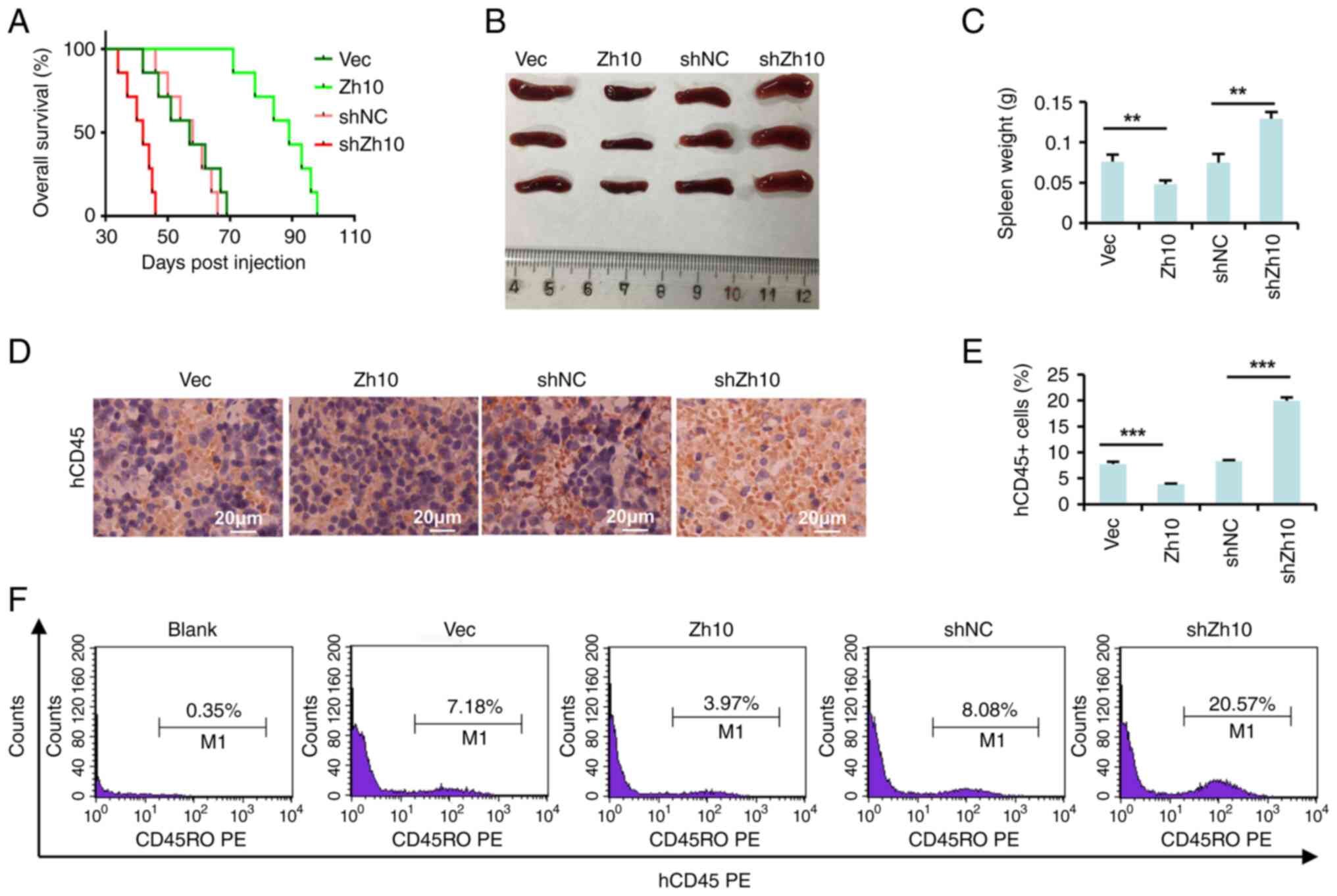
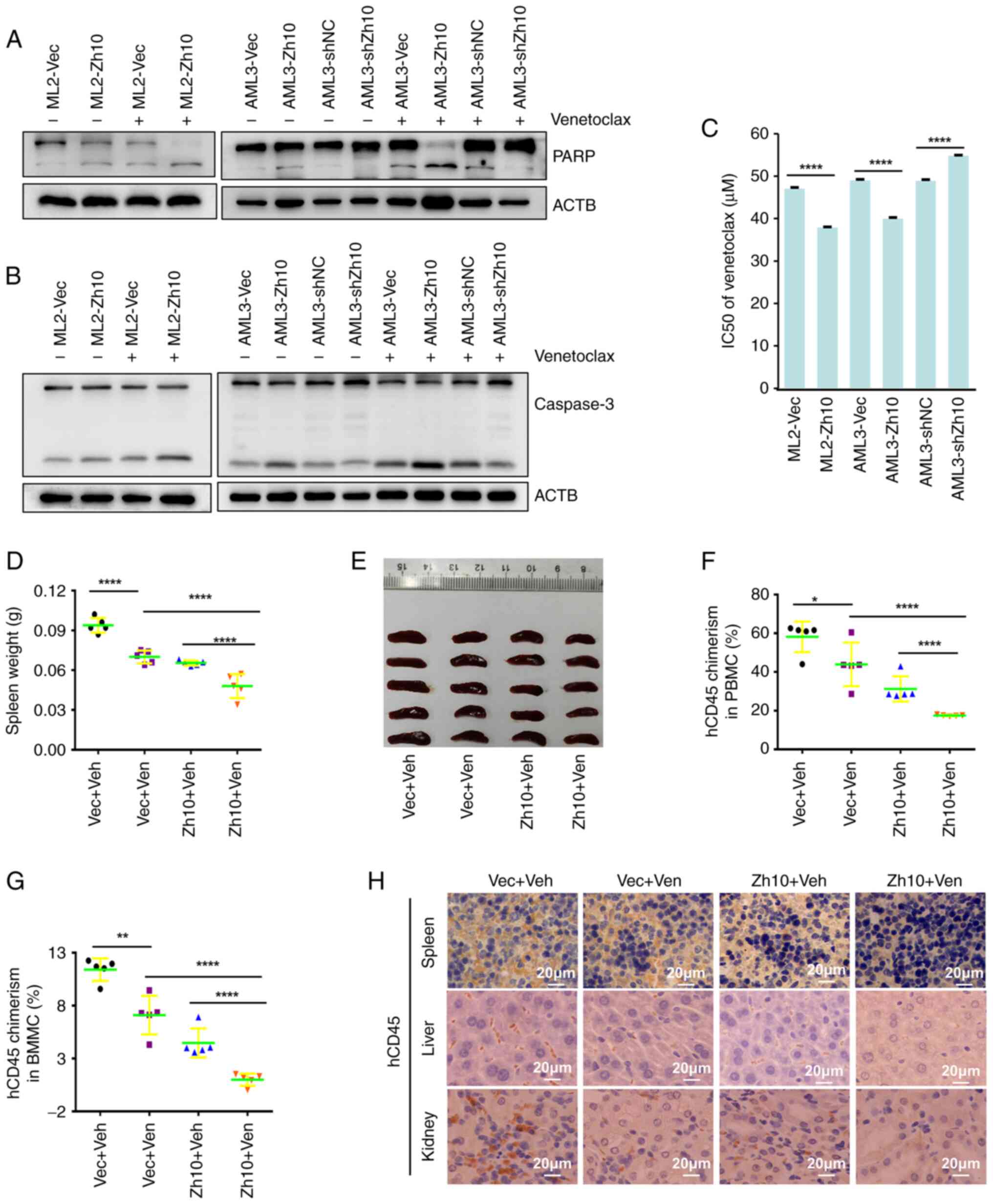
|
1
|
Chakraborty S and Park CY: Pathogenic
mechanisms in acute myeloid leukemia. Curr Treat Options Oncol.
