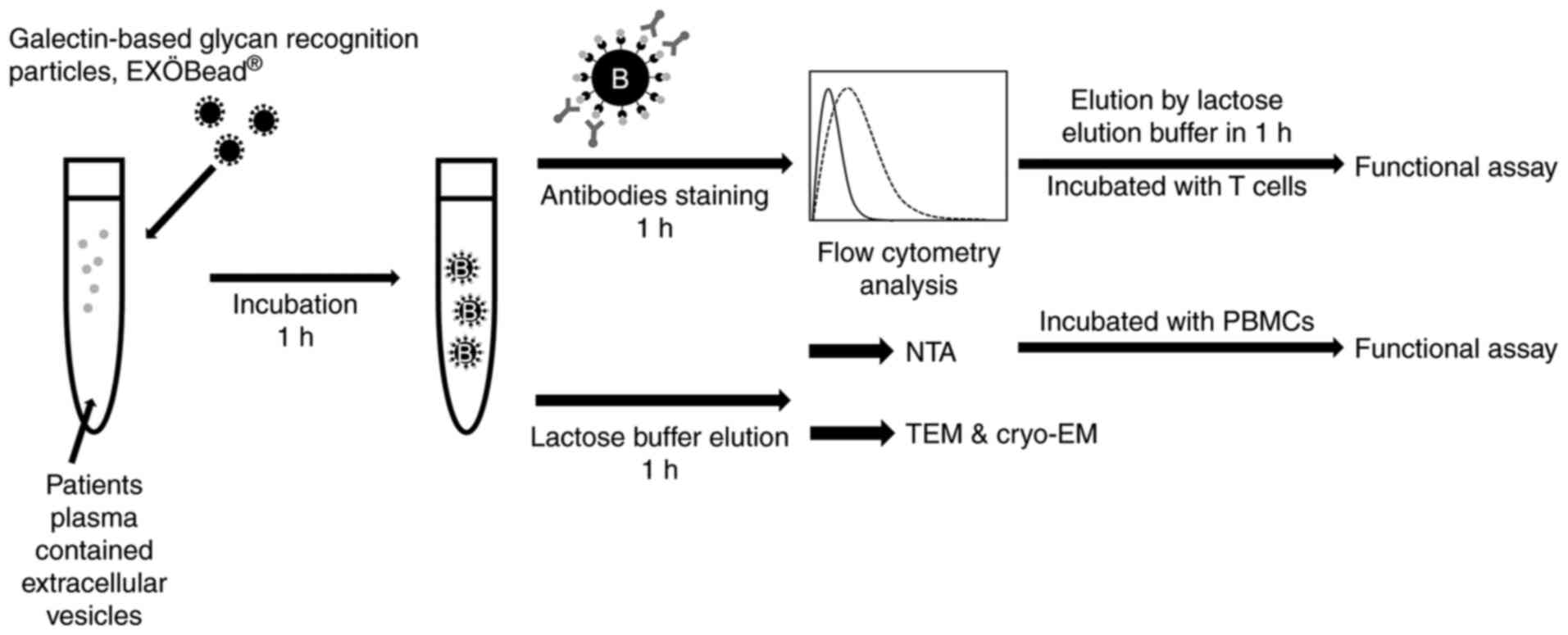
|
1
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopatina T, Favaro E, Danilova L, Fertig
EJ, Favorov AV, Kagohara LT, Martone T, Bussolati B, Romagnoli R,
Albera R, et al: Extracellular vesicles released by tumor
endothelial cells spread immunosuppressive and transforming signals
through various recipient cells. Front Cell Dev Biol. 8:6982020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao C, Song F, Zheng YL, Lv J, Wang QF
and Xu N: Exosomes in head and neck squamous cell carcinoma. Front
Oncol. 9:8942019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muller L, Hong CS, Stolz DB, Watkins SC
and Whiteside TL: Isolation of biologically-active exosomes from
human plasma. J Immunol Methods. 411:55–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theodoraki MN, Hoffmann TK, Jackson EK and
Whiteside TL: Exosomes in HNSCC plasma as surrogate markers of
tumour progression and immune competence. Clin Exp Immunol.
