Introduction
Several decades have passed since the first
discovery and scientific description of EVs in 1983 as 'blebbling
of membranes'. Research in the following years showed that they
participate in intercellular communication and molecule exchange.
Cancer cells are also considered to make extensive use of this
novel form of vesicular communication. Therefore, EVs have become
the focus of interest in recent years as potential biomarkers for
cancer diagnosis, cancer progression, disease monitoring and
response to chemotherapeutic agents (1,2). EVs
are produced in large numbers by cancer cells, named tumor-derived
EVs, implicating a possible explanation for tumor spread and
induction of the immunosuppressive tumor microenvironment (3). Furthermore, several studies have
demonstrated the immunosuppressive nature of tumor EVs including
EVs derived from squamous cell carcinoma (4).
Head and neck squamous cell carcinoma (HNSCC) is the
6th most frequent cancer worldwide. Globally, 666,037 cases were
reported in 2018 (5). Although
great progress has been made in diagnosis and treatment, the 5-year
overall survival rate remains poor at 60% and even worse in the
more advanced stages. Half of the patients with an advanced HNSCC
suffer a recurrence within two years (6). The current standard diagnostic
approach (endoscopy with at times invasive biopsies and imaging)
requires vast medical experience, usually only available at medical
centers, and thus often leads to delayed diagnosis. Therefore,
there is an urgent need for additional parameters in diagnostics
and follow-up screening. As a consequence, nanoscale vesicles
containing certain HNSCC proteins were investigated as potential
circulating biomarkers (7-9). As previously described, EVs can be
isolated in a large number in patients suffering from head and neck
cancer (10,11).
Standard methods for EV isolation, such as
ultra-centrifugation (UC), size exclusion chromatography (SEC), or
affinity-based methods remain relatively time-consuming,
susceptible for disturbance and difficult to implement into
clinical setting (1,7,12,13)
Consequently, there is a need for a more efficient, reliable EV
isolation method that can be easily applied in clinical routine and
yield purified EVs with low contamination. Progress to improve
current isolation techniques has already been made by implementing
columns and refinements (7-9,14).
In the present study, the potential clinical
application of galectin-based glycan recognition particles
(EXÖBead®) were investigated to isolate EVs from the
blood of cancer patients. Previously, this technique was studied in
patients with vascular problems and it was revealed that the
functions of EVs were altered when treated with antihypertensive
drugs (15). Our aim was to
investigate a new and simplified isolation technique that
eliminates the need for laborious ultracentrifugation and that can
detect common EV markers. It was also investigated whether EVs
eluted from EXÖBead® still displayed the familiar EV
morphology. The clinical focus was on the isolation of EVs from the
plasma of patients with HNSCC, their subtyping using various
biomarkers and investigating their ability to immunosuppress. In
the medium and long term, in addition to an improved understanding
of the role of EVs in tumors, our investigations are intended to
allow earlier detection of initial diagnoses and recurrences.
Materials and methods
Patient sample collection
Patients (n=18) from the department of
otorhinolaryngology of the University hospital of Basel
(Switzerland) with HNSCC in early and advanced stages were included
as well as 3 healthy controls (Table
I). The categorization is based on the current TNM 8 (16). The classification is used to divide
malignant tumors into stages. The three main categories of the TNM
system correspond to the three letters: T=tumor, extent and
behavior of the primary tumor; N=nodus-absence or presence of
regional lymph node metastases; M=metastases, absence or presence
of distant metastases. Depending on this, a distinction is made
between low grade and high grade (WHO I + II vs. III + IV). Ethical
approval (approval no. 2020-02173) was issued by ethical commission
of the northwest and central Switzerland. All patients provided
written informed consent for research and consented to anonymous
processing of the blood samples collected for scientific purposes.
Blood collection was performed before the start of tumor therapy. A
standardized peripheral venous blood collection of 7.5 ml blood was
performed (EDTA; S-Monovette; Sarstedt). To remove cells and cell
debris, whole blood was further centrifuged at 800 g, 10 min at
room temperature. Platelet-rich plasma (PRP) was collected into new
tubes. Platelet-poor plasma (PPP) was generated from PRP by
additional 10,000 g centrifugation at 4°C for 30 min, aliquoted and
stored at −80°C (7,12).
 | Table IAge and sex distribution of the
samples. The samples were collected between June 2019 and March
2021. |
Table I
Age and sex distribution of the
samples. The samples were collected between June 2019 and March
2021.
|
Characteristics | Total (n=18) |
|---|
| Sex | |
| Male | 13 (72.2%) |
| Female | 5 (27,8%) |
| Age, years | |
| Mean (range) | 68.9 (52-90) |
| ≥65 | 9 (50%) |
| >65 | 9 (50%) |
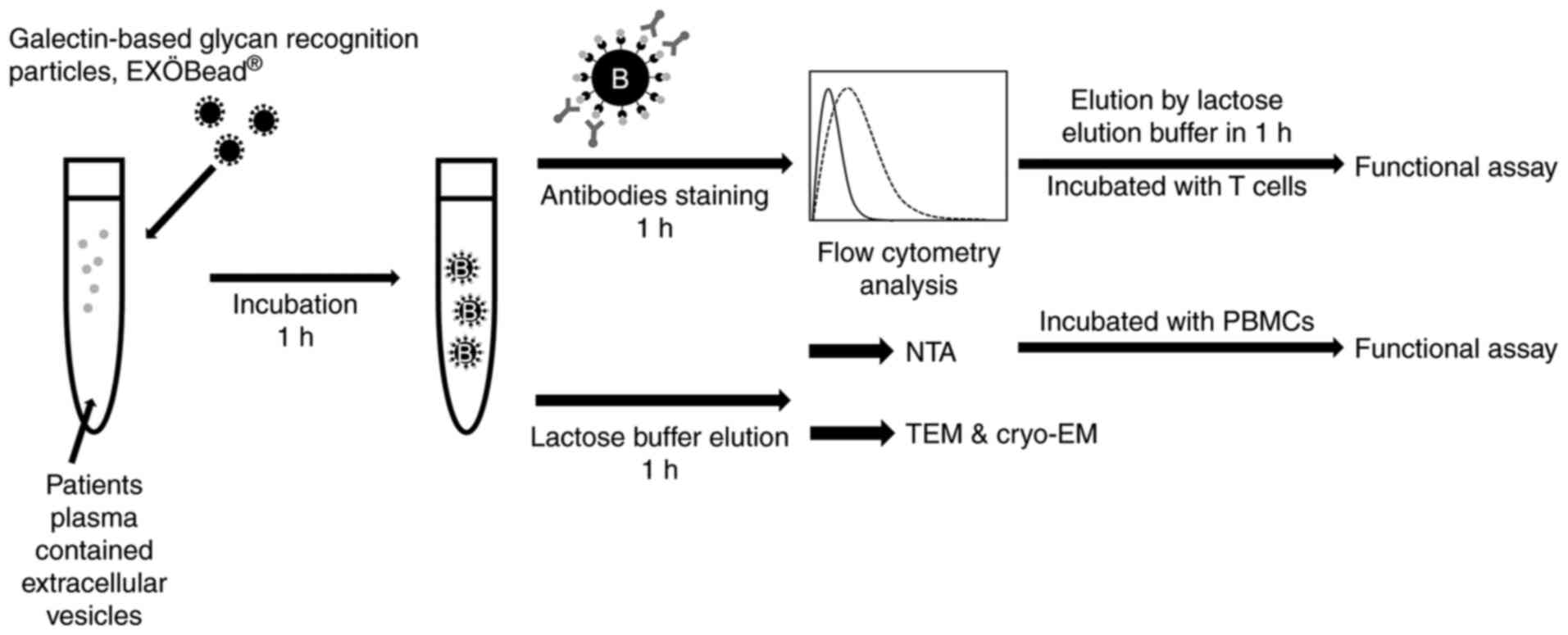
Galectin-based glycan recognition
particles EVs isolation, EXÖBead®
The EXÖBead® used are magnetic beads
coated with galectins for the isolation of EVs. The basis of this
was the detection of N-linked glycoproteins on EVs, in particular
galectin-3-binding protein (LGALS3BP), which was found on EVs from
ovarian cancer cells (17-19).
PPP (1 ml) was diluted directly in 0.9 ml of 0.5%
EV-free BSA (SERVA Electrophoresis GmbH) in PBS (100,000 g, 4°C for
overnight centrifugation) and incubated with 100 µl of
EXÖBead® (1 µm, 6×108 particles/ml,
Biovesicle Inc.) for 1 h at 25°C. EV-EXÖBead® complexes
were washed twice with 1 ml 0.5% EV-free BSA in PBS while magnetic
EXÖBead® were kept in the tube by using a magnet
(Biovesicle, Inc.). A total of 1 ml of 10% EV-free BSA in PBS was
also incubated with EXÖBead® as a negative control
(EF-EXÖBead® complex). Unbound plasma was also collected
as the non-EVs fraction. Non-EVs fractions were further used in
intracellular EV marker and non-EVs marker staining.
