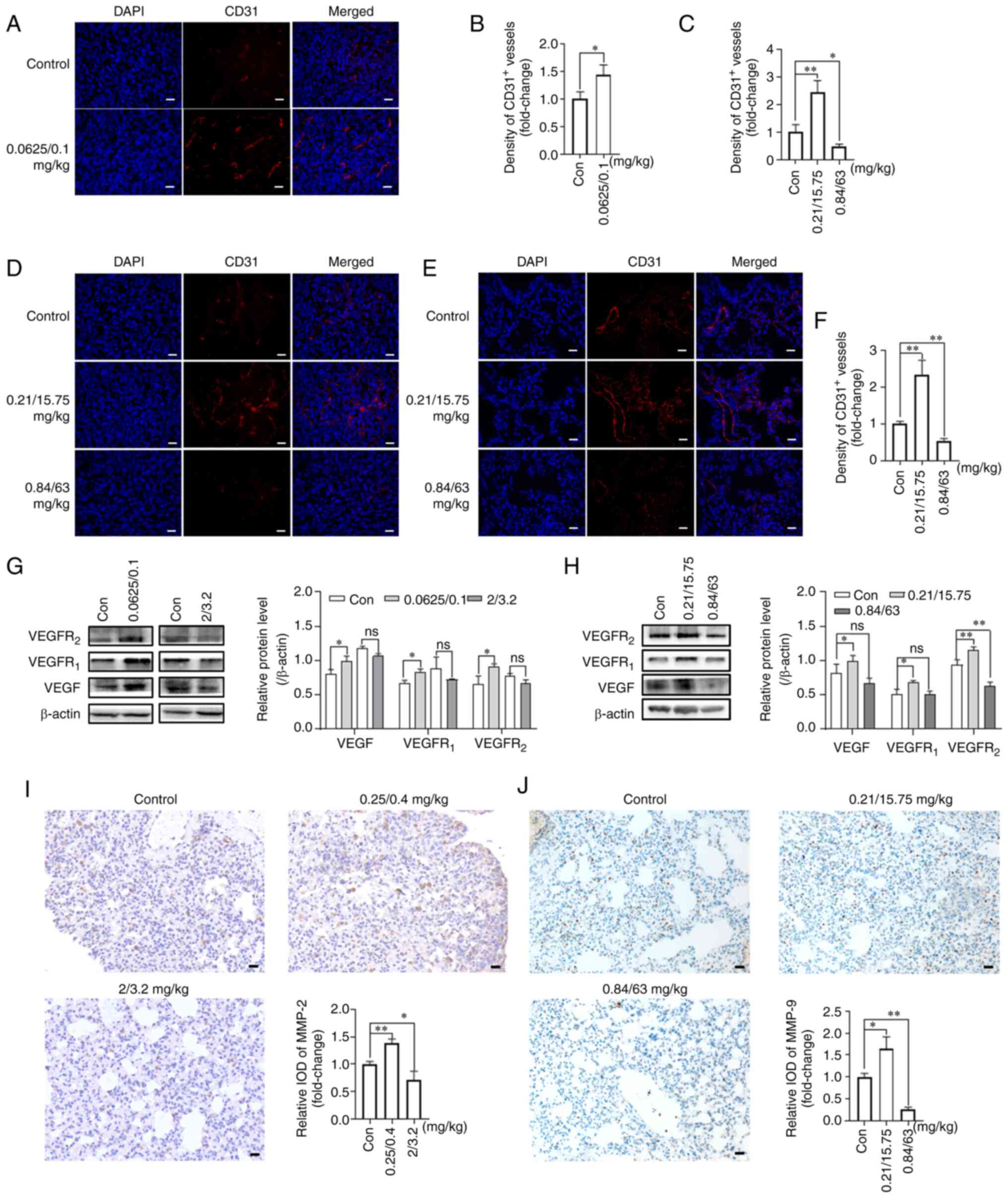
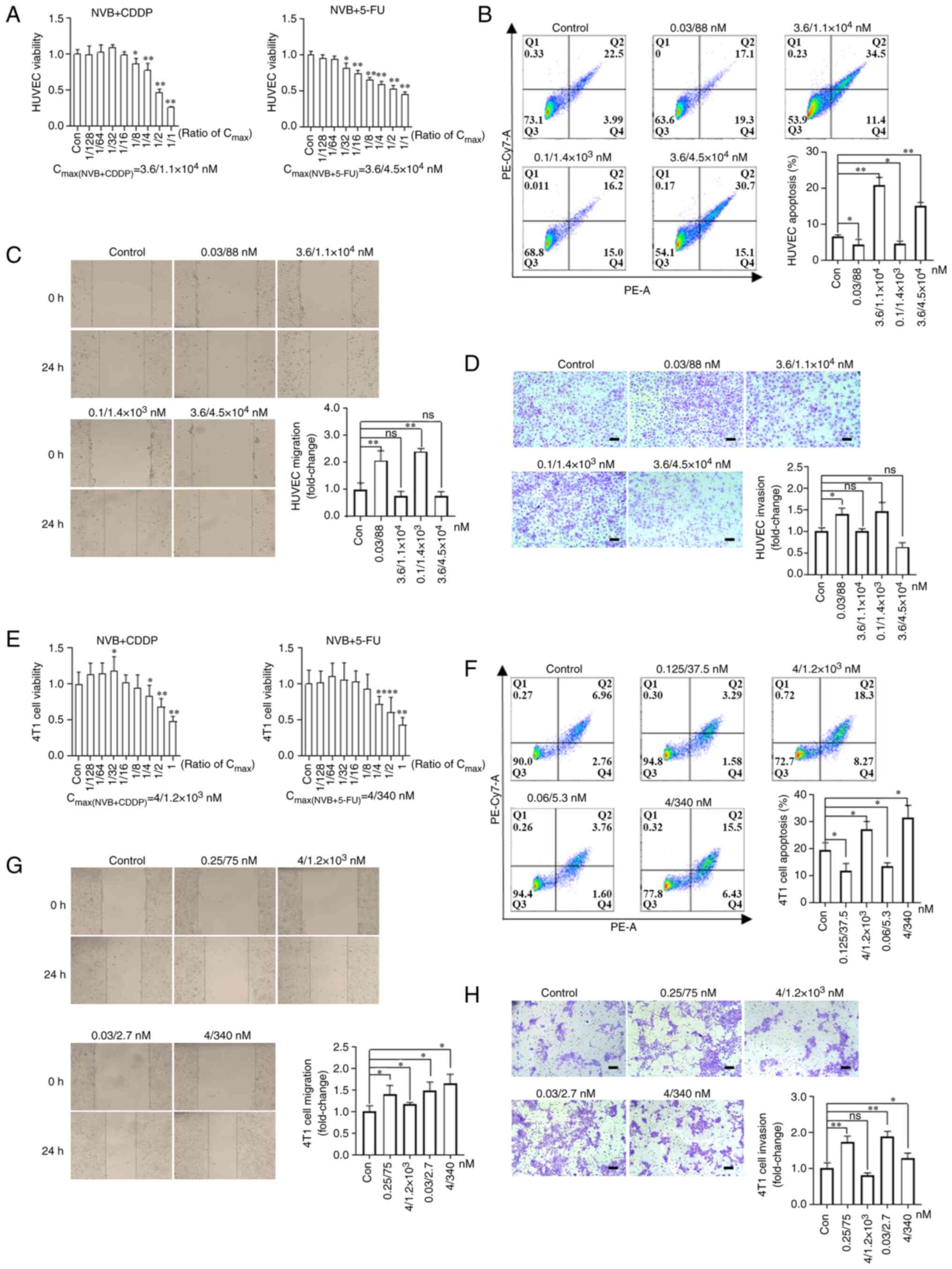
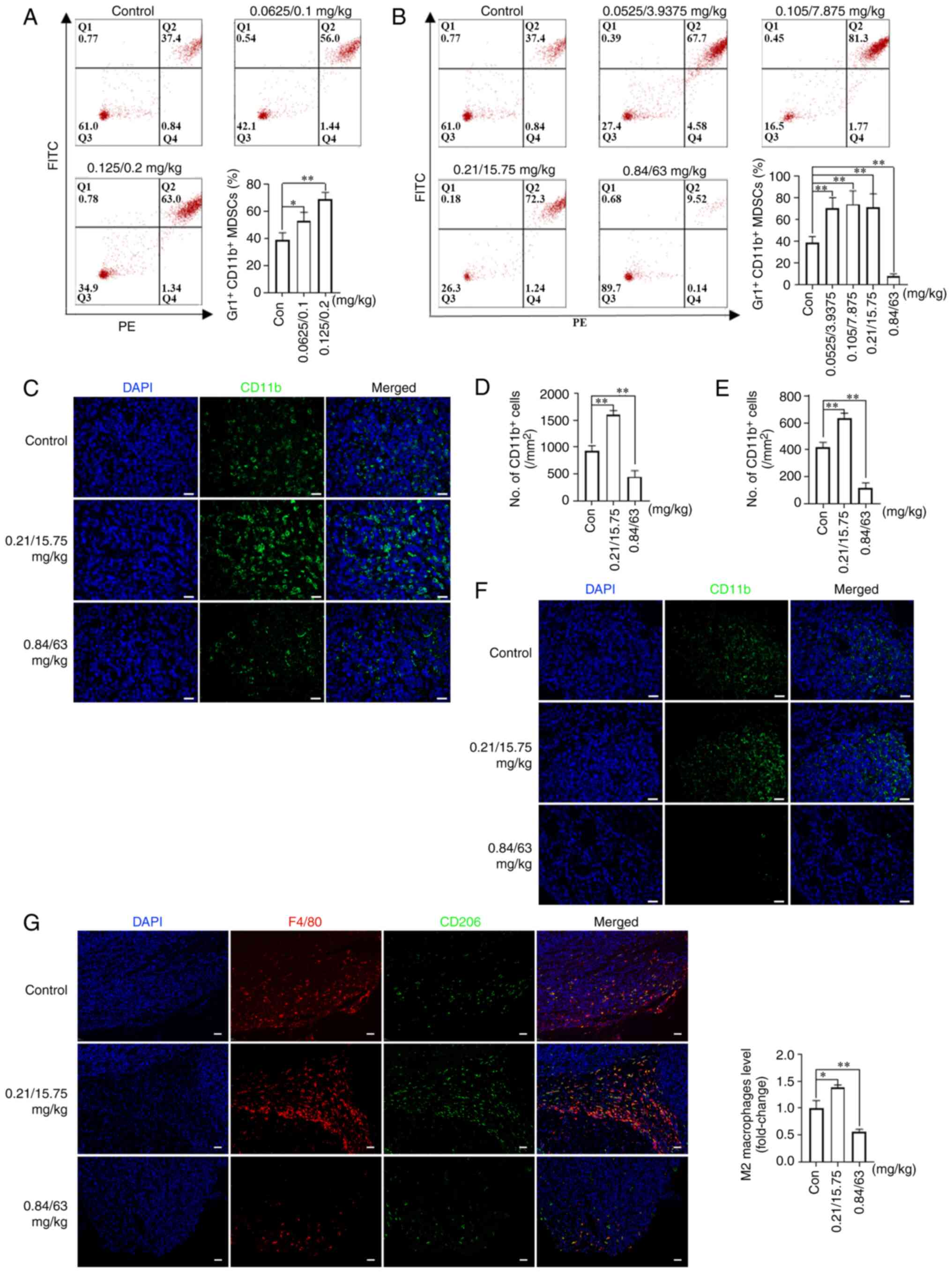
|
1
|
Wong HH and Halford S: Dose-limiting
toxicity and maximum tolerated dose: Still fit for purpose? Lancet
Oncol. 16:1287–1288. 2015.
|
|
2
|
Gasparini G: Metronomic scheduling: The
future of chemotherapy? Lancet Oncol. 2:733–740. 2001.
|
|
3
|
Pomeroy AE, Schmidt EV, Sorger PK and
Palmer AC: Drug independence and the curability of cancer by
combination chemotherapy. Trends Cancer. 8:915–929. 2022.
|
|
4
|
Wu J and Waxman DJ: Immunogenic
chemotherapy: Dose and schedule dependence and combination with
immunotherapy. Cancer Lett. 419:210–221. 2018.
|
|
5
|
Chen YL, Chang MC and Cheng WF: Metronomic
chemotherapy and immunotherapy in cancer treatment. Cancer Lett.
