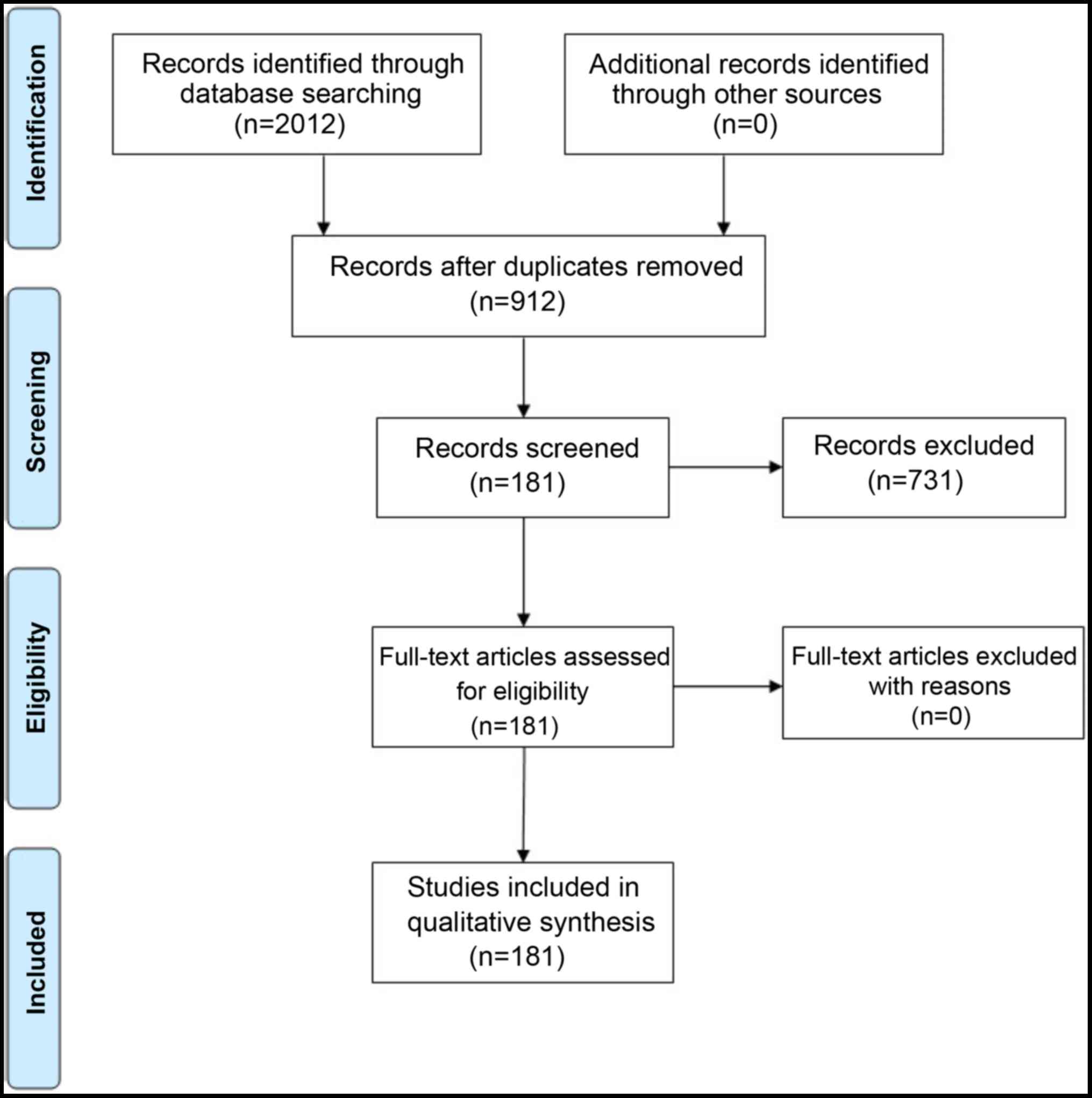
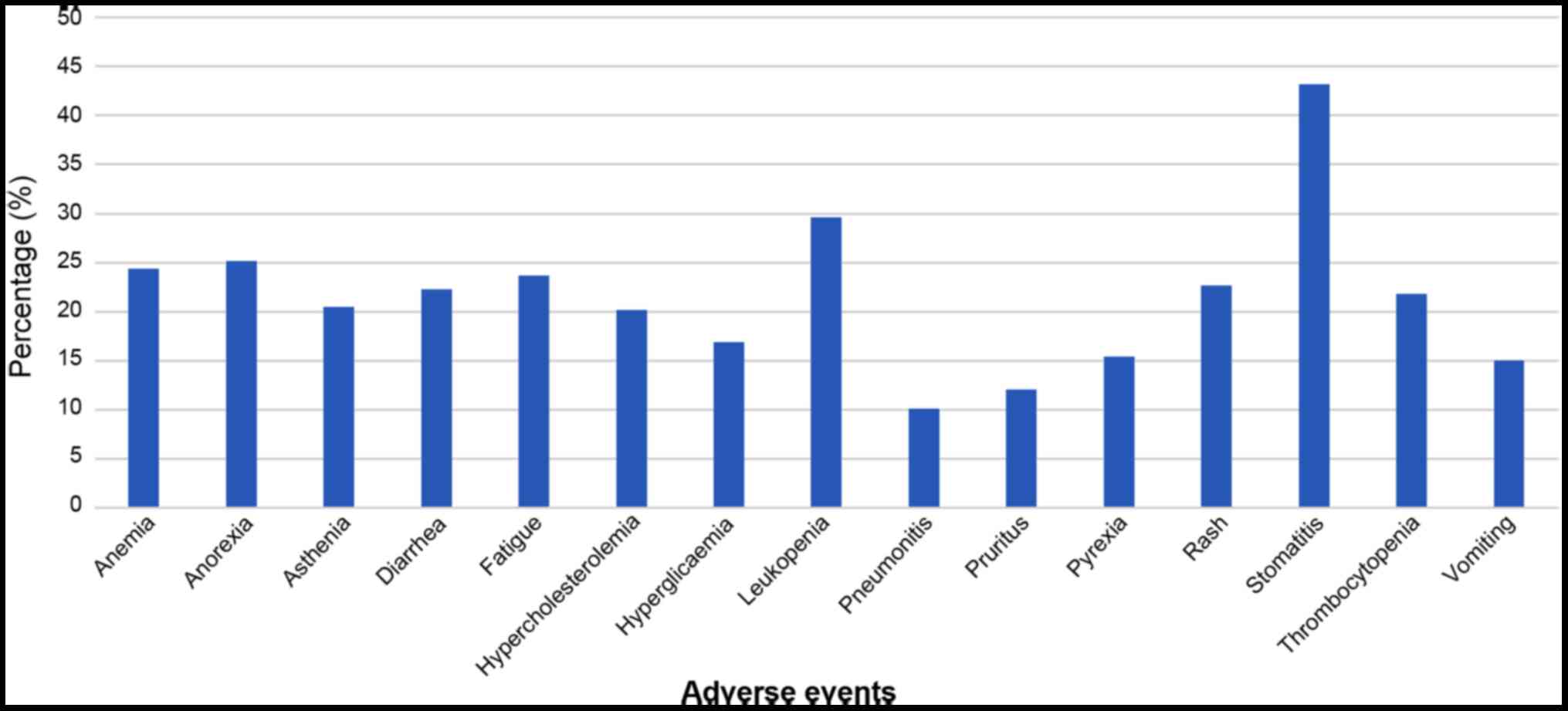
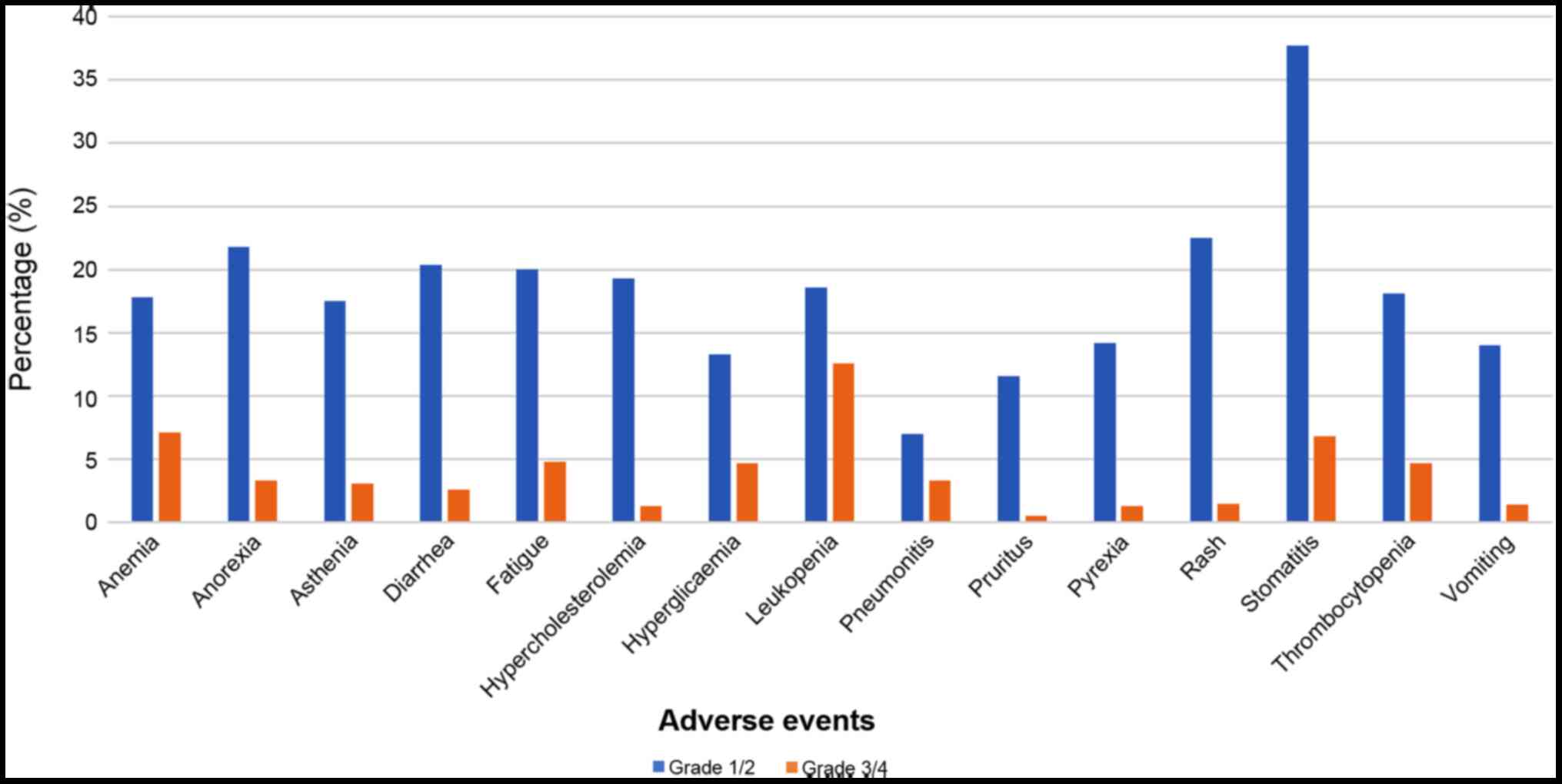
|
1
|
Keefe DM and Bateman EH: Tumor control
versus adverse events with targeted anticancer therapies. Nat Rev
Clin Oncol. 9:98–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. vii2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng LH and Zheng XF: Toward rapamycin
analog (rapalog)-based precision cancer therapy. Acta Pharmacol
Sin. 36:1163–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie J, Hao Y, Zhou ZY, Qi CZ, De G and
Glück S: Economic evaluations of everolimus versus other hormonal
therapies in the treatment of HR+/HER2- advanced breast cancer from
a US payer perspective. Clin Breast Cancer. 15:e263–e276. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madeddu C, Gramignano G, Astara G,
Demontis R, Sanna E, Atzeni V and Macciò A: Pathogenesis and
treatment options of cancer related anemia: perspective for a
targeted mechanism-based approach. Front Physiol. 9:12942018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar
|
|
7
|
Chocteau-Bouju D, Chakiba C, Mignot L,
Madranges N, Pierga JY, Beuzeboc P, Quenel-Tueux N, Dieras V,
Bonnefoi H, Debled M, et al: Efficacy and tolerance of everolimus
in 123 consecutive advanced ER positive, HER2 negative breast
cancer patients. A two center retrospective study. Breast.
