Breast cancer (BC) has gradually replaced lung
cancer as the most prevalent cancer type (1). BC is divided into four subtypes based
on the expression status of estrogen receptor (ER), progesterone
receptor (PR) and human epidermal growth factor receptor-2 (HER2)
and antigen Ki-67 detected by immunohistochemistry: Luminal A,
Luminal B, HER2-positive and triple-negative subtypes (2,3).
Luminal A BC (ER+ and/or PR+, and HER2-, Ki-67 <14%) is
characterized by high differentiation, slow growth and the best
prognosis (4). Luminal B BC (ER+
and/or PR+, and HER2+ or HER2-, Ki-67 >14%) is sensitive to
endocrine therapy and has a good prognosis (5). Triple-negative BC (ER- and PR-, and
HER2-) is associated with short overall survival (OS) and
unfavorable prognosis (6).
HER2-positive BC (ER- and PR- and HER2+) is characterized by high
aggressiveness, poor prognosis and chemotherapeutic resistance
(7,8). HER/erythroblastic leukemia viral
oncogene homolog (ERBB) is a member of the receptor tyrosine kinase
signaling family, which includes HER1/ERBB1, HER2/ERBB2, HER3/ERBB3
and HER4/ERBB4 (9). In numerous
types of malignant tumor, HER/ERBB family members exhibit
overexpression, amplification or mutation, with effects on cell
proliferation, migration, differentiation and apoptosis (10-13).
HER2-positive BC is attributed to ERBB2/neu amplification or HER2
transmembrane receptor protein overexpression, which affects 15-20%
of patients with BC (14).
Currently, trastuzumab is the primary treatment; other targeted
drugs [e.g., pertuzumab, neratinib, trastuzumab emtansine (T-DM1)
and trastuzumab deruxtecan (T-DXd)] are also used in clinical
treatment (15-18). However, drug resistance occurs in
numerous patients after treatment (19). Cancer cell escape from drug
treatment is related to the activities of BC stem cells (BCSCs),
which exhibit properties of self-renewal, infinite proliferation
and multidirectional differentiation capacities necessary for the
metastasis and recurrence of BC (20). In the present review, therapies for
HER2-positive BC and the roles of BCSCs in HER2-positive BC
treatment resistance were discussed. Recent research concerning
BCSCs and related signaling pathways that may serve as therapeutic
targets were also summarized, with the intention of providing a
basis for inhibiting tumorigenesis and the development of
HER2-positive BC.
In the clinical treatment of HER2-positive BC, three
main types of drugs are used: Monoclonal antibodies (e.g.,
trastuzumab and pertuzumab), small-molecule tyrosine kinase
inhibitors (TKIs; e.g., lapatinib, neratinib and tucatinib), and
antibody-drug conjugates (T-DM1 and T-DXd) (Table I) (21).
Trastuzumab, a humanized antibody that acts on the
extracellular domain IV region of the HER2 receptor (22), has demonstrated robust efficacy in
the targeted treatment of HER2-positive BC over the past 20 years
(23). Trastuzumab promotes cell
apoptosis by inhibiting HER2 exocytosis, blocking the PI3K/AKT
pathway and activating antibody-dependent cytotoxicity (24). Pertuzumab acts on region II of the
HER2 receptor to block ligand-dependent HER2 heterodimer formation,
thereby reducing HER2 intracellular signaling and inhibiting the
proliferation and invasion of tumor cells (25).
Lapatinib is a dual-target TKI that acts on HER1/2
to inhibit the activation of downstream effectors (MAPK and AKT),
leading to cell growth arrest and the acceleration of tumor cell
regression (26,27). In addition, neratinib is an oral
irreversible inhibitor that acts on HER1/2/4 to suppress the
phosphorylation of MAPK and AKT, thereby attenuating cancer cell
proliferation (28). Neratinib has
been proven to inhibit the trastuzumab-induced upregulation of HER4
and enhance sensitivity to trastuzumab by limiting the activity of
HER4 tyrosine kinase (29).
Furthermore, neratinib may improve the 2-year disease-free survival
in patients with early-stage HER2-positive BC (30). Another reversible, highly selective
TKI is tucatinib, which acts on the intracellular tyrosine kinase
region of the HER2 receptor (31)
to inhibit signal transduction downstream of HER2/3 via the MAPK
and PI3K/AKT pathways (32). A
phase III trial indicated that tucatinib plus capecitabine and
trastuzumab significantly prolonged progression-free survival and
overall survival in patients with HER2-positive BC (33).
T-DM1 is an antibody-drug conjugate formed by
conjugating trastuzumab to the cytotoxic drug emtansine (i.e., DM1)
using a linker (34). T-DM1
retains trastuzumab activity and simultaneously induces apoptosis
by delivering the microtubule inhibitor DM1 to HER2-overexpressing
tumor cells (35). T-DXd is a
novel antibody-drug conjugate composed of trastuzumab and the
topoisomerase type I inhibitor DXd using a linker (36). T-DXd has a high drug-to-antibody
ratio and favorable membrane permeability. In addition, DXd may
induce DNA fragmentation. Thus, T-DXd exhibits a robust killing
effect on HER2-overexpressing tumor cells (37).
Although various targeted drugs are effective,
numerous patients subsequently exhibit primary or acquired drug
resistance, leading to accelerated disease progression (38). Thus, there is considerable interest
in identifying effective therapies for the management of drug
resistance.
BCSCs may be characterized by the distribution of
biomarkers on the cell membrane, such as CD44, CD24, acetaldehyde
dehydrogenase (ALDH)1 and CD133 (44). The membrane glycoproteins CD44 and
CD24 are promising BCSC biomarkers. CD44 interacts with its primary
ligand hyaluronic acid to activate various signaling pathways,
which participate in cell proliferation and invasion (45,46).
Due to its rarity, CD24 expression in BCSCs is usually assessed in
combination with CD44 expression. The
CD44+/CD24−/low phenotype is a classical BCSC
biomarker that may be used to assess distant metastasis, recurrence
and prognosis (47). Furthermore,
the plasticity of BCSCs enables them to switch between
epithelial-mesenchymal transition (EMT, mesenchymal-like state) and
mesenchymal-epithelial transition (epithelial-like state), leading
to tumor invasion and metastasis (48). It has been reported that
mesenchymal-like CD44+/CD24−/low cells may be
responsible for the resistance of HER2-positive BC to trastuzumab
(49). ALDH1, a cellular lipase
present in cells capable of self-renewal and multilineage
differentiation, is an important BCSC biomarker (50). Liu et al (51) demonstrated that ALDH1 expression
was positively correlated with breast tumor growth. BCSCs exhibit
dormant and proliferative states; dormant BCSCs are more resistant
to antimitotic drugs (52).
Another study indicated that mesenchymal BCSCs with high
CD44+/CD24− expression were in the dormant
state, whereas epithelioid BCSCs with high ALDH+
expression were in the proliferative state (48). In the past 10 years,
CD44+CD24−/low ALDH+ expression
has been used as a specific BCSC biomarker, particularly for
HER2-positive BC (53). The
population of CD44+/CD24−/low phenotype BCSCs
significantly increases in HER2-positive MDA-MB-435 cells than
other cell lines (54).
CD133+, also known as prominin-1, is associated with
poor prognosis, angiogenesis, lymph node metastasis and HER2
positivity in BC (55,56). EPHA5−, a receptor
tyrosine kinase, is able to increase BCSC properties and increase
the resistance of HER2-positive BC to trastuzumab (56). Collectively, BCSC phenotypes are
closely connected to the development of HER2-positive BC. Thus,
specific phenotypic BCSC-targeted therapies may be a promising
approach to overcome BC and treatment resistance.
