Osteosarcoma (OS) typically originates from the long
bones, including the distal femur (30%), proximal tibia (15%) and
proximal humerus (15%). As a primary malignant bone tumor of
mesenchymal origin, OS exhibits osteoblastic differentiation and
malignant osteoid (1). The
clinical treatment of OS primarily includes surgical intervention,
radiotherapy, and chemotherapy. Although a combination of
chemotherapy and surgical intervention has resulted in an ~60%
increase in the survival rate of patients with OS, the 5-year
survival rate and prognosis of patients with OS remain
unsatisfactory due to a lack of early diagnostic and effective
treatment methods (1–3). In particular, OS is prone to
multidrug resistance and lung metastasis (4,5).
Tumor suppressor genes p53 and Retinoblastoma (Rb) are typically
observed to be mutated in clinical samples of patients with OS and
are closely associated with susceptibility to OS (6). The molecular pathogenesis of OS
involves the Janus kinase (JAK)/signal transducer and activator of
transcription (STAT)3, nuclear factor κB (NF-κB),
phosphatidylinositol 3 kinase (PI3K)/serine/threonine kinase (AKT)
and Wingless/Integrated (Wnt)/β-catenin signaling pathways, among
others. These signaling pathways play crucial roles during the
onset and development of OS (7–10).
Non-coding RNAs (ncRNAs) are a class of RNAs that
lack the ability to encode proteins. Over the course of the past
decade, ncRNAs have been found to play vital roles in gene
expression regulation. ncRNAs can be classified based on their
length as follows: small RNAs, which are defined as those that are
<200 nt in length, and long non-coding RNAs (lncRNAs), which are
those that are ≥200 nt (11).
Small RNAs can be further classified into small interfering RNAs
(siRNAs), microRNAs (miRNAs/miRs), Piwi-interacting RNAs (piRNAs),
etc. miRNAs are ~22 nt in length and regulate gene expression by
binding to response elements [also known as miRNA response elements
(MREs)] on target RNAs (12).
Circular RNAs (circRNAs) are covalently closed RNA molecules formed
through back-splicing, a process that differs from the classical
splicing mechanism. These molecules lack a 5′ end cap structure and
3′ end polyadenylate tail, rendering them resistant to degradation
by endonucleases and conferring a longer half-life (>48 h)
(13). In addition, circRNAs are
expressed in specific cells and tissues, and a number of them are
evolutionarily conserved (14,15).
Owing to their unique and novel biological structure and function,
circRNAs have emerged as a prominent area of research following
miRNAs and lncRNAs.
Several studies have reported that circRNAs play
critical roles in the pathogenesis of various diseases by
regulating various cellular processes, including the cell cycle,
tumorigenesis, invasion, metastasis, apoptosis and angiogenesis
(16–20). circRNAs have shown promise as novel
biomarkers for diagnosis and as therapeutic targets for various
diseases (21). Functional
analyses on circRNAs have demonstrated that circRNAs regulate the
expression of particular mRNAs by adsorbing their corresponding
binding miRNAs from targeted sites (22). Additionally, circRNAs also directly
modulate the functions of proteins by binding to RNA-binding
proteins (RBPs) (23). Moreover,
certain circRNAs are directly translated into proteins/peptides,
thereby exerting their functions in the form of proteins/peptides
(24). For examples, protein
C-E-Cad encoded by the circular E-cadherin RNA (circ-E-Cad)
facilitates the proliferation and migration of gastric cells by the
PI3K/AKT pathway (25). Protein
EIF6-224aa translated from circRNA Circ-EIF6 contributes to the
breast cancer progression via stabilizing MYH9 and activating the
Wnt/beta-catenin pathway (26). In
two recently related reviews, ~30 proteins/peptides translated from
circRNAs were experimentally validated to have importantly
physiological and pathological functions (27,28).
Numerous aberrantly expressed circRNAs have been identified and
shown to be involved in the occurrence and development of OS
(29,30). Although an increasing number of
aberrantly expressed circRNAs have been identified in OS, their
functions and molecular mechanisms remain unclear. Therefore, the
present review provides an overview of the aberrantly expresssed
circRNAs, the primary types of circRNAs, the functions and
mechanisms of circRNAs, and the potential clinical application of
circRNAs in OS, in an aim to provide new insight into the diagnosis
and treatment of OS.
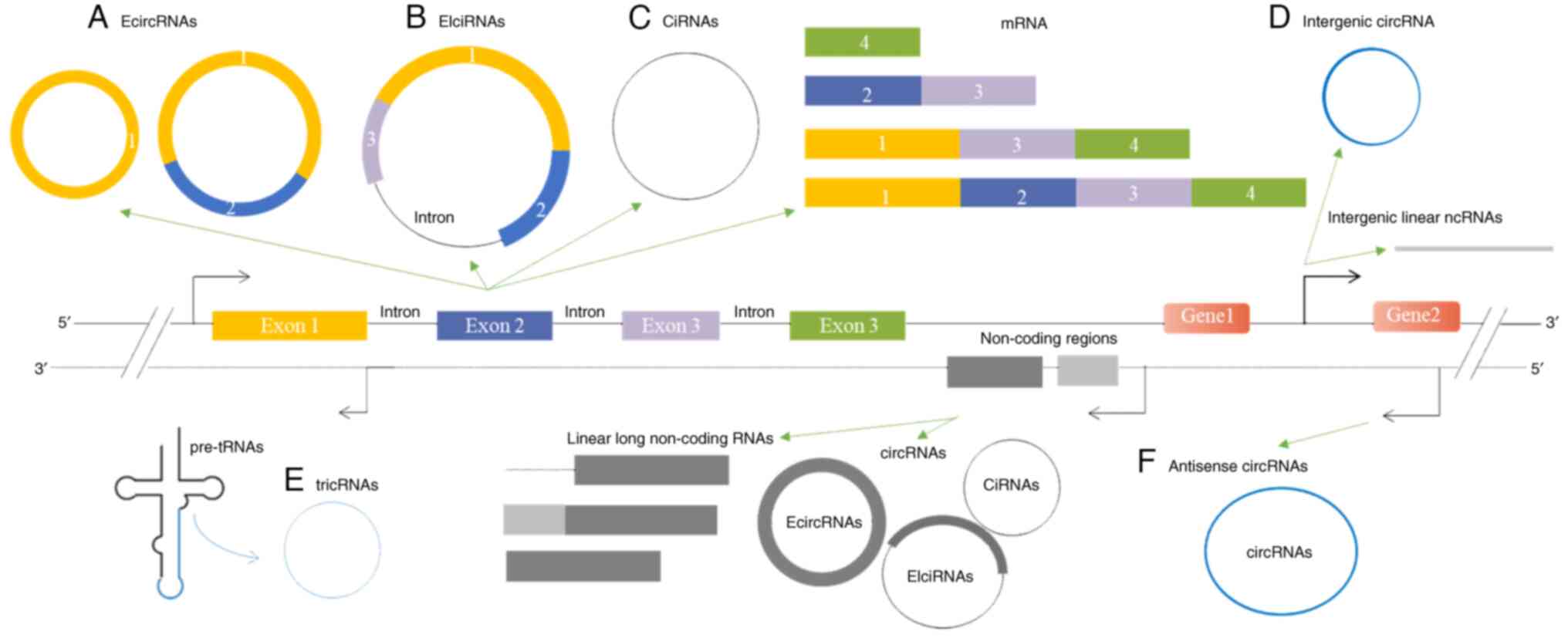
The biogenesis of ecircRNAs is closely associated
with their host pre-mRNAs. When the splicing receptor of the
upstream exon of a pre-mRNA and its downstream exon donor are in
close proximity, a lasso structure containing both exons and
introns is produced. As the introns in the lasso structure are
excised, ecircRNAs are formed through the formation of phosphate
ester bonds. Another mechanism through which ecircRNAs are produced
involves RBPs. Specifically, RBPs facilitate the interaction
between the upstream and downstream introns of pre-mRNAs, thereby
enabling the formation of ecircRNAs (44). The production of EIciRNAs is
primarily associated with circularization driven by intron pairing.
In this process, the downstream intron splicing donor and the
upstream intron splicing receptor are paired, based on the Alu
complementary base sequences. Subsequently, introns are either
retained or excised. Finally, EIciRNAs or ecircRNAs may be produced
through a third mechanism (37).
ciRNAs contain only introns, and their biogenesis primarily relies
on a 7-nt GU-rich sequence proximal to the 5′ splicing site and an
11-nt C-rich sequence proximal to the branching point site, which
serves to prevent degradation by exonucleases, with ciRNAs forming
after cyclization (39).
