Introduction
In conventional chemotherapy, antineoplastic drugs
are administered at a maximum tolerated dose (MTD), derived from
the maximum survivable dose or minimum lethal dose, with the aim of
eliminating as many proliferative tumor cells as possible (1). Accumulating evidence has indicated
that the heterogeneity of tumor tissues can result in tumor cells
that have different responses to the same chemotherapeutic regimen,
indicating that treatment regimens should be based upon a
combination of antineoplastic agents (2,3).
Moreover, the current combinations of effective MTD regimens are
still falling short of expectations for disease control and/or
regression, frequently due to severe toxicity. There have been
significant efforts to identify the optimal strategy for achieving
disease control while reducing toxicity, as drug doses and
schedules were found to be the determinants of chemotherapeutic
efficacy (4).
Metronomic chemotherapy (MCT) involves the
continuous administration of antineoplastic drugs at relatively
low, effective, minimally toxic doses and without extended
drug-free intervals (5). The mild
toxicity of MCT may enable more effective and tolerable
chemotherapy combinations, increasing the selectivity of
therapeutic strategies for cancer (6,7).
The purpose of combining low-dose antineoplastic drugs with
different modes of action is to achieve a synergy to yield a
sufficient antitumor efficacy with lower doses, and to reduce the
incidence and intensity of side effects. Nevertheless, determining
the optimal metronomic combination regimen has been challenging due
to low dosages and drug-free intervals covering numerous possible
combinations. There have been some attempts to develop metronomic
combination regimens, but they were empirical in terms of
determining the appropriate dosage and interval for administering
antineoplastic agents due to the lack of a specific definition of
MCT and standardized guidance (8,9).
Furthermore, emerging evidence has indicated that low-dose
antineoplastic agents, such as gemcitabine and cyclophosphamide,
may promote tumor growth or metastasis, suggesting a potential risk
associated with the empirical administration of chemotherapy drugs
in MCT (10,11). Therefore, metronomic combination
chemotherapy has only theoretical advantages, but presents a number
of risks and challenges in clinical practice. Further research is
urgently required to determine the optimal doses of combination
regimens, despite the proven efficacy and tolerability of
metronomic combination chemotherapy (12,13).
Vinorelbine (NVB) is an anti-tubulin agent, and one
of the most frequently used chemotherapy drugs in MCT, either in
monotherapy or in combination with other drugs, including cisplatin
(CDDP), fluorouracil (5-FU) and targeted agents such as tyrosine
kinase inhibitors and immune checkpoint inhibitors (14,15). Although NVB-based metronomic
combination therapy has yielded inconsistent clinical outcomes, the
details about this inconsistency remain unclear (16,17). CDDP and 5-FU are extensively
utilized chemotherapeutic drugs that have an antitumor effect on
various types of cancer, such as breast and colorectal cancer, by
inducing DNA lesions or obstructing DNA synthesis, respectively
(18,19). In the present study, the
dose-response associations and mechanisms of NVB-based combination
regimens were investigated, specifically NVB + CDDP and NVB + 5-FU,
both in vivo and in vitro.
Materials and methods
Chemicals
NVB (cat. no. 19990278) and CDDP (cat. no. 20040813)
were provided by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. 5-FU
(cat. no. 31020593) was purchased from Shanghai Xudong Haipu
Pharmaceutical Co., Ltd. The chemicals were prepared in 0.9% sodium
chloride solution (cat. no. 19994067), which was obtained from
Shanghai Baxter Medical Supplies Co., Ltd.
Cell lines
The human umbilical vein endothelial cell (HUVEC)
line and the 4T1 breast cancer cell line were obtained from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. The HUVEC line was cultured in DMEM media (Gibco; Thermo
Fisher Scientific, Inc.) and the 4T1 cell line was cultured in
RPMI-1640 media (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin, in a culture
chamber with 5% CO2 at 37°C.
Tumor models
Animal experiments were approved by The Biomedical
Ethics Committee of Xi'an Jiaotong University (Xi'an, China).
Furthermore, the animal experiments were performed in accordance
with the guide for the care and use of laboratory animals during
the treatment period (20).
Female BALB/c mice (6-8 weeks old; 18-22 g; GemPharmatech Co.,
Ltd.) were housed under a capacious and controlled environment with
constant humidity, temperature (20-22°C) and a 12-h light cycle, as
well as with unlimited access to chow and water to prevent death
caused by environmental and feeding factors.
Mouse models of tumor growth or metastasis were
established by injecting 1×106 in 0.1 ml 4T1 tumor cells
into the subcutaneous groin or tail vein of each mouse,
respectively. In the present study, the experimental doses were
determined based on published research and preliminary experiments
(21,22). All mice were randomly grouped into
the control or treatment groups (6 mice/group), and i.p. injected
with either 0.9% sodium chloride solution (control), NVB + CDDP
(0.03125/0.05, 0.0625/0.1, 0.125/0.2, 0.25/0.4, 0.5/0.8, 1/1.6 or
2/3.2 mg/kg) or NVB + 5-FU (0.0525/3.9375, 0.105/7.875, 0.21/15.75,
0.42/31.5 or 0.84/63 mg/kg) in a volume of 0.1 ml/10 g body weight,
24 h after tumor cell inoculation; this dosing regimen was repeated
every other day for 2 weeks. The health and behavior of the mice
and the size of the tumors were monitored daily. Notably, the diet
of the animals was closely monitored due to the common
gastrointestinal reactions such as nausea, vomiting and anorexia,
associated with these chemotherapeutic drugs (23). The tumor burden did not exceed 5%
of the initial body weight of the mouse, which is commonly
considered a humane endpoint in mouse models. At the end of the
study, the mice were anesthetized by intraperitoneal injection of
20% (1 g/kg) urethane solution and sacrificed by cervical
dislocation. The blood, tumors and lungs of the tumor-bearing mice
were collected for further examination.
Cell-counting kit-8 (CCK-8) and MTT
assays
HUVEC and 4T1 cell viability was measured by CCK-8
or MTT assays. The maximal concentration (Cmax) of NVB +
CDDP or NVB + 5-FU was determined using the following formula:
Cmax(NVB + CDDP)=IC50(NVB) +
IC50(CDDP) or Cmax(NVB +
5-FU)=IC50(NVB) + IC50(5-FU). Next, the
cells were seeded at 2.5×103 cells/well in a 96-well
plate and treated with a series of concentrations of NVB + CDDP and
NVB + 5-FU. The cells were incubated with CCK-8 (cat. no. AR1160;
Boster Biological Technology) or MTT (cat. no. 1334MG250; BioFroxx;
neoFroxx) reagent for 1 or 4 h, respectively, before measuring the
absorbance using a Multimode Reader (Synergy LX; BioTek) at 450 nm
for CCK-8 or 490 nm for MTT.
Cell apoptosis
Briefly, 1×105 HUVECs or 4T1 tumor cells
per well were seeded in a 6-well plate and harvested by
centrifugation (1,000 × g, 5 min,) 4°C) following treatment with
antineoplastic agents. The HUVECs or 4T1 cells were treated with
NVB + CDDP (0.03/88 and 3.6/1.1×104 nM; 0.125/37.5 and
4/1.2×103 nM) and NVB + 5-FU (0.1/1.4×103 and
3.6/4.5×104 nM; 0.06/5.3 nM and 4/340 nM). The harvested
cells were processed using an Annexin V-PE/7-AAD Apoptosis
Detection Kit (cat. no. G1512-50; Wuhan Servicebio Technology Co.,
Ltd.), following the manufacturer's instructions. Next, cell
apoptosis was measured by Flow Cytometry (FACSCanto™ II; BD
Biosciences) and analyzed by FlowJo version 10 software (FlowJo
LLC).
Wound healing assay and Transwell
assay
The HUVECs or 4T1 cells were treated with NVB + CDDP
(0.03/88 and 3.6/1.1×104 nM; 0.25/75 and
4/1.2×103 nM) and NVB + 5-FU (0.1/1.4×103 and
3.6/4.5×104 nM; 0.03/2.7 nM and 4/340 nM) in
vitro, respectively.
For wound healing assay, HUVECs or 4T1 cells were
seeded and cultured in a 6-well plate with media supplemented with
10% FBS until 80-90% confluency was reached. The cell layer was
then wounded using a 200-μl pipette tip and washed with PBS
to remove the non-adherent cells. Images were captured using a
light microscope (XD-202; Nanjing Jiangnan Novel Optics Co., Ltd.)
with ×100 magnification before and after the cells were incubated
with serum-free media containing antineoplastic agents for 24 h at
37°C. The relative change of the wound area was analyzed using
Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.).
