Circular RNA (circRNA) is a type of non-coding RNA
composed of a covalently closed cyclic structure that lacks a 5′
cap and 3′ polyadenylate tail, and typically forms through the
back-splicing of a single pre-mRNA. Due to the nature of its
structure, circRNA is resistant to RNase R-mediated degradation and
exhibits stable expression in multiple tissues and organs, such as
the brain (1,2). Nearly 10% of transcribed genes in
cells are potential sources of circRNA. Based on the different
looping sequences of parent genes, circRNAs can be classified into
four broad categories: Exon circRNA (ecRNA), intron circRNA
(ciRNA), exon-intron circRNA (elciRNA) and others, including
viruses, tRNA, rRNA and small nuclear RNA (3-6).
The functions of circRNA are diverse, including: i) Acting as
microRNA (miRNA) sponges, typically inhibiting miRNA activity
(7-9); ii) binding to RNA binding proteins
(RBPs) and regulating their interaction with target RNA molecules
(10-13); iii) being translated into peptide
segments (14-17); iv) influencing the transcription
of host genes (4,5); and v) impacting gene splicing
processes (18-20). A large number of circRNAs have
been shown to play important regulatory roles in the occurrence and
development of tumors, with some serving as potential clinical
serum markers that can provide meaningful information for clinical
treatment and prognosis.
Gastrointestinal cancers include esophageal cancer
(EC), gastric cancer (GC), hepatocellular carcinoma (HCC),
cholangiocarcinoma (CCA), pancreatic cancer (PC) and colorectal
cancer (CRC). EC is among the most fatal cancer types, ranking as
the sixth leading cause of cancer-related death globally and
exhibiting a higher prevalence in men (21). In total, ~90% of EC is esophageal
squamous cell carcinoma (ESCC), which is mainly induced by drinking
and smoking. Being overweight and gastroesophageal reflux are
recognized as the principal risk factors for esophageal
adenocarcinoma (21). GC ranks as
the third leading cause of cancer-related death globally, with
>1 million estimated new cases annually (22). Infection with Helicobacter
pylori remains a significant risk factor for GC. Liver cancer
is the sixth most common cancer and the fourth leading cause of
cancer-related mortality worldwide, including HCC (~80% of cases),
intrahepatic CCA (ICC; ~10% of cases), combined hepatocellular and
CCA, as well as other types (21). Long-term infection of hepatitis B
and C viruses, heavy drinking, smoking and extended periods of
consumption of foods containing aflatoxin are all risk factors for
liver cancer. PC is a highly malignant digestive tract tumor,
typically presenting with non-specific symptoms such as abdominal
pain and weight loss, as the first clinical manifestation (23). Most patients with PC are diagnosed
at an advanced stage soon after the symptoms appear, missing the
optimal timing for surgical intervention and resulting in an
extremely poor prognosis (24).
The triggers for PC are complex but smoking and a family history of
chronic pancreatitis are considered to be the main factors
(25). The incidence rate of CRC
is typically related to the lower development level or low economic
level of the country, mainly due to differences in living standards
and dietary patterns (26).
Numerous studies have shown that circRNA is
abundantly expressed in digestive system tumors, and its unique a
loop structure, resistance to degradation and ability to enter body
fluids make it a potential target for effective cancer treatment
(27-29). A comprehensive understanding of
current research progress on circRNA in gastrointestinal cancer
will therefore help to further explore its potential and establish
a foundation for the clinical translation of technologies that
target circRNA.
circRNAs from individual genes are mostly produced
in the form of multiple isomers, through a process termed 'reverse
splicing', which involves spliceosome machinery. Certain genes can
generate a greater variety of circRNAs than linear RNAs, such as
CAMSAP1, CYP24A1 and CRIM1 (30).
ecRNA is primarily located in the cytoplasm and is formed by
connecting the 5′ donor site downstream of the exon to the 3′
splice acceptor site through a single or multi-level jump. This
process forms a lasso-driven cyclization model, followed by intron
removal via splicing (6,31). ciRNA is predominantly located in
the nucleus and is an intron that depends on cleavage-catalyzed
cyclization, involving the insertion of the lariat tail (32). elciRNA is a double-stranded RNA
composed of introns located on both sides of an exon, which have
complementary sequences and ultimately produce a circRNA containing
both introns and exons through variable splicing (31). Thus far, the formation process of
circRNA has not been fully understood. For instance, the specific
mechanisms of catalyzing the cyclization of cord tail insertion
have not yet been completely elucidated and further research is
needed.
circRNA is a common competing endogenous RNA
(ceRNA), which can act as an miRNA sponge to regulate the
expression of miRNA target genes through complementary base
pairing. For instance, exosomal circUHRF1 has been shown to inhibit
natural killer cell-derived IFN-γ and TNF-α secretion through the
miR-449c-5p/T cell immunoglobulin and mucin-domain containing-3
axis and promotes the malignant progression of HCC (33). In CRC, circCAMSAP1 acts as a
sponge for miR-328-5p, thus abrogating its ability to suppress the
expression of the transcription factor, E2F1 (34). In addition, circNRIP1 acts as an
miR-149-5p sponge to promote GC progression via the AKT1/mammalian
target of rapamycin (mTOR) pathway (35). Thus, the miRNA sponge
functionality of circRNAs can have important implications for
cancer. Moreover, a single circRNA may contain multiple different
miRNA binding sites and may even target multiple conserved sites of
a single miRNA (8,36). circRNAs that act as miRNA sponges
are primarily ecRNA and elciRNA. In fact, miRNA sponge activity is
currently the most widely studied biological function of
circRNAs.
circRNA can promote protein stability and
transportation by binding to multiple RBPs and even forming
intricate circular and linear complexes. For instance, cerebellar
degeneration-related protein 1 antisense RNA (also known as ciRS-7)
acts as a molecular miRNA sponge by forming the RNA-induced
silencing complex with miR-7 and Argonaute2 proteins (9). Furthermore, circCTNNB1, an intronic
circRNA derived from the CTNNB1 gene, has been shown to bind to
DEAD-box polypeptide 3 and facilitate its interaction with
transcription factor Yin Yang 1 (YY1), triggering the
transactivation of YY1 and promoting β-catenin activity (37). Additionally, the expression level
of circFoxo3 can regulate the formation of ternary complexes with
p21 and CDK2, thereby controlling cell cycle progression (38).
Eukaryotic protein-coding mRNAs typically require a
5′-terminal cap structure. However, growing evidence suggests that
circRNAs that contain an internal ribosome entry site (IRES) can be
translated into polypeptides in vivo, through the IRES
cis-regulatory element (16,39-41). circZNF609 contains an open reading
frame (ORF) at the start codon with an in-frame stop codon,
allowing its translation into a protein product (12). In addition to mediating circRNA
translation through IRESs and ORFs, RNA methylation can also
mediate the initiation of circRNA-encoded protein translation
(17). The peptides/proteins
encoded by circRNAs are typically <100 amino acids, and these
functional proteins may play an important role in the progression
of diseases. FBXW7-185aa, a functional protein encoded by
circ-FBXW7, has been shown to inhibit the proliferation and cell
cycle progression in glioblastoma by degrading c-Myc (14). CircPPP1R12A-73aa, a protein
encoded by circPPP1R12A, has been reported to promote colon cancer
progression through the modulation of the Hippo-yes-associated
protein (YAP) pathway (42).
Due to the nuclear localization of ciRNA and
elciRNA, these types of circRNA can effectively regulate the
transcription of parental genes. For instance, ci-ankrd52, which is
formed from an intron of ankyrin repeat domain 50, can localize at
the transcription start site of the parent gene, exerting positive
regulation on RNA polymerase II (Pol II) and promoting gene
transcription (5). elciRNAs can
also enhance gene transcription by binding to Pol II nucleosides
upstream of the transcription start site of the gene (4).
The specific cyclization process of circRNA
inevitably affects the linear splicing of precursor mRNA (43). In both humans and fruit flies, the
Muscleblind (Mbl) protein binds to specific sites within the
introns that flank its own pre-mRNA. This interaction directly
competes with the splicing of the precursor mRNA, influencing the
balance between the production of linear mRNA and the formation of
circMbl, a circular RNA derived from the Mbl gene (18). During the formation of cDNA in
mice, Fmn, a new type of circRNA containing transcription
initiation sites, has been identified (19). This circRNA causes 'mRNA traps' by
separating transcription initiation sites, leading to the loss of
functional protein products. However, this circRNA may partially
reverse this physiological phenomenon post-translation.
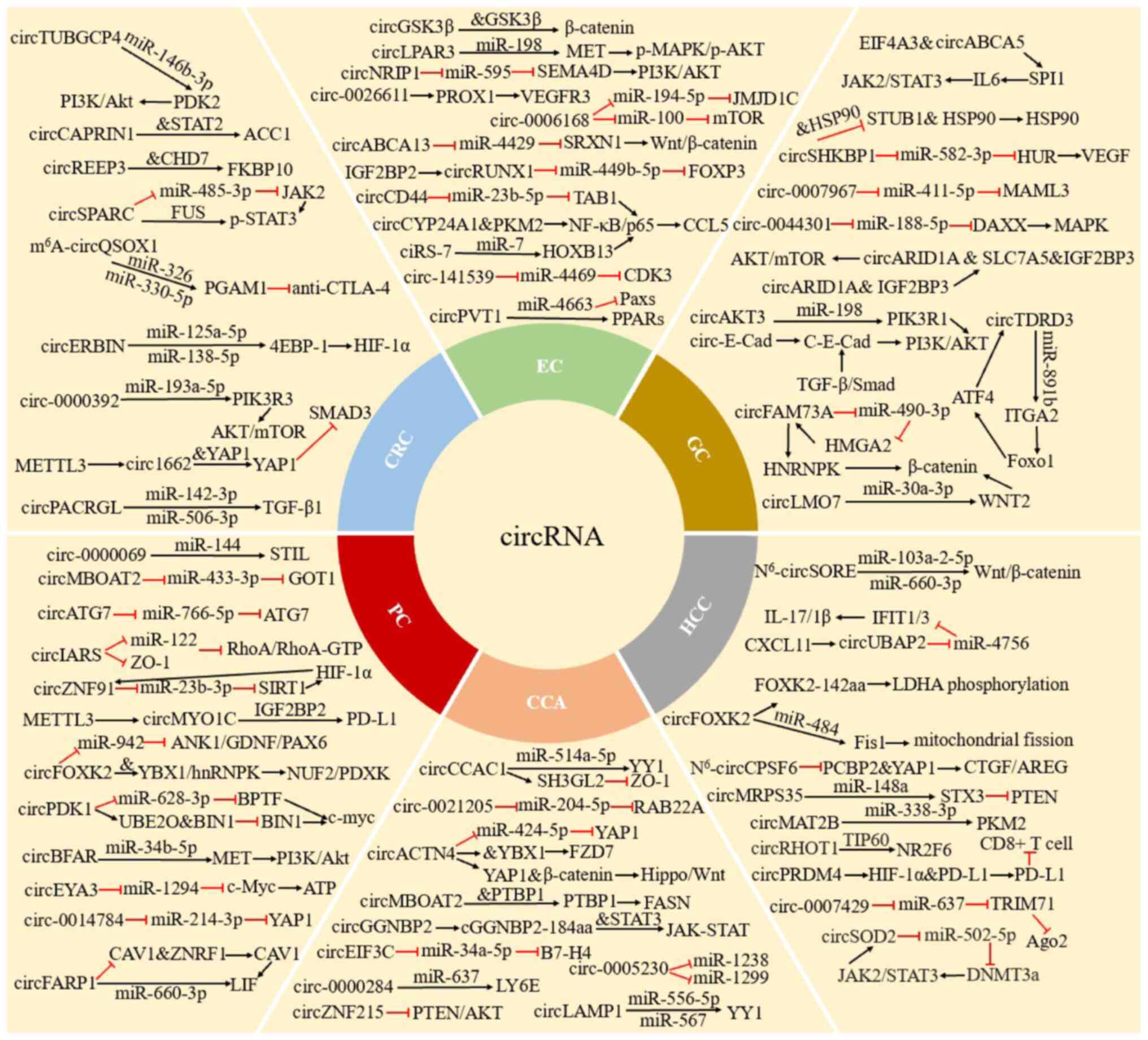
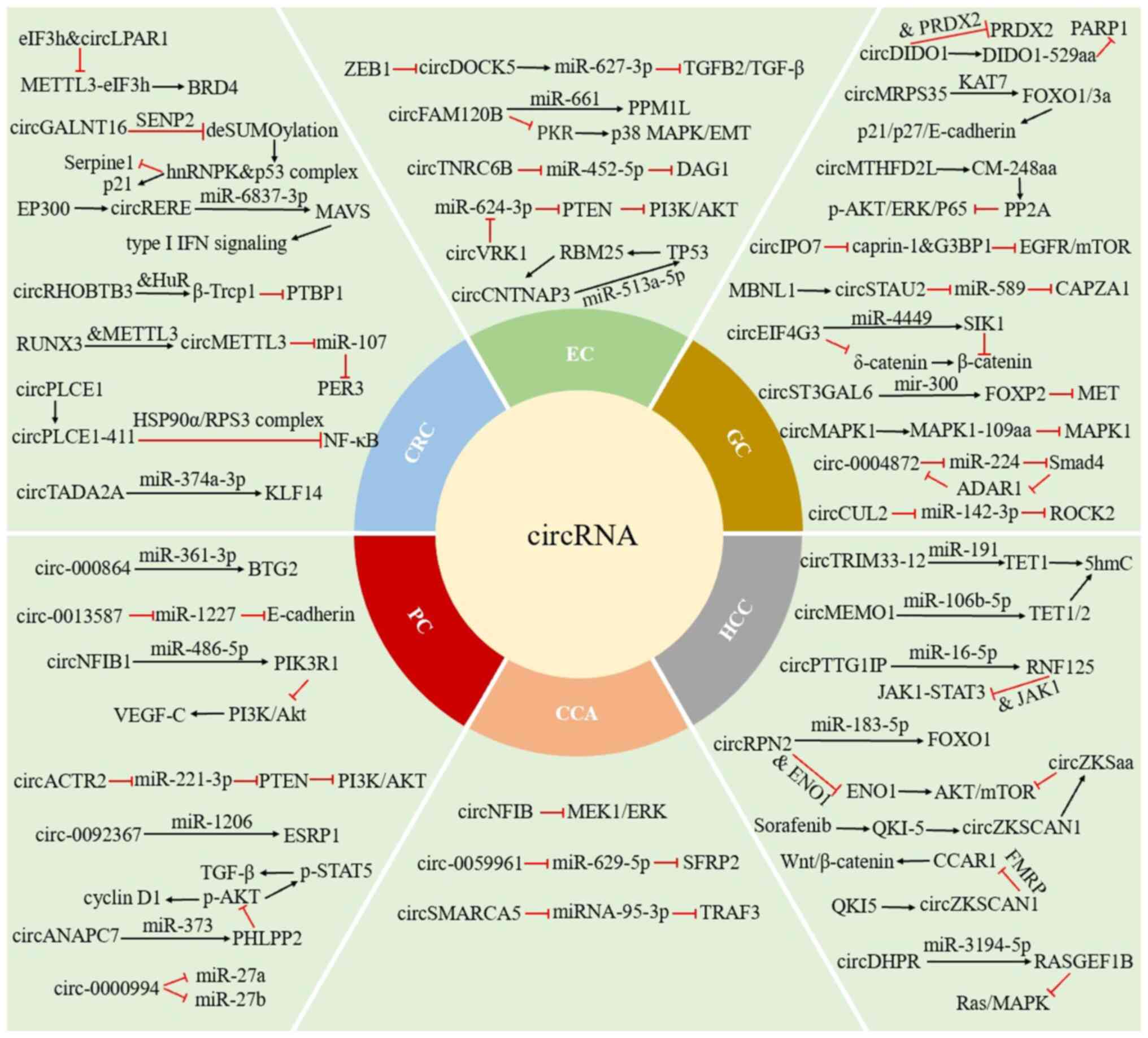
Among circRNAs that are upregulated in EC, circGSK3β
promotes the malignant progression of ESCC via direct inhibition of
GSK-3β. Furthermore, plasma circGSK3β levels can serve as a
biomarker for the detection of early-stage ESCC (44). circLPAR3 upregulates MET gene
expression through sponging miR-198 and activates the RAS/MAPK and
PI3K/AKT pathways, promoting ESCC cell migration, invasion and
metastasis in vitro and in vivo (45). circ-141539 upregulation is
positively associated with advanced TNM staging, low histological
grade and poor prognosis in patients with ESCC. Furthermore,
circ-141539 promotes the malignant progression of EC by sponging
miR-4469 and activating CDK3 expression (46). circPVT1 is significantly
upregulated in EC tissues and cancer cell lines and can reverse the
inhibitory effect of miR-4663 on tumors (47). ciRS-7 is significantly upregulated
in ESCC tissue and is associated with poor patient survival.