24:718–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez Fructuoso A, Ruiz San Millán JC,
Calvo N, Rodrigo E, Moreno MA, Cotorruelo J, Conesa J,
Gómez-Alamillo C, Arias M and Barrientos A: Evaluation of the
efficacy and safety of the conversion from a calcineurin inhibitor
to an everolimus-based therapy in maintenance renal transplant
patients. Transplant Proc. 39:2148–2150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Belle SJ and Cocquyt V: Impact of
haemoglobin levels on the outcome of cancers treated with
chemotherapy. Crit Rev Oncol Hematol. 47:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilreath JA, Stenehjem DD and Rodgers GM:
Diagnosis and treatment of cancer-related anemia. Am J Hematol.
89:203–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bohlius J, Weingart O, Trelle S and Engert
A: Cancer-related anemia and recombinant human erythropoietin - an
updated overview. Nat Clin Pract Oncol. 3:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nozawa M, Nonomura N, Ueda T, Nishimura K,
Kanayama HO, Miki T, Nakatani T, Tomita Y, Azuma H, Yoshioka T, et
al: Adverse event profile and dose modification of everolimus for
advanced renal cell carcinoma in real-world Japanese clinical
practice. Jpn J Clin Oncol. 43:1132–1138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gross ME, Dorff TB, Quinn DI, Diaz PM,
Castellanos OO and Agus DB: Safety and efficacy of docetaxel,
bevacizumab, and everolimus for castration-resistant prostate
cancer (CRPC). Clin Genitourin Cancer. Jul 14–2017.Epub ahead of
print. PubMed/NCBI
|
|
14
|
Amitani M, Asakawa A, Amitani H and Inui
A: Control of food intake and muscle wasting in cachexia. Int J
Biochem Cell Biol. 45:2179–2185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisen T, Sternberg CN, Robert C, Mulders
P, Pyle L, Zbinden S, Izzedine H and Escudier B: Targeted therapies
for renal cell carcinoma: Review of adverse event management
strategies. J Natl Cancer Inst. 104:93–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
Accessed November 27, 2017.
|
|
17
|
Niu Q, Wang W, Li Y, Qin S, Wang Y, Wan G,
Guan J and Zhu W: Cord blood-derived cytokine-induced killer cells
biotherapy combined with second-line chemotherapy in the treatment
of advanced solid malignancies. Int Immunopharmacol. 11:449–456.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moscetti L, Vici P, Gamucci T, Natoli C,
Cortesi E, Marchetti P, Santini D, Giuliani R, Sperduti I, Mauri M,
et al: Safety analysis, association with response and previous
treatments of everolimus and exemestane in 181 metastatic breast
cancer patients: A multicenter Italian experience. Breast.
29:96–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González Barón M, Feyjóo M, Carulla
Torrent J, Camps C, Escobar Y and Belda-Iniesta C: Study of the
prevalence of tumour-related asthenia in Spanish cancer patients.