The Transwell assay was performed using a 24-well
plate with 8-μm pore chambers (MilliporeSigma), as
previously described (24). The
top chamber was precoated with Matrigel Matrix (cat. no. 354234;
Corning, Inc.) for 4 h at 37°C. Next, 5×105 cells were
suspended with serum-free media and seeded in each chamber, and
complete media containing antineoplastic agents were placed in the
bottom wells. After 24 h incubation, the non-invaded cells were
wiped away and the invaded cells were fixed with 4%
paraformaldehyde for 10 min, followed by staining with 0.1% crystal
violet dye for 10 min, both at room temperature. Images were
captured using a light microscope (XD-202; Nanjing Jiangnan Novel
Optics Co., Ltd.) with ×100 magnification.
Hematoxylin & eosin (H&E)
staining, immunohistochemistry and immunofluorescence
The tissues, including tumor and lung tissues, were
fixed by immersing in a 4% paraformaldehyde solution for >24 h
at room temperature. After fixation, the tissues were embedded in
paraffin and cut into slices (5-μm thickness). The lung
slices were deparaffinized by a xylene and ethanol series for 5-10
min and stained with hematoxylin (1-2 min) and eosin (5 min) at
room temperature to analyze the tumor metastatic nodules in the
lung tissues. The images were digitized using a 3D Histech Scanner
System (DX12; 3DHISTECH Ltd.).
Immunohistochemical and immunofluorescence staining
were conducted as previously reported (25). Briefly, the dewaxed tissue slice
(treated as aforementioned) was incubated with 3%
H2O2 to block the endogenous peroxidase
activity, immersed in sodium citrate for antigen retrieval
(95-100°C for 15 min) and blocked with 5% bovine serum albumin
(cat. no. AR0004; Boster Biological Technology) for 30 min at 37°C.
For immunohistochemistry, the slice was incubated with osteopontin
(OPN; 1:1,000; cat. no. ab283656; Abcam), matrix metalloproteinase
(MMP)-2 (1:1,000; cat. no. GB11130; Wuhan Servicebio Technology
Co., Ltd.) and MMP-9 (1:5,000; cat. no. ab283575; Abcam) primary
antibodies overnight at 4°C, incubated with a biotin conjugated IgG
(cat. no. BA1003; Boster Biological Technology) secondary antibody
for 30 min at 37°C, stained with DAB and then counterstained with
hematoxylin for 1-2 min at room temperature. For
immunofluorescence, the slice was incubated with CD31 (1:800; cat.
no. GB11063-2; Wuhan Servicebio Technology Co., Ltd.), CD11b
(1:1,000; cat. no. 133357; Abcam), F4/80 (1:1,000; cat. no. 300421;
Abcam) and CD206 (1:400; cat. no. GB113497-100; Wuhan Servicebio
Technology Co., Ltd.) primary antibodies overnight at 4°C. The
slice was then incubated with Cy3 (1:1,000; cat. no. GB21303; Wuhan
Servicebio Technology Co., Ltd.) or Alexa Fluor® 488
(1:1,000; cat. no. GB25303; Wuhan Servicebio Technology Co.,
Ltd.)-conjugated IgG for 50 min at 37°C. Finally, the slice was
stained with DAPI (cat. no. AR1177; Boster Biological Technology)
for 10 min at room temperature. Images were obtained using a
fluorescent Zeiss Axio Imager (Carl Zeiss AG).
Western blotting
The tumor tissues were lysed with RIPA lysis buffer
(cat. no. R0020; Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with 1% protease and 1% phosphatase inhibitors.
The protein concentrations were quantified using a BCA protein
quantification kit (cat. no. G2026; Wuhan Servicebio Technology
Co., Ltd.). Equal amounts of 15 μg denatured protein per
lane were then separated on 10% SDS-PAGE gels and transferred to
PVDF membranes (MilliporeSigma). The membranes were immersed in 5%
(w/v) non-fat milk for 2 h at room temperature to block
non-specific binding and were then cut horizontally according to
the different molecular weights. The cut membranes were then
incubated with the following targeted primary antibodies overnight
at 4°C: β-actin (1:5,000; cat. no. bs-0061R; Bioss), VEGF (1:800;
cat. no. bs-0279R; Bioss), VEGFR1 (1:1,000; cat. no.
32152; Abcam), VEGFR2 (1:1,000; cat. no. 9698s; CST
Biological Reagents Co., Ltd.), NF-kB (1:1,000; cat. no. 8242; CST
Biological Reagents Co., Ltd.), caspase-3 (1:1,000; cat. no. 9662;
CST Biological Reagents Co., Ltd.), cleaved-caspase-3 (1:1,000;
cat. no. 9661; CST Biological Reagents Co., Ltd.), caspase-8
(1:1,000; cat. no. 4790T; CST Biological Reagents Co., Ltd.) and
cleaved-caspase-8 (1:1,000; cat. no. 8592T; CST Biological Reagents
Co., Ltd.), followed by incubation with an HRP-conjugated secondary
antibody (1:8,000; cat. no. BA1054; Boster Biological Technology)
for 2 h at 37°C. The signal was examined using Clarity Western ECL
Substrates (Bio-Rad Laboratories, Inc.) and a ChemiDoc™ XRS+ System
(Bio-Rad Laboratories, Inc.). The analysis was performed using
Image Lab software version 3.0 (Bio-Rad Laboratories, Inc.).
Flow cytometry
The blood collected from the tumor-bearing mice was
lysed with ammonium-chloride-potassium lysis buffer (cat. no.
00-4333-57; Thermo Fisher Scientific, Inc.) and washed with
pre-cooled PBS to harvest cells by centrifugation (1,000 × g, 5 min
at 4°C). The cells were incubated with PE anti-mouse Ly-6G/Ly-6C
(Gr1; 0.25 μg/μl; cat. no. 108407; BioLegend, Inc.),
FITC anti-mouse CD11b (0.25 μg/μl; cat. no. 101205;
BioLegend, Inc.) and APC anti-mouse Ki-67 (0.25
μg/μl; cat. no. 652405; BioLegend, Inc.) antibodies
for 40 min at 4°C. The positive cells were measured using a
FACSCanto II Flow Cytometry System (BD Biosciences) and analyzed by
FlowJo version 10 (FlowJo LLC).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 19.0; IBM Corp.). Data are presented as the mean
± SD. Comparisons of two or more groups were conducted using an
unpaired sample t-test or one-way ANOVA with the Bonferroni post
hoc test, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dual-directional effect of NVB combined
with CDDP or 5-FU on tumor growth and metastasis
The dose-response association of NVB combined with
CDDP or 5-FU on tumor growth was evaluated using a breast cancer
mouse model, which involved the subcutaneous injection of 4T1 tumor
cells into the inguinal area of BALB/c mice. The tumor weight
increased by 37.32, 40.09 and 54.09% (P<0.01, P<0.01 and
P<0.05, respectively) following treatment with 0.03125/0.05,
0.0625/0.1 and 0.125/0.2 mg/kg NVB + CDDP, respectively, but
decreased by 37.17, 45.60 and 72.20% (all P<0.01) following
treatment with 0.5/0.8, 1/1.6 and 2/3.2 mg/kg NVB + CDDP,
respectively, compared with the control (Fig. 1A and B). One mouse in the
0.03125/0.05 mg/kg NVB + CDDP treatment group died on day 6 due to
internal bleeding caused by an accident during the subcutaneous
injection process. A similar result was observed in the NVB + 5-FU
treatment groups (Fig. 1C), where
the tumor growth increased by 67.17, 60.55 and 58.34% (P<0.01,
P<0.01 and P<0.05, respectively) following treatment with
0.0525/3.9375, 0.105/7.875 and 0.21/15.75 mg/kg NVB + 5-FU,
respectively, while tumor growth decreased by 28.79% (P<0.05)
following treatment with 0.84/63 mg/kg NVB + 5-FU. As shown in
Fig. 1D and E, in the group
receiving 0.84/63 mg/kg NVB + 5-FU, one out of the six mice
exhibited weight loss (11.05%) and experienced mild diarrhea (wet
and soft stool), ultimately resulting in death on day 12, possibly
due to an adverse intestinal reaction (26).
 | Figure 1Effects of NVB combined with CDDP or
5-FU on tumor growth or metastasis in vivo. Analysis of the
4T1 tumor weight of BALB/c mice (n=6) treated with (A) low or (B)
high doses of NVB + CDDP, or (C) NVB + 5-FU, administered once
every other day for 2 weeks. (D) Survival curve of BALB/c mice
treated with NVB + 5-FU. (E) Weight change of the mouse treated
with 0.84/63 mg/kg NVB + 5-FU that died on day 12. (F)
Representative images and (G) analysis of pulmonary metastatic
nodules of BALB/c mice (n=3) treated with NVB + CDDP for 2 weeks,
as well as the (H) analysis and (I) images of the NVB + 5-FU
treatment groups. Scale bar, 1,000 μm.
