The simultaneous treatment of both primary and
metastatic tumors is the main treatment option following tumor
metastasis. However, the treatment for the primary tumor often has
limited efficacy on the metastasis (1), resulting in different responses.
Metastatic and primary tumors show varying degrees of resistance
after several treatments (2,3).
Compared with the metastatic sites of the primary tumors, they
often exhibit a more malignant progression state (4). Accompanying this is the rapid loss
of patient symptom management and the failure of antitumor
treatment. The differential response of primary tumors and
metastases to the treatment may be related to the heterogeneity in
their tumor microenvironments (TMEs) (5,6).
The TMEs are the internal and external
micro-landscape of tumor cells formed by the response of normal
organs to evolving cancer cells, mainly composed of tumor cells,
infiltrating immune cells, cancer-associated stromal cells, such as
cancer-associated fibroblasts (CAFs), endothelial cells and
lipocytes, along with the extracellular matrix (ECM) and multiple
signaling molecules (7). These
environmental factors play a key role in both the development of
tumors and their response to therapy (5). CAFs, as key components of the tumor
microenvironment, have been found to be closely associated with the
heterogeneity between primary tumors and metastases. This
heterogeneity may affect the drug resistance of primary and
metastatic sites (8).
CAFs promote tumor proliferation, therapeutic
resistance and immune rejection by secreting growth factors,
inflammatory ligands and ECM proteins (9-11).
Previously, CAFs were considered cell populations that were not
single but complex subclusters with different functions (9). Significant heterogeneity in the
subsets of CAFs associated with primary tumors and metastases
(pCAFs and mCAFs, respectively) have been shown to exhibit
different sensitivities to treatment (8). The heterogeneity of pCAFs and mCAFs
may be the key to the different treatment responses of primary
tumors and metastases.
Compared with pCAFs, mCAFs generally have a stronger
ability to shape the ECM, and in immunosuppression and angiogenesis
(12-16). The results of preclinical studies
suggest that targeting mCAFs can alleviate the progression of
metastatic cancer and mitigate therapeutic resistance, indicating
that mCAFs are a promising target for metastatic cancer (17). Therefore, comparing the
heterogeneity between mCAFs and pCAFs from the biological
characteristics is necessary to find opportunities to target mCAFs.
Among them, the identification of the cells of origin in CAF
subtypes is a central question, as it may partially determine the
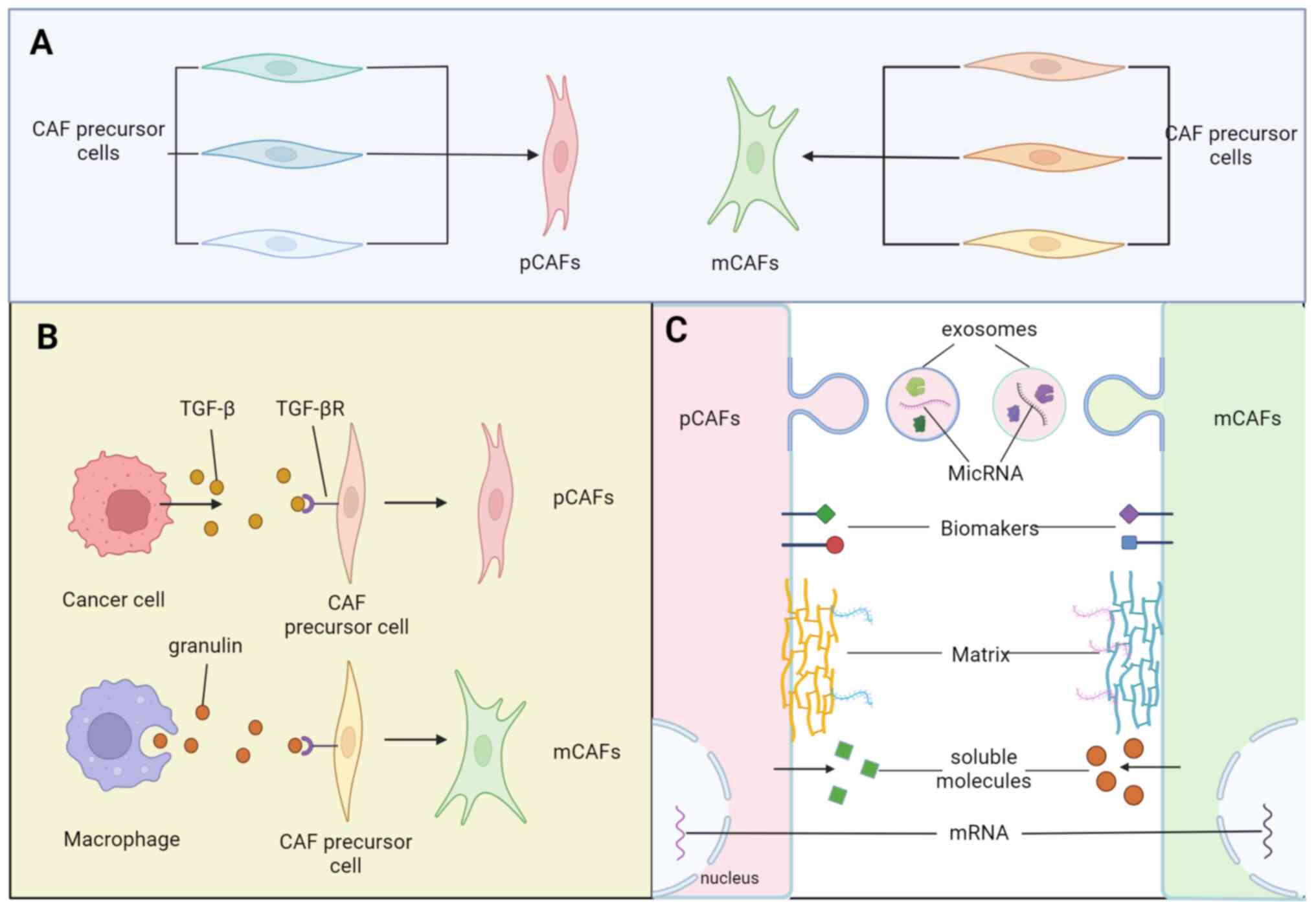
functions of distinct CAF populations (18) (Fig.
1A). Furthermore, fibroblasts heterogeneity can be partly
explained by variable activation levels of the resident fibroblasts
(RFs) with organ-specific features (19). The phenotypic heterogeneity of
activated CAFs can be manifested by a wide range of biological
markers with selective expression patterns in the context of
specific TMEs (10) (Fig. 1B). Moreover, the biological
heterogeneity of CAFs is also reflected in secreted molecules,
exosomes, transcriptional features and matreotypes (20,21) (Fig.
1C). The present study attempted to make sense of the different
treatment outcomes between primary tumors and metastases from the
perspective of heterogeneity and treatment resistance of pCAFs and
mCAFs, identifying treatment opportunities for metastases.
Any cell with properties associated with 'activated'
fibroblasts, myofibroblasts and mesenchymal stem cells (MSCs) can
be defined as fibroblasts (21).
In several experimental models of human tumors, validation at the
transcriptional and protein levels revealed that differences in the
spatial separation of CAF subclasses could be attributed to their
respective sources (22,23), which is called lineage-dependent
heterogeneity (18). In tumors,
various cells, such as RFs, MSCs, including bone marrow-derived
MSCs (BM-MSCs), pancreatic stellate cells (PSCs), hepatic stellate
cells (HSCs), smooth muscle cells, pericytes, adipocytes,
monocytes, mesothelial cells, epithelial cells and endothelial
cells, are activated into CAFs through different mechanisms
(24-31).
The present study summarized the functional
convergence of CAFs from the perspective of their sources and CAFs
from different sources may also have functional synergy (Table I). HSCs and PSCs share homology
and have similar morphologies and functions (32), CAFs derived from HSCs and PSCs are
prone to stromal deposition (33-35), similar to their function in
mediating tissue fibrosis in non-malignant diseases (36,37). Tumor-resident MSCs also function
in ECM production and remodeling after activation as CAFs and could
partially promote angiogenesis (38-40). CAFs derived from BM-MSCs may be
involved in angiogenesis and the maintenance of an inflammatory
environment in tumors (41,42). Pericytes are cells embedded
between the capillary basement membrane and endothelial cells that
physiologically regulate vasoconstriction and source of neovascular
walls (43), CAFs from this
source may be predominantly involved in tumor angiogenesis
(44). CAFs undergoing
mesenchymal transformation change their adhesion properties, which
is closely related to the enhancement of tumor aggressiveness
(45); due to differences in
organ anatomy and physiology, RFs activate CAFs in different ways
via different pathways, differentiating into elusive functional
phenotypes (46-49).
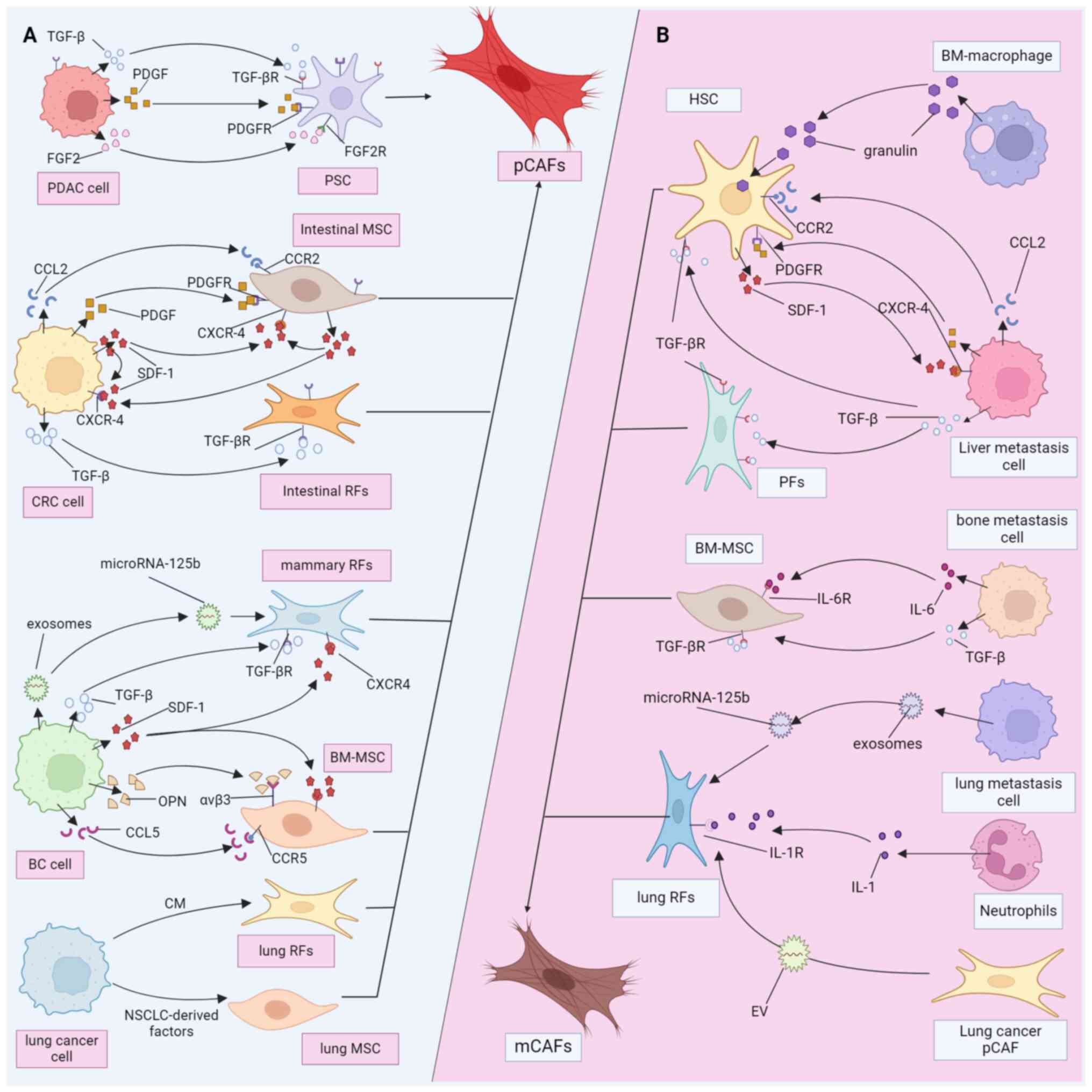
Pancreatic cancer (PDAC), colorectal cancer (CRC),
breast cancer (BC) and lung cancer are malignant tumors
characterized by high enrichment of CAFs and stromal hyperplasia
and they have received a great deal of attention in the field of
CAF research (50). Here, we take
these several types of cancer as examples to compare the
heterogeneity of pCAFs (Fig. 2A)
and mCAFs (Fig. 2B) sources. It
is of great significance in describing the overall picture of CAF
composition and understanding the microenvironment heterogeneity
between primary and metastatic tumors. The screening and ablation
of CAF precursor cells of specific origins may be an effective
means of improving the sensitivity of secondary tumors to drugs
(17).
