Introduction
Basal cell carcinoma (BCC) is the most common skin
tumor worldwide, accounting for ~80% of non-melanoma skin cancer
cases (1-3). BCC is generally well-differentiated
and exhibits a slow growth pattern, often resulting in skin nodules
and ulcers, and metastasis in rare cases (4). The most appropriate treatment for BCC
is determined according to the size and location of the tumor, and
the degree of infiltration. Interventions currently include
surgical resection, photodynamic therapy and molecular targeted
therapy (5,6). Previous studies demonstrated that the
increased incidence and mortality of BCC are due to the decreased
effectiveness of current treatments (3,7).
Therefore, the development of safe and reliable treatment
strategies to reduce the mortality rate is required.
Cold atmospheric plasma (CAP) is a near room
temperature ionized gas composed of charged particles, neutral
particles and electrons (8). In
the past two decades, CAP has been demonstrated to stimulate
coagulation and to promote wound healing (9,10).
An increasing number of studies have demonstrated the significant
anticancer effect of CAP in several cancer cell lines, including
glioblastoma, malignant melanoma, breast cancer and lung cancer
cell lines, in vitro (11,12).
From research on the anti-cancer mechanism of CAP, it was
identified that among some reactive oxygen species (ROS) and
reactive nitrogen species (RNS) produced by CAP, ROS, such as
hydrogen peroxide, hydroxide, ozone and peroxide ion, play a major
role in inducing tumor cell apoptosis (13,14).
A number of previous in vitro experiments demonstrated that
intracellular ROS concentrations were significantly increased
following treatment with CAP, resulting in damage to the
intracellular antioxidant system (15,16).
This phenomenon was also observed in a previous study (17). There are currently two different
perspectives regarding the mechanisms underlying this phenomenon.
One is that ROS originating from CAP enter the cells via
transmembrane transporters and directly disrupts cellular
homeostasis due to the increased concentration of ROS, eventually
leading to apoptosis. Alternatively, activated plasma may trigger
the expression of a series of signaling molecules in the cells by
acting on related protein targets on the cell membrane (18,19).
Either mechanism will lead to an imbalance between intracellular
ROS and cell defense systems, leading to apoptosis.
CAP comes into contact with the solution medium to
produce physical and chemical changes, and further acts on cells to
promote apoptosis in vitro (20). Based on this phenomenon, the
anticancer effect of CAP activating solution has gradually been
recognized (21-23). Compared with CAP direct
irradiation, CAP activating solution has the advantage of
convenient storage and transportation. The media used to prepare
the plasma activating solution (PAS) must be biocompatible, and
include cell culture medium, PBS and Ringer's equilibrium solution
(21,24). Yan et al (25) identified that CAP-stimulated medium
and CAP-stimulated buffered solution exerted anticancer effects on
pancreatic adenocarcinoma and glioblastoma cells in vitro.
In addition, the dilution of the solution is an important factor
that affects the anticancer effect (25). Tanaka et al (24) observed that plasma-activated
Ringer's solution exhibited good anticancer effects in vitro
and in vivo. These previous results demonstrated significant
progress in the use of PAS in clinical applications.
Ishaq et al (26) identified that CAP selectively
induced cancer by stimulating the oxidative stress-induced tumor
necrosis factor (TNF)-apoptosis signal regulating kinase 1
(ASK1)-JNK/p38-caspase-3/7 apoptotic pathway in melanoma.
Furthermore, CAP-induced NADPH oxidases generated intracellular
ROS, which induced apoptosis in colorectal cancer cells in
vitro by activating the ASK1-mediated apoptotic pathways
(19). The CAP-induced apoptotic
pathway in cancer cells is triggered by DNA and mitochondrial
damage (27). The majority of
tumor cells show different degrees of DNA double strand breaks
(DSBs) after treatment with CAP, and ataxia telangiectasia-mutated
(ATM) is an important marker of DSBs. This factor activates several
cell cycle arrest-associated proteins, including p53, as well as
the expression of apoptotic signals through phosphorylation
(28). The activation of p53
expression has been demonstrated in a variety of CAP-treated cancer
cell lines (16,29).
MAPKs are a group of serine-threonine protein
kinases that are activated by different extracellular stimuli,
including cytokines, neurotransmitters, hormones and cellular
stress (30). MAPKs can be divided
into four subfamilies: ERKs, p38, c-JNKs and ERK5, of which p38
plays a major role in mediating inflammation and apoptosis
(30-32). In the present study, the BCC cell
line TE354T was used to evaluate the effect of DMEM and PBS on
proliferation after treatment with CAP. Furthermore, the changes in
expression of factors in related apoptotic pathways were detected
by RNA-sequencing (RNA-seq) technology. Differential expression
analysis was performed on CAP-treated TE354T cells to identify
changes in the MAPK and TNF pathways in PAS-induced TE354 cell
apoptosis.
Materials and methods
Cell culture
The TE354T BCC and HaCaT keratinocyte cell lines
were acquired from The American Type Culture Collection, and were
maintained in DMEM/High Glucose (HyClone; GE Healthcare Life
Sciences; pH 7.0-7.4; buffer salt including sodium phosphate
monobasic, sodium bicarbonate and HEPES) with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.; 100 U/ml penicillin and 100 mg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells were
cultured at 37˚C and 5% CO2 in a humidified
environment.