*P<0.05, **P<0.01 vs. control. 5-FU,
fluorouracil; CDDP, cisplatin; Con, control; NVB, vinorelbine. |
Based on the aforementioned findings, the effect of
NVB + CDDP or NVB + 5-FU on tumor metastasis was further
investigated in a BALB/c metastasis mouse model where 4T1 cells
were injected into the tail vein. The H&E results demonstrated
that the number of pulmonary metastatic nodules increased by 43.75%
(P<0.05) following treatment with 0.25/0.4 mg/kg NVB + CDDP and
increased by 81.25% (P<0.01) and 28.13% (P<0.05) following
0.21/15.75 and 0.42/31.5 mg/kg NVB + 5-FU treatment, respectively,
compared with the untreated group (Fig. 1F-I). However, the number of
pulmonary metastatic nodules decreased by 59.38% (P<0.01)
following 0.84/63 mg/kg NVB + 5-FU treatment, compared with the
untreated group (Fig. 1F-I).
Notably, although 2/3.2 mg/kg NVB + CDDP significantly inhibited
tumor growth, this dose led to an insignificant reduction in
pulmonary metastases.
Collectively, these results indicated that
metronomic administration of NVB + CDDP or NVB + 5-FU induced a
possible dual-directional action of promoting and suppressing tumor
growth and metastasis at different doses.
Dual-directional effect of NVB combined
with CDDP or 5-FU on angiogenesis
To explore whether angiogenesis was involved in the
dual-directional effect of metronomic combined regimens on tumor
growth and metastasis, microvessel density (MVD) was observed in
tumor and lung samples from the statistically significant groups by
staining with a CD31 antibody, which is a surface marker of
neovascular endothelial cells. The MVD significantly increased in
tumor tissues by 44.05% (P<0.05) following treatment with
0.0625/0.1 mg/kg NVB + CDDP, compared with the control group
(Fig. 2A and B). Meanwhile, the
MVD increased by 145.99% (P<0.01) following 0.21/15.75 mg/kg NVB
+ 5-FU treatment and decreased by 51.77% (P<0.05) following
0.84/63 mg/kg NVB + 5-FU treatment, compared with the control group
(Fig. 2C and D). In the lungs
with metastatic tumors, 0.21/15.75 mg/kg NVB + 5-FU treatment
resulted in a significant increase (132.78%) in MVD and 0.84/63
mg/kg NVB + 5-FU treatment led to a 47.60% decrease, compared with
the untreated group (both P<0.01; Fig. 2E and F).
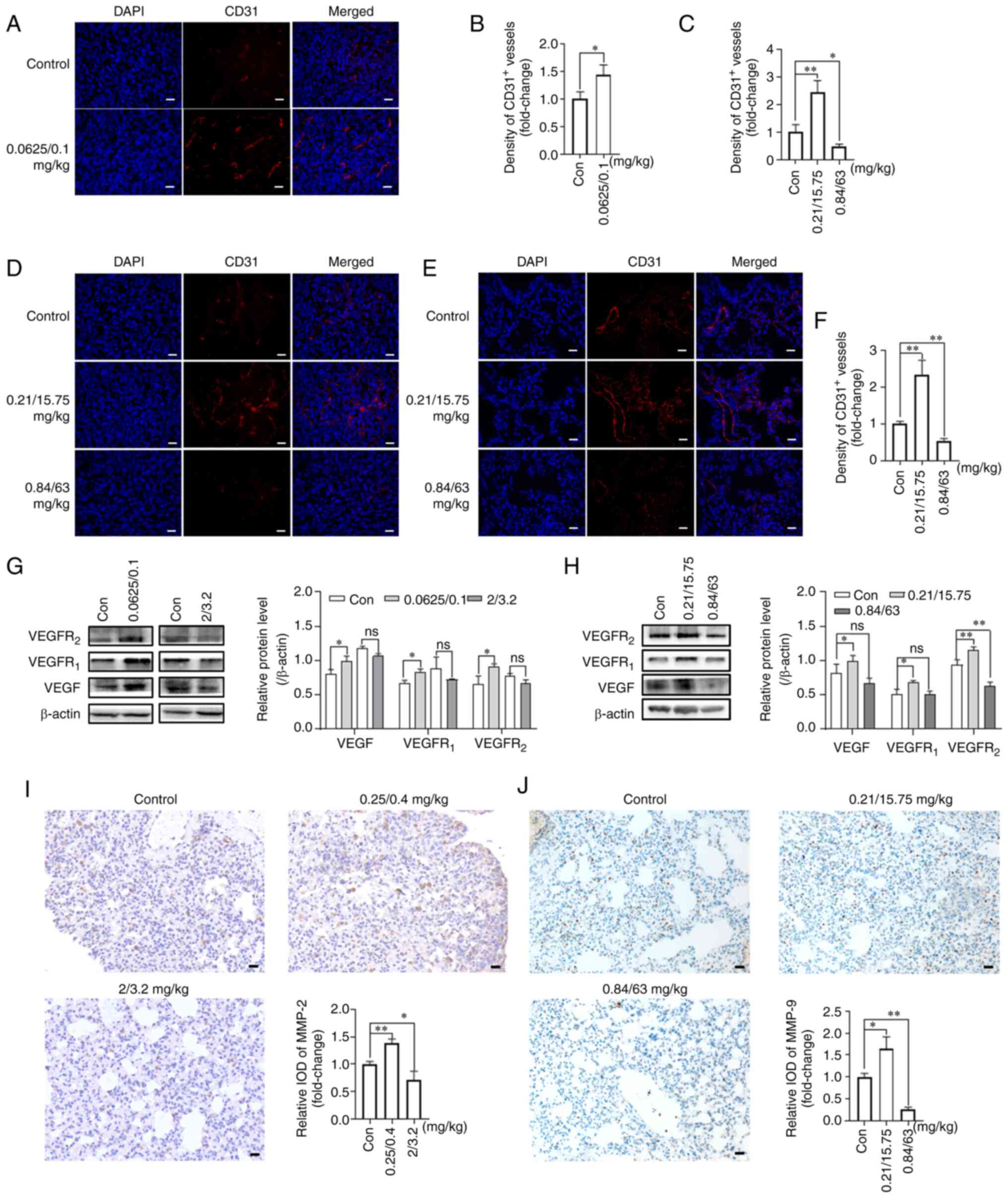 | Figure 2Effects of NVB combined with CDDP or
5-FU on angiogenesis. (A) Representative images and (B) analysis of
CD31 (red) in 4T1 tumors treated with 0.0625/0.1 mg/kg NVB + CDDP
using immunofluorescence. The cell nuclei were counterstained with
DAPI (blue). (C) Analysis and (D) representative images of CD31 in
tumors of BALB/c mice treated with NVB + 5-FU, as well as (E)
images and (F) analysis of CD31 in the lungs. VEGF, VEGFR1 and
VEGFR2 protein levels were detected in 4T1 tumors following
treatment with (G) NVB + CDDP or (H) NVB + 5-FU using western
blotting. Detection of (I) MMP-2 and (J) MMP-9 in the lungs of
metastatic BALB/c mice treated with NVB + CDDP or NVB + 5-FU using
immunohistochemistry. n=3. Scale bar, 20 μm.
*P<0.05, **P<0.01 vs. control. 5-FU,
fluorouracil; CDDP, cisplatin; Con, control; IOD, integral optical
density; MMP, matrix metalloproteinase; ns, not significant; NVB,
vinorelbine; ns, not significant. |
The expression levels of pro-angiogenic proteins,
VEGF, VEGFR1 and VEGFR2, in the tumors were
also determined by western blotting. As shown in Fig. 2G, 0.0625/0.1 mg/kg NVB + CDDP
significantly increased the VEGF, VEGFR1 and
VEGFR2 protein expression levels by 22.69, 23.90 and
37.57% (all P<0.05), respectively, compared with the untreated
group. However, the higher dose of 2/3.2 mg/kg NVB + CDDP reduced
the expression of VEGF, VEGFR1 and VEGFR2 by
9.08, 18.35 and 15.11%, respectively, compared with the untreated
group, but the changes were not statistically significant.
Similarly, the VEGF, VEGFR1 and VEGFR2
expression levels were significantly upregulated by 29.00, 34.32
and 23.85%, respectively (P<0.05, P<0.05 and P<0.01,
respectively) in the 0.21/15.75 mg/kg NVB + 5-FU treatment group
compared with the untreated group (Fig. 2H). However, the VEGF and
VEGFR1 expression levels were non-significantly reduced
by 18.36 and 0.50%, respectively, and the VEGFR2
expression level was significantly reduced by 32.20% (P<0.01),
following 0.84/63 mg/kg NVB + 5-FU, compared with the untreated
group (Fig. 2H).
The expression levels of angiogenesis-related
proteins, MMP-2 and MMP-9, in the lung tissues were also assessed.