400:282–292. 2017.
|
|
6
|
Scharovsky OG, Rico MJ, Mainetti LE,
Perroud HA and Rozados VR: Achievements and challenges in the use
of metronomics for the treatment of breast cancer. Biochem
Pharmacol. 175:1139092020.
|
|
7
|
Cox MC and Bocci G: Metronomic
chemotherapy regimens and targeted therapies in non-Hodgkin
lymphoma: The best of two worlds. Cancer Lett. 524:144–150.
2022.
|
|
8
|
Bocci G and Kerbel RS: Pharmacokinetics of
metronomic chemotherapy: A neglected but crucial aspect. Nat Rev
Clin Oncol. 13:659–673. 2016.
|
|
9
|
Lien K, Georgsdottir S, Sivanathan L, Chan
K and Emmenegger U: Low-dose metronomic chemotherapy: A systematic
literature analysis. Eur J Cancer. 49:3387–3395. 2013.
|
|
10
|
de Ruiter J, Cramer SJ, Smink T and van
Putten LM: The facilitation of tumour growth in the lung by
cyclophosphamide in artificial and spontaneous metastases models.
Eur J Cancer. 15:1139–1149. 1979.
|
|
11
|
Chen Y, Liu H, Zheng Q, Li H, You H, Feng
Y and Feng W: Promotion of tumor progression induced by continuous
low-dose administration of antineoplastic agent gemcitabine or
gemcitabine combined with cisplatin. Life Sci. 306:1208262022.
|
|
12
|
Dellapasqua S, Bertolini F, Bagnardi V,
Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch
A, Rocca A, et al: Metronomic cyclophosphamide and capecitabine
combined with bevacizumab in advanced breast cancer. J Clin Oncol.
26:4899–4905. 2008.
|
|
13
|
Montagna E, Palazzo A, Maisonneuve P,
Cancello G, Iorfida M, Sciandivasci A, Esposito A, Cardillo A,
Mazza M, Munzone E, et al: Safety and efficacy study of metronomic
vinorelbine, cyclophosphamide plus capecitabine in metastatic
breast cancer: A phase II trial. Cancer Lett. 400:276–281.
2017.
|
|
14
|
Rossi D: Metronomic oral vinorelbine and
lung cancer therapy during the COVID 19 pandemic: A single-center
experience. Lung Cancer. 145:83–84. 2020.
|
|
15
|
Xu B, Sun T, Wang S and Lin Y: Metronomic
therapy in advanced breast cancer and NSCLC: Vinorelbine as a
paradigm of recent progress. Expert Rev Anticancer Ther. 21:71–79.
2021.
|
|
16
|
Riesco-Martinez M, Parra K, Saluja R,
Francia G and Emmenegger U: Resistance to metronomic chemotherapy
and ways to overcome it. Cancer Lett. 400:311–318. 2017.
|
|
17
|
Munzone E and Colleoni M: Clinical
overview of metronomic chemotherapy in breast cancer. Nat Rev Clin
Oncol. 12:631–644. 2015.
|
|
18
|
Romani AMP: Cisplatin in cancer treatment.
Biochem Pharmacol. 206:1153232022.
|
|
19
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003.
|
|
20
|
National Research Council Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
The National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. National Academy of Sciences, National Academies Press;
Washington, DC: 2011
|
|
21
|
Aston WJ, Hope DE, Nowak AK, Robinson BW,
Lake RA and Lesterhuis WJ: A systematic investigation of the
maximum tolerated dose of cytotoxic chemotherapy with and without
supportive care in mice. BMC Cancer. 17:6842017.
|
|
22
|
Saif MW and von Borstel R: 5-Fluorouracil
dose escalation enabled with PN401 (triacetyluridine): Toxicity
reduction and increased antitumor activity in mice. Cancer
Chemother Pharmacol. 58:136–142. 2006.
|
|
23
|
Navari RM and Aapro M: Antiemetic
prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J
Med. 374:1356–1367. 2016.
|
|
24
|
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou
K, Wang L, Cao Y, Sun P and Wang T: The FTO/miR-181b-3p/ARL5B
signaling pathway regulates cell migration and invasion in breast
cancer. Cancer Commun (Lond). 40:484–500. 2020.
|
|
25
|
Magaki S, Hojat SA, Wei B, So A and Yong
WH: An introduction to the performance of immunohistochemistry.