Furthermore, ciRS-7 adsorbs miR-7 and reactivates its downstream
homeobox B13-mediated NF-κB/p65 pathway, which promotes the
malignant progression of ESCC (48). High expression of circ-0006168 is
positively associated with lymph node metastasis and an advanced
TNM stage in patients with ESCC, and it promotes the expression of
mTOR through sponging miR-100 (49). In addition, circ-0006168 can also
facilitate Taxol resistance in ESCC by regulating the
miR-194-5p/jumonji domain containing 1C axis (50). circNRIP1 promotes proliferation
and invasion of ESCC cells by targeting the miR-595/semaphorin 4D
axis and inhibiting the PI3K/AKT signaling pathway (51). circCYP24A1 binds pyruvate kinase
M2 (PKM2) to regulate NF-κB-induced C-C motif ligand 5 secretion in
the progression of ESCC (52).
Insulin-like growth factor 2 (IGF2BP2) mediates circRUNX1-enhanced
FOXP3 expression by acting as a competitive miRNA sponge to inhibit
miR-449b-5p activity, thus promoting ESCC cell proliferation and
metastasis in vitro and in vivo (53). circCD44 functions as a sponge for
miR-23b-5p, activating TGF-β-activated kinase 1/NF-κB signaling and
promoting ESCC cell migration, invasion and proliferation (54). circ-0026611 is highly expressed in
ESCC cells and exosomes, and exosomal circ-0026611 promotes ESCC
lymphangiogenesis by interacting with N-α-acetyltransferase 10 and
inhibiting prospero homeobox 1 acetylation and ubiquitination
(55). High circABCA13 expression
predicts a poor prognosis in patients with ESCC as it upregulates
sulfiredoxin 1 and subsequently activates the Wnt/β-catenin pathway
by acting as a sponge for miR-4429 in ESCC (56). Functional artificial circRNAs
prevent miRNA binding to downstream target genes by adsorbing
endogenous prooncogenic miRNAs, significantly inhibiting the growth
and migration of EC cells, allowing a new avenue for the
construction of therapeutic circRNAs (57).
In a study, compared with matched adjacent normal
tissues, circCNTNAP3 was more significantly downregulated in three
pairs of ESCC tissues (58).
circCNTNAP3 promotes expression of the tumor suppressor gene, p53,
through sponging miR-513a-5p and, in turn, p53/RNA binding motif
protein 25 mediates the regulation of circCNTNAP3 biosynthesis,
resulting in a positive feedback loop for the expression of
circCNTNAP3 and p53 in ESCC (58). circVRK1 is downregulated in ESCC
tissues and cell lines, exerting its tumor suppressor effect.
circVRK1 acts as a molecular sponge for miR-624-3p, inactivating
the PI3K/AKT signaling pathway by positively regulating PTEN
expression and ultimately reversing the radiation resistance of
ESCC (59). Zinc finger E-box
binding homeobox 1 inhibits eukaryotic translation initiation
factor 4A3 (EIF4A3) promoter activity, thereby repressing the
biogenesis of circDOCK5. Downregulated circDOCK5 expression
enhances the migration and invasion of ESCC cells by forming a
positive feedback loop with TGF-β, altering the miR-627-3p/TGFB2
signaling pathway (60). Tissue
microarray analysis identified that circTRPS1-2 was downregulated
in ESCC tissues, which was correlated with poor prognosis.
Furthermore, circTRPS1-2 inhibits the progression of ESCC by
decreasing ribosome biogenesis (61). The downregulation of circTNRC6B
expression in ESCC tissue is an independent risk factor for poor
prognosis. circTNRC6B exerts a tumor-suppressing effect in ESCC by
regulating the miR-452-5p/dystroglycan 1 axis (62). Downregulation of circFAM120B
expression in cancer tissues and patient plasma is associated with
poor prognosis and tumor progression in ESCC. However, circFAM120B
inhibits the tumorigenicity of ESCC by adsorbing miR-661 to
stabilize the expression of protein phosphatase 1L (63). In addition, circFAM120B decreases
the stability of protein kinase R by binding to it and promoting
its polyubiquitination and degradation, ultimately inhibiting the
p38 MAPK-mediated epithelial-mesenchymal transition (EMT) pathway
(63).
The relationship between circRNA and the malignant
hallmarks of EC is summarized in Table I. circRNAs play a significant role
in the development and progression of EC. Upregulated circRNAs such
as circGSK3β, circLPAR3, circ-141539, circPVT1, ciRS-7,
circ-0006168, circNRIP1, circ-0026611 and circABCA13, promote
malignant progression by competitive inhibition of miRNAs and the
consequent activation of various signaling pathways. Conversely,
downregulated circRNAs, including circCNTNAP3, circVRK1,
circTRPS1-2, circTNRC6B and circFAM120B, act as tumor suppressors
by regulating miRNA targets and inhibiting key signaling pathways.
Understanding the dysregulation of circRNAs in EC may provide
valuable insights for identifying potential biomarkers and
therapeutic strategies in the future.
The expression of circSHKBP1 in GC tissues is
significantly higher than that in normal tissues, and increased
expression of circSHKBP1 is related to advanced TNM stage, vascular
infiltration and poor survival. The level of circSHKBP1 in the
serum exosomes of patients with GC is ~6 times higher than that in
tumors (64). Exosomal circSHKBP1
can regulate the miR-582-3p/HuR/VEGF pathway and directly interact
with HSP90 to inhibit the ubiquitination of HSP90 by STIP1 homology
and U-box containing protein 1, ultimately accelerating GC
progression (64). The expression
of circAKT3 is significantly increased in cisplatin-resistant GC
tissues and cells, which can serve as a biomarker of cisplatin
resistance. Mechanistically, circAKT3 promotes
phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) expression
and activates the PI3K/AKT signaling pathway through sponging
miR-198, ultimately promoting cisplatin resistance in GC cells
(65). Heterogeneous nuclear
ribonucleoprotein L (HNRNPL) can bind to the flanking introns of
circLMO7 exons to promote the self-cyclization of circLMO7 in GC.
circLMO7 then promotes the development of GC through the
circLMO7/miR-30a-3p/WNT2 axis (66). High expression of circFAM73A
indicates a poor prognosis in patients with GC. circFAM73A, as a
sponge for miR-490-3p, relieves the inhibition of high mobility
group A2 (HMGA2), and HMGA2 can further enhance the activities of
E2F1 and HNRNPL, which in turn promote circFAM73A expression and
form a positive feedback loop (67). Additionally, circFAM73A can also
directly interact with heterogeneous nuclear ribonucleoprotein K
(hnRNPK) to facilitate β-catenin stabilization, ultimately
promoting the cancer stem cell-like properties and malignancy of GC
cells (67). circARID1A is
upregulated in GC tissues, promoting the interaction between
IGF2BP3 and solute carrier family 7 member 5 (SLC7A5) by directly
binding to IGF2BP3 (68). This
forms a circARID1A-IGF2BP3-SLC7A5 RNA-protein ternary complex to
increase SLC7A5 mRNA stability and activate the AKT/mTOR pathway,
ultimately promoting GC proliferation. circ-0007967 upregulates the
expression of mastermind-like transcriptional coactivator 3 through
sponge adsorption of miR-411-5p, promoting the proliferation of GC
cells (69). circ-0044301 is
significantly upregulated in GC tissues compared with non-cancerous
tissues and is positively correlated with poor patient prognosis.
circRNA-0044301 participates in the progression of GC by regulating
the miR-188-5p/death domain associated protein/MAPK axis (70). circABCA5 upregulates SPI1
expression and promotes nuclear translocation by directly binding
to EIF4A3, promoting the proliferation, invasion and migration of
GC cells by activating IL6/janus kinase 2 (JAK2)/STAT3 signaling
(71). C-E-Cad is significantly
upregulated in GC tissues compared with adjacent benign tissues.
circ-E-Cad encodes the C-E-Cad protein, while the TGF-β/SMAD
pathway enhances the expression level of C-E-Cad, ultimately
promoting the malignant progression of GC cells by upregulating the
PI3K/AKT pathway (72). circTDRD3
is upregulated in GC tissues and is correlated with tumor
progression and poor prognosis. circTDRD3 promotes GC cell
progression by regulating the miR-891b/integrin (ITG)A2 axis and
the AKT signaling pathway (73).
circCUL2 has low expression in GC tissues and cells.
circCUL2 inhibits the growth and metastasis of GC through the
miR-142-3p/Rho associated coiled-coil containing protein kinase 2
axis, while mediating the autophagy activation of GC cells and
increasing their sensitivity to cisplatin (74). circMRPS35 is significantly
downregulated in GC tissues compared with normal tissues, and high
expression of circMRPS35 is negatively correlated with advanced TNM
staging, lymph node metastasis and tumor size in GC. circMRPS35 is
mainly located in the nucleus of GC cells and can recruit lysine
acetyltransferase 7 to the promoters of FOXO1 and FOXO3a genes,
increase the acetylation level of H4K5 in the promoter region of
these genes, activate the transcription of FOXO1/3a and trigger
downstream target gene expression responses, including p21, p27 and
E-cadherin, inhibiting GC cell proliferation and invasion (75). circ-0004872 is significantly
downregulated in GC tissue, and sponges miR-224 to regulate the
expression of p21 and SMAD4, thus inhibiting the proliferation,
invasion and migration of GC cells. SMAD4 can also promote the
expression of circ-0004872 by directly binding to the adenosine
deaminase RNA specific 1 promoter region, forming a negative
regulatory loop (76). circDIDO
is considered beneficial in GC and is formed by the reverse
splicing of exons 2-6 of DIDO1. circDIDO encodes a 529 amino acid
tumor suppressor protein, which interacts with poly (ADP-ribose)
polymerase 1 and inhibits its activity (77). circDIDO1 also specifically binds
to peroxiredoxin 2 (PRDX2), promoting RBX1-mediated ubiquitination
and degradation of PRDX2, leading to the inactivation of its
downstream pathways to exert tumor inhibitory effects (77). circMAPK1 in GC tissue encodes a
novel protein, MAPK1-109aa, which inhibits the phosphorylation of
MAPK1 by competitively binding to MEK1, thereby suppressing the
activation of MAPK1 and its downstream factors in the MAPK pathway
and inhibiting the malignant biological behavior of GC cells
(78). circEIF4G3 is
downregulated in GC tissues compared with adjacent normal tissues.
Notably, lower circEIF4G3 expression predicts poor survival in
patients with GC. circEIF4G3 binds to δ-catenin protein to promote
tripartite motif containing (TRIM)25-mediated δ-catenin ubiquitin
degradation and inactivates β-catenin signaling in GC cells. In
addition, circEIF4G3 can interact with miR-4449 to upregulate salt
inducible kinase 1 expression and also inhibit β-catenin signaling
(79). Ultimately, circEIF4G3
inhibits the progression of GC. circST3GAL6 expression levels are
negatively associated with tumor stage and size. circST3GAL6, as
the ceRNA of miR-300, alleviates the inhibitory effect of miR-300
on its target gene, FOXP2, which transcriptionally inhibits MET and
regulates the AKT/mTOR pathway, thereby promoting apoptosis and
autophagy of GC (80). circSTAU2
and Mbl-like splicing regulator 1 (MBNL1) colocalize in the
cytoplasm, and MBNL1 promotes the expression of circSTAU2.
Exosome-delivered circSTAU2 promotes the expression of capping
actin protein muscle Z-line α1 via sponge adsorption of miR-589 and
suppresses GC cell proliferation, invasion and migration in
vitro and in vivo (81). As a tumor suppressor, circMTHFD2L
RNA-encoded CM-248aa competitively targets the acidic domain of SET
nuclear oncogene, which restores the activity of protein
phosphatase 2A and further mediates the dephosphorylation and
inactivation of AKT, ERK and p65 in GC (82). Low expression of circIPO7 is an
independent risk factor for poor prognosis in GC. Overexpressed
circIPO7 directly binds to captin-1, reducing the interaction
between captin-1 and Ras GTPase-activating protein-binding protein
1 (G3BP1), dissociating captin-1 from the ribosome and suppressing
the translation of its target mRNA, and reducing the activation of
the PI3K/AKT/mTOR pathway (83).
The relationship between circRNA and the malignant
hallmarks of GC is summarized in Table II. The expression levels of
circSHKBP1, circAKT3, circLMO7, circFAM73A, circARID1A,
circ-0007967, circ-0044301, circABCA5, circ-E-Cad and circTDRD3 are
significantly increased in GC tissues, and circCUL2, circMRPS35,
circ-0004872, circDIDO, circMAPK1, circEIF4G3, circST3GAL6,
circSTAU2, circMTHFD2L and circIPO7 are downregulated in GC
tissues. Hence, circRNAs play various roles in promoting or
inhibiting GC progression through different mechanisms. Certain
circRNAs can accelerate GC progression by promoting cancer stem
cell-like properties, enhancing mRNA stability or activating
proliferation and invasion. Conversely, other circRNAs inhibit
tumor formation and progression by influencing autophagy, gene
transcription, protein degradation and protein phosphorylation.