Preparation of CAP activation liquid
CAP equipment was provided by The Institute of
Plasma Physics, Chinese Academy of Sciences (Fig. 1). The air plasma device includes a
DC voltage input, a copper rod array and a ballast resistor. The
electrode consists of six arrays of thin copper rods with a radius
of 1 mm. The ballast resistors are 30 Ω, and the discharge current
is limited to ~5 mA. The device is activated by a 10-kV DC voltage
and generates plasma through a copper rod electrode. DMEM or PBS
was added to a 35-mm cell culture dish and irradiated with plasma
to prepare the PAS. Each dish was filled with 2 ml liquid (DMEM or
PBS), the plasma electrode was placed ~10 mm from the liquid
surface, and plasma activation liquids of different concentrations
were prepared by irradiation for different times (60, 120, 180, 240
and 300 sec). The doses of plasma-activated liquid are defined as
the different processing times of the liquid under the same
discharge parameters. The prepared activated solution was placed in
an ice water bath or stored at 4˚C for further experiments.
Cell morphology
TE354T cells were seeded in 6-well plates
(2x105 cells per well) and incubated at constant
temperature for 24 h. The medium was aspirated and 2 ml of 180 sec
plasma-activated DMEM solution was added to each well for
incubation for 6 and 12 h. The morphological changes of the cells
were observed directly under a light microscope (magnification,
x400). A Hoechst 33342 Staining kit was purchased from Beyotime
Institute of Biotechnology. The sterilized coverslips were placed
in 6-well plates and inoculated with TE354T cell culture. After the
cells had grown to 50-80% of the coverslip area, the medium was
aspirated and the cells were treated with 2 ml 180 sec
plasma-activated DMEM solution for 2 h. DMEM was aspirated again
and the fixing solution (cat. no. C0003-1; Beyotime Insitute of
Biotechnology) was added at 4˚C for 10 min. Subsequently, the cells
were rinsed twice with PBS and 0.5 ml Hoechst 33342 staining
solution was added for incubation at room temperature for 5 min in
the dark. The cells were washed twice with PBS and imaged under a
fluorescent microscope for observation (magnification, x1,000). The
images were taken using an IX-71 Olympus microscope (Olympus
Corporation).
Cell viability assay
Cell viability was measured by an MTT assay. All
experiments used logarithmic growth phase cells collected by
digestion, which were counted and inoculated in a 96-well culture
plate for 24 h (10,000 cells; 100 µl culture medium per well). The
cells were fully adherent at the start of experimental treatment.
The cells were stimulated with plasma-activated fluid for 2 h.
Thereafter, the activation fluid was replaced with fresh DMEM and
incubation was continued for 24, 48 or 72 h. In the 96-well plates,
100 µl MTT solution was added to each well for 4 h. Thereafter, the
formazan was dissolved in DMSO and the absorbance was measured at
570 nm using a microplate reader (SpectraMax i3x; Molecular
Devices, LLC).
Detection of intracellular ROS
An ROS assay kit was purchased from Beyotime
Institute of Biotechnology. HaCat and TE354T cells in the
logarithmic growth phase were inoculated on 96-well culture plates
(10,000 cells per well), and the experiment was performed after the
cells were completely adherent. Cells were loaded with the
fluorescent probe dichloro-dihydro-fluorescein diacetate and
stimulated with plasma-activated DMEM or PBS, and changes in the
concentration of ROS were measured after 2 h. Fluorescence was
measured using a fluorescence plate reader (SpectraMax i3x;
Molecular Devices) at an excitation wavelength of 488 nm and an
emission wavelength of 525 nm to calculate intracellular ROS
levels.
Reactive species and pH in solution
A H2O2 assay kit was purchased
from Beyotime Institute of Biotechnology. The cell-free DMEM and
PBS solutions were placed in 2-ml culture dishes, and were exposed
to CAP treatment for 60, 120, 180, 240 and 300 sec, respectively.
H2O2 detection reagent was added and a
microplate reader (SpectraMax i3x; Molecular Devices) was used to
measure A560 to calculate the H2O2
concentration in the liquid. The pH changes in different
experimental groups were detected using a pH meter.
NO3− in the PBS after plasma treatment was
evaluated using PhotoLab 6100 (Merck KGaA) with nitrate detection
kit (Merck KGaA).
Apoptosis assay
The Annexin V-FITC/propidium iodide (PI) apoptosis
kit was purchased from Best-Bio(http://bestbio.bioon.com.cn/), and a flow cytometer
was provided by The Institute of Technology Biology, Chinese
Academy of Sciences. Cells were innoculated in 6-well plates and
were cultured until fully adherent, and then stimulated for 2 h in
PAS; thereafter, the medium was replaced with fresh medium and
samples were placed in the incubator for 24 h. The cells were
collected for staining. Cells were suspended in Annexin V-FITC
binding solution at a concentration of ~1x106/ml.
Subsequently, 5 µl Annexin V-FITC staining solution was added for
incubation for 15 min. A total of 10 µl PI staining solution was
then added for incubation for 5 min, protected from light at 2-8˚C.
The apoptosis rate of each group of cells was analyzed using a flow
cytometer (BD Biosciences). The data were analyzed using FlowJo
software (v10.0.7; FlowJo LLC).
Western blotting
Western blot analysis was performed at 0, 3, 6 and
12 h after treating the cells with PAS. The cells were harvested by
trypsinization and the resulting cell suspension was washed twice
with PBS. RIPA (Beyotime Institute of Biotechnology) lysis buffer
was added, and the supernatant was collected after centrifugation
(4˚C; 10,000 x g; 5 min). Protein quantification was performed
using a bicinchoninic acid assay kit (Thermo Fisher Scientific,
Inc.). Loading buffer was mixed with protein and the mixture was
boiled for 3 min. Subsequently, proteins (10 µl per lane) were
separated by SDS-PAGE on 10% gels for 90 min, and transferred to a
polyvinylidene fluoride film (Bio-Rad Laboratories, Inc.) at a
temperature of 4˚C. The membrane was blocked with 5% skim milk
powder in TBS with Tween-20 (TBST) for 1 h at 24˚C. Antibodies
against Cas3 (cat. no. 9665T; Cell Signaling Technology, Inc.;
1:1,000), cleaved Cas3 (cat. no. 9664T; Cell Signaling Technology,
Inc.; 1:1,000), Cas9 (cat. no. 9508T; Cell Signaling Technology,
Inc.; 1:1,000), cleaved Cas9 (cat. no. 7237T; Cell Signaling
Technology, Inc.; 1:1,000) and β-actin (cat. no. TA-09; OriGene
Technologies, Inc.; 1:1,000) were added and incubated overnight at
4˚C. The films were then washed three times with TBST, and
secondary antibodies conjugated with horseradish peroxidase
(1:10,000) were added for incubation for 1 h at room temperature.