Anti-circRNAs, including antisense ecircRNAs, antisense EIciRNAs,
and antisense ciRNAs, are transcribed from non-coding gene loci on
the antisense strand (15,45).
tricRNAs are a specific type of intronic circRNAs
that have been exclusively detected in Archaea and
Drosophila. During the pre-tRNA splicing process, the
conserved tRNA sequence and splicing enzymes are required. The
enzymes cleaves pre-tRNAs into two parts: tricRNAs are generated
from 3′ to 5′ phosphate bonds, and the other portion gives rise to
tRNAs (41,45). Intergenic circRNAs are formed by
the cyclization of intergenic sequences situated between two
protein-coding genes (15).
circRNAs are primarily classified into six
categories based on their origin and are notably plentiful.
According to the latest records available on circRNADb and
TRCircle, there are at least 90,000 circRNAs in human cells
(42,43). However, the majority of aberrantly
expressed circRNAs in OS were identified between 2018 and 2022.
Furthermore, to date, the functions of only ~20 circRNAs have been
reported (46–81) (Table
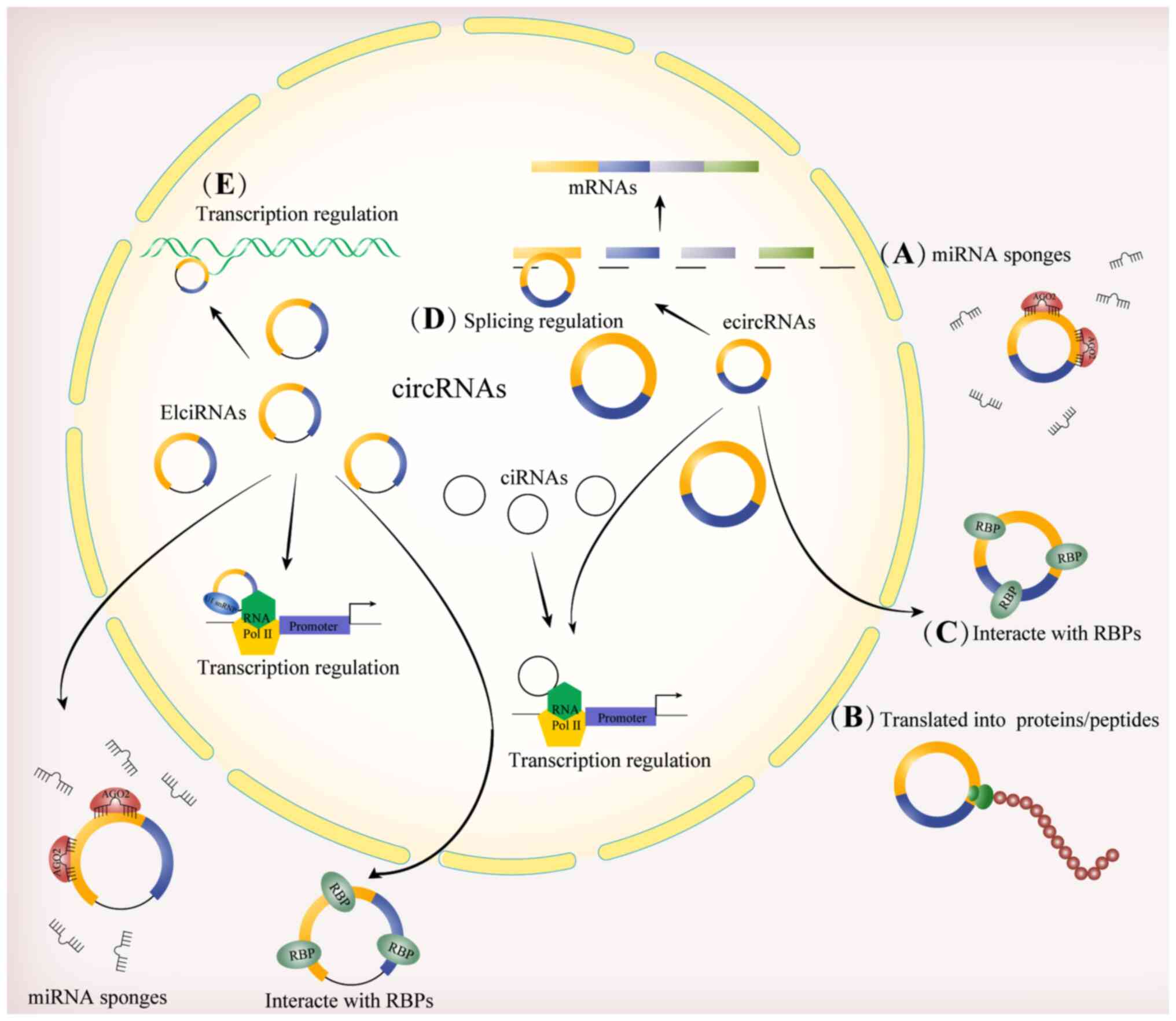
I). Functional analyses on circRNAs in OS have beeb primarily
centered around miRNA sponges (Fig.
2A). miRNA sponges, also referred to as competitive endogenous
RNAs (ceRNAs), are RNA transcripts that share MREs to regulate gene
expression by competing with miRNAs. Theoretically, any transcripts
containing MREs, including mRNAs, lncRNAs, pseudogene RNAs and
circRNAs, have the potential to function as ceRNAs (82). For example, upregulated
Circ_0005909 functions as a sponge for miR-936, thereby rendering
the translation of high mobility group protein 1 (HMGB1), which is
targeted by this miRNA, impervious to its effects. Hence, OS
growth, proliferation, epithelial-mesenchymal transformation (EMT),
migration and invasion are promoted (55). A given circRNA can be targeted by
multiple distinct miRNA pairs. Additionally, a single miRNA can
target various circRNAs. Circ_0001649 and Circ_0001649 have been
reported as sponges of various miRNAs that inhibit cell
proliferation (62,83). In OS, circ-XPO1 and its host gene
Exportin 1 (XPO1) are upregulated (84). The adsorption of miR-23a-3p,
miR-23b-3p, miR-23c and miR-130a-5p by circ-XPO1 leads to the
upregulation of XPO1 protein. Therefore, inhibiting the expression
of circ-XPO1 or XPO1 may be a novel therapeutic strategy for OS
(84). Certain cytoplasmic
circRNAs exhibit open reading frames and ribosomal binding sites,
indicating their potential for translation (85). Owing to the absence of an m7G cap
structure at the 5′ end and a polyadenylated tail at the 3′ end,
which are essential for linear mRNA translation, circRNAs are
translated in a cap-independent manner (Fig. 2B). circRNAs are primarily
translated through three mechanisms as follows: Internal ribosome
entry site (IRES)-mediated translation, m6A-dependent translation
and rolling circle amplification (RCA) translation. One of the
translation mechanisms that has gained wide recognition involves
the direct recognition of circRNAs possessing natural IRESs by
non-standard eukaryotic translation initiation factor 4 gamma
(eIF4G) proteins (eIF4G2 or DAP-5). eIF4G2 contains eukaryotic
translation initiation factor 4A (eIF4A) and eukaryotic translation
initiation factor 3 (eIF3) binding regions, but lacks the
eukaryotic translation initiation factor 4E (eIF4E) binding site
(86–89). IRESs assemble the eIF4 complex in
the absence of eIF4E and thereby, directly initiate circRNA
translation (90). In glioblastoma
cells, LINC-PINT, which is a long-chain gene spacer non-coding RNA
molecule, is translated into a peptide consisting of 87 amino acids
through IRES translation (91).
m6A is one of the most prevalent base modifications in RNAs,
exhibiting a notable enrichment in circRNAs. The translation of
circRNA is facilitated by the initiation factor eukaryotic
translation initiation factor 4 gamma 2 (eIF4G2) and the m6A reader
YTH N6-methyladenosine (m6A) RNA binding protein F3,
whereby the ribosome operates in a manner similar to IRES. A single
m6A site has been observed to be sufficient to drive translation
initiation (92). This mode of
translation of circRNAs can be enhanced by the methyltransferase
methyltransferase 3, N6-adenosine-methyltransferase
complex catalytic subunit (METTL)3/14, whereas it can be inhibited
by the demethylase alpha-ketoglutarate dependent dioxygenase
(17,92). During RCA translation, the length
of circRNAs should be a multiple of three, with the inclusion of
the starting codon AUG, but without IRES or termination codons.