LMs: Mouse experiments have shown that 95% of liver
myCAFs originate from HSCs and portal fibroblasts (PFs) (63). In some benign liver diseases, HSCs
and PFs are the main sources of myofibroblasts (64). HSCs play a critical role in
hepatic fibrosis (65), while PFs
are the first responders in biliary fibrosis (66). Through gene tracking, scRNA-seq
and Cre-lox mediated gene deletion methods, Bhattacharjee et
al (35) showed that the CAFs
of PDAC LMs mainly originate from HSCs. Xie et al (67) found the exosomal CD44v6/C1QBP
complex is delivered to the plasma membrane of HSCs, resulting in
the phosphorylation of insulin-like growth factor 1 (IGF-1),
leading to HSC activation and liver fibrosis. In another RNA-Seq
analysis of CRC LMs mCAFs, comparing ECM-CAFs subtypes functioning
in ECM remodeling and collagen (COL) production with fibroblast
gene features in the normal or cirrhotic liver, found that ECM-CAFs
significantly overlap with scar-associated MSCs (SAMes), which
express PFs markers. The Ctr-CAF-I subtype (with contractile
function), which expresses PLN and variants of actin gamma 2, may
originate from vascular smooth muscle cells (VSMCs); while the
Ctr-CAF-II subtype (the average CAF phenotype) may originate from
HSCs (68).
The most common metastatic site for PDAC and CRC is
the liver and some studies have found that the fate of this
directed metastasis appears to be mediated by fibroblasts before
the metastatic event occurs (34,69-71). When genome-wide transcription data
on the heterogeneity of human fibroblasts between organs were
acquired through cDNA microarray analysis of human skin fibroblast
cultures from different ages and anatomical locations, the
fibroblasts were clustered next to other fibroblasts from the same
site rather than cells from the same individual, which indicates
that fibroblasts have positional memory characterized by gene
expression patterns (72). LMs
have unique and similar morphological characteristics in PDAC and
CRC (35). This may be largely
attributed to liver RFs, including HSCs (73), which have conserved
transcriptional programs in different tumors and upon tumor
progression (18). These
persistent TMEs builders ultimately construct microenvironments
that differ from the primary tumor in LMs.
Lineage-dependent heterogeneity of CRC pCAFs and LMs
mCAFs is easily observable, however, in PDAC; PSCs and HSCs are
known to be homologous and the primary tumors are characterized by
abundant desmoplasia, constituting up to 90% of the total tumor
volume (58), exceeding the
proportion of stroma in LMs (74). One explanation for this is the
presence of other stroma-producing cells in the pancreas (32). Helms et al (75). conducted fluorescence tracing on
PSCs and found that they only produced a small portion of myCAFs
and PSC ablation still generated a high abundance of
α-S-adenosylmethionine (SAM) + CAFs, suggesting that PSCs are not
the only source of myCAFs They speculated that other myCAFs in PDAC
may originate from other RFs or the bone marrow (75). Through lineage tracing, Garcia
et al (76) found that
resident Gli1+ fibroblasts, which are distinct from PSCs in healthy
pancreas, may a source of myCAF populations, providing clues for
this hypothesis.
The lung cancer pCAFs mainly originate from lung RFs
and MSCs. Enhancing the expression of hypoxia inducible factor-1 in
fibroblasts via hypoxia induced the conversion of normal
fibroblasts into CAFs (77).
Research indicates that fibroblasts activated by lung cancer cell
conditioned media lead to an increase in interleukin (IL)-6
production, inducing epithelial-mesenchymal transition and
cisplatin resistance in non-small cell lung cancer (NSCLC) cells
(78). Treatment with
NSCLC-derived factors induce a CAF phenotype in both normal lung
resident MSCs and lung cancer-associated MSCs (79). One of the most common sites of
metastasis in lung cancer is intrapulmonary metastasis to the
ipsilateral or contralateral lung (80). A study showed that CAF
extracellular vesicles (EVs) can activate lung fibroblasts and
induce the formation of pre-metastatic niches in the lungs
(81). Another study shows that
the metastatic cells can bring stromal components from the primary
site to the lungs (82).
BC CAFs have a wide range of sources, primarily
including RFs, BM-fibroblasts, MSCs (including BM-MSCs) and
adipocytes (83,84). RFs and BM-MSCs are two more
important sources of CAFs in BC. RNA-seq of pCAFs extracted from a
mouse orthotopic transplantation model showed that podoplanin+ CAFs
had a transcription pattern similar to normal mammary fat pad
fibroblasts, while fibroblast-specific protein 1 (FSP1) was not
expressed in normal fibroblasts. Therefore, FPS1+ CAFs may have
been recruited from the periphery (85). A previous study on adoptive bone
marrow transplantation confirmed that BC recruits large numbers of
MSCs that do not express platelet-derived growth factor (PDGFRα)
from the bone marrow into the primary tumor and lung metastases and
found that CAFs derived from resident CAFs and BM-MSCs had
different abilities in inducing angiogenesis and recruiting
macrophages (41).
BC bone metastasis mCAFs are mainly derived from
BM-MSCs and primary tumor pCAFs. Specifically, pCAFs from
triple-negative BC (TNBC) produce stromal cell-derived factor 1
(SDF-1) and IGF-1 to induce bone metastasis of cancer cells with
high Src activity (86), where
pCAFs are transferred to bone marrow together with BC cells under
the mediation of osteopontin (OPN) (87). Tumor cells continue to evolve
after metastasis, but they no longer depend on the primary tumor.
They maintain a state of reduced information exchange due to
physical and chemical barriers, especially in organs such as the
brain and bone (88). The
influence of the bone marrow microenvironment on bone metastasis is
undoubtedly great. BM-MCSs, as the most important stromal cells in
the bone marrow, differentiate into different cell subsets such as
osteoblasts, adipocytes, fibroblasts and pericytes (89), playing a key role in tumor cell
homing, bone marrow colonization and tumor cell dormancy (90). The deleting of Rictor gene reduces
the secretion of IL-6, receptor activator of nuclear factor-kappa B
ligand (RANKL) and TGF-β and inhibits the transition from BM-MCSs
to CAFs, resulting in lower chemotaxis and less proliferation in
TM40D cells (42).
CAFs in BC lung metastases are mainly derived from
lung RFs and BM-MSCs. One study found that the expression patterns
of specific genes in the lung CAFs of mice with transgenic BC
dynamically changed at different metastatic stages (91). Houthuijzen et al (71) reported that a RF population only
found in the lungs was transformed into CAFs, promoting the lung
metastasis of BC cells. Raz et al (41) used β-actin-GFP-PyMT double
transgenic mice generated via adoptive bone marrow transplantation
as donors, transplanted their bone marrow into non-transgenic
control mice and found that GFP-labeled CAFs were specifically
recruited in the primary BC tumors and lung metastases.
Lymph nodes are transit stations for tumor
metastasis, but there have been few reports on the source of CAFs
during lymph node metastasis (Met-LNs). Immunohistochemistry (IHC)
assays showed that pCAFs in BC has similar biomarkers with mCAFs in
matching Met-LNs (92) and only
small differences were found in their transcription profiles
(93,94). Such results suggest that Met-LN
mCAFs were derived directly from pCAFs.
CAFs exist in the TEMs as quiescent precursor cells
and are abnormally activated to serve tumor proliferation,
migration and drug resistance (Table
II). In addition to lineage-dependent heterogeneity, the
heterogeneity between pCAFs and mCAFs is also reflected in their
mode of activation. First, fibroblasts in metastatic sites can be
activated differently from CAFs in primary tumors (18). For example, macrophages promote
the activation of HSCs into αSMA+ myCAFs which secrete high levels
of periosteal proteins in the LMs of PDAC by producing granulin
(96). CRC exosomal HSPC111
regulates the lipid metabolism of HSCs and promotes the formation
of pre-metastatic liver niches (97). Furthermore, even identical CAF
subtypes may exhibit different activation levels in primary and
secondary sites. For example, the liver colonization of tumors is
extremely dependent on TGF-β signaling in the liver stroma
(98), which is compatible with
the high ECM stress of the liver. This is because TGF-β is present
in the ECM and binds to latent TGF binding proteins; its activation
and release require mechanical forces acting on integrin-specific
domains (99). TGF-β released
under the high ECM stress in LMs participates in ECM remodeling and
EMT through classical and non-classical pathways and plays a
decisive role in cancer cell migration and invasion (100,101). The positive feedback loop of
TGF-β secretion is established by TGF-β-activated CAFs through the
SDF-1/C-X-C chemokine receptor 4 (CXCR4) signaling pathway
(69,102). Some studies have shown that LMs
express more CXCR4 than the primary tumor (103,104), which confirms the differential
activation of pCAFs and mCAFs.
Notably, while the differences in activation modes
between primary and metastatic CAFs are constantly being
discovered, different CAF activation methods within the same site
are also found in the complex microenvironment. In PDAC, IL-1
induces leukemia inhibitory factor expression and activates the
downstream JAK/STAT pathway to transform PSCs into iCAFs, while
TGF-β antagonizes this process by downregulating IL-1R1 expression
and promoting myCAF generation (105). This indicates that there may be
mutual antagonistic between different activation modes for CAFs,
which may be the reason for the differential spatial distribution
of these CAF subtypes in PDAC. The ability to dynamically change
with different environmental stimuli reflects the extremely strong
plasticity of CAFs, which may be one of the reasons why the source
of CAFs so far remains elusive (106).
In most cases, CAF markers used are not considered
to be representative of their functional heterogeneity and this is
because CAFs lack unique markers, as most of them are also
expressed in other cells (107).
The classification of CAFs with known markers only compensates for
the current lack of understanding on this topic, although
combinatorial labeling improves the sorting accuracy of CAFs in
complex environments (20). It
should be noted that the classification of functional CAF subsets
using markers is not as clear as that of immune cells.
Additionally, due to the presence of different functional subtypes
of CAFs, a single marker is not sufficient to distinguish them
effectively. When attempting to generalize the prognostic value of
the complete CAF population without distinguishing the effects of
the heterogeneous CAF subgroups, contradictory results may be
obtained (106). Nevertheless, a
number of studies have investigated the prognostic value of
commonly used CAF biomarkers in various types of cancer (108). Similarly, the differential
expression of common markers in mCAFs and pCAFs has also been
reported (8,109,110).
FAP is a type II transmembrane serine protease with
both dipeptidyl peptidase and endopeptidase activity. It is
expressed in ~90% of CAFs and is a hallmark of CAF activation
(111). Studies have shown that
FAP+ CAFs promote tumor progression, ECM degradation, tumor
invasion, angiogenesis and immune suppression (112,113). Brain metastases from multiple
types of primary tumors uniformly show high levels of FAP
expression and no association of histological type (114), therefore, FAP may serve as a
broad therapeutic target for brain metastasis.
As the most promising therapeutic target and
radiotracer, FAP has become the focus of CAFs research in recent
years (115,116). FAP inhibitors (FAPi) based on
quinoline have been used as tracers for positron emission
tomography and computerized tomography (PET-CT) diagnosis. FAPi can
specifically bind to FAP and be internalized by CAFs. 68Ga, 177LU
or 18F can chelate it and be fed back as image information through
imaging systems for the diagnosis and identification of tumors
(111). FAPi is characterized by
high intratumoral uptake and rapid in vivo clearance.
Various types of FAPi have since been developed and compared
head-to-head with fluorodeoxyglucose (FDG) PET-CT, showing that
FAPi tracers are more advantageous in discovering some primary,
lymph node, or metastatic tumors (112,115,117). However, the sample sizes of
these studies are very limited and there is still no convincing
evidence of which tumor types or which metastatic organs are more
appropriate to FAPi. However, it is important to improve the
detection rate of early tumors and accurately determine the tumor
stage to positively influence clinical decisions. Serfling et
al (118) established a
positive correlation and significance between FAP targeted
expression and FAPi PET standardized uptake values (SUVs). In their
study, 15 patients underwent 68Ga-FAPi-46 PET-CT scans to determine
the biodistribution of 68Ga-FAPi-46 in various tissues (117). After subsequent surgical
treatment, FAP expression was scored on the excised samples and the
results showed a positive correlation between FAP IHC scores and
the 68Ga-FAPi-46 SUVmax and SUVmean (119). In addition, Serfling et
al (118) suggested that
FAPα Met-LNs expression was correlated with lesion size. Sollini
et al (120) reasoned
that the relatively low performance of 68Ga-FAPi in detecting
Met-LNs reported in some studies may be related to the low
enrichment of CAFs within the lymph nodes. The SUVs of FAPi PET/CT
reflects the expression of FAP in CAFs to a certain extent. This is
an important means to compare marker heterogeneity between pCAFs
and mCAFs. Data on the SUVs of primary tumors and metastatic tumors
regarding FAPi PET-CT were collected and compared in order to find
partial correlations. Disappointingly, research on FAPi tracers is
still in its infancy. Moreover, factors such as their wide variety,
insufficient sample size and the majority of studies being
pan-cancer samples prevent the comparison of the data
comprehensively. Although some meta-analyses have demonstrated the
diagnostic advantages of FAPi tracers (120), this is not the focus of the
present study. However, the present study still showed some results
suggesting the possibility of differential expression of FAP
between pCAFs and mCAFs (Table
III).