The secondary antibodies used for Cas3, cleaved Cas3 and cleaved
Cas9 were goat rabbit anti-lgG (cat. no. ZB2301; OriGene
Technologies, Inc.); the secondary antibodies used for Cas9 were
goat mouse anti-IgG (cat. no. ZB2305; OriGene Technologies, Inc.).
Signals were detected with using an enhanced chemiluminescence kit
(Amersham ECL plus; GE Healthcare Life Sciences). β-actin (was used
as an internal control. Density was analyzed using the Fino-do X6
analysis system (Tanon Science and Technology, Co., Ltd.). ImageJ
software (v1.46; National Institutes of Health) was used to
quantify the protein bands of interest.
RNA-seq
Cells were treated with PAS for 0, 4 or 8 h,
followed by total RNA extraction using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The cell lysate was
loaded into an RNase-free centrifuge tube and RNA-seq was performed
by Shenzhen Huada Gene Technology Co., Ltd. The sequencing results
and analysis of differential gene expression functions were
performed by Shenzhen Huada Gene Technology Co., Ltd. The
sequencing results were provided by Shenzhen Huada Gene Technology
Co., Ltd. The analysis results report has not been shared to a
public database at the time of publication.
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Statistical significance was determined
using an independent Student's t-test and ANOVA. Least Significant
Difference post hoc test and Dunnett's post hoc test were used
where appropriate. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using GraphPad Prism (version 5.01; GraphPad Software,
Inc.) software.
Results
PAS leads to morphological changes in
TE354T cells
Compared with untreated cells, cells treated with
PAS for 6 h exhibited reduced intracellular volume and shrunken
cell membranes. Furthermore, certain cells had detached from the
bottom of the culture dish, and appeared small and round (Fig. 2). These effects became more
apparent after treatment for 12 h. Hoechst staining revealed bright
blue cells after PAS treatment, indicating that the nucleus was
condensed, and that the cells had begun to undergo apoptosis.
Evaluating the effect of PAS on BCC cell
viability
Increased PAS treatment time resulted in a gradual
decrease in the cell viability rate and this effect was more
pronounced in the PBS group compared with the DMEM group (Fig. 3). Furthermore, plasma-activated
DMEM and PBS stored for 24 h prior to treatment induced cell death.
It is worth noting that after PAS storage for 24 h, compared with
the control group, the CAP-treated DMEM and PBS for 60 sec showed a
proliferation-promoting effect on the cells.
As presented in Fig.
4, following treatment with PAS, TE354T cells were cultured for
24, 48 and 72 h under standard conditions. The survival rate of
each group was then measured. It was observed that the
proliferation of TE354T cells was inhibited within 72 h of PAS
treatment. In the 300 sec group, after TE354T cells were treated
with CAP-activated DMEM and CAP-activated PBS, the cell survival
rate was ~30%, and this inhibition continued to be significant for
72 h. Furthermore, PAS demonstrated dose- and time-dependent
inhibitory effects on the proliferation of TE354T cells.
PAS significantly decreased the proliferation of
TE354T cells compared with HaCat cells (Fig. 5). In the absence of malignant
epidermal keratinocytes, the viability of cells within the same
groups was significantly higher than those of the BCC cell groups.
In the DMEM group, even at the highest dose of PAS treatment, the
survival rate of HaCat cells was ~75%, while that of TE354T cells
in the same group was <40%. In the PBS group, this difference
was less pronounced. Compared with DMEM, the apoptotic effect of
PBS following plasma irradiation was stronger, but the selectivity
was relatively weak.
Intracellular ROS levels are increased
after PAS treatment
The intracellular ROS levels in HaCat and TE354T
cells were examined before and after PAS treatment (Fig. 6). After treating cells with
CAP-activated DMEM, ROS levels increased by ~1.7-fold in TE354T
cells in the 300 sec group, while those in HaCat cells only
increased by ~1.4-fold. After treating cells with CAP-irradiated
PBS, ROS levels in TE354T cells were increased by a maximum of
~2.6-fold. The basal ROS concentration in HaCat cells was lower
than that in TE354T cells, and the increase in ROS levels in HaCat
cells was lower after PAS treatment.
