Introduction
Plant extracts are becoming increasingly important,
as a prominent source of active compounds, which are able to
interfere with biological activities in eukaryotic and prokaryotic
cells (1). In traditional
medicine, the crude extracts of different parts of plants were
formerly used, as a folk remedy for a large variety of pathologies,
which is due to the biological proprieties of the molecules present
in the extracts (2).
Juglans regia L. (J. regia), the
common walnut, belongs to the Juglandaceae family and is rich, in
all parts, in various chemical products, with antimicrobial,
anti-biofilm, anti-inflammatory and anti-oxidant activities
(3,4). Accordingly, the fresh green fruit,
the peel, the skin, the leaves, the bark and the root have been
widely used in food, cosmetic and pharmaceutical indus-tries
(5). The brown and thin leathery
covering of the kernel contains a high concentration of phenolic
compounds and protects the kernel from microbial attack (6). The antimicrobial activity of walnut
tree branches and walnut pellicle extract has been shown (4,7).
Furthermore, the anticancer activity of phenolic compounds and, in
particular, the anti-proliferative effect of J. regia
extracts on human breast and oral cancer cell lines has been
demonstrated (8,9). However, despite the proven antitumor
effects on different cancer cell lines, the efficacy of walnut
extract on glioblastoma cells is still currently unknown.
Brain cancers are a heterogeneous group of tumors
deriving from neoplastic transformation of brain cells (primary
tumor) or from the invasion of cancer cells originating from
another part of the body (secondary tumor). Data regarding the
incidence rates of primary tumors of the central nervous system are
not encouraging. Specifically, glioblastoma, the most aggressive
form of astrocytoma, accounts for 50% of all gliomas and in 2018,
it was responsible for 2.5% cancer-associated death, worldwide
(10-13). Using molecular and histological
techniques, it is possible to recognize different hallmarks of
malignancy, such as intense proliferation, cell heterogeneity,
genomic point aberrations, high vascularization and invasion
(14,15).
The development of innovative treatments and novel
therapeutic strategies are areas of active research, aimed at
improving the quality of life and life expectancy of patients with
cancer (16-20). However, despite the enormous
progress in this field, even with the introduction of immunotherapy
(21), brain tumors are still
associated with high mortality rates (22). The standard therapy for
glioblastoma requires three different steps: surgical resection,
external beam radiation therapy and chemotherapy (23).
It is worth noting that one of the complications of
antitumor therapy is infectious diseases, due to immunosuppression
in patients with cancer. In this regard, it has been reported that
concomitant radio- and chemotherapy treatment causes neutropenia
and lymphocytopenia, thus promoting opportunistic infection by
Pneumocystis carinii pneumonia in patients with grade III
anaplastic astrocytoma and grade IV glioblastoma multiforme
(24). Therefore, the emergence of
opportunistic infections in patients with cancer depends on the
cytotoxic effects of chemotherapeutic agents, that prevent the
active reproduction of proliferating cells, including cells in the
immune system. This leads to a reduction in the host defense system
(25).
Several studies have demonstrated the beneficial
effects of walnut consumption for the brain. In particular, in both
animal and human studies it was demonstrated that a walnut diet
reduces the brain-accumulation of damaged proteins and reduces
brain inflammation associated with aging (26-28).
A previous study has highlighted the strong antioxidant effects of
walnut septum extracts (29),
suggesting a possible role of such extracts in the maintenance of
brain homeostasis (30,31). Therefore, these studies have
demonstrated that walnut consumption or walnut extracts are
protective for brain aging (32),
paving the way for further studies investigating brain tumors.
Thus, the aim of the present study was to
investigate the effect of the walnut septum extract against one of
the most aggressive brain tumors. Using high-performance liquid
chromatography-diode array detection (HPLC/DAD) and
HPLC-electrospray ionization tandem mass spectrometry (HPLC/ESI-MS)
analysis, the phytochemical composition of Sicilian walnut fruit
septum ethanolic extract was obtained. The potential cytostatic and
cytotoxic properties of the extract against human glioblastoma cell
line and the antibacterial activity against different Gram-positive
and Gram-negative bacterial strains were also investigated. Using
the Prediction of Activity Spectra for Substances (PASS) analysis,
the possible bioactive compounds responsible for the biological
effect of walnut septum extract was also investigated.
Materials and methods
Chemicals
Unless otherwise stated, all reagents and solvents
were of analytical grade and used without further purification.
Pure reference standards: gallic acid, vanillic acid, ellagic acid,
p-coumaric acid and quercetin 3-O-glucoside were purchased from
Sigma Aldrich (Merck KGaA), while flavan-3-ols catechin,
epigallocatechin and epigallocatechingallate were obtained from
Extrasynthese. HPLC grade water, acetonitrile and methanol were
purchased from VWR International, LLC (Avantor).
Plant material and preparation of the
extract
Walnuts were collected in Trecastagni (Catania,
Italy). The specimen was authenticated by the Botanist Prof.
Salvatore Ragusa, Department of Health Sciences, University of
Catanzaro, (Catania, Italy). A voucher specimen of walnut was
deposited in the herbarium of the same Department. The preparation
of walnuts septum extract was conducted as previously described by
Acquaviva et al (4).
Briefly, 10 g of dried J. regia septum were ground using a
pestle and mortar. Ethanolic extract was obtained by maceration of
10 g pulverized walnut septum in 50 ml 96% ethanol (Merck KGaA) for
48 h, under constant shaking at room temperature. The extraction
process was repeated four times. The four aliquots were combined
together (200 ml), filtered and evaporated to a dry product, under
reduced pressure with a rotatory evaporator (Stuart RE300; Thermo
Fisher Scientific, Inc.). The weight of the dried extract was 0.55
g and it was stored at 4°C in an airtight glass vial until further
use. The extract obtained was then solubilized in 96% ethanol and
used for the experiments.
HPLC/DAD and HPLC/ESI-MS analyses
Chromatographic analyses were performed using an
Ultimate3000 UHPLC focused instrument equipped with a binary
high-pressure pump, a Photodiode Array detector, a Thermostatted
Column Compartment and an Automated Sample Injector (Thermo Fisher
Scientific, Inc.). The collected data was processed using a
Chromeleon Chromatography Information Management System v6.80
(Thermo Fisher Scientific, Inc.). Chromatographic runs were
performed using a reverse-phase column (Gemini C18; 250 × 4.6 mm; 5
µm particle size) equipped with a guard column (Gemini C18 4
× 3.0 mm; 5 µm particle size) (both from Phenomenex, Inc.).
Walnut fruit septum polyphenols were eluted using a gradient of B
(2.5% formic acid in acetonitrile) in A (2.5% formic acid in
water): 0 min: 5% B; 10 min: 15% B; 30 min: 25% B; 35 min:
30% B; 50 min: 90% B; 57 min then held for a further 7 min,
100% B. The solvent flow rate was 1 mL/min, the temperature was
maintained at 25°C and the injector volume selected was 10
µl. Quantification was performed at 280 nm for organic acids
(protocatechuic and vanillic acid) using vanillic acid as the
reference (R2, 0.9999), while gallic acid and its derivatives
[including hexahydroxydiphenol (HHDP) derivatives] were quantified
at the same wavelength using gallic acid (R2, 0.9998) as an
external standard. Similarly, quantification of flavan-3-ols was
performed at 280 nm using catechin (R2, 0.9999), epigallocatechin
(R2, 0,9999) and epigallocatechin gallate (R2, 0.9998) as
references, whilst p-coumaric acid was quantified at 330 nm using
the corresponding commercially available material (R2, 0.9999).
Quercetin 3-O-glucoside (R2, 0.9998) was used to quantify all
flavonols present in the extract; ellagic acid (R2, 0.9997) was
used as the reference for the quantification of its own derivatives
and valoneic acid dilactone. Flavonols, ellagic acids and valoneic
acid derivatives were all quantified at 350 nm. To unambiguously
identify the chromatographic signals and to confirm peak
assignments, HPLC/ESI-MS analyses were also performed. The HPLC
apparatus used was the same as aforementioned, whilst ESI MS
spectra were acquired using a Thermo Scientific Exactive Plu
Orbitra MS (Thermo Fisher Scientific, Inc.), using a heated
electrospray ionization (HESI II) interface. MS spectra were
recorded operating in negative ion mode, in the m/z range
120-1,500, at a resolving power of 25,000
(full-width-at-half-maximum, at m/z 200,
full-width-at-half-maximum, resulting in a scan rate of >1.5
scans/sec when using automatic gain control target of
1.0×106 and a C-trap inject time of 250 ms, under the
following conditions: Capillary temperature, 300°C; nebulizer gas
(nitrogen) with a flow rate 60 arbitrary units; auxiliary gas flow
rate 10 arbitrary units; source voltage 3 kV; capillary voltage
82.5 V; tube lens voltage 85 V. The Orbitrap MS system was tuned
and calibrated in positive modes, by infusion of solutions of a
standard mixture of SDS (Mr 265.17 Da), sodium taurocholate (Mr
514.42 Da) and Ultramark (Mr 1621 Da). Data acquisition and
analyses were performed using the Excalibur software v4.3 (Thermo
Fisher Scientific, Inc.). Analyses were all performed in
triplicate.
Cell culture and treatment
Human glioblastoma cells (A172), were purchased from
American Type Culture Collection (ATCC) and maintained in
Dulbecco's modified Eagle's medium (DMEM; ATCC®
30-2002™; ATTC) containing 4 mM L-glutamine, 4,500 mg/l
glucose, 1 mM sodium pyruvate and 1,500 mg/l sodium bicarbonate,
supplemented with 10% heat-inactivated fetal bovine serum
(FBS; Sigma-Aldrich; Merck KGaA) and 1%
penicillin/streptomycin, at 37°C in a humidified incubator with
5% CO2. Human foreskin fibroblasts (HFF-1) were
also from ATCC and were used as a normal control, as previously
described (33). Cells were
cultured in DMEM (ATCC® 30-2002™; ATCC)
supplemented with 15% heat-inactivated FBS (Sigma-Aldrich;
Merck KGaA) and 1% penicillin/streptomycin, at 37°C in a
humidified incubator with 5% CO2. Cells were
passaged once a week following trypsinization and replaced with new
medium twice weekly.
The A172 and HFF-1 cell lines were cultured in
presence or absence (control) of increasing concentrations of
walnut septum extract, ranging from 8.75 to 140 µg/ml, for
24 and 48 h. All the treatments were performed using culture medium
containing 1% FBS (starvation conditions) to minimize cell
proliferation, induced by the medium (34). The final ethanol concentration
(used for extract solubilization) in the culture medium was 0.05%.
This low concentration excludes any possible effect of the vehicle
(ethanol) on treated cells (35).