Therefore, in theory, once the translation of circRNAs begins, the
elongated portion can rotate around the ring multiple times,
thereby yielding high molecular weight proteins (93). Circ-EGFR, which arises from the
aberrant activation of epidermal growth factor receptor (EGFR) in
adult glioblastomas, is translated into repetitive amino acid
sequences through RCA, ultimately resulting in the formation of a
new polymeric protein complex (94). Generally, the reported quantity of
circRNAs with functional proteins/peptide is ~30 (27,28).
Herein, several peptides/proteins with critical physiological and
pathological functions encoded by circRNAs are summarized (Table II). Owing to the fact that a
number of circRNAs contain some of the same codons as their host
genes, proteins/peptides translated from circRNAs are usually
truncated and their functions are mostly similar to the full-length
proteins encoded by their host genes (92,95).
However, the functions of certain proteins/peptides encoded by
circRNAs are independent of their corresponding host gene products.
In fact, several functions are opposite to those of the proteins
encoded by the host genes (96). A
list of functional peptides/proteins encoded by circRNAs under
different physiological and pathological states is presented in
Table II (96–107).
Since circRNAs interact with proteins, they
primarily bind to RBPs, thereby affecting cellular processes such
as proliferation, metastasis and apoptosis (Fig. 2C). For example, circRNA poly(A)
binding protein nuclear 1 (PABPN1; circPABPN1), which is produced
from the precursor mRNA of PABPN1, PABPN1 translation by
blocking the binding of RBP HuR to PABPN1 mRNA, thereby attenuating
the proliferation of tumor cells (108). Circular RNA forkhead box (Fox) O3
(Circ-Foxo3) is primarily distributed in the cytoplasm and is
highly expressed in cardiac tissue samples from geriatric patients
and mice. Circ-Foxo3 promotes cardiac aging by enhancing its
interaction with the anti-aging protein inhibitor of DNA binding 1
(ID-1), E2F transcription factor 1, anti-stress protein focal
adhesion kinase (FAK) and hypoxia inducible factor 1 subunit α
(109). RBPs interacting with
circRNAs regulate the expression of circRNAs. Nuclear factor of
activated T-cells 90 kDa (NF90) and its subtype NF110 bind to
intron pairs within the nucleus to form regions of ecircRNA,
thereby promoting the production of circRNAs. However, during viral
infections, NF90/NF110 is rapidly transported into the cytoplasm,
where it binds to viral RNA with consequent reduction in the
generation of corresponding circRNAs (110).
In addition to sponging miRNAs, undergoing
translation and interacting with RBPs, circRNAs regulate
alternative splicing and gene transcription (Fig. 2D and E). Although the majority of
circRNAs are distributed in the cytoplasm, EIciRNAs and ciRNAs have
been observed primarily in the nucleus (111). Both EIciRNAs and ciRNAs regulate
the expression of host genes in cis within the nucleus. For
example, the EIciRNAs circEIF3J and circPAIP2 interact with the Pol
II transcription complex on the promoter of their host genes via U1
small nuclear RNA, thereby ultimately promoting the transcription
of their host genes (111). The
ciRNA ci-ankrd52 aggregates at its host gene transcription site,
thereby forming RNA:DNA hybrids that regulate the Pol II elongation
process.
As this circRNA is a positive regulator of Pol II
transcription, its downregulation decreases its host gene
expression (39). In addition to
regulating the transcription of host genes, nuclear circRNAs
directly participate in the processing and maturation of their host
genes. For example, nuclear circRNA derived from exon 6 of
SEPALLATA3 (SEP3) regulates splicing of its linear precursor host
mRNA (112). Although circRNAs
enriched in the nucleus can pair with genomic DNA to form RNA:DNA
hybrids, whether they affect DNA replication remains unknown.
circRNAs exhibit functional diversity based on their
distinct subcellular locations. Nuclear circRNAs are primarily
associated with the transcriptional regulation and the processing
or/and maturation of mRNAs. Cytoplasmic circRNAs are primarily
associated with post-transcriptional regulation. Mitochondrial
circRNAs are closely associated with the function of mitochondria.
Exosome circRNAs typically serve as signal molecules (113). In addition to serving as miRNA
sponges, circRNAs interact with RBPs, regulate gene splicing or
transcription, and undergo translation to give rise to proteins or
small peptides (36,44). However, functional studies of
circRNAs have mostly centered around miRNA sponges. The functions
of circRNAs are dynamically regulated under various physiological
and pathological conditions. Thus, the functions and mechanisms of
circRNAs in OS remain unclear, and additional investigations are
required to enhance our understanding of them.
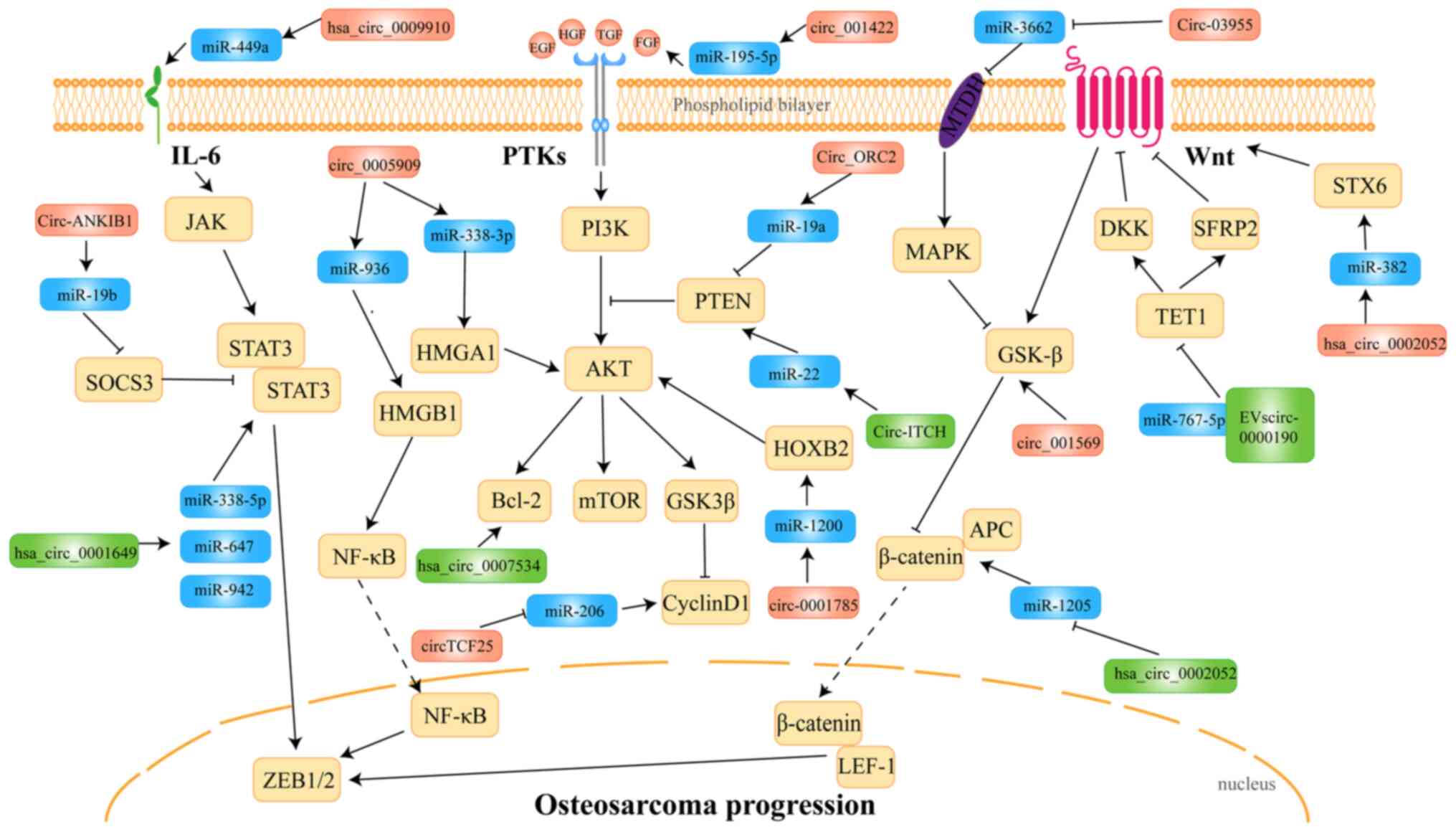
circRNAs exhibit versatility by engaging in
interactions with DNA, RNA and RBPs during the pathogenesis and
development of OS. Mechanistic analyses have revealed that circRNAs
function by primarily participating in key signaling pathways
within the cell, including the JAK/STAT3, NF-κB, PI3K/AKT and
Wnt/β-catenin signaling pathways (30) Herein, these pathways are summarized
and emphasized (Fig. 3).