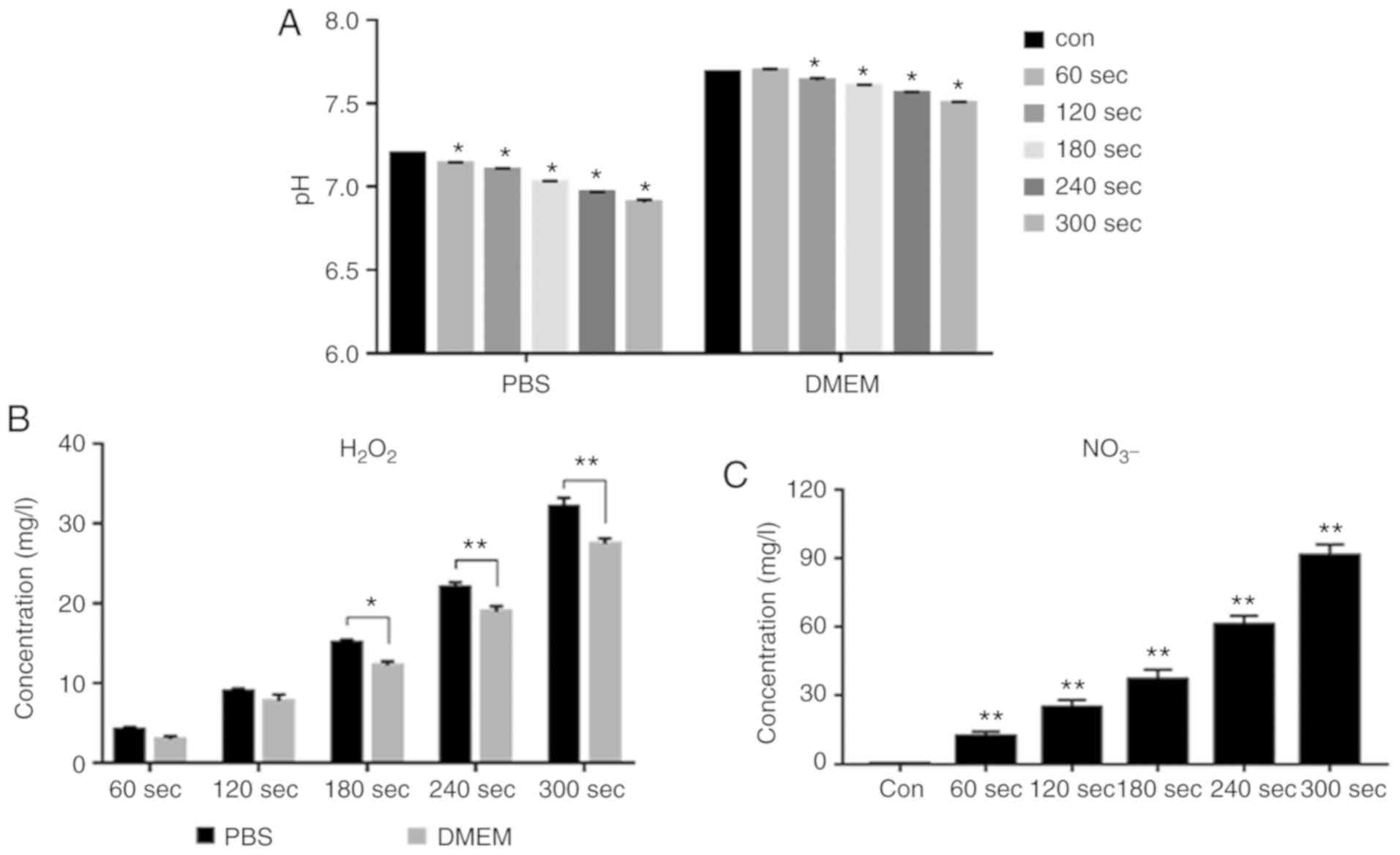
Reactive species in the solution increase
and the pH decreases after CAP treatment
After 60, 120, 180, 240 and 300 sec of CAP
treatment, the pH of both DMEM and PBS solutions demonstrated a
downward trend (Fig. 7A). After
CAP treatment, H2O2 in both solutions
increased. This growth trend was more pronounced in PBS solution
after receiving CAP treatment at the same time. After 300 sec of
CAP treatment, the H2O2 concentration
measured in PBS solution was ~33 mg/l. At the same time, it was ~28
mg/l in DMEM (Fig. 7B). As the
color of DMEM will affect the detection of NO3-, only
the change of NO3- concentration in PBS solution after
CAP treatment was measured. No NO3- concentration was
measured in the blank control group With the increase of CAP
treatment time, the concentration of NO3- in PBS
solution increased (Fig. 7C).
After 300 sec of treatment, the measured NO3-
concentration in the solution was ~90 mg/l.
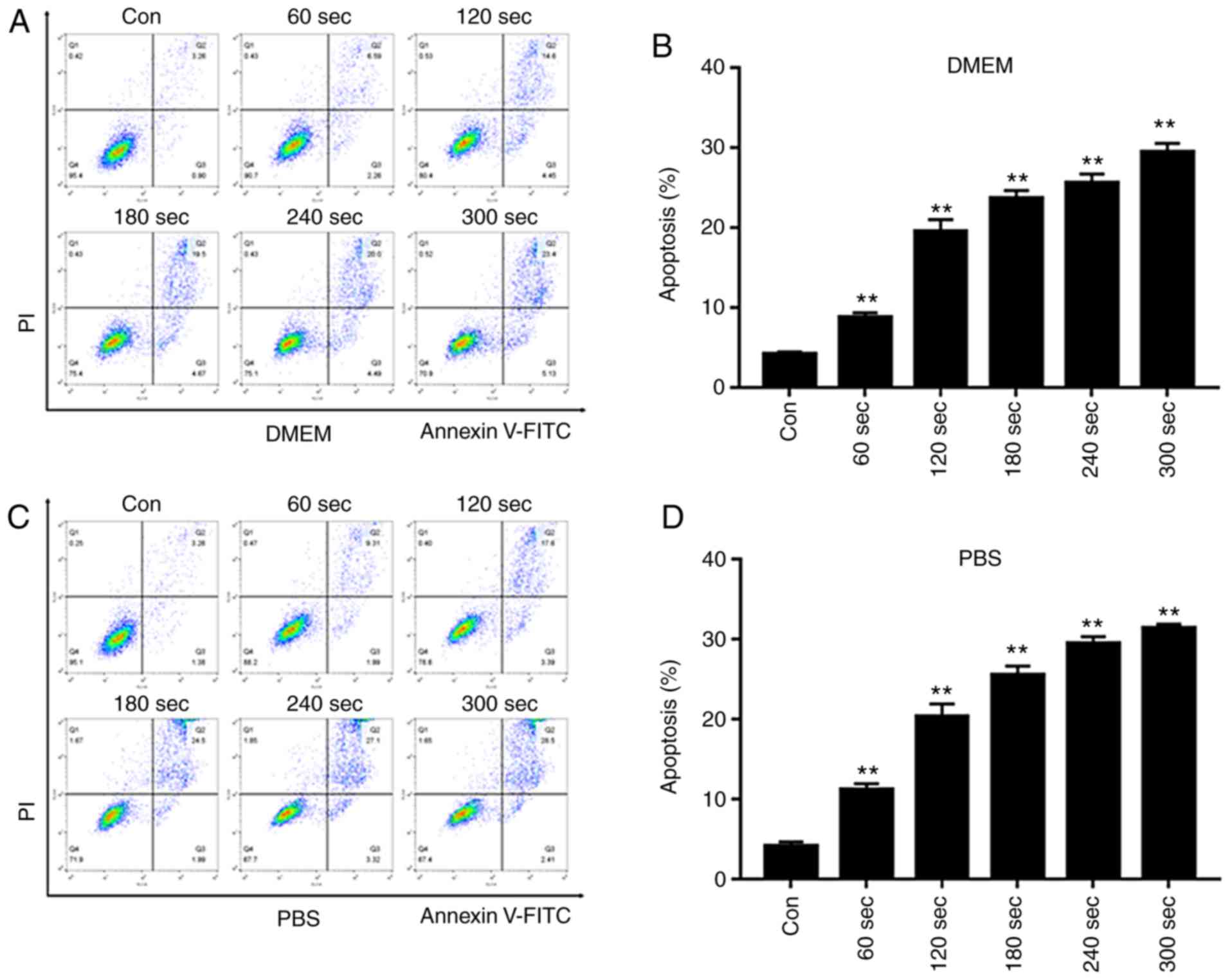
PAS induces apoptosis of BCC cells
Cell staining with Annexin V-FITC/PI can indicate
the rate and time of apop-tosis in cells. In the flow cytometry
plots, the lower and upper right quadrants indicate early and late
apoptosis, respectively. As presented in Fig. 8, the apoptotic rate was
significantly increased following PAS treatment compared with the
untreated control groups. Both early and late apoptosis exhibited
an upward trend, which was more pronounced in the PBS-treated
group.
Western blot analysis of
apoptosis-related protein expression
Cleaved-caspase (cle-cas) is the activated form of
caspase (cas). The relative activity of cas9 and cas3 was
significantly increased following PAS treatment, suggesting that
PAS induced the intrinsic apoptotic pathway in TE354T cells. The
ratio of cle-cas to cas was calculated, and the ratio increased
significantly following PAS treatment. After 12 h, the activity of
cle-cas9 demonstrated a downward trend, possibly due to the
necrosis of cells (Fig. 9).
Detection of PAS-induced apoptosis by
RNA-seq
Differentially expressed genes (DEGs) were analyzed
in untreated control cells and BCC cells stimulated with PAS for 4
or 8 h. An increase in the expression of 754 genes, and a decrease
in the expression of 281 genes, were observed after stimulation
with PAS for 4 h. After 8 h, the number of upregulated DEGs
increased to 1,763 (Fig. 10A).
Based on these results, the genes were classified according to Gene
Ontology (GO) terms: Molecular function, cellular component and
biological process (Fig. 10B).
Kyoto Encyclopedia of Genes and Genomes (KEGG) biological pathway