MTT assay
To verify the ability of the natural extract to
affect A172 and HFF-1 cell viability, the MTT assay was used
(Thermo Fisher Scientific, Inc.). Cell lines were seeded in 96-well
plates, at a density of 1.5×104 per well and incubated
overnight at 37°C prior to experimentation. Following which, cells
were treated with scalar concentrations of walnut septum extract
(8.75, 17.5, 35, 70, 140 µg/ml) for 24 and 48 h then, 10
µl MTT reagent (5 mg/ml) was added to each well and the
cells were incubated for 3 h at 37°C. The formazan crystals were
solubilized with 100 µl DMSO and plates were shaken for 10
min. The absorbance was measured at 570 nm using a plate reader
(Synergy 2-BioTek; Agilent Technologies, Inc.).
Cell proliferation
Proliferation of the human glioblastoma cell line
was determined using crystal violet (Merck KGaA) staining assay.
Briefly, A172 cells were cultured in 96-well plates, at a density
of 1.5×104 per well and incubated for 18 h at 37°C to
enable adhesion of cells to the wells. Subsequently, cells were
treated with 70 µg/ml walnut septum extract for 24 h and 48
h. At the appropriate time point, the medium was removed, and the
cells were washed twice with PBS. After washing, control and
treated cells were observed using a phase contrast optical
microscope and images were obtained using an inverted Leica DM IRB
microscope equipped with a CCD camera (Leica Microsystems, Inc.).
Subsequently, 100 µl 0.5% crystal violet staining
solution was added to each well and then the cells were incubated
for 10 min at room temperature. Following three washes with PBS,
the plate was air-dried, without the lid for 2 h at room
temperature. After the addition of 200 µl 0.1% SDS
solution, the plates were shaken for 10 min and read at 570 nm
using a plate reader (Synergy 2-BioTek; Agilent Technologies,
Inc.).
Cell migration
The migration ability of the A172 cell line was
measured using a standard wound-healing assay, performed as
previously described (14).
Migration was captured using an inverted Leica DM IRB microscope
equipped with a CCD camera (Leica Microsystems, Inc.). The ability
of the A172 cells migrate into the wound was evaluated by
determining the percentage of growth area into the wound compared
with the initial starting point at time 0 (t0). According to Ammann
et al (36), to calculate
the percentage of growth, the areas were measured by tracing the
boundary of growth with the ImageJ software (ImageJ bundled with
64-bit Java v1.8.0_112; National Institutes of Health). Time 0
represents the time after which the wound was created for all
conditions: control (cells grown with 1% FBS for 24 and 48
h) and treated cells (cells grown with 1% FBS and 70
µg/ml walnut septum extract for 24 and 48 h). The percentage
migration was deter-mined using the following calculation:
Percentage migration = [(Ainitial −
Amigration)/Ainitial × 100, where
Ainitial was the initial wound area and
Amigration was the wound area following cell
migration.
Caspase-3 colorimetric protease
assay
Caspase-3 activity was analyzed using A172 cell
lysates with a colorimetric protease assay (Thermo Fisher
Scientific, Inc.) as previously described (37). The absorbance was read using a
microplate reader (Synergy 2-BioTek; Agilent Technologies, Inc.) at
400 nm.
Flow cytometery analysis
The A172 cell line was seeded in 6-well plates, at a
density of 3×104 cells. Following treatment with 70
µg/ml walnut septum extract for 24 and 48 h, cells were
collected and washed with PBS, then subsequently stained with
Annexin V-FITC/propidium iodide (PI), in Annexin-V binding buffer
(Sigma-Aldrich; Merck KGaA) for 10 min at 20°C (protected from
light), according to the manufacturer's instructions. Samples were
analyzed immediately using an Amnis®
FlowSight® flow cytometer (Luminex Corporation). A 488
nm laser was used for excitation. Bright field (430-480 nm),
Annexin V-FITC (505-560 nm) and PI (595-642 nm) analysis was
focused on at least 5,000 cell events per sample.
INSPIRE® software (vMark II) was used to setup,
calibrate and obtain spectral compensation, while IDEAS®
software (v6.0) (both EMD Millipore; Merck KGaA) was used to
quantify the number of cell subpopulations (healthy, apoptotic and
necrotic cells).
Bacterial strains
In vitro assays were performed against 32
clinical isolates (16 Gram-positive and 16 Gram-negative strains),
from the bacterial library of the Department of Biomedical and
Biotechnological Sciences (University of Catania, Catania, Italy).
Staphylococcus aureus (S. aureus; ATCC 29213),
Staphylococcus epidermidis (S. epidermidis; ATCC
14990), Enterococcus faecalis (E. faecalis; ATCC
29212), Enterococcus faecium (E. faecium; ATCC
700221), Escherichia coli (E. coli; ATCC 35218),
Pseudomonas aeruginosa (ATCC 27853), Klebsiella
pneumoniae (K. pneumoniae; ATCC 700630) and Proteus
mirabilis (P. mirabilis; ATCC 7002) were purchased from
ATCC and used as reference strains.
Antimicrobial susceptibility testing
The Minimal Inhibitory Concentration (MIC) of J.
regia L. extract was tested using the broth microdilution
method, with reference to the standard procedures of the Clinical
and Laboratory Standards Institute (38). The dry extract was solubilized in
ethanol and diluted in cation adjusted Mueller-Hinton broth (CAMHB)
(Becton, Dickinson and company) with a 1:100 ratio. The stock
solution was filtered using a 0.22 µm filter (EMD Millipore)
and serial two-fold dilutions were made at concentrations ranging
from 0.53 to 275.00 µg/ml in sterile 96-well microplates
(Corning, Inc.) containing CAMHB. Isolated colonies on Mueller
Hinton agar plates were suspended in 0.85% sodium chloride,
to achieve a turbidity equivalent to 0.5 McFarland Standard.
Turbidity evaluation was performed using a spectrophotometer, at
600 nm (Synergy 2-BioTek; Agilent Technologies, Inc.). After a
dilution with a 1:100 ratio, bacterial suspensions were added to
each well for a final concentration of 5×105 colony
forming units/ml. The MIC was defined as the lowest concentration
at which there was no visible growth following incubation at 37°C
without CO2 for 18-24 h (39). The broad-spectrum antibiotic
ciprofloxacin (Sigma-Aldrich; Merck KGaA), at concentrations
ranging from 0.06 to 32.00 µg/ml, was used as an
antibacterial positive control. Each test included a positive
growth control and a negative sterility control (culture broth
without bacteria). Results are expressed as the mean form 4
experiments.
PASS analysis
PASS is an online platform for the prediction of
biological and pharmacological effects, based on the structure of
drug-like compounds (40-42). The analysis also hypothesizes a
possible mechanism of action of the studied molecules. Based on
this potential, a PASS analysis on HPLC detected compounds,
available on the PubChem platform, was performed (43). For each molecule, the Pa (probable
activity) and Pi (probable inactivity) values, both ranging from
0,000 to 1,000, were reported. Higher values of Pa are associated
with a higher probability to obtain that effect experimentally,
although some real activities could be lost (44). The study reported only the
cytostatic, cytotoxic and antimicrobial activities of the selected
compounds, with a Pa>0,700.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three independent experiments, performed in triplicate.
Statistical significance between two groups was analyzed using an
unpaired Student's t-test. One-way analysis of variance (ANOVA),
followed by Tukey's post-hoc test, was used to compare the means
for multiple groups.
Results
Polyphenol profile and content of
Sicilian walnut (J. regia) fruit septum
To characterize the phenolic profile and content of
the fruit septum from Sicilian walnuts, a series of HPLC/Uv-vis-DAD
and HPLC/ESI-MS analyses was performed and the corresponding DAD
chromatogram, visualized at 280 nm, is depicted in Fig. 1. A total of 30 peaks were
tentatively identified, in the range between 0 and 30 minutes;
identification was made on the basis of their relative retention
times, UV-visible (UV-vis) and mass spectral data (data not shown).
Injection with pure analytical standards was used when available
and comparison with literature data on similar matrices
corroborated the assignments from a preliminary analysis. The
majority of the thirty peaks showed an UV-vis spectrum with a
single peak in the range between 265 and 280 nm (Table I), which was attributed to gallic
acid-based metabolites. Mass spectra analyses, particularly
extracted ion chromatograms provided a pivotal contribution in the
tentative identification of these peaks. Furthermore, the UV-vis
and mass spectral data show a series of mono, di-, tri-, tetra- and
pentagalloyl-hexose isomers (Fig.
S1), among which pedunculagine, peak number 3 (p.n. 3) and
tellimagradine I (p.n. 30) were found. The p.ns. 8, 11 and 13 were
identified using the corresponding available commercial standards,
such as epigallocatechin, catechin and epicatechin, respectively;
mass spectra analyses confirmed the attribution. The p.ns. 4 and 9
exhibited a strange UV-vis spectra (two bands at 292 and 255 nm),
which is typical of catechol chromophore; commercial reference and
mass analyses also assisted with identification, which was found to
be protocatechuic acid (p.n. 4) and vanillic acid (p.n. 9). The
p.n. 10 was identified as the sole detecTable hydroxycinnamic acid
present in the extract and reported as p-coumaric acid hexoside
from its UV-vis and mass spectra data. The p.ns. 23 and 26 showed a
UV-vis spectra (absorptions at 255 and 350 nm; strange spectrum
shape) that was similar to quercetin derivatives; following mass
analyses these peaks were identified as quercetin 3-O-glucoside
(p.n. 23) and quercitrin (quercetin 3-O-rhamnoside; p.n. 26).
Another common constituent of the hydrolysable tannins group,
ellagic acid, itself is derived from the intramolecular
lactonization of HHDP, which confers to this molecule a strange
shaped UV-vis spectrum with two absorption bands at 255 and 263-264
nm. This allowed the identification of peaks 20, 22 and 28 to be
determined as ellagic acid derivatives; mass spectral data
confirmed the attribution as two ellagic acid hexoside isomers
(p.ns. 20 and 22) and ellagic acid pentoside (p.n. 28). The p.n. 29
showed a UV-vis spectrum, which was very similar to that of ellagic
acid; however, it was not identical, with a more intense peak at
~290 nm (Fig. S2). A literature
search on a walnut seed (45,46)
or similar seeds (47) identified
the compound at p.n. 29 as a valoneic acid dilactone derivative
(molecular weight, 632). With respect to quantitative analysis, the
primary compound in the extract was at peak 22 (ellagic acid
hexoside isomer 2; 6.16 mg/100 mg extract) followed by valoneic
acid dilactone with 5.63 mg/100 mg extract and gallotannin
pedunculagin 3 with 3.50 mg/100 mg extract (Table I). Despite the lower number of
compounds with respect to gallotannins, ellagic acid derivatives
account for ca. 50% of the total metabolites in the extract
(13.39 mg vs. 32.9 mg; Table I),
with total gallo-tannins accounting for 13.51 mg/100 mg extract.