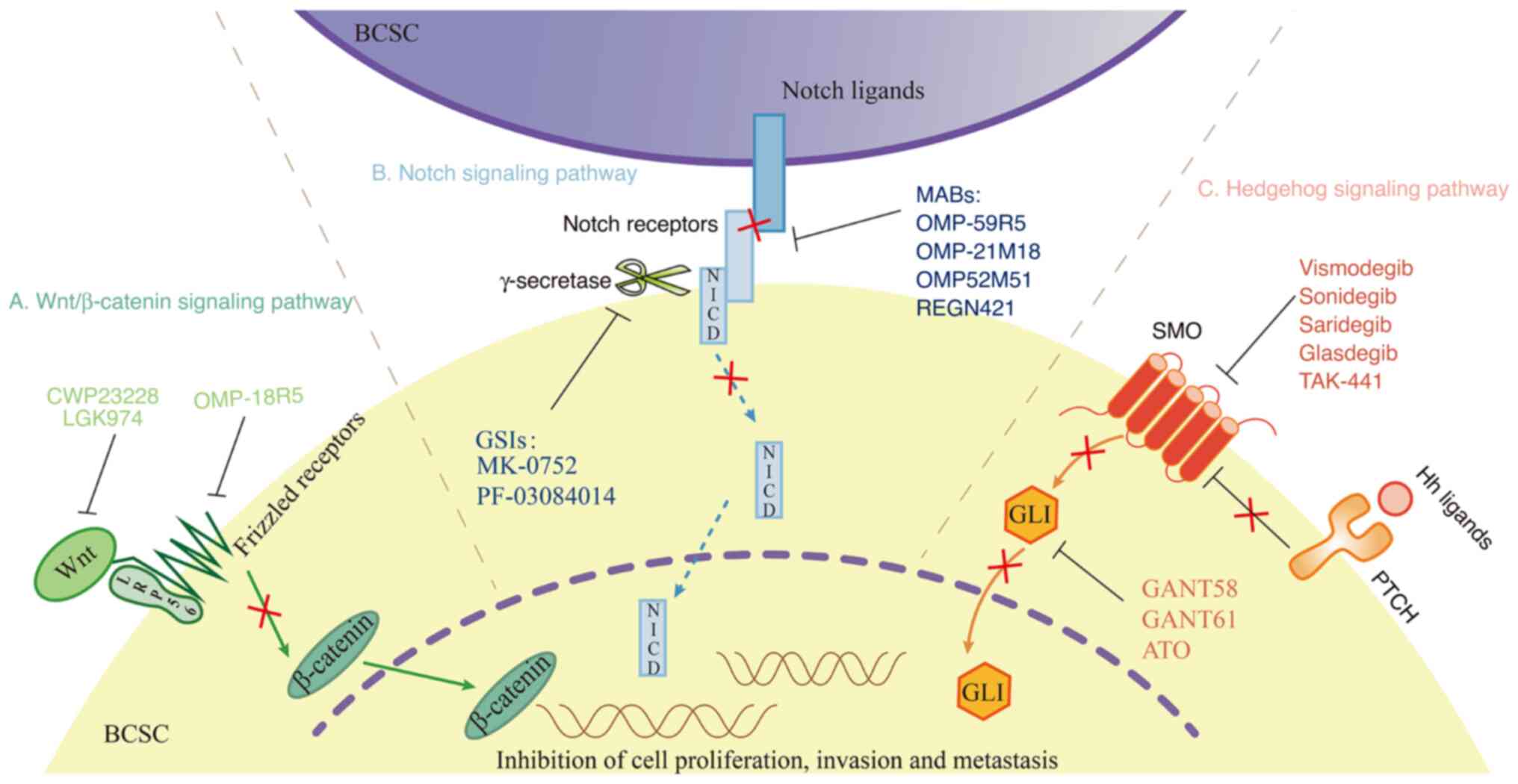
Several signaling pathways are involved in shaping
the properties of BCSCs, including the Wnt/β-catenin, Notch and
Hedgehog pathways. Pathway dysregulation or aberrant activation
induces abnormal BCSC proliferation, leading to reduced sensitivity
to drug therapy and enhancement of BC development (Fig. 1). Thus, a deep understanding of
these pathways may lead to the discovery of novel targeted
therapies.
The Wnt/β-catenin signaling pathway is associated
with the proliferation, migration and chemotherapeutic resistance
of BCSCs. Wnt proteins usually bind to Frizzled receptors (i.e.,
G-protein-coupled receptors) and low-density-lipoprotein
receptor-related protein 5 or 6 (LRP5/6) to form Wnt-FZD-LRP5/6
trimeric complexes in an autocrine or paracrine manner, leading to
catenin stabilization (Fig. 1A)
(57). Activation of the
Wnt/β-catenin pathway promotes EMT, treatment resistance and
self-renewal in BCSCs (58). Wu
et al (59) suggested that
upregulated Wnt3 activated the Wnt/β-catenin signaling pathway that
may lead to trastuzumab resistance in HER2-positive BC cells.
Cyclin-dependent kinase 12 may induce proliferation and tumor
recurrence in BCSCs through effects on the Wnt/β-catenin pathway,
leading to low trastuzumab efficacy in the treatment of
HER2-positive BC (60).
Furthermore, high RNA expression levels of Wnt in BCSCs led to an
increased metastatic rate and shortened the overall survival of
patients (61).
The Notch signaling pathway has four receptors
(Notch1-4) and five associated ligands [Jagged-1-2 and Delta-like
ligand (DLL)-1-4] (Fig. 1B)
(62). The Notch pathway is
closely associated with BC occurrence and progression. Through
ligand-receptor binding interactions, aberrant Notch activation
promotes aggressiveness and drug resistance in BCSCs. Baker et
al (63) found that Notch-1
maintained BCSC survival by inhibiting phosphatase and tensin
homolog, which led to drug resistance in HER2-positive BC cells.
Pandya et al (64) reported
that protein kinase C-α reversed trastuzumab resistance in
HER2-positive BC by inhibiting Jagged-1-mediated notch
signaling.
The Hedgehog signaling pathway consists of three
ligands (Sonic, desert and Indian hedgehog), two receptors [Patched
(PTCH) and smoothened (SMO)], and the glioma-associated oncogene
transcription factors (GLI)1-3 (Fig.
1C). He et al (65)
found that PTCH, SMO, GLI1 and GLI2 were significantly upregulated
in BCSC-enriched MCF-7 mammosphere cells. High GLI1 expression is
associated with trastuzumab resistance and poor prognosis in
HER2-positive BC (66). Gupta
et al (67) demonstrated
that silencing of the GLI2 gene inhibited HER2-positive BC invasion
and metastasis. Doheny et al (68) reported that knockdown of SMO
inhibited BCSC growth, suggesting that Hedgehog pathway inhibitors
may be useful in BCSC-targeted therapy. In addition, further signal
transduction pathways, including the Hippo (69), TGF-β (70), JAK2/STAT3 (71) and PI3K/AKT/mTOR (72) pathways, are closely associated with
BCSCs through their effects on BC occurrence and progression.
Increasing evidence has indicated that BCSCs
accelerate BC progression due to their stem cell properties, drug
resistance and immune evasion (73). In the following chapter, the
mechanisms of the involvement of BCSCs in the treatment resistance
of HER2-positive BC is discussed. There are several possible
mechanisms BCSCs participate in to induce HER2-positive BC
resistance, including the tumor microenvironment, ABC transporters
and non-coding RNAs.
The BCSC microenvironment mainly consists of
cytokines, the extracellular matrix (ECM), vascular
microenvironment and bone marrow microenvironment. Cytokines (e.g.,
IL-6, IL-8 and TGF-β), are secreted by cancer-associated
fibroblasts (CAFs), endothelial cells (ECs), mesenchymal stem cells
(MSCs) and tumor-associated macrophages, regulating drug resistance
by activating BCSC-related signaling pathways (74-76).
Mao et al (77)
demonstrated that CAFs induce trastuzumab resistance by secreting
IL-6 to expand BCSCs and activate multiple pathways in
HER2-positive BC (Fig. 2A). The
ECM forms a protective membrane at the periphery of a cluster of
cancer cells; this physical barrier weakens drug penetration and
protects BCSCs from drug elimination (78). Collagen is the main structural
protein in the ECM, and collagen type I α1 (COL1A1) promotes cell
proliferation and drug resistance in BC (Fig. 2B) (79). Hanker et al (80) indicated high COL1A2 expression was
related to lower clinical response to trastuzumab by regulating
PI3K/AKT signaling in patients with HER2-positive BC. The ECM also
regulates the ability of BCSCs to boost growth and survival,
thereby contributing to therapeutic resistance (81). Through their multidirectional
differentiation potential, BCSCs may differentiate into ECs, which
allows participation in angiogenesis and alteration of the vascular
microenvironment (Fig. 2C)
(82). Hori et al (83) found that HER2-positive BC cells
exhibit vasculogenic mimicry in the angiogenic microenvironment
after complete trastuzumab resistance. Additional studies have
demonstrated that increased expression of stemness markers, such as
octamer-binding transcription factor 4 (Oct4), aldehyde
dehydrogenase 1 (ALDH1) and CD44 in BCSCs promote BC cell growth
and treatment resistance (84-86).