EVs-EXÖBead® complexes and EF-EXÖBead®
complex were further analyzed regarding EV surface marker,
intracellular EV marker and non-EV marker staining. To dissolve EVs
from the beads, EVs-EXÖBead® complexes were incubated
with 200 µl of 0.3 M lactose in PBS with
EV-EXÖBead® complexes at room temperature for 1 h.
EF-EXÖBead® complex was also incubated with 0.3 M
lactose in PBS as an elution buffer negative control. Particles
from EF-EXÖBead® complex were too low to detect (data
not shown). Eluted EVs were further analyzed by nanoparticle
Tracking Analysis (NTA), transmission electron microscopy (TEM),
cryogenic TEM (cryo-TEM) and peripheral blood mononuclear cells
(PBMCs) functional assay (Fig.
1).
EVs surface marker staining
Antibody master mix (200 µl) was incubated
each with plasma EV-EXÖBead® complexes as well as with
EV-free (EF)-EXÖBead® complexes (1 ml of 10% EV-free BSA
in PBS). Incubation lasted for 1 h at 25°C. After antibody
incubation, the EV-EXÖBead® and EF-EXÖBead®
complexes were washed twice with 1 ml of 0.5% EV-free BSA in PBS,
while the magnetic EXÖBead® were held in the tube using
a magnet (Biovesicle, Inc.). Plasma EV-EXÖBead® and
EF-EXÖBead® complexes stained with antibodies were
subsequently prepared on the BD LSR Fortessa™ (BD Biosciences) and
the data were analyzed using FlowJo software (Tree Star, Inc.).
EF-EXÖBead® complexes served as a non-EV control. Plasma
EV-EXÖBead® complexes were also stained with IgG
antibodies. The background signal of EF-EXÖBead®
complexes with test master mix antibodies and plasma
EVs-EXÖBead® complexes with IgG antibodies was
comparable (data not shown). EF-EXÖBead® complexes were
used as the gating basis of the flow cytometry data. Our antibody
master mix contained: 2.5 µg/ml of PE/Cyanine7 anti-human
CD63 (1:80; clone: H5C6), FITC anti-human CD81 (1:80; clone: 5A6),
APC anti-human CD9 (1:80; clone: HI9a; all from Biolegend, Inc.)
and eFluor 450 anti-human programmed death-ligand 1 (PD-L1; 1:80;
clone: MIH1; Thermo Fisher Scientific, Inc.). For the EV
subpopulation gating strategy, CD9+ or Neg and
CD81+or Neg were first gated on Plasma
EV-EXÖBead® complexes which based on the background
signal from EF-EXÖBead® complexes. These four-population
included CD9+ CD81Neg, CD9+
CD81+, CD9Neg CD81+ and
CD9Neg CD81Neg plasma EV-EXÖBead®
complexes. These four populations were further gated with
CD63+ or Neg and PD-L1+ or Neg based on the
signal of EF-EXÖBead® complexes. Significance was
calculated by two-way ANOVA with Šídák's multiple comparisons
test.
HNSCC biomarkers staining
Due to limited fluorescence channels, the PanEV
antibody was used as a positive EV marker instead of three
individual antibodies. PanEV antibody (3 µl) was premixed
with 1 µl of PE anti-CD63: H5C6 clone, 1 µl of
PE-CD81: 5A6 clone and 1 µl of PE-CD9: HI9a clone (1:66.7;
Biolegend, Inc.). The antibody master mix contained the PanEV
marker, three disease-specific biomarkers, and a common leukocyte
antigen CD45. The mix was set up of 3 µl of PE-PanEV
antibodies (1:66.7), 4 µl of Alexa Fluor® 488
anti-Pan Cytokeratin: C-11 (10 µg/ml; 1:50), 5 µl of
Brilliant Violet 421™ anti-human CD45 Antibody: HI30 (1:40; all
from Biolegend, Inc.), 5 µl of APC anti-PD-L1 antibody: MIH1
(1:40; Thermo Fisher Scientific, Inc.) and 5 µl of APC-Cy7
Mouse Anti-Human CD326 antibody: 9C4 (APC-Cy7 Mouse Anti-Human
CD326 antibody; 1:40). To avoid a multiple plasma
EV-EXÖBead® aggregation signal, individual beads from
FSC-A and SSC-A were first gated. For the single positive EV
subpopulation gating strategy, 'PanEV+ and CD45+ or
Neg', 'PanEV+ and epithelial cell adhesion
molecule (EpCAM)+ or Neg', 'PanEV+ and
PD-L1+ or Neg' or 'PanEV+ and pan-cytokeratin
(PanCK)+ or Neg' were further gated on Plasma
EV-EXÖBead® complexes which based on the background
signal from EF-EXÖBead® complexes. The final populations
were expressed as a percentage of single beads. For the double
positive EV subpopulation gating strategy, PanEV+ and
CD45Neg were gated on plasma EV-EXÖBead®
complexes based on the background signal from
EF-EXÖBead® complexes. PanEV+
CD45Neg plasma EV-EXÖBead® complexes were
further gated EpCAM+/Neg and PD-L1+/Neg also
based on the background signal from EF-EXÖBead®
complexes. The final four double positive populations of
PanEV+ CD45Neg EpCAM+
PD-L1Neg, PanEV+ CD45Neg
EpCAM+ PD-L1+, PanEV+
CD45Neg EpCAMNeg PD-L1+ and
PanEV+ CD45Neg EpCAMNeg
PD-L1Neg were expressed as percentages of single beads.
For the triple positive EV subpopulation gating strategy,
PanEV+ and CD45+/neg were gated on Plasma
EV-EXÖBead® complexes based on the background signal
from EF-EXÖBead® complexes. Two populations of
PanEV+ CD45Neg and PanEV+
CD45+ EV-EXÖBead® complexes were further
gated with EpCAM+/Neg and PanCK+/Neg also
based on background signal from EF-EXÖBead® complexes.
These four populations of PanEV+ CD45Neg with
EpCAM+/Neg and PD-L1+/Neg were further gated
by PD-L1+ population based on background signal from
EF-EXÖBead® complexes. The final four triple positive
populations of PanEV+ CD45Neg
EpCAM+ PanCKNeg PD-L1+,
PanEV+ CD45Neg EpCAM+
PanCK+ PD-L1+, PanEV+
CD45Neg EpCAMNeg PanCK+
PD-L1+ and PanEV+ CD45Neg
EpCAMNeg PanCKNeg PD-L1+ were
expressed as percentages of single beads. Another final four triple
positive population of PanEV+ CD45+
EpCAM+ PanCKNeg PD-L1+,
PanEV+ CD45+ EpCAM+
PanCK+ PD-L1+, PanEV+
CD45+ EpCAMNeg PanCK+
PD-L1+ and PanEV+ CD45+
EpCAMNeg PanCKNeg PD-L1+ also used
the same gating strategy and were expressed as percentages of
single beads. Significance was calculated by an unpaired t-test
with Welch's correction. The specific test was selected based on
number of groups, number of samples, and normality.
Intracellular EV marker and non-EVs
marker staining
Non-EV fractions were collected after PPP incubation
with EXÖBead®. To compare the non-EVs fraction to the
plasma EV-EXÖBead® complexes, the non-EVs fraction were
coupled to the same amount, number, and material of magnetic beads.
The coupling steps consisted of first activating the magnetic
particles with carboxyl groups (Chemicell GmbH) through incubation
with 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride
(EDC; Merck KGaA) in 0.1 M 2-(N-Morpholino) ethane sulfonic acid
(MES; cat. no. M8250; Merck KGaA) at pH 5.0 for 30 min at 25°C.