High expression of exosomal circRNA-100338 in serum
was considered an adverse factor for metastasis and poor prognosis
in patients with HCC who underwent curative hepatectomy.
circ-100338 can affect the angiogenesis and vasculogenic mimicry
formation ability of human umbilical vein endothelial cells,
ultimately promoting the proliferation and metastasis of HCC
(84). circSORE is highly
expressed in sorafenib-resistant HCC cells and is modified with
N6-methyladenosine (m6A). circSORE is a
sponge for miR-103a-2-5p and miR-660-3p, which can competitively
activate the WNT2b/β-catenin pathway, thus inducing the resistance
of HCC to sorafenib treatment, laying a theoretical foundation for
clinical research of sorafenib resistant HCC (85). Histone writers, E1A binding
protein p300 (EP300) and WD repeat domain 5, bind to the circSOD2
promoter and trigger its promoter H3K27ac and H3K4me3 modification,
respectively, which further activates circSOD2 expression in HCC.
circSOD2 sponges miR-502-5p to upregulate the expression of DNA
methyltransferase 3a (DNMT3a) in HCC cells. Upregulated DNMT3a
decreases suppressor of cytokine signaling 3 (SOCS3) expression by
increasing SOCS3 promoter DNA methylation and accelerates
JAK2/STAT3 signaling pathway activation (86). circRHOT1 is mainly distributed in
the nucleus and interacts directly with TIP60, recruiting TIP60 to
the nuclear receptor subfamily 2 group F member 6 (NR2F6) promoter
and initiating the transcription of NR2F6, promoting the growth,
migration and invasion of HCC cells (87). circMAT2B promotes glycolysis and
malignant progression of HCC by activating the miR-338-3p/PKM2 axis
under hypoxia (88). In HCC
tissues, CXC motif chemokine ligand 11 secreted by
cancer-associated fibroblasts (CAFs) upregulates the expression of
circUBAP2, competitively upregulating interferon induced protein
with tetratricopeptide repeats (IFIT)1/IFIT3 levels and promoting
the expression of IL-17 and IL-1β via sponge adsorption of
miR-4756, promoting the progression of HCC (89). m6A-modified circCPSF6
competitively interacts with poly(rC)-binding protein 2 to prevent
its conjugation to YAP mRNA, resulting in enhanced YAP1 mRNA
stability and increased HCC malignancy (90). circMRPS35 is highly expressed in
patients with HCC and upregulates the expression of syntaxin 3
through sponging miR-148a, thereby promoting the ubiquitination and
degradation of PTEN (91). Upon
cisplatin treatment, circMRPS35-168aa is significantly upregulated,
contributing to enhanced cell survival and reduced apoptosis in HCC
cells. This phenomenon underscores the pivotal role of the peptide
in mediating chemoresistance, thereby advancing understanding of
the molecular intricacies involved in HCC progression and the
development of therapeutic resistance (91). Upregulation of circFOXK2 indicates
poor prognosis of HCC. However, circFOXK2-encoded FOXK2-142aa
interacts with lactate dehydrogenase A (LDHA) to activate its
phosphorylation (92). The
phosphorylation of LDHA plays a pivotal role in reprogramming
glucose metabolism towards the Warburg effect, facilitating HCC
progression and highlighting the potential of targeting this
pathway for therapeutic intervention. In addition, circFOXK2 can
also induce mitochondrial fission by regulating the miR-484/Fis1
pathway, promoting tumor progression and activating the Warburg
effect in HCC cells (92).
Upregulation of circPRDM4 in patients with HCC treated with
anti-programmed cell death protein-1 therapy facilitates tumor
growth and immune escape. Mechanistically, circPRDM4 recruits
hypoxia-inducible factor (HIF)-1α to the CD274 promoter to boost
programmed death-ligand 1 (PD-L1) expression and inhibit the
CD8+ T cell-mediated antitumor immune response under
hypoxic conditions (93).
circ-0007429 is aberrantly upregulated in HCC tissues and is
positively correlated with poor clinical outcomes. circ-0007429
acts as a sponge targeting the tumor suppressor, miR-637, to
promote TRIM71 expression and affect the expression of its
downstream molecule, argonaute-2, which promotes HCC progression
and aerobic glycolysis in vitro and in vivo (94).
The RNA-splicing protein, Quaking (QK) 5, regulates
the low expression of circZKSCAN1 in HCC, and circZKSCAN1 blocks
the binding between fragile X mental retardation protein (FMRP) and
cell division cycle and apoptosis regulator 1 mRNA by competitively
binding FMRP, subsequently restraining the transcriptional activity
of the Wnt/β-catenin signaling pathway and negatively regulating
cell stemness, which inhibits the malignant behavior of HCC
(95). Downregulation of
circTRIM33-12 expression is an independent risk factor for the
overall survival and recurrence free survival rates of patients
with HCC after surgery. circTRIM33-12 significantly reduces the
level of 5-hydroxymethylcytosine (5hmC) in HCC cells by sponging
miR-191 and enhancing Tet methylcytosine dioxygenase 1 (TET1)
expression, resulting in the impairment of tumor immune evasion
(96). circMEMO1 is significantly
downregulated in HCC tissues and can modulate the promoter
methylation and gene expression of transcription factor 21; it
regulates EMT through the miR-106b-5p/TET1/5hmC axis, resulting in
negative regulation of HCC progression and increased sensitivity of
HCC cells to sorafenib treatment (97). circPABPC1 not only inhibits cell
adhesion and migration by downregulating ITGB1 but also directly
connects ITGB1 to the 26S proteasome, resulting in
ubiquitin-independent ITGB1 degradation (98). Decreased circRPN2 expression in
HCC is correlated with poor prognosis. circRPN2 binds to enolase 1
(ENO1) and accelerates ubiquitin/proteasome-dependent ENO1
degradation, activating the AKT/mTOR pathway to regulate aerobic
glycolysis reprogramming in HCC cells. circRPN2 also upregulates
FOXO1 expression by sponging miR-183-5p to suppress HCC glycolysis
and metastasis (99). As a
potential therapeutic target for HCC, circVAMP3 has been found to
bind to the cell cycle-associated protein 1 (CAPRIN1) and G3BP1
complex by directly interacting with CAPRIN1, promoting stress
granule formation in cells and negatively regulating the
proliferation and metastasis of HCC cells in vitro and in
vivo (100). As a newly
identified tumor suppressor circRNA in HCC, low expression of
circPTTG1IP is associated with the number of tumors, tumor
encapsulation, microvascular invasion and TNM stage, and is an
independent factor of overall survival and recurrence in patients
with HCC (101). Mechanistic
studies have determined that downregulation of circPTTG1P
sequesters miR-16-5p by acting as a miRNA sponge. This
competitively regulates the ring finger protein 125 (RNF125)
expression level, and RNF125 interacts with and degrades JAK1
protein to inhibit the JAK1/STAT3 pathway (101). In addition, the study also
demonstrated that the JAK1-selective inhibitor, Figotinib, can
markedly inhibit the recruitment of tumor-associated macrophages
and M2 polarization triggered by the circPTTG1IP/JAK1 axis, to
remodel the tumor microenvironment and improve patient prognosis.
Patients with HCC with low circDHPR expression have shorter overall
survival and disease-free survival times. circDHPR acts as a ceRNA
for miR-3194-5p, which increases RasGEF domain family member 1B
expression to promote tumor growth and metastasis (102). Sorafenib-resistance in HCC is a
challenging clinical problem. As such, elucidating the underlying
mechanism of sorafenib treatment is crucial for identifying new
therapeutic targets for HCC. Sorafenib regulates the expression of
circZKSCAN1 via upregulation of QKI-5. circZKSCAN1 can enhance the
anti-tumorigenesis effect of sorafenib in HCC cells by encoding the
circZKSaa peptide. circZKSCAN1 can also interact with F-box and WD
repeat domain containing 7 to promote the ubiquitination of mTOR,
thereby inhibiting activation of the PI3K/AKT/mTOR pathway and
making HCC cells sensitive to sorafenib (103).
The relationship between circRNA and the malignant
hallmarks of HCC is summarized in Table III. Overall, several circRNAs
are dysregulated in HCC, either upregulated or downregulated, and
have critical roles in HCC progression and sensitivity to
treatment. Upregulated circRNAs such as circ-100338, circSORE,
circSOD2, circRHOT1, circMAT2B, circUBAP2, circCPSF6, circMRPS35,
circFOXK2, circPRDM4 and circ-0007429, are associated with various
adverse factors, including metastasis, resistance to treatment and
poor prognosis. By contrast, downregulated circRNAs such as
circZKSCAN1, circTRIM33-12, circMEMO1, circPABPC1, circRPN2,
circVAMP3, circPTTG1IP and circZKSCAN1 are involved in inhibiting
malignant behavior, immune evasion, HCC progression and promoting
sensitivity to sorafenib treatment. Understanding the dysregulation
of circRNAs in HCC may provide valuable insights into the molecular
mechanisms underlying HCC development and potentially guide the
development of new therapeutic strategies for this disease.
circCCAC1 levels are increased in cancerous
bile-resident extracellular vesicles and tissues. circCCAC1
upregulates YY1 through sponge adsorption of miR-514a-5p, and YY1
directly binds to the promoter of calcium modulating ligand to
activate its transcription, ultimately promoting the proliferation
and invasion of CAA. Meanwhile, circCCAC1 in extracellular vesicles
can enter human umbilical vein endothelial cells to disrupt the
vascular endothelial barrier and induce angiogenesis (104). In addition, circCCAC1 enhances
endothelial cell monolayer permeability by regulating the SH3
domain containing GRB2 like-2, endophilin A1/ZO-1/Occludin
signaling pathway, accelerating occurrence and metastasis of CCA
(104). circ-0000284 not only
promotes the progression of CCA through the miR-637/lymphocyte
antigen 6 family member E axis but also directly transfers from CCA
cells to surrounding normal cells through exosomes, stimulating the
migration and proliferation of surrounding normal cells (105). Microarray analysis was used to
identified 171 differentially expressed circRNAs in six paired
distal CCA tumor and adjacent normal tissue samples, including 132
upregulated and 39 downregulated circRNAs. Upregulated circ-0000673
expression was associated with tumor invasion, poor cell
differentiation and residual tumor. The area under the receiver
operating characteristic (ROC) curve of circ-0000673 was 0.85,
demonstrating a good ability to distinguish distal CCA from normal
tissue (106). circ-0005230 is
highly expressed in CCA tissues and cells, promoting the growth and
metastasis of CCA cells through sponging miR-1238 and miR-1299
(107). circLAMP1 is upregulated
in CCA and is associated with poor prognosis. circLAMP1 upregulates
YY1 expression via sponging miR-556-5p and miR-567, playing a
tumorigenic role in CCA (108).
circ-0021205 expression is enhanced in CCA tissues and is
correlated with tumor size and advanced TNM stage. circ-0021205
upregulates RAB22A expression by targeting miR-204-5p, thereby
facilitating CCA progression (109). circACTN4 is upregulated in ICC
tissues and upregulates YAP1 expression by acting as a molecular
sponge for miR-424-5p. circACTN4 also transcriptionally activates
frizzled class receptor 7 by interacting with Y-box binding protein
1 (YBX1). Furthermore, circACTN4 enhances the interaction between
YAP1 and β-catenin and activates the Wnt/Hippo signaling pathways,
ultimately promoting the proliferation and metastasis of ICC
(110). circEIF3C promotes ICC
progression and immune evasion through the miR-34a-5p/B7-H4 axis
(111). circGGNBP2 induced by
IL-6 can encode cGGNBP2-184aa protein, which directly interacts
with STAT3 and promotes STAT3Tyr705 phosphorylation,
nuclear translocation and activation of the JAK/STAT pathway in ICC
(112). circMBOAT2 is an
upregulated circRNA associated with lipid metabolism in ICC and is
correlated with an unfavorable prognosis. CircMBOAT2 interacts with
polypyrimidine tract binding protein 1 (PTBP1) in ICC cells and
protects PTBP1 from ubiquitin/proteasome-dependent degradation,
promoting PTBP1-mediated cytoplasmic export of fatty acid synthase
(FASN) mRNA (113). Overall,
circMBOAT2 promotes lipid metabolism reprogramming in ICC via the
circMBOAT2/PTBP1/FASN axis, especially unsaturated lipids,
ultimately affecting cell membrane composition, energy metabolism
and redox homeostasis, leading to the progression of ICC (113). circZNF215 expression is notably
increased in ICC tissues, and high circZNF215 expression predicts a
poor prognosis. circZNF215 promotes oxidation-induced inactivation
of PTEN and AKTSer473/Thr308 phosphorylation by blocking
the interaction between peroxiredoxin 1 (PRDX1) and PTEN through
competitively binding to PRDX1 (114).
circNFIB is downregulated in ICC tissues and is
associated with a worse prognosis. circNFIB competitively interacts
with MEK1, which induces the dissociation between MEK1 and ERK2,
thereby inhibiting ERK phosphorylation and suppressing ICC growth
and metastasis. Moreover, circNFIB serves as a promising
therapeutic molecule in ICC as it has been shown to enhance the
antitumor effects of trametinib in vitro and in vivo
(115). circ-0059961 is
downregulated in CCA tissues and carcinoma cells, such as CCLP-1
and QBC939, and overexpressed circ-0059961 inhibits CCA
proliferation, migration and invasion. Mechanistically,
circ-0059961 acts as a sponge for miR-629-5p to upregulate secreted
frizzled related protein 2 (116). circSMARCA5 is downregulated in
CCA, and mechanistic investigations have demonstrated that
circSMARCA5 inhibits cell growth and promotes apoptosis by sponging
miRNA-95-3p to regulate the expression of TNF receptor associated
factor 3 (117).
The relationship between circRNA and the malignant
hallmarks of CCA is summarized in Table IV. In CCA, upregulated or
downregulated circRNAs influence the progression of the disease.
Upregulated circRNAs such as circCCAC1, promote the growth and
invasion of CCA cells, disrupt blood vessels and induce the
formation of new blood vessels. Other upregulated circRNAs such as
circ-0000284, circ-0005230, circLAMP1, circ-0021205, circACTN4,
circEIF3C, circGGNBP2, circMBOAT2 and circZNF215, also contribute
to CCA progression via various mechanisms. However, certain
circRNAs are downregulated in CCA such as circNFIB, circ-0059961
and circSMARCA5, which inhibit the growth and metastasis of CCA
cells. Understanding the role of these circRNAs in CCA could aid
with finding new biomarkers for the prognosis and treatment of
CCA.
circIARS in the plasma exosomes of patients with
metastatic PC can enter human microvascular vein endothelial cells
and upregulate RhoA and RhoA-GTP levels through the miR-122/ZO-1
signaling axis, ultimately enhancing endothelial monolayer
permeability and promoting tumor invasion and metastasis (118). circFOXK2 not only acts as a
sponge for miR-942 to promote the expression of ankyrin 1, glial
cell line-derived neurotrophic factor and paired box 6, but it can
also enhance the expression of oncogene NUF2 and pyridoxal kinase
through interaction with YBX1 and hnRNPK, ultimately promoting the
progression of pancreatic ductal adenocarcinoma (PDAC) (119). circBFAR is positively correlated
with lymph node metastasis and advanced TNM stage in PDAC,
indicating a poor prognosis in patients. circBFAR upregulates the
expression of MET and activates the PI3K/AKT signaling pathway by
sponging miR-34b-5p, ultimately promoting the malignant progression
of PDAC (120). circ-0000069 is
markedly upregulated in PC tissues and cell lines, and its area
under the ROC curve in cancer tissues is 0.8944, demonstrating
diagnostic value. circ-000069 can promote the growth of PC cells
through the miR-144/STIL axis, and circ-0000069 in exosomes can be
transferred from SW1990 cells to human pancreatic duct epithelial
cells, thereby enhancing the malignant transformation of PC
(121). circMBOAT2 is highly
expressed in PC tissues and cells and modulates tumor development
and glutamine catabolism through the
miR-433-3p/glutamic-oxaloacetic transaminase 1 axis (122). circEYA3 is elevated in PDAC
tissues and cells, and high expression of circEYA3 is associated
with advanced lymph node invasion and tumor metastasis, predicting
a poor prognosis in patients. Mechanistically, circEYA3 exerts a
carcinogenic effect on PDAC by increasing ATP synthesis through the
miR-1294/c-Myc axis (123).
circZNF91 in hypoxic exosomes of PC cells competitively binds to
miR-23b-3p, upregulates sirtuin 1 expression and enhances the
deacetylation-dependent stability of HIF-1α, leading to glycolysis
and gemcitabine chemoresistance in recipient PC cells. Meanwhile,
transcription of HIF-1α also increases the expression of circZNF91
in hypoxic exosomes, forming a positive feedback loop (124). circPDK1 is upregulated in PC
tissues and serum exosomes and is associated with poor prognosis.