In the bone marrow microenvironment, extracellular vesicles
released from MSCs may be internalized by BCSCs, promoting drug
resistance in BC cells (87). Kim
et al (88) reported that
the IL-6-JAK1-STAT3-Oct-4 signaling pathway in the bone marrow
microenvironment was able to convert non-BCSCs into BCSCs by
regulating BCSC-associated Oct-4 gene expression. In addition, the
hypoxia environment increased the population of BCSCs and induced
trastuzumab resistance in HER2-positive BC cells (89,90).
Lee et al (91) found that
hypoxia-inducible factor-1α promoted BCSC aggregation and tumor
recurrence. Furthermore, the expression levels of multiple BCSC
biomarkers [e.g., ATP-binding cassette G member 2 (ABCG2),
sex-determining region Y-box 2, Krüppel-like factor 4 and
CD44+/CD24−/low] are upregulated under
hypoxic conditions, contributing to increased drug resistance in BC
cells (Fig. 2D) (92).
ABC transporter overexpression is an important
factor that contributes to multidrug resistance in HER2-positive BC
(93). Through the drug discharge
pump mechanism, ABC transporters mediate intracellular drug outflow
and help to decrease intracellular drug concentrations, thereby
enhancing drug resistance in BCSCs (94). ABCG2, a representative member of
the ABC transporter family, has a vital role in the development of
multidrug resistance in HER2-positive BC (Fig. 2E) (95). Němcová-Fürstová et al
(96) indicated higher expression
of ABCG2 protein in paclitaxel-resistant SK-BR-3 cells.
Furthermore, inhibition of the Wnt pathway may attenuate ABCG2
expression (97). Overall, the
drug pump effects of ABC transporters facilitate drug resistance
among BCSCs in HER2-positive BC.
In the past 10 years, the involvement of non-coding
RNAs in HER2-positive BC resistance via regulation of BCSCs
(Fig. 2F) has attracted
considerable attention. Ye et al (98) found that microRNA (miR)-221 was
able to induce BCSC proliferation, thereby reducing the sensitivity
of HER2-positive BC to drug therapy. Elevated expression of long
non-coding RNAs [lncRNAs; e.g., LINC00578, LINC00668 and SEMA3B
antisense RNA 1 (SEMA3B-AS1)] in HER2-positive BC enhanced BCSC
stemness (99). In addition, the
expression levels of lncRNA H19 (100), lung cancer-associated transcript
1 (101) and terminal
differentiation-induced non-coding RNA (102) were observed to be higher in
HER2-positive BC tissues than in normal breast tissues. Conversely,
miR-375 and lncRNA growth-arrest-specific 5 attenuated the
proliferation and drug resistance capacities of tumor cells
(103,104). Numerous metabolic factors are
associated with BCSC involvement in HER2-positive BC resistance.
For instance, group XVI phospholipase A2, a promoter associated
with phospholipid metabolism, contributes to the maintenance of
BCSC characteristics and may serve as a BCSC biomarker (105). Pyruvate dehydrogenase kinase 1,
produced during glycolysis, significantly increases the numbers of
ALDH+ BCSCs and promotes BC progression (106). Fox et al (107) found that targeted HER2 therapy
led to the activation of nuclear factor erythroid 2-related factor
2 in dormant tumor cells by modulating redox potential and
nucleotide metabolism. DNA damage repair (DDR) is a prevalent
phenomenon in BCSCs, where it facilitates repair after reactive
oxygen species-mediated damage to DNA (108,109). Overexpression of poly[ADP-ribose]
polymerase 1 was reported to enhance tolerability to DNA damage in
trastuzumab-resistant HER2-positive BC (110). In addition, certain DNA damage
sensor proteins, such as the DNA-dependent protein kinase catalytic
subunit, the ataxia-telangiectasia-mutated kinase and the
ataxia-telangiectasia and Rad3-related kinase, are also involved in
the DDR (111). Therefore, the
inhibition of DDR signaling may enhance BCSC sensitivity to
chemotherapy and substantially improve the prognosis of
patients.
Mutations in the Notch signaling pathway regulate
the development of drug resistance among BCSCs. There are two main
types of Notch inhibitor: Notch receptor cleavage inhibitors [e.g.,
γ-secretase inhibitors (GSIs)] and monoclonal antibodies that
interfere with receptor-ligand binding. GSIs mainly include MK-0752
and PF-03084014. MK-0752 and PF-03084014 have demonstrated good
efficacy in clinical trials on the treatment of advanced BC
(118,119). Treatment with GSIs plus docetaxel
led to a reduction in the number of BCSCs, downregulation of
CD44+/CD24− and ALDH+ biomarkers
and a decrease in BC volume (118). Monoclonal antibodies against
Notch receptors or ligands include OMP-59R5, OMP-21M18, OMP-52M51
and REGN421, and their targets are Notch2/3, DLL-4, Notch1 and
DLL-4, respectively (119). These
drugs enhance antitumor activity when combined with typical
targeted agents (120-123). Li et al (56) demonstrated that
erythropoietin-producing hepatocellular receptor A5 inhibited BCSC
self-renewal via the Notch1 signaling pathway, thereby reducing the
risk of trastuzumab resistance in HER2-positive BC.
Hedgehog signaling pathway inhibitors may be
categorized as SMO inhibitors (vismodegib, sonidegib, saridegib,
glasdegib and TAK-441) and GLI inhibitors (GANT58, GANT61 and
arsenic trioxide) (124).
Vismodegib and sonidegib have been approved by the Food and Drug
Administration for the therapy of metastatic or recurrent basal
cell carcinoma; they significantly inhibit the spread of metastatic
cells and improve median patient survival (125). GANT58 and GANT61 are also in
preclinical studies (126). Liu
et al (127) reported that
cordycepin inhibited SMO receptors and GLI transcription factors,
thereby limiting BC cell growth and metastasis. Although several
inhibitors remain in the preclinical stage of investigation, these
new approaches may enhance the effectiveness of BCSC-targeted
resistance (128).
TAZ and YAP, two core transcription factors in the
Hippo signaling pathway, have essential roles in BC occurrence and
development. Inhibitors targeting TAZ/YAP may restrict BCSC
proliferation and tumorigenesis. Statins may inhibit TAZ/YAP
activity and block signaling transduction in the Hippo pathway
(129). Furthermore, numerous
preclinical studies on TGF-β inhibitors are underway, including the
investigation of recombinant RNA technology that may interfere with
TGF-β signaling to inhibit the proliferation and invasion of BC
cells (130). In addition, Wang
et al (131) demonstrated
that inhibition of the JAK2/STAT3 pathway led to downregulation of
the expression of key fatty acid β-oxidation enzymes in BCSCs,
restoring their sensitivity to chemotherapy. As drugs that target a
single signaling pathway may be insufficient for clinical needs,
diverse multitarget strategies are required for future treatment of
HER2-positive BC.
Dormant BCSCs may evade drug treatment and undergo
plastic transformation with proliferating cells, leading to
recurrence and metastasis. Non-coding RNAs may be involved in
converting dormant BCSCs into proliferative BCSCs (132). LncRNA-Na+-sulfate
cotransporter 1 is upregulated in dormant mesenchymal-like BCSCs,
where it contributes to a prolonged dormancy period and reduces
tumorigenicity (133). Similarly,
the combined effects of Src family kinase inhibitors and MEK1/2
inhibitors may extend dormancy in BCSCs and induce apoptosis to
prevent BC recurrence (134). In
addition, a Tet methylcy-tosine dioxygenase 2-targeted strategy was
observed to be able to transform dormant cells into active
proliferating cells, thus restoring chemotherapeutic sensitivity
(135). Therefore, therapeutic
exploitation of BCSC status involves directly eliminating dormant
cells or suppressing cell transition from dormancy to
proliferation.