Then non-EV fractions were covalent-coupled to the same number and
same material of EDC activated carboxyl-group magnetic particles by
incubation for 2 h at 25°C. Non-EV-bead complex were further
blocked by 0.5% EV-free BSA in PBS. A total of 1 ml of 10% EV-free
BSA in PBS was coupled with EDC-activated carboxyl-group magnetic
particles as a negative control. Plasma EV-EXÖBead®
complexes, EF-EXÖBead® complexes, non-EV fractions-bead
complexes and EF-bead complexes were then fixed by 200 µl of
4% paraformaldehyde in PBS for 10 min at 25°C, penetrated by 200
µl of 0.1% Tween-20 in PBS for 10 min at 25°C and blocked by
200 µl of 10% EV-free FBS in PBS (100,000 g, 4°C for
overnight centrifugation) for 30 min at 25°C. Fixed/Penetrated
plasma EV-EXÖBead® complexes, EF-EXÖBead®
complexes, non-EV fractions-bead complexes and EF-bead complexes
were further stained with 200 µl of 0.5% EV-free BSA in PBS
with antibodies master mix at 25°C for 1 h incubation. Antibodies
master mix contained 2.5 µg/ml of Alexa Fluor®
488 anti-apolipoprotein A1/ApoA1 (1:40; clone: 2083A; R&D
Systems, Inc.) and 5 µg/ml of PE anti-TSG101 (1:40; clone:
EPR7130(B), Abcam). Antibodies-stained plasma
EV-EXÖBead® complexes, EF-EXÖBead® complexes,
non-EV fractions-bead complexes and EF-bead complexes were
visualized by BD LSRFortessa™ (BD Biosciences) and data was
analyzed with FlowJo V10.8 software (Tree Star, Inc.). The
geometric mean fluorescence intensity (MFI) was used for the
calculation. The initial MFIs of EF-EXÖBead® complexes
and EF bead complexes were similar (data not shown). For the group
of plasma EV-EXÖBead® complexes, the reduced MFI of the
negative control was the MFI of the plasma EV-EXÖBead®
complexes minus the MFI of the EF-EXÖBead® complexes.
For the non-EV fraction bead complexes, the reduced MFI of the
negative control was the MFI of the non-EV fraction bead complexes
minus the EF bead complexes. Significance was calculated using an
unpaired t-test.
Size-exclusion chromatography (SEC) was used qEV
original 70 nm (IZON Science, Ltd.). EV fractions were collected
from fraction 7 to 10 as recommends by the manual. A total of 500
µl of solution was collected per fraction. A total of 7 to
10 fractions were then pooled together as SEC-EVs (2 ml). To
understand the total isolated components of SEC isolation, SEC was
not concentrated by further steps such as ultracentrifugation or
ultrafiltration. SEC EVs (2 ml) were coupled with the same amount,
number and material of magnetic beads. SEC EVs were further coupled
to 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) activated
carboxyl group magnetic particles (Chemicell GmbH). A total of 1 ml
of 10% EV-free BSA in PBS was also coupled with EDC-activated
carboxyl group magnetic particles as a negative control. In the
comparative study, plasma EV-EXÖBead® complexes,
EF-EXÖBead® complexes, non-EV fraction bead complexes,
and EF bead complexes were incubated with an antibody master mix in
200 µl of 0.5% EV-free BSA for 1 h at 25°C. The antibody
master mix contained 3 µl PE-PanEV (premixed CD9, CD63 and
CD81 antibodies; 1:66) and 5 µl apolipoprotein A-I/ApoA1
Alexa Fluor® 488-conjugated antibodies (ApoA1; 1:40;
cat. no. EP1368Y; Abcam). The initial MFIs of
EF-EXÖBead® complexes and EF bead complexes were similar
(data not shown). Plasma EV-EXÖBead® complexes,
EF-EXÖBead® complexes, EF-bead complexes, and
SEC-EV-bead complexes were visualized on BD LSRFortessa™ (BD
Biosciences) and the data were analyzed using FlowJo software (Tree
Star, Inc.). For the gating strategy, a gating population of beads
was first determined based on FSC-A and SSC-A. Then, the
PanEV+/Neg and ApoA1+/Neg populations were
gated based on EF control. Significance was calculated using an
unpaired t-test.
NTA
EV numbers and sizes were determined by
ZetaView® Nanoparticle Tracking Analyzer PMX 110
(Particle Metrix GmbH). EVs were diluted in PBS to a final volume
of 1 ml. For each measurement, two measurement cycles were
performed by scanning 11 positions each and acquiring 60 frames per
second under the following settings: pre-acquisition parameters
were set to a sensitivity of 80; Shutter was set to 70. Cell
temperature was set to 25°C and trace length to 15 (20). After recording, the videos were
analyzed with the built-in ZetaView software 8.05.11 SP1 (Particle
Metrix GmbH) with specific analysis parameters: minimum particle
brightness: 20; minimum size of 5 pixels, maximum size of 1,000
pixels and PSD nm/class of 10 and PSD classes/decade of 10.
TEM
A total of 5 µl of the undiluted sample were
adsorbed for 60 sec to glow-discharged parlodion/carbon-coated
copper grids. The grids were then blotted, washed 3 times with
double-distilled water and negatively stained on two droplets of 2%
uranyl acetate solution. Samples were imaged using a FEI Talos
F200C TEM (FEI) operated at 120 kV. Electron micrographs were
recorded on a Veleta Camera (EMSIS GmbH).
Cryo-TEM
A total of 4 µl aliquot of sample was
adsorbed onto holey carbon-coated grid (Ted Pella, Inc.) blotted
with Whatman 1 filter paper and vitrified into liquid ethane at
-178°C using a Leica GP plunger (Leica Microsystems GmbH). Frozen
grids were transferred onto a Talos electron microscope (FEI) using
a Gatan 626 cryo-holder. Electron micrographs were recorded at an
accelerating voltage of 200 kV, using a low-dose system (20 e-/Å2)
and keeping the sample at low temperature. Micrographs were
recorded on a CETA camera (Thermo Fisher Scientific, Inc.).
Functional T cell assay
The functional assay was performed in
CD4+ immune cells of 3 patient blood sample in 3
replicates. CD4+ cell extraction was performed from
buffy coats obtained from the Blood Donation Center (Basel
District) according to the manufacturer's protocol using the
StraightFromTM Buffy Coat CD4 Micro Bead kit (product no;
130-114-980 MACS Miltenyi Biotec, Inc.). The T-cell activation was
conducted according to the manual as described in the T Cell
Activation/Expansion kit (product no. 130-091-441; MACS Miltenyi
Biotec, Inc.) (21,22). A total of 30 out of 200 µl
eluted EVs isolated from tumor patient plasma were added to each
well and gently mixed for 24 h at 37°C. Cells were further stained
with an antibody master mix containing 2.5 µl anti-body/test
of CD152 Monoclonal Antibody APC (Clone: 14D3), CD4 Monoclonal
Antibody eFluor® 450 (Clone: SK3) and CD69 Monoclonal
Antibody FITC: FN50 (all from Thermo Fisher Scientific, Inc.) for
50 min at 37°C. Analytical assay was performed using flow cytometry
(CytoFlex; Beckman Coulter, Inc.). Significance was calculated by
non-parametric Kruskal-Wallis test with Dunn's multiple comparisons
test.
Functional PBMCs assay
The functional assay was performed in PBMCs of
different HNSCC patients' plasma EVs in technological triplicates.
PBMCs were isolated from buffy coats received by healthy donors.
PBMCs activation was conducted according to the manual as described
in the T Cell Activation/Expansion kit (product no. 130-091-441;
MACS Miltenyi Biotec, Inc.). Eluted EVs (5×107) isolated
from tumor patient plasma were added to each well with
1×106 PBMCs/ml in 1 ml culture medium and gently mixed
for 24 h at 37°C in the incubator. Lactose elution buffer (0.3 M
lactose in PBS) was used as a negative control and as a gating
base. To avoid the effect of the elution buffer, the total amount
of CD69 was also examined in the elution buffer treatment group and
in the group with only activated T cells. The total CD69 amount did
not differ between elution buffer with T-cell activation
stimulation and T-cell activation stimulation only (data not
shown). Cells were first stained at 100 µl at a 1:100 ratio
with the cell viability dye Zombie NIR™ for 30 min at 25°C
(Biolegend, Inc.). After two washing steps, cells were stained with
100 µl of 1:100 antibody master mix, CD4 Monoclonal Antibody
eFluor® 450 (Clone: SK3, Thermo Fisher Scientific,
Inc.), APC anti-PD-L1 antibody: MIH1 (Thermo Fisher Scientific,
Inc.), PE anti-human CD279 (PD-1) Antibody: EH12.2H7 (Biolegend
Inc.) and CD69 Monoclonal Antibody FITC: FN50 (Thermo Fisher
Scientific, Inc.) for 30 min at 4°C. Analytical assay was performed
using flow cytometry: BD LSRFortessa™ (BD Biosciences) and flow
data were analyzed by FlowJo software (Tree Star, Inc.). First, the
lymphocyte population was recorded based on FSC-A and SSC-A. Then,
the population of individual cells was detected based on FSC-A and
FSC-H. To identify live cells, the zombie NIR negative population
was gated. To examine live T cells, the CD4+ population
was then gated. The final gate was gated to CD69+/Neg
with PD1+/Neg or CD69+/Neg with
PD-L1+/Neg. Significance was calculated by
Brown-Forsythe and Welch's ANOVA test with Dunnett's T3 multiple
comparisons test.