The MMP-2 expression level was higher (38.20%; P<0.01) in the
0.25/0.4 mg/kg NVB + CDDP group and lower (29.43%; P<0.05) in
the 2/3.2 mg/kg NVB + CDDP group compared with that in the control
group (Fig. 2I). In addition,
0.21/15.75 mg/kg NVB + 5-FU increased the level of MMP-9 by 64.33%
(P<0.05), whereas 0.84/63 mg/kg NVB + 5-FU decreased the level
of MMP-9 by 73.65% (P<0.01), compared with the untreated group
(Fig. 2J).
Collectively, these findings indicated that
angiogenesis likely played a role in regulating tumor growth and
metastasis in the present study, and the enhancement of
angiogenesis was particularly notable at low doses of combined
treatment.
Promotion of apoptosis in tumors treated
with low and high doses of NVB combined with CDDP or 5-FU
To observe the impact of metronomic NVB-based
combination regimens on apoptosis, the expression levels of
apoptosis-related proteins in tumor tissues were measured using
western blotting. The levels of cleaved-caspase-3, caspase-3 and
caspase-8, but not cleaved-caspase-8, were increased by 30.04,
29.22 and 45.72% (P<0.01, P<0.01 and P<0.05),
respectively, following treatment with 0.84/63 mg/kg NVB + 5-FU,
compared with the untreated group (Fig. 3A). Notably, low-dose 0.21/15.75
mg/kg NVB + 5-FU also significantly increased the expression levels
of cleaved-caspase-3, cleaved-caspase-8 and caspase-8 by 53.52,
44.69 and 32.48% (P<0.01, P<0.01 and P<0.05),
respectively, compared with the control group. Similar results were
obtained in the low-dose NVB + CDDP treatment groups. 0.0625/0.1
mg/kg NVB + CDDP significantly increased the expression levels of
cleaved-caspase-3, caspase-3, cleaved-caspase-8 and caspase-8 by
29.44, 35.06, 51.98 and 27.04% (P<0.05, P<0.01, P<0.01 and
P<0.01), respectively, compared with the untreated group
(Fig. 3B). These findings
suggested that either low or high dose treatments enhanced the
expression of apoptotic proteins. Meanwhile, the inhibition of
tumor growth induced by high-dose treatment was related to the
upregulation of apoptosis-related proteins.
 | Figure 3Effects of NVB combined with CDDP or
5-FU on apoptosis and Ki67 expression. Detection of the
cleaved-caspase-3, caspase-3, cleaved-caspase-8 and caspase-8 in
4T1 tumors following treatment with (A) NVB + 5-FU or (B) NVB +
CDDP by western blotting. (C) NF-κB protein levels in 4T1 tumors
treated with or NVB + CDDP. (D) Detection of OPN (brown) in 4T1
tumors following treatment with low dose and (E) high dose of NVB +
CDDP using immunohistochemical staining. (F) Representative images
and (G) analysis of OPN in 4T1 tumors in the NVB + 5-FU treatment
group. (H) Analysis and (I) images of Ki67+ cells in the
blood of tumor metastatic mice after treatment with NVB + CDDP
using flow cytometry, as well as the (J) analysis and (K) images
after NVB + 5-FU treatment. (L) Detection of Ki67+ cells
in the lungs of metastatic BALB/c mice treated with NVB + 5-FU.
n=3. Scale bar, 20 μm. *P<0.05,
**P<0.01 vs. control. 5-FU, fluorouracil; CDDP,
cisplatin; Con, control; IOD, integral optical density; ns, not
significant; NVB, vinorelbine; OPN, osteopontin; ns, not
significant. |
Previous studies suggest that, despite its known
role in tumor suppression, apoptosis may stimulate tumor growth
through the participation of NF-κB and OPN (27,28). In the present study, it was found
that the expression level of NF-κB was higher (25.45%; P<0.05)
in the 0.0625/0.1 mg/kg NVB + CDDP group than in the control group,
and lower (14.42%;) in the 2/3.2 mg/kg NVB + CDDP group, but this
result was not significant (Fig.
3C). Meanwhile, 0.0625/0.1 mg/kg NVB + CDDP increased the OPN
expression level by 51.39% (P<0.01), whereas 2/3.2 mg/kg NVB +
CDDP decreased the OPN expression level by 49.52% (P<0.05),
compared with the untreated group (Fig. 3D and E). Furthermore, the OPN
expression level increased by 29.7% in the 0.21/15.75 mg/kg NVB +
5-FU group and decreased by 41.35% in the 0.84/63 mg/kg NVB + 5-FU
group, compared with the untreated group (both P<0.05; Fig. 3F and G). These findings
demonstrated that the role of apoptosis in tumor growth may be
influenced by NF-κB and OPN, at low treatment doses.
The Ki67 index, a well-recognized marker for the
evaluation of cell proliferation, independently predicts the
malignancy of tumors (29,30).
Therefore, the effect of NVB-based combination regimens on Ki67
expression was investigated in the blood and lungs of mice with
metastatic tumors. Although no significant differences were
identified in the percentage of Ki67+ cells in the blood
of different subgroups treated with NVB + CDDP or NVB + 5-FU
(Fig. 3H-K), the number of
Ki67+ cells in the lungs increased following treatment
with 0.21/15.75 mg/kg NVB + 5-FU but decreased following 0.84/63
mg/kg NVB + 5-FU (Fig. 3L). These
results suggested that the number of Ki67+ cells in
tissues may be more valuable in the assessment of cell
proliferation rates than the levels in peripheral blood.
Dual-directional effect of NVB combined
with CDDP or 5-FU on the apoptosis of endothelial and tumor cells
in vitro
To explore the direct effect of NVB combined with
CDDP or 5-FU on endothelial and tumor cells, cell viability,
apoptosis, migration and invasion rates were assessed in
vitro. The 50% inhibitory concentration (IC50) of
NVB, CDDP and 5-FU were 3.6, 1.1×104 and
4.5×104 nM for HUVECs, respectively. HUVEC viability was
significantly suppressed following treatment with NVB + CDDP
[concentrations ≥1/8 of the Cmax(NVB + CDDP)] and NVB +
5-FU [concentrations ≥1/32 of the Cmax(NVB + 5-FU)]
(Fig. 4A). Meanwhile, HUVEC
apoptosis significantly increased by 220.66 and 133.81% (both
P<0.01) following exposure to 3.6/1.1×104 nM NVB +
CDDP and 3.6/4.5×104 nM NVB + 5-FU, whereas it was
inhibited by 32.89 and 28.31% (both P<0.05) following treatment
with 0.03/88 nM NVB + CDDP and 0.1/1.4×103 nM NVB +
5-FU, compared with untreated cells (Fig. 4B). In addition, the migration and
invasion levels of HUVECs were significantly upregulated by 107.75%
(P<0.01) and 40.18% (P<0.05), respectively, following
exposure to 0.03/88 nM NVB + CDDP, and by 141.11% (P<0.01) and
46.72% (P<0.05), respectively, following treatment with
0.1/1.4×103 nM NVB + 5-FU, compared with the respective
control cells (Fig. 4C and D).
However, higher concentrations of 3.6/1.1×104 nM NVB +
CDDP or 3.6/4.5×104 nM NVB + 5-FU slightly inhibited
migration and invasion, compared with the control cells, but these
changes were not statistically significant.