As mentioned above, the BCSC microenvironment
participates in the onset of treatment resistance; therefore,
strategies targeting the BCSC microenvironment may be useful.
COL1A1 knockdown reduces cell proliferation and invasion, leading
to decreased expression of stemness markers (e.g., sex-determining
region Y-box2, octamer-binding transcription factor 4 and CD133)
that inhibit EMT and stem cell activity (136). Furthermore, abnormalities in the
vascular microenvironment may hinder therapeutic effects. Chen
et al (137) indicated
that erlotinib was able to normalize the tumor vascular system,
improve perfusion and oxygenation, and enhance the chemotherapeutic
effects of nanodrugs in a mouse model of BC. In addition, Kim et
al (138) reported that
AzCDF, a small molecule drug, was able to target BCSCs in a hypoxic
environment, blocking tumor growth and lowering tumorigenesis
rates. The inhibition of TGFβ-inducible protein expression improved
hypoxia and tumor angiogenesis, thereby reducing the number of
BCSCs and inhibiting cancer cell metastasis (139).
Apatinib significantly down-regulates the expression
of ABCG2 to inhibit BCSC proliferation (140). Wu et al (141) demonstrated that progesterone
increased BCSC sensitivity to drug treatment by modulating ABCG2
transcriptional activity, which led to decreased drug efflux.
Lapatinib was found to block ABCG2-mediated efflux in HER2-positive
BC cells (142). Elacridar, the
ABCG2-transporter inhibitor, enhances the therapeutic effect of
lapatinib on HER2-positive advanced and metastatic BC (143). Yi et al (144) indicated that pyrotinib was able
to inhibit the expression of ABCG2 to restore the sensitivity of
drug-resistant HER2-positive BC cells.
Metformin, an anti-diabetes drug, is able to
selectively kill BCSCs by inhibiting the PI3K/AKT/mTOR pathway and
improving BC sensitivity to drug therapy (105,145,146). The expression levels of IL-8 were
positively associated with BCSC activity; inhibition of the
chemokine receptors C-X-C motif chemokine receptor (CXCR)1/2 was
able to reduce the level of IL-8 (147). Therefore, small molecule
antagonists of CXCR1/2, in combination with HER2-targeted therapy,
have the potential to inhibit BCSC activity and prolong the
survival of patients with HER2-positive BC (148). The DDR is activated to repair DNA
damage in BCSCs and ATR is a major regulator of the DDR. Kim et
al (149) demonstrated that
AZD6738, an ATR inhibitor, considerably reduced DDR efficiency and
weakened BCSC formation in HER2-positive BC. Several types of DDR
inhibitors are currently in development.
Increasing evidence indicates that BCSCs have
critical roles in the treatment resistance and recurrence of
HER2-targeted therapy. An improved understanding of the mechanism
by which BCSCs contribute to drug resistance will help to prevent
breast tumor recurrence and drug resistance. The mechanism of drug
resistance of BCSCs is complex. Factors such as abnormal signaling
pathway activation, BCSC microenvironment, ABC transporters and
BCSC repair capacity may lead to BCSC proliferation and the onset
of drug resistance in HER2-positive BC. The development of
BCSCs-targeted treatment approaches is expected to improve the
effectiveness of HER2-positive BC. A variety of therapeutic
strategies have been implemented to eliminate or reduce BCSCs,
which may restore trastuzumab sensitivity in vitro and in
vivo. However, most of the therapies are still restricted to
laboratory investigation. Therefore, in future studies, it is
necessary to clarify new biological characteristics and molecular
mechanisms of BCSCs and develop combination therapy or multi-target
therapy to overcome and reverse the drug resistance of BCSCs,
ultimately improving the cure rate and reducing the recurrence rate
of HER2-positive BC.
Not applicable.
LX and QL were responsible for the
conceptualization, methodology and writing-original draft
preparation. FH and XS were responsible for supervision,
writing-reviewing and editing and funding acquisition. LZ, WD, KZ,
CK and NH were responsible for data curation and investigation.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by grants from the National Natural
Science Foundation of China (grant nos. 32101029, 82170865 and
81870593), Natural Science Foundation of Shandong Province of China
(grant no. ZR2020QB164) and Special Funds for Taishan Scholars
Project of Shandong Province (grant no. tsqn202211365) and Yuandu
scholars (grant no. 20212022).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hugh J, Hanson J, Cheang MC, Nielsen TO,
Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et
al: Breast cancer subtypes and response to docetaxel in
node-positive breast cancer: Use of an immunohistochemical
definition in the BCIRG 001 trial. J Clin Oncol. 27:1168–1176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prat A, Cheang MC, Martín M, Parker JS,
Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen
TO, et al: Prognostic significance of progesterone
receptor-positive tumor cells within immunohistochemically defined
luminal A breast cancer. J Clin Oncol. 31:203–209. 2013. View Article : Google Scholar
|
|
5
|
Raj-Kumar PK, Liu J, Hooke JA, Kovatich
AJ, Kvecher L, Shriver CD and Hu H: PCA-PAM50 improves consistency
between breast cancer intrinsic and clinical subtyping
reclassifying a subset of luminal A tumors as luminal B. Sci Rep.
9:79562019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagini S: Breast cancer: Current molecular
therapeutic targets and new players. Anticancer Agents Med Chem.
17:152–163. 2017. View Article : Google Scholar
|
|
7
|
Burstein HJ: The distinctive nature of
HER2-positive breast cancers. N Engl J Med. 353:1652–1654. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pernas S, Barroso-Sousa R and Tolaney SM:
Optimal treatment of early stage HER2-positive breast cancer.
Cancer. 124:4455–4466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pellat A, Vaquero J and Fouassier L: Role
of ErbB/HER family of receptor tyrosine kinases in cholangiocyte
biology. Hepatology. 67:762–773. 2018. View Article : Google Scholar
|
|
10
|
Reschke M, Mihic-Probst D, van der Horst
EH, Knyazev P, Wild PJ, Hutterer M, Meyer S, Dummer R, Moch H and
Ullrich A: HER3 is a determinant for poor prognosis in melanoma.
Clin Cancer Res. 14:5188–5197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saglam O, Xiong Y, Marchion DC, Strosberg
C, Wenham RM, Johnson JJ, Saeed-Vafa D, Cubitt C, Hakam A and
Magliocco AM: ERBB4 expression in ovarian serous carcinoma
resistant to platinum-based therapy. Cancer Control. 24:89–95.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z: ErbB receptors and cancer. Methods
Mol Biol. 1652:3–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe S, Yonesaka K, Tanizaki J,
Nonagase Y, Takegawa N, Haratani K, Kawakami H, Hayashi H, Takeda
M, Tsurutani J and Nakagawaet K: Targeting of the HER2/HER3
signaling axis overcomes ligand-mediated resistance to trastuzumab
in HER2-positive breast cancer. Cancer Med. 8:1258–1268. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cronin KA, Harlan LC, Dodd KW, Abrams JS
and Ballard-Barbash R: Population-based estimate of the prevalence
of HER-2 positive breast cancer tumors for early stage patients in
the US. Cancer Invest. 28:963–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab Emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar
|
|
16
|
Saura C, Oliveira M, Feng YH, Dai MS, Chen
SW, Hurvitz SA, Kim SB, Moy B, Delaloge S, Gradishar W, et al:
Neratinib plus capecitabine versus lapatinib plus capecitabine in
HER2-positive metastatic breast cancer previously treated with ≥2
HER2-directed regimens: Phase III NALA trial. J Clin Oncol.
38:3138–3149. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piccart M, Procter M, Fumagalli D, de
Azambuja E, Clark E, Ewer MS, Restuccia E, Jerusalem G, Dent S,
Reaby L, et al: Adjuvant Pertuzumab and trastuzumab in early
HER2-positive breast cancer in the APHINITY trial: 6 Years'
follow-up. J Clin Oncol. 39:1448–1457. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nader-Marta G, Martins-Branco D and de
Azambuja E: How we treat patients with metastatic HER2-positive
breast cancer. ESMO Open. 7:1003432022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Figueroa-Magalhães MC, Jelovac D, Connolly
R and Wolff AC: Treatment of HER2-positive breast cancer. Breast.