Exosomal circPDK1 is activated by HIF-1α at the transcriptional
level during hypoxia and upregulates bromodomain PHD finger
transcription factor expression by competitively binding to
miR-628-3p (125). In addition,
circPDK1 may serve as a scaffold to enhance the binding of
ubiquitin conjugating enzyme E2 O (UBE2O) and bridging integrator 1
(BIN1), thus facilitating the effects of UBE2O on the
ubiquitination and degradation of BIN1 (125). circATG7 in the cytoplasm of PC
cells increases the autophagy related 7 (ATG7) mRNA level by
sponging miR-766-5p, and nuclear circATG7 increases the stability
of ATG7 mRNA by recruiting HuR, which promotes the proliferation,
metastasis and autophagy of PC (126). circFARP1 expression in CAFs is
positively correlated with gemcitabine chemoresistance in advanced
PDAC. In addition, circFARP1 enhances leukemia inhibitory factor
(LIF) expression by functioning as a miR-660-3p sponge in CAFs.
circFARP1 also directly interacts with caveolin 1 (CAV1) and blocks
the interaction of CAV1 and E3 ubiquitin-protein ligase zinc and
ring finger 1 to inhibit CAV1 degradation, which enhances LIF
secretion. Overall, circFARP1 enhances the expression and secretion
of LIF in CAFs to induce chemoresistance (127). circMYO1C mediated by the
m6A methyltransferase, methyltransferase 3
N6-adenosine-methyltransferase complex catalytic subunit (METTL3),
is highly expressed in PDAC tissues. circMYO1C targets the
m6A site of PD-L1 mRNA to enhance PD-L1 mRNA stability
by cooperating with the m6A reader, IGF2BP2, thereby
accelerating PDAC immune escape (128). Upregulation of circ-0014784
promotes the invasion, proliferation, EMT and angiogenesis of PC by
regulating miR-214-3p/YAP1 signaling (129).
circNFIB1 is downregulated in PDAC tissues and is
negatively correlated with lymph node metastasis in patients.
circNFIB1 inhibits the PI3K/AKT pathway and decreases the
expression of VEGF-C by sponging miR-486-5p and upregulating PIK3R1
expression, ultimately suppressing lymphangiogenesis and lymph node
metastasis in PDAC (130). The
expression level of circ-000864 is lower in PC tumor tissues
compared with adjacent tissues. circ-000864 inhibits the migration
and invasion of PC cells by upregulating the expression of BTG
anti-proliferation factor 2 through sponging miR-361-3p (131). The expression of circ-0013587 is
significantly decreased in erlotinib-resistant AsPC-1 cells, and
circ-0013587 can reverse erlotinib resistance in PC cells by
increasing the levels of E-cadherin through suppressing the
expression of miR-1227 (132).
Cytoplasmic circ-0092367 sponges miR-1206 and decreases its
expression and increases the expression of epithelial splicing
regulatory protein 1 (a target gene of miR-766-5p), thereby
inhibiting EMT and enhancing the sensitivity of PC cells to
gemcitabine treatment (133).
circANAPC7, a tumor suppressor, promotes the expression of PH
domain and leucine rich repeat protein phosphatase 2 (PHLPP2) by
binding to miR-373. circANAPC7 is involved in the ZIP4-mediated
cAMP response element-binding protein/miR-373/PHLPP2 feed-forward
loop, leading to AKT dephosphorylation and downregulation of cyclin
D1 and TGF-β, suppressing tumor growth and muscle wasting in PC
(134). circ-0000994 inhibits
the proliferation, migration and invasion of PC cells through
spongingmiR-27a and miR-27b (135). circACTR2 is significantly
downregulated in gemcitabine-resistant PC cell lines, and high
expression of circACTR2 is associated with improved survival in
patients with PC. circACTR2 directly represses miR-221-3p levels
and thus upregulates the expression of PTEN, thereby inhibiting the
PI3K/AKT signaling pathway and enhancing the sensitivity of PC
cells to gemcitabine treatment (136).
The relationship between circRNA and the malignant
hallmarks of PC is summarized in Table V. Several circRNAs such as
circIARS, circFOXK2, circBFAR, circ-0000069, circMBOAT2, circEYA3,
circZNF91, circPDK1, circATG7, circFARP1, circMYO1C and
circ-0014784, have been found to be upregulated in PC and
contribute to tumor progression by regulating various pathways and
molecules. Conversely, circNFIB1, circ-000864, circ-0013587,
circ-0092367, circANAPC7, circ-0000994 and circACTR2 are
downregulated in PC and function as tumor suppressors by inhibiting
proliferation and invasion and by promoting drug sensitivity via
their interaction with specific miRNAs. These findings suggest that
circRNAs have a critical role in the development and progression of
PC.
circNSUN2 is significantly upregulated in the tumor
tissue and serum samples of patients with CRC with liver
metastasis. The m6A modification of circNSUN2 promotes
the formation of a ternary complex with IGF2BP2 and HMGA2 in the
cytoplasm, enhancing the stability of HMGA2 mRNA and promoting the
progression of liver metastasis in CRC (137). As a novel CRC-derived exosomal
circRNA, circPACRGL is significantly upregulated in CRC cells.
circPACRGL serves as a sponge for miR-142-3p/miR-506-3p to enhance
the expression of TGF-β1, which promotes the proliferation,
migration and invasion of CRC cells, as well as differentiation of
neutrophils from N1 to N2 (138). circERBIN enhances the expression
of eukaryotic initiation factor 4E-binding protein 1 (4EBP-1) by
sponging miR-125a-5p and miR-138-5p and increases HIF-1α protein
expression via 4EBP-1, significantly promoting the angiogenesis of
CRC (139). circ-0000392
distinguishes CRC cases from healthy controls with an area under
the curve (AUC) of 0.713, and the expression of circ-0000392 is
significantly correlated with pathological stage, lymph node
metastasis and distant metastasis. circ-0000392 acts as a ceRNA
against miR-193a-5p, which directly targets PIK3R3, affecting
activation of the AKT-mTOR pathway in CRC cells (140). The expression level of circ1662
is positively correlated with the malignant progression of CRC and
is associated with poor survival. METTL3 promotes the expression of
circ1662 by binding to its flanking sequences and installing
m6A modifications. circ1662 directly binds to YAP1 and
accelerates its nuclear accumulation to inhibit the expression of
SMAD3, enhancing CRC invasion and migration (141). circSPARC is highly abundant in
CRC tumor tissues and plasma and shows promising diagnostic
performance in distinguishing CRC from normal tissues (AUC=0.8613).
circSPARC serves as a sponge for miR-485-3p to facilitate JAK2
expression and promote the accumulation of phosphorylated
(p)-STAT3. In addition, circSPARC can recruit FUS and promote
nuclear translocation of p-STAT3, ultimately enhancing the
proliferation and migration of CRC (142). circQSOX1 is highly expressed and
predicts poor prognosis in patients with CRC.
m6A-modified circQSOX1 facilitates CRC tumorigenesis by
sponging miR-326 and miR-330-5p to upregulate phosphoglycerate
mutase 1, thereby activating glycolysis and inactivating the
anti-cytotoxic T-lymphocyte associated protein-4 therapy response
of CRC (143). circREEP3 is
frequently upregulated in tumor tissues from patients with CRC and
predicts poorer patient survival. circREEP3 interacts with
chromodomain helicase DNA binding protein 7 to activate FKBP prolyl
isomerase 10 transcription and promote the proliferation and
metastasis of CRC cells. In addition, circREEP3 can restrict
antitumor immunity via suppression of retinoic acid-inducible gene
1 signaling (144). circCAPRIN1
is upregulated in the tissues of patients with CRC. Furthermore,
high expression of circCAPRIN1 is associated with advanced TNM
stage and poor survival. Mechanistically, circCAPRIN1 interacts
with STAT2 to transcriptionally activate acetyl-CoA carboxylase 1,
thereby promoting lipid synthesis and facilitating CRC
tumorigenesis (145). Exosomal
circTUBGCP4 can induce the tip cell formation, angiogenesis and
tumor metastasis of CRC cells. circTUBGCP4 sponges miR-146b-3p to
upregulate pyruvate dehydrogenase kinase isoform 2 expression and
ultimately contribute to the accumulation of p-AKT (146).
circTADA2A is significantly downregulated in CRC
compared with normal adjacent tissues and cell lines. circTADA2A
inhibits glycolysis and the cell cycle and potentiates the
apoptosis of CRC cells by acting as a sponge for miR-374a-3p and
increasing Krüppel-like factor 14 expression (147). circGALNT16 is downregulated in
CRC tissues and can significantly inhibit the proliferation,
invasion and migration of CRC cells. Mechanistically, circGALNT16
inhibits the progression of CRC by specifically binding to the KH3
domain of hnRNPK, and it can also enhance the interaction between
hnRNPK and p53 by inhibiting SUMO specific peptidase 2-mediated
hnRNPK deSUMOylation. circGALNT16 attenuates serpin family E member
1 and enhances the p21 mRNA expression level by regulating the
sequence-specific DNA-binding ability of the hnRNPK-p53 complex
(148). circRHOBTB3 binds to HuR
and promotes β-transducin repeat-containing protein 1-mediated
ubiquitination of HuR, thereby reducing the expression level of the
downstream target, PTBP1, and inhibiting the invasion and migration
of CRC cells (149). The low
expression of circPLCE1 in CRC tissue can encode a new protein
termed circPLCE1-411. circPLCE1-411 promotes the
ubiquitin-dependent degradation of ribosomal protein S3 (RPS3) by
directly binding to the HSP90α/RPS3 complex to reduce NF-κB nuclear
translocation in CRC cells, which inhibits NF-κB signaling and CRC
cell proliferation and migration (150). Exosomal circLPAR1 shows cancer
specificity in a CRC diagnosis, with an area under the ROC curve of
0.875 when combined with carcinoembryonic antigen and carbohydrate
antigen 19-9. Exosomal circLPAR1 suppresses CRC tumorigenesis by
regulating bromodomain containing 4 levels upon interaction with
eukaryotic translation initiation factor 3 (151). circMETTL3 is downregulated in
CRC tissues and cells, and circMETTL3 expression is negatively
correlated with advanced TNM stages, increased lymph node and
distant metastasis of CRC tumors. circMETTL3 serves as a tumor
suppressor in CRC in vivo. Mechanistically, RUNX family
transcription factor 3 directly binds to METTL3 promoter region and
activates its transcription, and circMETTL3 directly binds with
miR-107 and positively regulates its downstream gene, period
circadian regulator 3, expression (152). circRERE, regulated by EP300,
exerts antitumor immunity and restrains CRC development by acting
as a miR-6837-3p sponge to regulate mitochondrial antiviral
signaling protein expression and activate the type I IFN pathway
(153).
The relationship between circRNA and the malignant
hallmarks of CRC is summarized in Table VI. Several circRNAs such as
circNSUN2, circPACRGL, circERBIN, circ-0000392, circ1662,
circSPARC, circQSOX1, circREEP3, circCAPRIN1 and exosomal
circTUBGCP4, are significantly upregulated in CRC and contribute to
tumor progression, metastasis, angiogenesis and immune regulation.
Conversely, circTADA2A, circGALNT16, circRHOBTB3, circPLCE1,
exosomal circLPAR1, circMETTL3 and circRERE are downregulated in
CRC and exhibit tumor-suppressive effects by inhibiting glycolysis,
cell cycle progression, invasion, migration and NF-κB signaling,
and enhancing apoptosis and antitumor immunity. These findings
provide insights into the dysregulation of circRNAs in CRC and
their potential as diagnostic markers and therapeutic targets.