Flavonoids are the third more represented subclass of polyphenols
with only 2.56 total mg/100 mg extract.
 | Table IPeak list, diagnostics and
quantitative data of the metabolites from the J. regia fruit
septum leaves ethanolic extract. |
Table I
Peak list, diagnostics and
quantitative data of the metabolites from the J. regia fruit
septum leaves ethanolic extract.
| Peak no. | Retention time,
mina | Tentative compound
identification | Biochemical
class | UV-vis, nmb | MW | ESI, m/z | Extract weight,
mg/100 mg | Dry vegetable
matrix weight, mg/g |
|---|
| 1 | 4.57 | Gallic acidd | Organic acids | 270.7 | 170 | 269c (M-H) | 0.413 | 0.108 |
| 2 | 5.41 | Galloyl hexose
isomer 1 | Gallotannins | 265.4 | 332 | 331 (M-H) | 0.284 | 0.074 |
| 3 | 6.13 | Pedunculagin (bis
HHDP- hexose) | Gallotannins | 281.2 | 784 | 783 (M-H).
481c | 3.500 | 0.913 |
| 4 | 6.79 | Protocatechuic
acid | Organic acids | 294.259 | 154 | 153c (M-H) | 0.299 | 0.078 |
| 5 | 7.25 | Galloyl hexose
isomer 2 | Gallotannins | 269.5 | 332 | 331c (M-H). 271 | 0.459 | 0.120 |
| 6 | 7.56 | Galloyl HHDP
hexose | Gallotannins | 276.4 | 634 | 633 (M-H).
463c | 0.138 | 0.036 |
| 7 | 7.84 | Digalloyl hexose
isomer 1 | Gallotannins | 275.3 | 484 | 483 (M-H) | 0.091 | 0.024 |
| 8 | 8.22 |
Epigallocatechind | Flavan-3-ols | 276.2 | 306 | 305 (M-H) | 0.159 | 0.041 |
| 9 | 8.59 | Vanillic
acidd | Organic acids | 310. 279 | 168 | 167 (M-H) | 0.340 | 0.089 |
| 10 | 9.18 | P-coumaric acid
hexoside | Hydroxycinnamic
acids | 310.6. 295 sh | 326 | 325 (M-H).
191c | 0.592 | 0.154 |
| 11 | 9.75 | Catechind | Flavan-3-ols | 276.7 | 290 | 289 (M-H). 245 | 1.461 | 0.381 |
| 12 | 10.24 | Galloyl HHDP-
hexose isomer | Gallotannins | 282.1 | 634 | 633c (M-H) | 0.078 | 0.020 |
| 13 | 11.04 | Epicatechin | Flavan-3-ols | 277.1 | 290 | 289 (M-H) | 0.068 | 0.018 |
| 14 | 11.37 | Digalloyl hexose
isomer 2 | Gallotannins | 285.8 | 484 | 483c (M-H). 331 | 0.246 | 0.064 |
| 15 | 11.63 | Trigalloyl hexose
isomer 1 | Gallotannins | 282.9 | 636 | 635 (M-H).
465c | 0.657 | 0.171 |
| 16 | 11.94 | Trigalloyl hexose
isomer 2 | Gallotannins | 280.1 | 636 | 635 (M-H) | 0.159 | 0.041 |
| 17 | 13.83 |
Epigallocatechingallated | Flavan-3-ols | 276.8 | 458 | 457 (M-H).
289c | 0.515 | 0.134 |
| 18 | 14.38 | Trigalloyl hexose
isomer 3 | Gallotannins | 279.8 | 636 | 635 (M-H).
465c.423 | 2.993 | 0.780 |
| 19 | 17.79 | Tetragalloyl hexose
isomer 1 | Gallotannins | 278.7 | 788 | 787 (M-H).
617c | 3.204 | 0.836 |
| 20 | 18.69 | Ellagic acid
hexoside isomer 1 | Ellagic acid
derivatives | 360.3. 301sh.
252.6 | 464 | 463
(M-H).301c | 1.513 | 0.395 |
| 21 | 19.40 | Tetragalloyl hexose
isomer 2 | Gallotannins | 284.2 | 788 | 787 (M-H) | 0.151 | 0.039 |
| 22 | 20.16 | Ellagic acid
hexoside isomer 2 | Ellagic acid
derivatives | 365.5. 300sh.
252.8 | 464 | 463c (M-H). 301 | 6.162 | 1.607 |
| 23 | 21.14 | Quercetin
3-O-glucosided | Flavonols | 354. 256.2 | 464 | 463c (M-H). 301 | 0.402 | 0.105 |
| 24 | 21.76 | HHDP-hexose
isomer | Gallotannins | 281.5 | 482 | 481c (M-H). 301 | 0.512 | 0.134 |
| 25 | 22.54 | Pentagalloyl hexose
isomer | Gallotannins | 280.7 | 940 | 939 (M-H) | 0.185 | 0.048 |
| 26 | 24.78 | Quercetin
3-O-rhamnoside (quercitrin) | Flavonols | 349.3. 256.2 | 448 | 447c (M-H). 301 | 2.163 | 0.564 |
| 27 | 25.72 | Digalloyl hexose
isomer 3 | Gallotannins | 282.8 | 484 | 483 (M-H).
331c | 0.205 | 0.053 |
| 28 | 26.51 | Ellagic acid
pentoside | Ellagic acid
derivatives | 365.1. 300sh.
262.8 | 434 | 433c (M-H). 301 | 0.084 | 0.022 |
| 29 | 28.67 | Valoneic acid
dilactone derivative | Ellagic acid
derivatives | 364.9. 290sh.
263.8 | 632 | 631(M-H).
469c. 301 | 5.632 | 1.469 |
| 30 | 29.39 | Tellimagrandin I
(digalloyl-HHDP-hexose) | Gallotannins | 272.3 | 786 | 785 (M-H). 633 | 0.239 | 0.062 |
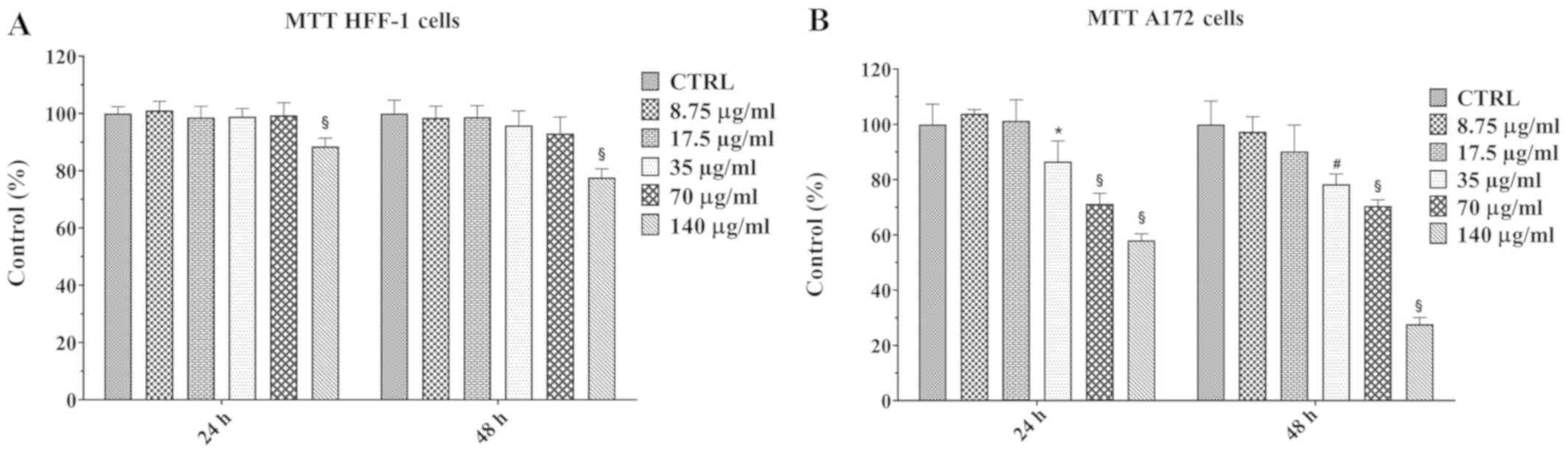
Viability of HFF-1 and A172 cells,
cultured in the presence of increasing concentrations of walnut
septum extract
Prior to investigating the anti-proliferative effect
of the natural extract on human A172 glioblastoma cells, the
possible cytotoxic effect of the extract on A172 cells and a
non-cancerous cell line (HFF-1) was performed using MTT assay. The
A172 and HFF-1 cell lines were cultured in absence (control cells)
or in presence of increasing concentrations of walnut septum
extract, ranging from 8.75 to 140 µg/ml, for 24 and 48 h
(Fig. 2A and B). The natural
extract was solubilized in ethanol and, subsequently, diluted in
medium at 0.05% final concentration. The treatment with
vehicle alone (ethanol) did not cause any change in viability in
both cell lines, at the two time points (data not shown). A
significant reduction of HFF-1 cell viability occurred only in
presence of the highest concentration of the extract (140
µg/ml), at 24, as well as 48 h (Fig. 2A). In A172 cells the treatment with
lower doses of the natural extract (8.75 and 17.5 µg/ml) did
not produce any effect on cell viability, at both 24 and 48 h.
However, a dose-dependent reduction in cell viability was observed
when A172 cells were treated with higher doses (from 35 to 140
µg/ml) of the walnut septum extract (Fig. 2B). The concentration of 70
µg/ml, inducing a significant reduction of A172 cell
viability, without affecting HFF-1 cell viability, was selected for
subsequent experiments on human glioblastoma cells.
Effect of walnut septum extract on A172
cell proliferation
Proliferation of A172 cells, cultured in the
presence or absence of walnut septum extract (70 µg/ml), for
24 and 48 h was evaluated using a crystal violet assay and the cell
micro-graphs of control and treated cells are shown in Fig. 3A. The quantification of
proliferation following crystal violet staining assay demonstrated
that the treatment with the natural extract significantly reduced
A172 cell proliferation by 20 and 42% at 24 and 48 h,
respectively, compared with that in the control group (Fig. 3B).
Effect of walnut septum extract on A172
cell migration
A172 cell migration was investigated using the
wound-healing assay. The migration of cells was monitored for 24
and 48 h following creation of the wound and the representative
images at 0, 24 and 48 h time points are shown in Fig. 4A. The untreated A172 cells were
able to migrate across the wound and close it at 48 h. Conversely,
the treatment of cells with 70 µg walnut septum extract
significantly reduced migration of the cells, at 24 and 48 h,
compared with that in untreated cells. The results were analyzed
quantitatively, and cell migration was significantly decreased by
42% at 24 h and by 50% at 48 h in cells treated with
walnut septum extract, compared with that in the control groups
(Fig. 4B).