Statistical analysis
Flow cytometry data including gating were performed
by FlowJo version 10.8 software (Tree Star, Inc.). Data were
statistically analyzed with Prism version 8 (GraphPad Software,
Inc.). The specific test used is described in the figure legend.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphology and particle number of eluted
EVs by magnetic galectin-based glycan recognition particles
(EXÖBead®) isolation
To compare whether the eluted EVs were similar in
size to those reported in the literature (23) the morphology was examined after
isolation of EXÖBead® and elution in lactose-PBS buffer
in conventional TEM, where they were observed as intact,
cup-shaped, membrane-bound vesicles with a size of 30-200 nm
(Fig. 2A and B). Eluted EVs were
observed as a double lipid layer of vesicles in cryo-EM with a size
of 30 nm-250 nm (Fig. 2C and D).
ZetaView (Particle Metrix, Inning am), a NTA instrument, was used
to measure the yield and size of nanoparticles from three
individual donors as biological triplicates (Fig. 2E-G) and three technological
triplicates of a same donor (Fig.
2H). The medium size of eluted EVs from three technological
triplicates was 81.6±2.43 nm (CV: 2.97%; Fig. 2E-G). The total particle number from
1 ml plasma of 3 technological triplicates was
1.57×108±2.8×107 (CV: 13.29%; Fig. 2H). The eluted EVs from
EXÖBead® isolation represented reproducible and high
similarity as mentioned in the literature (23).
EV surface marker, intracellular marker
and non-EV marker
The recovered EVs were analyzed and checked for
established EV surface markers by bead-based flow cytometry.
CD63+, CD9+ and CD81+ were
reported as common and MISEV compliant EV surface markers (23). Geometric MFI of CD63, CD9 and CD81
was expressed on recovered EXÖBead® isolates and was
increased in HNSCC patients compared with healthy donors for all
surface markers (Figs. 3A and
S1). MFI of CD9+ was
significantly higher in patients with HNSCC compared with the
healthy controls (P=0.0097; Figs.
3A and S1A). In addition, MFI
of PD-L1+, an important diagnostic cancer and surface
marker (24) was increased in
HNSCC tumor patients compared with the healthy controls. Relative
ratio of PD-L1+ MFI versus CD81+ MFI
(P=0.0004) was significantly higher in patients with HNSCC compared
with the healthy controls (Fig.
3B) in both cases. PD-L1+ CD9+
CD63+ CD81Neg EVs-EXÖBead® complex
from HNSCC patients had also higher relative scores (P<0.0001;
Figs. 3C and S1B-E). However, PD-L1+
CD9+ CD63+ CD81+,
PD-L1+ CD9+ CD63Neg
CD81+ and PD-L1+ CD9+
CD63Neg CD81Neg EVs-EXÖBead®
complex showed no difference between the two groups (Figs. 3C and S1B-E). These population changes
suggested that PD-L1+ CD9+ CD63+
EVs may play an important role in HNSCC, but further research is
required to support or refute this assumption. In addition to
extracellular markers, TSG101 was additionally measured as typical
intracellular EV marker (23). The
experiments were well feasible and TSG101 could be detected in EVs
of HNSCC after isolation and recovery by EXÖBead®. The
levels of the isolates were significantly increased in the
EXÖBead® fraction compared with the unbound patient
plasma (P=0.015; Figs. 4A and
S2A). A frequent problem of EV
isolation is the contamination by protein aggregates or other
aggregates/particles from blood samples, due to the complexity of
blood composition (23). A
well-established marker for contamination in EV preparations is
ApoA1 (23). The ApoA1
concentration was high in residual plasma with MFI 7000 compared
with almost none in the EXÖBead® fraction (P=0.0002;
Figs. 4A and S2A). To gain an improved understanding
of the specificity of EV isolation, SEC was compared with
EXÖBead® in terms of PanEV and ApoA1 protein presence.
PanEV+ ApoA1neg population was significantly
higher in the EV-EXÖBead® complex compared with the
EV-SEC beads complex (P=0.019; Figs.
4B and S2B). While
PanEVneg ApoA1+ population was significantly
higher in EV-SEC beads complex compared with EV-EXÖBead®
complex (P=0.0061; Figs. 4B and
S2B). This flow cytometric result
of EV-EXÖBead® complex suggested that measurable
lipoprotein contamination may be lower with EXÖBead®
isolation of plasma EVs than with isolation by SEC method. Notably,
PanEV+ ApoA1+ population may be detected by
both isolation methods with no significant difference (23,25).
HNSCC specific exosomal markers
A specific set of serum markers for diagnosis and
therapeutic purpose in HNSCC patients is currently lacking
(26). CD45, EpCAM, PanCK and
PD-L1 have been reported as potential biomarkers in HNSCC patients
and their isolated EVs/exosomes (11,24).
However, the expression level of CD45, EpCAM, PanCK and PD-L1 on
EVs in HNSCC patients remains unknown. EXÖBead® was used
to isolate EVs from plasma which were then stained with the master
mix of antibodies containing PanEV, CD45, EpCAM, PanCK and PD-L1.
The expression level of EpCAM+ in HNSCC patients was
significantly higher than in healthy controls (P=0.023) while there
was no significant difference in the other markers when considered
independently (Figs. 5A and
S3A). To accurately determine the
percentage of tumor-specific EVs in the circulation, single EV bead
complexes were next gated to exclude multiple EV bead complexes and
examine the difference between HNSCC patients and healthy controls.
In the antibody staining of the obtained EVs, the results of
EV-EXÖBead® complex showed that there was a significant
difference between CD45neg PanEV+,
EpCam+ PanEV+ and PDL1+
PanEV+ between patients with HNSCC and healthy controls
(CD45neg PanEV+, P=0.026; EpCAM+
PanEV+, P=0.004; PD-L1+ PanEV+,
P=0.007) (Figs. 5B-D and S3B-D). The EV-EXÖBead®
complex stained with PanCK showed no significant difference between
HNSCC and control (Figs. 5E and
S3E). The EV-EXÖBead®
complex population of CD45neg EVs that was
EpCAM+ and PD-L1+ was significantly increased
in HNSCC patients compared with the control group (P=0.002;
Fig. 5F and Fig. S3F-I). The same was found for the
group of EV-EXÖBead® complex which was
CD45neg, EpCAM+ and PD-L1neg
(P=0.003; Figs. 5F and S4F-I). For this purpose,
CD45neg, EpCAM+/neg, PD-L1+/neg
and PanEV+ were gated on a single EV beads complex
(Fig. 5F and S3F-I). In addition, the triple positive
population on the EV-EXÖBead® complex was examined. It
was found that CD45neg EV-EXÖBead® complexes
that were double or triple positive for the markers EpCAM, PD-L1
and PanCK were significantly more expressed in HNSCC than in
healthy controls [(EpCAM+, PD-L1+ and
PanCKneg; P=0.0005) (EpCAM+,
PD-L1+ and PanCK+; P=0.02; Figs. 5G and S3J-M)]. Notably, it was also identified
that CD45+ EV-EXÖBead® complexes with
EpCAM+, PD-L1+, but PanCK− were
detectable to a significantly higher extent in HNSCCs than in
healthy controls (P=0.025; Figs.
5H and S3N-Q). These
significantly altered populations of EV-EXÖBead® complex
suggested that single positive EVs, double positive EVs or triple
positive EVs may play an important role in the progression of
HNSCC. Further studies need to be performed. To demonstrate yet
again that EVs were specifically isolated with the method described
in the present study, particle size and number of particles were
checked in parallel with the analysis of specific markers on the
EVs with NTA. Similar particle sizes were found in both groups
(HNSCC: 125.2±9.1 nm; control: 123.32±7.42 nm). The particle number
was comparable (HNSCC: 2.54×108±1.27×108;
control group: 5.58×108±3.17×108) (Fig. 5I).
Functional in vitro competence of
plasma-derived EVs
To evaluate the biological activity and functional
in vitro competence of EVs isolated and recovered from
plasma of tumor patients, EVs obtained by EXÖBead® as
well as EVs obtained by polyethylene glycol (PEG)-based
precipitation method was co-incubated with activated T cells
(CD4+ T cells) and compared with activated T cells
without EV co-incubation. The aim was to measure the in
vitro immunosuppressive capacity of EVs isolated by the
established PEG-based precipitation method (27,28)
and compare it to the immunosuppressive capacity of EVs isolated by
EXÖBead®. In the present study, the purity of
CD4+ T cells was at least 90%, analogous to analyses in
older proprietary trials (6).