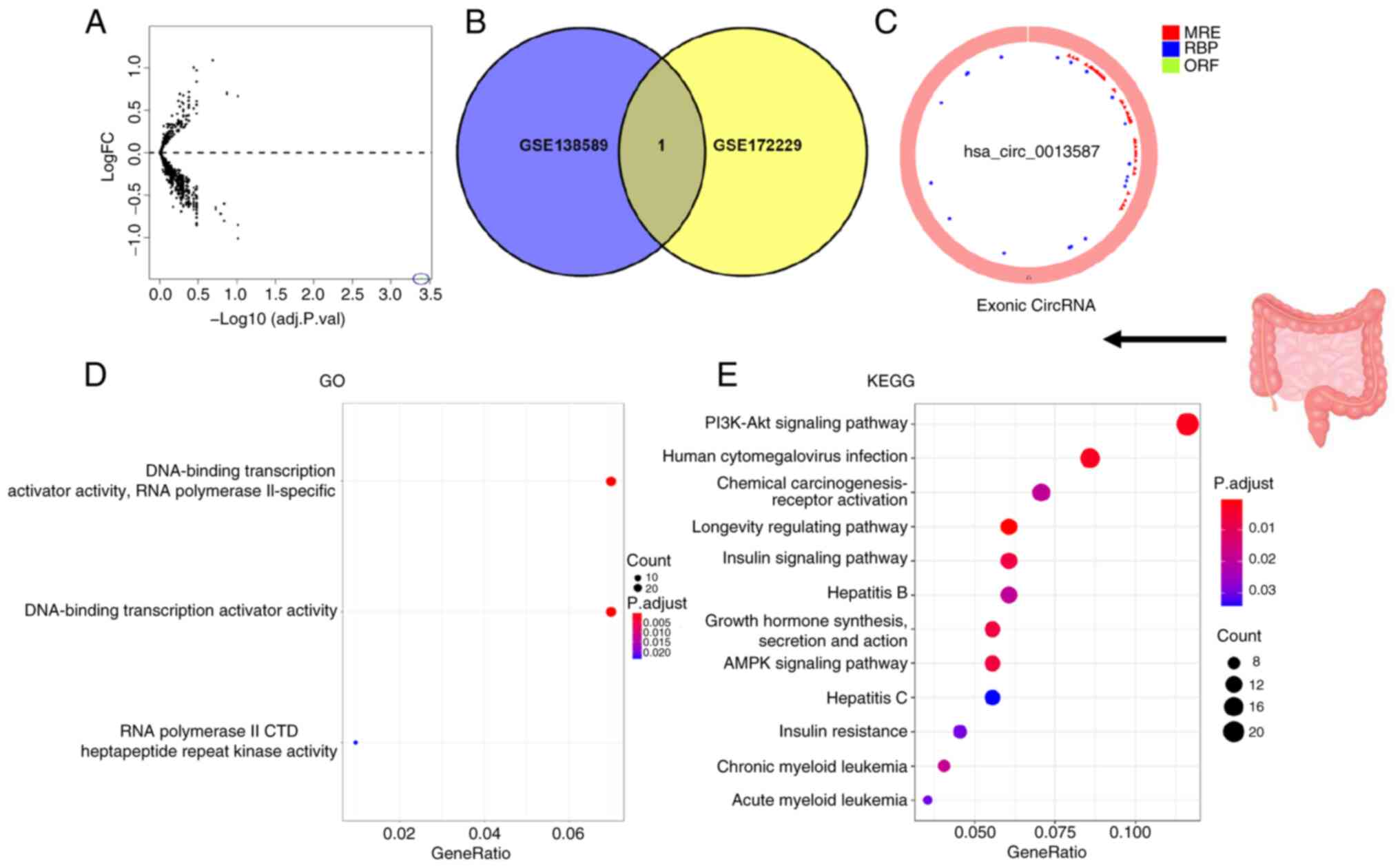
In total, two independent circRNA expression
profiles were downloaded for each type of digestive tumor from the
Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Bioinformatics
data mining was conducted utilizing R (version 4.0.3; https://www.r-project.org/) for the comprehensive
analysis of differentially expressed circRNAs (DEcircRNAs) from a
combined standardized dataset derived from two distinct data
matrices in the GEO database. The process encompassed data
preprocessing, normalization and differential expression analysis,
adhering to the criteria of an absolute value of log2 fold change
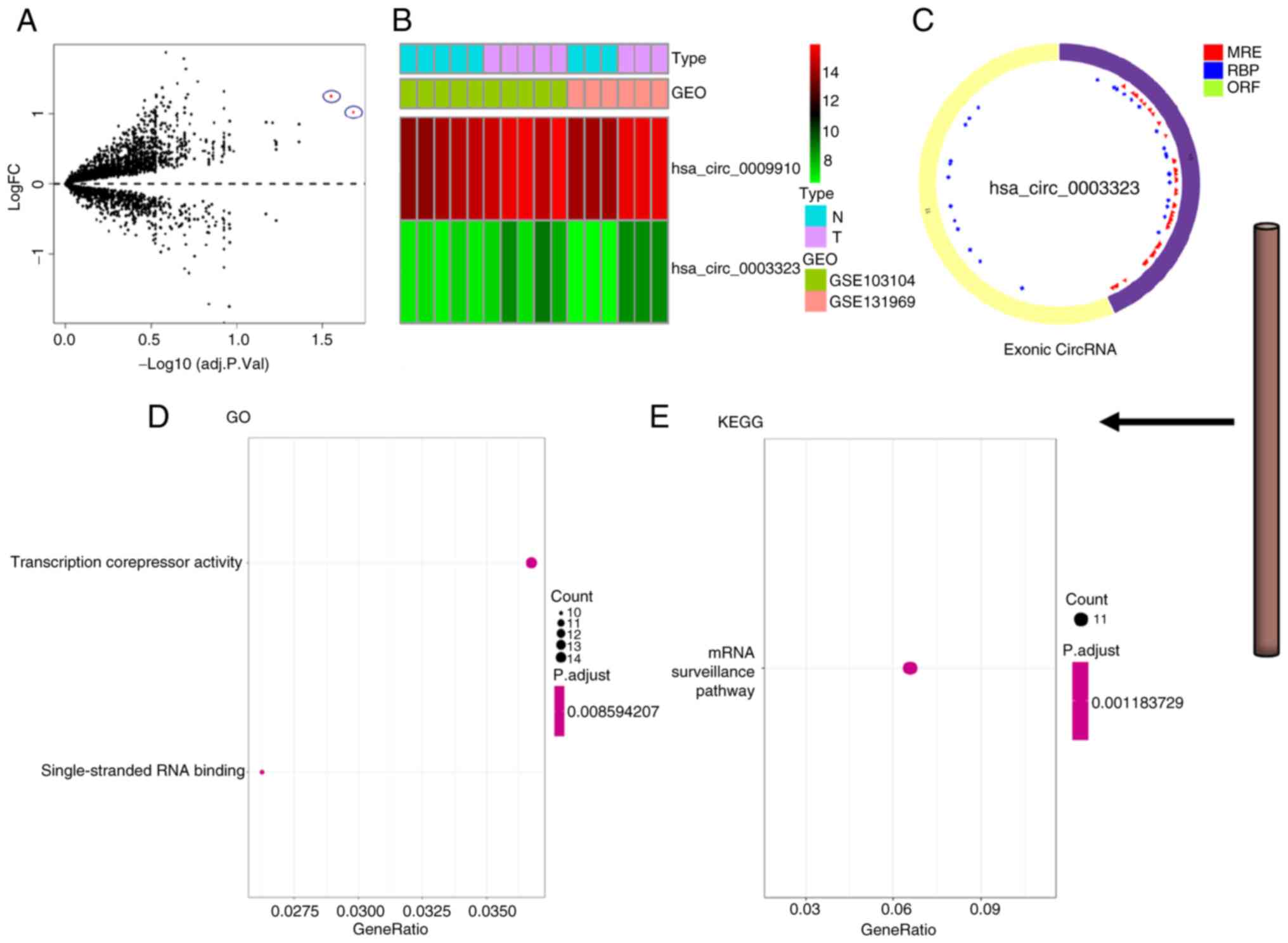
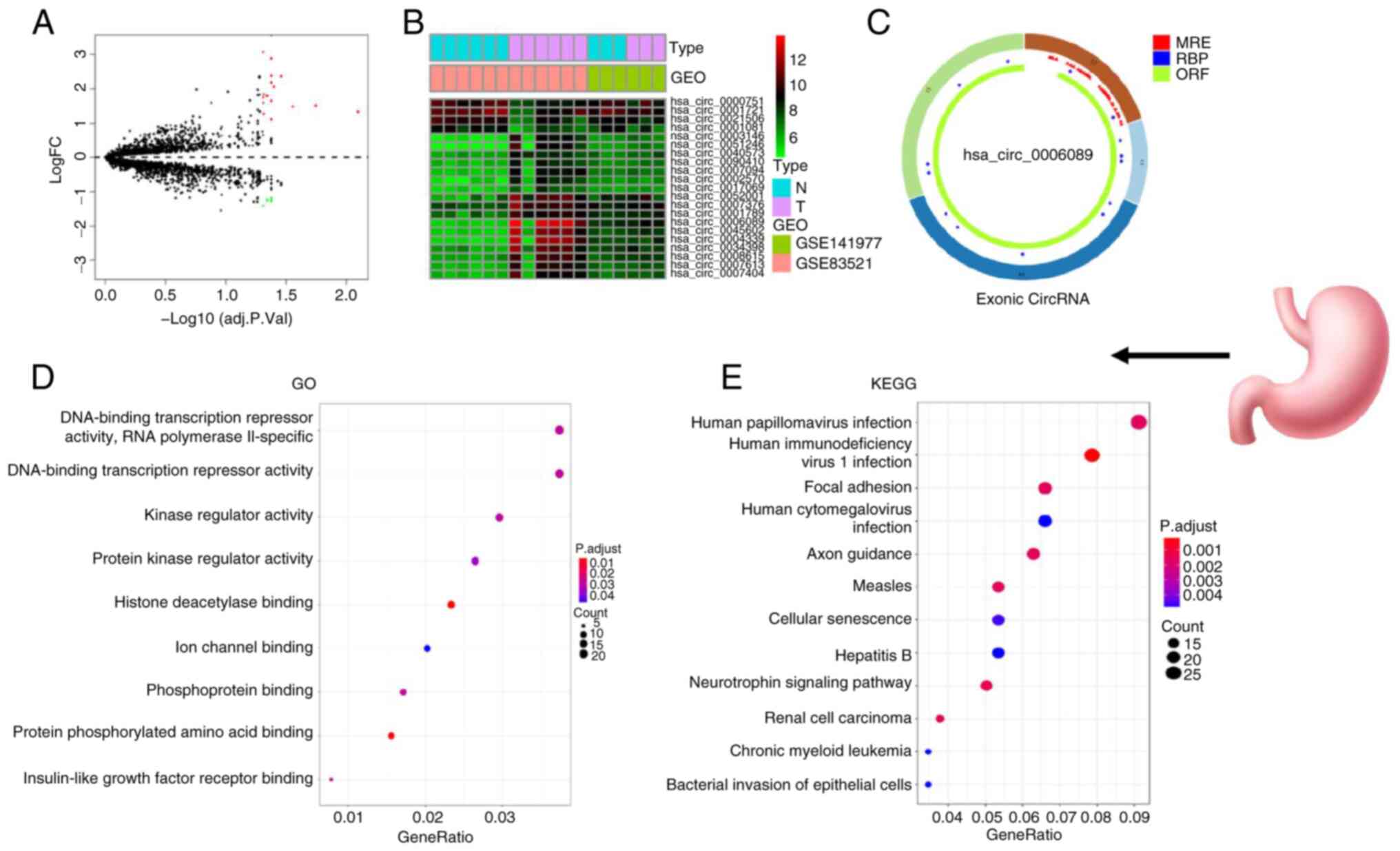
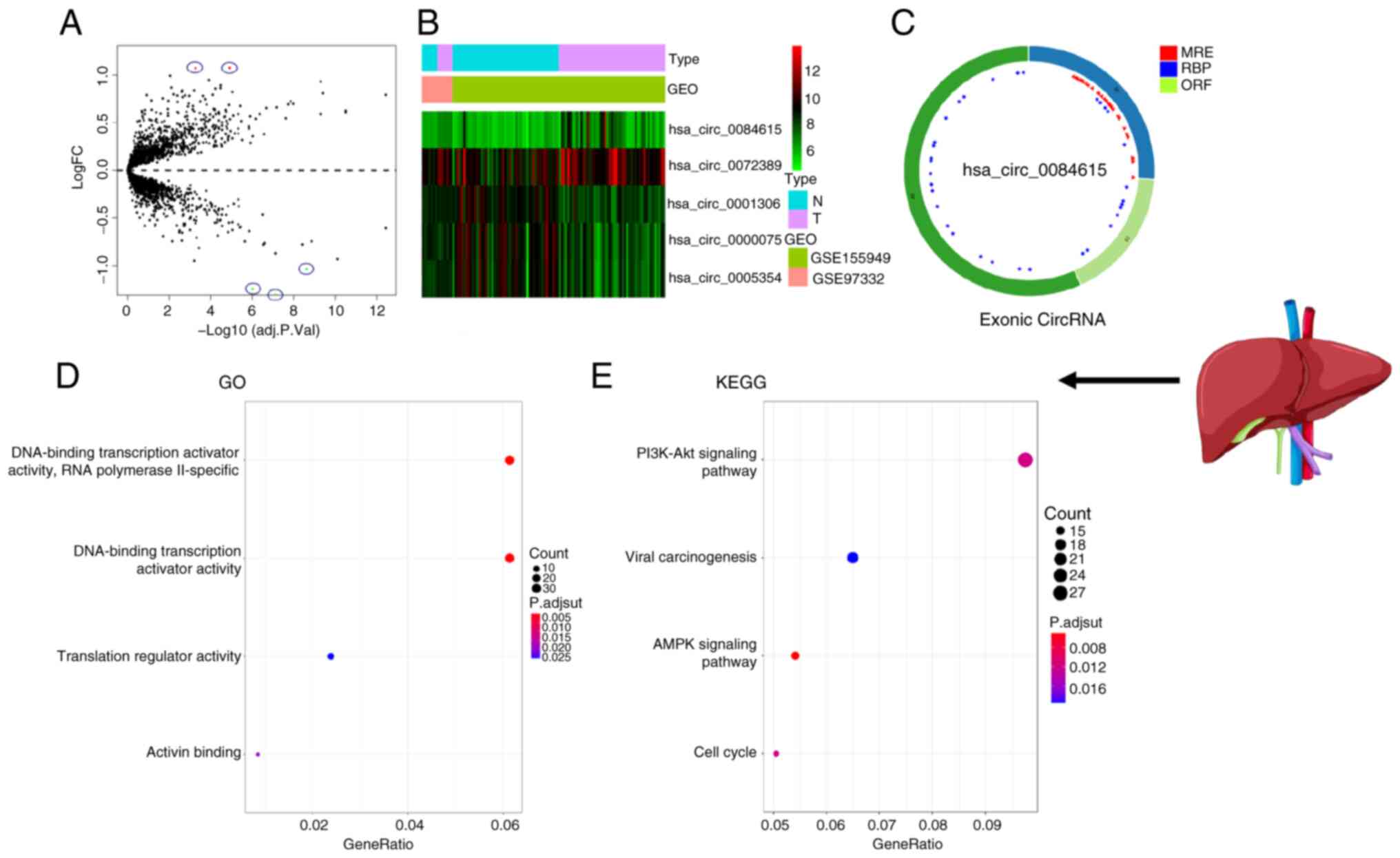
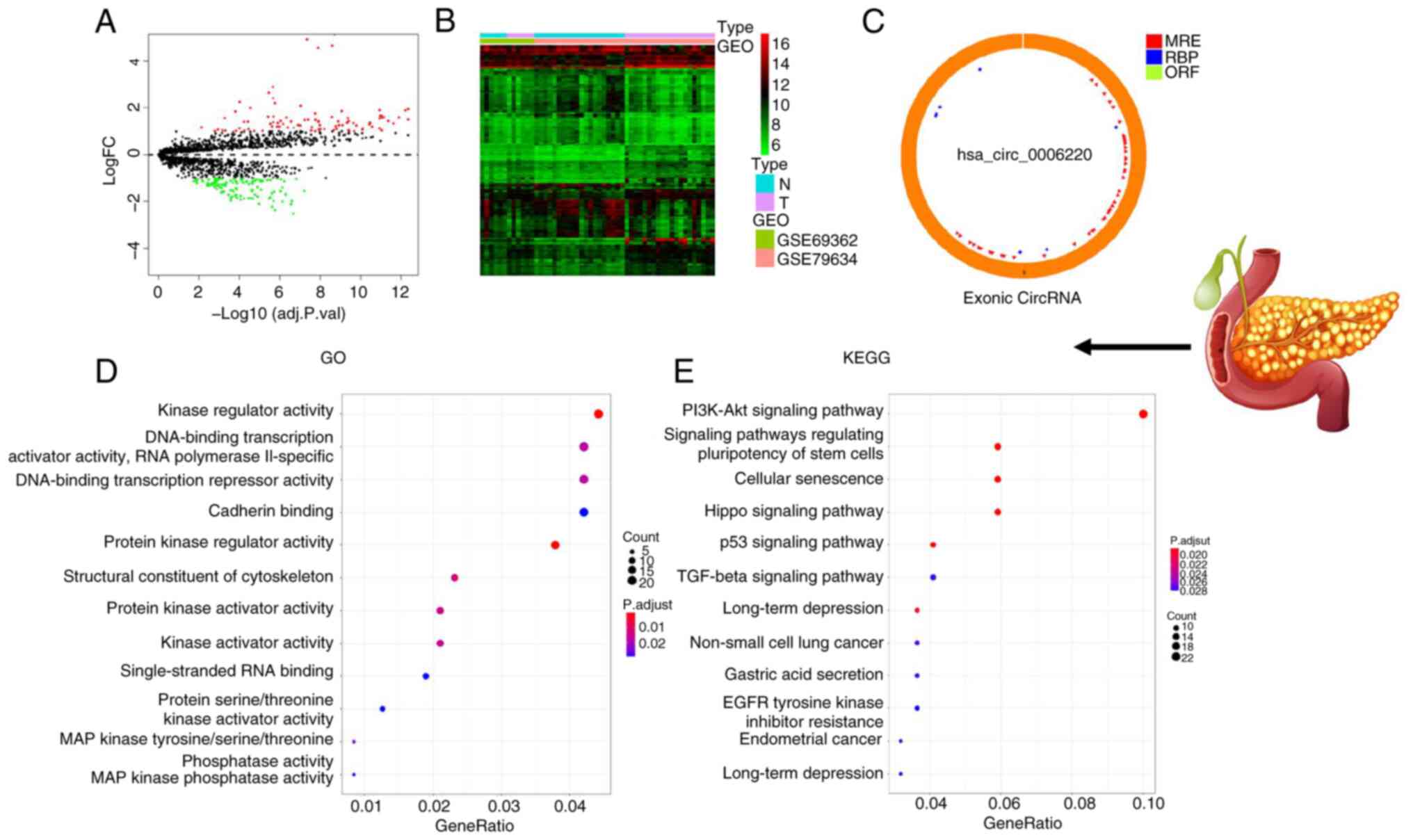
>2 and P<0.05. The circle diagram of the most significantly
upregulated circRNA for each tumor type and its targeted miRNAs
were performed using the Cancer-Specific CircRNA website (154). TargetScan (155), miRTarBase (156) and miRDB (157) databases were used to predict
miRNA-targeted mRNA. Gene Ontology functional enrichment analysis
(http://geneontology.org) and Kyoto Encyclopedia
of Genes and Genomes pathway enrichment analysis (https://www.genome.jp/kegg/) of these targeted mRNAs
were conducted to identify the potential functions of the
identified circRNA using R.