Effect of walnut septum extract on A172
cell apoptosis
The caspase-3 assay was performed on the human A172
glioblastoma cell line to investigate the ability of walnut septum
extract to induce apoptotic death (Fig. 5). A172 cells were cultured in the
presence or absence of 70 µg/ml walnut septum extract, for
24 and 48 h. A significant induction in caspase-3 activity was
found in the presence of 70 µg/ml walnut extract compared
with that in untreated cells, at both time points. Notably, at the
48-h time point, the activity of the enzyme was double that in A172
treated cells compared with that in the control group.
To further analyze the pro-apoptotic effect of
walnut septum extract on the human A172 glioblastoma cell line,
flow cytometry evaluation of Annexin-V/PI staining was performed
(Fig. 6A and B). The flow
cytometry plots are displayed, for each experimental condition, and
the distribution of A172 cells in four different quadrants,
depending on their staining with Annexin-V and PI (Fig. 6A). A172 cells, cultured without
walnut septum extract for 24 and 48 h, were double negative for
staining (Annexin-V and PI), therefore are considered as healthy
(Fig. 6A, panels 1 and 2). The
analysis revealed that treatment with 70 µg/ml walnut septum
extract at both 24 and 48 h increased early and late apoptosis of
A172 cells (Fig. 6A, panels 3 and
4). There was a moderate increase in the number of necrotic cells
observed in A172 cells cultured in the presence of 70 µg/ml
walnut septum extract (Fig. 6A,
panels 3 and 4) compared with that in the control group, at 24 and
48 h (Fig. 6A, panels 1 and 2).
The percentages of the single cell subpopulation (healthy, early
apoptotic, late apoptotic and necrotic cells), for each
experimental condition, are shown in Fig. 6B. The treatment of A172 cells with
70 µg/ml walnut septum extract caused a significant
reduction of healthy cells, followed by a concomitant increment of
apoptotic and necrotic cells, which was more evident at 48 h.
Notably, the increase in late apoptotic cells cultured with walnut
septum extract at 48 h was higher compared with that in cells at 24
h. The results suggested that, at 70 µg/ml, the natural
extract could cause apoptotic death of human glioblastoma
cells.
Antibacterial activity of walnut septum
extract
The antibacterial activity of walnut septum extract
was also determined using the standard broad-spectrum drug,
ciprofloxacin (Table II). The
natural extract exhibited an antibacterial effect on Gram-positive
bacteria; however, the effect was found at varying degrees. The
MICs were in the ranges of 8.59-275 µg/ml against S.
aureus, S. epidermidis, E. faecalis and E.
faecium. Gram-negative strains were the least sensitive, with
MIC values of 275 μg/ml against E. coli, K. pneumoniae,
P. aeruginosa and P. mirabilis. The extract was more
effective against P. aeruginosa compared with that in other
Gram-negative bacterial strains (MIC 137.5 µg/ml).
 | Table IIAntimicrobial activity of Juglans
regia L. against Gram-positive and Gram-negative bacterial
strains. |
Table II
Antimicrobial activity of Juglans
regia L. against Gram-positive and Gram-negative bacterial
strains.
A, Gram-positive
bacterial strains
|
|---|
| Strain no.a | Name | Source | MIC, µg/ml
| ICb |
|---|
| Juglans regia
L. | Cip |
|---|
| 001/040 | S. aureus
ATCC 29213 | Standard | 68.75 | 0.50 | S |
| 002/040 | S.
aureus | Endophtalmitis | 17.18 | 0.25 | S |
| 003/040 | S.
aureus | Pneumonia | 275.00 | 4.00 | R |
| 004/040 | S.
aureus | Pneumonia | 8.59 | 0.25 | S |
| 005/040 | S.
aureus | Endophtalmitis | 17.18 | 0.50 | S |
| 006/040 | S.
epidermidis ATCC 14990 | Standard | 8.59 | 0.12 | S |
| 007/040 | S.
epidermidis | Osteomyelitis | 8.59 | 0.03 | S |
| 008/040 | S.
epidermidis | Septicemia | ORC | 8.00 | R |
| 009/040 | S.
epidermidis | Endophtalmitis | 68.75 | 0.01 | S |
| 010/040 | S.
epidermidis | Septicemia | 275.00 | 8.00 | R |
| 011/040 | E. faecalis
ATCC 29212 | Standard | 34.37 | 0.50 | S |
| 012/040 | E.
faecalis | Abscess | 8.59 | 0.50 | S |
| 013/040 | E.
faecalis | Septicemia | 34.37 | 0.50 | S |
| 014/040 | E.
faecalis | Pneumonia | 34.37 | 1.00 | S |
| 015/040 | E.
faecalis | Abscess | 17.18 | 0.25 | S |
| 016/040 | E. faecium
ATCC 700221 | Standard | ORC | ORC | R |
| 017/040 | E.
faecium | Cholecystitis | ORC | ORC | R |
| 018/040 | E.
faecium | Catheter
cystitis | 275.00 | 8.00 | R |
| 019/040 | E.
faecium | Catheter
cystitis | 8.59 | 1.00 | S |
| 020/040 | E.
faecium | Cholecystitis | 275.00 | 16.00 | R |
|
| B, Gram-negative
bacterial strains |
|
| Strain no.a | Name | Source | MIC, µg/ml
| ICb |
| Juglans regia
L. | Cip |
|
| 021/040 | E. coli ATCC
35218 | Standard | 275.00 | 0.01 | S |
| 022/040 | E. coli | Septicemia | 275.00 | 0.01 | S |
| 023/040 | E. coli | Septicemia | ORC | ORC | R |
| 024/040 | E. coli | Cystitis | 275.00 | 4.00 | R |
| 025/040 | E. coli | Cystitis | 275.00 | 8.00 | R |
| 026/040 | P.
aeruginosa ATCC 27853 | Standard | 275.00 | 0.25 | S |
| 027/040 | P.
aeruginosa | Septicemia | 137.50 | 0.06 | S |
| 028/040 | P.
aeruginosa | Septicemia | 275.00 | 4.00 | R |
| 029/040 | P.
aeruginosa | Pneumonia | 275.00 | 0.12 | S |
| 030/040 | P.
aeruginosa | Pneumonia | 275.00 | 16.00 | R |
| 031/040 | K.
pneumoniae ATCC 700630 | Standard | 275.00 | 0.25 | S |
| 032/040 | K.
pneumoniae | Nephritis | ORC | 4.00 | R |
| 033/040 | K.
pneumoniae | Pneumonia | ORC | 32.00 | R |
| 034/040 | K.
pneumoniae | Pneumonia | ORC | 8.00 | R |
| 035/040 | K.
pneumoniae | Nephritis | 275 | 4.00 | R |
| 036/040 | P. mirabilis
ATCC 7002 | Standard | 275 | 0.25 | S |
| 037/040 | P.
mirabilis | Cystitis | ORC | 1.00 | S |
| 038/040 | P.
mirabilis | Cystitis | 275.00 | 0.01 | S |
| 039/040 | P.
mirabilis | Cystitis | 275.00 | 0.01 | S |
| 040/040 | P.
mirabilis | Cystitis | ORC | 8.00 | R |
Furthermore, treatment with the highest doses of
natural extract (275 µg/ml) was able to affect the growth of
Gram-positive and Gram-negative bacterial strains, most of which
were resistant to the antibiotic Ciprofloxacin.
Predicted cytostatic, cytotoxic and
antimicrobial activity of walnut septum extract: PASS analysis
To determine which molecules could be responsible
for the biological effects of walnut septum extract, PASS analysis
was performed. The PubChem platform was used to verify the
predicted activities of HPLC identified compounds (43). The results showed an activity
spectrum for each molecule, including antineoplastic,
antimutagenic, cytostatic, anti-infective, and antiseptic effects
(Table III).
 | Table IIIPrediction of Activity Spectra for
Substances analysis for walnut septum extract. |
Table III
Prediction of Activity Spectra for
Substances analysis for walnut septum extract.
| Peak no. | Compound | Probable
activity | Probable
inactivity | Predicted
biological activity |
|---|
| 1 | Gallic acid | 0.828 | 0.005 | Anti-infective |
| 3 | Pedunculagin (bis
HHDP-hexose) | 0.918 | 0.005 | Antineoplastic |
| 0.860 | 0.005 | Apoptosis
agonist |
| 0.817 | 0.006 | Cytostatic |
| 0.898 | 0.005 | TP53 expression
enhancer |
| 4 | Protocatechuic
acid | 0.776 | 0.005 | Anti-infective |
| 0.834 | 0.003 | Antimutagenic |
| 0.906 | 0.003 | Antiseptic |
| 8 |
Epigallocatechin | 0.815 | 0.005 |
Anticarcinogenic |
| 0.953 | 0.001 | Antimutagenic |
| 0.742 | 0.019 | Antineoplastic |
| 0.712 | 0.005 | Antiviral
(Influenza) |
| 0.759 | 0.010 | Apoptosis
agonist |
| 0.710 | 0.005 | Proliferative
diseases treatment |
| 0.963 | 0.003 | TP53 expression
enhancer |
| 9 | Vanillic acid | 0.708 | 0.007 | Anti-infective |
| 0.834 | 0.003 | Antimutagenic |
| 0.898 | 0.003 | Antiseptic |
| 0.713 | 0.024 | TP53 expression
enhancer |
| 10 | P-coumaric acid
hexoside | 0.887 | 0.003 |
Anticarcinogenic |
| 0.875 | 0.004 | Anti-infective |
| 0.759 | 0.017 | Antineoplastic |
| 0.751 | 0.004 | Antiviral
(Influenza) |
| 0.813 | 0.005 | Caspase 3
stimulant |
| 0.744 | 0.005 | Cell adhesion
molecule inhibitor |
| 0.955 | 0.002 | G-protein-coupled
receptor kinase inhibitor |
| 0.811 | 0.003 | Proliferative
diseases treatment |
| 0.828 | 0.009 | TP53 expression
enhancer |
| 11 | Catechin | 0.795 | 0.005 |
Anticarcinogenic |
| 0.959 | 0.003 | TP53 expression
enhancer |
| 13 | Epicatechin | 0.795 | 0.005 |
Anticarcinogenic |
| 0.959 | 0.003 | TP53 expression
enhancer |
| 17 |
Epigallocatechingallate | 0.841 | 0.004 |
Anticarcinogenic |
| 0.926 | 0.002 | Antimutagenic |
| 0.771 | 0.003 | Antiviral
(Influenza) |
| 0.741 | 0.012 | Apoptosis
agonist |
| 0.937 | 0.004 | TP53 expression
enhancer |
| 23 | Quercetin
3-O-glucoside | 0.965 | 0.001 |
Anticarcinogenic |
| 0.714 | 0.009 | Antifungal |
| 0.726 | 0.006 | Anti-infective |
| 0.763 | 0.004 | Antimutagenic |
| 0.833 | 0.008 | Antineoplastic |
| 0.715 | 0.005 | Antiviral
(Influenza) |
| 0.792 | 0.009 | Apoptosis
agonist |
| 0.801 | 0.005 | Caspase 3
stimulant |
| 0.825 | 0.006 | Cytostatic |
| 0.921 | 0.002 | Proliferative
diseases treatment |
| 0.959 | 0.003 | TP53 expression
enhancer |
| 26 | Quercetin
3-O-rhamnoside (quercitrin) | 0.943 | 0.002 |
Anticarcinogenic |
| 0.740 | 0.008 | Antifungal |
| 0.748 | 0.005 | Antimutagenic |
| 0.854 | 0.007 | Antineoplastic |
| 0.814 | 0.007 | Apoptosis
agonist |
| 0.803 | 0.005 | Caspase 3
stimulant |
| 0.751 | 0.008 | Cytostatic |
| 0.890 | 0.002 | Proliferative
diseases treatment |
| 0.928 | 0.004 | TP53 expression
enhancer |
| 30 | Tellimagrandin I
(digalloyl-HHDP-hexose) | 0.706 | 0.007 | Anti-infective |
| 0.790 | 0.012 | TP53 expression
enhancer |
Discussion
Increasing scientific evidence highlights the
crucial role of diet (48),
probiotics (49) and nutraceutical
(50) products on biological
processes (pathogen resistance, xenobiotic and drug metabolism,
delay in the aging process, prevention of chronic diseases,
increase in life expectancy, or support in the structure or
function of the body) and, consequently, on human health and
diseases, including cancer (51-55).