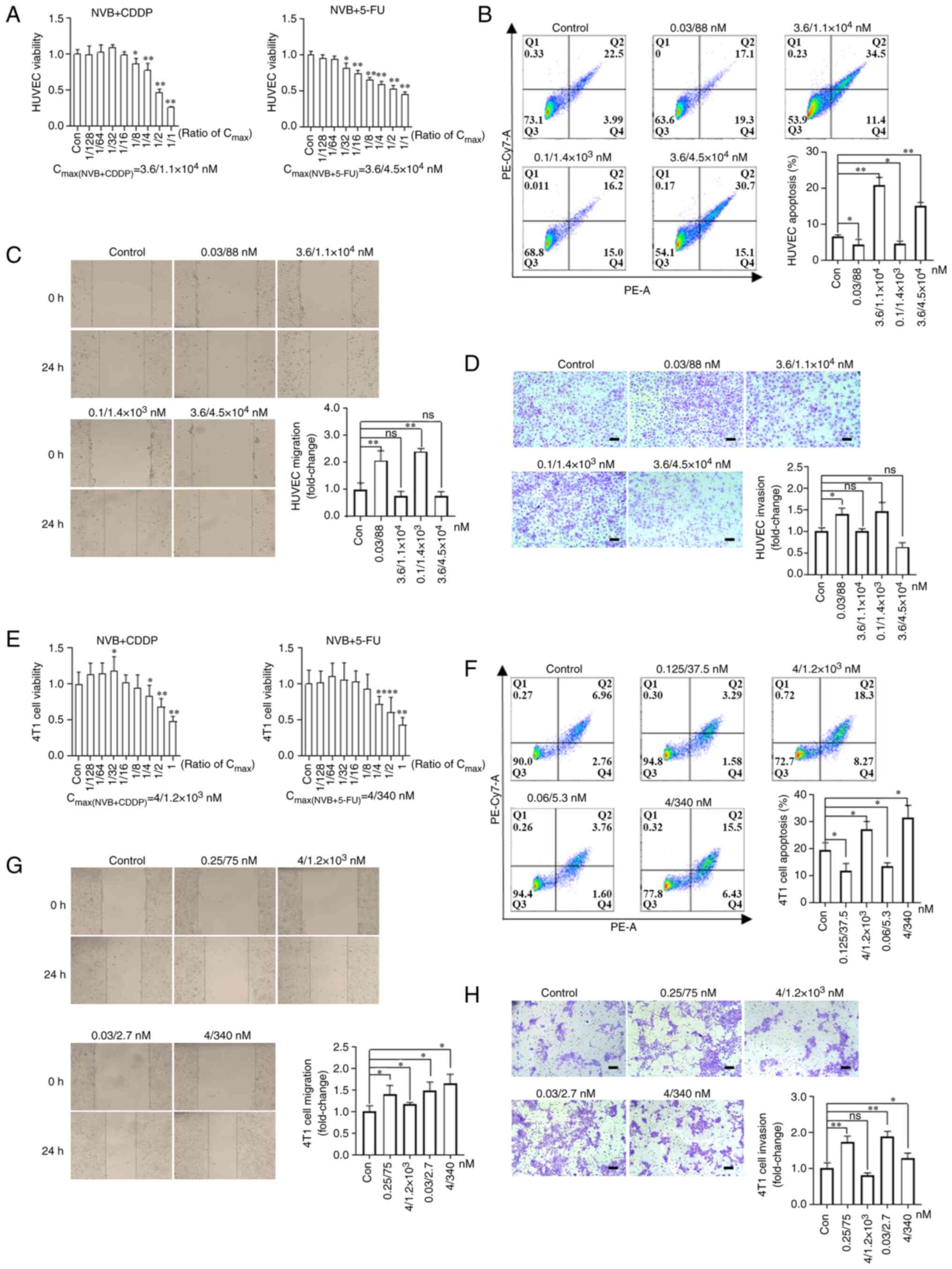 | Figure 4Effects of NVB combined with CDDP or
5-FU on the functions of endothelial and 4T1 tumor cells. (A)
Analysis of HUVEC viability following treatment with NVB + CDDP or
NVB + 5-FU for 48 h using an MTT assay (n=6). (B) Detection of
HUVEC apoptosis induced by NVB + CDDP or NVB + 5-FU by staining
with Annexin V-PE and 7-AAD (n=3). HUVEC (C) migration and (D)
invasion were investigated following treatment with NVB + CDDP or
NVB + 5-FU by wound healing and Transwell assays, respectively
(n=3). (E) Cell viability, (F) apoptosis, (G) migration and (H)
invasion of 4T1 tumor cells following treatment with NVB + CDDP or
NVB + 5-FU. Scale bar, 100 μm. *P<0.05,
**P<0.01 vs. control. 5-FU, fluorouracil; CDDP,
cisplatin; Con, control; HUVEC, human umbilical vein endothelial
cell; ns, not significant; NVB, vinorelbine; ns, not
significant. |
For 4T1 tumor cells, the IC50 values of
NVB, CDDP and 5-FU were 4, 1.2×103 and 340 nM,
respectively. NVB + CDDP and NVB + 5-FU had a significant
inhibitory effect on cell viability following exposure to
concentrations of ≥1/4 of the Cmax, and a significant
enhancement effect was observed following exposure to 1/32 of the
Cmax of NVB + CDDP, compared with the untreated cells
(Fig. 4E). The apoptosis levels
of 4T1 cells were significantly increased by 40.38 and 61.86%
following treatment with 4/1,200 nM NVB + CDDP and 4/340 nM NVB +
5-FU, respectively, but were decreased by 39.74 and 30.41%
following treatment with 0.125/37.5 nM NVB + CDDP and 0.06/5.3 nM
NVB + 5-FU, respectively, compared with untreated cells (all
P<0.05; Fig. 4F). Furthermore,
treatment with 0.25/75 nM NVB + CDDP significantly increased
migration and invasion by 39.87% (P<0.05) and 73.19%
(P<0.01), respectively, and treatment with 0.03/2.7 nM NVB +
5-FU significantly increased migration and invasion by 48.36%
(P<0.05) and 88.19% (P<0.01), respectively, compared with the
respective controls (Fig. 4G and
H). Notably, a high concentration of 4/340 nM NVB + 5-FU
increased cell migration and invasion by 65.10 and 28.09% (both
P<0.05), compared with untreated cells, and 4/1.2×103
nM NVB + CDDP increased migration by 18.03% (P<0.05), compared
with untreated cells, but no marked effect on invasion was
observed.
Collectively, the results indicated that low
concentration NVB-based combination treatments suppressed the
apoptosis of endothelial and tumor cells, and stimulated migration
and invasion. Moreover, high concentrations had an
antiproliferative and apoptotic effect on both endothelial and
tumor cells but induced inconsistent effects on cell migration and
invasion.
Dual-directional effect of NVB combined
with CDDP or 5-FU on myeloid-derived suppressor cells (MDSCs) and
macrophages
MDSCs are regarded as important contributors to
immunosuppression of the tumor microenvironment (31). To observe the effect of metronomic
NVB-based combination regimens on immune cells, MDSC levels were
initially measured in the blood and tumors of mice from the tumor
growth model, as well as in the lungs of mice from the tumor
metastasis model. It was found that the percentage of
Gr1+CD11b+ MDSCs in the peripheral blood
increased by 36.50% (P<0.05) and 77.44% (P<0.01) in the
0.0625/0.1 and 0.125/0.2 mg/kg NVB + CDDP treatment groups,
respectively, compared with the control (Fig. 5A). In addition, treatments with
0.0525/3.9375, 0.105/7.875 and 0.21/15.75 mg/kg NVB + 5-FU
increased the percentage of Gr1+CD11b+ MDSCs
by 80.59, 90.75 and 83.87% (all P<0.01), respectively, while
0.84/63 mg/kg NVB + 5-FU decreased the percentage of
Gr1+CD11b+ MDSCs by 80.32% (P<0.01),
compared with the control (Fig.
5B). In tumor tissues, the levels of CD11b+ MDSCs
were higher (71.43%) following treatment with 0.21/15.75 mg/kg NVB
+ 5-FU, and lower (51.79%) following 0.84/63 mg/kg NVB + 5-FU,
compared with those in untreated mice (both P<0.01; Fig. 5C and D). In lung tissues,
0.21/15.75 mg/kg NVB + 5-FU significantly increased the number of
CD11b+ MDSCs by 52.00%, and 0.84/63 mg/kg NVB + 5-FU
decreased the number of CD11b+ MDSCs by 72.00% (both
P<0.01; Fig. 5E and F).