23:128–136. 2014. View Article : Google Scholar
|
|
20
|
Qiu Y, Yang L, Liu H and Luo X: Cancer
stem cell-targeted therapeutic approaches for overcoming
trastuzumab resistance in HER2-positive breast cancer. Stem Cells.
39:1125–1136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y: The root cause of drug resistance
in HER2-positive breast cancer and the therapeutic approaches to
overcoming the resistance. Pharmacol Ther. 218:1076772021.
View Article : Google Scholar :
|
|
22
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lambertini M, Pondé NF, Solinas C and de
Azambuja E: Adjuvant trastuzumab: A 10-year overview of its
benefit. Expert Rev Anticancer Ther. 17:61–74. 2017. View Article : Google Scholar
|
|
24
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: Mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCormack PL: Pertuzumab: A review of its
use for first-line combination treatment of HER2-positive
metastatic breast cancer. Drugs. 73:1491–1502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H,
Rusnak DW, Owens G, Alligood KJ and Spector NL: Anti-tumor activity
of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation
of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene.
21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hegde PS, Rusnak D, Bertiaux M, Alligood
K, Strum J, Gagnon R and Gilmer TM: Delineation of molecular
mechanisms of sensitivity to lapatinib in breast cancer cell lines
using global gene expression profiles. Mol Cancer Ther.
6:1629–1640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rabindran SK, Discafani CM, Rosfjord EC,
Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R,
Overbeek E, et al: Antitumor activity of HKI-272, an orally active,
irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res.
64:3958–3965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohd Nafi SN, Generali D, Kramer-Marek G,
Gijsen M, Strina C, Cappelletti M, Andreis D, Haider S, Li JL,
Bridges E, et al: Nuclear HER4 mediates acquired resistance to
trastuzumab and is associated with poor outcome in HER2 positive
breast cancer. Oncotarget. 5:5934–5949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kourie HR, Chaix M, Gombos A, Aftimos P
and Awada A: Pharmacodynamics, pharmacokinetics and clinical
efficacy of neratinib in HER2-positive breast cancer and breast
cancer with HER2 mutations. Expert Opin Drug Metab Toxicol.
12:947–957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borges VF, Ferrario C, Aucoin N, Falkson
C, Khan Q, Krop I, Welch S, Conlin A, Chaves J, Bedard PL, et al:
Tucatinib combined with Ado-trastuzumab emtansine in advanced
ERBB2/HER2-positive metastatic breast cancer: A phase 1b clinical
trial. JAMA Oncol. 4:1214–1220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kulukian A, Lee P, Taylor J, Rosler R, de
Vries P, Watson D, Forero-Torres A and Peterson S: Preclinical
activity of HER2-selective tyrosine kinase inhibitor tucatinib as a
single agent or in combination with trastuzumab or docetaxel in
solid tumor models. Mol Cancer Ther. 19:976–987. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murthy RK, Loi S, Okines A, Paplomata E,
Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, et
al: Tucatinib, trastuzumab, and capecitabine for HER2-positive
metastatic breast cancer. N Engl J Med. 382:597–609. 2020.
View Article : Google Scholar
|
|
34
|
Junttila TT, Li G, Parsons K, Phillips GL
and Sliwkowski MX: Trastuzumab-DM1 (T-DM1) retains all the
mechanisms of action of trastuzumab and efficiently inhibits growth
of lapatinib insensitive breast cancer. Breast Cancer Res Treat.
128:347–356. 2011. View Article : Google Scholar
|
|
35
|
Li G, Guo J, Shen BQ, Yadav DB, Sliwkowski
MX, Crocker LM, Lacap JA and Phillips G: Mechanisms of acquired
resistance to trastuzumab emtansine in breast cancer cells. Mol
Cancer Ther. 17:1441–1453. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagai Y, Oitate M, Shiozawa H and Ando O:
Comprehensive preclinical pharmacokinetic evaluations of
trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug
conjugate, in cynomolgus monkeys. Xenobiotica. 49:1086–1096. 2019.
View Article : Google Scholar
|
|
37
|
Ogitani Y, Aida T, Hagihara K, Yamaguchi
J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, et
al: DS-8201a, A novel HER2-targeting ADC with a Novel DNA
topoisomerase I inhibitor, demonstrates a promising antitumor
efficacy with differentiation from T-DM1. Clin Cancer Res.
22:5097–5108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Metzger-Filho O, Vora T and Awada A:
Management of metastatic HER2-positive breast cancer progression
after adjuvant trastuzumab therapy-current evidence and future
trends. Expert Opin Investig Drugs. 19(Suppl 1): S31–S39. 2010.
View Article : Google Scholar
|
|
39
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim YJ, Sung D, Oh E, Cho Y, Cho TM,
Farrand L, Seo JH and Kim JY: Flubendazole overcomes trastuzumab
resistance by targeting cancer stem-like properties and HER2
signaling in HER2-positive breast cancer. Cancer Lett. 412:118–130.
2018. View Article : Google Scholar
|
|
43
|
Seo AN, Lee HJ, Kim EJ, Jang MH, Kim YJ,
Kim JH, Kim SW, Ryu HS, Park IA, Im SA, et al: Expression of breast
cancer stem cell markers as predictors of prognosis and response to
trastuzumab in HER2-positive breast cancer. Br J Cancer.
114:1109–1116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
et al: Intrinsic resistance of tumorigenic breast cancer cells to
chemotherapy. J Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bourguignon L: Matrix hyaluronan-CD44
interaction activates MicroRNA and LncRNA signaling associated with
chemoresistance, invasion, and tumor progression. Front Oncol.
9:4922019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Y, Song J, Jiang Y, Yu C and Ma Z:
Predictive value of CD44 and CD24 for prognosis and chemotherapy
response in invasive breast ductal carcinoma. Int J Clin Exp
Pathol. 8:11287–11295. 2015.PubMed/NCBI
|
|
48
|
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu
Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, et al:
Breast cancer stem cells transition between epithelial and
mesenchymal states reflective of their normal counterparts. Stem
Cell Reports. 2:78–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Oliveras-Ferraros C, Vazquez-Martin A,
Martin-Castillo B, Cufí S, Del Barco S, Lopez-Bonet E, Brunet J and
Menendez JA: Dynamic emergence of the mesenchymal
CD44(pos)CD24(neg/low) phenotype in HER2-gene amplified breast
cancer cells with de novo resistance to trastuzumab (Herceptin).
Biochem Biophys Res Commun. 397:27–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
51
|
Liu C, Qiang J, Deng Q, Xia J, Deng L,
Zhou L, Wang D, He X, Liu Y, Zhao B, et al: ALDH1A1 activity in
tumor-initiating cells remodels myeloid-derived suppressor cells to
promote breast cancer progression. Cancer Res. 81:5919–5934. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Talukdar S, Bhoopathi P, Emdad L, Das S,
Sarkar D and Fisher PB: Dormancy and cancer stem cells: An enigma
for cancer therapeutic targeting. Adv Cancer Res. 141:43–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Duru N, Fan M, Candas D, Menaa C, Liu HC,
Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al:
HER2-associated radiore-sistance of breast cancer stem cells
isolated from HER2-negative breast cancer cells. Clin Cancer Res.
18:6634–6647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shao J, Fan W, Ma B and Wu Y: Breast
cancer stem cells expressing different stem cell markers exhibit
distinct biological characteristics. Mol Med Rep. 14:4991–4998.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Barzegar Behrooz A, Syahir A and Ahmad S:
CD133: Beyond a cancer stem cell biomarker. J Drug Target.