Among 64 patients with lung squamous cell carcinoma,
47 had a high level of PDPN expression in the primary stroma but 27
patients had a high level of PDPN in Met-LNs; univariate analysis
found that only high PDPN expression in Met-LNs was significantly
associated with prognosis (132). Koo et al performed
immunohistochemical staining on different metastatic sites of BC
and observed that FSP1 expression was significantly elevated in
bone metastases while it was significantly reduced in LMs. The
expression of PDPN was significantly elevated in bone metastasis
and PDGFR expression was elevated in bone and lung metastases, but
significantly reduced in LMs (109). These results prove that markers
are expressed in different proportions in different metastatic
sites. In one study, TNBC 4T1 cells were injected in situ into the
breast fat pad of immunocompetent BALB/c mice, at week 4, it was
observed that the proportion of PDPN+ CAFs, which originally
accounted for 70% of the total CAFs, was reduced to 23% in the
primary tumor, while FSP1+ CAFs, which originally accounted for
30%, increased to 77%. Notably, two FSP1+ CAF subtypes that were
not observed in the primary tumor appeared in lung metastases and
were shown to express IL6 and CXC chemokine ligand 1 (CXCL1),
respectively (85).
Recent advances in scRNA-seq have allowed for a
comprehensive profiling of the complexity and heterogeneity within
the CAF subpopulations across various tumor entities (Table IV). Some reviews have examined
the organ-specific features of CAFs and summarized the
transcriptomic information of CAFs in different organs with
ECM-remodeling, inflammation and immunity and antigen presentation
(133,134). However, CAF subtypes do not
exhibit universality across different tumor types, even though the
classical myCAF and iCAF subtypes have been described in PDAC, BC
and cervical cancer (59,135,136). scRNA-seq technology has the
drawback of losing spatial information, resulting in incomplete
information on the correlation between different CAF subtypes and
the anatomical location of tumors. At the same time, the loss of
temporal information makes it difficult to present the evolutionary
trajectory of CAF subtypes. The integration of scRNA-seq and
microarray-based spatial transcriptomics methods and pseudotime
inference might be able to partially solve these issues (137,138). It is hypothesized that a more
comprehensive understanding of the heterogeneity of CAFs will
provide innovative solutions for cancer treatment and enable
clinical applications.
The differences in transcriptional levels between
mCAFs and pCAFs are being described as RNA-seq technology matures
(23,139). The transcription profiles of
MSCs obtained from bone metastases in BC are significantly
different from those of CAFs obtained from the primary site
(93). RNA-Seq for BC primary
tumors and paired brain metastases yielded 48 differential gene
expression signatures, most of which were those of immunity and
fibroblasts (139). Similarly,
another study noted the differential upregulation of genes
associated with ECM remodeling and BM-derived cell recruitment in
lung mCAFs compared with BC pCAFs (41). Within PDAC samples, Liu et
al (140) found large
changes in fibroblast subclasses at succeeding stages of PDAC
progression, with the emergence of specific subclasses when cancer
trespasses stroma to metastasize to proximal lymph nodes (stage IIA
to IIB) and gene expression analysis showed increased expression of
cytoskeletal protein and inflammatory cytokines when transition to
IIB, indicating that tumor growth and metastasis are strictly
regulated by genes.
MicroRNAs (miRNAs or miRs) are non-coding RNAs which
can disrupt mRNA expression (141). Tumor cells can deliver miRNAs to
CAFs through exosomes, promoting the malignant phenotype of CAFs
(142). As expected, there are
also differences between the miRNA profiles of pCAFs and mCAFs.
Upon exposure to estrogen, the number of miRNAs upregulated or
downregulated in skin mCAFs in BC is three times that in pCAFs
(143), but the biological
effects of these differentially expressed miRNAs need further
verification. In advanced CRC, the differential expression of
miR-21 between the center of the primary tumor and distant
metastases is common (144).
There were five upregulated and six downregulated miRNAs in the
exosomes of mCAFs with peritoneal metastasis in ovarian cancer,
among which miR-29c-3p downregulation was the most significant and
positively correlated with patient overall survival (OS) (145).
CAFs continuously release soluble molecules into the
ECM to provide information feedback and functionally regulate the
microenvironment (18,20). The biological characteristics of
primary tumors and metastases are inseparable from cytokines
secreted by pCAFs and mCAFs. CXCR4 is a G-protein-coupled receptor,
which is little expressed if at all in normal cells, but
dysregulated and aberrant in a number of tumors (146). The best characterized ligand
that binds and activates CXCR4 is stromal SDF-1 (147). SDF-1/CXCR4 signaling has serious
consequences on cancer cell differentiation, proliferation,
invasion, metastasis and angiogenesis (148). Several studies have found that
high expression of SDF-1/CXCR4 signaling is associated with high
density of CAFs in tumor stroma (103,149). Tan et al (69) observed that there were more CXCR4+
cells at the LMs tissues Compared with the CRC primary sites.
Maintenance of the SDF-1 gradient by the BC primary tumor is
independently controlled by both miR-126 and miR-126*, which show a
significantly lower expression in metastatic tissue compared with
primary tumor tissue (150). Dai
et al (149) detected a
higher CAFs density in metastatic lesions than those in primary
tumor site from human ovarian cancer tissues, however, no
significant difference of SDF-1α production from CAFs was found
between primary and metastatic lesions. This may require further
validation of CXCR4 expression and regulation through methylation
and acetylation (146). CAFs
secrete leucine-rich α-2-glycoprotein 1 (LRG1) through the
IL-6/Janus tyrosine kinase 2/signal transducer and activator of
transcription 3 (IL-6/JAK2/STAT3) pathway (151). The expression of LRG1 is
significantly upregulated in CRC LMs, making LMs more aggressive
(151). In another study,
increased aggressiveness was also found to be associated with high
phosphoribosyltransferase expression in LMs (152). The secretome of mCAFs in
peritoneal metastases of CRC mainly comprises insulin-like growth
factor binding protein 2, CXCL2 and SDF-1, while pCAFs secrete
higher levels of matrix metalloproteinases (MMP), chemokine (c-c
motif) ligand 8 (CCL8) and CCL11 (153). Proteomic analysis showed that,
compared with primary ovarian cancer tumors, 62 proteins in omentum
metastases were significantly up- or downregulated, among which the
expression of N-methyltransferase (NNMT) was significantly altered
(104). NNMT transfers an active
methyl group from SAM to nicotinamide to produce
S-adenosylhomocysteine, the loss of which leads to decreased
histone methylation, which affects gene expression (104). In another experiment, the
difference between ovarian cancer primary tumors and metastases was
also validated, where mCAFs were hypothesized to express higher
levels of Jagged1 and cause peritoneal metastases to produce more
vascular endothelial growth factor (VEGFA) and cyclin-dependent
kinase inhibitor p21 (CDKN1A) (154). The methylation of metabolic
genes NQO1 and ALDH1a3 induced in LMs downregulate the mRNA
expression of metabolic genes in CAFs, however, compared with
normal lung fibroblasts, the gene methylation levels of NQO-1 and
ALDH1a3 in fibroblasts isolated from lung metastases remained at
baseline levels (155).
In a cross-comparison analysis conducted via
RNA-seq, the differential expression of ECM-related genes is the
main feature of transcriptome heterogeneity in inter-organ
fibroblasts (156). Different
types of COL and glycoproteins crosslink with each other to form a
stable ECM network. The post-translational modification of matrix
components in various organs via hydroxylation, glycosylation,
transglutamination, sulfation, crosslinking, cleavage and
degradation further modulates these features (14), such changes happen dynamically on
a time scale from seconds to minutes. In this way, the various
matrix components expressed through time and space are called the
matreotype of the tissue (157).
In the PDAC mouse model, after the fibrogenic gene Sonic hedgehog
was deleted, the tumor stroma was significantly reduced, but the
tumor acquired enhanced angiogenesis and invasive capabilities
(158). This suggests that in
early stages of tumorigenesis, fibroblastic reactions orchestrated
by CAFs within the TMEs envelop the tumor cells, inhibiting their
growth and spread. However, as tumor stromal components continue to
evolve during the course of tumorigenesis, the further modification
of the behavior of CAFs helps their tumor-promoting properties
(159). Notably, the
tumor-promoting and tumor-restraining abilities of CAF at different
stages of tumor progression can both be induced by TGF-β (9). In the early stages of tumors, TGF-β
primarily promotes fibroblast proliferation to inhibit metastasis
and as CAFs evolve, TGF-β can also induce CAFs to promote
metastatic events such as EMT (160).
The heterogeneity between pCAFs and mCAFs in the
plasma membrane, cytoplasm, exosomes and the nucleus has been
extensively characterized, deepening our understanding of the
differences in the microenvironments of primary tumors and
metastases. However, most of the data collected focused on the
differences between NFs and CAFs or CAFs in metastatic and
nonmetastatic primary tumors (85,173-175), which is conducive to
highlighting metastasis-inducing factors. Pseudo-time RNA-seq
analysis using matched normal tissue around the tumor, primary
tumor and metastatic tumor to simulate pre-tumorigenesis,
early-stage tumors and advanced-stage tumors, respectively, has
shortcomings in effectively simulating the dynamic changes of CAFs
during tumor evolution (18).
However, it is an excellent model to describe the spatial
heterogeneity of pCAFs and mCAFs after metastasis occurs, which
helps to explore more about this in the future.
We have previously discussed the differences
between pCAFs and mCAFs from multiple aspects of their biological
characteristics and their abilities in shaping the microenvironment
of primary and metastatic tumors. When facing treatment pressure,
the microenvironment of metastatic tumors provides more efficient
protection for the survival of tumor cells (8). mCAFs may make metastatic tumors
relatively more drug-resistant through EMT or sustaining cancer
stemness (Fig. 3B). Gui et
al (8) isolated eight CAFs
from normal tissues, primary tumors and multiple metastatic tumors
of patients with BC Co-culture in vitro has shown that mCAFs
can enhance the proliferation, migration and invasion of BC cell
lines. The team further verified the resistance of mCAFs to
treatment and their results showed that tumor cells co-cultured
with mCAFs exhibited stronger doxorubicin resistance than pCAFs and
observed that mCAFs induced tumor cells to undergo EMT and express
more tumor stem cell markers (8),
which may be one of the mechanisms underlying the stronger drug
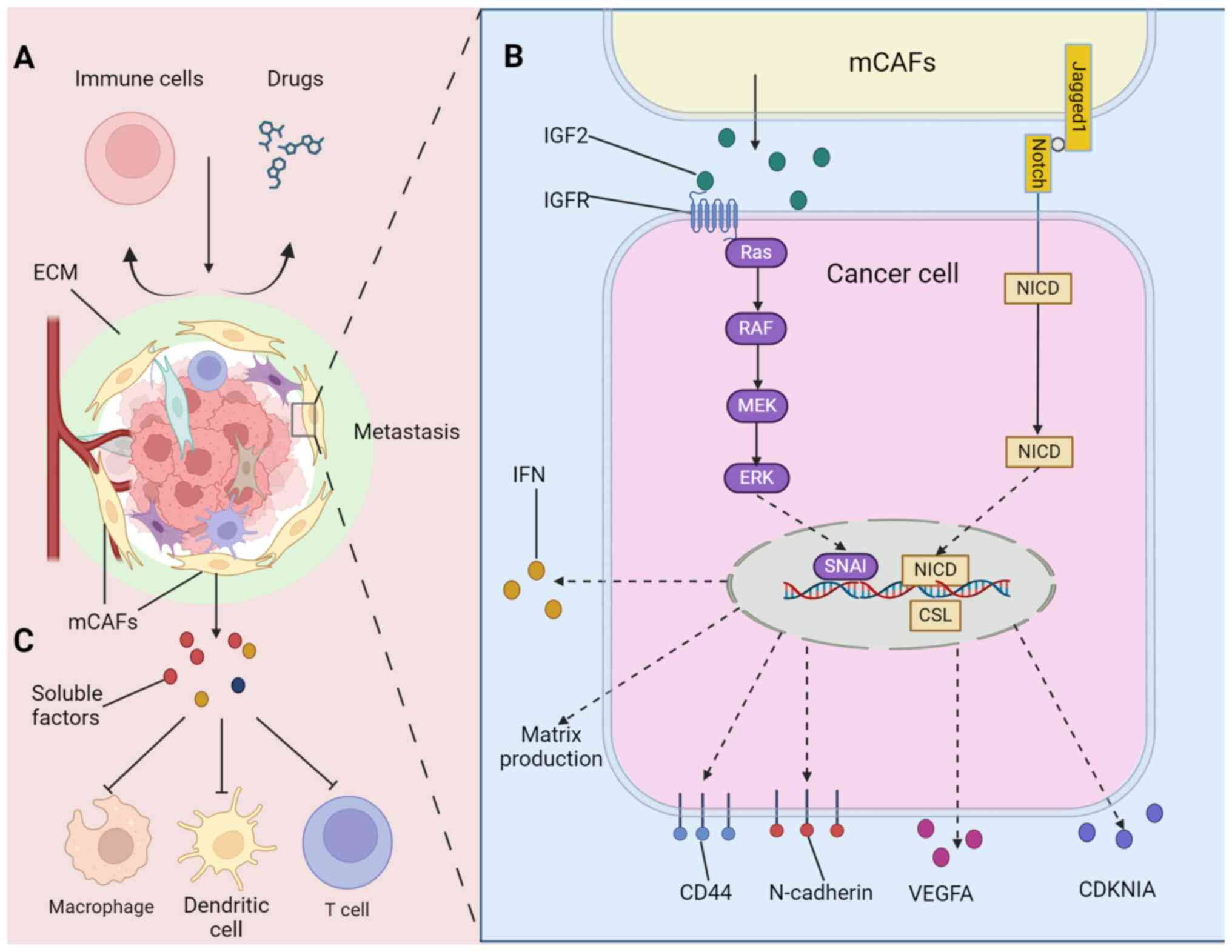
resistance in metastases (176,177). Comparison of the gene expression
profiles of pCAFs and mCAFs revealed multiple significantly
differentially expressed genes, of which IGF2 was the most
significantly enriched (8). The
overexpression of IGF2 was shown to play a key role in chemotherapy
resistance (178). Mukherjee
et al (154) discovered
that when CAFs differentially overexpressing Jagged1 were
co-cultured with ovarian cancer cells, the Notch3 signal increased
with the increase in the expression level of Jagged1. Peritoneal
metastasis mCAFs obtained from ascites have been shown to express
higher levels of Jagged1 than the primary tumor (154). Further experiments show that
CAFs affect the expression of VEGFA and CDKN1A through
Jagged1/Notch3, increasing the proportion of tumor stem cells and
resistance to cisplatin (154).