Notably, bad dietary and lifestyle habits can induce
both genetic and epigenetic changes (for example, DNA methylation,
histone post-translational modification, such as acetylation,
ubiquitination, sumoylation, phosphorylation and ADP-ribosylation)
(56), which can significantly
impact the health status of individuals, inducing genetic mutations
or the alteration in the expression levels of micro(mi)RNAs, which
are known to be involved in the pathogenesis of different tumors.
For example, upregulation (such as hsa-miR-196a-5p, hsa-miR-503-5p,
hsa-miR-7-5p, hsa-miR-542-5p, hsa-miR-142-5p, hsa-miR-19a-3p,
hsa-miR-18a-5p, hsa-miR-19b-3p, hsa-miR-32-5p, hsa-miR-196b-5p,
hsa-miR-33b-5p, hsa-miR-34b-3p, from 1.55 to 8.1 fold change) and
downregulation (such as hsa-miR-195-5p, hsa-miR-378a-5p,
hsa-miR-363-3p, hsa-miR-100-5p, hsa-miR-328-5p, hsa-miR-99a-5p,
hsa-miR-218-5p, hsa-miR-432-5p, hsa-miR-379-5p, hsa-miR-154-5p,
hsa-miR-133a-3p, hsa-miR-487b-5p, hsa-miR-135a-5p, hsa-miR-411-5p
hsa-miR-1-3p) of miRNAs have been demonstrated in oral cancers.
Furthermore, has-miR-514a-3p, hsa-miR-508-3p, hsa-miR-509-3-5p,
has-miR-513c-5p, has-miR-513a-5p were downregulated, while
has-miR-592 and has-miR-199a-5p were upregulated in patients with
high-grade human melanoma compared with that in patients with
low-grade disease (57-60). On the other hand, it has been
widely demonstrated that some micro- and macronutrients, including
those contained in walnuts, have beneficial effects for
individuals. In particular, it has been demonstrated that the
consumption of 18% of dietary calories from walnuts
significantly reduced the growth rate of MDA-MB 231 human breast
cancer cells implanted in mice (61). Another study demonstrated that
walnut consumption also prevented cancer development in a
transgenic mouse genetically programmed to develop cancer (62). The effects on cancer suppression
were associated with the walnut content of phenolic compounds,
which could be distinguished into flavonoids, phenolic acids,
stilbenes, coumarins, lignans, and tannins (63). Active molecules are used in
Traditional Chinese Medicine, Ayurveda, Kampo, Traditional Korean
Medicine, and in Unani (2). Over
the last ten years the number of reports focusing on the biological
activity of natural extracts has increased. Among the biological
properties, the antiproliferative and antimicrobial effect of
natural extracts have been widely reported (64-68).
Walnut extracts exerted a potent anticancer effect against
different cancer cell lines, such as human colorectal
adenocarcinoma, breast cancer and oral squamous carcinoma cancer
(69). In particular, the
dose-dependent antiproliferative effect of walnut septum extract on
human A549 lung adenocar-cinoma, human T47D-KBluc and MCF-7 breast
cancer cell lines has been demonstrated (70). In addition, walnut consumption or
walnut extracts showed antioxidant, anti-inflammatory and
anti-aging properties in the brain (26,29,32).
However, the antitumor activity of walnut septum extract against
one of the most aggressive brain tumors, glioblastoma, has not been
investigated.
In the present study, the chemical composition of
the septum extract of Sicilian walnuts was analyzed. Walnuts are
considered as a type of 'superfood', with high nutritional content,
as they contain numerous essential unsaturated fatty acids,
tocopherols, sterols, fiber and polyphenols. With respect to
biosynthesis, the majority of the known hydrolysable tannins
originate from the different coupling possibilities of the same few
building blocks: gallic acid, its dimer hexahydroxydiphenyl HHDP
(sharing a similar chromophore) and a polyol, usually glucose or
quinic acid (45). Walnut
poly-phenols comprise several subclasses of molecules, including
flavan-3-ols, flavonols and hydrolizable tannins, which have been
reported to dominate the phenolic profile of J. regia fruit
(45,46,71).
With respect to the walnut fruit septum (the wooden diaphragm
inside the kernel), it has been used for a long time as a folk
remedy in Traditional Chinese Medicine, with the name of
Diaphragma juglandis fructus (72); however, there have been few studies
investigating the compounds inside. A recent study by Li et
al (73), bridged this gap by
reporting a detailed characterization of phenols present in the
walnut fruit septum from a Chinese cultivar using ultra performance
liquid chromatography-MS analyses; the authors found 75 different
compounds, including flavonoids (flavan-3-ols and flavanols),
ellagic acid derivatives and gallotannins, thus confirming the
precise compositional similarity of this part with the
corresponding fruit (45).
The results of the chemical analysis in the present
study are similar to those reported by previous studies (45,73),
with polyphenols being the majority type of metabolites found in
walnut by-products and hydrolyzable tannins are the most abundant
polyphenol subclass. The presence of a valoneic acid dilactone
derivative in considerable amounts in the extract in the present
study was in disagreement with the study by Li et al
(73), which reported several
ellagic acid derivatives but not valoneic acid.
Furthermore, Li et al (73) identified the presence of several
hydroxycinnamic acids and 11 different quercetin derivatives,
whereas the presence of p-coumaric hexoside and glucoside and
rhamnoside derivatives were found in the present study. These
discrepancies could be due to both the different provenance of the
plant material and to the different extraction solvent used.
Notably, a high content of gallotannins, ellagic acid derivatives
and flavanols were identified which have been associated with
different biological proprieties, including cytostatic and
antimicrobial activities (74-76).
Based on the chemical analysis, the cytostatic and
cytotoxic effects of walnut septum extract on the human A172
glioblastoma cell line was investigated. The treatment of A172
cells with walnut septum extract resulted in a significant
reduction of their viability, in a dose-dependent manner. Notably,
only the highest dose of the extract was able to reduce the
viability of non-cancerous cells. On the other hand, the viability
of A172 cells was affected at the intermedia dose (35
µg/ml). However, since this dose showed a modest effect, 70
µg/ml was chosen for all the experiments. Furthermore, the
cytostatic activity was further evaluated using proliferation and
migration assays, and the results demonstrated a reduced ability of
treated cells to proliferate and migrate compared with that in the
control group. The results of the present study are supported by
data reporting the cytostatic activity of walnut extracts against
different cancer cell lines. For example, the antiproliferative
effect of walnut seed, green husk and leaf methanolic extracts on
two human renal (A-498 and 769-P) and one colon (Caco2) cancer cell
line has been reported (77).
However, a concentration of 500 µg/ml of walnut methanolic
extracts was used to treat human renal and colon cells, which is
much higher compared with that used in in the present study. This
could suggest either a higher sensitivity of the human A172
glioblastoma cells compared with that in the human renal and colon
cell lines to the extract action, or to a greater activity of
walnut septum extract compared to the extracts from other parts of
the walnut.
The ability of the walnut septum extract to
decrease A172 cell proliferation and migration led to the
hypothesis that it could exert a pro-apoptotic action or act
against proteases and proteins that favor cell invasion and
migration. This has been supported from preliminary animal studies,
which found that walnut extracts were effective in reducing the
concentration of matrix metalloproteinases at the mRNA and protein
level, that are well-known to be involved in tumor invasion
(78-80). In the same manner, several studies
have demonstrated the pro-apoptotic action of walnut septum
polyphenols for example, in human breast cancer cell lines
implanted in nude mice, and in mammary gland, prostate, colon, and
renal cancers tumors in transgenic mouse models (62,81).
The results in the present study revealed that human glioblastoma
cells underwent apoptosis, induced by the treatment with natural
walnut septum extract. It is well-known that caspase-3 mediates
programmed cell death, as it catalyzes the cleavage of numerous key
cellular target proteins, such as the DNA repair enzyme poly
(ADP-ribose) polymerase, the retino-blastoma protein and the
DNA-dependent protein kinase catalytic subunit (82,83).
Phytochemical analysis of walnut septum extract revealed the
presence of the two flavonoids, epigallocathecin and
epigallocathecingallate, both have been found to exhibit antitumor
activity in in vivo and in vitro models of human
breast cancer (84). Notably, it
has also been reported that epigallocathecingallate induced
apoptosis of human T98G and U87MG glioblastoma cell lines, but not
in human normal astrocytes, by activating the pro-apoptotic caspase
3 protein (85). In the present
study, a significant increase in caspase-3 activity was found,
following treatment of A172 cells with 70 µg/ml walnut
septum extract, which primarily occurred after 48 h. These findings
were consistent with the results obtained by flow cytometry
analyses, in which there was a significant reduction in the number
of healthy cells, followed by a significant increase in the number
of apoptotic cells, in A172 treated cells, compared with that in
the control group, particularly after 48 h incubation. In
particular, following treatment for 48 h, there was an increase in
the number of late apoptotic cells, compared with that at 24 h,
indicating that a prolonged treatment period with walnut extract
induces an irreversible apoptotic death of glioblastoma cells. The
increase in the number of glioblastoma apoptotic cells confirmed
the cytotoxic activity of the walnut septum extract against one of
the most malignant tumors, for which therapy remains ineffective.