27:257–269. 2019. View Article : Google Scholar
|
|
56
|
Li Y, Chu J, Feng W, Yang M, Zhang Y,
Zhang Y, Qin Y, Xu J, Li J, Vasilatos SN, et al: EPHA5 mediates
trastuzumab resistance in HER2-positive breast cancers through
regulating cancer stem cell-like properties. FASEB J. 33:4851–4865.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
Arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wei B, Cao J, Tian JH, Yu CY, Huang Q, Yu
JJ, Ma R, Wang J, Xu F and Wang LB: Mortalin maintains breast
cancer stem cells stemness via activation of Wnt/GSK3β/β-catenin
signaling pathway. Am J Cancer Res. 11:2696–2716. 2021.
|
|
59
|
Wu Y, Ginther C, Kim J, Mosher N, Chung S,
Slamon D and Vadgama JV: Expression of Wnt3 activates Wnt/β-catenin
pathway and promotes EMT-like phenotype in trastuzumab-resistant
HER2-overexpressing breast cancer cells. Mol Cancer Res.
10:1597–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Choi HJ, Jin S, Cho H, Won HY, An HW,
Jeong GY, Park YU, Kim HY, Park MK, Son T, et al: CDK12 drives
breast tumor initiation and trastuzumab resistance via WNT and
IRS1-ErbB-PI3K signaling. EMBO Rep. 20:e480582019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
El Abbass KA, Abdellateif MS, Gawish AM,
Zekri AN, Malash I and Bahnassy AA: The role of breast cancer stem
cells and some related molecular biomarkers in metastatic and
nonmetastatic breast cancer. Clin Breast Cancer. 20:e373–e384.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shen Q and Reedijk M: Notch signaling and
the breast cancer microenvironment. Adv Exp Med Biol. 1287:183–200.
2021. View Article : Google Scholar
|
|
63
|
Baker A, Wyatt D, Bocchetta M, Li J,
Filipovic A, Green A, Peiffer DS, Fuqua S, Miele L, Albain KS and
Osipo C: Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast
cancer cell proliferation and stem cell survival. Oncogene.
37:4489–4504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pandya K, Wyatt D, Gallagher B, Shah D,
Baker A, Bloodworth J, Zlobin A, Pannuti A, Green A, Ellis IO, et
al: PKCα attenuates Jagged-1-mediated notch signaling in
ErbB-2-positive breast cancer to reverse trastuzumab resistance.
Clin Cancer Res. 22:175–186. 2016. View Article : Google Scholar
|
|
65
|
He M, Fu Y, Yan Y, Xiao Q, Wu H, Yao W,
Zhao H, Zhao L, Jiang Q, Yu Z, et al: The Hedgehog signalling
pathway mediates drug response of MCF-7 mammosphere cells in breast
cancer patients. Clin Sci (Lond). 129:809–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu S, Duan X, Xu L, Ye J, Cheng Y, Liu Q,
Zhang H, Zhang S, Zhu S, Li T and Liu Y: Nuclear Gli1 expression is
associated with pathological complete response and event-free
survival in HER2-positive breast cancer treated with
trastuzumab-based neoadjuvant therapy. Tumour Biol. 37:4873–4881.
2016. View Article : Google Scholar
|
|
67
|
Gupta P, Gupta N, Fofaria NM, Ranjan A and
Srivastava SK: HER2-mediated GLI2 stabilization promotes anoikis
resistance and metastasis of breast cancer cells. Cancer Lett.
442:68–81. 2019. View Article : Google Scholar
|
|
68
|
Doheny D, Sirkisoon S, Carpenter RL,
Aguayo NR, Regua AT, Anguelov M, Manore SG, Arrigo A, Jalboush SA,
Wong GL, et al: Combined inhibition of JAK2-STAT3 and
SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor
growth, and metastasis. Oncogene. 39:6589–6605. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Guo Z, Guo A and Zhou C: Breast cancer
stem cell-derived ANXA6-containing exosomes sustain paclitaxel
resistance and cancer aggressiveness in breast cancer. Front Cell
Dev Biol. 9:7187212021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yousefnia S, Seyed Forootan F, Seyed
Forootan S, Nasr Esfahani MH, Gure AO and Ghaedi K: Mechanistic
pathways of malignancy in breast cancer stem cells. Front Oncol.
10:4522020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhao Q, Liu Y, Wang T, Yang Y, Ni H, Liu
H, Guo Q, Xi T and Zheng L: MiR-375 inhibits the stemness of breast
cancer cells by blocking the JAK2/STAT3 signaling. Eur J Pharmacol.
884:1733592020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu Y, Guo R, Wei J, Zhou Y, Ji W, Liu J,
Zhi X and Zhang J: Effects of PI3K inhibitor NVP-BKM120 on
overcoming drug resistance and eliminating cancer stem cells in
human breast cancer cells. Cell Death Dis. 6:e20202015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xing F, Kobayashi A, Okuda H, Watabe M,
Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, et al:
Reactive astrocytes promote the metastatic growth of breast cancer
stem-like cells by activating Notch signalling in brain. EMBO Mol
Med. 5:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou N, Zhang Y, Zhang X, Lei Z, Hu R, Li
H, Mao Y, Wang X, Irwin DM, Niu G and Tan H: Exposure of
tumor-associated macrophages to apoptotic MCF-7 cells promotes
breast cancer growth and metastasis. Int J Mol Sci. 16:11966–11982.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ko YS, Rugira T, Jin H, Joo YN and Kim HJ:
Radiotherapy-resistant breast cancer cells enhance tumor
progression by enhancing premetastatic niche formation through the
HIF-1α-LOX. Axis Int J Mol Sci. 21:80272020. View Article : Google Scholar
|
|
77
|
Mao Y, Zhang Y, Qu Q, Zhao M, Lou Y, Liu
J, huang O, Chen X, Wu J and Shen K: Cancer-associated fibroblasts
induce trastuzumab resistance in HER2 positive breast cancer cells.
Mol Biosyst. 11:1029–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brown Y, Hua S and Tanwar PS:
Extracellular matrix-mediated regulation of cancer stem cells and
chemoresistance. Int J Biochem Cell Biol. 109:90–104. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du
CW and Zhang GJ: Collagen 1A1 (COL1A1) promotes metastasis of
breast cancer and is a potential therapeutic target. Discov Med.
25:211–223. 2018.PubMed/NCBI
|
|
80
|
Hanker AB, Estrada MV, Bianchini G, Moore
PD, Zhao J, Cheng F, Koch JP, Gianni L, Tyson DR, Sánchez V, et al:
Extracellular matrix/integrin signaling promotes resistance to
combined inhibition of HER2 and PI3K in HER2+ Breast
Cancer. Cancer Res. 77:3280–3292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jokela TA and LaBarge MA: Integration of
mechanical and ECM microenvironment signals in the determination of
cancer stem cell states. Curr Stem Cell Rep. 7:39–47. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li F, Xu J and Liu S: Cancer stem cells
and neovascularization. Cells. 10:10702021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hori A, Shimoda M, Naoi Y, Kagara N, Tanei
T, Miyake T, Shimazu K, Kim SJ and Noguchi S: Vasculogenic mimicry
is associated with trastuzumab resistance of HER2-positive breast
cancer. Breast Cancer Res. 21:882019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bussolati B, Grange C, Sapino A and
Camussi G: Endothelial cell differentiation of human breast tumour
stem/progenitor cells. J Cell Mol Med. 13:309–319. 2009. View Article : Google Scholar
|
|
85
|
McClements L, Yakkundi A, Papaspyropoulos
A, Harrison H, Ablett MP, Jithesh PV, McKeen HD, Bennett R, Donley
C, Kissenpfennig A, et al: Targeting treatment-resistant breast
cancer stem cells with FKBPL and its peptide derivative, AD-01, via
the CD44 pathway. Clin Cancer Res. 19:3881–3893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li M, Pan M, You C, Zhao F, Wu D, Guo M,
Xu H, Shi F, Zheng D and Dou J: MiR-7 reduces the BCSC subset by
inhibiting XIST to modulate the miR-92b/Slug/ESA axis and inhibit
tumor growth. Breast Cancer Res. 22:262020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sandiford OA, Donnelly RJ, El-Far MH,
Burgmeyer LM, Sinha G, Pamarthi SH, Sherman LS, Ferrer AI, DeVore
DE, Patel SA, et al: Mesenchymal stem cell-secreted extracellular
vesicles instruct stepwise dedifferentiation of breast cancer cells
into dormancy at the bone marrow perivascular region. Cancer Res.