Percentage of CTLA4+ CD69neg T responder
cells was measured by flow cytometry (29,30)
after co-incubation with EVs isolated by the two different methods.
A highly significant difference was observed between untreated
activated T cells and T cells treated with 30 out of 200 µl
EXÖBead®-isolated EVs in terms of CTLA4 and CD69
expression (P=0.025; Fig. 6A and
S4A). Notably, this effect was
not observed when activated T cells were treated with EVs obtained
by PEG-based isolation (Figs. 6A
and S4A). There was no difference
of single CTLA4+ CD4+ T cells within these
three groups (Fig. S4B). To
address the question of whether EVs isolated from plasma of
patients with HNSCC using EXÖBead® retain their
biological activity, EVs from different patients were examined for
immunosuppression. The present data showed that PD-L1+
CD69neg CD4+ T cells were significantly
increased after co-incubation with 5×107 plasma EVs from
HNSCC patients (n=13, with technological triplicate) compared with
co-incubation with elution buffer (technological triplicate)
(P=0.0011) and healthy donors (n=3, with technological triplicate)
(P=0.0045) as controls (Figs. 6B
and S4C). It was also found that
PD-L1+ CD69neg CD4+ T cells were
also higher in treatment with healthy controls' EVs compared with
controls (P=0.0404; Figs. 6B and
S4C). Notably, it was also found
that PD1+ CD69neg CD4+ T cells
were also significantly increased in patients' group compared with
the elution buffer group (P=0.0344), but there was no difference
between healthy controls and elution buffer group (Fig. S4D). No difference was observed
between the groups of patients and controls and elution buffer from
signal positive of CD69, PD1 and PDL1 CD4+ T cells
(Fig. S4E). Thus, by isolating
EVs with EXÖBead® it appeared to be possible to isolate
functionally competent EVs from plasma of HNSCC patients.
Discussion
Over the past decade, interest in EVs as biomarkers
for tumor disease has markedly increased. This is also true for
malignancies of the head and neck, particularly the most common
entity of HNSCC. Unfortunately, numerous patients with HNSCC are
already at an advanced stage at the time of diagnosis, so a tool
for earlier initial diagnosis, as well as for recurrence detection
and therapy monitoring, would be of great clinical value. There is
still a long way to go before EVs are regularly used clinically as
tumor markers, although much has already been learnt about their
biomarker potential (31).
One of the problems being faced in integrating into
clinical practice is the development of a simple, rapid, and
reproducible method to reliably isolate pure EVs from patient
plasma.
In the present study, a novel galectin-coupled
magnetic bead method was used to isolate pure EVs from human
plasma. Intact eluted EVs were examined in detail by TEM imaging
and cryo-EM, and the characteristic EV shell shape was confirmed.
Size and concentration measurements by NTA showed low variability
and high reproducibility in all process triplets. In accordance
with MISEV 2018 guidelines, extracellular and -intracellular EV
protein markers and non-EV markers were fully validated by flow
cytometry (23).
Our result firstly showed that galectin-coupled
magnetic beads (EXÖBead®) isolated EVs from plasma with
low contamination of lipoprotein. In a second step, lactose-based
elution buffer was used to successfully obtain functionally intact
EVs after elution. Galectins are glycan-binding proteins and
contain C-terminal carbohydrate recognition domains that
preferentially bind β-galactoside-rich glycoprotein (32).
Polylactosamine, α2,6-linked sialic acid, high
mannose N-glycan, and complex-type N-glycan were previously shown
to be present on the surface of EVs in enriched form (33,34).
Previous studies showed that EVs from ovarian cancer cells
accumulate galectin-3-binding protein (LGALS3BP), which contains a
large amount of sialylated complex-type N-glycans (17,19).
In our view, this highlights the potential application of an
isolation method for EVs based on galectin-based glycan
recognition.
Research conducted both in-house and by other
research group indicated that protein levels of EVs isolated from
plasma of HNSCC patients were significantly higher than those from
healthy donors (10,11). Ludwig et al (9) also showed that the increase in total
plasma EVs correlated well with disease activity. However, in
numerous of these studies, SEC was used as a purification method to
isolate EVs from patient plasma. Meanwhile, it is known that SEC is
not only used to isolate EVs, but also to purify high amounts of
lipoprotein (25). This fact may
prove somewhat problematic in the future, as Chen et al
(35) demonstrated that only
exosomal biomarkers show a correlation with disease progression.
Therefore, the specificity of EV isolation is of particular
importance in this context. Using an isolation method for EVs based
on galectin-based glycan recognition (EXÖBead®),
measurable lipoprotein contamination was markedly lower when
isolating exosomes from plasma than when isolating them using the
SEC method. This was reflected in the higher concentration of
PanEV+ ApoA1neg population in the
EXÖBead® isolation fraction compared with the SEC
isolation fraction while it was the opposite in PanEVneg
ApoA1+ population. It was also observed that particle
numbers from SEC isolation were 100-200 times higher than
EXÖBead® isolation from our unpublished results. When
the particle number and bead-based flow cytometric data were
combined, it was considered that SEC isolates a larger amount of
lipoprotein compared with EXÖBead®. Fluorescent NTA and
antibody-stained EV flow cytometry was used to determine which
particles are pure EVs and which particles are lipoproteins in SEC
and EXÖBead®. Notably, the PanEV+
ApoA1+ population could be detected by both isolation
methods without significant difference. PanEV+
ApoA1+ may indicate that small EVs carry ApoA1 protein
or that high-density lipoprotein carries PanEV proteins. It has
already been revealed that EVs isolated from plasma can be covered
with low-density lipoprotein (36-38).
In addition, lipoproteins such as ApoA, ApoB and ApoE could be
detected on EVs of pigment cells (39), making it difficult to distinguish
between lipoprotein particle contaminants and EV-associated
lipoproteins (25). Thus, a
precise classification of the function of the PanEV+
ApoA1+ population is not yet possible. To demonstrate
PanEV+ ApoA1+ population, further preparation
of 'pure EVs isolation from cell cultures' and 'pure EVs isolation
from lipoprotein overexpression cell cultures' will be required in
future studies. Another limitation is that most of EVs studies did
not measure lipoprotein level before EV isolation. The lipoprotein
level should be also considered before EV isolation in the future.
Therefore, particularly in this still early phase, purity in the
workup is considered to be particularly important for diagnostic
and prognostic statements. Our research to date has shown that
lipoprotein levels can be significantly reduced by isolation based
on galectin-based glycan recognition compared with unbound
plasma.
An interesting and important observation in the
present study was that a T-cell activation assay could show that
EVs maintained their functional activity throughout the isolation
process with EXÖBead®. Eluted EVs from patients showed a
stronger suppressive character after isolation based on
galectin-based glycan recognition than with the conventional
isolation method using PEG. Since our EVs were obtained from the
same patients at the same time point, it was postulated that they
lose a greater proportion of their functional activity during
conventional isolation. This difference may play an important role
in functional assays of EVs. While the concentration and expression
patterns of tumor-derived EVs already provide us with important
information, functional assays play an important role in
understanding how tumors interact with the immune system.
It is now known that EVs can tell us a lot about
their mother cells by their expression pattern (11). However, the interaction between
tumor and immune system is markedly more complex to be
characterized by surface patterns alone. Our understanding can
become more improved, as closer it gets to the 'in vivo
activity' of EVs. Thus, isolated EVs should retain their biological
activity as much as possible. It is considered that the
galectin-based glycan recognition method provides a good basis for
this, although further and broader studies will be needed. For
example, it was identified that CTLA4+
CD69neg increases to a greater extent in activated T
cells in co-incubation experiments with EXÖBead® than in
co-incubation experiments with a conventional assay (PEG
isolation). Since only 30 out of 200 µl eluted EVs were
used, fixed particles number/T cells for CTLA4+/Neg
CD69+/neg T cells experiment will also be examined in
the future. The results showed that PD-L1+
CD69+ T cells were significantly higher in treatment
with patient's plasma EVs (EV versus PBMCs ratio: 50:1) compared
with healthy controls and elution buffer only. Theodoraki et
al (24) showed that
PD-L1+ exosomes were highly correlated with disease
progression (24).
CD69+ CD8+ T cells were significantly lower
when treatment with PD-L1+ high exosomes compared with
PD-L1+ low exosomes (24).
The present data showed the similar suppression
effect on increasing of PD-L1+ CD4+ T cells
in treating with patients' plasma EVs compared with controls.
However, no changes of CD69+ CD4+ T cells
were observed between treatment with patients' EVs, controls' EVs
and elution buffer only. One possibility may be that HNSCC patients
were not separated into different stages. Further studies will need
to demonstrate if CD69+ CD4+ T cells are
reduced by treatment with later stages of patients' plasma EVs.