In addition, CAFs and immune cells are the main
supporting cell populations in solid tumors and there is extensive
functional interaction between them (7). Although pCAFs and mCAFs can
crosstalk with immune cells in various ways, affecting their
chemotaxis and polarization, and regulating the immune response in
the TMEs (60,179), mCAFs may achieve protective
effects against metastases through stronger immunosuppressive
effects (Fig. 3C). A RF
population that highly expresses cyclooxygenase-2 has been shown to
exist only in the lung, where it promotes the lung metastasis of BC
cells and inhibits the antigen presentation function of BM
dendritic cells (71). CRC
peritoneal metastasis mCAFs have a different secretome from pCAFs
as the macrophages displayed expression profiles associated with T
cell biology with a pronounced shift to a type 2 immune response
and T cell tolerance (153).
Similarly, peritoneal metastases mCAFs have been found in mouse
models of gastric cancer to induce macrophage M2 polarization,
resulting in low infiltration of CD8+ T cells (16).
Most anti-tumor resistance studies focus on
exploring the underlying molecular mechanisms. However, the limited
distribution of drugs in tumors is often ignored. Drug penetration
efficiency directly can affect drug efficacy and its influencing
factors involve the ECM, vascular structure and hemodynamics
(180). It is well known that
the ECM forms a physical barrier that greatly impedes drug delivery
(180). Some studies have
described the heterogeneity in physical properties between the
primary and metastatic stroma and the difference in the matreotype
may be the key to the differences in the response of different
metastatic organs to anti-tumor treatment (12,74,181) (Fig. 3A).
Studies have proven that stromal mechanical stress
can guide the directed differentiation of naïve MSCs (182). For example, vascular progenitor
cells with low and high stiffness differentiate into endothelial
and smooth muscle cells, respectively, under the mediation of
integrin (181). In another
study, the effects of load initiation time, magnitude and mode of
mechanical force on the formation of microvascular networks were
also simulated (183). This
means that primary tumors and metastases produce different the
microvascular system in different matreotypes. For example, despite
tumor vascularization, irregular vascular networks enhance blood
fluid resistance in mouse models of LMs, leading to capillary
collapse within metastases and limiting the tumor perfusion of
drugs (13). Furthermore, stromal
hyperplasia and a lack of blood supply have been shown to lead to a
series of malignant events, such as hypoxia, in the deep tumor
(182). Hypoxia has been
previously found to be an important cause of treatment resistance
(184).
In addition, the deposition of ECM traps immune
cells in the tumor stroma and increases their resistance to
infiltration into the tumor parenchyma (14). Gertych et al (15) observed that there was a difference
in the proportion of CAFs in primary tumors and metastases and the
differences in the matreotypes between the two were determined by
COL11A1+ CAFs, although there is high CD8+ T cell infiltration in
metastases, they are excluded by the proliferating ECM, resulting
in a lower survival rate.
Notably, some studies have found that high
intratumoral drug concentrations do not improve anti-tumor
efficiency (185,186). Hence, the role of the TMEs as a
physical barrier for drug delivery becomes worth re-examining.
Hessmann et al (74)
observed that KPC tumors had more CAFs and stromal hyperplasia than
LMs and they found that CAFs captured
2′,2′-difluorodeoxycytidine-5′-triphosphate (dFdCTP), an active
metabolic component of gemcitabine, thereby reducing the chance of
gemcitabine contact with tumors, resulting in PDAC primary tumors
with lower sensitivity to chemotherapy than matched LMs. This
demonstrates that the response of CAFs to the highly selective
pressure exerted by chemotherapy is pluralistic, which may explain
why successful treatments targeting the ECM are difficult.
Metastases are almost incurable and are the
underlying cause of mortality in most patients with cancer
(10). The fact that mCAFs cause
metastasis treatment resistance makes it a promising therapeutic
target (17). Eliminating or
balancing the differences between mCAFs and pCAFs may be an
effective treatment method (8,154). As aforementioned, most of the
differences between primary tumors and metastases are 'more and
less' rather than 'presence or absence', which means that
treatments targeting the differences between them not only improve
the sensitivity of metastases also benefit primary tumors. Although
targeting mCAFs in mouse models or cell experiments is emphasized,
when these drugs are used in human trials, there is more hope of
achieving simultaneous remission of both the primary and metastatic
tumors. Therefore, the present study did not emphasize whether the
new therapeutic agents of clinical trials are mCAFs or pCAFs.
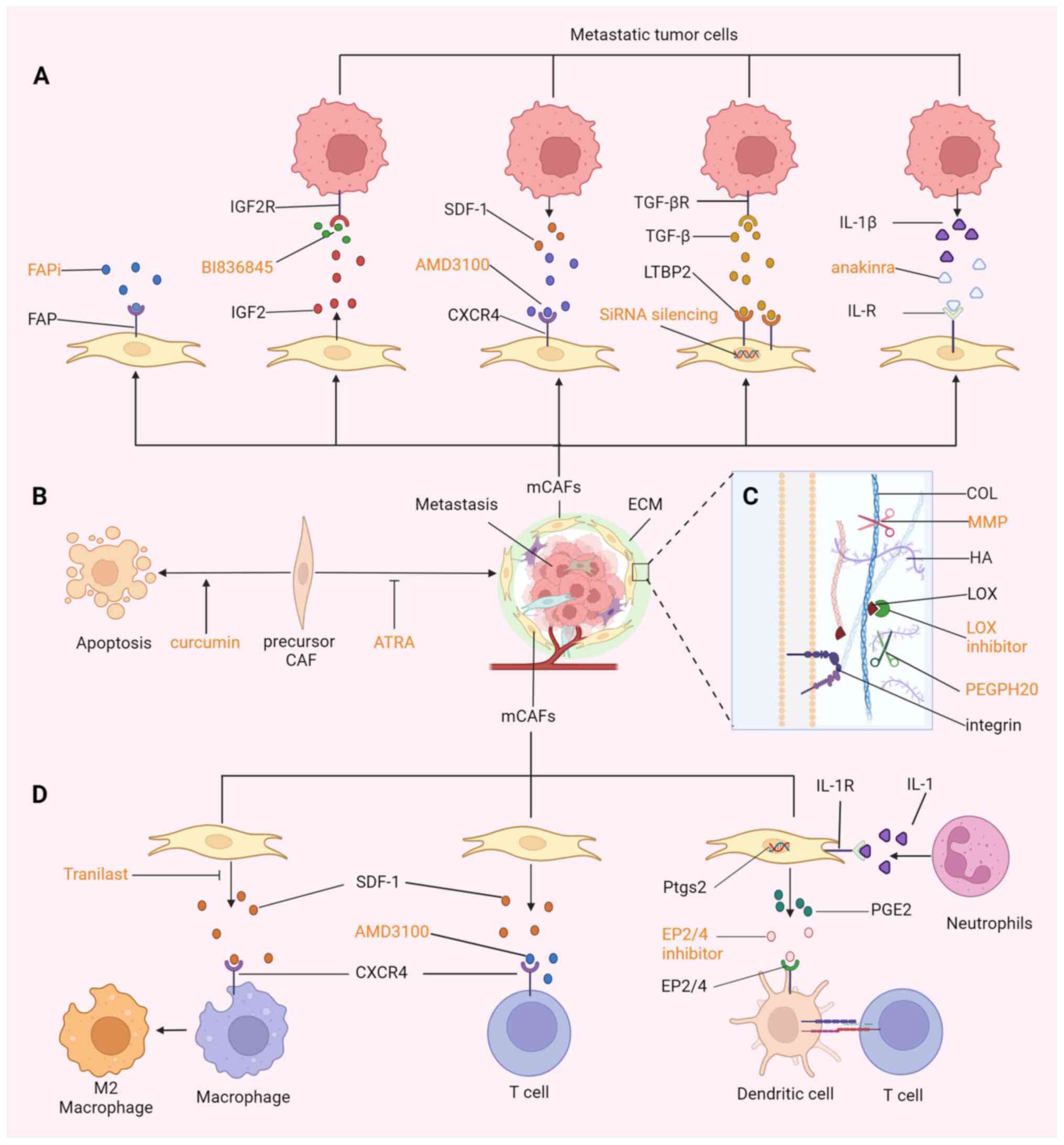
Different metastatic organs have specific sources
of CAF precursor cells; therefore, directly killing these precursor
cells or restoration of their quiescence in the target organ can
eliminate or attenuate their malignant effect on the metastatic
microenvironment (10,187) (Fig. 4B). Bhattacharjee et al
(35) depleted HSCs in triple
transgenic mice through the injection of diphtheria toxin (DT) and
the TdTomato reporter gene showed that 97% of the HSCs were
depleted. In the PDAC and CRC mouse models, the depletion of HSCs
led to a significant reduction in the area of LMs. The natural
compound curcumin induces HSC senescence by activating peroxisome
proliferator-activated receptor γ and promoting P53 expression
(188). In a recent study, the
targeted biomimetic nanoparticle-based delivery of
all-trans-retinoic acid in resting HSCs improved artificially
induced liver fibrosis in mice (189). Although numerous chemicals,
herbs and their bioactive extracts have been proven to promote the
apoptosis of HSCs, at present, no recognized HSC-depletion drugs
have been approved for clinical use (187). Hence, there is still a long way
to go in developing mCAF-derived treatments for metastases.
Identifying the proportional expression of markers
in primary tumors and metastases is a prerequisite for accurately
targeting metastases. However, sufficient epidemiological evidence
or clear molecular mechanisms regarding independent prognostic
markers are necessary. FAP is probably the most reliable marker, as
large studies have shown that the high expression of FAP is an
independent prognostic marker for poor prognosis in ovarian cancer,
lung cancer, PDAC, hepatocarcinoma and CRC (113,190). Therapeutic strategies for FAP,
including FAP-activating drugs, DNA vaccines, anti-FAP chimeric
antigen receptor redirected T cells, radionuclide-based approaches
and FAP antibodies conjugated with toxins, have been reported to be
effective in clinical and preclinical studies (111,113,115,191) (Fig. 4A). FAP-4-1BBL has bispecific
antibody activity that can act on both FAP and the co-stimulatory
molecule 4-1BBL and has been designed to provide costimulatory
signals to immune effector cells selectively within the tumor
(192). FAP-4-1BBL co-stimulates
T cells in ex vitro in patient-derived tumor tissues, additionally
the combination of carcinoembryonic antigen-targeted T cell
bispecific antibody and FAP-4-1BBL in mouse models can induce the
infiltration of CD8+ T cells and tumor regression (192). Subsequently, the first-in-human
study of the FAP-4-1BB agonist RO7122290 was initiated in patients
with advanced solid tumors, however, the study was not designed to
demonstrate differences between single-agent and combination
therapy (193). A case report
described a patient with BC and brain metastases who experienced a
decrease in the intensity of headaches after 4 weeks of FAPi
targeted radiotherapy (194). In
other studies, radiotherapy strategies based on the high expression
of FAP and FAPi may create a new integrated tumor
diagnosis/treatment model in the future. High FAPi expression on
FAPi-46 PET-CT was a criterion of consideration for
peptide-targeted radionuclide therapy following two cycles of
177Lu-FAPi-46 targeted therapy. In the selected
patients, 12 of the 18 advanced patients were stable disease with
no significant change in clinical condition but the remaining six
progressed (195). Another group
conducted a similar study (196). Unfortunately, these studies did
not provide the information on local response status in each
patient, so it is unknown whether the therapeutic benefit was
correlated to the SUVs and whether low FAP expression implies
resistance to FAP-targeting therapy.