81:1567–1582. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim SY, Kang JW, Song X, Kim BK, Yoo YD,
Kwon YT and Lee YJ: Role of the IL-6-JAK1-STAT3-Oct-4 pathway in
the conversion of non-stem cancer cells into cancer stem-like
cells. Cell Signal. 25:961–969. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rodríguez CE, Berardi DE, Abrigo M, Todaro
LB, Bal de Kier Joffé ED and Fiszman GL: Breast cancer stem cells
are involved in Trastuzumab resistance through the HER2 modulation
in 3D culture. J Cell Biochem. 119:1381–1391. 2018. View Article : Google Scholar
|
|
90
|
Maroufi NF, Amiri M, Dizaji BF, Vahedian
V, Akbarzadeh M, Roshanravan N, Haiaty S, Nouri M and Rashidi MR:
Inhibitory effect of melatonin on hypoxia-induced vasculogenic
mimicry via suppressing epithelial-mesenchymal transition (EMT) in
breast cancer stem cells. Eur J Pharmacol. 881:1732822020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee KM, Giltnane JM, Balko JM, Schwarz LJ,
Guerrero-Zotano AL, Hutchinson KE, Nixon MJ, Estrada MV, Sánchez V,
Sanders ME, et al: MYC and MCL1 cooperatively promote
chemotherapy-resistant breast cancer stem cells via regulation of
mitochondrial oxidative phosphorylation. Cell Metab. 26:633–647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Park SJ, Kim JG, Kim ND, Yang K, Shim JW
and Heo K: Estradiol, TGF-β1 and hypoxia promote breast cancer
stemness and EMT-mediated breast cancer migration. Oncol Lett.
11:1895–1902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Takegawa N, Nonagase Y, Yonesaka K, Sakai
K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K and
Tsurutani J: DS-8201a, a new HER2-targeting antibody-drug conjugate
incorporating a novel DNA topoisomerase I inhibitor, overcomes
HER2-positive gastric cancer T-DM1 resistance. Int J Cancer.
141:1682–1689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen K, Huang YH and Chen JL:
Understanding and targeting cancer stem cells: Therapeutic
implications and challenges. Acta Pharmacol Sin. 34:732–740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang YS, Yang C, Han L, Liu L and Liu YJ:
Expression of BCRP/ABCG2 Protein in invasive breast cancer and
response to neoadjuvant chemotherapy. Oncol Res Treat. 45:94–101.
2022. View Article : Google Scholar
|
|
96
|
Němcová-Fürstová V, Kopperová D,
Balušíková K, Ehrlichová M, Brynychová V, Václavíková R, Daniel P,
Souček P and Kovář J: Characterization of acquired paclitaxel
resistance of breast cancer cells and involvement of ABC
transporters. Toxicol Appl Pharm. 310:215–228. 2016. View Article : Google Scholar
|
|
97
|
Shi RZ, He YF, Wen J, Niu YN, Gao Y, Liu
LH, Zhang XP, Wang Y, Zhang XL, Zhang HF, et al: Epithelial cell
adhesion molecule promotes breast cancer resistance
protein-mediated multidrug resistance in breast cancer by inducing
partial epithelial-mesenchymal transition. Cell Biol Int.
45:1644–1653. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ye X, Bai W, Zhu H, Zhang X, Chen Y, Wang
L, Yang A, Zhao J and Jia L: MiR-221 promotes
trastuzumab-resistance and metastasis in HER2-positive breast
cancers by targeting PTEN. BMB Rep. 47:268–273. 2014. View Article : Google Scholar :
|
|
99
|
Li X, Li Y, Yu X and Jin F: Identification
and validation of stemness-related lncRNA prognostic signature for
breast cancer. J Transl Med. 18:3312020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Müller V, Oliveira-Ferrer L, Steinbach B,
Pantel K and Schwarzenbach H: Interplay of lncRNA H19/miR-675 and
lncRNA NEAT1/miR-204 in breast cancer. Mol Oncol. 13:1137–1149.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zheng A, Song X, Zhang L, Zhao L, Mao X,
Wei M and Jin F: Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis
regulates breast cancer stemness via Wnt/β-catenin pathway. J Exp
Clin Cancer Res. 38:3052019. View Article : Google Scholar
|
|
102
|
Xu S, Kong D, Chen Q, Ping Y and Pang D:
Oncogenic long noncoding RNA landscape in breast cancer. Mol
Cancer. 16:1292017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pickard MR and Williams GT: Regulation of
apoptosis by long non-coding RNA GAS5 in breast cancer cells:
Implications for chemotherapy. Breast Cancer Res Treat.
145:359–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ye XM, Zhu HY, Bai WD, Wang T, Wang L,
Chen Y, Yang AG and Jia LT: Epigenetic silencing of miR-375 induces
trastuzumab resistance in HER2-positive breast cancer by targeting
IGF1R. BMC Cancer. 14:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu S, Sun Y, Hou Y, Yang L, Wan X, Qin Y,
Liu Y, Wang R, Zhu P, Teng Y and Liuet M: A novel lncRNA
ROPM-mediated lipid metabolism governs breast cancer stem cell
properties. J Hematol Oncol. 14:1782021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Peng F, Wang JH, Fan WJ, Meng YT, Li MM,
Li TT, Cui B, Wang HF, Zhao Y, An F, et al: Glycolysis gatekeeper
PDK1 repro-grams breast cancer stem cells under hypoxia. Oncogene.
37:1062–1074. 2018. View Article : Google Scholar
|
|
107
|
Fox DB, Garcia N, McKinney BJ, Lupo R,
Noteware LC, Newcomb R, Liu J, Locasale JW, Hirschey MD and Alvarez
JV: NRF2 activation promotes the recurrence of dormant tumour cells
through regulation of redox and nucleotide metabolism. Nat Metab.
2:318–334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Najafi M, Mortezaee K and Majidpoor J:
Cancer stem cell (CSC) resistance drivers. Life Sci.
234:1167812019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Abad E, Graifer D and Lyakhovich A: DNA
damage response and resistance of cancer stem cells. Cancer Lett.
474:106–117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Oh KS, Nam AR, Bang JH, Seo HR, Kim JM,
Yoon J, Kim TY and Oh DY: A synthetic lethal strategy using PARP
and ATM inhibition for overcoming trastuzumab resistance in
HER2-positive cancers. Oncogene. 41:3939–3952. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wengner AM, Scholz A and Haendler B:
Targeting DNA damage response in prostate and breast cancer. Int J
Mol Sci. 21:82732020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Torres VI, Godoy JA and Inestrosa NC:
Modulating Wnt signaling at the root: Porcupine and Wnt acylation.
Pharmacol Ther. 198:34–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yang Y, Li X, Wang T, Guo Q, Xi T and
Zheng L: Emerging agents that target signaling pathways in cancer
stem cells. J Hematol Oncol. 13:602020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jang GB, Hong IS, Kim RJ, Lee SY, Park SJ,
Lee ES, Park JH, Yun CH, Chung JU, Lee KJ, et al: Wnt/β-Catenin
small-molecule inhibitor CWP232228 preferentially inhibits the
growth of breast cancer stem-like cells. Cancer Res. 75:1691–1702.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gurney A, Axelrod F, Bond CJ, Cain J,
Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et
al: Wnt pathway inhibition via the targeting of Frizzled receptors
results in decreased growth and tumorigenicity of human tumors.
Proc Natl Acad Sci USA. 109:11717–11722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mu J, Hui T, Shao B, Li L, Du Z, Lu L, Ye
L, Li S, Li Q, Xiao Q, et al: Dickkopf-related protein 2 induces
G0/G1 arrest and apoptosis through suppressing Wnt/β-catenin
signaling and is frequently methylated in breast cancer.
Oncotarget. 8:39443–39459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
An SM, Ding Q, Zhang J, Xie J and Li L:
Targeting stem cell signaling pathways for drug discovery: Advances
in the Notch and Wnt pathways. Sci China Life Sci. 57:575–580.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Schott AF, Landis MD, Dontu G, Griffith
KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT,
et al: Preclinical and clinical studies of gamma secretase
inhibitors with docetaxel on human breast tumors. Clin Cancer Res.