Knowing that CTLA4 and PD1/PD-L1 is a suppressive marker and CD69
is an activating marker, it can be assumed that EVs isolated with
EXÖBead® have a suppressive character and thus probably
an improved-preserved functionality. This allows reliable
co-incubation experiments with EVs from tumor patients and immune
cells, which is considered necessary to elucidate tumor-immune
interaction via EVs in an improved way. Understanding which types
of regulatory T cells were induced by patient's plasma EVs will
also be an interesting research subject. Further study will be
needed to identify by different regulatory T cells markers, such as
CD25 and Foxp3 (40).
In patients with tumor disease, 2 major producers
of EVs have been previously identified. One is the metabolically
active tumor cells themselves and the other is the immune cells
(41-43). To classify these different groups
of EVs based on their surface markers, plasma EVs were first
divided into two main subgroups. First, those that are mainly
tumor-associated released (CD45neg) and second, EVs
released by immune cells (CD45+). Immunologically hot
tumors-i.e., those with a high number of tumor-infiltrating
lymphocytes (TILs)-tend to have an improved response to primarily
immunotherapies (44,45). To infer from this that there are
good immune cell-derived EVs and bad tumor cell-derived EVs would
fall far short. Not least because immune cell derived EVs-like
immune cells themselves-appear to play a dual role in tumorigenesis
and progression (46). Several
studies on TILs suggested that they play an important role in HNSCC
progression (47,48). As early as 2007, Rajjoub et
al (49) published studies on
the prognostic significance of CD3low TILs in oropharyngeal cancer.
An association between this subset of T cells and a high rate of
metastasis was revealed. By contrast, Badoual et al
(50) showed that the enrichment
of tumor-infiltrating CD4+CD69+ T cells
correlates with improved survival. Thus, how exactly TILs
contribute to HNSCC progression is not yet well understood and it
is considered that isolation and analysis of EVs coming from TILs
may contribute to an improved understanding of HNSCC progression.
According to our literature search, studies regarding the role of
TIL-derived EVs and their role in HNSCC are still very sparse. In
the present study, the group of CD45+ EVs that were
simultaneously positive for EpCAM and PD-L1 was significantly
increased in the HNSCC group compared with healthy controls.
Although further and more in-depth studies are needed, this triple
staining may contribute to an improved understanding and, in
perspective, may be an option for monitoring disease
progression.
In the bead-based flow cytometric analysis of EVs
isolated from human plasma using galectin-based glycan recognition
as isolation method, 3 common EV surface markers (CD9, CD63, and
CD81), an intracellular EV marker (TSG101) and 3 HNSCC biomarkers
(PD-L1, PanCK, and EpCAM) were searched for. The bead-based flow
cytometry result showed PD-L1+ CD9+
CD63+ CD81Neg EVs-EXÖBead® complex
was significantly higher in HNSCC patients compared with healthy
controls. However, it was only tested in small samples size and
these antibodies pair shall be examined with a large samples size
in the future. The bead-based flow cytometry suggested that ApoA1
were low in the EV-EXÖBead® complex, whereas these were
detectable to a markedly higher extent in the unbound plasma. In
the present choice of biomarkers for HNSCC, it was not possible to
rely on any established serum biomarker to date, such as exists
with prostate specific antigen in prostate cancer or thyroglobulin
in thyroid cancer. Nevertheless, there are a number of molecules in
HNSCC that have received increasing attention in previous years and
may have prognostic value (51-53).
These considerations formed the basis for the choice of the markers
selected and their combination. The bead-based flow cytometry
suggested 5 biomarker panels to be the future of EV-based liquid
biopsy and some potential was observed in the selected markers
based on the present data. Beads-based flow cytometry and
sub-population gating strategy can help to easily/fast understand
the expression profiles of each marker and sub-populations of EVs.
However, antibodies-stained EV-EXÖBead® complex was
analyzed instead of EVs directly. Further researches will be needed
in the future, such as EV flow cytometry next-generation sequencing
and proteomics.
For numerous years, PD-1 or PD-L1 has received
marked attention in cancer research (54). For HNSCC, for example, it has been
identified that there is an association between PD-1 and PD-L1
overexpression and tumor progression (55). For numerous years, target therapy
with checkpoint inhibitors in HNSCC was mainly experimental in
nature. PD-1/PD-L1 inhibitors were taken out of this niche by the
Keynote-048 study, which showed that the checkpoint inhibitor
pembrolizumab is superior to the previously established EXTREME
study (cetuximab, cisplatin, 5 FU) in metastatic and/or relapsed
HNSCC in combination or as monotherapy (56,57).
However, since not all patients respond equally to this approach,
PD-L1 status is used to subtype patient groups. For this purpose,
immunohistochemical examinations are performed on tissue samples
and scores are generated to predict response to immunotherapy.
Although there is a certain correlation, the predictive character
of these examinations still needs improvement. Since the tumor is
constantly changing during the course of the disease and
particularly under the selection pressure of tumor therapy, good
therapy monitoring requires regular analysis of the tumor
expression pattern. However, frequent biopsies are associated with
increased morbidity for patients. Regular characterization of the
tumor should therefore be performed by blood sampling. In our
opinion, exosomal PD-L1 is suitable for this purpose and can be
determined quickly and reliably using the EXÖBead®
isolation technique.
Notably, pretreatment high exoPD-L1 levels but not
soluble PD-L1 levels were shown to be associated with disease
progression (24) in patients with
HNSCC. A similar observation was made by Chen et al
(35) in studies of patients with
metastatic melanoma. When examining the levels of different types
of PD-L1 (total soluble, micro vesicular, exosomal, and secreted or
excreted PD-L1) before and after immunotherapy, the levels of
exoPD-L1, but not other forms of PD-L1, differed between patients
who would respond to PD1 blockade. Early after initiation of
immunotherapy, the magnitude of the increase in exoPD-L1 was
significantly higher in responders, whereas a non-significant
difference was observed for the other types of PDL1 (35). Whether this increase in exoPD-L1
was due to the release of exosomes from tumor cells or immune
cells, or even both, requires further investigation of plasma EVs.
These findings, together with a simplified and reliable isolation
technique, may allow a more precise selection of patients who will
benefit from immunotherapy in the future.
However, other molecules also have the potency of
prognostic relevance. EpCAM is a known tumor-associated antigen
that is highly overexpressed on various squamous cell carcinomas
including HNSCC (48). The
expression level of EpCAM on isolated EVs was significantly higher
in HNSCC patients than in healthy controls, whereas there was no
significant difference in the other markers when considered
independently. The present findings confirmed data published by
Theodoraki et al (58),
which showed significantly higher EpCAM expression on
CD45neg-EVs by separation of CD45 antibody beads base
isolation. Analyses of the MFI of EpCAM, a number of complexes also
showed a significantly higher percentage in patients with HNSCC
than in healthy patients.
In our studies, it was observed that the quadruplex
of EVs that were CD45neg, PanEV+,
EpCAM+, PanCK+, and PD-L1+ was
significantly higher in HNSCC patients compared with EVs from
healthy controls. This fact allowed to hypothesize that this may be
the population of tumor-derived EVs and thus the combination of
different markers may allow higher accuracy in subdividing
different subsets of EVs. Notably, it was also found that
CD45+ EVs, which were EpCAM+,
PD-L1+ but PanCKneg were significantly more
detectable in HNSCCs than in healthy controls. This may indicate
that these are EVs from tumor-infiltrating lymphocytes.
While some prognostic potential was observed for
individual markers (PD-L1 and EpCAM) when considered separately, it
is the combination of the various markers with the gating strategy
used that appears to allow more accurate differentiation between
the various EV subpopulations.
Further studies with larger numbers of patients
will be needed to examine this hypothesis in more depth, but it is
hypothesized that in such a validation cohort the combination of
markers may increase the prognostic potential in HNSCC. Because the
membrane-bound proteins are also a reflection of the cancer and can
be obtained from a simple blood sample, they also provide a source
for functional studies to understand in an improved way the
biological mechanisms of exosomal membrane-bound proteins in HNSCC
and cancer in general.
Using a galectin-based glycan recognition
(EXÖBead®) as isolation method with a 5-antibody
staining panel, it was possible to identify tumor-specific EV
populations. A distinction between infection/inflammation and tumor
is also conceivable, although this requires considerably more
investigation. However, if this assumption is confirmed it could be
of great help in clinical practice particularly in the
differentiation of post-therapeutic inflammation and tumor
recurrence. There are also certain limitations. At the moment,
EXÖBead® is only available for small plasma amount from
250 µl to 2 ml plasma. Maximal EVs production have to test
or improve in the future. At present, the authors of the present
study remain in a small team with limited funding and patient
samples and they are testing/planning in different types of cancer
by using EXÖBead®.