The penetration efficiency of drugs in tumors
directly affects the drug concentration in contact with tumors. The
means of targeting the ECM mainly include adjusting the
modification state to inhibit ECM deposition, reducing the
production of COL to soften tumors and directly shearing the ECM
(Fig. 4C). For example,
inhibiting citrullination can reduce LMs growth in CRC (163). As an important active enzyme for
ECM crosslinking, LOX can also achieve tumor anti-fibrosis by
inhibiting it (197). Hepatic
fibroblasts express angiotensin II (AngII), a component of the RAS
system. AngII activates the AngII type I receptor (AT1R) to undergo
liver fibrosis through downstream JAK2 signaling (198). Using patient samples and atomic
force microscopy, Shen et al (12) found that tissue stiffness is
higher in LMs than in primary CRC. Highly activated mCAFs increase
tissue stiffness, which enhances angiogenesis and anti-angiogenic
therapy resistance. Drugs targeting the LMs mCAFs RAS system
inhibit fibroblast contraction and ECM deposition, thereby reducing
LMs stiffening and increasing the anti-angiogenic effects of
bevacizumab (12). The use of
valsartan in the treatment of spontaneous lung metastases of BC in
mouse models inhibits the production of fibronectin and vimentin
and reduces the occurrence of lung metastases (199). Another phase II clinical trial
of FOLFIRINOX in combination with losartan has also achieved
promising results as a neoadjuvant therapy for locally advanced
unresectable PDAC (200). These
studies demonstrate the anti-tumor efficacy of reduction of ECM
stiffness, however, there seems to be little therapeutic
breakthrough in clinical trials involving the direct degradation of
the ECM. PEGPH20, is a polyethylene glycol hyaluronidase and early
research has found that it can increase the distribution
concentration of antitumor drugs in primary and metastatic tumors
by degrading HA in the ECM (201). However, PEGPH20 combined with
standard regimens for advanced PDAC has failed in multiple clinical
trials. Some hypothesize that using only one ECM-degrading enzyme
may be the reason for not meeting the expected clinical outcomes
(202). However, this does not
explain the negative results of the phase IB/II trial of PEGPH20 in
combination with FOLFIRINOX in patients with metastatic PDAC
(203). This is more likely due
to the fact that ECM degradation products have a similar structure
to some growth factors and they can bind to the corresponding
receptors and activate downstream signaling pathways (14). Another clinical trial investigated
AG in combination with PEGPH20 in the treatment of advanced PDAC.
Although the combination group showed an increased objective
response rate, it did not show improved OS or progression-free
survival (204). Meanwhile, it
was shown to increase the risk of thromboembolism, which requires
prophylaxis with heparin. Hence, from a number of perspectives,
PEGPH20 is not an optimum treatment choice.
Therapies targeting the matrix can also enhance the
invasion of immune cells in metastases, which can be achieved by
blocking the SDF-1/CXCR4 signaling axis in the case of BC
metastasis (103) (Fig. 4D). Through modifications, some
drugs with direct tissue penetration have also been developed
(205), including lipophilic
liposomes, albumin preparations, water-soluble prodrug preparations
and nanocrystals (206). These
modifications have been shown to enhance the precise delivery of
drugs in the complex ECM environment of metastases.
Directly targeting the up- or downstream pathways
of mCAFs is another method to relieve the drug tolerance of
metastases, which is related to the crosstalk between mCAFs and
metastatic tumor cells (Fig. 4A).
Gene silencing or receptor blocking of IGF2 can effectively inhibit
the promoting effect of mCAFs on the growth of metastatic tumors
(8). CXCR4 is highly expressed in
LMs from BC and CRC and the CXCR4 inhibitor AMD3100 has been shown
to alleviate desmoplasia in metastases (69,103). CXCR4 blocking has also been
observed to sensitize the mBC tumors to immune checkpoint blockers
(103). Similarly, silencing
LRG1, which is highly expressed in LMs, can significantly reduce
tumor migration and invasion (151). IL-1R knockout mice have
demonstrated that IL-1β secreted by tumors can induce bone mCAFs to
secrete SDF-1, promoting bone metastasis and this effect can be
blocked by the IL-1 inhibitor anakinra (207). ECM-CAFs make up a high
proportion in LMs, especially at the center of LMs, and promote
vascular growth and tumor proliferation by secreting LTBP2;
siRNA-mediated silencing of LTBP2 expression can regulate the
phenotype of ECM-CAFs (68).
Tranilast inhibits the production of SDF-1 in the myCAF cell line
LmcMF in a mouse peritoneal metastasis model of gastric cancer,
reducing the infiltration of M2 macrophages and leading to
apoptosis of cancer cells by an immune response (208). However, the LmcMF cell line used
in that study completed peritoneal implantation via intraperitoneal
injection and may not represent the true source of mCAFs in
peritoneal metastases. Although a large number of positive results
have been obtained in laboratory studies, large-scale clinical
trials are needed to provide direct evidence for blocking the
upstream and downstream signals of mCAFs to improve the control
rate of metastases and reveal a new avenue for advanced treatment
in various tumors.
The reason why the present study emphasized the
heterogeneity of pCAF and mCAF is because CAFs are the main
cellular components in ECM and the frequent information exchange
between different 'personalities'. CAFs with tumor cells result in
great differences between metastasic and primary TMEs, ultimately
showing different resistance in treatment. The present study
described these differences in terms of origin, activation
patterns, markers, matreotype, cytokines and transcriptome
profiles.
In fact, there are a number of differences between
pCAFs and mCAFs that may explain the low treatment responsiveness
of metastases and some studies eliminated these differences to
enhance the sensitivity of metastases to treatment options
(8,12). However, one should pay attention
to the fact that differences in the distribution of various CAF
subsets exist even within the primary tumor, the best examples
being the tumor center and the invasion front (124). Furthermore, CAFs have a very
clear stage-dependent heterogeneity and the identity and prevalence
of the various CAF subtypes present in a tumor or metastatic site
change in response to normal, inflammatory, precancerous and
malignant states, including anticancer treatment (18). These characteristics of CAFs also
change as tumor growth progresses (85). The heterogeneity of pCAFs and
mCAFs presented in the present review is only a cross-section at a
certain point in time. Therefore, it is necessary to rely on new
culture methods and observation methods to comprehensively and
clearly describe the succession process and role of CAFs in the
progression of the entire tumor.
Therapies targeting CAFs are currently being
developed, including methods such as blocking ECM deposition and
remodeling, directly targeting tumor-promoting CAFs, or using the
plasticity of CAFs to engineer them into tumor-suppressing
phenotypes (10). Most of these
treatment strategies have failed because the heterogeneity of CAFs
in different cancer types, tumor stages and metastasis sites makes
these treatment methods one-sided (106), requiring a more comprehensive
understanding of the role of CAFs within tumors. Future treatment
options for advanced tumors may not only consider the molecular
type of the tumor but also comprise more elaborate individualized
treatment strategies which consider the heterogeneity of pCAFs and
mCAFs. Some challenging questions lie ahead, such as the criteria
for identifying the heterogeneity between mCAFs and pCAFs.
Additional treatment for metastases will inevitably increase
patient intolerance, so that screening for highly effective,
low-toxicity sensitizers becomes particularly important. In
addition, the timing of metastasis treatment and the choice of
local or systemic treatment still needs to be solved urgently.
In conclusion, considering the biological
heterogeneity of pCAFs and mCAFs, the present study provided a new
perspective on the differential outcomes of primary and metastasis
tumor treatment, revealing their key role in shaping different
TMEs. It also explored possible means to improve the clinical
treatment of metastases, providing new ideas for advanced
anti-tumor treatments.
Not applicable.
CS and QZ conceived and designed the present study.
ZK and CL contributed to data analysis and wrote the manuscript. WZ
was responsible for the figures. LL supervised the study, aided by
QZ and CS. Data authentication is not applicable. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 82305000, 81973677
and 82174222), Natural Science Foundation of Shandong Province
(grant no. ZR2021LZY015), Traditional Chinese Medicine Science and
Technology Project of Shandong Province (grant no. Q-2023205) and
Weifang Science and Technology Development Plan (grant no.
2022GX008).
|
1
|
Garcia-Vicién G, Mezheyeuski A, Bañuls M,
Ruiz-Roig N and Molleví DG: The Tumor microenvironment in liver
metastases from colorectal carcinoma in the context of the
histologic growth patterns. Int J Mol Sci. 22:15442021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo F, Li J, Wu S, Wu X, Chen M, Zhong X
and Liu K: Comparative profiling between primary colorectal
carcinomas and metastases identifies heterogeneity on drug
resistance. Oncotarget. 7:63937–63949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Z, Li Z, Ma Z and Curtis C:
Multi-cancer analysis of clonality and the timing of systemic
spread in paired primary tumors and metastases. Nat Genet.
52:701–708. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korentzelos D, Clark AM and Wells A: A
perspective on therapeutic pan-resistance in metastatic cancer. Int
J Mol Sci. 21:73042020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirata E and Sahai E: Tumor
microenvironment and differential responses to therapy. Cold Spring
Harb Perspect Med. 7:a0267812017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiménez-Sánchez A, Memon D, Pourpe S,
Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O,
Konner J, et al: Heterogeneous tumor-immune microenvironments among
differentially growing metastases in an ovarian cancer patient.
Cell. 170:927–938.e20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gui Y, Aguilar-Mahecha A, Krzemien U,
Hosein A, Buchanan M, Lafleur J, Pollak M, Ferrario C and Basik M:
Metastatic breast carcinoma-associated fibroblasts have enhanced
protumorigenic properties related to increased IGF2 expression.
Clin Cancer Res. 25:7229–7242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar
|
|
11
|
Park D, Sahai E and Rullan A: SnapShot:
Cancer-associated fibroblasts. Cell. 181:486–486.e1. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, Wang X, Lu J, Salfenmoser M,
Wirsik NM, Schleussner N, Imle A, Freire Valls A, Radhakrishnan P,
Liang J, et al: Reduction of liver metastasis stiffness improves
response to bevacizumab in metastatic colorectal cancer. Cancer
Cell. 37:800–817.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ziemys A, Simic V, Milosevic M, Kojic M,
Liu YT and Yokoi K: Attenuated microcirculation in small metastatic
tumors in murine liver. Pharmaceutics. 13:7032021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cox TR: The matrix in cancer. Nat Rev
Cancer. 21:217–238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gertych A, Walts AE, Cheng K, Liu M, John
J, Lester J, Karlan BY and Orsulic S: Dynamic changes in the
extracellular matrix in primary, metastatic, and recurrent ovarian
cancers. Cells. 11:73692022. View Article : Google Scholar
|
|
16
|
Fujimori D, Kinoshita J, Yamaguchi T,
Nakamura Y, Gunjigake K, Ohama T, Sato K, Yamamoto M, Tsukamoto T,
Nomura S, et al: Established fibrous peritoneal metastasis in an
immunocompetent mouse model similar to clinical immune
microenvironment of gastric cancer. BMC Cancer. 20:10142020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Liu J, Huang H, Ye M, Li X, Wu R,
Liu H and Song Y: Metastasis-associated fibroblasts: an emerging
target for metastatic cancer. Biomark Res. 9:472021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biffi G and Tuveson DA: Diversity and
biology of cancer-associated fibroblasts. Physiol Rev. 101:147–176.
2021. View Article : Google Scholar :
|
|
19
|
Miyashita N and Saito A: Organ specificity
and heterogeneity of cancer-associated fibroblasts in colorectal
cancer. Int J Mol Sci. 22:109732021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ganguly D, Chandra R, Karalis J, Teke M,
Aguilera T, Maddipati R, Wachsmann MB, Ghersi D, Siravegna G, Zeh
HJ III, et al: Cancer-associated fibroblasts: versatile players in
the tumor microenvironment. Cancers (Basel). 12:26522020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bu L, Baba H, Yoshida N, Miyake K, Yasuda
T, Uchihara T, Tan P and Ishimoto T: Biological heterogeneity and
versatility of cancer-associated fibroblasts in the tumor
microenvironment. Oncogene. 38:4887–4901. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartoschek M, Oskolkov N, Bocci M, Lövrot
J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson
G, et al: Spatially and functionally distinct subclasses of breast
cancer-associated fibroblasts revealed by single cell RNA
sequencing. Nat Commun. 9:51502018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Potenta S, Zeisberg E and Kalluri R: The
role of endothelial-to-mesenchymal transition in cancer
progression. Br J Cancer. 99:1375–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dulauroy S, Di Carlo SE, Langa F, Eberl G
and Peduto L: Lineage tracing and genetic ablation of ADAM12(+)
perivascular cells identify a major source of profibrotic cells
during acute tissue injury. Nat Med. 18:1262–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rinkevich Y, Mori T, Sahoo D, Xu PX,
Bermingham JR Jr and Weissman IL: Identification and prospective
isolation of a mesothelial precursor lineage giving rise to smooth
muscle cells and fibroblasts for mammalian internal organs, and
their vasculature. Nat Cell Biol. 14:1251–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L
and Yin R: Cancer-associated fibroblasts: An emerging target of
anti-cancer immunotherapy. J Hematol Oncol. 12:862019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bielczyk-Maczynska E: White adipocyte
plasticity in physiology and disease. Cells. 8:15072019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X, He C, Hua X, Kan A, Mao Y, Sun S,
Duan F, Wang J, Huang P and Li S: Oxidative stress induces
monocyte-to-myofibroblast transdifferentiation through p38 in
pancreatic ductal adenocarcinoma. Clin Transl Med. 10:e412020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto G, Taura K, Iwaisako K, Asagiri
M, Ito S, Koyama Y, Tanabe K, Iguchi K, Satoh M, Nishio T, et al:
Pancreatic stellate cells have distinct characteristics from
hepatic stellate cells and are not the unique origin of
collagen-producing cells in the pancreas. Pancreas. 46:1141–1151.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bachem MG, Schünemann M, Ramadani M, Siech
M, Beger H, Buck A, Zhou S, Schmid-Kotsas A and Adler G: Pancreatic
carcinoma cells induce fibrosis by stimulating proliferation and
matrix synthesis of stellate cells. Gastroenterology. 128:907–921.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erez N: Fibroblasts form a hospitable
metastatic niche in the liver. Nat Cell Biol. 18:465–466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhattacharjee S, Hamberger F, Ravichandra
A, Miller M, Nair A, Affo S, Filliol A, Chin L, Savage TM, Yin D,
et al: Tumor restriction by type I collagen opposes tumor-promoting
effects of cancer-associated fibroblasts. J Clin Invest.