classification and enrichment analysis was performed (Fig. 10C). Based on the degree of
enrichment and the number of DEGs, 'MAPK signaling pathway', 'TNF
signaling pathway' and 'IL-17 signaling pathway' were the KEGG
pathways most signifi-cantly associated with PAS-induced
apoptosis.
Discussion
Previous studies have typically used CAP to directly
treat cells in standard culture. In this process, the active
substance produced by CAP is first incubated with the liquid
medium, ROS/RNS are dissolved in solution, and physical and
chemical changes are induced by high concentrations of these
components (33,34). In the present study, CAP-activated
PBS and DMEM solutions were prepared separately, in order to
compare the effects of the two different activating fluids on BCC
TE354T cells. PBS, which has a simple composition, more effectively
inhibited the viability and promoted the apoptosis of TE354T cells
following CAP treatment compared with DMEM. By contrast, the
selective effect of PBS was not as prominent as DMEM when incubated
with HaCat cells. The H2O2 concentration in
the solution was found to be higher in the PBS solution under the
same processing conditions. This may be associated with the simpler
composition of the PBS solution. Yan et al (21) identified that cysteine and
methionine in solution are the main factors leading to the
inactivation of active substances. However, due to the absence of
these specific amino acids, fewer physical and chemical changes in
PBS were observed after CAP treatment, suggesting that it exerts
more potent anticancer activity and can be stored for longer
periods. This phenomenon is consistent with the results observed in
the present study.
As a new strategy for cancer therapy, the shelf life
of PAS presents a challenge that needs to be addressed. The effects
of two PAS samples after 24 h storage and those of two fresh PAS
samples were compared in the present study. After storage at 4˚C,
both fresh and aged PAS inhibited TE354T cell viability after 24 h.
It is worth noting that after 24 h of storage, the group with the
lowest exposure time exhibited increased cell viability. In
previous studies, low doses of CAP were demonstrated to promote
cell proliferation and wound healing (35,36).
In our previous study, plasma-activated medium successfully
inhibited the proliferation of TE354T and human skin squamous cell
carcinoma A431 cells after storage for 2 weeks at room temperature
(~28˚C), 4˚C and -20˚C (Yang et al, unpublished).
Increased intracellular ROS is the most common
cellular response to CAP treatment (18,37,38).
However, the magnitude of this increase may differ based on a
phenomenon known as CAP selective action (39,40).