Methods Mol Biol. 1897:289–298. 2019.
|
|
26
|
Zhang S, Liu Y, Xiang D, Yang J, Liu D,
Ren X and Zhang C: Assessment of dose-response relationship of
5-fluorouracil to murine intestinal injury. Biomed Pharmacother.
106:910–916. 2018.
|
|
27
|
Deng J, Yang H, Haak VM, Yang J, Kipper
FC, Barksdale C, Hwang SH, Gartung A, Bielenberg DR, Subbian S, et
al: Eicosanoid regulation of debris-stimulated metastasis. Proc
Natl Acad Sci USA. 118:e21077711182021.
|
|
28
|
Chang J, Bhasin SS, Bielenberg DR,
Sukhatme VP, Bhasin M, Huang S, Kieran MW and Panigrahy D:
Chemotherapy-generated cell debris stimulates colon carcinoma tumor
growth via osteopontin. FASEB J. 33:114–125. 2019.
|
|
29
|
Yin Y, Zeng K, Wu M, Ding Y, Zhao M and
Chen Q: The levels of Ki-67 positive are positively associated with
lymph node metastasis in invasive ductal breast cancer. Cell
Biochemistry Biophysics. 70:1145–1151. 2014.
|
|
30
|
Bruey JM, Kantarjian H, Estrov Z, Zhang Z,
Ma W, Albitar F, Abdool A, Thomas D, Yeh C, O'Brien S and Albitar
M: Circulating Ki-67 protein in plasma as a biomarker and
prognostic indicator of acute lymphoblastic leukemia. Leuk Res.
34:173–176. 2010.
|
|
31
|
Safarzadeh E, Orangi M, Mohammadi H,
Babaie F and Baradaran B: Myeloid-derived suppressor cells:
Important contributors to tumor progression and metastasis. J Cell
Physiol. 233:3024–3036. 2018.
|
|
32
|
Cassetta L and Pollard JW: Targeting
macrophages: Therapeutic approaches in cancer. Nature reviews Drug
Discovery. 17:887–904. 2018.
|
|
33
|
Chen Y, Zhang S, Wang Q and Zhang X:
Tumor-recruited M2 macrophages promote gastric and breast cancer
metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol
Oncol. 10:362017.
|
|
34
|
Barbolosi D, Ciccolini J, Lacarelle B,
Barlési F and André N: Computational oncology-mathematical
modelling of drug regimens for precision medicine. Nat Rev Clin
Oncol. 13:242–254. 2016.
|
|
35
|
Ebos JM and Kerbel RS: Antiangiogenic
therapy: Impact on invasion, disease progression, and metastasis.
Nat Rev Clin Oncol. 8:210–221. 2011.
|
|
36
|
Zhang T, Zhang L, Gao Y, Wang Y, Liu Y,
Zhang H, Wang Q, Hu F, Li J, Tan J, et al: Role of aneuploid
circulating tumor cells and CD31+ circulating tumor
endothelial cells in predicting and monitoring anti-angiogenic
therapy efficacy in advanced NSCLC. Mol Oncol. 15:2891–2909.
2021.
|
|
37
|
Chatterjee S, Heukamp LC, Siobal M,
Schöttle J, Wieczorek C, Peifer M, Frasca D, Koker M, König K,
Meder L, et al: Tumor VEGF:VEGFR2 autocrine feed-forward loop
triggers angiogenesis in lung cancer. J Clin Invest. 123:1732–1740.
2013.
|
|
38
|
Freire Valls A, Knipper K, Giannakouri E,
Sarachaga V, Hinterkopf S, Wuehrl M, Shen Y, Radhakrishnan P, Klose
J, Ulrich A, et al: VEGFR1+ Metastasis-associated
macrophages contribute to metastatic angiogenesis and influence
colorectal cancer patient outcome. Clin Cancer Res. 25:5674–5685.