Clin Transl Oncol. 10:351–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moioli M, Barra F, Maramai M, Valenzano
Menada M, Vellone VG, Costantini S and Ferrero S: Mucinous ovarian
cancer: Current therapeutic targets, preclinical progress, and
experimental drugs. Expert Opin Investig Drugs. 28:1025–1029. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bajetta E, Catena L, Fazio N, Pusceddu S,
Biondani P, Blanco G, Ricci S, Aieta M, Pucci F, Valente M, et al:
Everolimus in combination with octreotide long-acting repeatable in
a first-line setting for patients with neuroendocrine tumors: An
ITMO group study. Cancer. 120:2457–2463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Besse B, Leighl N, Bennouna J,
Papadimitrakopoulou VA, Blais N, Traynor AM, Soria JC, Gogov S,
Miller N, Jehl V, et al: Phase II study of everolimus-erlotinib in
previously treated patients with advanced non-small-cell lung
cancer. Ann Oncol. 25:409–415. 2014. View Article : Google Scholar
|
|
23
|
Benson AB III, Ajani JA, Catalano RB,
Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP,
O'Dorisio TM, Vokes EE, et al: Recommended guidelines for the
treatment of cancer treatment-induced diarrhea. J Clin Oncol.
22:2918–2926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
et al: Common toxicity criteria: version 2.0. an improved reference
for grading the acute effects of cancer treatment: impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stein J and Mann J: Specialty pharmacy
services for patients receiving oral medications for solid tumors.
Am J Health Syst Pharm. 73:775–796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franz DN, Belousova E, Sparagana S, Bebin
EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, et
al: Everolimus for subependymal giant cell astrocytoma in patients
with tuberous sclerosis complex: 2-year open-label extension of the
randomised EXIST-1 study. Lancet Oncol. 15:1513–1520. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Irvine D, Vincent L, Graydon JE, Bubela N
and Thompson L: The prevalence and correlates of fatigue in
patients receiving treatment with chemotherapy and radiotherapy. A
comparison with the fatigue experienced by healthy individuals.
Cancer Nurs. 17:367–378. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohandas H, Jaganathan SK, Mani MP, Ayyar
M and Rohini Thevi GV: Cancer-related fatigue treatment: An
overview. J Cancer Res Ther. 13:916–929. 2017.PubMed/NCBI
|
|
29
|
Motzer RJ, Alyasova A, Ye D, Karpenko A,
Li H, Alekseev B, Xie L, Kurteva G, Kowalyszyn R, Karyakin O, et
al: Phase II trial of second-line everolimus in patients with
metastatic renal cell carcinoma (RECORD-4). Ann Oncol. 27:441–448.
2016. View Article : Google Scholar :
|
|
30
|
Sarkaria JN, Galanis E, Wu W, Peller PJ,
Giannini C, Brown PD, Uhm JH, McGraw S, Jaeckle KA and Buckner JC:
North Central Cancer Treatment Group Phase I trial N057K of
everolimus (RAD001) and temozolomide in combination with radiation
therapy in patients with newly diagnosed glioblastoma multiforme.
Int J Radiat Oncol Biol Phys. 81:468–475. 2011. View Article : Google Scholar
|
|
31
|
Tian W, Yao Y, Fan G, Zhou Y, Wu M, Xu D
and Deng Y: Changes in lipid profiles during and after
(neo)adjuvant chemotherapy in women with early-stage breast cancer:
A retrospective study. PLoS One. 14:e02218662019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Capdevila J, Sevilla I, Alonso V, Antón
Aparicio L, Jiménez Fonseca P, Grande E, Reina JJ, Manzano JL,
Alonso Lájara JD, Barriuso J, et al: Evaluation of the efficacy and
safety of lanreotide in combination with targeted therapies in
patients with neuroendocrine tumours in clinical practice: A
retrospective cross-sectional analysis. BMC Cancer. 15:4952015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun JM, Kim JR, Do IG, Lee SY, Lee J, Choi
YL, Ahn JS, Ahn MJ and Park K: A phase-1b study of everolimus plus
paclitaxel in patients with small-cell lung cancer. Br J Cancer.
109:1482–1487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tobinai K, Ogura M, Maruyama D, Uchida T,
Uike N, Choi I, Ishizawa K, Itoh K, Ando K, Taniwaki M, et al:
Phase I study of the oral mammalian target of rapamycin inhibitor
everolimus (RAD001) in Japanese patients with relapsed or
refractory non-Hodgkin lymphoma. Int J Hematol. 92:563–570. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hwangbo Y and Lee EK: Acute hyperglycemia
associated with anti-cancer medication. Endocrinol Metab (Seoul).