In the present review, a summary of the role and
potential mechanisms of different circRNAs in various malignant
tumors of the digestive tract was provided (Figs. 6 and 7). Research into the diverse biological
functions of circRNAs has elucidated the significance of previously
unknown circRNAs in gastrointestinal tumors, as well as their
possible molecular mechanisms. Despite being studied for over a
decade, the understanding of circRNA remains incomplete. At
present, research on circRNA primarily focuses on its ceRNA
mechanism. The characterization of other mechanisms, such as the
regulatory effect of ciRNA on parental genes and the encoding of
functional proteins, has been less extensive. Despite numerous
studies confirming the clinical potential of circRNAs as a
biomarker, their integration into clinical testing has not been
realized. The balance between these mechanisms, where some circRNAs
function as miRNA sponges or interact with proteins at multiple
sites involving different or even opposing pathways, is maintained
through complex regulatory processes. In conclusion, circRNAs have
a crucial regulatory role in the formation and development of
digestive tumors. They not only influence coding and non-coding
RNAs but also have measurable implications on multiple signaling
pathways and the biological characteristics of tumors. An
increasing number of circRNAs are being identified as ideal serum
biomarkers, offering new targets for the clinical diagnosis and
treatment of digestive tumors. In the future, there are undoubtedly
more circRNAs to be discovered and characterized.
The data generated in the present study may be
requested from the corresponding author.
CZ conceived and designed the manuscript. MQ
summarized the data and wrote the manuscript. YC conducted the
bioinformatics analysis and drew the figures. All authors have read
and approved the final version of the manuscript. MQ and YC confirm
the authenticity of all the raw data.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by the Young Talent Research and
Cultivation Fund of the First Affiliated Hospital of Nanchang
University (grant no. YFYPY202265).
|
1
|
Venø MT, Hansen TB, Venø ST, Clausen BH,
Grebing M, Finsen B, Holm IE and Kjems J: Spatio-temporal
regulation of circular RNA expression during porcine embryonic
brain development. Genome Biol. 16:2452015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rybak-Wolf A, Stottmeister C, Glažar P,
Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss
R, et al: Circular RNAs in the mammalian brain are highly abundant,
conserved, and dynamically expressed. Mol Cell. 58:870–885. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piwecka M, Glažar P, Hernandez-Miranda LR,
Memczak S, Wolf SA, Rybak-Wolf A, Filipchyk A, Klironomos F, Cerda
Jara CA, Fenske P, et al: Loss of a mammalian circular RNA locus
causes miRNA deregulation and affects brain function. Science.
357:eaam85262017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conn SJ, Pillman KA, Toubia J, Conn VM,
Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA and
Goodall GJ: The RNA binding protein quaking regulates formation of
circRNAs. Cell. 160:1125–1134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zang J, Lu D and Xu A: The interaction of
circRNAs and RNA binding proteins: An important part of circRNA
maintenance and function. J Neurosci Res. 98:87–97. 2020.
View Article : Google Scholar
|
|
12
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 is a circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang F, Hu A, Li D, Wang J, Guo Y, Liu Y,
Li H, Chen Y, Wang X, Huang K, et al: Circ-HuR suppresses HuR
expression and gastric cancer progression by inhibiting CNBP
transactivation. Mol Cancer. 18:1582019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel Role of FBXW7
Circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar :
|
|
15
|
Chen CY and Sarnow P: Initiation of
protein synthesis by the eukaryotic translational apparatus on
circular RNAs. Science. 268:415–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao CW, Chan DC, Kuo A and Leder P: The
mouse formin (Fmn) gene: Abundant circular RNA transcripts and
gene-targeted deletion analysis. Mol Med. 4:614–628. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gualandi F, Trabanelli C, Rimessi P,
Calzolari E, Toffolatti L, Patarnello T, Kunz G, Muntoni F and
Ferlini A: Multiple exon skipping and RNA circularisation
contribute to the severe phenotypic expression of exon 5 dystrophin
deletion. J Med Genet. 40:e1002003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar
|
|
23
|
Martínez-Bosch N, Cristóbal H, Iglesias M,
Gironella M, Barranco L, Visa L, Calafato D, Jiménez-Parrado S,
Earl J, Carrato A, et al: Soluble AXL is a novel blood marker for
early detection of pancreatic ductal adenocarcinoma and
differential diagnosis from chronic pancreatitis. EBioMedicine.
75:1037972022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abe T, Blackford AL, Tamura K, Ford M,
McCormick P, Chuidian M, Almario JA, Borges M, Lennon AM, Shin EJ,
et al: Deleterious germline mutations are a risk factor for
neoplastic progression among high-risk individuals undergoing
pancreatic surveillance. J Clin Oncol. 37:1070–1080. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thanikachalam K and Khan G: Colorectal
cancer and nutrition. Nutrients. 11:1642019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li R, Jiang J, Shi H, Qian H, Zhang X and
Xu W: CircRNA: A rising star in gastric cancer. Cell Mol Life Sci.
77:1661–1680. 2020. View Article : Google Scholar
|
|
28
|
Li J, Xu Q, Huang ZJ, Mao N, Lin ZT, Cheng
L, Sun B and Wang G: CircRNAs: A new target for the diagnosis and
treatment of digestive system neoplasms. Cell Death Dis.
12:2052021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dragomir MP, Kopetz S, Ajani JA and Calin
GA: Non-coding RNAs in GI cancers: From cancer hallmarks to
clinical utility. Gut. 69:748–763. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
32
|
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL
and Yang L: Complementary sequence-mediated exon circularization.
Cell. 159:134–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ,
Shi GM, Cai JB and Ke AW: Cancer cell-derived exosomal circUHRF1
induces natural killer cell exhaustion and may cause resistance to
anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer.
19:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou C, Liu HS, Wang FW, Hu T, Liang ZX,
Lan N, He XW, Zheng XB, Wu XJ, Xie D, et al: circCAMSAP1 promotes
tumor growth in colorectal cancer via the miR-328-5p/E2F1 axis. Mol
Ther. 28:914–928. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Wang S, Wang H, Cao J, Huang X,
Chen Z, Xu P, Sun G, Xu J, Lv J and Xu Z: Circular RNA circNRIP1
acts as a microRNA-149-5p sponge to promote gastric cancer
progression via the AKT1/mTOR pathway. Mol Cancer. 18:202019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kristensen LS, Okholm T, Venø MT and Kjems
J: Circular RNAs are abundantly expressed and upregulated during
human epidermal stem cell differentiation. RNA Biol. 15:280–291.
2018. View Article : Google Scholar :
|
|
37
|
Yang F, Fang E, Mei H, Chen Y, Li H, Li D,
Song H, Wang J, Hong M, Xiao W, et al: Cis-Acting circ-CTNNB1
promotes β-catenin signaling and cancer progression via
DDX3-mediated transactivation of YY1. Cancer Res. 79:557–571. 2019.
View Article : Google Scholar
|
|
38
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marques R, Lacerda R and Romão L: Internal
ribosome entry site (IRES)-mediated translation and its potential
for novel mRNA-based therapy development. Biomedicines.
10:18652022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan X, Yang Y, Chen C and Wang Z:
Pervasive translation of circular RNAs driven by short IRES-like
elements. Nat Commun. 13:37512022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen LL: The expanding regulatory
mechanisms and cellular functions of circular RNAs. Nat Rev Mol
Cell Biol. 21:475–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z,
Xu B, Wu C, Zhou Q, Hu W, Wu C and Jiang J: A novel protein encoded
by a circular RNA circPPP1R12A promotes tumor pathogenesis and
metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer.
18:472019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kelly S, Greenman C, Cook PR and
Papantonis A: Exon skipping is correlated with exon
circularization. J Mol Biol. 427:2414–2417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu X, Wu D, He X, Zhao H, He Z, Lin J,
Wang K, Wang W, Pan Z, Lin H and Wang M: circGSK3β promotes
metastasis in esophageal squamous cell carcinoma by augmenting
β-catenin signaling. Mol Cancer. 18:1602019. View Article : Google Scholar
|
|
45
|
Shi Y, Fang N, Li Y, Guo Z, Jiang W, He Y,
Ma Z and Chen Y: Circular RNA LPAR3 sponges microRNA-198 to
facilitate esophageal cancer migration, invasion, and metastasis.
Cancer Sci. 111:2824–2836. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu ZH, Yang SZ, Li WY, Dong SY, Zhou SY
and Xu S: circRNA_141539 can serve as an oncogenic factor in
esophageal squamous cell carcinoma by sponging miR-4469 and
activating CDK3 gene. Aging (Albany NY). 13:12179–12193. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhong R, Chen Z, Mo T, Li Z and Zhang P:
Potential role of circPVT1 as a proliferative factor and treatment
target in esophageal carcinoma. Cancer Cell Int. 19:2672019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li RC, Ke S, Meng FK, Lu J, Zou XJ, He ZG,
Wang WF and Fang MH: CiRS-7 promotes growth and metastasis of
esophageal squamous cell carcinoma via regulation of miR-7/HOXB13.
Cell Death Dis. 9:8382018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi Y, Guo Z, Fang N, Jiang W, Fan Y, He
Y, Ma Z and Chen Y: hsa_circ_0006168 sponges miR-100 and regulates
mTOR to promote the proliferation, migration and invasion of
esophageal squamous cell carcinoma. Biomed Pharmacother.
117:1091512019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qu F, Wang L, Wang C, Yu L, Zhao K and
Zhong H: Circular RNA circ_0006168 enhances Taxol resistance in
esophageal squamous cell carcinoma by regulating miR-194-5p/JMJD1C
axis. Cancer Cell Int. 21:2732021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou S, Guo Z, Zhou C, Zhang Y and Wang S:
circ_NRIP1 is oncogenic in malignant development of esophageal
squamous cell carcinoma (ESCC) via miR-595/SEMA4D axis and PI3K/AKT
pathway. Cancer Cell Int. 21:2502021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gu L, Sang Y, Nan X, Zheng Y, Liu F, Meng
L, Sang M and Shan B: circCYP24A1 facilitates esophageal squamous
cell carcinoma progression through binding PKM2 to regulate
NF-kappaB-induced CCL5 secretion. Mol Cancer. 21:2172022.
View Article : Google Scholar
|
|
53
|
Wang C, Zhou M, Zhu P, Ju C, Sheng J, Du
D, Wan J, Yin H, Xing Y, Li H, et al: IGF2BP2-induced circRUNX1
facilitates the growth and metastasis of esophageal squamous cell
carcinoma through miR-449b-5p/FOXP3 axis. J Exp Clin Cancer Res.
41:3472022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Meng F, Zhang X, Wang Y, Lin J, Tang Y,
Zhang G, Qiu B, Zeng X, Liu W and He X: Hsa_circ_0021727
(circ-CD44) promotes ESCC progression by targeting miR-23b-5p to
activate the TAB1/NFκB pathway. Cell Death Dis. 14:92023.
View Article : Google Scholar
|
|
55
|
Yao W, Jia X, Zhu L, Xu L, Zhang Q, Xia T
and Wei L: Exosomal circ_0026611 contributes to lymphangiogenesis
by reducing PROX1 acetylation and ubiquitination in human lymphatic
endothelial cells (HLECs). Cell Mol Biol Lett. 28:132023.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luo J, Tian Z, Zhou Y, Xiao Z, Park SY,
Sun H, Zhuang T, Wang Y, Li P and Zhao X: CircABCA13 acts as a
miR-4429 sponge to facilitate esophageal squamous cell carcinoma
development by stabilizing SRXN1. Cancer Sci. 114:2835–2847. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Z, Ma K, Cheng Y, Abraham JM, Liu X,
Ke X, Wang Z and Meltzer SJ: Synthetic circular multi-miR sponge
simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma.
Lab Invest. 99:1442–1453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang H, Song X, Wang Y, Yin X, Liang Y,
Zhang T, Xu L, Jiang F and Dong G: CircCNTNAP3-TP53-positive
feedback loop suppresses malignant progression of esophageal
squamous cell carcinoma. Cell Death Dis. 11:10102020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
He Y, Mingyan E, Wang C, Liu G, Shi M and
Liu S: CircVRK1 regulates tumor progression and radioresistance in
esophageal squamous cell carcinoma by regulating
miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int J Biol Macromol.
125:116–123. 2019. View Article : Google Scholar
|
|
60
|
Meng L, Zheng Y, Liu S, Ju Y, Ren S, Sang
Y, Zhu Y, Gu L, Liu F, Zhao Y, et al: ZEB1 represses biogenesis of
circ-DOCK5 to facilitate metastasis in esophageal squamous cell
carcinoma via a positive feedback loop with TGF-β. Cancer Lett.
519:117–129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao R, Chen P, Qu C, Liang J, Cheng Y,
Sun Z and Tian H: Circular RNA circTRPS1-2 inhibits the
proliferation and migration of esophageal squamous cell carcinoma
by reducing the production of ribosomes. Cell Death Discov.
9:52023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xu R, Ding P, Zhao X, Li Z, Liu F, Gu L,
Zheng Y, Sang M and Meng L: Circular RNA circ-TNRC6B inhibits the
proliferation and invasion of esophageal squamous cell carcinoma
cells by regulating the miR-452-5p/DAG1 axis. Mol Oncol.