2019.
|
|
39
|
Liu W, Xu J, Wang M, Wang Q, Bi Y and Han
M: Tumor-derived vascular endothelial growth factor (VEGF)-a
facilitates tumor metastasis through the VEGF-VEGFR1 signaling
pathway. Int J Oncol. 39:1213–1220. 2011.
|
|
40
|
Riedel F, Götte K, Schwalb J, Bergler W
and Hörmann K: Expression of 92-kDa type IV collagenase correlates
with angiogenic markers and poor survival in head and neck squamous
cell carcinoma. Int J Oncol. 17:1099–1105. 2000.
|
|
41
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010.
|
|
42
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944. 2006.
|
|
43
|
Browder T, Butterfield CE, Kräling BM, Shi
B, Marshall B, O'Reilly MS and Folkman J: Antiangiogenic scheduling
of chemotherapy improves efficacy against experimental
drug-resistant cancer. Cancer Res. 60:1878–1886. 2000.
|
|
44
|
Silva F, Padin-Iruegas ME, Caponio VCA,
Lorenzo-Pouso AI, Saavedra-Nieves P, Chamorro-Petronacci CM,
Suarez-Penaranda J and Perez-Sayans M: Caspase 3 and cleaved
Caspase 3 expression in tumorogenesis and its correlations with
prognosis in head and neck cancer: A systematic review and
meta-analysis. Int J Mol Sci. 23:119372022.
|
|
45
|
Tummers B and Green DR: Caspase-8:
regulating life and death. Immunol Rev. 277:76–89. 2017.
|
|
46
|
Haak VM, Huang S and Panigrahy D:
Debris-stimulated tumor growth: A Pandora's box? Cancer Metastasis
Rev. 40:791–801. 2021.
|
|
47
|
Huang JS, Yang CM, Wang JS, Liou HH, Hsieh
IC, Li GC, Huang SJ, Shu CW, Fu TY, Lin YC, et al: Caspase-3
expression in tumorigenesis and prognosis of buccal mucosa squamous
cell carcinoma. Oncotarget. 8:84237–84247. 2017.
|
|
48
|
Weigert A, Mora J, Sekar D, Syed S and
Brüne B: Killing is not enough: How apoptosis hijacks
tumor-associated macrophages to promote cancer progression. Adv Exp
Med Biol. 930:205–239. 2016.
|
|
49
|
Revesz L: Effect of tumour cells killed by
x-rays upon the growth of admixed viable cells. Nature.
178:1391–1392. 1956.
|
|
50
|
Donato AL, Huang Q, Liu X, Li F, Zimmerman
MA and Li CY: Caspase 3 promotes surviving melanoma tumor cell
growth after cytotoxic therapy. J Invest Dermatol. 134:1686–1692.
2014.
|
|
51
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019.
|
|
52
|
Fianco G, Contadini C, Ferri A, Cirotti C,
Stagni V and Barilà D: Caspase-8: A novel target to overcome
resistance to chemotherapy in glioblastoma. Int J Mol Sci.
19:37982018.
|
|
53
|
Zhu CC, Chen C, Xu ZQ, Zhao JK, Ou BC, Sun
J, Zheng MH, Zong YP and Lu AG: CCR6 promotes tumor angiogenesis
via the AKT/NF-κB/VEGF pathway in colorectal cancer. Biochim
Biophys Acta Mol Basis Dis. 1864:387–397. 2018.
|
|
54
|
Lang S, Bruderek K, Kaspar C, Höing B,
Kanaan O, Dominas N, Hussain T, Droege F, Eyth C, Hadaschik B and
Brandau S: Clinical relevance and suppressive capacity of human
Myeloid-derived suppressor cell subsets. Clin Cancer Res.
24:4834–4844. 2018.
|
|
55
|
Udumula MP, Sakr S, Dar S, Alvero AB,
Ali-Fehmi R, Abdulfatah E, Li J, Jiang J, Tang A, Buekers T, et al:
Ovarian cancer modulates the immunosuppressive function of
CD11b+Gr1+ myeloid cells via glutamine
metabolism. Mol Metab. 53:1012722021.
|
|
56
|
Nielsen SR and Schmid MC: Macrophages as
Key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017:96247602017.
|
|
57
|
Beury DW, Parker KH, Nyandjo M, Sinha P,
Carter KA and Ostrand-Rosenberg S: Cross-talk among myeloid-derived
suppressor cells, macrophages, and tumor cells impacts the
inflammatory milieu of solid tumors. J Leukoc Biol. 96:1109–1118.
2014.
|