32:23–29. 2017. View Article : Google Scholar
|
|
36
|
Nathan DM, Buse JB, Davidson MB,
Ferrannini E, Holman RR, Sherwin R and Zinman B; American Diabetes
Association; European Association for Study of Diabetes: Medical
management of hyperglycemia in type 2 diabetes: a consensus
algorithm for the initiation and adjustment of therapy: a consensus
statement of the American Diabetes Association and the European
Association for the Study of Diabetes. Diabetes Care. 32:193–203.
2009. View Article : Google Scholar :
|
|
37
|
Nathan DM, Buse JB, Davidson MB,
Ferrannini E, Holman RR, Sherwin R and Zinman B; American Diabetes
Association; European Association for the Study of Diabetes:
Medical management of hyperglycaemia in type 2 diabetes mellitus: a
consensus algorithm for the initiation and adjustment of therapy: a
consensus statement from the American Diabetes Association and the
European Association for the Study of Diabetes. Diabetologia.
52:17–30. 2009. View Article : Google Scholar
|
|
38
|
Mita M, Mita A and Rowinsky EK: mTOR
inhibition for cancer therapy: past, present and future.
Springer-Verlag; Paris: 2015
|
|
39
|
Molina AM, Feldman DR, Voss MH, Ginsberg
MS, Baum MS, Brocks DR, Fischer PM, Trinos MJ, Patil S and Motzer
RJ: Phase 1 trial of everolimus plus sunitinib in patients with
metastatic renal cell carcinoma. Cancer. 118:1868–1876. 2012.
View Article : Google Scholar
|
|
40
|
Hassan B, Yusoff Z and Othman: A close
look at neutropenia among cancer patients - risk factor and
management. Updates on Cancer Treatment. IntechOpen.
2015.https://www.intechopen.com/books/updates-on-cancer-treatment/a-close-look-at-neutropenia-among-cancer-patients-risk-factor-and-management.
Accessed October 28, 2015. View
Article : Google Scholar
|
|
41
|
Ju Y, Hu Y, Sun S, Wang J and Jiao S:
Toxicity and adverse effects of everolimus in the treatment of
advanced nonsmall cell lung cancer pretreated with chemotherapy -
Chinese experiences. Indian J Cancer. 52(Suppl 1): e32–e36. 2015.
View Article : Google Scholar
|
|
42
|
Kanesvaran R, Watt K, Turnbull JD,
Armstrong AJ, Wolkowiez MC and George DJ: A single-arm phase 1b
study of everolimus and sunitinib in patients with advanced renal
cell carcinoma. Clin Genitourin Cancer. 13:319–327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Amato RJ, Jac J, Giessinger S, Saxena S
and Willis JP: A phase 2 study with a daily regimen of the oral
mTOR inhibitor RAD001 (everolimus) in patients with metastatic
clear cell renal cell cancer. Cancer. 115:2438–2446. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuter DJ: Managing thrombocytopenia
associated with cancer chemotherapy. Oncology (Williston Park).
29:282–284. 2015.
|
|
45
|
Nishino M, Brais LK, Brooks NV, Hatabu H,
Kulke MH and Ramaiya NH: Drug-related pneumonitis during mammalian
target of rapamycin inhibitor therapy in patients with
neuroendocrine tumors: A radiographic pattern-based approach. Eur J
Cancer. 53:163–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torrisi JM, Schwartz LH, Gollub MJ,
Ginsberg MS, Bosl GJ and Hricak H: CT findings of
chemotherapy-induced toxicity: What radiologists need to know about
the clinical and radiologic manifestations of chemotherapy
toxicity. Radiology. 258:41–56. 2011. View Article : Google Scholar
|
|
47
|
Limper AH: Chemotherapy-induced lung
disease. Clin Chest Med. 25:53–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vlahovic G, Meadows KL, Uronis HE, Morse
MA, Blobe GC, Riedel RF, Zafar SY, Alvarez-Secord A, Gockerman J,
Starodub AN, et al: A phase I study of bevacizumab, everolimus and
panitumumab in advanced solid tumors. Cancer Chemother Pharmacol.
70:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weisshaar E, Fleischer AB Jr, Bernhard JD,
et al: Pruritus and dysesthesia. Dermatology. Bolognia JL, Jorizzo
JL and Schaffer JV: Elsevier Saunders; pp. 111–125. 2012
|
|
50
|
Jóźwiak S, Kotulska K, Berkowitz N,
Brechenmacher T and Franz DN: Safety of everolimus in patients
younger than 3 years of age: results from EXIST-1, a randomized,
controlled clinical trial. J Pediatr. 172:151–155.e1. 2016.
View Article : Google Scholar
|
|
51
|
Castagnola E, Fontana V, Caviglia I,
Caruso S, Faraci M, Fioredda F, Garrè ML, Moroni C, Conte M,
Losurdo G, et al: A prospective study on the epidemiology of
febrile episodes during chemotherapy-induced neutropenia in
children with cancer or after hemopoietic stem cell
transplantation. Clin Infect Dis. 45:1296–1304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tan P, Tiong IS, Fleming S, Pomilio G,
Cummings N, Droogleever M, McManus J, Schwarer A, Catalano J, Patil
S, et al: The mTOR inhibitor everolimus in combination with
azacitidine in patients with relapsed/refractory acute myeloid
leukemia: A phase Ib/II study. Oncotarget. 8:52269–52280. 2016.
View Article : Google Scholar
|
|
53
|
Amato RJ, Flaherty AL and Stepankiw M:
Phase I trial of everolimus plus sorafenib for patients with
advanced renal cell cancer. Clin Genitourin Cancer. 10:26–31. 2012.
View Article : Google Scholar
|
|
54
|
Aapro M, Andre F, Blackwell K, Calvo E,
Jahanzeb M, Papazisis K, Porta C, Pritchard K and Ravaud A: Adverse
event management in patients with advanced cancer receiving oral
everolimus: Focus on breast cancer. Ann Oncol. 25:763–773. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Quek R, Wang Q, Morgan JA, Shapiro GI,
Butrynski JE, Ramaiya N, Huftalen T, Jederlinic N, Manola J, Wagner
AJ, et al: Combination mTOR and IGF-1R inhibition: Phase I trial of
everolimus and figitumumab in patients with advanced sarcomas and
other solid tumors. Clin Cancer Res. 17:871–879. 2011. View Article : Google Scholar
|
|
56
|
Staves KL and Ramchandran KJ: Prevention
and treatment options for mTOR inhibitor-associated stomatitis.
JCSO. 15:74–81. 2017. View Article : Google Scholar
|
|
57
|
Lacouture M and Sibaud V: Toxic Side
Effects of Targeted Therapies and Immunotherapies Affecting the
Skin, Oral Mucosa, Hair, and Nails. Am J Clin Dermatol. 19(Suppl
1): 31–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Watters AL, Epstein JB and Agulnik M: Oral
complications of targeted cancer therapies: A narrative literature
review. Oral Oncol. 47:441–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Raber-Durlacher JE, Elad S and Barasch A:
Oral mucositis. Oral Oncol. 46:452–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Priestman T: Cancer chemotherapy in
clinical practice. Springer; New York, NY: 2012, View Article : Google Scholar
|
|
61
|
Razmara F and Khayamzadeh M: An
Investigation into the prevalence and treatment of oral mucositis
after cancer treatment. Int J Cancer Manag. 12:e884052019.
View Article : Google Scholar
|
|
62
|
Naidu MU, Ramana GV, Rani PU, Mohan IK,
Suman A and Roy P: Chemotherapy-induced and/or radiation
therapy-induced oral mucositis - complicating the treatment of
cancer. Neoplasia. 6:423–431. 2004. View Article : Google Scholar : PubMed/NCBI
|