23:1522–1534. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boutelle AM and Attardi LD: p53 and tumor
suppression: It takes a network. Trends Cell Biol. 31:298–310.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen XM, Xu ZJ, Jin Y, Xia PH, Ma JC, Qian
W, Lin J and Qian J: Association analyses of TP53 mutation with
prognosis, tumor mutational burden, and immunological features in
acute myeloid leukemia. Front Immunol. 12:7175272021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abramowitz J, Neuman T, Perlman R and
Ben-Yehuda D: Gene and protein analysis reveals that p53 pathway is
functionally inactivated in cytogenetically normal acute myeloid
leukemia and acute promyelocytic leukemia. BMC Med Genomics.
10:182017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quintas-Cardama A, Hu C, Qutub A, Qiu YH,
Zhang X, Post SM, Zhang N, Coombes K and Kornblau SM: p53 pathway
dysfunction is highly prevalent in acute myeloid leukemia
independent of TP53 mutational status. Leukemia. 31:1296–1305.
2017. View Article : Google Scholar
|
|
7
|
Chen Q, Deng S, Deng M, Shi Y, Zhong M,
Ding L, Jiang Y, Zhou Y, Carter BZ and Xu B: Therapeutic synergy of
triptolide and MDM2 inhibitor against acute myeloid leukemia
through modulation of p53-dependent and -independent pathways. Exp
Hematol Oncol. 11:232022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang DD, Tang Q, Kong Y, Rong T, Wang Q,
Li N, Fang X, Gu J, Xiong D, Yin Y, et al: MDM2 inhibitor APG-115
exerts potent antitumor activity and synergizes with
standard-of-care agents in preclinical acute myeloid leukemia
models. Cell Death Discov. 7:902021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan R, Ruvolo V, Mu H, Leverson JD,
Nichols G, Reed JC, Konopleva M and Andreeff M: Synthetic lethality
of combined Bcl-2 inhibition and p53 activation in AML: Mechanisms
and superior antileukemic efficacy. Cancer Cell. 32:748–760.e6.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ning Y, Hui N, Qing B, Zhuo Y, Sun W, Du
Y, Liu S, Liu K and Zhou J: ZCCHC10 suppresses lung cancer
progression and cisplatin resistance by attenuating MDM2-mediated
p53 ubiquitination and degradation. Cell Death Dis. 10:4142019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Beau MM, Espinosa R III, Neuman WL,
Neuman WL, Stock W, Roulston D, Larson RA, Keinanen M and Westbrook
CA: Cytogenetic and molecular delineation of the smallest commonly
deleted region of chromosome 5 in malignant myeloid diseases. Proc
Natl Acad Sci USA. 90:5484–5488. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Treppendahl MB, Qiu X, Søgaard A, Yang X,
Nandrup-Bus C, Hother C, Andersen MK, Kjeldsen L, Möllgård L,
Hellström-Lindberg E, et al: Allelic methylation levels of the
noncoding VTRNA21 located on chromosome 5q31.1 predict outcome in
AML. Blood. 119:206–216. 2012. View Article : Google Scholar :
|
|
13
|
Boumber YA, Kondo Y, Chen X, Shen L,
Gharibyan V, Konishi K, Estey E, Kantarjian H, Garcia-Manero G and
Issa JP: RIL, a LIM gene on 5q31, is silenced by methylation in
cancer and sensitizes cancer cells to apoptosis. Cancer Res.
67:1997–2005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bake V, Roesler S, Eckhardt I, Belz K and
Fulda S: Synergistic interaction of Smac mimetic and IFNα to
trigger apoptosis in acute myeloid leukemia cells. Cancer Lett.
355:224–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peña-Martinez P, Eriksson M, Ramakrishnan
R, Chapellier M, Högberg C, Orsmark-Pietras C, Richter J, Andersson
A, Fioretos T and Järås M: Interleukin 4 induces apoptosis of acute
myeloid leukemia cells in a Stat6-dependent manner. Leukemia.
32:588–596. 2018. View Article : Google Scholar :
|
|
16
|
Tyner JW, Tognon CE, Bottomly D, Wilmot B,
Kurtz SE, Savage SL, Long N, Schultz AR, Traer E, Abel M, et al:
Functional genomic landscape of acute myeloid leukaemia. Nature.