Deficiencies in the immune system of patients undergoing
chemotherapy deter-mine the reactivation and invasion of latent
microorganisms, with an increased risk for patients with cancer of
opportunistic infections (25).
Microbiological studies demonstrated the ability of different plant
extracts to prevent bacterial and fungal growth (1,86-90).
For example, in our previous study, the ability of the pellicle
extract to inhibit the growth and biofilm formation of 7
coagulase-negative staphylococci strains and to eradicate the
biofilm, previously formed by bacteria, was demonstrated (4). The antibacterial activity of walnut
septum extract against several Gram-positive and Gram-negative
bacterial strains was also investigated. The selection of the
strains was based on their clinical relevance, being responsible
for the most common nosocomial infections and presenting a
different spectrum of antibiotic susceptibility (Table II).
Notably, Gram-positive bacteria was found to be
more sensitive to the action of walnut septum extract compared with
that in Gram-negative bacterial strains, as indicated by the lower
MIC values, which could be due to a different cell wall composition
and structure of the two types of micro-organisms (91). The Gram-positive cell wall is
composed of peptidoglycan, teichoic and lipoteichoic acids, while
in Gram-negative bacteria it is constituted of a thin layer of
peptidoglycan, enveloped by an external membrane containing
lipopolysaccharides and lipoproteins (92). This further layer reduces membrane
permeability, providing selective movement of molecules, and is
known to be responsible for the antibiotic therapy failure in
Gram-negative bacteria-related infections (92-95).
This could explain the efficacy of the natural extract in reducing
the Gram-positive bacteria growth already at low concentrations
(from 8.59 to 34.37 µg/ml).
However, according to the study by Saraiva et
al (96), walnut septum
extract was found to be an active antimicrobial agent for S.
aureus and P. aeruginosa, as the MIC values ranged from
100 to 500 µg/ml.
The biological properties found in the walnut
septum extract could be due to the presence of specific active
compounds, in which PASS analysis (Table III) predicted cytostatic,
cytotoxic and antimicrobial effects. Furthermore, some molecules,
such as p-coumaric acid hexoside (p.n. 10) (97), quercetin 3-O-glucoside (p.n. 23)
(98), quercetin 3-O-rhamnoside
(quercitrin) (p.n. 26) (99) were
predicted pro-apoptotic agents by stimulating caspase-3 expression.
The compounds [pedunculagin (bis HHDP-hexose) (p.n. 3) (62), epigallocatechin (p.n. 8),
epigallocatechingallate (p.n. 17) (85), quercetin 3-O-rhamnoside
(quercitrin) (p.n. 26) (100)]
were predicted to promote apoptotic death. Therefore, the results
highlight a dual role of walnut septum extract, supporting a
possible use as a co-adjuvant in glioblastoma treatment, as it is
able to counteract both tumor cell proliferation and bacterial
growth. These results will pave the way for additional functional
and molecular studies to elucidate the molecular mechanisms
responsible for the anti-proliferative effects mediated by walnut
septum extracts. The development of novel molecular and proteomic
high-throughput technologies has enabled the detection of small
changes in the expression levels of cancer hallmarks, including
DNA, microRNAs, proteins, small molecules (such as cell lipids,
metabolites, organic compounds), circulating tumor cells, and
extracellular vesicles (11,101,102). Therefore, the administration of
nutraceutical compounds coupled with highly sensitive diagnostic
and prognostic techniques represents a promising strategy for the
management of patients with glioblastoma. Further validation of the
data in the present study will be performed in additional different
tumor cell lines, including tumor organoids, as well as in a
clinical setting.
In conclusion, phytochemical analysis of walnut
septum extract revealed a high content of active compounds, such as
gallotannins, ellagic acid derivatives and flavanols. Subsequently,
the ability of walnut septum extract to reduce cell viability,
proliferation and migration in human A172 glioblastoma cell line
was found; however, the extract did not affect the viability of the
primary human HFF-1 foreskin fibroblast-1 cell line. Furthermore,
caspase-3 activity showed a significant increase following
incubation for 48 h. The anti-microbial analysis of walnut septum
extract highlighted the ability of the extract to counteract
bacterial growth, particularly in Gram-positive bacteria. The dual
function shown by the walnut septum extract could be due to the
presence of a cocktail of biologically active compounds that can
alter cell physiology, producing cytostatic, cytotoxic and
antibacterial effects, as identified using PASS analysis. The
promising results could provide a novel prospective on the possible
and future applications of walnut septum extract as co-adjuvant
treatment in cancer therapy.
Supplementary Data
Funding
This research was funded by a National Grant from
Ministry of Education, University and Research (grant no. PRIN
2015JXE7E8) and by the Piano triennale per la Ricerca Linea
Intervento 2, University of Catania, Italy.
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG, MTC, GL, CDA and MS made substantial
contributions to the conception and design of the study. FD, CG,
APA, LS, LP, GAM acquired, analyzed and interpreted the data. GL,
CDA, and MS drafted the article and revised it critically for
important intellectual content. All authors read and approved the
final manuscript. All authors agree to be held accounT-able for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Salvatore
Ragusa, a botanist at the Department of Health Sciences, Magna
Graecia University of Catanzaro (Catania, Italy) for the
authentication of the specimens.
References
|
1
|
Genovese C, Acquaviva R, Ronsisvalle S,
Tempera G, Antonio Malfa G, D'Angeli F, Ragusa S and Nicolosi D: In
vitro evaluation of biological activities of Orobanche crenata
Forssk. leaves extract. Nat Prod Res. View Article : Google Scholar : 2697
|
|
2
|
Yuan H, Ma Q, Ye L and Piao G: The
Traditional medicine and modern medicine from natural products.
Molecules. 21:212016. View Article : Google Scholar
|
|
3
|
Jahanban-Esfahlan A, Ostadrahimi A,
Tabibiazar M and Amarowicz R: A Comparative review on the
extraction, anti-oxidant content and antioxidant potential of
different parts of walnut (Juglans regia L.) fruit and tree.
Molecules. 24:242019. View Article : Google Scholar
|
|
4
|
Acquaviva R, D'Angeli F, Malfa GA,
Ronsisvalle S, Garozzo A, Stivala A, Ragusa S, Nicolosi D, Salmeri
M and Genovese C: Antibacterial and anti-biofilm activities of
walnut pellicle extract (Juglans regia L.) against
coagulase-negative staphylococci. Nat Prod Res. View Article : Google Scholar
|
|
5
|
Britton MTLC, Dandekar AM, McGranahan GH
and Caboni E: Persian Walnut. Compendium Transgenic Crop Plants.
4:285–300. 2009.
|
|
6
|
Ara I, Shinwari MMA, Rashed SA and MA B:
Evaluation of antimicrobial properties of two different extracts of
Juglans regia tree bark and search for their compounds using gas
chromatohraphy-mass spectrum. International Journal of Biology.
View Article : Google Scholar
|
|
7
|
Wei Q, Ma XH and Dong JE: Preparation,
chemical constituents and antimicrobial activity of pyroligneous
acids from walnut tree branches. J Anal Appl Pyrolysis. 87:24–28.
2010. View Article : Google Scholar
|
|
8
|
Rywaniak J, Luzak B, Podsedek A, Dudzinska
D, Rozalski M and Watala C: Comparison of cytotoxic and
anti-platelet activities of polyphenolic extracts from Arnica
montana flowers and Juglans regia husks. Platelets. 26:168–176.
2015. View Article : Google Scholar
|
|
9
|
Salimi M, Ardestaniyan MH, Mostafapour
Kandelous H, Saeidnia S, Gohari AR, Amanzadeh A, Sanati H, Sepahdar
Z, Ghorbani S and Salimi M: Anti-proliferative and apoptotic
activities of constituents of chloroform extract of Juglans regia
leaves. Cell Prolif. 47:172–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanif F, Muzaffar K, Perveen K, Malhi SM
and Simjee ShU: Glioblastoma multiforme: A review of its
epidemiology and pathogenesis through clinical presentation and
treatment. Asian Pac J Cancer Prev. 18:3–9. 2017.PubMed/NCBI
|
|
11
|
Silantyev AS, Falzone L, Libra M, Gurina
OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias
PD and Tsatsakis A: Current and future trends on diagnosis and
prognosis of glioblastoma: From molecular biology to proteomics.
Cells. 8:82019. View Article : Google Scholar
|
|
12
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rock K, McArdle O, Forde P, Dunne M,
Fitzpatrick D, O'Neill B and Faul C: A clinical review of treatment
outcomes in glio-blastoma multiforme--the validation in a non-trial
population of the results of a randomised Phase III clinical trial:
Has a more radical approach improved survival? Br J Radiol.
85:e729–e733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motta C, D'Angeli F, Scalia M, Satriano C,
Barbagallo D, Naletova I, Anfuso CD, Lupo G and Spina-Purrello V:
PJ-34 inhibits PARP-1 expression and ERK phosphorylation in
glioma-conditioned brain microvascular endothelial cells. Eur J
Pharmacol. 761:55–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Tellingen O, Yetkin-Arik B, de Gooijer
MC, Wesseling P, Wurdinger T and de Vries HE: Overcoming the
blood-brain tumor barrier for effective glioblastoma treatment.
Drug Resist Updat. 19:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Matteis V, Rizzello L, Ingrosso C,
Liatsi-Douvitsa E, De Giorgi ML, De Matteis G and Rinaldi R:
Cultivar-dependent anticancer and antibacterial properties of
silver nanoparticles synthesized using leaves of different Olea
Europaea trees. Nanomaterials (Basel). 9:92019. View Article : Google Scholar
|
|
17
|
Menzl I, Witalisz-Siepracka A and Sexl V:
CDK8-novel therapeutic opportunities. Pharmaceuticals (Basel).
12:122019. View Article : Google Scholar
|
|
18
|
Candido S, Lupo G, Pennisi M, Basile MS,
Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra
M, et al: The analysis of miRNA expression profiling datasets
reveals inverse microRNA patterns in glioblastoma and Alzheimer's
disease. Oncol Rep. 42:911–922. 2019.PubMed/NCBI
|
|
19
|
Naletova I, Cucci LM, D'Angeli F, Anfuso
CD, Magrì A, La Mendola D, Lupo G and Satriano C: A tunable
nanoplatform of nanogold functionalised with angiogenin peptides
for anti-angiogenic therapy of brain tumours. Cancers (Basel).
11:112019. View Article : Google Scholar
|
|
20
|
Falzone L, Salomone S and Libra M:
Evolution of Cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christofi T, Baritaki S, Falzone L, Libra
M and Zaravinos A: Current perspectives in cancer immunotherapy.