STATs are highly conserved in eukaryotes and
essential for various biological processes, such as embryonic
development, immunity, hematopoiesis and cell migration (114). STAT3, a member of the STAT
family, is ubiquitously expressed in the cytoplasm in an inactive
form. During the signal cascade process, STAT3 is rapidly activated
by the interleukin (IL)-6 family members, EGF and platelet derived
growth factor. Upon activation, STAT3 undergoes dimerization,
translocates to the nucleus and subsequently regulates downstream
gene transcription (44). A high
expression of STAT3 usually indicates a poor prognosis in OS, and
the transformation, proliferation, tumorigenesis, invasion,
metastasis and drug resistance of OS cells (115). The circRNA hsa_circ_0009910 is
overexpressed in OS cells and tissues (49). This circRNA promotes the expression
of IL-6R by sponging miR-449a, which targets IL-6R. Therefore, the
activation of the JAK1/STAT3 signal downstream of IL-6R by
hsa_circ_0009910 leads to the development of OS (49). Circ_ANKIB1 sponges miR-19b, which
targets suppressor of cytokine signaling 3 (SOCS3), thereby
activating the STAT3 pathway downstream of SOCS3 (57). In addition to activating the STAT3
pathway, circRNAs in OS also exert adverse effects through this
pathway. The low expression of Circ_0001649 in OS is associated
with the increased expression of apoptotic peptidase activating
factor 1, cleaved caspase-3 and caspase-9, which results in the
apoptosis of OS cells. Mechanistic analyses have revealed that this
circRNAs sponges miR-338-5p, miR-647 and miR-942 to inhibit the
activation of STAT3/5 (62).
NF-κB is a member of a family of transcription
factors that not only play crucial roles in the regulation of
immune response and inflammation, but have also been increasingly
validated to play vital roles in tumorigenesis (116). The increased expression of EGFR,
insulin growth factor receptor and tumor necrosis factor receptor
family members, and the activation of Ras/mitogen-activated protein
kinase (MAPK) and PI3K/AKT leads to the activation of NF-κB
(117). Upon activation, NF-κB
triggers downstream signaling pathways, thereby resulting in the
aberrant expression of various genes associated with tumor cell
proliferation, migration and apoptosis, ultimately leading to the
occurrence and development of tumors. For example, HMGB1 activates
the Ras oncogene by binding to the RAGE receptor, leading to the
activation of MAPK-mediated NF-κB inflammatory pathway, thereby
promoting the expression of related downstream proinflammatory
cytokines, and ultimately promoting cancer progression (118). In OS, the aberrant upregulation
of Circ_0005909, which sponges miR-936, prevents the targeting of
HMGB1 by miR-936. Consequently, HMGB1 induces the persistent
activation of the downstream NF-κB pathway, ultimately leading to
cell growth, migration, invasion, and EMT in OS (55).
The PI3K/AKT pathway, which is negatively regulated
by phosphatase and tensin homolog (PTEN) by mediating AKT
dephosphorylation, activates the protein activity of many
downstream genes by phosphorylation, such as mammalian target of
rapamycin (mTOR). This pathway plays crucial roles in many
physiological and pathological processes (119). Upon activation of the PI3K/AKT
signaling pathway, PI3K triggers the conversion of
phosphatidylinositol bisphosphate (PIP2) to phosphatidylinositol
trisphosphate (PIP3). Subsequently, PIP3 phosphorylates and
activates AKT in a pyruvate dehydrogenase kinase 1-dependent
manner, with mTORC2 also contributing to AKT activation. Activated
AKT increases Ras homolog enriched in brain-GTP levels by
inhibiting the formation of the tuberous sclerosis complex 1
(TSC1)/TSC1 heterodimers, thereby activating mTORC1. Subsequently,
mTORC1 mediates the phosphorylation of S6 kinase and eukaryotic
translation initiation factor 4E binding protein 1, which promotes
the release of eIF4E, thereby increasing the rate of protein
synthesis and leading to more rapid cell growth. In addition, an
increase in inhibitor of kappa B kinase (IKK) activity induced by
AKT promotes inhibitor of NF-κB (IκB) degradation, thereby
resulting in the release of NF-κB and its translocation into the
nucleus for gene transcription. AKT also negatively regulates
glycogen synthase kinase 3β (GSK3β) and FOXO1, thereby enhancing
cyclin-dependent kinase expression to promote cell cycle
progression. In addition, AKT inhibits cell apoptosis by
upregulating Bcl-xL, Bcl-2 and Mcl1, and downregulating Bad, Bax
and p53 (10). In OS, the
expression of PTEN is typically decreased or absent, resulting in
the activation of the PI3K/AKT signaling pathway (120,121). The expression of hsa_circ_0007534
is positively associated with p-AKT and p-GSK3β in OS. By
regulating the AKT/GSK3β signaling pathway, this circRNA promotes
OS progression (46). The
overexpression of Circ_001422 promotes the activation of PI3K/AKT
signal transduction and subsequent OS progression by sponging
miR-195-5p, the target of which is fibroblast growth factor 2
(63). Similarly, highly expressed
Circ_0001785 adsorbs miR-1200, the target of which is the gene
HOXB2, leading to the activation of the Bcl-2 family and the
PI3k/AKT/mTOR signaling pathway (52). It has been shown that high mobility
group AT-hook 1 (HMGA1) activates the MAPK/ERK1/2 and PI3K/AKT
signaling pathways (122).
Moreover, both the MAPK/ERK1/2 and PI3K/AKT signaling pathways
exhibit increased activation in OS (10). Circ_0005909 adsorbs miR-338-3p, the
target of which is HMGA1, thereby leading to the development and
progression of OS (64). These
findings suggest that Circ_0005909 may lead to OS progression
through the MAPK/ERK1/2 and PI3K/AKT signaling pathways. Notably,
Circ_ORC2 enhances the inhibitory effects of miR-19a on PTEN
expression by binding to miR-19a, thereby activating the PI3K/AKT
signaling pathway and promoting the proliferation and invasion of
OS cells (51). Of note, the
majority of circRNAs that function as miRNA sponges typically
protect miRNA targets. The circRNA Circ_ORC2 has been discovered to
exhibit an additional function as an miRNA sponge, thereby
exhibiting a novel role of circRNAs. In addition to highly
expressed circRNAs, the downregulated expression of circRNA
circ-ITCH decreases cell viability, proliferation, migration and
invasion by inhibiting the PTEN/PI3K/AKT and Sp1 transcription
factor signaling pathways (53).
Wnt-mediated intracellular signaling pathways
include the classical Wnt/β-catenin pathway, Wnt/Ca2+
pathway, Wnt/PCP pathway and Wnt/PKA pathway (123). It has been demonstrated that
Wnt-mediated signal transduction pathways regulate various cellular
processes, including cell growth, proliferation and differentiation
(124). In OS, the aberrant
expression of a number of Wnt components, including Wnt ligands and
Frizzled and lipoprotein receptor-related protein (LRP) receptors,
affects the development and progression of OS (125). In OS, various circRNAs have been
found to inhibit or activate the Wnt/β-catenin signaling pathway.
Circ_001569 (hsa_circ_0000677) is significantly overexpressed in OS
tissue and its upregulation promotes the resistance of OS cells to
chemotherapy (48). The knockdown
of Circ_001569 in OS cell lines has been shown to reduce p-GSK3β
and β-catenin expression, but to increase GSK3β expression.