131:e1469872021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Omary MB, Lugea A, Lowe AW and Pandol SJ:
The pancreatic stellate cell: A star on the rise in pancreatic
diseases. J Clin Invest. 117:50–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kisseleva T: The origin of fibrogenic
myofibroblasts in fibrotic liver. Hepatology. 65:1039–1043. 2017.
View Article : Google Scholar
|
|
38
|
Klopp AH, Spaeth EL, Dembinski JL,
Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M and Marini FC:
Tumor irradiation increases the recruitment of circulating
mesenchymal stem cells into the tumor microenvironment. Cancer Res.
67:11687–11695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spaeth EL, Dembinski JL, Sasser AK, Watson
K, Klopp A, Hall B, Andreeff M and Marini F: Mesenchymal stem cell
transition to tumor-associated fibroblasts contributes to
fibrovascular network expansion and tumor progression. PLoS One.
4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mi Z, Bhattacharya SD, Kim VM, Guo H,
Talbot LJ and Kuo PC: Osteopontin promotes CCL5-mesenchymal stromal
cell-mediated breast cancer metastasis. Carcinogenesis. 32:477–487.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Raz Y, Cohen N, Shani O, Bell RE,
Novitskiy SV, Abramovitz L, Levy C, Milyavsky M, Leider-Trejo L,
Moses HL, et al: Bone marrow-derived fibroblasts are a functionally
distinct stromal cell population in breast cancer. J Exp Med.
215:3075–3093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Wang H, He J, Yuan X and Sun W:
Rictor ablation in BMSCs inhibits bone metastasis of TM40D cells by
attenuating osteolytic destruction and CAF formation. Int J Biol
Sci. 15:2448–2460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang Z, Zhou J, Li L, Liao S, He J, Zhou
S and Zhou Y: Pericytes in the tumor microenvironment. Cancer Lett.
556:2160742023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hosaka K, Yang Y, Seki T, Fischer C, Dubey
O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y, et al:
Pericyte-fibroblast transition promotes tumor growth and
metastasis. Proc Natl Acad Sci USA. 113:E5618–E5627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sharon Y, Raz Y, Cohen N, Ben-Shmuel A,
Schwartz H, Geiger T and Erez N: Tumor-derived osteopontin
reprograms normal mammary fibroblasts to promote inflammation and
tumor growth in breast cancer. Cancer Res. 75:963–973. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vu LT, Peng B, Zhang DX, Ma V,
Mathey-Andrews CA, Lam CK, Kiomourtzis T, Jin J, McReynolds L,
Huang L, et al: Tumor-secreted extracellular vesicles promote the
activation of cancer-associated fibroblasts via the transfer of
microRNA-125b. J Extracell Vesicles. 8:15996802019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gong Z, Li Q, Shi J, Wei J, Li P, Chang
CH, Shultz LD and Ren G: Lung fibroblasts facilitate pre-metastatic
niche formation by remodeling the local immune microenvironment.
Immunity. 55:1483–1500.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
D'Arcangelo E, Wu NC, Cadavid JL and
McGuigan AP: The life cycle of cancer-associated fibroblasts within
the tumour stroma and its importance in disease outcome. Br J
Cancer. 122:931–942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koliaraki V, Pallangyo CK, Greten FR and
Kollias G: Mesenchymal cells in colon cancer. Gastroenterology.
152:964–979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hawinkels LJAC, Paauwe M, Verspaget HW,
Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman
JHN, Mesker W, ten Dijke P and Sier CFM: Interaction with colon
cancer cells hyperactivates TGF-β signaling in cancer-associated
fibroblasts. Oncogene. 33:97–107. 2014. View Article : Google Scholar
|
|
53
|
Kobayashi H, Gieniec KA, Lannagan TRM,
Wang T, Asai N, Mizutani Y, Iida T, Ando R, Thomas EM, Sakai A, et
al: The origin and contribution of cancer-associated fibroblasts in
colorectal carcinogenesis. Gastroenterology. 162:890–906. 2022.
View Article : Google Scholar
|
|
54
|
Shinagawa K, Kitadai Y, Tanaka M, Sumida
T, Kodama M, Higashi Y, Tanaka S, Yasui W and Chayama K:
Mesenchymal stem cells enhance growth and metastasis of colon
cancer. Int J Cancer. 127:2323–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Flier SN, Tanjore H, Kokkotou EG, Sugimoto
H, Zeisberg M and Kalluri R: Identification of epithelial to
mesenchymal transition as a novel source of fibroblasts in
intestinal fibrosis. J Biol Chem. 285:20202–20212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Apte MV, Park S, Phillips PA, Santucci N,
Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA,
et al: Desmoplastic reaction in pancreatic cancer: Role of
pancreatic stellate cells. Pancreas. 29:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Houg DS and Bijlsma MF: The hepatic
pre-metastatic niche in pancreatic ductal adenocarcinoma. Mol
Cancer. 17:952018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Öhlund D, Handly-Santana A, Biffi G,
Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA,
Lee EJ, et al: Distinct populations of inflammatory fibroblasts and
myofibroblasts in pancreatic cancer. J Exp Med. 214:579–596. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Elyada E, Bolisetty M, Laise P, Flynn WF,
Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS,
et al: Cross-species single-cell analysis of pancreatic ductal
adenocarcinoma reveals antigen-presenting cancer-associated
fibroblasts. Cancer Discov. 9:1102–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsoumakidou M: The advent of immune
stimulating CAFs in cancer. Nat Rev Cancer. 23:258–269. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dominguez CX, Müller S, Keerthivasan S,
Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW,
Castiglioni A, et al: Single-cell RNA sequencing reveals stromal
evolution into LRRC15+ myofibroblasts as a determinant
of patient response to cancer immunotherapy. Cancer Discov.
10:232–253. 2020. View Article : Google Scholar
|
|
62
|
Huang H, Wang Z, Zhang Y, Pradhan RN,
Ganguly D, Chandra R, Murimwa G, Wright S, Gu X, Maddipati R, et
al: Mesothelial cell-derived antigen-presenting cancer-associated
fibroblasts induce expansion of regulatory T cells in pancreatic
cancer. Cancer Cell. 40:656–673.e7. 2022. View Article : Google Scholar :
|
|
63
|
Iwaisako K, Jiang C, Zhang M, Cong M,
Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, et al:
Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl
Acad Sci USA. 111:E3297–E3305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lua I, Li Y, Zagory JA, Wang KS, French
SW, Sévigny J and Asahina K: Characterization of hepatic stellate
cells, portal fibroblasts, and mesothelial cells in normal and
fibrotic livers. J Hepatol. 64:1137–1146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
O'Hara SP and LaRusso NF: Portal
fibroblasts: A renewable source of liver myofibroblasts.
Hepatology. 76:1240–1242. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xie Z, Gao Y, Ho C, Li L, Jin C, Wang X,
Zou C, Mao Y, Wang X, Li Q, et al: Exosome-delivered CD44v6/C1QBP
complex drives pancreatic cancer liver metastasis by promoting
fibrotic liver microenvironment. Gut. 71:568–579. 2022. View Article : Google Scholar
|
|
68
|
Giguelay A, Turtoi E, Khelaf L, Tosato G,
Dadi I, Chastel T, Poul MA, Pratlong M, Nicolescu S, Severac D, et
al: The landscape of cancer-associated fibroblasts in colorectal
cancer liver metastases. Theranostics. 12:7624–7639. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tan HX, Gong WZ, Zhou K, Xiao ZG, Hou FT,
Huang T, Zhang L, Dong HY, Zhang WL, Liu Y and Huang ZC:
CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into
carcinoma-associated fibroblasts and promoted liver metastasis of
colon cancer. Cancer Biol Ther. 21:258–268. 2020. View Article : Google Scholar
|
|
70
|
Mukaida N, Zhang D and Sasaki SI:
Emergence of cancer-associated fibroblasts as an indispensable
cellular player in bone metastasis process. Cancers (Basel).
12:28962020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Houthuijzen JM and de Visser KE: The lung
fibroblast as 'soil fertilizer' in breast cancer metastasis.
Immunity. 55:1336–1339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chang HY, Chi JT, Dudoit S, Bondre C, van
de Rijn M, Botstein D and Brown PO: Diversity, topographic
differentiation, and positional memory in human fibroblasts. Proc
Natl Acad Sci USA. 99:12877–12882. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Apte MV, Wilson JS, Lugea A and Pandol SJ:
A starring role for stellate cells in the pancreatic cancer
microenvironment. Gastroenterology. 144:1210–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hessmann E, Patzak MS, Klein L, Chen N,
Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, et
al: Fibroblast drug scavenging increases intratumoural gemcitabine
accumulation in murine pancreas cancer. Gut. 67:497–507. 2018.
View Article : Google Scholar
|
|
75
|
Helms EJ, Berry MW, Chaw RC, DuFort CC,
Sun D, Onate MK, Oon C, Bhattacharyya S, Sanford-Crane H, Horton W,
et al: Mesenchymal lineage heterogeneity underlies nonredundant
functions of pancreatic cancer-associated fibroblasts. Cancer
Discov. 12:484–501. 2022. View Article : Google Scholar
|
|
76
|
Garcia PE, Adoumie M, Kim EC, Zhang Y,
Scales MK, El-Tawil YS, Shaikh AZ, Wen HJ, Bednar F, Allen BL, et
al: Differential contribution of pancreatic fibroblast subsets to
the pancreatic cancer stroma. Cell Mol Gastroenterol Hepatol.
10:581–599. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Y, Bian Y, Wang Y, Wang Y, Duan X,
Han Y, Zhang L, Wang F, Gu Z and Qin Z: HIF-1α is necessary for
activation and tumour-promotion effect of cancer-associated
fibroblasts in lung cancer. J Cell Mol Med. 25:5457–5469. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shintani Y, Fujiwara A, Kimura T, Kawamura
T, Funaki S, Minami M and Okumura M: IL-6 secreted from
cancer-associated fibroblasts mediates chemoresistance in NSCLC by
increasing epithelial-mesenchymal transition signaling. J Thorac
Oncol. 11:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gottschling S, Granzow M, Kuner R, Jauch
A, Herpel E, Xu EC, Muley T, Schnabel PA, Herth FJ and Meister M:
Mesenchymal stem cells in non-small cell lung cancer-different from
others? Insights from comparative molecular and functional
analyses. Lung Cancer. 80:19–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang H, Shui L, Chen R and Chen Y, Guo J
and Chen Y: Occurrence and prognosis of lung cancer metastasis to
major organs: A population-based study. Eur J Cancer Prev.
32:246–253. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kong J, Tian H, Zhang F, Zhang Z, Li J,
Liu X, Li X, Liu J, Li X, Jin D, et al: Extracellular vesicles of
carcinoma-associated fibroblasts creates a pre-metastatic niche in
the lung through activating fibroblasts. Mol Cancer. 18:1752019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Duda DG, Duyverman AM, Kohno M, Snuderl M,
Steller EJ, Fukumura D and Jain RK: Malignant cells facilitate lung
metastasis by bringing their own soil. Proc Natl Acad Sci USA.
107:21677–21682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Buchsbaum RJ and Oh SY: Breast
cancer-associated fibroblasts: Where We Are And Where We Need To
Go. Cancers (Basel). 8:192016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P,
Zhuo W, Hu Y, Chen C, Chen L, et al: Cancer-associated fibroblasts
in breast cancer: Challenges and opportunities. Cancer Commun
(Lond). 42:401–434. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Friedman G, Levi-Galibov O, David E,
Bornstein C, Giladi A, Dadiani M, Mayo A, Halperin C,
Pevsner-Fischer M, Lavon H, et al: Cancer-associated fibroblast
compositions change with breast cancer progression linking the
ratio of S100A4+ and PDPN+ CAFs to clinical
outcome. Nat Cancer. 1:692–708. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang XHF, Jin X, Malladi S, Zou Y, Wen
YH, Brogi E, Smid M, Foekens JA and Massagué J: Selection of bone
metastasis seeds by mesenchymal signals in the primary tumor
stroma. Cell. 154:1060–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kuo MC, Kothari AN, Kuo PC and Mi Z:
Cancer stemness in bone marrow micrometastases of human breast
cancer. Surgery. 163:330–335. 2018. View Article : Google Scholar
|
|
88
|
Ban J, Fock V, Aryee DNT and Kovar H:
Mechanisms, diagnosis and treatment of bone metastases. Cells.
10:29442021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Haider MT, Smit DJ and Taipaleenmäki H:
The endosteal niche in breast cancer bone metastasis. Front Oncol.