194:67–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ludwig S, Floros T, Theodoraki MN, Hong
CS, Jackson EK, Lang S and Whiteside TL: Suppression of lymphocyte
functions by plasma exosomes correlates with disease activity in
patients with head and neck cancer. Clin Cancer Res. 23:4843–4854.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong CS, Funk S, Muller L, Boyiadzis M and
Whiteside TL: Isolation of biologically active and morphologically
intact exosomes from plasma of patients with cancer. J Extracell
Vesicles. 5:292892016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beccard IJ, Hofmann L, Schroeder JC,
Ludwig S, Laban S, Brunner C, Lotfi R, Hoffmann TK, Jackson EK,
Schuler PJ and Theodoraki MN: Immune suppressive effects of
plasma-derived exosome populations in head and neck cancer. Cancers
(Basel). 12:19972020. View Article : Google Scholar
|
|
12
|
Thery C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol. Chapter
3: Unit 3.22. 2006. View Article : Google Scholar
|
|
13
|
Bianco NR, Kim SH, Morelli AE and Robbins
PD: Modulation of the immune response using dendritic cell-derived
exosomes. Methods Mol Biol. 380:443–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theodoraki MN, Yerneni SS, Brunner C,
Theodorakis J, Hoffmann TK and Whiteside TL: Plasma-derived
exosomes reverse epithelial-to-mesenchymal transition after
photodynamic therapy of patients with head and neck cancer.
Oncoscience. 5:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SJ, Tsui PF, Chuang YP, Chiang DML,
Chen LW, Liu ST, Lin FY, Huang SM, Lin SH, Wu WL, et al: Carvedilol
ameliorates experimental atherosclerosis by regulating cholesterol
efflux and exosome functions. Int J Mol Sci. 20:52022019.
View Article : Google Scholar :
|
|
16
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. John Wiley & Sons,
Inc; Chichester, West Sussex, UK ; Hoboken, NJ: 2017
|
|
17
|
Gomes J, Gomes-Alves P, Carvalho SB,
Peixoto C, Alves PM, Altevogt P and Costa J: Extracellular vesicles
from ovarian carcinoma cells display specific glycosignatures.
Biomolecules. 5:1741–1761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capello M, Vykoukal JV, Katayama H, Bantis
LE, Wang H, Kundnani DL, Aguilar-Bonavides C, Aguilar M, Tripathi
SC, Dhillon DS, et al: Exosomes harbor B cell targets in pancreatic
adenocarcinoma and exert decoy function against complement-mediated
cytotoxicity. Nat Commun. 10:2542019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kugeratski FG, Hodge K, Lilla S, McAndrews
KM, Zhou X, Hwang RF, Zanivan S and Kalluri R: Quantitative
proteomics identifies the core proteome of exosomes with syntenin-1
as the highest abundant protein and a putative universal biomarker.
Nat Cell Biol. 23:631–641. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bachurski D, Schuldner M, Nguyen PH, Malz
A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M
and von Strandmann EP: Extracellular vesicle measurements with
nanoparticle tracking analysis-An accuracy and repeatability
comparison between NanoSight NS300 and ZetaView. J Extracell
Vesicles. 8:15960162019. View Article : Google Scholar
|
|
21
|
Muller L, Mitsuhashi M, Simms P, Gooding
WE and Whiteside TL: Tumor-derived exosomes regulate expression of
immune function-related genes in human T cell subsets. Sci Rep.
6:202542016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller L, Simms P, Hong CS, Nishimura MI,
Jackson EK, Watkins SC and Whiteside TL: Human tumor-derived
exosomes (TEX) regulate Treg functions via cell surface signaling
rather than uptake mechanisms. Oncoimmunology. 6:e12612432017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thery C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Arche F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
24
|
Theodoraki MN, Yerneni SS, Hoffmann TK,
Gooding WE and Whiteside TL: Clinical significance of PD-L1(+)
exosomes in plasma of head and neck cancer patients. Clin Cancer
Res. 24:896–905. 2018. View Article : Google Scholar
|
|
25
|
Karimi N, Cvjetkovic A, Jang SC,
Crescitelli R, Feizi MAH, Nieuwland R, Lötvall J and Lässer C:
Detailed analysis of the plasma extracellular vesicle proteome
after separation from lipoproteins. Cell Mol Life Sci.
