and growth of orthotopic anaplastic thyroid
carcinoma xenografts in nude mice. Mol Cancer Ther. 6:1785–1792.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
D'Haene N, Koopmansch C, Van Eycke YR,
Hulet F, Allard J, Bouri S, Rorive S, Remmelink M, Decaestecker C,
Maris C and Salmon I: The prognostic value of the combination of
low VEGFR-1 and High VEGFR-2 expression in endothelial cells of
colorectal cancer. Int J Mol Sci. 19:pii: E35362018. View Article : Google Scholar
|
|
31
|
Chen G, Nicula D, Renko K and Derwahl M:
Synergistic anti-proliferative effect of metformin and sorafenib on
growth of anaplastic thyroid cancer cells and their stem cells.
Oncol Rep. 33:1994–2000. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Zhang C, Ning Z, Xu L, Zhu X and
Meng Z: Bufalin enhances anti-angiogenic effect of sorafenib via
AKT/VEGF signaling. Int J Oncol. 48:1229–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higashiyama T, Ito Y, Hirokawa M,
Fukushima M, Uruno T, Miya A, Matsuzuka F and Miyauchi A: Induction
chemotherapy with weekly paclitaxel administration for anaplastic
thyroid carcinoma. Thyroid. 20:7–14. 2010. View Article : Google Scholar
|
|
34
|
Onoda N, Sugino K, Higashiyama T, Kammori
M, Toda K, Ito K, Yoshida A, Suganuma N, Nakashima N, Suzuki S, et
al: The safety and efficacy of weekly paclitaxel administration for
anaplastic thyroid cancer patients: A nationwide prospective study.
Thyroid. 26:1293–1299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jing C, Gao Z, Wang R, Yang Z, Shi B and
Hou P: Lenvatinib enhances the antitumor effects of paclitaxel in
anaplastic thyroid cancer. Am J Cancer Res. 7:903–912.
2017.PubMed/NCBI
|
|
36
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar
|
|
37
|
Gunda V, Gigliotti B, Ndishabandi D, Ashry
T, McCarthy M, Zhou Z, Amin S, Freeman GJ, Alessandrini A and
Parangi S: Combinations of BRAF inhibitor and anti-PD-1/PD-L1
antibody improve survival and tumour immunity in an immunocompetent
model of orthotopic murine anaplastic thyroid cancer. Br J Cancer.
119:1223–1232. 2018. View Article : Google Scholar : PubMed/NCBI
|