Conversely, the upregulation of Circ_001569 has been shown to
increase p-GSK3β and β-catenin expression, but decrease GSK3β
expression (48). Tet
methylcytosine dioxygenase 1 (TET1) inhibits the Wnt/β-catenin
signaling pathway by activating dickkopf WNT signaling pathway
inhibitor 1 and secreted frizzled related protein 2, thereby
preventing the EMT of OS cells (126). Hsa_circ_0002052 is significantly
downregulated in OS tissues and cell lines. This circRNA inhibits
the Wnt/β-catenin signaling pathway by sponging miR-767-5p, the
target of which is TET1. Furthermore, this circRNA also sponges
miR-1205, the target of which is adenomatosis polyposis coli 2
(APC2), a negative regulator of the Wnt/β-catenin signaling
pathway. Hence, hsa_circ_0002052 functions as an inhibitor of the
Wnt/β-catenin signaling pathway by promoting TET1 and APC2
expression via miRNA sponging, ultimately resulting in the delayed
development of OS (50). However,
a recent study demonstrated that hsa_circ_0002052 was upregulated
in OS and activated the Wnt/β-catenin pathway by sponging miR-382,
the target of which is syntaxin 6 (56). These findings demonstrate the
complex nature of OS, underscoring the requirement for
comprehensive studies on circRNAs in OS.
It has been widely reported that aberrantly
expressed circRNAs are more abundant and stable than linear RNAs,
and are closely associated with the occurrence and development of
various diseases. Therefore, they could be highly valuable in
clinical diagnosis and treatment. As regards tumor molecular
markers, circRNAs exhibit more promising potential than existing
markers. The area under the receiver operating characteristic curve
(AUC) of Circ_0008717 in OS is 0.73. The diagnostic sensitivity and
specificity of this circRNA are 80.00 and 73.33%, respectively
(127). CircRNA-PVT1 is a more
representative molecule that is significantly upregulated in OS
tissue, serum, and drug-resistant cell lines. It exhibits an AUC of
0.871, which is comparable to the level of lactate dehydrogenase
(LDH) (0.852) but superior to that of alkaline phosphatase (ALP)
(0.673), which are commonly used diagnostic biomarkers of OS in
clinical settings (128).
Furthermore, the AUC of hsa_circ_0003074 is 0.93, which is
significantly superior to that of LDH and ALP in the diagnosis of
OS (129).
In addition to diagnostic biomarkers, aberrantly
expressed circRNAs in OS may be used as therapeutic molecules or
targets. For example, hsa_circ_0003074 is highly expressed in OS
tissues, peripheral blood, and cell lines (129). However, following surgical
intervention or chemotherapy, this circRNA is substantially reduced
in the peripheral blood of patients with OS. Furthermore,
hsa_circ_0003074 is closely associated with tumor size, lung
metastasis, Enneking stage, chemotherapy resistance and other
prognostic factors (129). Hence,
targeting this circRNA may be beneficial for patients with OS.
Similarly, circPVT1 is significantly upregulated in OS tissues,
serum and drug-resistant cells, and its increased expression is
significantly associated with the Enneking stage, lung metastasis
and chemotherapeutic resistance. The expression of circPVT1 is
higher in patients with lung metastasis or resistance to
chemotherapy than in those who do not exhibit lung metastasis or
those who are sensitive to chemotherapy. The downregulation of
circPVT1 expression reduces the expression of the drug
resistance-related gene ABCB1, thereby increasing the
sensitivity of OS cells to cisplatin and doxorubicin. Additionally,
Kaplan-Meier survival analysis has revealed that patients with high
expression of circPVT1 exhibit a shorter overall survival period
than those with low expression (128). The overexpression of Circ_001569
activates the Wnt/β-catenin signaling pathway, thereby increasing
the half maximal inhibitory concentration value of cisplatin,
doxorubicin, or methotrexate for OS (48). Therefore, the detection of these
aberrantly expressed circRNAs in OS can indicate the effect of OS
treatment and prognosis. Furthermore, these circRNAs can also
potentially serve as therapeutic targets in OS.
Although chemotherapy combined with surgery has made
tremendous progress in the treatment of OS, this disease remains
difficult to be diagnosed and treated. Therefore, it is of utmost
urgency to conduct in-depth research into the molecular mechanisms
responsible for its occurrence and development (130). With the rapid development of high
throughput sequencing technologies, an increasing number of
abnormally expressed circRNAs are found to be critical during the
pathogenesis of OS. circRNAs can regulate various pathological
processes of OS by participating in cellular proliferation,
apoptosis, migration, invasion, drug resistance, stemness, EMT,
angiogenesis and cell cycle arrest (30). Although circRNAs functioning as
miRNA sponges have been extensively studied, studies have indicated
that circRNAs can also function via other mechanisms, such as for
example the regulation of gene expression at the transcriptional
level, or affect functions of RBPs by binding to them, and even
directly be translated into proteins/polypeptides (36,44).
In addition, circRNAs can also function in the tumor
microenvironment (131). This
provides a theoretical basis for its potential applications as
molecular markers, therapeutic molecules or target genes in the
clinical diagnosis and treatment of OS.
Although circRNAs have been well documented in
different diseases, functional and mechanistic studies on circRNAs
in OS are just beginning and a number of issues remain to be
elucidated. First, the biogenesis of circRNAs and their
corresponding regulatory mechanisms have not yet been fully
elucidated (132). Secondly,
circRNAs in OS related to metastasis, drug resistance, prognosis
and survival are mostly clinically observed, and their function and
mechanism studies are few and relatively unclear (133). Thirdly, functional and
mechanistic analyses on circRNAs in OS, as well as in other
diseases mainly focus on miRNA sponges. Constructing an RNA
interaction network using circRNAs may aid in the identification of
related miRNAs, although their interactions still need to be
determined using more accurate methods (134,135). Fourthly, it is difficult to
obtain a large number of OS samples due to its low incidence rate.
Hence, a greater number of clinical medical centers are required to
cooperate and share resources with each other.
Not applicable.
The present study was supported by the Training Programs for
Innovation and Entrepreneurship (grant no. 202110413017), the Key
Laboratory of Prevention and Treatment of Cardiovascular and
Cerebrovascular of Ministry of Education of Gannan Medical
University (grant no. XN202013), the Science and Technology
Research Project of Jiangxi Provincial Department of Education
(grant no. GJJ201528), and the Startup Foundation for Advanced
Talents of Gannan Medical University (grant no. QD202124).
Not applicable.
WJN, FX and XML conceived the study, LZ and LL were
involved in literature search, collection and analysis. LZ, WJN and
XML were involved in the acquisition of funding. LZ, LL, WJN, FX
and XML were involved in the design of the tables and figures. XML
was involved in project administration. FX and XML supervised the
study. LZ and LL were involved in the writing of the original
draft. WJN, FX and XML were involved in the writing, reviewing and
editing of the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Link MP, Goorin AM, Miser AW, Green AA,
Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick
JA, et al: The effect of adjuvant chemotherapy on relapse-free
survival in patients with osteosarcoma of the extremity. N Engl J
Med. 314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer
Data Base report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers PA, Healey JH, Chou AJ, Wexler LH,
Merola PR, Morris CD, Laquaglia MP, Kellick MG, Abramson SJ and
Gorlick R: Addition of pamidronate to chemotherapy for the
treatment of osteosarcoma. Cancer. 117:1736–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaspar N, Occean B, Pacquement H, Bompas
E, Bouvier C, Brisse HJ, Castex MP, Cheurfa N, Corradini N, Delaye
J, et al: Results of methotrexate-etoposide-ifosfamide based
regimen (M-EI) in osteosarcoma patients included in the French
OS2006/sarcome-09 study. Eur J Cancer. 88:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadykova LR, Ntekim AI, Muyangwa-Semenova
M, Rutland CS, Jeyapalan JN, Blatt N and Rizvanov AA: Epidemiology
and risk factors of osteosarcoma. Cancer Invest. 38:259–269. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kushlinskii NE, Fridman MV and Braga EA:
Molecular mechanisms and microRNAs in osteosarcoma pathogenesis.
Biochemistry (Mosc). 81:315–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saraf AJ, Fenger JM and Roberts RD:
Osteosarcoma: Accelerating progress makes for a hopeful future.
Front Oncol. 8:42018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chong ZX, Yeap SK and Ho WY: Unraveling
the roles of miRNAs in regulating epithelial-to-mesenchymal
transition (EMT) in osteosarcoma. Pharmacol Res. 172:1058182021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Yu X, Yan Y, Wang C and Wang W:
PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 444:182–192.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
ENCODE Project Consortium, . Birney E,
Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH,
Weng Z, Snyder M, Dermitzakis ET, et al: Identification and
analysis of functional elements in 1% of the human genome by the
ENCODE pilot project. Nature. 447:799–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L and Yang L: Regulation of circRNA
biogenesis. Rna Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang G, Liang M, Liu H, Huang J, Li P,
Wang C, Zhang Y, Lin Y and Jiang X: CircRNA hsa_circRNA_104348
promotes hepatocellular carcinoma progression through modulating
miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell
Death Dis. 11:10652020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X, Huang M, Xing L, Yang R, Wang X,
Jiang R, Zhang L and Chen J: The circRNA circSEPT9 mediated by E2F1
and EIF4A3 facilitates the carcinogenesis and development of
triple-negative breast cancer. Mol Cancer. 19:732020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Lu K, Qu H, Wang H, Chen Y, Shan
T, Ge X, Wei Y, Zhou P and Xia J: CircRBM33 regulates IL-6 to
promote gastric cancer progression through targeting miR-149.