10:3352020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Croucher PI, McDonald MM and Martin TJ:
Bone metastasis: The importance of the neighbourhood. Nat Rev
Cancer. 16:373–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cassell K, Thomas-Lopez D, Kjelsø C and
Uldum S: Provincial trends in Legionnaires' disease are not
explained by population structure in Denmark, 2015 to 2018. Euro
Surveill. 26:20000362021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mundim FGL, Pasini FS, Nonogaki S, Rocha
RM, Soares FA, Brentani MM and Logullo AF: Breast
carcinoma-associated fibroblasts share similar biomarker profiles
in matched lymph node metastasis. Appl Immunohistochem Mol Morphol.
24:712–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Del Valle PR, Milani C, Brentani MM,
Katayama ML, de Lyra EC, Carraro DM, Brentani H, Puga R, Lima LA,
Rozenchan PB, et al: Transcriptional profile of fibroblasts
obtained from the primary site, lymph node and bone marrow of
breast cancer patients. Genet Mol Biol. 37:480–489. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Puram SV, Tirosh I, Parikh AS, Patel AP,
Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, et
al: Single-cell transcriptomic analysis of primary and metastatic
tumor ecosystems in head and neck cancer. Cell. 171:1611–1624.e24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Han L, Wu Y, Fang K, Sweeney S, Roesner
UK, Parrish M, Patel K, Walter T, Piermattei J, Trimboli A, et al:
The splanchnic mesenchyme is the tissue of origin for pancreatic
fibroblasts during homeostasis and tumorigenesis. Nat Commun.
14:12023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Nielsen SR, Quaranta V, Linford A, Emeagi
P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, et al:
Macrophage-secreted granulin supports pancreatic cancer metastasis
by inducing liver fibrosis. Nat Cell Biol. 18:549–560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang C, Wang XY, Zhang P, He TC, Han JH,
Zhang R, Lin J, Fan J, Lu L, Zhu WW, et al: Cancer-derived exosomal
HSPC111 promotes colorectal cancer liver metastasis by
reprogramming lipid metabolism in cancer-associated fibroblasts.
Cell Death Dis. 13:572022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Calon A, Espinet E, Palomo-Ponce S,
Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung
P, Zhang XH, et al: Dependency of colorectal cancer on a
TGF-β-driven program in stromal cells for metastasis initiation.
Cancer Cell. 22:571–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T
and Springer TA: Latent TGF-β structure and activation. Nature.
474:343–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Massagué J: TGFbeta signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar
|
|
101
|
Su J, Morgani SM, David CJ, Wang Q, Er EE,
Huang YH, Basnet H, Zou Y, Shu W, Soni RK, et al: TGF-β
orchestrates fibrogenic and developmental EMTs via the RAS effector
RREB1. Nature. 577:566–571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kojima Y, Acar A, Eaton EN, Mellody KT,
Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg
RA and Orimo A: Autocrine TGF-beta and stromal cell-derived
factor-1 (SDF-1) signaling drives the evolution of tumor-promoting
mammary stromal myofibroblasts. Proc Natl Acad Sci USA.
107:20009–20014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen IX, Chauhan VP, Posada J, Ng MR, Wu
MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R and Jain RK:
Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte
infiltration, and improves immunotherapy in metastatic breast
cancer. Proc Natl Acad Sci USA. 116:4558–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Eckert MA, Coscia F, Chryplewicz A, Chang
JW, Hernandez KM, Pan S, Tienda SM, Nahotko DA, Li G, Blaženović I,
et al: Proteomics reveals NNMT as a master metabolic regulator of
cancer-associated fibroblasts. Nature. 569:723–728. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Biffi G, Oni TE, Spielman B, Hao Y, Elyada
E, Park Y, Preall J and Tuveson DA: IL1-induced JAK/STAT signaling
is antagonized by TGFβ to shape CAF heterogeneity in pancreatic
ductal adenocarcinoma. Cancer Discov. 9:282–301. 2019. View Article : Google Scholar
|
|
106
|
Chen Y, McAndrews KM and Kalluri R:
Clinical and therapeutic relevance of cancer-associated
fibroblasts. Nat Rev Clin Oncol. 18:792–804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Maehira H, Miyake T, Iida H, Tokuda A,
Mori H, Yasukawa D, Mukaisho KI, Shimizu T and Tani M: Vimentin
expression in tumor microenvironment predicts survival in
pancreatic ductal adenocarcinoma: Heterogeneity in fibroblast
population. Ann Surg Oncol. 26:4791–4804. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Paulsson J and Micke P: Prognostic
relevance of cancer-associated fibroblasts in human cancer. Semin
Cancer Biol. 25:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim HM, Jung WH and Koo JS: Expression of
cancer-associated fibroblast related proteins in metastatic breast
cancer: An immunohistochemical analysis. J Transl Med. 13:2222015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Koerber SA, Staudinger F, Kratochwil C,
Adeberg S, Haefner MF, Ungerechts G, Rathke H, Winter E, Lindner T,
Syed M, et al: The role of 68Ga-FAPI PET/CT for patients
with malignancies of the lower gastrointestinal tract: First
clinical experience. J Nucl Med. 61:1331–1336. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Altmann A, Haberkorn U and Siveke J: The
latest developments in imaging of fibroblast activation protein. J
Nucl Med. 62:160–167. 2021. View Article : Google Scholar
|
|
112
|
Imlimthan S, Moon ES, Rathke H,
Afshar-Oromieh A, Rösch F, Rominger A and Gourni E: New frontiers
in cancer imaging and therapy based on radiolabeled fibroblast
activation protein inhibitors: A rational review and current
progress. Pharmaceuticals (Basel). 14:10232021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhao L, Chen J, Pang Y, Fu K, Shang Q, Wu
H, Sun L, Lin Q and Chen H: Fibroblast activation protein-based
theranostics in cancer research: A state-of-the-art review.
Theranostics. 12:1557–1569. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zubaľ M, Výmolová B, Matrasová I, Výmola
P, Vepřková J, Syrůček M, Tomáš R, Vaníčková Z, Křepela E, Konečná
D, et al: Fibroblast activation protein as a potential theranostic
target in brain metastases of diverse solid tumours. Pathology.
55:806–817. 2023. View Article : Google Scholar
|
|
115
|
Peltier A, Seban RD, Buvat I, Bidard FC
and Mechta-Grigoriou F: Fibroblast heterogeneity in solid tumors:
From single cell analysis to whole-body imaging. Semin Cancer Biol.
86:262–272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Taralli S, Lorusso M, Perrone E, Perotti
G, Zagaria L and Calcagni ML: PET/CT with fibroblast activation
protein inhibitors in breast cancer: Diagnostic and theranostic
application-A literature review. Cancers (Basel). 15:9082023.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dong Y, Zhou H, Alhaskawi A, Wang Z, Lai
J, Yao C, Liu Z, Hasan Abdullah Ezzi S, Goutham Kota V, Hasan
Abdulla Hasan Abdulla M and Lu H: The superiority of fibroblast
activation protein inhibitor (FAPI) PET/CT versus FDG PET/CT in the
diagnosis of various malignancies. Cancers (Basel). 15:11932023.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Serfling S, Zhi Y, Schirbel A, Lindner T,
Meyer T, Gerhard-Hartmann E, Lapa C, Hagen R, Hackenberg S, Buck AK
and Scherzad A: Improved cancer detection in Waldeyer's tonsillar
ring by 68Ga-FAPI PET/CT imaging. Eur J Nucl Med Mol
Imaging. 48:1178–1187. 2021. View Article : Google Scholar
|
|
119
|
Mona CE, Benz MR, Hikmat F, Grogan TR,
Lueckerath K, Razmaria A, Riahi R, Slavik R, Girgis MD, Carlucci G,
et al: Correlation of 68Ga-FAPi-46 PET biodistribution
with FAP expression by immunohistochemistry in patients with solid
cancers: Interim analysis of a prospective translational
exploratory study. J Nucl Med. 63:1021–1026. 2022. View Article : Google Scholar :
|
|
120
|
Sollini M, Kirienko M, Gelardi F, Fiz F,
Gozzi N and Chiti A: State-of-the-art of FAPI-PET imaging: A
systematic review and meta-analysis. Eur J Nucl Med Mol Imaging.
48:4396–4414. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yazdani S, Bansal R and Prakash J: Drug
targeting to myofibroblasts: Implications for fibrosis and cancer.
Adv Drug Deliv Rev. 121:101–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Becker LM, O'Connell JT, Vo AP, Cain MP,
Tampe D, Bizarro L, Sugimoto H, McGow AK, Asara JM, Lovisa S, et
al: Epigenetic reprogramming of cancer-associated fibroblasts
deregulates glucose metabolism and facilitates progression of
breast cancer. Cell Rep. 31:1077012020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Muchlińska A, Nagel A, Popęda M, Szade J,
Niemira M, Zieliński J, Skokowski J, Bednarz-Knoll N and Żaczek AJ:
Alpha-smooth muscle actin-positive cancer-associated fibroblasts
secreting osteopontin promote growth of luminal breast cancer. Cell
Mol Biol Lett. 27:452022. View Article : Google Scholar
|
|
124
|
Kwak Y, Lee HE, Kim WH, Kim DW, Kang SB
and Lee HS: The clinical implication of cancer-associated
microvasculature and fibroblast in advanced colorectal cancer
patients with synchronous or metachronous metastases. PLoS One.
9:e918112014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Itou RA, Uyama N, Hirota S, Kawada N, Wu
S, Miyashita S, Nakamura I, Suzumura K, Sueoka H, Okada T, et al:
Immunohistochemical characterization of cancer-associated
fibroblasts at the primary sites and in the metastatic lymph nodes
of human intrahepatic cholangiocarcinoma. Hum Pathol. 83:77–89.
2019. View Article : Google Scholar
|
|
126
|
Borriello L, Nakata R, Sheard MA,
Fernandez GE, Sposto R, Malvar J, Blavier L, Shimada H, Asgharzadeh
S, Seeger RC and DeClerck YA: Cancer-associated fibroblasts share
characteristics and protumorigenic activity with mesenchymal
stromal cells. Cancer Res. 77:5142–5157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang J, Chen L, Liu X, Kammertoens T,
Blankenstein T and Qin Z: Fibroblast-specific protein
1/S100A4-positive cells prevent carcinoma through collagen
production and encapsulation of carcinogens. Cancer Res.
73:2770–2781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Shindo K, Aishima S, Ohuchida K, Fujiwara
K, Fujino M, Mizuuchi Y, Hattori M, Mizumoto K, Tanaka M and Oda Y:
Podoplanin expression in cancer-associated fibroblasts enhances
tumor progression of invasive ductal carcinoma of the pancreas. Mol
Cancer. 12:1682013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kubouchi Y, Yurugi Y, Wakahara M, Sakabe
T, Haruki T, Nosaka K, Miwa K, Araki K, Taniguchi Y, Shiomi T, et
al: Podoplanin expression in cancer-associated fibroblasts predicts
unfavourable prognosis in patients with pathological stage IA lung
adenocarcinoma. Histopathology. 72:490–499. 2018. View Article : Google Scholar
|
|
130
|
Zhou Q, Wang Z, Zeng H, Zhang H, Liu Z,
Huang Q, Wang J, Chang Y, Bai Q, Liu L, et al: Identification and
validation of poor prognosis immunoevasive subtype of
muscle-invasive bladder cancer with tumor-infiltrating
podoplanin+ cell abundance. Oncoimmunology.
9:17473332020. View Article : Google Scholar
|
|
131
|
Wang P, Song L, Ge H, Jin P, Jiang Y, Hu W
and Geng N: Crenolanib, a PDGFR inhibitor, suppresses lung cancer
cell proliferation and inhibits tumor growth in vivo. Onco Targets
Ther. 7:1761–1768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Matsuwaki R, Ishii G, Zenke Y, Neri S,
Aokage K, Hishida T, Yoshida J, Fujii S, Kondo H, Goya T, et al:
Immunophenotypic features of metastatic lymph node tumors to
predict recurrence in N2 lung squamous cell carcinoma. Cancer Sci.
105:905–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lavie D, Ben-Shmuel A, Erez N and
Scherz-Shouval R: Cancer-associated fibroblasts in the single-cell
era. Nat Cancer. 3:793–807. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang X, Zhu R, Yu D, Wang J, Yan Y and Xu
K: Single-cell RNA sequencing to explore cancer-associated
fibroblasts heterogeneity: 'Single' vision for 'heterogeneous'
environment. Cell Prolif. e135922023.Epub ahead of print.
View Article : Google Scholar
|
|
135
|
Li C, Wu H, Guo L, Liu D, Yang S, Li S and
Hua K: Single-cell transcriptomics reveals cellular heterogeneity
and molecular stratification of cervical cancer. Commun Biol.
5:12082022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B,
Li X, Zhou W, Li J, Li Z, et al: Single-cell RNA sequencing reveals
a pro-invasive cancer-associated fibroblast subgroup associated
with poor clinical outcomes in patients with gastric cancer.
Theranostics. 12:620–638. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Guo W, Zhou B, Yang Z, Liu X, Huai Q, Guo
L, Xue X, Tan F, Li Y, Xue Q, et al: Integrating microarray-based
spatial transcriptomics and single-cell RNA-sequencing reveals
tissue architecture in esophageal squamous cell carcinoma.