In the present study, focus was primarily addressed
on the isolation of EVs from the blood of cancer patients and their
analysis. The focus was primarily on potential diagnostic use.
However, the field of therapeutic use of EVs has also grown rapidly
in recent years.
One of the advantages is that EVs transport
proteins and nucleic acids and protect their contents from
proteases and RNAses through their double membrane. They are
histocompatible, not recognized by the complement system, do not
trigger unwanted immune reactions due to their endogenous origin,
and are less rapidly removed by the mononuclear phagocyte system
due to their nanoscale size (59)
This predisposes them as carriers for various biological
therapeutics, including short interfering RNA (siRNA) and
recombinant proteins.
Different techniques are used to cargo EVs with the
desired drug load. A distinction is made between exogenous and
endogenous loading. The successful use of EVs for targeted delivery
of siRNA was first demonstrated in 2011 by Alvarez-Erviti et
al (60). Herein, isolated EVs
were loaded with siRNA against an important protein in Alzheimer's
Disease pathogenesis (BACE1) and injected systemically. This
resulted in a decrease (55%) of the deleterious protein β-amyloid
1-42 in the brain. Particularly in cancers where target mutations
are known, this may be a promising approach. An additional
possibility is to overcome chemotherapy resistance by means of EVs.
Paclitaxel-loaded EVs have been shown to increase cytotoxicity in
multidrug-resistant neoplasms almost 50-fold compared with
paclitaxel without EVs (61). A
similar approach exists with doxorubicin (62). Particularly for these therapeutic
uses, it is considered that is necessary to have a fast and
reliable isolation method available that preserves biological
activity, as in the case of EXÖBead®. However, it
remains to be clarified what effect the loading process has.
Although knowledge of EVs has multiplied in recent years,
overseeing the complexities remains far. To date, the effects of
modifications on their biological behavior cannot be predicted with
sufficient reliability. Great potential in EVs is also observed in
the field of cancer immunotherapy due to their ability to induce
tumor-specific immunity, they are being traded as potential cancer
vaccines, with animal and clinical studies already conducted
(63). Particularly in this
context, it is essential that the biological characteristics of EVs
are not altered during the isolation process, hence advantages are
observed in the less aggressive elution process using lactose
buffer. Nevertheless, it is important to keep in mind that due to
the dual properties of EVs (they can both inhibit and promote
cancer development), a particularly good comprehension of the
underlying mechanisms is required. In summary, the therapeutic use
of EVs is a promising one, where the unaltered preservation of
biological activity is probably even more important than in the
context of diagnostic purposes.
To conclude, the EXÖBead® technique has
been shown to provide reliable results in the isolation of EVs in
the present study, which proved to be functional. It was possible
to perform the isolation with a significantly reduced time (~2 h),
since pre-isolation and ultracentrifugation are obsolete here.
Hereby, coming from the temporal expenditure into ranges, which
make an integration into the clinical routine examination
imaginable. Basic requirements for clinical use, such as
reliability, time efficiency and manageable costs, are fulfilled as
far as manageable to date. In addition to continuous monitoring of
cancer development in the clinical setting, the
EXÖBead®-based isolation technique can also provide a
valuable simplification of sample preparation for research purposes
to understand the role of EVs in cancer in an improved way.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed in the current
study are available to us in their complete form and are available
upon request from the corresponding author.
Authors' contributions
LB, EE, DMC, MWP and LM conceptualized the present
study. DMC, LB and EE curated data. DMC, LB, and LM performed
formal analysis. LM acquired funding. Investigation Methodology:
DMC, LB, EE, MWP and LM provided investigation methodology. LM
performed project administration. LM, DMC and MWP provided
resources. DMC, LB and LM performed software analysis. LM and MWP
supervised the study. LB, DMC, MWP and LM conducted data
validation. LB, DMC and LM performed visualization. LB and EE wrote
the original manuscript. LB and DMC revised and finalized the
manuscript. LM provided supervision, reviewed and edited the
finalized manuscript. MWP reviewed and edited the finalized
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2020-02173) by the ethical commission of the northwest and central
Switzerland. All patients provided written informed consent for
research and consented to anonymous processing of the blood samples
collected for scientific purposes.
Patient consent for publication
Not applicable.
Competing interests
DC is the founder of Biovesicle, Inc. All
experiments were conducted without financial contribution, simply
scientific advices were obtained. LM and MWP are scientific advisor
of Biovesicle, Inc. and did not receive profit.
Acknowledgments
The authors would like to thank the imaging
facility of the department of biomedicine University Basel,
particularly Carola Alampi and Mohamed Chami for acquiring the TEM
images.
Funding
The present study was supported by the 'pro patient' grant
(grant no. pp18-08) and Krebsliga 462 beider Basel (grant no.
18-463 2016).
References
|
1
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopatina T, Favaro E, Danilova L, Fertig
EJ, Favorov AV, Kagohara LT, Martone T, Bussolati B, Romagnoli R,
Albera R, et al: Extracellular vesicles released by tumor
endothelial cells spread immunosuppressive and transforming signals
through various recipient cells. Front Cell Dev Biol. 8:6982020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao C, Song F, Zheng YL, Lv J, Wang QF
and Xu N: Exosomes in head and neck squamous cell carcinoma. Front
Oncol. 9:8942019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muller L, Hong CS, Stolz DB, Watkins SC
and Whiteside TL: Isolation of biologically-active exosomes from
human plasma. J Immunol Methods. 411:55–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theodoraki MN, Hoffmann TK, Jackson EK and
Whiteside TL: Exosomes in HNSCC plasma as surrogate markers of
tumour progression and immune competence. Clin Exp Immunol.
194:67–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ludwig S, Floros T, Theodoraki MN, Hong
CS, Jackson EK, Lang S and Whiteside TL: Suppression of lymphocyte
functions by plasma exosomes correlates with disease activity in
patients with head and neck cancer. Clin Cancer Res. 23:4843–4854.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong CS, Funk S, Muller L, Boyiadzis M and
Whiteside TL: Isolation of biologically active and morphologically
intact exosomes from plasma of patients with cancer. J Extracell
Vesicles. 5:292892016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beccard IJ, Hofmann L, Schroeder JC,
Ludwig S, Laban S, Brunner C, Lotfi R, Hoffmann TK, Jackson EK,
Schuler PJ and Theodoraki MN: Immune suppressive effects of
plasma-derived exosome populations in head and neck cancer. Cancers
(Basel). 12:19972020. View Article : Google Scholar
|
|
12
|
Thery C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol. Chapter
3: Unit 3.22. 2006. View Article : Google Scholar
|
|
13
|
Bianco NR, Kim SH, Morelli AE and Robbins
PD: Modulation of the immune response using dendritic cell-derived
exosomes. Methods Mol Biol. 380:443–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theodoraki MN, Yerneni SS, Brunner C,
Theodorakis J, Hoffmann TK and Whiteside TL: Plasma-derived
exosomes reverse epithelial-to-mesenchymal transition after
photodynamic therapy of patients with head and neck cancer.
Oncoscience. 5:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SJ, Tsui PF, Chuang YP, Chiang DML,
Chen LW, Liu ST, Lin FY, Huang SM, Lin SH, Wu WL, et al: Carvedilol
ameliorates experimental atherosclerosis by regulating cholesterol
efflux and exosome functions. Int J Mol Sci. 20:52022019.
View Article : Google Scholar :
|
|
16
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. John Wiley & Sons,
Inc; Chichester, West Sussex, UK ; Hoboken, NJ: 2017
|
|
17
|
Gomes J, Gomes-Alves P, Carvalho SB,
Peixoto C, Alves PM, Altevogt P and Costa J: Extracellular vesicles
from ovarian carcinoma cells display specific glycosignatures.
Biomolecules. 5:1741–1761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capello M, Vykoukal JV, Katayama H, Bantis
LE, Wang H, Kundnani DL, Aguilar-Bonavides C, Aguilar M, Tripathi
SC, Dhillon DS, et al: Exosomes harbor B cell targets in pancreatic
adenocarcinoma and exert decoy function against complement-mediated
cytotoxicity. Nat Commun. 10:2542019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kugeratski FG, Hodge K, Lilla S, McAndrews
KM, Zhou X, Hwang RF, Zanivan S and Kalluri R: Quantitative
proteomics identifies the core proteome of exosomes with syntenin-1
as the highest abundant protein and a putative universal biomarker.