Biomed Pharmacother. 125:1098762020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou P, Xie W, Huang HL, Huang RQ, Tian C,
Zhu HB, Dai YH and Li ZY: circRNA_100859 functions as an oncogene
in colon cancer by sponging the miR-217-HIF-1α pathway. Aging
(Albany NY). 12:13338–13353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Liu Y, Cheng P, Wang C, Liu Y, Zhou
W, Xu Y and Ji G: CircRNA_0000392 promotes colorectal cancer
progression through the miR-193a-5p/PIK3R3/AKT axis. J Exp Clin
Cancer Res. 39:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arnaiz E, Sole C, Manterola L,
Iparraguirre L, Otaegui D and Lawrie CH: CircRNAs and cancer:
Biomarkers and master regulators. Semin Cancer Biol. 58:90–99.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Yang L and Chen L: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F, Tang H, Zhao S, Gao X, Yang L and Xu
J: Circ-E-Cad encodes a protein that promotes the proliferation and
migration of gastric cancer via the TGF-β/Smad/C-E-Cad/PI3K/AKT
pathway. Mol Carcinog. 62:360–368. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Wang Z, Su P, Liang Y, Li Z, Zhang
H, Song X, Han D, Wang X, Liu Y, et al: circ-EIF6 encodes
EIF6-224aa to promote TNBC progression via stabilizing MYH9 and
activating the Wnt/beta-catenin pathway. Mol Ther. 30:415–430.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng J, Li G, Wang W, Stovall DB, Sui G
and Li D: Circular RNAs with protein-coding ability in oncogenesis.
Biochim Biophys Acta Rev Cancer. 1878:1889092023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sinha T, Panigrahi C, Das D and Chandra
PA: Circular RNA translation, a path to hidden proteome. Wiley
Interdiscip Rev RNA. 13:e16852022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Li X, Xu D, Chen X, Li S, Zhang L,
Chan MTV and Wu WKK: An update on the roles of circular RNAs in
osteosarcoma. Cell Prolif. 54:e129362021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kolakofsky D: Isolation and
characterization of Sendai virus DI-RNAs. Cell. 8:547–555. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nigro JM, Cho KR, Fearon ER, Kern SE,
Ruppert JM, Oliner JD, Kinzler KW and Vogelstein B: Scrambled
exons. Cell. 64:607–613. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Capel B, Swain A, Nicolis S, Hacker A,
Walter M, Koopman P, Goodfellow P and Lovell-Badge R: Circular
transcripts of the testis-determining gene Sry in adult mouse
testis. Cell. 73:1019–1030. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cocquerelle C, Mascrez B, Hétuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Solé C and Lawrie CH: Circular RNAs and
cancer: Opportunities and challenges. Adv Clin Chem. 99:87–146.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Noto JJ, Schmidt CA and Matera AG:
Engineering and expressing circular RNAs via tRNA splicing. Rna
Biol. 14:978–984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Talhouarne GJS and Gall JG: Lariat
intronic RNAs in the cytoplasm of vertebrate cells. Proc Natl Acad
Sci USA. 115:E7970–E7977. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu Z, Filonov GS, Noto JJ, Schmidt CA,
Hatkevich TL, Wen Y, Jaffrey SR and Matera AG: Metazoan tRNA
introns generate stable circular RNAs in vivo. RNA. 21:1554–1565.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Han P, Zhou T, Guo X, Song X and
Li Y: circRNADb: A comprehensive database for human circular RNAs
with protein-coding annotations. Sci Rep. 6:349852016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang Z, Li X, Zhao J, Qian F, Feng C, Li
Y, Zhang J, Jiang Y, Yang Y, Wang Q and Li C: TRCirc: A resource
for transcriptional regulation information of circRNAs. Brief
Bioinform. 20:2327–2333. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao X, Cai Y and Xu J: Circular RNAs:
Biogenesis, mechanism, and function in human cancers. Int J Mol
Sci. 20:39262019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hansen TB, Wiklund ED, Bramsen JB,
Villadsen SB, Statham AL, Clark SJ and Kjems J: miRNA-dependent
gene silencing involving Ago2-mediated cleavage of a circular
antisense RNA. EMBO J. 30:4414–4422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li B and Li X: Overexpression of
hsa_circ_0007534 predicts unfavorable prognosis for osteosarcoma
and regulates cell growth and apoptosis by affecting AKT/GSK-3β
signaling pathway. Biomed Pharmacother. 107:860–866. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu B, Yang T, Wang Z, Zhang Y, Liu S and
Shen M: CircRNA CDR1as/miR-7 signals promote tumor growth of
osteosarcoma with a potential therapeutic and diagnostic value.
Cancer Manag Res. 10:4871–4880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang H, Yan J, Lang X and Zhuang Y:
Expression of circ_001569 is upregulated in osteosarcoma and
promotes cell proliferation and cisplatin resistance by activating
the Wnt/β-catenin signaling pathway. Oncol Lett. 16:5856–5862.
2018.PubMed/NCBI
|
|
49
|
Deng N, Li L, Gao J, Zhou J, Wang Y, Wang
C and Liu Y: Hsa_circ_0009910 promotes carcinogenesis by promoting
the expression of miR-449a target IL6R in osteosarcoma. Biochem
Biophys Res Commun. 495:189–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Z, Shi W and Jiang C: Overexpressing
circular RNA hsa_circ_0002052 impairs osteosarcoma progression via
inhibiting Wnt/β-catenin pathway by regulating miR-1205/APC2 axis.
Biochem Biophys Res Commun. 502:465–471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Sun XH, Xu HY, Pan HS, Liu Y and He
L: Circ_ORC2 enhances the regulatory effect of miR-19a on its
target gene PTEN to affect osteosarcoma cell growth. Biochem
Biophys Res Commun. 514:1172–1178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li S, Pei Y, Wang W, Liu F, Zheng K and
Zhang X: Circular RNA 0001785 regulates the pathogenesis of
osteosarcoma as a ceRNA by sponging miR-1200 to upregulate HOXB2.
Cell Cycle. 18:1281–1291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ren C, Liu J, Zheng B, Yan P, Sun Y and
Yue B: The circular RNA circ-ITCH acts as a tumour suppressor in
osteosarcoma via regulating miR-22. Artif Cells Nanomed Biotechnol.
47:3359–3367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Z, Deng M, Chen L, Wang W, Liu G, Liu
D, Han Z and Zhou Y: Circular RNA Circ-03955 promotes
epithelial-mesenchymal transition in osteosarcoma by regulating
miR-3662/metadherin pathway. Front Oncol. 10:5454602020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ding S, Zhang G, Gao Y, Chen S and Cao C:
Circular RNA hsa_circ_0005909 modulates osteosarcoma progression
via the miR-936/HMGB1 axis. Cancer Cell Int. 20:3052020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang PR, Ren J, Wan JS, Sun R and Li Y:
Circular RNA hsa_circ_0002052 promotes osteosarcoma via modulating
miR-382/STX6 axis. Hum Cell. 33:810–818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Du YX, Guo LX, Pan HS, Liang YM and Li X:
Circ_ANKIB1 stabilizes the regulation of miR-19b on SOCS3/STAT3
pathway to promote osteosarcoma cell growth and invasion. Hum Cell.
33:252–260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gao Y, Ma H, Gao Y, Tao K, Fu L, Ren R, Hu
X, Kou M, Chen B, Shi J and Wen Y: CircRNA Circ_0001721 promotes
the progression of osteosarcoma through miR-372-3p/MAPK7 axis.
Cancer Manag Res. 12:8287–8302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu YP, Wan J, Long F, Tian J and Zhang C:
circPVT1 facilitates invasion and metastasis by regulating
miR-205-5p/c-FLIP axis in osteosarcoma. Cancer Manag Res.