75:2873–2886. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teng Y, Gao L, Loveless R, Rodrigo JP,
Strojan P, Willems SM, Nathan CA, Mäkitie AA, Saba NF and Ferlito
A: The hidden link of exosomes to head and neck cancer. Cancers
(Basel). 13:58022021. View Article : Google Scholar
|
|
27
|
Whiteside TL: Immune modulation of T-cell
and NK (natural killer) cell activities by TEXs (tumour-derived
exosomes). Biochem Soc Trans. 41:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T lymphocytes. J
Immunol. 183:3720–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Canavan JB, Afzali B, Scotta C, Fazekasova
H, Edozie FC, Macdonald TT, Hernandez-Fuentes MP, Lombardi G and
Lord GM: A rapid diagnostic test for human regulatory T-cell
function to enable regulatory T-cell therapy. Blood. 119:e57–e66.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruitenberg JJ, Boyce C, Hingorani R,
Putnam A and Ghanekar SA: Rapid assessment of in vitro expanded
human regulatory T cell function. J Immunol Methods. 372:95–106.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ebnoether E and Muller L: Diagnostic and
therapeutic applications of exosomes in cancer with a special focus
on head and neck squamous cell carcinoma (HNSCC). Int J Mol Sci.
21:43442020. View Article : Google Scholar :
|
|
32
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krishnamoorthy L, Bess JW Jr, Preston AB,
Nagashima K and Mahal LK: HIV-1 and microvesicles from T cells
share a common glycome, arguing for a common origin. Nat Chem Biol.
5:244–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams C, Royo F, Aizpurua-Olaizola O,
Pazos R, Boons GJ, Reichardt NC and Falcon-Perez JM: Glycosylation
of extracellular vesicles: Current knowledge, tools and clinical
perspectives. J Extracell Vesicles. 7:14429852018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sodar BW, Kittel A, Pálóczi K, Vukman KV,
Osteikoetxea X, Szabó-Taylor K, Németh A, Sperlágh B, Baranyai T,
Giricz Z, et al: Low-density lipoprotein mimics blood
plasma-derived exosomes and microvesicles during isolation and
detection. Sci Rep. 6:243162016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Otahal A, Kuten-Pella O, Kramer K,
Neubauer M, Lacza Z, Nehrer S and Luna AD: Functional repertoire of
EV-associated miRNA profiles after lipoprotein depletion via
ultracentrifugation and size exclusion chromatography from
autologous blood products. Sci Rep. 11:58232021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Borg EGF, Liaci AM, Vos HR and
Stoorvogel W: A novel three step protocol to isolate extracellular
vesicles from plasma or cell culture medium with both high yield
and purity. J Extracell Vesicles. 9:17914502020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Niel G, Bergam P, Di Cicco A, Hurbain
I, Cicero AL, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers
LJ, et al: Apolipoprotein E regulates amyloid formation within
endosomes of pigment cells. Cell Rep. 13:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Jiang P, Wei S, Xu X and Wang J:
Regulatory T cells in tumor microenvironment: New mechanisms,
potential therapeutic strategies and future prospects. Mol Cancer.
19:1162020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan W and Jiang S: Immune cell-derived
exosomes in the cancer-immunity cycle. Trends Cancer. 6:506–517.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li I and Nabet BY: Exosomes in the tumor
microenvironment as mediators of cancer therapy resistance. Mol
Cancer. 18:322019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang C and Robbins PD: The roles of
tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol.
2011:8428492011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Paijens ST, Vledder A, de Bruyn M and
Nijman HW: Tumor-infiltrating lymphocytes in the immunotherapy era.