ROS is an important intracellular secondary messenger under normal
physiological conditions. However, under pathological conditions,
when intracellular ROS accumulation is excessive or the
intracellular ROS scavenging system is damaged, it can cause damage
to intracellular macromolecules, leading to a series of changes
that ultimately induce necrosis or apoptosis (41,42).
In the present study, ROS levels in TE354T cells increased in a
time-dependent manner after incubation with CAP-irradiated
solution. This increase corresponded to the rate of necrotic
apoptosis in the cells. Furthermore, under the same conditions, the
increase in ROS in TE354T cells was higher than that in HaCat
cells. Previous studies have suggested that hydrogen peroxide,
hydroxyl radical and the peroxide ion are the main ROS produced by
incubation with CAP (14,43,44).
As the active substance from which CAP originates is a polar or
charged ion, specific channels and transport proteins on the cell
membrane are required for the transmembrane diffusion of these
activated substances (45,46). Previous studies demonstrated that
an increased number of aquaporins on the surface of cancer cells
are the main cause of this significant increase in intracellular
ROS levels (47,48). This helps to explain why the ROS
levels in TE354T cells were higher than those in HaCat cells under
the same conditions.
It is now well known that after CAP treatment,
apoptotic pathways are triggered by DNA and mitochondrial damage in
cancer cells (39). RNA-seq
technology was used to identify DEGs in TE354T cells before and
after PAS treatment in the present study. The TNF and MAPK
signaling pathways were significantly upregulated in TE354T cells
after PAS stimulation. These two pathways are major pathways
associated with cancer cell apoptosis (26,49).
Ishaq et al (26)
investigated CAP-treated malignant melanoma cells and identified
that the TNF receptor-based apoptotic pathway was activated by an
increase in intracellular ROS. Xiang et al (49) identified that the MAPK signaling
pathway plays a major role in the apoptosis of breast cancer cells
induced by low temperature plasma. The hypothesis is that after ROS
stimulation, the cells immediately develop DNA damage, particularly
DNA DSBs. ATM is an important marker of DSBs that activates several
cell cycle arrest-associated factors, including p53, as well as the
expression of apoptotic signals through phosphorylation (28). Activation of the MAPK signaling
pathway and the release of cytochrome c into the mitochondria lead
to activation of the caspase family (49), which ultimately induced apoptosis
in TE354T cells in the present study. Whether this effect is
observed in all cancer cells treated with CAP requires further
verification.
In the present study, CAP-irradiated DMEM and PBS
inhibited the viability and promoted the apoptosis of BCC TE354T
cells. This phenomenon was most likely due to an increase in
intracellular ROS levels. Using RNA-seq technology, the TNF and
MAPK signaling pathways were identified to be upregulated, and may
play an important role in mediating cell necrosis and apoptosis.
The results obtained in the present study provided a theoretical
basis for the further study of the anticancer mechanisms of
CAP.
Funding
The present study was supported by The Foundation of
The Second Affiliated Hospital of Anhui Medical University (grant
no. 2015hhjh04); The National Natural Science Foundation of China
(grant nos. 51777206, 3160 0680, 81673099, 31670860, 81602083 and
31800702); Anhui Provincial Natural Science Foundation (grant nos.
1608085QH216 and 1708085MA13); and Grants for Scientific Research
of BSKY (grant no. XJ201505) from Anhui Medical University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
This study was conceived and designed by CY. The
main experiment was conducted by XY, LW and ZC. YW also conducted
some experiments. As an expert in the field of cold atmospheric
plasma, CC proposed the concept of this study together with CY and
participated in the drafting of the manuscript. YZ contributed to
the experimental design of the present study, and analyzed the
experimental data during the experiment. GZ participated in the
completion of apoptotic cell flow detection, western blotting, RNA
sequencing and other related experiments, and made significant
contributions to the analysis and interpretation of experimental
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Xianbin Cao and
Dr Pengbo Wen (Anhui Province Key Laboratory of Environmental
Toxicology & Pollution Control Technology, Hefei Institutes of
Physical Science, Chinese Academy of Sciences) for their help in
related experiments.
Abbreviations:
|
ATM
|
ataxia telangiectasia-mutated
|
|
BCC
|
basal cell carcinoma
|
|
CAP
|
cold atmospheric plasma
|
|
DEG
|
differentially expressed gene
|
|
DSB
|
double strand break
|
|
PAS
|
plasma-activated solution
|
|
RNS
|
reactive nitrogen species
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Verkouteren JAC, Ramdas KHR, Wakkee M and
Nijsten T: Epidemiology of basal cell carcinoma: Scholarly review.
Br J Dermatol. 177:359–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muzic JG, Schmitt AR, Wright AC, Alniemi
DT, Zubair AS, Olazagasti Lourido JM, Sosa Seda IM, Weaver AL and
Baum CL: Incidence and trends of basal cell carcinoma and cutaneous
squamous cell carcinoma: A population-based study in olmsted
county, minnesota, 2000 to 2010. Mayo Clin Proc. 92:890–898. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcovich S, Colloca G, Sollena P, Andrea
B, Balducci L, Cho WC, Bernabei R and Peris K: Skin cancer
epidemics in the elderly as an emerging issue in geriatric
oncology. Aging Dis. 8:643–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pyne JH, Myint E, Barr EM, Clark SP, David
M, Na R and Hou R: Superficial basal cell carcinoma: A comparison
of superficial only subtype with superficial combined with other
subtypes by age, sex and anatomic site in 3150 cases. J Cutan
Pathol. 44:677–683. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lipson EJ, Lilo MT, Ogurtsova A, Esandrio
J, Xu H, Brothers P, Schollenberger M, Sharfman WH and Taube JM:
Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor
regression after PD-1 blockade. J Immunother Cancer. 5:232017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandel VD, Arginelli F, Pellacani G and
Greco M: Combined carbon dioxide laser with photodynamic therapy
for the treatment of nodular and infiltrative basal cell carcinoma.