17:1437–1452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Song H, Tian D, Sun J, Mao X, Kong W, Xu
D, Ji Y, Qiu B, Zhan M and Wang J: circFAM120B functions as a tumor
suppressor in esophageal squamous cell carcinoma via the
miR-661/PPM1L axis and the PKR/p38 MAPK/EMT pathway. Cell Death
Dis. 13:3612022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F,
Ma P, Jiang H, Wu X, Shu Y and Xu T: Exosomal circSHKBP1 promotes
gastric cancer progression via regulating the miR-582-3p/HUR/VEGF
axis and suppressing HSP90 degradation. Mol Cancer. 19:1122020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang X, Li Z, Zhang Q, Wang W, Li B, Wang
L, Xu Z, Zeng A, Zhang X, Zhang X, et al: Circular RNA AKT3
upregulates PIK3R1 to enhance cisplatin resistance in gastric
cancer via miR-198 suppression. Mol Cancer. 18:712019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cao J, Zhang X, Xu P, Wang H, Wang S,
Zhang L, Li Z, Xie L, Sun G, Xia Y, et al: Circular RNA circLMO7
acts as a microRNA-30a-3p sponge to promote gastric cancer
progression via the WNT2/β-catenin pathway. J Exp Clin Cancer Res.
40:62021. View Article : Google Scholar
|
|
67
|
Xia Y, Lv J, Jiang T, Li B, Li Y, He Z,
Xuan Z, Sun G, Wang S, Li Z, et al: CircFAM73A promotes the cancer
stem cell-like properties of gastric cancer through the
miR-490-3p/HMGA2 positive feedback loop and HNRNPK-mediated
β-catenin stabilization. J Exp Clin Cancer Res. 40:1032021.
View Article : Google Scholar
|
|
68
|
Ma Q, Yang F, Huang B, Pan X, Li W, Yu T,
Wang X, Ran L, Qian K, Li H, et al: CircARID1A binds to IGF2BP3 in
gastric cancer and promotes cancer proliferation by forming a
circARID1A-IGF2BP3-SLC7A5 RNA-protein ternary complex. J Exp Clin
Cancer Res. 41:2512022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zha Q, Wu X, Zhang J, Xu T, Shi Y, Sun Y,
Fang Y, Gu Y, Ma P, Shu Y and Tian S: Hsa_circ_0007967 promotes
gastric cancer proliferation through the miR-411-5p/MAML3 axis.
Cell Death Discov. 8:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang F, Liu G, Chen X, Li Q, Fang F and
Shen X: Hsa_circ_0044301 regulates gastric cancer cell's
proliferation, migration, and invasion by modulating the
Hsa-miR-188-5p/DAXX axis and MAPK pathway. Cancers (Basel).
14:41832022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hou G, Zuo H, Shi J, Dai D, Wang H, Song
X, Xu G and Tao G: EIF4A3 induced circABCA5 promotes the gastric
cancer progression by SPI1 mediated IL6/JAK2/STAT3 signaling. Am J
Cancer Res. 13:602–622. 2023.PubMed/NCBI
|
|
72
|
Li F, Tang H, Zhao S, Gao X, Yang L and Xu
J: Circ-E-Cad encodes a protein that promotes the proliferation and
migration of gastric cancer via the TGF-β/Smad/C-E-Cad/PI3K/AKT
pathway. Mol Carcinog. 62:360–368. 2023. View Article : Google Scholar
|
|
73
|
Zhou P, Qu H, Shi K, Chen X, Zhuang Z,
Wang N, Zhang Q, Liu Z, Wang L, Deng K, et al: ATF4-mediated
circTDRD3 promotes gastric cancer cell proliferation and metastasis
by regulating the miR-891b/ITGA2 axis and AKT signaling pathway.
Gastric Cancer. 26:565–579. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Peng L, Sang H, Wei S, Li Y, Jin D, Zhu X,
Li X, Dang Y and Zhang G: circCUL2 regulates gastric cancer
malignant transformation and cisplatin resistance by modulating
autophagy activation via miR-142-3p/ROCK2. Mol Cancer. 19:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jie M, Wu Y, Gao M, Li X, Liu C, Ouyang Q,
Tang Q, Shan C, Lv Y, Zhang K, et al: CircMRPS35 suppresses gastric
cancer progression via recruiting KAT7 to govern histone
modification. Mol Cancer. 19:562020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ma C, Wang X, Yang F, Zang Y, Liu J, Wang
X, Xu X, Li W, Jia J and Liu Z: Circular RNA hsa_circ_0004872
inhibits gastric cancer progression via the miR-224/Smad4/ADAR1
successive regulatory circuit. Mol Cancer. 19:1572020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Y, Jiang J, Zhang J, Shen H, Wang M,
Guo Z, Zang X, Shi H, Gao J, Cai H, et al: CircDIDO1 inhibits
gastric cancer progression by encoding a novel DIDO1-529aa protein
and regulating PRDX2 protein stability. Mol Cancer. 20:1012021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jiang T, Xia Y, Lv J, Li B, Li Y, Wang S,
Xuan Z, Xie L, Qiu S, He Z, et al: A novel protein encoded by
circMAPK1 inhibits progression of gastric cancer by suppressing
activation of MAPK signaling. Mol Cancer. 20:662021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zang X, Jiang J, Gu J, Chen Y, Wang M,
Zhang Y, Fu M, Shi H, Cai H, Qian H, et al: Circular RNA EIF4G3
suppresses gastric cancer progression through inhibition of
β-catenin by promoting δ-catenin ubiquitin degradation and
upregulating SIK1. Mol Cancer. 21:1412022. View Article : Google Scholar
|
|
80
|
Xu P, Zhang X, Cao J, Yang J, Chen Z, Wang
W, Wang S, Zhang L, Xie L, Fang L, et al: The novel role of
circular RNA ST3GAL6 on blocking gastric cancer malignant
behaviours through autophagy regulated by the FOXP2/MET/mTOR axis.
Clin Transl Med. 12:e7072022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang C, Wei G, Zhu X, Chen X, Ma X, Hu P,
Liu W, Yang W, Ruan T, Zhang W, et al: Exosome-delivered circSTAU2
inhibits the progression of gastric cancer by targeting the
miR-589/CAPZA1 axis. Int J Nanomedicine. 18:127–142. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu H, Fang D, Zhang C, Zhao Z, Liu Y,
Zhao S, Zhang N and Xu J: Circular MTHFD2L RNA-encoded CM-248aa
inhibits gastric cancer progression by targeting the SET-PP2A
interaction. Mol Ther. 31:1739–1755. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu J, Niu L, Hao J, Yao Y, Yan M and Li
H: circIPO7 dissociates caprin-1 from ribosomes and inhibits
gastric cancer cell proliferation by suppressing EGFR and mTOR.
Oncogene. 42:980–993. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Huang XY, Huang ZL, Huang J, Xu B, Huang
XY, Xu YH, Zhou J and Tang ZY: Exosomal circRNA-100338 promotes
hepatocellular carcinoma metastasis via enhancing invasiveness and
angiogenesis. J Exp Clin Cancer Res. 39:202020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L,
Gorshkov K, Mao Q, Xia S, Cen D, et al:
N6-methyladenosine-modified CircRNA-SORE sustains
sorafenib resistance in hepatocellular carcinoma by regulating
β-catenin signaling. Mol Cancer. 19:1632020. View Article : Google Scholar
|
|
86
|
Zhao Z, Song J, Tang B, Fang S, Zhang D,
Zheng L, Wu F, Gao Y, Chen C, Hu X, et al: CircSOD2 induced
epigenetic alteration drives hepatocellular carcinoma progression
through activating JAK2/STAT3 signaling pathway. J Exp Clin Cancer
Res. 39:2592020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang L, Long H, Zheng Q, Bo X, Xiao X and
Li B: Circular RNA circRHOT1 promotes hepatocellular carcinoma
progression by initiation of NR2F6 expression. Mol Cancer.
18:1192019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li Q, Pan X, Zhu D, Deng Z, Jiang R and
Wang X: Circular RNA MAT2B promotes glycolysis and malignancy of
hepatocellular carcinoma through the miR-338-3p/PKM2 axis under
hypoxic stress. Hepatology. 70:1298–1316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu G, Sun J, Yang ZF, Zhou C, Zhou PY,
Guan RY, Sun BY, Wang ZT, Zhou J, Fan J, et al: Cancer-associated
fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell
migration and tumor metastasis through the
circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. 12:2602021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen Y, Ling Z, Cai X, Xu Y, Lv Z, Man D,
Ge J, Yu C, Zhang D, Zhang Y, et al: Activation of YAP1 by
N6-methyladenosine-modified circCPSF6 drives malignancy
in hepatocellular carcinoma. Cancer Res. 82:599–614. 2022.
View Article : Google Scholar
|
|
91
|
Li P, Song R, Yin F, Liu M, Liu H, Ma S,
Jia X, Lu X, Zhong Y, Yu L, et al: circMRPS35 promotes malignant
progression and cisplatin resistance in hepatocellular carcinoma.
Mol Ther. 30:431–447. 2022. View Article : Google Scholar :
|
|
92
|
Zheng J, Yan X, Lu T, Song W, Li Y, Liang
J, Zhang J, Cai J, Sui X, Xiao J, et al: CircFOXK2 promotes
hepatocellular carcinoma progression and leads to a poor clinical
prognosis via regulating the Warburg effect. J Exp Clin Cancer Res.
42:632023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen ZQ, Zuo XL, Cai J, Zhang Y, Han GY,
Zhang L, Ding WZ, Wu JD and Wang XH: Hypoxia-associated circPRDM4
promotes immune escape via HIF-1α regulation of PD-L1 in
hepatocellular carcinoma. Exp Hematol Oncol. 12:172023. View Article : Google Scholar
|
|
94
|
Fan L, Xia P, Wang J, Xu S, Qiu Z, Wu Y,
Feng M, Zhao Q, Wang H and Li X: Circ_0007429/miR-637/TRIM71/Ago2
axis participates in the regulation of proliferation, migration,
invasion, apoptosis, and aerobic glycolysis of HCC. Mol Carcinog.
62:820–832. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu YJ, Zheng B, Luo GJ, Ma XK, Lu XY, Lin
XM, Yang S, Zhao Q, Wu T, Li ZX, et al: Circular RNAs negatively
regulate cancer stem cells by physically binding FMRP against CCAR1
complex in hepatocellular carcinoma. Theranostics. 9:3526–3540.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang PF, Wei CY, Huang XY, Peng R, Yang
X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, et al: Circular RNA
circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress
hepatocellular carcinoma progression. Mol Cancer. 18:1052019.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dong ZR, Ke AW, Li T, Cai JB, Yang YF,
Zhou W, Shi GM and Fan J: CircMEMO1 modulates the promoter
methylation and expression of TCF21 to regulate hepatocellular
carcinoma progression and sorafenib treatment sensitivity. Mol
Cancer. 20:752021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shi L, Liu B, Shen DD, Yan P, Zhang Y,
Tian Y, Hou L, Jiang G, Zhu Y, Liang Y, et al: A tumor-suppressive
circular RNA mediates uncanonical integrin degradation by the
proteasome in liver cancer. Sci Adv. 7:eabe50432021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li J, Hu ZQ, Yu SY, Mao L, Zhou ZJ, Wang
PC, Gong Y, Su S, Zhou J, Fan J, et al: CircRPN2 inhibits aerobic
glycolysis and metastasis in hepatocellular carcinoma. Cancer Res.
82:1055–1069. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen S, Cao X, Zhang J, Wu W, Zhang B and
Zhao F: circVAMP3 drives CAPRIN1 phase separation and inhibits
hepatocellular carcinoma by suppressing c-Myc translation. Adv Sci
(Weinh). 9:e21038172022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Peng R, Cao J, Su BB, Bai XS, Jin X, Wang
AQ, Wang Q, Liu RJ, Jiang GQ, Jin SJ, et al: Down-regulation of
circPTTG1IP induces hepatocellular carcinoma development via
miR-16-5p/RNF125/JAK1 axis. Cancer Lett. 543:2157782022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Guo Z, Xie Q, Wu Y, Mo H, Zhang J, He G,
Li Z, Gan L, Feng L, Li T, et al: Aberrant expression of circular
RNA DHPR facilitates tumor growth and metastasis by regulating the
RASGEF1B/RAS/MAPK axis in hepatocellular carcinoma. Cell Oncol
(Dordr). 46:1333–1350. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Song R, Ma S, Xu J, Ren X, Guo P, Liu H,
Li P, Yin F, Liu M, Wang Q, et al: A novel polypeptide encoded by
the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR.
Mol Cancer. 22:162023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xu Y, Leng K, Yao Y, Kang P, Liao G, Han
Y, Shi G, Ji D, Huang P, Zheng W, et al: A circular RNA,
cholangiocarcinoma-associated circular RNA 1, contributes to
cholangiocarcinoma progression, induces angiogenesis, and disrupts
vascular endothelial barriers. Hepatology. 73:1419–1435. 2021.
View Article : Google Scholar
|
|
105
|
Wang S, Hu Y, Lv X, Li B, Gu D, Li Y, Sun
Y and Su Y: Circ-0000284 arouses malignant phenotype of
cholangiocarcinoma cells and regulates the biological functions of
peripheral cells through cellular communication. Clin Sci (Lond).
133:1935–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhao X, Zhang X, Zhang Z, Liu Z, Zhu J,
Lyu S, Li L, Lang R and He Q: Comprehensive circular RNA expression
profiling constructs a ceRNA network and identifies
hsa_circ_0000673 as a novel oncogene in distal cholangiocarcinoma.
Aging (Albany NY). 12:23251–23274. 2020.PubMed/NCBI
|
|
107
|
Xu Y, Yao Y, Liu Y, Wang Z, Hu Z, Su Z, Li
C, Wang H, Jiang X, Kang P, et al: Elevation of circular RNA
circ_0005230 facilitates cell growth and metastasis via sponging
miR-1238 and miR-1299 in cholangiocarcinoma. Aging (Albany NY).
11:1907–1917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Xu Y, Gao P, Wang Z, Su Z, Liao G, Han Y,
Cui Y, Yao Y and Zhong X: Circ-LAMP1 contributes to the growth and
metastasis of cholangiocarcinoma via miR-556-5p and miR-567
mediated YY1 activation. J Cell Mol Med. 25:3226–3238. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tu J, Chen W, Zheng L, Fang S, Zhang D,
Kong C, Yang Y, Qiu R, Zhao Z, Lu C, et al: Circular RNA
Circ0021205 promotes cholangiocarcinoma progression through
MiR-204-5p/RAB22A axis. Front Cell Dev Biol. 9:6532072021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen Q, Wang H, Li Z, Li F, Liang L, Zou
Y, Shen H, Li J, Xia Y, Cheng Z, et al: Circular RNA ACTN4 promotes
intrahepatic cholangiocarcinoma progression by recruiting YBX1 to
initiate FZD7 transcription. J Hepatol. 76:135–147. 2022.