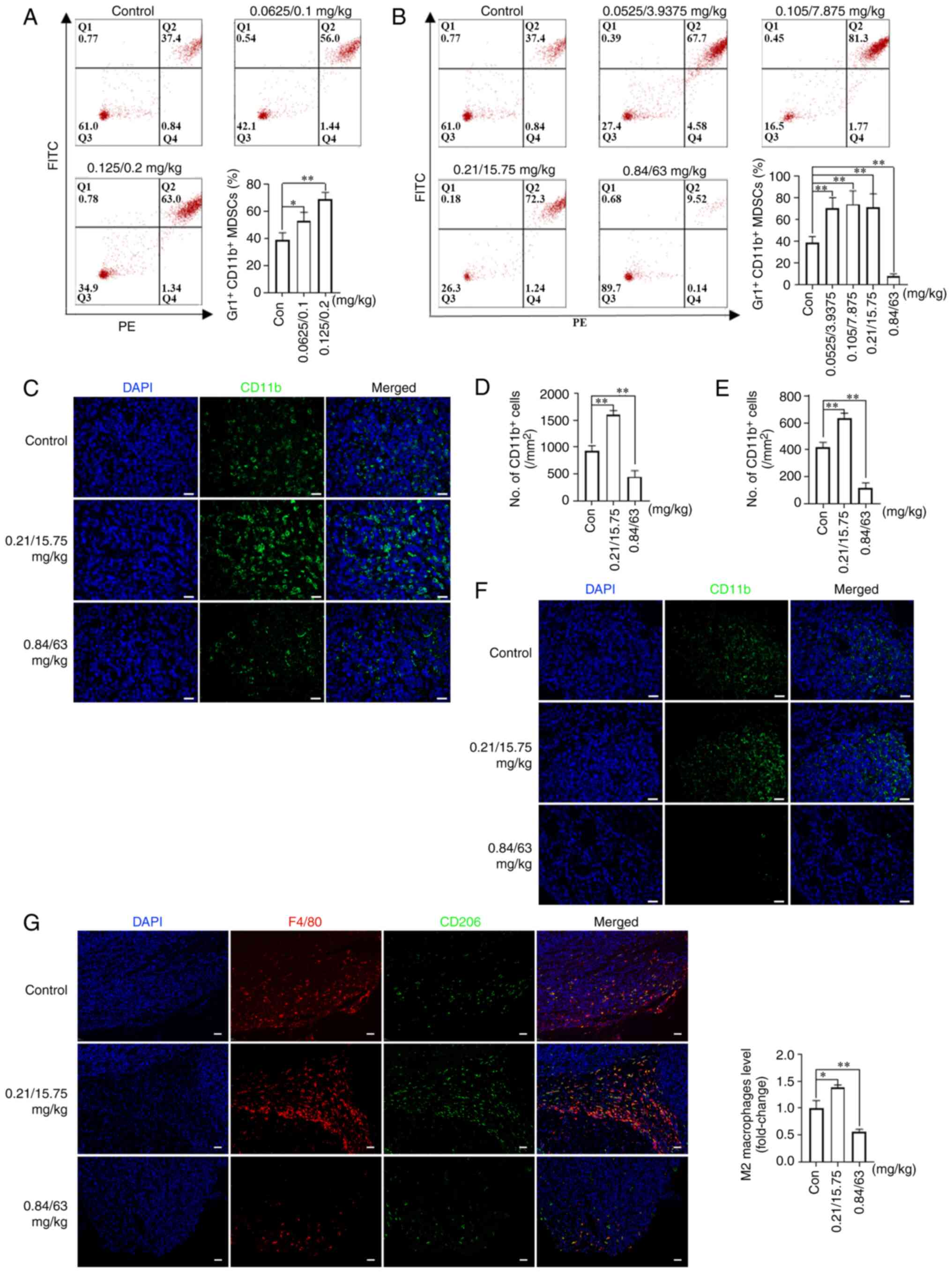 | Figure 5Effects of NVB combined with CDDP or
5-FU on MDSCs and macrophages. The percentage of MDSCs in the
peripheral blood of BALB/c mice following treatment with (A) NVB +
CDDP or (B) NVB + 5-FU was measured by staining with Gr-1 and CD11b
antibodies. (C) Representative images and (D) analysis of CD11b+
MDSCs (green) in the tumors of BLAB/c mice treated with NVB + 5-FU
using immunofluorescence, as well as the (E) analysis and (F)
images in the lungs. (G) Detection of total macrophages (F4/80,
red) and M2 macrophages (CD206, green) in tumors treated with NVB +
5-FU. Cell nuclei were counterstained with DAPI (blue) (n=3). Scale
bar, 50 μm. *P<0.05, **P<0.01
vs. control. Gr-1, PE anti-mouse Ly-6G/Ly-6C; 5-FU, fluorouracil;
CDDP, cisplatin; Con, control; MDSCs, myeloid-derived suppressor
cells; ns, not significant; NVB, vinorelbine. |
A number of studies have revealed that M2
macrophages are involved in tumor growth and metastasis (32,33). To further explore the potential
role of macrophages in tumor growth, the levels of total and M2
macrophages were investigated in 4T1 tumor tissues of BALB/c mice
from the tumor growth model. The levels of F4/80+ total
and CD206+ M2 macrophages increased following treatment
with 0.21/15.75 mg/kg NVB + 5-FU, while the levels decreased
following 0.84/63 mg/kg NVB + 5-FU (Fig. 5G).
Collectively, the results of the present study
demonstrated a dual-directional action of NVB combined with CDDP or
5-FU on tumor growth and metastasis. Moreover, the dual-directional
action induced by the metronomic combination chemotherapy may have
been the result of a mutual interaction of several mechanisms
rather than any single specific mechanism (Fig. 6).
Discussion
There is a great divide between ideal clinical
demands and practical applications in cancer chemotherapy,
primarily due to the difficulty in achieving an optimal
efficacy-toxicity balance (34).
A thorough understanding of the dose-response association of
metronomic combination chemotherapy is crucial for the success of
this chemotherapeutic strategy. In the present study, a
dose-dependent dual-directional pharmacological phenomenon was
discovered in 4T1 tumor-bearing BALB/c mice treated with NVB + CDDP
and NVB + 5-FU at the regular interval of every other day. The
results demonstrated that tumor growth and metastases were
stimulated following low-dose treatment and was suppressed
following higher doses. A previous study also demonstrated that
gemcitabine + CDDP at certain low doses facilitated tumor formation
and growth in a xenograft mouse model of melanoma (11). These results suggested that
inappropriate metronomic combination regimens, particularly when
empirically determining metronomic regimens, may lead to unexpected
outcomes associated with the use of low doses. In addition, the
present study demonstrated that the doses that promote or inhibit
tumor growth may differ from those that promote or inhibit tumor
metastasis in the same drug combination. The modification of
antineoplastic agent dosages or their schedules may target
different cellular and molecular pathways, but the dosage might be
a critical factor in influencing the pharmacological actions of
antineoplastic agents administered at the same frequency based on
the findings of the present study.
Blood vessels within tumor tissues provide oxygen
and nutrients for tumor growth and serve as a route for the
circulation of tumor and metastatic cells (35). The results of the present study
demonstrated that the level of CD31, a surface marker of
neovascular endothelial cells, in tumor or lung tissues increased
after the low-dose treatments and decreased after the higher dose
treatments, which was in line with the dual-directional effect on
tumor growth or metastasis (36).
VEGF binding to VEGFR2 is an important pathway for the
functions of endothelial cells and angiogenesis (37). In the present study, western
blotting demonstrated that low doses of NVB + CDDP or NVB + 5-FU
increased the expression levels of VEGF and VEGFR2,
suggesting that metronomic combination chemotherapy promoted tumor
growth by enhancing tumor angiogenesis and the
VEGF/VEGFR2 pathway. VEGFR1, which is
required for metastasis, has an important role in the biological
process of angiogenesis (38).
The upregulation of VEGFR1 has been revealed to be
associated with the promotion of tumor angiogenesis and metastasis
(11,39). In the present study, low doses of
NVB + CDDP or NVB + 5-FU increased the expression of
VEGFR1, suggesting that VEGFR1 might be an
important activator of angiogenesis at low doses of these drugs.
However, in the present study, there was no statistically
significant reduction in the levels of pro-angiogenic proteins in
the high-dose treatments that led to the inhibition of tumor
growth, and more research is needed to clarify this aspect. MMPs,
including MMP-2 and MMP-9, participate in multiple stages of tumor
progression, and are positively correlated with microvessel density
and angiogenic markers, such as VEGF (40,41). Tumorigenesis and tumor progression
may be mediated by angiogenic processes, such as the increase of
vascularization and upregulation of VEGF, MMP-2 and MMP-9 (11,42). The results of the present study
demonstrated that the expression levels of MMP-2 and MMP-9
increased in the lungs following low-dose treatments, indicating
that angiogenesis participated in the mechanism of promoting tumor
metastasis. Unlike conventional chemotherapy targeting
fast-dividing tumor cells, endothelial cells responsible for tumor
neovascularization have always been regarded as the primary target
of MCT (43). In the present
study, NVB combined with CDDP or 5-FU suppressed the apoptosis of
endothelial cells and stimulated their migration and invasion at
low concentrations. However, at higher concentrations, NVB combined
with CDDP or 5-FU enhanced apoptosis and suppressed proliferation.
These results suggested that the survival and function of
endothelial cells directly contributed to the acceleration of tumor
growth at low drug doses, while apoptosis and anti-proliferation
primarily lead to the inhibition of tumor growth.