G Ital Dermatol Venereol. 152:672–674. 2017.PubMed/NCBI
|
|
7
|
Wong CS, Strange RC and Lear JT: Basal
cell carcinoma. BMJ. 327:794–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SJ and Chung TH: Cold atmospheric
plasma jet-generated RONS and their selective effects on normal and
carcinoma cells. Sci Rep. 6:203322016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rashmei Z, Bornasi H and Ghoranneviss M:
Evaluation of treatment and disinfection of water using cold
atmospheric plasma. J Water Health. 14:609–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinlin J, Morfill G, Landthaler M, Stolz
W, Isbary G, Zimmermann JL, Shimizu T and Karrer S: Plasma
medicine: Possible applications in dermatology. J Dtsch Dermatol
Ges. 8:968–976. 2010.In English, German. PubMed/NCBI
|
|
11
|
Wang M, Holmes B, Cheng X, Zhu W, Keidar M
and Zhang LG: Cold atmospheric plasma for selectively ablating
metastatic breast cancer cells. PLoS One. 8:e737412013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keidar M, Walk R, Shashurin A, Srinivasan
P, Sandler A, Dasgupta S, Ravi R, Guerrero-Preston R and Trink B:
Cold plasma selectivity and the possibility of a paradigm shift in
cancer therapy. Br J Cancer. 105:1295–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ratovitski EA, Cheng X, Yan D, Sherman JH,
Canady J, Trink B and Keidar M: Anti-cancer therapies of 21st
century: Novel approach to treat human cancers using cold
atmospheric plasma. Plasma Proc Polymers. 11:1128–1137. 2014.
View Article : Google Scholar
|
|
14
|
Kalghatgi S, Kelly CM, Cerchar E, Torabi
B, Alekseev O, Fridman A, Friedman G and Azizkhan-Clifford J:
Effects of non-thermal plasma on mammalian cells. PLoS One.
6:e162702011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaushik NK, Kaushik N, Park D and Choi EH:
Altered antioxidant system stimulates dielectric barrier discharge
plasma-induced cell death for solid tumor cell treatment. PLoS One.
9:e1033492014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandamme M, Robert E, Lerondel S, Sarron
V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B,
Pouvesle JM and Pape AL: ROS implication in a new antitumor
strategy based on non-thermal plasma. Int J Cancer. 130:2185–2194.
2012. View Article : Google Scholar
|
|
17
|
Wang L, Yang X, Yang C, Gao J, Zhao Y,
Cheng C, Zhao G and Liu S: The inhibition effect of cold
atmospheric plasma-activated media in cutaneous squamous carcinoma
cells. Future Oncol. 15:495–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun JK, Joh HM and Chung TH: Production of
intracellular reactive oxygen species and change of cell viability
induced by atmospheric pressure plasma in normal and cancer cells.
Appl Phys Lett. 103:1537052013. View Article : Google Scholar
|
|
19
|
Ishaq M, Evans MD and Ostrikov KK:
Atmospheric pressure gas plasma-induced colorectal cancer cell
death is mediated by Nox2-ASK1 apoptosis pathways and oxidative
stress is mitigated by Srx-Nrf2 anti-oxidant system. Biochim
Biophys Acta. 1843:2827–2837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen NH, Park HJ, Yang SS, Choi KS and
Lee JS: Anti-cancer efficacy of nonthermal plasma dissolved in a
liquid, liquid plasma in heterogeneous cancer cells. Sci Rep.
6:290202016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan D, Nourmohammadi N, Bian K, Murad F,
Sherman JH and Keidar M: Stabilizing the cold plasma-stimulated
medium by regulating medium's composition. Sci Rep. 6:260162016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan D, Sherman JH, Cheng X, Ratovitski E,
Canady J and Keidar M: Controlling plasma stimulated media in
cancer treatment application. Appl Phys Lett. 105:2241012014.
View Article : Google Scholar
|
|
23
|
Adachi T, Tanaka H, Nonomura S, Hara H,
Kondo S and Hori M: Plasma-activated medium induces A549 cell
injury via a spiral apoptotic cascade involving the
mitochondrial-nuclear network. Free Radic Biol Med. 79:28–44. 2015.
View Article : Google Scholar
|
|
24
|
Tanaka H, Nakamura K, Mizuno M, Ishikawa
K, Takeda K, Kajiyama H, Utsumi F, Kikkawa F and Hori M:
Non-thermal atmospheric pressure plasma activates lactate in
Ringer's solution for anti-tumor effects. Sci Rep. 6:362822016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan D, Cui H, Zhu W, Nourmohammadi N,
Milberg J, Zhang LG, Sherman JH and Keidar M: The specific
vulnerabilities of cancer cells to the cold atmospheric
plasma-stimulated solutions. Sci Rep. 7:44792017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishaq M, Kumar S, Varinli H, Han ZJ, Rider
AE, Evans MD, Murphy AB and Ostrikov K: Atmospheric gas
plasma-induced ROS production activates TNF-ASK1 pathway for the
induction of melanoma cancer cell apoptosis. Mol Biol Cell.
25:1523–1531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keidar M: Plasma for cancer treatment.
Post Communist Economies. 24:2015.
|
|
28
|
Xu H, Klas M, Liu Y, Stack MS and
Ptasinska S: DNA damage in oral cancer cells induced by nitrogen
atmospheric pressure plasma jet. Appl Phys Lett. 102:644–654.
2013.
|
|
29
|
Chang JW, Kang SU, Shin YS, Kim KI, Seo
SJ, Yang SS, Lee JS, Moon E, Baek SJ, Lee K and Kim CH: Non-thermal
atmospheric pressure plasma induces apoptosis in oral cavity
squamous cell carcinoma: Involvement of DNA-damage-triggering
sub-G(1) arrest via the ATM/p53 pathway. Arch Biochem Biophys.