12:1229–1240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z,
Chen S, Yang Y, Wang S, Shen P, et al: CircMYO10 promotes
osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to
enhance the transcriptional activity of β-catenin/LEF1 complex via
effects on chromatin remodeling. Mol Cancer. 18:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li S, Pei Y, Wang W, Liu F, Zheng K and
Zhang X: Extracellular nanovesicles-transmitted circular RNA
has_circ_0000190 suppresses osteosarcoma progression. J Cell Mol
Med. 24:2202–2214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun D and Zhu D: Circular RNA
hsa_circ_0001649 suppresses the growth of osteosarcoma cells via
sponging multiple miRNAs. Cell Cycle. 19:2631–2643. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang B, Li L, Tong G, Zeng Z, Tan J, Su Z,
Liu Z, Lin J, Gao W, Chen J, et al: Circular RNA circ_001422
promotes the progression and metastasis of osteosarcoma via the
miR-195-5p/FGF2/PI3K/Akt axis. J Exp Clin Cancer Res. 40:2352021.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang C, Na N, Liu L and Qiu Y: CircRNA
hsa_circ_0005909 promotes cell proliferation of osteosarcoma cells
by targeting miR-338-3p/HMGA1 axis. Cancer Manag Res. 13:795–803.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bai Y, Li Y, Bai J and Zhang Y:
Hsa_circ_0004674 promotes osteosarcoma doxorubicin resistance by
regulating the miR-342-3p/FBN1 axis. J Orthop Surg Res. 16:5102021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li S, Liu F, Zheng K, Wang W, Qiu E, Pei
Y, Wang S, Zhang J and Zhang X: CircDOCK1 promotes the
tumorigenesis and cisplatin resistance of osteogenic sarcoma via
the miR-339-3p/IGF1R axis. Mol Cancer. 20:1612021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shi Z, Wang K, Xing Y and Yang X:
CircNRIP1 encapsulated by bone marrow mesenchymal stem cell-derived
extracellular vesicles aggravates osteosarcoma by modulating the
miR-532-3p/AKT3/PI3K/AKT axis. Front Oncol. 11:6581392021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Feng ZH, Zheng L, Yao T, Tao SY, Wei XA,
Zheng ZY, Zheng BJ, Zhang XY, Huang B, Liu JH, et al:
EIF4A3-induced circular RNA PRKAR1B promotes osteosarcoma
progression by miR-361-3p-mediated induction of FZD4 expression.
Cell Death Dis. 12:10252021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li JJ, Xiong MY, Sun TY, Ji CB, Guo RT, Li
YW and Guo HY: CircFAM120B knockdown inhibits osteosarcoma
tumorigenesis via the miR-1205/PTBP1 axis. Aging (Albany NY).
13:23831–23841. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gao Y, Liu C, Zhao X, Liu C, Bi W and Jia
J: hsa_circ_0000006 induces tumorigenesis through miR-361-3p
targeting immunoglobulin-like domains protein 1 (LRIG1) in
osteosarcoma. Ann Transl Med. 9:12422021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang M, Yu GY, Liu G and Liu WD: Circular
RNA circ_0002137 regulated the progression of osteosarcoma through
regulating miR-433-3p/IGF1R axis. J Cell Mol Med. 26:1806–1816.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang W, Wang J, Li Y and Zhao Y:
Circ_0051079 silencing inhibits the malignant phenotypes of
osteosarcoma cells by the TRIM66/Wnt/β-catenin pathway in a
miR-625-5p-dependent manner. J Bone Oncol. 35:1004362022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Y, Yuan J, Zhang Q, Ren Z, Li G and
Tian R: Circ_0016347 modulates proliferation, migration, invasion,
cell cycle, and apoptosis of osteosarcoma cells via the
miR-661/IL6R axis. Autoimmunity. 55:264–274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu Y, Qiu G, Luo Y, Li S, Xu Y, Zhang Y,
Hu J, Li P, Pan H and Wang Y: Circular RNA ROCK1, a novel circRNA,
suppresses osteosarcoma proliferation and migration via altering
the miR-532-5p/PTEN axis. Exp Mol Med. 54:1024–1037. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Peng L, Liu Q, Wu T, Li P, Cai Y, Wei X,
Zeng Y, Ye J, Chen P, Huang J and Lin H: Hsa_circ_0087302, a
circular RNA, affects the progression of osteosarcoma via the
Wnt/β-catenin signaling pathway. Int J Med Sci. 19:1377–1387. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang D, Wang Y, Wang H, Yang Y, Li L, Liu
Y and Yin X: Hsa_circ_0000591 drives osteosarcoma glycolysis and
progression by sequestering miR-194-5p and elevating HK2
expression. Clin Exp Pharmacol Physiol. 50:463–475. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Z, Zheng L, Yang L, Chen D, Ren G, Yan
X and Pu J: Hsa_circ_0020378 targets miR-556-5p/MAPK1 to regulate
osteosarcoma cell proliferation and migration. Gene.
856:1471352023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gong Z, Shen P, Wang H, Zhu J, Liang K,
Wang K, Mi Y, Shen S, Fang X and Liu G: A novel circular RNA
circRBMS3 regulates proliferation and metastasis of osteosarcoma by
targeting miR-424-eIF4B/YRDC axis. Aging (Albany NY). 15:1564–1590.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Trang NTN, Lai CY, Tsai HC, Huang YL, Liu
SC, Tsai CH, Fong YC, Tzeng HE and Tang CH: Apelin promotes
osteosarcoma metastasis by upregulating PLOD2 expression via the
Hippo signaling pathway and hsa_circ_0000004/miR-1303 axis. Int J
Biol Sci. 19:412–425. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Luo Y, Yang B, Yuan X and Zheng J:
Silencing circUSP48 suppresses osteosarcoma progression by
regulating the miR-335/smad nuclear interacting protein 1 pathway.
J Clin Lab Anal. 37:e248282023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu L, Duan J, Li M, Zhou C and Wang Q:
Circ_0000253 promotes the progression of osteosarcoma via the
miR-1236-3p/SP1 axis. J Pharm Pharmacol. 75:227–235. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu T, Song Z and Gai Y: Circular RNA
circ_0001649 acts as a prognostic biomarker and inhibits NSCLC
progression via sponging miR-331-3p and miR-338-5p. Biochem Biophys
Res Commun. 503:1503–1509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jiang Y, Hou J, Zhang X, Xu G, Wang Y,
Shen L, Wu Y, Li Y and Yao L: Circ-XPO1 upregulates XPO1 expression
by sponging multiple miRNAs to facilitate osteosarcoma cell
progression. Exp Mol Pathol. 117:1045532020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 is a circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Imataka H, Olsen HS and Sonenberg N: A new
translational regulator with homology to eukaryotic translation
initiation factor 4G. EMBO J. 16:817–825. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Morino S, Imataka H, Svitkin YV, Pestova
TV and Sonenberg N: Eukaryotic translation initiation factor 4E
(eIF4E) binding site and the middle one-third of eIF4GI constitute
the core domain for cap-dependent translation, and the C-terminal
one-third functions as a modulatory region. Mol Cell Biol.
20:468–477. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liberman N, Gandin V, Svitkin YV, David M,
Virgili G, Jaramillo M, Holcik M, Nagar B, Kimchi A and Sonenberg
N: DAP5 associates with eIF2β and eIF4AI to promote internal
ribosome entry site driven translation. Nucleic Acids Res.
43:3764–3775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lamphear BJ, Kirchweger R, Skern T and
Rhoads RE: Mapping of functional domains in eukaryotic protein
synthesis initiation factor 4G (eIF4G) with picornaviral proteases.