Cancers (Basel). 11:112019. View Article : Google Scholar
|
|
22
|
Abarca-Merlin DM, Maldonado-Bernal C and
Alvarez-Arellano L: Toll-like receptors as therapeutic targets in
central nervous system tumors. BioMed Res Int. 2019:52863582019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oberheim Bush NA, Hervey-Jumper SL and
Berger MS: Management of glioblastoma, present and future. World
Neurosurg. 131:328–338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stupp R, Dietrich PY, Ostermann Kraljevic
S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G,
Miralbell R, et al: Promising survival for patients with newly
diagnosed glio-blastoma multiforme treated with concomitant
radiation plus temozolomide followed by adjuvant temozolomide. J
Clin Oncol. 20:1375–1382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taplitz RA, Kennedy EB, Bow EJ, et al:
Antimicrobial prophylaxis for adult patients with cancer-related
immunosuppression: ASCO and IDSA clinical practice guideline
update. J Clin Oncol. 36:3043–3054. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poulose SM, Bielinski DF and Shukitt-Hale
B: Walnut diet reduces accumulation of polyubiquitinated proteins
and inflammation in the brain of aged rats. J Nutr Biochem.
24:912–919. 2013. View Article : Google Scholar
|
|
27
|
Arab L and Ang A: A cross sectional study
of the association between walnut consumption and cognitive
function among adult US populations represented in NHANES. J Nutr
Health Aging. 19:284–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chauhan A and Chauhan V: Beneficial
effects of walnuts on cognition and brain health. Nutrients.
12:122020. View Article : Google Scholar
|
|
29
|
Rusu ME, Georgiu C, Pop A, Mocan A, Kiss
B, Vostinaru O, Fizesan I, Stefan MG, Gheldiu AM, Mates L, et al:
Antioxidant effects of walnut (Juglans regia L.) kernel and walnut
septum extract in a D-galactose-induced aging model and in
naturally aged rats. Antioxidants. 9:92020. View Article : Google Scholar
|
|
30
|
Chauhan N, Wang KC, Wegiel J and Malik MN:
Walnut extract inhibits the fibrillization of amyloid beta-protein,
and also defibrillizes its preformed fibrils. Curr Alzheimer Res.
1:183–188. 2004. View Article : Google Scholar
|
|
31
|
Muthaiyah B, Essa MM, Chauhan V and
Chauhan A: Protective effects of walnut extract against amyloid
beta peptide-induced cell death and oxidative stress in PC12 cells.
Neurochem Res. 36:2096–2103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poulose SM, Miller MG and Shukitt-Hale B:
Role of walnuts in maintaining brain health with age. J Nutr.
144(Suppl): 561S–566S. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heckler MM and Riggins RB: ERRβ splice
variants differentially regulate cell cycle progression. Cell
Cycle. 14:31–45. 2015. View Article : Google Scholar
|
|
34
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Timm M, Saaby L, Moesby L and Hansen EW:
Considerations regarding use of solvents in in vitro cell based
assays. Cytotechnology. 65:887–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ammann KR, DeCook KJ, Li M and Slepian MJ:
Migration versus proliferation as contributor to in vitro wound
healing of vascular endothelial and smooth muscle cells. Exp Cell
Res. 376:58–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
D'Angeli F, Scalia M, Cirnigliaro M,
Satriano C, Barresi V, Musso N, Trovato-Salinaro A, Barbagallo D,
Ragusa M, Di Pietro C, et al: PARP-14 promotes survival of
mammalian α but not β pancreatic cells following cytokine
treatment. Front Endocrinol (Lausanne). 10:2712019. View Article : Google Scholar
|
|
38
|
Clinical and Laboratory Standards
Institute: Performance standards for antimicrobial susceptibility
testing; Twenty-seventh informational supplement M100-S28. 28th
edition. CLSI; Wayne, PA: 2018
|
|
39
|
Li W, Li V, Ward C, Müller W and Ackermann
M: Comparative antiproliferative and antiangiogenic activity of
cacao preparations (P06-048-19). Curr Dev Nutr. View Article : Google Scholar : P06-048-19
|
|
40
|
Poroikov VV and Filimonov DA: How to
acquire new biological activities in old compounds by computer
prediction. J Comput Aided Mol Des. 16:819–824. 2002. View Article : Google Scholar
|
|
41
|
Filimonov DA, Lagunin AA, Gloriozova TA,
Rudik AV, Druzhilovskii DS, Pogodin PV and Poroikov VV: Prediction
of the biological activity spectra of organic compounds using the
pass online web resource. Chem Heterocycl Compd. 50:444–457. 2014.
View Article : Google Scholar
|
|
42
|
Goel RK, Singh D, Lagunin A and Poroikov
V: PASS-assisted exploration of new therapeutic potential of
natural products. Med Chem Res. 20:1509–1514. 2011. View Article : Google Scholar
|
|
43
|
Kim S, Thiessen PA, Bolton EE, Chen J, Fu
G, Gindulyte A, Han L, He J, He S, Shoemaker BA, et al: PubChem
substance and compound databases. Nucleic Acids Res. 44(D1):
D1202–D1213. 2016. View Article : Google Scholar :
|
|
44
|
Lagunin AA, Dubovskaja VI, Rudik AV,
Pogodin PV, Druzhilovskiy DS, Gloriozova TA, Filimonov DA, Sastry
NG and Poroikov VV: CLC-Pred: A freely available web-service for in
silico prediction of human cell line cytotoxicity for drug-like
compounds. PLoS One. 13:e01918382018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Regueiro J, Sánchez-González C,
Vallverdú-Queralt A, Simal-Gándara J, Lamuela-Raventós R and
Izquierdo-Pulido M: Comprehensive identification of walnut
polyphenols by liquid chromatography coupled to linear ion
trap-Orbitrap mass spectrometry. Food Chem. 152:340–348. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li L, Tsao R, Yang R, Liu C, Zhu H and
Young JC: Polyphenolic profiles and antioxidant activities of
heartnut (Juglans ailan-thifolia Var. cordiformis) and Persian
walnut (Juglans regia L.). J Agric Food Chem. 54:8033–8040. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iasnaia Maria de Carvalho T, Nogueira TYK,
Mauro MA, Gómez-Alonso S, Gomes E, Da-Silva R, Hermosín-Gutiérrez I
and Lago-Vanzela ES: Dehydration of jambolan [Syzygium cumini (L.)]
juice during foam mat drying: Quantitative and qualitative changes
of the phenolic compounds. Food Res Int. 102:32–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Norheim F, Gjelstad IM, Hjorth M, Vinknes
KJ, Langleite TM, Holen T, Jensen J, Dalen KT, Karlsen AS, Kielland
A, et al: Molecular nutrition research: The modern way of
performing nutritional science. Nutrients. 4:1898–1944. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Day RL, Harper AJ, Woods RM, Davies OG and
Heaney LM: Probiotics: Current landscape and future horizons.
Future Sci OA. 5:FSO3912019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nasri H, Baradaran A, Shirzad H and
Rafieian-Kopaei M: New concepts in nutraceuticals as alternative
for pharmaceuticals. Int J Prev Med. 5:1487–1499. 2014.
|
|
51
|
Vivarelli S, Salemi R, Candido S, Falzone
L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G and Libra
M: Gut microbiota and cancer: From pathogenesis to therapy. Cancers
(Basel). 11:112019. View Article : Google Scholar
|
|
52
|
Amiot-Carlin MJ: Fruit and vegeTable
consumption: What benefits, what risks? Rev Prat. 69:139–142.
2019.In French. PubMed/NCBI
|
|
53
|
Prokopiou E, Kolovos P, Georgiou C,
Kalogerou M, Potamiti L, Sokratous K, Kyriacou K and Georgiou T:
Omega-3 fatty acids supplementation protects the retina from
age-associated degeneration in aged C57BL/6J mice. BMJ Open
Ophthalmol. 4:e0003262019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ciofu O, Smith S and Lykkesfeldt J:
Antioxidant supplementation for lung disease in cystic fibrosis.
Cochrane Database Syst Rev. 10:CD0070202019.PubMed/NCBI
|
|
55
|
Banna GL, Torino F, Marletta F, Santagati
M, Salemi R, Cannarozzo E, Falzone L, Ferraù F and Libra M:
Lactobacillus rhamnosus GG: An overview to explore the rationale of
its use in cancer. Front Pharmacol. 8:6032017. View Article : Google Scholar :
|
|
56
|
Tiffon C: The impact of nutrition and
environmental epigenetics on human health and disease. Int J Mol
Sci. 19:192018. View Article : Google Scholar
|
|
57
|
Filetti V, Falzone L, Rapisarda V,
Caltabiano R, Eleonora Graziano AC, Ledda C and Loreto C:
Modulation of microRNA expression levels after naturally occurring
asbestiform fibers exposure as a diagnostic biomarker of
mesothelial neoplastic transformation. Ecotoxicol Environ Saf.
198:1106402020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Falzone L, Lupo G, La Rosa GRM, Crimi S,
Anfuso CD, Salemi R, Rapisarda E, Libra M and Candido S:
Identification of novel microRNAs and their diagnostic and
prognostic significance in oral cancer. Cancers (Basel). 11:112019.
View Article : Google Scholar
|
|
59
|
Falzone L, Romano GL, Salemi R, Bucolo C,
Tomasello B, Lupo G, Anfuso CD, Spandidos DA, Libra M and Candido
S: Prognostic significance of deregulated microRNAs in uveal
melanomas. Mol Med Rep. 19:2599–2610. 2019.PubMed/NCBI
|
|
60
|
Falzone L, Scola L, Zanghì A, Biondi A, Di
Cataldo A, Libra M and Candido S: Integrated analysis of colorectal
cancer microRNA datasets: Identification of microRNAs associated
with tumor development. Aging (Albany NY). 10:1000–1014. 2018.
View Article : Google Scholar
|
|
61
|
Hardman WE and Ion G: Suppression of
implanted MDA-MB 231 human breast cancer growth in nude mice by
dietary walnut. Nutr Cancer. 60:666–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hardman WE: Walnuts have potential for
cancer prevention and treatment in mice. J Nutr. 144(Suppl):
555S–560S. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Alasalvar C, Salvadó JS and Ros E:
Bioactives and health benefits of nuts and dried fruits. Food Chem.
314:1261922020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tibullo D, Caporarello N, Giallongo C,
Anfuso CD, Genovese C, Arlotta C, Puglisi F, Parrinello NL,
Bramanti V, Romano A, et al: Antiproliferative and antiangiogenic
effects of Punica granatum juice (PGJ) in multiple myeloma (MM).
Nutrients. 8:82016. View Article : Google Scholar
|
|
65
|
Acquaviva R, Menichini F, Ragusa S,
Genovese C, Amodeo A, Tundis R, Loizzo MR and Iauk L: Antimicrobial
and antioxidant properties of Betula aetnensis Rafin. (Betulaceae)
leaves extract. Nat Prod Res. 27:475–479. 2013. View Article : Google Scholar
|
|
66
|
Acquaviva R, Genovese C, Amodeo A,
Tomasello B, Malfa G, Sorrenti V, Tempera G, Addamo AP, Ragusa S,
Rosa T, et al: Biological activities of Teucrium flavum L.,
Teucrium fruticans L., and Teucrium siculum Rafin crude extracts.