562:526–531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chuang MK, Chiu YC, Chou WC, Hou HA, Tseng
MH, Kuo YY, Chen Y, Chuang EY and Tien HF: An mRNA expression
signature for prognostication in de novo acute myeloid leukemia
patients with normal karyotype. Oncotarget. 6:39098–39110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Sun Y, Wei G, Luo H, Wu W, Skogerbø G, Luo
J and Chen R: The long noncoding RNA SNHG1 promotes tumor growth
through regulating transcription of both local and distal genes.
Oncogene. 36:6774–6783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Chen Q, Tsai HJ, Wang G, Hong X,
Zhou Y, Zhang C, Liu C, Liu R, Wang H, et al: Maternal
preconception body mass index and offspring cord blood DNA
methylation: Exploration of early life origins of disease. Environ
Mol Mutagen. 55:223–230. 2014. View Article : Google Scholar
|
|
21
|
Bao XL, Zhang L and Song WP: LncRNA SNHG1
overexpression regulates the proliferation of acute myeloid
leukemia cells through miR-488-5p/NUP205 axis. Eur Rev Med
Pharmacol Sci. 23:5896–5903. 2019.PubMed/NCBI
|
|
22
|
Tian M, Gong W and Guo J: Long non-coding
RNA SNHG1 indicates poor prognosis and facilitates disease
progression in acute myeloid leukemia. Biol Open. 8:bio0464172019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu M, Chen X, Lin K, Zeng K, Liu X, Pan B,
Xu X, Xu T, Hu X, Sun L, et al: The long noncoding RNA SNHG1
regulates colorectal cancer cell growth through interactions with
EZH2 and miR-154-5p. Mol Cancer. 17:1412018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong KK, Lawrie CH and Green TM: Oncogenic
roles and inhibitors of DNMT1, DNMT3A, and DNMT3B in acute myeloid
leukaemia. Biomarker Insights. 14:11772719198464542019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei Y, Cao Y, Sun R, Cheng L, Xiong X, Jin
X, He X, Lu W and Zhao M: Targeting Bcl-2 proteins in acute myeloid
leukemia. Front Oncol. 10:5849742020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Short NJ, Dombret H, Adès L and Kantarjian
H: The evolution of research and therapy with hypomethylating
agents in acute myeloid leukemia and myelodysplastic syndrome: New
directions for old drugs. Cancer J. 28:29–36. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stomper J, Rotondo JC, Greve G and Lubbert
M: Hypomethylating agents (HMA) for the treatment of acute myeloid
leukemia and myelodysplastic syndromes: Mechanisms of resistance
and novel HMA-based therapies. Leukemia. 35:1873–1889. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thin KZ, Tu JC and Raveendran S: Long
non-coding SNHG1 in cancer. Clin Chim Acta. 494:38–47. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C, Gao Q, Wang M and Xin H: LncRNA
SNHG1 contributes to the regulation of acute myeloid leukemia cell
growth by modulating miR-489-3p/SOX12/Wnt/β-catenin signaling. J
Cell Physiol. 236:653–663. 2020. View Article : Google Scholar
|
|
30
|
Vaddavalli PL and Schumacher B: The p53
network: Cellular and systemic DNA damage responses in cancer and
aging. Trends Genet. 38:598–612. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohira T, Kojima H, Kuroda Y, Aoki S,
Inaoka D, Osaki M, Wanibuchi H, Okada F, Oshimura M and Kugoh H:
PITX1 protein interacts with ZCCHC10 to regulate hTERT mRNA
transcription. PLoS One. 14:e02176052019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma ZH, Shi PD and Wan BS: MiR-410-3p
activates the NF-κB pathway by targeting ZCCHC10 to promote
migration, invasion and EMT of colorectal cancer. Cytokine.
140:1554332021. View Article : Google Scholar
|
|
33
|
Lehmann C, Friess T, Birzele F, Kiialainen
A and Dangl M: Superior anti-tumor activity of the MDM2 antagonist
idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type
acute myeloid leukemia models. J Hematol Oncol. 9:502016.
View Article : Google Scholar : PubMed/NCBI
|