Cell Mol Immunol. 18:842–859. 2021. View Article : Google Scholar :
|
|
45
|
Spector ME, Bellile E, Amlani L, Zarins K,
Smith J, Brenner JC, Rozek L, Nguyen A, Thomas D, McHugh JB, et al:
Prognostic value of tumor-infiltrating lymphocytes in head and neck
squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg.
145:1012–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peltanova B, Raudenska M and Masarik M:
Effect of tumor micro-environment on pathogenesis of the head and
neck squamous cell carcinoma: A systematic review. Mol Cancer.
18:632019. View Article : Google Scholar
|
|
47
|
Uppaluri R, Dunn GP and Lewis JS Jr: Focus
on TILs: Prognostic significance of tumor infiltrating lymphocytes
in head and neck cancers. Cancer Immun. 8:162008.PubMed/NCBI
|
|
48
|
Andratschke M, Hagedorn H and Nerlich A:
Expression of the epithelial cell adhesion molecule and cytokeratin
8 in head and neck squamous cell cancer. A comparative study
Anticancer Res. 35:3953–3960. 2015.
|
|
49
|
Rajjoub S, Basha SR, Einhorn E, Cohen MC,
Marvel DM and Sewell DA: Prognostic significance of
tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose
Throat J. 86:506–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Badoual C, Hans S, Rodriguez J, Peyrard S,
Klein C, Agueznay NEH, Mosseri V, Laccourreye O, Bruneval P,
Fridman WH, et al: Prognostic value of tumor-infiltrating CD4+
T-cell subpopulations in head and neck cancers. Clin Cancer Res.
12:465–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hofmann L, Ludwig S, Vahl JM, Brunner C,
Hoffmann TK and Theodoraki MN: The emerging role of exosomes in
diagnosis, prognosis, and therapy in head and neck cancer. Int J
Mol Sci. 21:40722020. View Article : Google Scholar :
|
|
52
|
Economopoulou P, de Bree R, Kotsantis I
and Psyrri A: Diagnostic tumor markers in head and neck squamous
cell carcinoma (HNSCC) in the clinical setting. Front Oncol.
9:8272019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dahiya K and Dhankhar R: Updated overview
of current biomarkers in head and neck carcinoma. World J Methodol.
6:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Daassi D, Mahoney KM and Freeman GJ: The
importance of exosomal PDL1 in tumour immune evasion. Nat Rev
Immunol. 20:209–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schneider S, Kadletz L, Wiebringhaus R,
Kenner L, Selzer E, Füreder T, Rajky O, Berghoff AS, Preusser M and
Heiduschka G: PD-1 and PD-L1 expression in HNSCC primary cancer and
related lymph node metastasis-impact on clinical outcome.
Histopathology. 73:573–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland A, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Theodoraki MN, Matsumoto A, Beccard I,
Hoffmann TK and Whiteside TL: CD44v3 protein-carrying tumor-derived
exosomes in HNSCC patients' plasma as potential noninvasive
biomarkers of disease activity. Oncoimmunology. 9:17477322020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kooijmans SA, Vader P, van Dommelen SM,
van Solinge WW and Schiffelers RM: Exosome mimetics: A novel class
of drug delivery systems. Int J Nanomedicine. 7:1525–1541.
2012.PubMed/NCBI
|
|
60
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar
|
|
62
|
Tian Y, Li S, Song J, Ji T, Zhu M,
Anderson GJ, Wei J and Nie G: A doxorubicin delivery platform using
engineered natural membrane vesicle exosomes for targeted tumor
therapy. Biomaterials. 35:2383–2390. 2014. View Article : Google Scholar
|
|
63
|
Scholl JN, de Fraga Dias A, Pizzato PR,
Lopes DV, Moritz CEJ, Jandrey EHF, Souto GD, Colombo M, Rohden F,
Sévigny J, et al: Characterization and antiproliferative activity
of glioma-derived extracellular vesicles. Nanomedicine (Lond).
15:1001–1018. 2020. View Article : Google Scholar
|