19:1512–1524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar :
|
|
120
|
Yen WC, Fischer MM, Axelrod F, Bond C,
Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, et al:
Targeting Notch signaling with a Notch2/Notch3 antagonist
(tarextumab) inhibits tumor growth and decreases tumor-initiating
cell frequency. Clin Cancer Res. 21:2084–2095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Huang J, Hu W, Hu L, Previs RA, Dalton HJ,
Yang XY, Sun Y, McGuire M, Rupaimoole R, Nagaraja AS, et al: Dll4
inhibition plus aflibercept markedly reduces ovarian tumor growth.
Mol Cancer Ther. 15:1344–1352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
McKeage MJ, Kotasek D, Markman B, Hidalgo
M, Millward MJ, Jameson MB, Harris DL, Stagg RJ, Kapoun AM, Xu L,
et al: Phase IB Trial of the Anti-cancer stem cell DLL4-binding
agent demcizumab with pemetrexed and carboplatin as First-line
treatment of metastatic non-squamous NSCLC. Target Oncol. 13:89–98.
2018. View Article : Google Scholar
|
|
123
|
Silkenstedt E, Arenas F, Colom-Sanmartí B,
Xargay-Torrent S, Higashi M, Giró A, Rodriguez V, Fuentes P,
Aulitzky WE, van der Kuip H, et al: Notch1 signaling in
NOTCH1-mutated mantle cell lymphoma depends on delta-like ligand 4
and is a potential target for specific antibody therapy. J Exp Clin
Cancer Res. 38:4462019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hui M, Cazet A, Nair R, Watkins DN,
O'Toole SA and Swarbrick A: The Hedgehog signalling pathway in
breast development, carcinogenesis and cancer therapy. Breast
Cancer Res. 15:2032013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Clara JA, Monge C, Yang Y and Takebe N:
Targeting signalling pathways and the immune microenvironment of
cancer stem cells-a clinical update. Nat Rev Clin Oncol.
17:204–232. 2020. View Article : Google Scholar
|
|
126
|
Bhateja P, Cherian M, Majumder S and
Ramaswamy B: The hedgehog signaling pathway: A viable target in
breast cancer. Cancers (Basel). 11:11262019. View Article : Google Scholar
|
|
127
|
Liu C, Qi M, Li L, Yuan Y, Wu X and Fu J:
Natural cordycepin induces apoptosis and suppresses metastasis in
breast cancer cells by inhibiting the Hedgehog pathway. Food Funct.
11:2107–2116. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
129
|
Sorrentino G, Ruggeri N, Specchia V,
Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio
R, Piazza S, et al: Metabolic control of YAP and TAZ by the
mevalonate pathway. Nat Cell Biol. 16:357–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Haque S and Morris JC: Transforming growth
factor-β: A therapeutic target for cancer. Hum Vaccin Immunother.
13:1741–1750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang T, Fahrmann JF, Lee H, Li YJ,
Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, et al:
JAK/STAT3-regulated fatty acid β-oxidation is critical for breast
cancer stem cell self-renewal and chemoresistance. Cell Metab.
27:136–150. 2018. View Article : Google Scholar
|
|
132
|
Patel JS, Hu M, Sinha G, Walker ND,
Sherman LS, Gallagher A and Rameshwar P: Non-coding RNA as
mediators in microenvironment-breast cancer cell communication.
Cancer Lett. 380:289–295. 2016. View Article : Google Scholar
|
|
133
|
Liu Y, Zhang P, Wu Q, Fang H, Wang Y, Xiao
Y, Cong M, Wang T, He Y, Ma C, et al: Long non-coding RNA NR2F1-AS1
induces breast cancer lung metastatic dormancy by regulating NR2F1
and ΔNp63. Nat Commun. 12:52322021. View Article : Google Scholar
|
|
134
|
El Touny LH, Vieira A, Mendoza A, Khanna
C, Hoenerhoff MJ and Green JE: Combined SFK/MEK inhibition prevents
metastatic outgrowth of dormant tumor cells. J Clin Invest.
124:156–168. 2014. View Article : Google Scholar :
|
|
135
|
Puig I, Tenbaum SP, Chicote I, Arqués O,
Martínez-Quintanilla J, Cuesta-Borrás E, Ramírez L, Gonzalo P, Soto
A, Aguilar S, et al: TET2 controls chemoresistant slow-cycling
cancer cell survival and tumor recurrence. J Clin Invest.
128:3887–3905. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ma HP, Chang HL, Bamodu OA, Yadav VK,
Huang TY, Wu A, Yeh CT, Tsai SH and Lee WH: Collagen 1A1 (COL1A1)
is a reliable biomarker and putative therapeutic target for
hepatocellular carcinogenesis and metastasis. Cancers (Basel).
11:7862019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen Q, Xu L, Chen J, Yang Z, Liang C,
Yang Y and Liu Z: Tumor vasculature normalization by orally fed
erlotinib to modulate the tumor microenvironment for enhanced
cancer nanomedicine and immunotherapy. Biomaterials. 148:69–80.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim JH, Verwilst P, Won M, Lee J, Sessler
JL, Han J and Kim JS: A small molecule strategy for targeting
cancer stem cells in hypoxic microenvironments and preventing
tumorigenesis. J Am Chem Soc. 143:14115–14124. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Fico F and Santamaria-Martínez A: TGFBI
modulates tumour hypoxia and promotes breast cancer metastasis. Mol
Oncol. 14:3198–3210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Jiang B, Zhu H, Tang L, Gao T, Zhou Y,
Gong F, Tan Y, Xie L, Wu X and Li Y: Apatinib inhibits stem
properties and malignant biological behaviors of breast cancer stem
cells by blocking wnt/β-catenin signal pathway through
down-regulating LncRNA ROR. Anticancer Agents Med Chem.
22:1723–1734. 2022. View Article : Google Scholar
|
|
141
|
Wu X, Zhang X, Sun L, Zhang H, Li L, Wang
X, Li W, Su P, Hu J, Gao P and Zhou G: Progesterone negatively
regulates BCRP in progesterone receptor-positive human breast
cancer cells. Cell Physiol Biochem. 32:344–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Vannini I, Zoli W, Fabbri F, Ulivi P,
Tesei A, Carloni S, Brigliadori G and Amadori D: Role of efflux
Pump activity in Lapatinib/Caelyx combination in breast cancer cell
lines. Anticancer Drugs. 20:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Karbownik A, Sobańska K, Płotek W,
Grabowski T, Klupczynska A, Plewa S, Grześkowiak E and Szałek E:
The influence of the coadministration of the p-glycoprotein
modulator elacridar on the pharmacokinetics of lapatinib and its
distribution in the brain and cerebrospinal fluid. Invest New
Drugs. 38:574–583. 2020. View Article : Google Scholar :
|
|
144
|
Yi J, Chen S, Yi P, Luo J, Fang M, Du Y,
Zou L and Fan P: Pyrotinib sensitizes 5-fluorouracil-resistant HER2
breast cancer cells to 5-fluorouracil. Oncol Res. 28:519–531. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cufi S, Corominas-Faja B, Vazquez-Martin
A, Oliveras-Ferraros C, Dorca J, Bosch-Barrera J, Martin-Castillo B
and Menendez JA: Metformin-induced preferential killing of breast
cancer initiating CD44+CD24-/low cells is sufficient to overcome
primary resistance to trastuzumab in HER2+ human breast cancer
xenografts. Oncotarget. 3:395–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Song CW, Lee H, Dings RP, Williams B,
Powers J, Santos TD, Choi BH and Park HJ: Metformin kills and
radiosensitizes cancer cells and preferentially kills cancer stem
cells. Sci Rep. 2:3622012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Singh JK, Simões BM, Clarke RB and Bundred
NJ: Targeting IL-8 signalling to inhibit breast cancer stem cell
activity. Expert Opin Ther Targets. 17:1235–1241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar
|
|
149
|
Kim HJ, Min A, Im SA, Jang H, Lee KH, Lau
A, Lee M, Kim S, Yang Y, Kim J, et al: Anti-tumor activity of the
ATR inhibitor AZD6738 in HER2 positive breast cancer cells. Int J
Cancer. 140:109–119. 2017. View Article : Google Scholar
|