View Article : Google Scholar
|
|
111
|
Zhong X, Ji C, Ren D, Ke A and Yang Z:
Circular RNA circEIF3C promotes intrahepatic cholangiocarcinoma
progression and immune evasion via the miR-34a-5p/B7-H4 axis. Genes
Dis. 10:370–372. 2022. View Article : Google Scholar
|
|
112
|
Li H, Lan T, Liu H, Liu C, Dai J, Xu L,
Cai Y, Hou G, Xie K, Liao M, et al: IL-6-induced cGGNBP2 encodes a
protein to promote cell growth and metastasis in intrahepatic
cholangiocarcinoma. Hepatology. 75:1402–1419. 2022. View Article : Google Scholar
|
|
113
|
Yu X, Tong H, Chen J, Tang C, Wang S, Si
Y, Wang S and Tang Z: CircRNA MBOAT2 promotes intrahepatic
cholangiocarcinoma progression and lipid metabolism reprogramming
by stabilizing PTBP1 to facilitate FASN mRNA cytoplasmic export.
Cell Death Dis. 14:202023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Liao W, Du J, Li L, Wu X, Chen X, Feng Q,
Xu L, Chen X, Liao M, Huang J, et al: CircZNF215 promotes tumor
growth and metastasis through inactivation of the PTEN/AKT pathway
in intrahepatic cholangiocarcinoma. J Exp Clin Cancer Res.
42:1252023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Du J, Lan T, Liao H, Feng X, Chen X, Liao
W, Hou G, Xu L, Feng Q, Xie K, et al: CircNFIB inhibits tumor
growth and metastasis through suppressing MEK1/ERK signaling in
intrahepatic cholangiocarcinoma. Mol Cancer. 21:182022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang X, Zhao Y, Wang W, Yu S, Liu L, Sun
D, Li W and Jiang X: Upregulation of circ_0059961 suppresses
cholangiocarcinoma development by modulating miR-629-5p/SFRP2 axis.
Pathol Res Pract. 234:1539012022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang G, Gao X, Sun Z, He T, Huang C, Li S
and Long H: Circular RNA SMARCA5 inhibits cholangiocarcinoma via
microRNA-95-3p/tumor necrosis factor receptor associated factor 3
axis. Anticancer Drugs. 34:1002–1009. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li J, Li Z, Jiang P, Peng M, Zhang X, Chen
K, Liu H, Bi H, Liu X and Li X: Circular RNA IARS (circ-IARS)
secreted by pancreatic cancer cells and located within exosomes
regulates endothelial monolayer permeability to promote tumor
metastasis. J Exp Clin Cancer Res. 37:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wong CH, Lou UK, Li Y, Chan SL, Tong JH,
To KF and Chen Y: CircFOXK2 promotes growth and metastasis of
pancreatic ductal adenocarcinoma by complexing with RNA-binding
proteins and sponging MiR-942. Cancer Res. 80:2138–2149. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Guo X, Zhou Q, Su D, Luo Y, Fu Z, Huang L,
Li Z, Jiang D, Kong Y, Li Z, et al: Circular RNA circBFAR promotes
the progression of pancreatic ductal adenocarcinoma via the
miR-34b-5p/MET/Akt axis. Mol Cancer. 19:832020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ye Z, Zhu Z, Xie J, Feng Z, Li Y, Xu X, Li
W and Chen W: Hsa_circ_0000069 knockdown inhibits tumorigenesis and
exosomes with downregulated hsa_circ_0000069 suppress malignant
transformation via inhibition of STIL in pancreatic cancer. Int J
Nanomedicine. 15:9859–9873. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou X, Liu K, Cui J, Xiong J, Wu H, Peng
T and Guo Y: Circ-MBOAT2 knockdown represses tumor progression and
glutamine catabolism by miR-433-3p/GOT1 axis in pancreatic cancer.
J Exp Clin Cancer Res. 40:1242021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rong Z, Shi S, Tan Z, Xu J, Meng Q, Hua J,
Liu J, Zhang B, Wang W, Yu X and Liang C: Circular RNA CircEYA3
induces energy production to promote pancreatic ductal
adenocarcinoma progression through the miR-1294/c-Myc axis. Mol
Cancer. 20:1062021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zeng Z, Zhao Y, Chen Q, Zhu S, Niu Y, Ye
Z, Hu P, Chen D, Xu P, Chen J, et al: Hypoxic exosomal
HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic
pancreatic cancer cells via enhancing glycolysis. Oncogene.
40:5505–5517. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lin J, Wang X, Zhai S, Shi M, Peng C, Deng
X, Fu D, Wang J and Shen B: Hypoxia-induced exosomal circPDK1
promotes pancreatic cancer glycolysis via c-myc activation by
modulating miR-628-3p/BPTF axis and degrading BIN1. J Hematol
Oncol. 15:1282022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
He Z, Cai K, Zeng Z, Lei S, Cao W and Li
X: Autophagy-associated circRNA circATG7 facilitates autophagy and
promotes pancreatic cancer progression. Cell Death Dis. 13:2332022.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hu C, Xia R, Zhang X, Li T, Ye Y, Li G, He
R, Li Z, Lin Q, Zheng S and Chen R: circFARP1 enables
cancer-associated fibroblasts to promote gemcitabine resistance in
pancreatic cancer via the LIF/STAT3 axis. Mol Cancer. 21:242022.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Guan H, Tian K, Luo W and Li M:
m6A-modified circRNA MYO1C participates in the tumor
immune surveillance of pancreatic ductal adenocarcinoma through
m6A/PD-L1 manner. Cell Death Dis. 14:1202023. View Article : Google Scholar
|
|
129
|
Liu B, Gong Y, Jiang Q, Wu S, Han B, Chen
F, Lin Q, Wang P and Yang D: Hsa_circ_0014784-induced YAP1 promoted
the progression of pancreatic cancer by sponging miR-214-3p. Cell
Cycle. 22:1583–1596. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kong Y, Li Y, Luo Y, Zhu J, Zheng H, Gao
B, Guo X, Li Z, Chen R and Chen C: circNFIB1 inhibits
lymphangiogenesis and lymphatic metastasis via the
miR-486-5p/PIK3R1/VEGF-C axis in pancreatic cancer. Mol Cancer.
19:822020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Huang L, Han J, Yu H, Liu J, Gui L, Wu Z,
Zhao X, Su S, Fu G and Li F: CircRNA_000864 upregulates B-cell
translocation gene 2 expression and represses migration and
invasion in pancreatic cancer cells by binding to miR-361-3p. Front
Oncol. 10:5479422020. View Article : Google Scholar
|
|
132
|
Xu H, Chen R, Shen Q, Yang D, Peng H, Tong
J and Fu Q: Overexpression of circular RNA circ_0013587 reverses
erlotinib resistance in pancreatic cancer cells through regulating
the miR-1227/E-cadherin pathway. Front Oncol. 11:7541462021.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yu S, Wang M, Zhang H, Guo X and Qin R:
Circ_0092367 inhibits EMT and gemcitabine resistance in pancreatic
cancer via regulating the miR-1206/ESRP1 axis. Genes (Basel).
12:17012021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Shi X, Yang J, Liu M, Zhang Y, Zhou Z, Luo
W, Fung KM, Xu C, Bronze MS, Houchen CW and Li M: Circular RNA
ANAPC7 inhibits tumor growth and muscle wasting via
PHLPP2-AKT-TGF-β signaling axis in pancreatic cancer.
Gastroenterology. 162:2004–2017.e2. 2022. View Article : Google Scholar
|
|
135
|
Liu J, Yuan W and Gong D: Hsa_circ_0000994
inhibits pancreatic cancer progression by clearing immune-related
miR-27a and miR-27b. J Oncol. 2022:72747942022.PubMed/NCBI
|
|
136
|
Xu C, Ye Q, Ye C and Liu S: circACTR2
attenuates gemcitabine chemoresiatance in pancreatic cancer through
PTEN mediated PI3K/AKT signaling pathway. Biol Direct. 18:142023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen RX, Chen X, Xia LP, Zhang JX, Pan ZZ,
Ma XD, Han K, Chen JW, Judde JG, Deas O, et al:
N(6)-methyladenosine modification of circNSUN2 facilitates
cytoplasmic export and stabilizes HMGA2 to promote colorectal liver
metastasis. Nat Commun. 10:46952019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Shang A, Gu C, Wang W, Wang X, Sun J, Zeng
B, Chen C, Chang W, Ping Y, Ji P, et al: Exosomal circPACRGL
promotes progression of colorectal cancer via the
miR-142-3p/miR-506-3p-TGF-β1 axis. Mol Cancer. 19:1172020.
View Article : Google Scholar
|
|
139
|
Chen LY, Wang L, Ren YX, Pang Z, Liu Y,
Sun XD, Tu J, Zhi Z, Qin Y, Sun LN and Li JM: The circular RNA
circ-ERBIN promotes growth and metastasis of colorectal cancer by
miR-125a-5p and miR-138-5p/4EBP-1 mediated cap-independent HIF-1α
translation. Mol Cancer. 19:1642020. View Article : Google Scholar
|
|
140
|
Xu H, Liu Y, Cheng P, Wang C, Liu Y, Zhou
W, Xu Y and Ji G: CircRNA_0000392 promotes colorectal cancer
progression through the miR-193a-5p/PIK3R3/AKT axis. J Exp Clin
Cancer Res. 39:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Chen C, Yuan W, Zhou Q, Shao B, Guo Y,
Wang W, Yang S, Guo Y, Zhao L, Dang Q, et al:
N6-methyladenosine-induced circ1662 promotes metastasis of
colorectal cancer by accelerating YAP1 nuclear localization.
Theranostics. 11:4298–4315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wang J, Zhang Y, Song H, Yin H, Jiang T,
Xu Y, Liu L, Wang H, Gao H, Wang R and Song J: The circular RNA
circSPARC enhances the migration and proliferation of colorectal
cancer by regulating the JAK/STAT pathway. Mol Cancer. 20:812021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Liu Z, Zheng N, Li J, Li C, Zheng D, Jiang
X, Ge X, Liu M, Liu L, Song Z, et al: N6-methyladenosine-modified
circular RNA QSOX1 promotes colorectal cancer resistance to
anti-CTLA-4 therapy through induction of intratumoral regulatory T
cells. Drug Resist Updat. 65:1008862022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen Z, He L, Zhao L, Zhang G, Wang Z, Zhu
P and Liu B: circREEP3 drives colorectal cancer progression via
activation of FKBP10 transcription and restriction of antitumor
immunity. Adv Sci (Weinh). 9:e21051602022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yang Y, Luo D, Shao Y, Shan Z, Liu Q, Weng
J, He W, Zhang R, Li Q, Wang Z and Li X: circCAPRIN1 interacts with
STAT2 to promote tumor progression and lipid synthesis via
upregulating ACC1 expression in colorectal cancer. Cancer Commun
(Lond). 43:100–122. 2023. View Article : Google Scholar
|
|
146
|
Chen C, Liu Y, Liu L, Si C, Xu Y, Wu X,
Wang C, Sun Z and Kang Q: Exosomal circTUBGCP4 promotes vascular
endothelial cell tipping and colorectal cancer metastasis by
activating Akt signaling pathway. J Exp Clin Cancer Res. 42:462023.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li Z, Yao H, Wang S, Li G and Gu X:
CircTADA2A suppresses the progression of colorectal cancer via
miR-374a-3p/KLF14 axis. J Exp Clin Cancer Res. 39:1602020.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Peng C, Tan Y, Yang P, Jin K, Zhang C,
Peng W, Wang L, Zhou J, Chen R, Wang T, et al: Circ-GALNT16
restrains colorectal cancer progression by enhancing the
SUMOylation of hnRNPK. J Exp Clin Cancer Res. 40:2722021.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chen J, Wu Y, Luo X, Jin D, Zhou W, Ju Z,
Wang D, Meng Q, Wang H, Fu X, et al: Circular RNA circRHOBTB3
represses metastasis by regulating the HuR-mediated mRNA stability
of PTBP1 in colorectal cancer. Theranostics. 11:7507–7526. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liang ZX, Liu HS, Xiong L, Yang X, Wang
FW, Zeng ZW, He XW, Wu XR and Lan P: A novel NF-κB regulator
encoded by circPLCE1 inhibits colorectal carcinoma progression by
promoting RPS3 ubiquitin-dependent degradation. Mol Cancer.
20:1032021. View Article : Google Scholar
|
|
151
|
Zheng R, Zhang K, Tan S, Gao F, Zhang Y,
Xu W, Wang H, Gu D, Zhu L, Li S, et al: Exosomal circLPAR1
functions in colorectal cancer diagnosis and tumorigenesis through
suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer.
21:492022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang F, Su T and Xiao M: RUNX3-regulated
circRNA METTL3 inhibits colorectal cancer proliferation and
metastasis via miR-107/PER3 axis. Cell Death Dis. 13:5502022.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ding N, You AB, Yang H, Hu GS, Lai CP, Liu
W and Ye F: A Tumor-suppressive molecular axis
EP300/circRERE/miR-6837-3p/MAVS activates type I IFN pathway and
antitumor immunity to suppress colorectal cancer. Clin Cancer Res.
29:2095–2109. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Xia S, Feng J, Chen K, Ma Y, Gong J, Cai
F, Jin Y, Gao Y, Xia L, Chang H, et al: CSCD: A database for
cancer-specific circular RNAs. Nucleic Acids Res. 46(D1):
D925–D929. 2018. View Article : Google Scholar :
|
|
155
|
Tan LP, Seinen E, Duns G, de Jong D, Sibon
OC, Poppema S, Kroesen BJ, Kok K and van den Berg A: A high
throughput experimental approach to identify miRNA targets in human
cells. Nucleic Acids Res. 37:e1372009. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46(D1): D296–D302.
2018. View Article : Google Scholar :
|
|
157
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43(Database Issue): D146–D152. 2015. View Article : Google Scholar :
|
|
158
|
Cai S, Zhang Y, Zhang X, Wang L, Wu Z,
Fang W and Chen X: A microarray expression profile and
bioinformatic analysis of circular RNA in human esophageal
carcinoma. J Gastrointest Oncol. 13:510–526. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang Y, Li J, Yu J, Liu H, Shen Z, Ye G,
Mou T, Qi X and Li G: Circular RNAs signature predicts the early
recurrence of stage III gastric cancer after radical surgery.
Oncotarget. 8:22936–22943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Han J, Thurnherr T, Chung AYF, Goh BKP,
Chow PKH, Chan CY, Cheow PC, Lee SY, Lim TKH, Chong SS, et al:
Clinicopathological-associated regulatory network of deregulated
circRNAs in hepatocellular carcinoma. Cancers (Basel). 13:27722021.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li H, Hao X, Wang H, Liu Z, He Y, Pu M,
Zhang H, Yu H, Duan J and Qu S: Circular RNA expression profile of
pancreatic ductal adenocarcinoma revealed by microarray. Cell
Physiol Biochem. 40:1334–1344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Qu S, Song W, Yang X, Wang J, Zhang R,
Zhang Z, Zhang H and Li H: Microarray expression profile of
circular RNAs in human pancreatic ductal adenocarcinoma. Genom
Data. 5:385–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Guo S, Xu X, Ouyang Y, Wang Y, Yang J, Yin
L, Ge J and Wang H: Microarray expression profile analysis of
circular RNAs in pancreatic cancer. Mol Med Rep. 17:7661–7671.
2018.PubMed/NCBI
|
|
165
|
Yang H, Li X, Meng Q, Sun H, Wu S, Hu W,
Liu G, Li X, Yang Y and Chen R: CircPTK2 (hsa_circ_0005273) as a
novel therapeutic target for metastatic colorectal cancer. Mol
Cancer. 19:132020. View Article : Google Scholar : PubMed/NCBI
|