Caspase-8 and its downstream protein, caspase-3, are
associated with apoptotic machinery and are considered reliable
biomarkers for cell apoptosis (44,45). In the present study, high dose NVB
+ 5-FU increased the expression of pro-apoptotic proteins in tumor
tissues, suggesting a positive effect of apoptosis on inhibiting
tumor growth. More notably, increased expression of pro-apoptotic
proteins was also observed in low-dose treatments that induced the
promotion of tumor growth. Emerging evidence has indicated that
cell apoptosis could facilitate tumor growth and is correlated with
poor prognosis and disease-free survival in certain types of cancer
such as breast, head and neck, and colon cancer (46,47). Chang et al (28) demonstrated that 5-FU-generated
tumor cell debris stimulated tumor growth by triggering the release
of OPN from tumor cells in subcutaneous and orthotopic models of
colon cancer. In the present study, it was found that the level of
OPN increased in low-dose treatments, which indirectly indicated
that cell apoptosis may be involved in the promotion of tumor
growth mediated by OPN. In addition, cell debris produced by cell
death (such as apoptosis and necrosis) promotes tumor growth
through the production of pro-inflammation cytokines (48,49). Caspase-3 and caspase-8 have been
suggested to stimulate tumor growth through various mechanisms
(50). For example, caspase-8 has
the non-apoptotic function of promoting the accumulation of NF-κB
and the expression of NF-κB-dependent cytokines (such as IL-1, IL-8
and VEGF) (45,51,52). Moreover, NF-κB could also promote
angiogenesis by upregulating the signaling pathways involved in
angiogenic factors, such as VEGF (53). The results of the present study
demonstrated an upregulation of NF-κB expression following low-dose
NVB + CDDP treatment, implying the potential positive action in
promotion of tumor growth. However, the role of apoptosis in
promoting tumor growth remains unclear and further research is
required to elucidate it. In the present study, in vitro,
low concentrations of NVB + CDDP or NVB + 5-FU suppressed apoptosis
and stimulated the migration and invasion of 4T1 tumor cells, which
was consistent with the promotion of these functions by low
concentrations of gemcitabine + CDDP in B16, MCF-7 and T-47D tumor
cells in vitro (11).
However, the results of 4T1 tumor cells treated with
high-concentration NVB combined with CDDP or 5-FU were
contradictory, as they revealed that proliferation was inhibited,
and apoptosis, migration and invasion were promoted. These results
suggested that the anti-apoptosis, migration and invasion of tumor
cells might directly stimulate tumor progression, while apoptosis
and anti-proliferation contribute to the inhibition of tumor
growth.
Clinical data have suggested that MDSCs are strongly
correlated with poor overall survival time (54). MDSCs, which can be mobilized into
the peripheral blood and recruited to tumor or lung tissues,
promote tumor growth and metastasis through angiogenesis, invasion
or the formation of pre-metastatic niches (31,55). In a bone marrow transplantation
mouse model, it was found that gemcitabine + CDDP treatment
promoted the mobilization of Gr1+CD11b+MDSCs
and enhanced the accumulation of bone marrow-derived cells in
tumors (11). In the present
study, MDSC levels increased in the blood, tumors and lungs of mice
following treatment with low-dose NVB + CDDP or NVB + 5-FU, but
were decreased following high doses, which suggested that the
mobilization and recruitment of MDSCs plays a role in the
dual-directional regulation of tumor growth and metastasis in
metronomic combination chemotherapy. In addition to MDSCs,
macrophages are another type of immune cell abundantly found in the
tumor microenvironment of various solid tumors such as pancreas,
breast and lung tumors, and this abundance has been found to be
correlated with poor survival time (56). Emerging evidence has revealed that
macrophages, specifically M2 macrophages, can promote tumor
initiation and progression by enhancing angiogenesis, stimulating
tumor cell functions, such as migration, invasion and
intravasation, and suppressing antitumor immunity (32). The present study found that both
total macrophage and M2 macrophage levels increased in the low-dose
NVB + 5-FU group, and decreased in the high-dose group, indicating
that macrophages may participate in the dual-directional biological
process of tumor growth induced by metronomic combination treatment
at different doses. Furthermore, it has been reported that the
cross-talk among MDSCs, macrophages and tumor cells affects
antitumor immunity by regulating the production of molecules,
including IL-6, IL-10, IL-12, TNF-α and NO (57).
In conclusion, the present study illustrated a
dual-directional mode of action of NVB-based metronomic combination
chemotherapy. The promotion of tumor growth and metastasis induced
by low doses of chemotherapeutic drugs highlighted that there is an
effective dose interval that varies with different drugs or
combinations in metronomic combination chemotherapy. Therefore,
more preclinical and clinical studies are needed to optimize
specific combined metronomic regimens, including the optimal drug
combination, dosage and intervals, prior to their widespread
implementation in clinical settings.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL performed the experiments and wrote the
manuscript; ML and JK analyzed the data; YL collected the data; HY
interpreted the data and reviewed the work critically for important
intellectual content; WF designed and supervised the research. HL,
ML, JK and WF confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments were approved by The Biomedical
Ethics Committee of Xi'an Jiaotong University (Xi'an, China;
approval no. 2020-1644).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 81372379).
References
|
1
|
Wong HH and Halford S: Dose-limiting
toxicity and maximum tolerated dose: Still fit for purpose? Lancet
Oncol. 16:1287–1288. 2015.
|
|
2
|
Gasparini G: Metronomic scheduling: The
future of chemotherapy? Lancet Oncol. 2:733–740. 2001.
|
|
3
|
Pomeroy AE, Schmidt EV, Sorger PK and
Palmer AC: Drug independence and the curability of cancer by
combination chemotherapy. Trends Cancer. 8:915–929. 2022.
|
|
4
|
Wu J and Waxman DJ: Immunogenic
chemotherapy: Dose and schedule dependence and combination with
immunotherapy. Cancer Lett. 419:210–221. 2018.
|
|
5
|
Chen YL, Chang MC and Cheng WF: Metronomic
chemotherapy and immunotherapy in cancer treatment. Cancer Lett.
400:282–292. 2017.
|
|
6
|
Scharovsky OG, Rico MJ, Mainetti LE,
Perroud HA and Rozados VR: Achievements and challenges in the use
of metronomics for the treatment of breast cancer. Biochem
Pharmacol. 175:1139092020.
|
|
7
|
Cox MC and Bocci G: Metronomic
chemotherapy regimens and targeted therapies in non-Hodgkin
lymphoma: The best of two worlds. Cancer Lett. 524:144–150.
2022.
|
|
8
|
Bocci G and Kerbel RS: Pharmacokinetics of
metronomic chemotherapy: A neglected but crucial aspect. Nat Rev
Clin Oncol. 13:659–673. 2016.
|
|
9
|
Lien K, Georgsdottir S, Sivanathan L, Chan
K and Emmenegger U: Low-dose metronomic chemotherapy: A systematic
literature analysis. Eur J Cancer. 49:3387–3395. 2013.
|
|
10
|
de Ruiter J, Cramer SJ, Smink T and van
Putten LM: The facilitation of tumour growth in the lung by
cyclophosphamide in artificial and spontaneous metastases models.
Eur J Cancer. 15:1139–1149. 1979.
|
|
11
|
Chen Y, Liu H, Zheng Q, Li H, You H, Feng
Y and Feng W: Promotion of tumor progression induced by continuous
low-dose administration of antineoplastic agent gemcitabine or
gemcitabine combined with cisplatin. Life Sci. 306:1208262022.
|
|
12
|
Dellapasqua S, Bertolini F, Bagnardi V,
Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch
A, Rocca A, et al: Metronomic cyclophosphamide and capecitabine
combined with bevacizumab in advanced breast cancer. J Clin Oncol.
26:4899–4905. 2008.
|
|
13
|
Montagna E, Palazzo A, Maisonneuve P,
Cancello G, Iorfida M, Sciandivasci A, Esposito A, Cardillo A,
Mazza M, Munzone E, et al: Safety and efficacy study of metronomic
vinorelbine, cyclophosphamide plus capecitabine in metastatic
breast cancer: A phase II trial. Cancer Lett. 400:276–281.
2017.
|
|
14
|
Rossi D: Metronomic oral vinorelbine and
lung cancer therapy during the COVID 19 pandemic: A single-center
experience. Lung Cancer. 145:83–84. 2020.
|
|
15
|
Xu B, Sun T, Wang S and Lin Y: Metronomic
therapy in advanced breast cancer and NSCLC: Vinorelbine as a
paradigm of recent progress. Expert Rev Anticancer Ther. 21:71–79.
2021.
|
|
16
|
Riesco-Martinez M, Parra K, Saluja R,
Francia G and Emmenegger U: Resistance to metronomic chemotherapy
and ways to overcome it. Cancer Lett. 400:311–318. 2017.
|
|
17
|
Munzone E and Colleoni M: Clinical
overview of metronomic chemotherapy in breast cancer. Nat Rev Clin
Oncol. 12:631–644. 2015.
|
|
18
|
Romani AMP: Cisplatin in cancer treatment.
Biochem Pharmacol. 206:1153232022.
|
|
19
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003.
|
|
20
|
National Research Council Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
The National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. National Academy of Sciences, National Academies Press;
Washington, DC: 2011
|
|
21
|
Aston WJ, Hope DE, Nowak AK, Robinson BW,
Lake RA and Lesterhuis WJ: A systematic investigation of the
maximum tolerated dose of cytotoxic chemotherapy with and without
supportive care in mice. BMC Cancer. 17:6842017.
|
|
22
|
Saif MW and von Borstel R: 5-Fluorouracil
dose escalation enabled with PN401 (triacetyluridine): Toxicity
reduction and increased antitumor activity in mice. Cancer
Chemother Pharmacol. 58:136–142. 2006.
|
|
23
|
Navari RM and Aapro M: Antiemetic
prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J
Med. 374:1356–1367. 2016.
|
|
24
|
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou
K, Wang L, Cao Y, Sun P and Wang T: The FTO/miR-181b-3p/ARL5B
signaling pathway regulates cell migration and invasion in breast
cancer. Cancer Commun (Lond). 40:484–500. 2020.
|
|
25
|
Magaki S, Hojat SA, Wei B, So A and Yong
WH: An introduction to the performance of immunohistochemistry.