Nat Cell Biol. 23:631–641. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bachurski D, Schuldner M, Nguyen PH, Malz
A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M
and von Strandmann EP: Extracellular vesicle measurements with
nanoparticle tracking analysis-An accuracy and repeatability
comparison between NanoSight NS300 and ZetaView. J Extracell
Vesicles. 8:15960162019. View Article : Google Scholar
|
|
21
|
Muller L, Mitsuhashi M, Simms P, Gooding
WE and Whiteside TL: Tumor-derived exosomes regulate expression of
immune function-related genes in human T cell subsets. Sci Rep.
6:202542016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller L, Simms P, Hong CS, Nishimura MI,
Jackson EK, Watkins SC and Whiteside TL: Human tumor-derived
exosomes (TEX) regulate Treg functions via cell surface signaling
rather than uptake mechanisms. Oncoimmunology. 6:e12612432017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thery C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Arche F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
24
|
Theodoraki MN, Yerneni SS, Hoffmann TK,
Gooding WE and Whiteside TL: Clinical significance of PD-L1(+)
exosomes in plasma of head and neck cancer patients. Clin Cancer
Res. 24:896–905. 2018. View Article : Google Scholar
|
|
25
|
Karimi N, Cvjetkovic A, Jang SC,
Crescitelli R, Feizi MAH, Nieuwland R, Lötvall J and Lässer C:
Detailed analysis of the plasma extracellular vesicle proteome
after separation from lipoproteins. Cell Mol Life Sci.
75:2873–2886. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teng Y, Gao L, Loveless R, Rodrigo JP,
Strojan P, Willems SM, Nathan CA, Mäkitie AA, Saba NF and Ferlito
A: The hidden link of exosomes to head and neck cancer. Cancers
(Basel). 13:58022021. View Article : Google Scholar
|
|
27
|
Whiteside TL: Immune modulation of T-cell
and NK (natural killer) cell activities by TEXs (tumour-derived
exosomes). Biochem Soc Trans. 41:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T lymphocytes. J
Immunol. 183:3720–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Canavan JB, Afzali B, Scotta C, Fazekasova
H, Edozie FC, Macdonald TT, Hernandez-Fuentes MP, Lombardi G and
Lord GM: A rapid diagnostic test for human regulatory T-cell
function to enable regulatory T-cell therapy. Blood. 119:e57–e66.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruitenberg JJ, Boyce C, Hingorani R,
Putnam A and Ghanekar SA: Rapid assessment of in vitro expanded
human regulatory T cell function. J Immunol Methods. 372:95–106.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ebnoether E and Muller L: Diagnostic and
therapeutic applications of exosomes in cancer with a special focus
on head and neck squamous cell carcinoma (HNSCC). Int J Mol Sci.
21:43442020. View Article : Google Scholar :
|
|
32
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krishnamoorthy L, Bess JW Jr, Preston AB,
Nagashima K and Mahal LK: HIV-1 and microvesicles from T cells
share a common glycome, arguing for a common origin. Nat Chem Biol.
5:244–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams C, Royo F, Aizpurua-Olaizola O,
Pazos R, Boons GJ, Reichardt NC and Falcon-Perez JM: Glycosylation
of extracellular vesicles: Current knowledge, tools and clinical
perspectives. J Extracell Vesicles. 7:14429852018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sodar BW, Kittel A, Pálóczi K, Vukman KV,
Osteikoetxea X, Szabó-Taylor K, Németh A, Sperlágh B, Baranyai T,
Giricz Z, et al: Low-density lipoprotein mimics blood
plasma-derived exosomes and microvesicles during isolation and
detection. Sci Rep. 6:243162016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Otahal A, Kuten-Pella O, Kramer K,
Neubauer M, Lacza Z, Nehrer S and Luna AD: Functional repertoire of
EV-associated miRNA profiles after lipoprotein depletion via
ultracentrifugation and size exclusion chromatography from
autologous blood products. Sci Rep. 11:58232021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Borg EGF, Liaci AM, Vos HR and
Stoorvogel W: A novel three step protocol to isolate extracellular
vesicles from plasma or cell culture medium with both high yield
and purity. J Extracell Vesicles. 9:17914502020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Niel G, Bergam P, Di Cicco A, Hurbain
I, Cicero AL, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers
LJ, et al: Apolipoprotein E regulates amyloid formation within
endosomes of pigment cells. Cell Rep. 13:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Jiang P, Wei S, Xu X and Wang J:
Regulatory T cells in tumor microenvironment: New mechanisms,
potential therapeutic strategies and future prospects. Mol Cancer.
19:1162020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan W and Jiang S: Immune cell-derived
exosomes in the cancer-immunity cycle. Trends Cancer. 6:506–517.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li I and Nabet BY: Exosomes in the tumor
microenvironment as mediators of cancer therapy resistance. Mol
Cancer. 18:322019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang C and Robbins PD: The roles of
tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol.
2011:8428492011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Paijens ST, Vledder A, de Bruyn M and
Nijman HW: Tumor-infiltrating lymphocytes in the immunotherapy era.
Cell Mol Immunol. 18:842–859. 2021. View Article : Google Scholar :
|
|
45
|
Spector ME, Bellile E, Amlani L, Zarins K,
Smith J, Brenner JC, Rozek L, Nguyen A, Thomas D, McHugh JB, et al:
Prognostic value of tumor-infiltrating lymphocytes in head and neck
squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg.
145:1012–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peltanova B, Raudenska M and Masarik M:
Effect of tumor micro-environment on pathogenesis of the head and
neck squamous cell carcinoma: A systematic review. Mol Cancer.
18:632019. View Article : Google Scholar
|
|
47
|
Uppaluri R, Dunn GP and Lewis JS Jr: Focus
on TILs: Prognostic significance of tumor infiltrating lymphocytes
in head and neck cancers. Cancer Immun. 8:162008.PubMed/NCBI
|
|
48
|
Andratschke M, Hagedorn H and Nerlich A:
Expression of the epithelial cell adhesion molecule and cytokeratin
8 in head and neck squamous cell cancer. A comparative study
Anticancer Res. 35:3953–3960. 2015.
|
|
49
|
Rajjoub S, Basha SR, Einhorn E, Cohen MC,
Marvel DM and Sewell DA: Prognostic significance of
tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose
Throat J. 86:506–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Badoual C, Hans S, Rodriguez J, Peyrard S,
Klein C, Agueznay NEH, Mosseri V, Laccourreye O, Bruneval P,
Fridman WH, et al: Prognostic value of tumor-infiltrating CD4+
T-cell subpopulations in head and neck cancers. Clin Cancer Res.
12:465–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hofmann L, Ludwig S, Vahl JM, Brunner C,
Hoffmann TK and Theodoraki MN: The emerging role of exosomes in
diagnosis, prognosis, and therapy in head and neck cancer. Int J
Mol Sci. 21:40722020. View Article : Google Scholar :
|
|
52
|
Economopoulou P, de Bree R, Kotsantis I
and Psyrri A: Diagnostic tumor markers in head and neck squamous
cell carcinoma (HNSCC) in the clinical setting. Front Oncol.
9:8272019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dahiya K and Dhankhar R: Updated overview
of current biomarkers in head and neck carcinoma. World J Methodol.
6:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Daassi D, Mahoney KM and Freeman GJ: The
importance of exosomal PDL1 in tumour immune evasion. Nat Rev
Immunol. 20:209–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schneider S, Kadletz L, Wiebringhaus R,
Kenner L, Selzer E, Füreder T, Rajky O, Berghoff AS, Preusser M and
Heiduschka G: PD-1 and PD-L1 expression in HNSCC primary cancer and
related lymph node metastasis-impact on clinical outcome.
Histopathology. 73:573–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland A, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Theodoraki MN, Matsumoto A, Beccard I,
Hoffmann TK and Whiteside TL: CD44v3 protein-carrying tumor-derived
exosomes in HNSCC patients' plasma as potential noninvasive
biomarkers of disease activity. Oncoimmunology. 9:17477322020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kooijmans SA, Vader P, van Dommelen SM,
van Solinge WW and Schiffelers RM: Exosome mimetics: A novel class
of drug delivery systems. Int J Nanomedicine. 7:1525–1541.
2012.PubMed/NCBI
|
|
60
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar
|
|
62
|
Tian Y, Li S, Song J, Ji T, Zhu M,
Anderson GJ, Wei J and Nie G: A doxorubicin delivery platform using
engineered natural membrane vesicle exosomes for targeted tumor
therapy. Biomaterials. 35:2383–2390. 2014. View Article : Google Scholar
|
|
63
|
Scholl JN, de Fraga Dias A, Pizzato PR,
Lopes DV, Moritz CEJ, Jandrey EHF, Souto GD, Colombo M, Rohden F,
Sévigny J, et al: Characterization and antiproliferative activity
of glioma-derived extracellular vesicles. Nanomedicine (Lond).
15:1001–1018. 2020. View Article : Google Scholar
|