Implications for cap-dependent and cap-independent translational
initiation. J Biol Chem. 270:21975–21983. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hellen CU and Sarnow P: Internal ribosome
entry sites in eukaryotic mRNA molecules. Gene Dev. 15:1593–1612.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei
P, Liu H, Xu J, Xiao F, Zhou H, et al: A peptide encoded by
circular form of LINC-PINT suppresses oncogenic transcriptional
elongation in glioblastoma. Nat Commun. 9:44752018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
He L, Man C, Xiang S, Yao L, Wang X and
Fan Y: Circular RNAs' cap-independent translation protein and its
roles in carcinomas. Mol Cancer. 20:1192021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu Y, Li Z, Zhang M, Zhou H, Wu X, Zhong
J, Xiao F, Huang N, Yang X, Zeng R, et al: Rolling-translated EGFR
variants sustain EGFR signaling and promote glioblastoma
tumorigenicity. Neuro Oncol. 23:743–756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ye F, Gao G, Zou Y, Zheng S, Zhang L, Ou
X, Xie X and Tang H: circFBXW7 inhibits malignant progression by
sponging miR-197-3p and encoding a 185-aa protein in
triple-negative breast cancer. Mol Ther Nucleic Acids. 18:88–98.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pan Z, Cai J, Lin J, Zhou H, Peng J, Liang
J, Xia L, Yin Q, Zou B, Zheng J, et al: A novel protein encoded by
circFNDC3B inhibits tumor progression and EMT through regulating
Snail in colon cancer. Mol Cancer. 19:712020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Weigelt CM, Sehgal R, Tain LS, Cheng J,
Eßer J, Pahl A, Dieterich C, Grönke S and Partridge L: An
insulin-sensitive circular RNA that regulates lifespan in
Drosophila. Mol Cell. 79:268–279.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Duan JL, Chen W, Xie JJ, Zhang ML, Nie RC,
Liang H, Mei J, Han K, Xiang ZC, Wang FW, et al: A novel peptide
encoded by N6-methyladenosine modified circMAP3K4 prevents
apoptosis in hepatocellular carcinoma. Mol Cancer. 21:932022.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Song R, Ma S, Xu J, Ren X, Guo P, Liu H,
Li P, Yin F, Liu M, Wang Q, et al: A novel polypeptide encoded by
the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR.
Mol Cancer. 22:162023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Peng Y, Xu Y, Zhang X, Deng S, Yuan Y, Luo
X, Hossain MT, Zhu X, Du K, Hu F, et al: A novel protein
AXIN1-295aa encoded by circAXIN1 activates the Wnt/β-catenin
signaling pathway to promote gastric cancer progression. Mol
Cancer. 20:1582021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jiang T, Xia Y, Lv J, Li B, Li Y, Wang S,
Xuan Z, Xie L, Qiu S, He Z, et al: A novel protein encoded by
circMAPK1 inhibits progression of gastric cancer by suppressing
activation of MAPK signaling. Mol Cancer. 20:662021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang C, Zhou X, Geng X, Zhang Y, Wang J,
Wang Y, Jing J, Zhou X and Pan W: Circular RNA hsa_circ_0006401
promotes proliferation and metastasis in colorectal carcinoma. Cell
Death Dis. 12:4432021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liang ZX, Liu HS, Xiong L, Yang X, Wang
FW, Zeng ZW, He XW, Wu XR and Lan P: A novel NF-κB regulator
encoded by circPLCE1 inhibits colorectal carcinoma progression by
promoting RPS3 ubiquitin-dependent degradation. Mol Cancer.
20:1032021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gao X, Xia X, Li F, Zhang M, Zhou H, Wu X,
Zhong J, Zhao Z, Zhao K, Liu D, et al: Circular RNA-encoded
oncogenic E-cadherin variant promotes glioblastoma tumorigenicity
through activation of EGFR-STAT3 signalling. Nat Cell Biol.
23:278–291. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xia X, Li X, Li F, Wu X, Zhang M, Zhou H,
Huang N, Yang X, Xiao F, Liu D, et al: A novel tumor suppressor
protein encoded by circular AKT3 RNA inhibits glioblastoma
tumorigenicity by competing with active phosphoinositide-dependent
kinase-1. Mol Cancer. 18:1312019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z,
Xu B, Wu C, Zhou Q, Hu W, Wu C and Jiang J: A novel protein encoded
by a circular RNA circPPP1R12A promotes tumor pathogenesis and
metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer.
18:472019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang X, Jian W, Luo Q and Fang L:
CircSEMA4B inhibits the progression of breast cancer by encoding a
novel protein SEMA4B-211aa and regulating AKT phosphorylation. Cell
Death Dis. 13:7942022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Abdelmohsen K, Panda AC, Munk R,
Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM,
Martindale JL and Gorospe M: Identification of HuR target circular
RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA
Biol. 14:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Du WW, Yang W, Chen Y, Wu ZK, Foster FS,
Yang Z, Li X and Yang BB: Foxo3 circular RNA promotes cardiac
senescence by modulating multiple factors associated with stress
and senescence responses. Eur Heart J. 38:1402–1412.
2017.PubMed/NCBI
|
|
110
|
Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin
QF, Wei J, Yao RW, Yang L and Chen LL: Coordinated circRNA
biogenesis and function with NF90/NF110 in viral infection. Mol
Cell. 67:214–227.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Conn VM, Hugouvieux V, Nayak A, Conos SA,
Capovilla G, Cildir G, Jourdain A, Tergaonkar V, Schmid M, Zubieta
C and Conn SJ: A circRNA from SEPALLATA3 regulates splicing of its
cognate mRNA through R-loop formation. Nat Plants. 3:170532017.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu CX and Chen LL: Circular RNAs:
Characterization, cellular roles, and applications. Cell.
185:2016–2034. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hirano T, Ishihara K and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu Y, Liao S, Bennett S, Tang H, Song D,
Wood D, Zhan X and Xu J: STAT3 and its targeting inhibitors in
osteosarcoma. Cell Prolif. 54:e129742021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gill J and Gorlick R: Advancing therapy
for osteosarcoma. Nat Rev Clin Oncol. 18:609–624. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xi Y and Chen Y: PTEN plays dual roles as
a tumor suppressor in osteosarcoma cells. J Cell Biochem.
118:2684–2692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liau SS, Jazag A and Whang EE: HMGA1 is a
determinant of cellular invasiveness and in vivo metastatic
potential in pancreatic adenocarcinoma. Cancer Res. 66:11613–11622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cai Y, Cai T and Chen Y: Wnt pathway in
osteosarcoma, from oncogenic to therapeutic. J Cell Biochem.
115:625–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: Implications for therapy
in osteosarcoma. Expert Rev Anticanc. 11:1223–1232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Guo Y, Zi X, Koontz Z, Kim A, Xie J,
Gorlick R, Holcombe RF and Hoang BH: Blocking Wnt/LRP5 signaling by
a soluble receptor modulates the epithelial to mesenchymal
transition and suppresses met and metalloproteinases in
osteosarcoma Saos-2 cells. J Orthop Res. 25:964–971. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Duan H, Yan Z, Chen W, Wu Y, Han J, Guo H
and Qiao J: TET1 inhibits EMT of ovarian cancer cells through
activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2.
Gynecol Oncol. 147:408–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhou X, Natino D, Qin Z, Wang D, Tian Z,
Cai X, Wang B and He X: Identification and functional
characterization of circRNA-0008717 as an oncogene in osteosarcoma
through sponging miR-203. Oncotarget. 9:22288–22300. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kun-Peng Z, Xiao-Long M and Chun-Lin Z:
Overexpressed circPVT1, a potential new circular RNA biomarker,
contributes to doxorubicin and cisplatin resistance of osteosarcoma
cells by regulating ABCB1. Int J Biol Sci. 14:321–330. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lei S and Xiang L: Up-regulation of
circRNA hsa_circ_0003074 expression is a reliable diagnostic and
prognostic biomarker in patients with osteosarcoma. Cancer Manag
Res. 12:9315–9325. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lin Y, Jewell BE, Gingold J, Lu L, Zhao R,
Wang LL and Lee DF: Osteosarcoma: Molecular pathogenesis and iPSC
modeling. Trends Mol Med. 23:737–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Corre I, Verrecchia F, Crenn V, Redini F
and Trichet V: The osteosarcoma microenvironment: A complex but
targetable ecosystem. Cells. 9:9762020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Meng X, Li X, Zhang P, Wang J, Zhou Y and
Chen M: Circular RNA: An emerging key player in RNA world. Brief
Bioinform. 18:547–557. 2017.PubMed/NCBI
|
|
133
|
Xi Y, Fowdur M, Liu Y, Wu H, He M and Zhao
J: Differential expression and bioinformatics analysis of circRNA
in osteosarcoma. Biosci Rep. 39:BSR201815142019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yanbin Z and Jing Z: CircSAMD4A
accelerates cell proliferation of osteosarcoma by sponging miR-1244
and regulating MDM2 mRNA expression. Biochem Biophys Res Commun.
516:102–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Xie C, Chen B, Wu B, Guo J, Shi Y and Cao
Y: CircSAMD4A regulates cell progression and epithelial-mesenchymal
transition by sponging miR-342-3p via the regulation of FZD7
expression in osteosarcoma. Int J Mol Med. 46:107–118.
2020.PubMed/NCBI
|