Methods Mol Biol. 1897:289–298. 2019.
|
|
26
|
Zhang S, Liu Y, Xiang D, Yang J, Liu D,
Ren X and Zhang C: Assessment of dose-response relationship of
5-fluorouracil to murine intestinal injury. Biomed Pharmacother.
106:910–916. 2018.
|
|
27
|
Deng J, Yang H, Haak VM, Yang J, Kipper
FC, Barksdale C, Hwang SH, Gartung A, Bielenberg DR, Subbian S, et
al: Eicosanoid regulation of debris-stimulated metastasis. Proc
Natl Acad Sci USA. 118:e21077711182021.
|
|
28
|
Chang J, Bhasin SS, Bielenberg DR,
Sukhatme VP, Bhasin M, Huang S, Kieran MW and Panigrahy D:
Chemotherapy-generated cell debris stimulates colon carcinoma tumor
growth via osteopontin. FASEB J. 33:114–125. 2019.
|
|
29
|
Yin Y, Zeng K, Wu M, Ding Y, Zhao M and
Chen Q: The levels of Ki-67 positive are positively associated with
lymph node metastasis in invasive ductal breast cancer. Cell
Biochemistry Biophysics. 70:1145–1151. 2014.
|
|
30
|
Bruey JM, Kantarjian H, Estrov Z, Zhang Z,
Ma W, Albitar F, Abdool A, Thomas D, Yeh C, O'Brien S and Albitar
M: Circulating Ki-67 protein in plasma as a biomarker and
prognostic indicator of acute lymphoblastic leukemia. Leuk Res.
34:173–176. 2010.
|
|
31
|
Safarzadeh E, Orangi M, Mohammadi H,
Babaie F and Baradaran B: Myeloid-derived suppressor cells:
Important contributors to tumor progression and metastasis. J Cell
Physiol. 233:3024–3036. 2018.
|
|
32
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nature reviews Drug
Discovery. 17:887–904. 2018.
|
|
33
|
Chen Y, Zhang S, Wang Q and Zhang X:
Tumor-recruited M2 macrophages promote gastric and breast cancer
metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol
Oncol. 10:362017.
|
|
34
|
Barbolosi D, Ciccolini J, Lacarelle B,
Barlési F and André N: Computational oncology-mathematical
modelling of drug regimens for precision medicine. Nat Rev Clin
Oncol. 13:242–254. 2016.
|
|
35
|
Ebos JM and Kerbel RS: Antiangiogenic
therapy: Impact on invasion, disease progression, and metastasis.
Nat Rev Clin Oncol. 8:210–221. 2011.
|
|
36
|
Zhang T, Zhang L, Gao Y, Wang Y, Liu Y,
Zhang H, Wang Q, Hu F, Li J, Tan J, et al: Role of aneuploid
circulating tumor cells and CD31+ circulating tumor
endothelial cells in predicting and monitoring anti-angiogenic
therapy efficacy in advanced NSCLC. Mol Oncol. 15:2891–2909.
2021.
|
|
37
|
Chatterjee S, Heukamp LC, Siobal M,
Schöttle J, Wieczorek C, Peifer M, Frasca D, Koker M, König K,
Meder L, et al: Tumor VEGF:VEGFR2 autocrine feed-forward loop
triggers angiogenesis in lung cancer. J Clin Invest. 123:1732–1740.
2013.
|
|
38
|
Freire Valls A, Knipper K, Giannakouri E,
Sarachaga V, Hinterkopf S, Wuehrl M, Shen Y, Radhakrishnan P, Klose
J, Ulrich A, et al: VEGFR1+ Metastasis-associated
macrophages contribute to metastatic angiogenesis and influence
colorectal cancer patient outcome. Clin Cancer Res. 25:5674–5685.
2019.
|
|
39
|
Liu W, Xu J, Wang M, Wang Q, Bi Y and Han
M: Tumor-derived vascular endothelial growth factor (VEGF)-a
facilitates tumor metastasis through the VEGF-VEGFR1 signaling
pathway. Int J Oncol. 39:1213–1220. 2011.
|
|
40
|
Riedel F, Götte K, Schwalb J, Bergler W
and Hörmann K: Expression of 92-kDa type IV collagenase correlates
with angiogenic markers and poor survival in head and neck squamous
cell carcinoma. Int J Oncol. 17:1099–1105. 2000.
|
|
41
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010.
|
|
42
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944. 2006.
|
|
43
|
Browder T, Butterfield CE, Kräling BM, Shi
B, Marshall B, O'Reilly MS and Folkman J: Antiangiogenic scheduling
of chemotherapy improves efficacy against experimental
drug-resistant cancer. Cancer Res. 60:1878–1886. 2000.
|
|
44
|
Silva F, Padin-Iruegas ME, Caponio VCA,
Lorenzo-Pouso AI, Saavedra-Nieves P, Chamorro-Petronacci CM,
Suarez-Penaranda J and Perez-Sayans M: Caspase 3 and cleaved
Caspase 3 expression in tumorogenesis and its correlations with
prognosis in head and neck cancer: A systematic review and
meta-analysis. Int J Mol Sci. 23:119372022.
|
|
45
|
Tummers B and Green DR: Caspase-8:
regulating life and death. Immunol Rev. 277:76–89. 2017.
|
|
46
|
Haak VM, Huang S and Panigrahy D:
Debris-stimulated tumor growth: A Pandora's box? Cancer Metastasis
Rev. 40:791–801. 2021.
|
|
47
|
Huang JS, Yang CM, Wang JS, Liou HH, Hsieh
IC, Li GC, Huang SJ, Shu CW, Fu TY, Lin YC, et al: Caspase-3
expression in tumorigenesis and prognosis of buccal mucosa squamous
cell carcinoma. Oncotarget. 8:84237–84247. 2017.
|
|
48
|
Weigert A, Mora J, Sekar D, Syed S and
Brüne B: Killing is not enough: How apoptosis hijacks
tumor-associated macrophages to promote cancer progression. Adv Exp
Med Biol. 930:205–239. 2016.
|
|
49
|
Revesz L: Effect of tumour cells killed by
x-rays upon the growth of admixed viable cells. Nature.
178:1391–1392. 1956.
|
|
50
|
Donato AL, Huang Q, Liu X, Li F, Zimmerman
MA and Li CY: Caspase 3 promotes surviving melanoma tumor cell
growth after cytotoxic therapy. J Invest Dermatol. 134:1686–1692.
2014.
|
|
51
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019.
|
|
52
|
Fianco G, Contadini C, Ferri A, Cirotti C,
Stagni V and Barilà D: Caspase-8: A novel target to overcome
resistance to chemotherapy in glioblastoma. Int J Mol Sci.
19:37982018.
|
|
53
|
Zhu CC, Chen C, Xu ZQ, Zhao JK, Ou BC, Sun
J, Zheng MH, Zong YP and Lu AG: CCR6 promotes tumor angiogenesis
via the AKT/NF-κB/VEGF pathway in colorectal cancer. Biochim
Biophys Acta Mol Basis Dis. 1864:387–397. 2018.
|
|
54
|
Lang S, Bruderek K, Kaspar C, Höing B,
Kanaan O, Dominas N, Hussain T, Droege F, Eyth C, Hadaschik B and
Brandau S: Clinical relevance and suppressive capacity of human
Myeloid-derived suppressor cell subsets. Clin Cancer Res.
24:4834–4844. 2018.
|
|
55
|
Udumula MP, Sakr S, Dar S, Alvero AB,
Ali-Fehmi R, Abdulfatah E, Li J, Jiang J, Tang A, Buekers T, et al:
Ovarian cancer modulates the immunosuppressive function of
CD11b+Gr1+ myeloid cells via glutamine
metabolism. Mol Metab. 53:1012722021.
|
|
56
|
Nielsen SR and Schmid MC: Macrophages as
Key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017:96247602017.
|
|
57
|
Beury DW, Parker KH, Nyandjo M, Sinha P,
Carter KA and Ostrand-Rosenberg S: Cross-talk among myeloid-derived
suppressor cells, macrophages, and tumor cells impacts the
inflammatory milieu of solid tumors. J Leukoc Biol. 96:1109–1118.
2014.
|




