Plant Biosyst. 152:720–727. 2018. View Article : Google Scholar
|
|
67
|
Genovese C, D'Angeli F, Attanasio F,
Caserta G, Scarpaci KS and Nicolosi D: Phytochemical composition
and biological activities of Orobanche crenata Forssk.: A review.
Nat Prod Res. View Article : Google Scholar
|
|
68
|
Malfa GA, Tomasello B, Acquaviva R,
Genovese C, La Mantia A, Cammarata FP, Ragusa M, Renis M and Di
Giacomo C: Betula etnensis raf. (Betulaceae) extract induced HO-1
expression and ferroptosis cell death in human colon cancer cells.
Int J Mol Sci. 20:202019. View Article : Google Scholar
|
|
69
|
Salimi M, Majd A, Sepahdar Z, Azadmanesh
K, Irian S, Ardestaniyan MH, Hedayati MH and Rastkari N:
Cytotoxicity effects of various Juglans regia (walnut) leaf
extracts in human cancer cell lines. Pharm Biol. 50:1416–1422.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rusu ME, Fizesan I, Pop A, Mocan A,
Gheldiu AM, Babota M, Vodnar DC, Jurj A, Berindan-Neagoe I, Vlase
L, et al: Walnut (Juglans regia L.) Septum: Assessment of bioactive
molecules and in vitro biological effects. Molecules. 25:252020.
View Article : Google Scholar
|
|
71
|
Castro-López C, Ventura-Sobrevilla JM,
González-Hernández MD, Rojas R, Ascacio-Valdés JA, Aguilar CN and
Martínez-Ávila GCG: Impact of extraction techniques on antioxidant
capacities and phytochemical composition of polyphenol-rich
extracts. Food Chem. 237:1139–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bi D, Zhao Y, Jiang R, Wang Y, Tian Y,
Chen X, Bai S and She G: Phytochemistry, bioactivity and potential
impact on health of juglans: The Original plant of walnut. Nat Prod
Commun. 11:869–880. 2016.PubMed/NCBI
|
|
73
|
Li L, Song L, Sun X, Yan S, Huang W and
Liu P: Characterisation of phenolics in fruit septum of Juglans
regia Linn. by ultra performance liquid chromatography coupled with
Orbitrap mass spectrometer. Food Chem. 286:669–677. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Alañón ME, Oliver-Simancas R,
Gómez-Caravaca AM, Arráez-Román D and Segura-Carretero A: Evolution
of bioactive compounds of three mango cultivars (Mangifera indica
L.) at different maturation stages analyzed by HPLC-DAD-q-TOF-MS.
Food Res Int. 125:1085262019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wafa BA, Makni M, Ammar S, Khannous L,
Hassana AB, Bouaziz M, Es-Safi NE and Gdoura R: Antimicrobial
effect of the Tunisian Nana variety Punica granatum L. extracts
against Salmonella enterica (serovars Kentucky and Enteritidis)
isolated from chicken meat and phenolic composition of its peel
extract. Int J Food Microbiol. 241:123–131. 2017. View Article : Google Scholar
|
|
76
|
Spatafora C and Tringali C:
Natural-derived polyphenols as potential anticancer agents.
Anticancer Agents Med Chem. 12:902–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Carvalho M, Ferreira PJ, Mendes VS, Silva
R, Pereira JA, Jerónimo C and Silva BM: Human cancer cell
antiproliferative and antioxidant activities of Juglans regia L.
Food Chem Toxicol. 48:441–447. 2010. View Article : Google Scholar
|
|
78
|
Napoli S, Scuderi C, Gattuso G, Bella VD,
Candido S, Basile MS, Libra M and Falzone L: Functional roles of
matrix metalloproteinases and their inhibitors in melanoma. Cells.
9:92020. View Article : Google Scholar
|
|
79
|
Park JS: Walnut husk ethanol extract
possess antioxidant activity and inhibitory effect of matrix
metalloproteinase-1 expression induced by tumor necrosis factor
alpha in human keratinocyte. Asian J Beauty Cosmetol. 11:715–719.
2013.
|
|
80
|
Loftus JP, Belknap JK and Black SJ: Matrix
metalloproteinase-9 in laminae of black walnut extract treated
horses correlates with neutrophil abundance. Vet Immunol
Immunopathol. 113:267–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ma S, Huang D, Zhai M, Yang L, Peng S,
Chen C, Feng X, Weng Q, Zhang B and Xu M: Isolation of a novel
bio-peptide from walnut residual protein inducing apoptosis and
autophagy on cancer cells. BMC Complement Altern Med. 15:4132015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jänicke RU, Ng P, Sprengart ML and Porter
AG: Caspase-3 is required for alpha-fodrin cleavage but dispensable
for cleavage of other death substrates in apoptosis. J Biol Chem.
273:15540–15545. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rosengren RJ: Catechins and the treatment
of breast cancer: Possible utility and mechanistic targets. IDrugs.
6:1073–1078. 2003.PubMed/NCBI
|
|
85
|
Das A, Banik NL and Ray SK: Flavonoids
activated caspases for apoptosis in human glioblastoma T98G and
U87MG cells but not in human normal astrocytes. Cancer.
116:164–176. 2010.
|
|
86
|
El Moussaoui A, Jawhari FZ, Almehdi AM,
Elmsellem H, Fikri Benbrahim K, Bousta D and Bari A: Antibacterial,
anti-fungal and antioxidant activity of total polyphenols of
Withania frutescens.L. Bioorg Chem. 93:1033372019. View Article : Google Scholar
|
|
87
|
Rhimi W, Ben Salem I, Immediato D, Saidi
M, Boulila A and Cafarchia C: Chemical composition, antibacterial
and antifungal activities of crude dittrichia viscosa (L.) Greuter
leaf extracts. Molecules. 22:222017. View Article : Google Scholar
|
|
88
|
Genovese C, Davinelli S, Mangano K,
Tempera G, Nicolosi D, Corsello S, Vergalito F, Tartaglia E,
Scapagnini G and Di Marco R: Effects of a new combination of plant
extracts plus d-mannose for the management of uncomplicated
recurrent urinary tract infections. J Chemother. 30:107–114. 2018.
View Article : Google Scholar
|
|
89
|
Nicolosi D, Tempera G, Genovese C and
Furneri PM: Anti-Adhesion activity of A2-type proanthocyanidins (a
Cranberry major component) on uropathogenic E. coli and P.
mirabilis strains. Antibiotics (Basel). 3:143–154. 2014. View Article : Google Scholar
|
|
90
|
Genovese C, Pulvirenti L, Cardullo N,
Muccilli V, Tempera G, Nicolosi D and Tringali C: Bioinspired
benzoxanthene lignans as a new class of antimycotic agents:
Synthesis and Candida spp. growth inhibition. Nat Prod Res.
34:1653–1662. 2018. View Article : Google Scholar
|
|
91
|
Amer AM: Antimicrobial effects of egyptian
local chicory, Cichorium endivia subsp. pumilum. Int J Microbiol.
View Article : Google Scholar : 2018.
|
|
92
|
Auer GK and Weibel DB: Bacterial cell
mechanics. Biochemistry. 56:3710–3724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Exner M, Bhattacharya S, Christiansen B,
Gebel J, Goroncy-Bermes P, Hartemann P, Heeg P, Ilschner C, Krame
A, Larson E, et al: Antibiotic resistance: What is so special about
multidrug-resistant Gram-negative bacteria? Gms Hyg Infect Contr.
View Article : Google Scholar
|
|
94
|
Salmeri M, Motta C, Mastrojeni S, Amodeo
A, Anfuso CD, Giurdanella G, Morello A, Alberghina M, Toscano MA
and Lupo G: Involvement of PKCα-MAPK/ERK-phospholipase A(2) pathway
in the Escherichia coli invasion of brain micro-vascular
endothelial cells. Neurosci Lett. 511:33–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Caporarello N, Olivieri M, Cristaldi M,
Scalia M, Toscano MA, Genovese C, Addamo A, Salmeri M, Lupo G and
Anfuso CD: Blood-brain barrier in a Haemophilus influenzae type a
in vitro infection: Role of adenosine receptors A2A and A2B. Mol
Neurobiol. 55:5321–5336. 2018. View Article : Google Scholar
|
|
96
|
Saraiva AM, Castro RHA, Cordeiro RP,
Sobrinho TJSDP, Castro VTNA, Amorim ELC, Xavier HS and Pisciottano
MNC: In vitro evaluation of antioxidant, antimicrobial and toxicity
properties of extracts of Schinopsis brasiliensis Engl.
(Anacardiaceae). Afr J Pharm Pharmacol. 5:1724–1731. 2011.
|
|
97
|
Peng J, Zheng TT, Liang Y, Duan LF, Zhang
YD, Wang LJ, He GM and Xiao HT: p-Coumaric acid protects human lens
epithelial cells against oxidative stress-induced apoptosis by MAPK
signaling. Oxid Med Cell Longev. 2018:85490522018. View Article : Google Scholar :
|
|
98
|
Sudan S and Rupasinghe HV:
Antiproliferative activity of long chain acylated esters of
quercetin-3-O-glucoside in hepatocellular carcinoma HepG2 cells.
Exp Biol Med (Maywood). 240:1452–1464. 2015. View Article : Google Scholar
|
|
99
|
Zhang Y, Guo Y, Wang M, Dong H, Zhang J
and Zhang L: Quercetrin from Toona sinensis leaves induces cell
cycle arrest and apoptosis via enhancement of oxidative stress in
human colorectal cancer SW620 cells. Oncol Rep. 38:3319–3326.
2017.PubMed/NCBI
|
|
100
|
Cincin ZB, Unlu M, Kiran B, Bireller ES,
Baran Y and Cakmakoglu B: Molecular mechanisms of
quercitrin-induced apoptosis in non-small cell lung cancer. Arch
Med Res. 45:445–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tuaeva NO, Falzone L, Porozov YB, Nosyrev
AE, Trukhan VM, Kovatsi L, Spandidos DA, Drakoulis N, Kalogeraki A,
Mamoulakis C, et al: Translational application of circulating DNA
in oncology: Review of the last decades achievements. Cells.
8:82019. View Article : Google Scholar
|
|
102
|
Salemi R, Falzone L, Madonna G, Polesel J,
Cinà D, Mallardo D, Ascierto PA, Libra M and Candido S: MMP-9 as a
Candidate marker of response to BRAF inhibitors in melanoma
patients with BRAFV600E mutation detected in circulating-free DNA.
Front Pharmacol. 9:8562018. View Article : Google Scholar :
|