545:133–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Utaipan T, Athipornchai A, Suksamrarn A,
Chunsrivirot S and Chunglok W: Isomahanine induces endoplasmic
reticulum stress and simultaneously triggers p38 MAPK-mediated
apoptosis and autophagy in multidrug-resistant human oral squamous
cell carcinoma cells. Oncol Rep. 37:1243–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menon MB, Gropengießer J, Fischer J,
Novikova L, Deuretzbacher A, Lafera J, Schimmeck H, Czymmeck N,
Ronkina N, Kotlyarov A, et al: p38MAPK/MK2-dependent
phosphorylation controls cytotoxic RIPK1 signalling in inflammation
and infection. Nat Cell Biol. 19:1248–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nadeem A, Ahmad SF, Al-Harbi NO, Fardan
AS, El-Sherbeeny AM, Ibrahim KE and Attia SM: IL-17A causes
depression-like symptoms via NFκB and p38MAPK signaling pathways in
mice: Implications for psoriasis associated depression. Cytokine.
97:14–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang SU, Cho JH, Chang JW, Shin YS, Kim
KI, Park JK, Yang SS, Lee JS, Moon E, Lee K and Kim CH: Nonthermal
plasma induces head and neck cancer cell death: The potential
involvement of mitogen-activated protein kinase-dependent
mitochondrial reactive oxygen species. Cell Death Dis. 5:e10562014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanaka H, Mizuno M, Toyokuni S, Maruyama
S, Kodera Y, Terasaki H, Adachi T, Kato M, Kikkawa F and Hori M:
Cancer therapy using non-thermal atmospheric pressure plasma with
ultra-high electron density. Phys Plasmas. 22:391–400. 2015.
View Article : Google Scholar
|
|
35
|
Arndt S, Unger P, Berneburg M, Bosserhoff
AK and Karrer S: Cold atmospheric plasma (CAP) activates
angiogenesis-related molecules in skin keratinocytes, fibroblasts
and endothelial cells and improves wound angiogenesis in an
autocrine and paracrine mode. J Dermatol Sci. 89:181–190. 2018.
View Article : Google Scholar
|
|
36
|
Haertel B, von Woedtke T, Weltmann KD and
Lindequist U: Non-thermal atmospheric-pressure plasma possible
application in wound healing. Biomol Ther (Seoul). 22:477–490.
2014. View Article : Google Scholar
|
|
37
|
Bekeschus S, Kolata J, Winterbourn C,
Kramer A, Turner R, Weltmann KD, Bröker B and Masur K: Hydrogen
peroxide: A central player in physical plasma-induced oxidative
stress in human blood cells. Free Radical Res. 48:542–549. 2014.
View Article : Google Scholar
|
|
38
|
Yan D, Talbot A, Nourmohammadi N, Sherman
JH, Cheng X and Keidar M: Toward understanding the selective
anticancer capacity of cold atmospheric plasma-a model based on
aquapo-rins (Review). Biointerphases. 10:408012015. View Article : Google Scholar
|
|
39
|
Yan D, Sherman JH and Keidar M: Cold
atmospheric plasma, a novel promising anti-cancer treatment
modality. Oncotarget. 8:15977–15995. 2017.
|
|
40
|
Canal C, Fontelo R, Hamouda I,
Guillem-Marti J, Cvelbar U and Ginebra MP: Plasma-induced
selectivity in bone cancer cells death. Free Radic Biol Med.
110:72–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao S, Xiong Z, Xiang M, Meng D, Lei Q,
Li Y, Deng P, Chen M, Tu M, Lu X, et al: Atmospheric pressure room
temperature plasma jets facilitate oxidative and nitrative stress
and lead to endoplasmic reticulum stress dependent apoptosis in
HepG2 cells. PLoS One. 8:e736652013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arjunan KP and Clyne AM: Non-thermal
dielectric barrier discharge plasma induces angiogenesis through
reactive oxygen species. Conf Proc IEEE Eng Med Biol Soc.
2011:2447–2450. 2011.
|
|
43
|
Sun JK, Chung TH, Bae SH and Leem SH:
Induction of apoptosis in human breast cancer cells by a pulsed
atmospheric pressure plasma jet. Appl Phys Lett. 97:237022010.
View Article : Google Scholar
|
|
44
|
Yan D, Talbot A, Nourmohammadi N, Cheng X,
Canady J, Sherman J and Keidar M: Principles of using cold
atmospheric plasma stimulated media for cancer treatment. Sci Rep.
5:183392015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Almasalmeh A, Krenc D, Wu B and Beitz E:
Structural determinants of the hydrogen peroxide permeability of
aquaporins. FEBS J. 281:647–656. 2014. View Article : Google Scholar
|
|
46
|
Bienert GP and Chaumont F:
Aquaporin-facilitated trans-membrane diffusion of hydrogen
peroxide. Biochim Biophys Acta. 1840:1596–1604. 2014. View Article : Google Scholar
|
|
47
|
Kawasaki T, Kusumegi S, Kudo A,
Sakanoshita T, Tsurumaru T, Sato A, Uchida G, Koga K and Shiratani
M: Effects of irradiation distance on supply of reactive oxygen
species to the bottom of a Petri dish filled with liquid by an
atmospheric O2/He plasma jet. J Appl Phys. 119:1733012016.
View Article : Google Scholar
|
|
48
|
Miller EW, Dickinson BC and Chang CJ:
Aquaporin-3 mediates hydrogen peroxide uptake to regulate
downstream intracellular signaling. Proc Natl Acad Sci USA.
107:15681–15686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiang L, Xu X, Zhang S, Cai D and Dai X:
Cold atmospheric plasma conveys selectivity on triple negative
breast cancer cells both in vitro and in vivo. Free Radic Biol Med.
124:205–213. 2018. View Article : Google Scholar : PubMed/NCBI
|