EBioMedicine. 84:1042812022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Buechler MB, Pradhan RN, Krishnamurty AT,
Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien R,
Roose-Girma M, et al: Cross-tissue organization of the fibroblast
lineage. Nature. 593:575–579. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Garcia-Recio S, Hinoue T, Wheeler GL,
Kelly BJ, Garrido-Castro AC, Pascual T, De Cubas AA, Xia Y,
Felsheim BM, McClure MB, et al: Multiomics in primary and
metastatic breast tumors from the AURORA US network finds
microenvironment and epigenetic drivers of metastasis. Nat Cancer.
4:128–147. 2023.
|
|
140
|
Liu S, Suhail Y, Novin A, Perpetua L and
Kshitiz: Metastatic transition of pancreatic ductal cell
adenocarcinoma is accompanied by the emergence of pro-invasive
cancer-associated fibroblasts. Cancers (Basel). 14:21972022.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Hill M and Tran N: MicroRNAs regulating
MicroRNAs in cancer. Trends Cancer. 4:465–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Vivacqua A, Muoio MG, Miglietta AM and
Maggiolini M: Differential MicroRNA landscape triggered by
estrogens in cancer associated fibroblasts (CAFs) of primary and
metastatic breast tumors. Cancers (Basel). 11:4122019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lee KS, Nam SK, Koh J, Kim DW, Kang SB,
Choe G, Kim WH and Lee HS: Stromal expression of MicroRNA-21 in
advanced colorectal cancer patients with distant metastases. J
Pathol Transl Med. 50:270–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Han Q, Tan S, Gong L, Li G, Wu Q, Chen L,
Du S, Li W, Liu X, Cai J and Wang Z: Omental cancer-associated
fibroblast-derived exosomes with low microRNA-29c-3p promote
ovarian cancer peritoneal metastasis. Cancer Sci. 114:1929–1942.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Alsayed RKME, Khan AQ, Ahmad F, Ansari AW,
Alam MA, Buddenkotte J, Steinhoff M, Uddin S and Ahmad A:
Epigenetic regulation of CXCR4 signaling in cancer pathogenesis and
progression. Semin Cancer Biol. 86:697–708. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Petit I, Jin D and Rafii S: The
SDF-1-CXCR4 signaling pathway: A molecular hub modulating
neo-angiogenesis. Trends Immunol. 28:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shi Y, Riese DJ II and Shen J: The role of
the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol.
11:5746672020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Dai JM, Sun K, Li C, Cheng M, Guan JH,
Yang LN and Zhang LW: Cancer-associated fibroblasts contribute to
cancer metastasis and apoptosis resistance in human ovarian cancer
via paracrine SDF-1α. Clin Transl Oncol. 25:1606–1616. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: miR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhong B, Cheng B, Huang X, Xiao Q, Niu Z,
Chen YF, Yu Q, Wang W and Wu XJ: Colorectal cancer-associated
fibroblasts promote metastasis by up-regulating LRG1 through
stromal IL-6/STAT3 signaling. Cell Death Dis. 13:162021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Xie H, Lei Y, Mao Y, Lan J, Yang J, Quan H
and Zhang T: FK866 inhibits colorectal cancer metastasis by
reducing NAD+ levels in cancer-associated fibroblasts.
Genes Genomics. 44:1531–1541. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Walterskirchen N, Müller C, Ramos C,
Zeindl S, Stang S, Herzog D, Sachet M, Schimek V, Unger L,
Gerakopoulos V, et al: Metastatic colorectal carcinoma-associated
fibroblasts have immunosuppressive properties related to increased
IGFBP2 expression. Cancer Lett. 540:2157372022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Mukherjee S, Sakpal A, Mehrotra M, Phadte
P, Rekhi B and Ray P: Homo and heterotypic cellular cross-talk in
epithelial ovarian cancer impart pro-tumorigenic properties through
differential activation of the notch3 pathway. Cancers (Basel).
14:33652022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Pan X, Zhou J, Xiao Q, Fujiwara K, Zhang
M, Mo G, Gong W and Zheng L: Cancer-associated fibroblast
heterogeneity is associated with organ-specific metastasis in
pancreatic ductal adenocarcinoma. J Hematol Oncol. 14:1842021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Muhl L, Genové G, Leptidis S, Liu J, He L,
Mocci G, Sun Y, Gustafsson S, Buyandelger B, Chivukula IV, et al:
Single-cell analysis uncovers fibroblast heterogeneity and criteria
for fibroblast and mural cell identification and discrimination.
Nat Commun. 11:39532020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ewald CY: The matrisome during aging and
longevity: A systems-level approach toward defining matreotypes
promoting healthy aging. Gerontology. 66:266–274. 2020. View Article : Google Scholar
|
|
158
|
Rhim AD, Oberstein PE, Thomas DH, Mirek
ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP,
Tattersall IW, et al: Stromal elements act to restrain, rather than
support, pancreatic ductal adenocarcinoma. Cancer Cell. 25:735–747.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Boyd LNC, Andini KD, Peters GJ, Kazemier G
and Giovannetti E: Heterogeneity and plasticity of
cancer-associated fibroblasts in the pancreatic tumor
microenvironment. Semin Cancer Biol. 82:184–196. 2022. View Article : Google Scholar
|
|
160
|
von Ahrens D, Bhagat TD, Nagrath D, Maitra
A and Verma A: The role of stromal cancer-associated fibroblasts in
pancreatic cancer. J Hematol Oncol. 10:762017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Deasy SK and Erez N: A glitch in the
matrix: organ-specific matrisomes in metastatic niches. Trends Cell
Biol. 32:110–123. 2022. View Article : Google Scholar
|
|
162
|
Elia I, Rossi M, Stegen S, Broekaert D,
Doglioni G, van Gorsel M, Boon R, Escalona-Noguero C, Torrekens S,
Verfaillie C, et al: Breast cancer cells rely on environmental
pyruvate to shape the metastatic niche. Nature. 568:117–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yuzhalin AE, Gordon-Weeks AN, Tognoli ML,
Jones K, Markelc B, Konietzny R, Fischer R, Muth A, O'Neill E,
Thompson PR, et al: Colorectal cancer liver metastatic growth
depends on PAD4-driven citrullination of the extracellular matrix.
Nat Commun. 9:47832018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Levental KR, Yu H, Kass L, Lakins JN,
Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et
al: Matrix crosslinking forces tumor progression by enhancing
integrin signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Er EE, Valiente M, Ganesh K, Zou Y,
Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, et al:
Pericyte-like spreading by disseminated cancer cells activates YAP
and MRTF for metastatic colonization. Nat Cell Biol. 20:966–978.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Che LH, Liu JW, Huo JP, Luo R, Xu RM, He
C, Li YQ, Zhou AJ, Huang P, Chen YY, et al: A single-cell atlas of
liver metastases of colorectal cancer reveals reprogramming of the
tumor microenvironment in response to preoperative chemotherapy.
Cell Discov. 7:802021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Whatcott CJ, Diep CH, Jiang P, Watanabe A,
LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD and Han H:
Desmoplasia in primary tumors and metastatic lesions of pancreatic
cancer. Clin Cancer Res. 21:3561–3568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Placencio-Hickok VR, Lauzon M, Moshayedi
N, Guan M, Kim S, Nissen N, Lo S, Pandol S, Larson BK, Gong J, et
al: Hyaluronan heterogeneity in pancreatic ductal adenocarcinoma:
Primary tumors compared to sites of metastasis. Pancreatology.
22:92–97. 2022. View Article : Google Scholar
|
|
169
|
Ueno H, Sekine S, Oshiro T, Kanemitsu Y,
Hamaguchi T, Shida D, Takashima A, Ishiguro M, Ito E, Hashiguchi Y,
et al: Disentangling the prognostic heterogeneity of stage III
colorectal cancer through histologic stromal categorization.
Surgery. 163:777–783. 2018. View Article : Google Scholar
|
|
170
|
Ao T, Kajiwara Y, Yonemura K, Shinto E,
Mochizuki S, Okamoto K, Aosasa S and Ueno H: Prognostic
significance of histological categorization of desmoplastic
reaction in colorectal liver metastases. Virchows Arch.
475:341–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Ao T, Kajiwara Y, Yonemura K, Shinto E,
Mochizuki S, Okamoto K, Kishi Y and Ueno H: Morphological
consistency of desmoplastic reactions between the primary
colorectal cancer lesion and associated metastatic lesions.
Virchows Arch. 477:47–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Mayorca-Guiliani AE, Madsen CD, Cox TR,
Horton ER, Venning FA and Erler JT: ISDoT: In situ
decellularization of tissues for high-resolution imaging and
proteomic analysis of native extracellular matrix. Nat Med.
23:890–898. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Yang H, Sun B, Fan L, Ma W, Xu K, Hall
SRR, Wang Z, Schmid RA, Peng RW, Marti TM, et al: Multi-scale
integrative analyses identify THBS2+ cancer-associated
fibroblasts as a key orchestrator promoting aggressiveness in
early-stage lung adenocarcinoma. Theranostics. 12:3104–3130. 2022.
View Article : Google Scholar :
|
|
174
|
Irum B, Asif M, Mumtaz B and Aslam N:
Effect of dental proximal restorations on periodontal health in
patients. J Ayub Med Coll Abbottabad. 34(Suppl 1): S987–S990. 2022.
View Article : Google Scholar
|
|
175
|
Machado RB, Aguiar LMS and Silva JMC:
Brazil: Plan for zero vegetation loss in the Cerrado. Nature.
615:2162023. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Belfiore A, Rapicavoli RV, Le Moli R,
Lappano R, Morrione A, De Francesco EM and Vella V: IGF2: A role in
metastasis and tumor evasion from immune surveillance?
Biomedicines. 11:2292023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Chhabra Y and Weeraratna AT: Fibroblasts
in cancer: Unity in heterogeneity. Cell. 186:1580–1609. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Minchinton AI and Tannock IF: Drug
penetration in solid tumours. Nat Rev Cancer. 6:583–592. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wong L, Kumar A, Gabela-Zuniga B, Chua J,
Singh G, Happe CL, Engler AJ, Fan Y and McCloskey KE: Substrate
stiffness directs diverging vascular fates. Acta Biomater.
96:321–329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Engler AJ, Sen S, Sweeney HL and Discher
DE: Matrix elasticity directs stem cell lineage specification.
Cell. 126:677–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ruehle MA, Eastburn EA, LaBelle SA,
Krishnan L, Weiss JA, Boerckel JD, Wood LB, Guldberg RE and Willett
NJ: Extracellular matrix compression temporally regulates
microvascular angiogenesis. Sci Adv. 6:eabb63512020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Jing X, Yang F, Shao C, Wei K, Xie M, Shen
H and Shu Y: Role of hypoxia in cancer therapy by regulating the
tumor microenvironment. Mol Cancer. 18:1572019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Alvarez R, Musteanu M, Garcia-Garcia E,
Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A,
Rodriguez-Pascual J, et al: Stromal disrupting effects of
nab-paclitaxel in pancreatic cancer. Br J Cancer. 109:926–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Kim H, Samuel S, Lopez-Casas P, Grizzle W,
Hidalgo M, Kovar J, Oelschlager D, Zinn K, Warram J and Buchsbaum
D: SPARC-independent delivery of nab-paclitaxel without depleting
tumor stroma in patient-derived pancreatic cancer xenografts. Mol
Cancer Ther. 15:680–688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Zhang X, Zeng Y, Zhao L, Xu Q, Miao D and
Yu F: Targeting hepatic stellate cell death to reverse hepatic
fibrosis. Curr Drug Targets. 24:568–583. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Jin H, Lian N, Zhang F, Chen L, Chen Q, Lu
C, Bian M, Shao J, Wu L and Zheng S: Activation of PPARγ/P53
signaling is required for curcumin to induce hepatic stellate cell
senescence. Cell Death Dis. 7:e21892016. View Article : Google Scholar
|
|
189
|
Xia S, Liu Z, Cai J, Ren H, Li Q, Zhang H,
Yue J, Zhou Q, Zhou T, Wang L, et al: Liver fibrosis therapy based
on biomimetic nanoparticles which deplete activated hepatic
stellate cells. J Control Release. 355:54–67. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zi F, He J, He D, Li Y, Yang L and Cai Z:
Fibroblast activation protein alpha in tumor microenvironment:
Recent progression and implications (review). Mol Med Rep.
11:3203–3211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Bughda R, Dimou P, D'Souza RR and
Klampatsa A: Fibroblast activation protein (FAP)-targeted CAR-T
cells: Launching an attack on tumor stroma. Immunotargets Ther.
10:313–323. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Claus C, Ferrara C, Xu W, Sam J, Lang S,
Uhlenbrock F, Albrecht R, Herter S, Schlenker R, Hüsser T, et al:
Tumor-targeted 4-1BB agonists for combination with T cell
bispecific antibodies as off-the-shelf therapy. Sci Transl Med.
11:eaav59892019. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Melero I, Tanos T, Bustamante M, Sanmamed
MF, Calvo E, Moreno I, Moreno V, Hernandez T, Martinez Garcia M,
Rodriguez-Vida A, et al: A first-in-human study of the fibroblast
activation protein-targeted, 4-1BB agonist RO7122290 in patients
with advanced solid tumors. Sci Transl Med. 15:eabp92292023.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Ballal S, Yadav MP, Kramer V, Moon ES,
Roesch F, Tripathi M, Mallick S, ArunRaj ST and Bal C: A
theranostic approach of [68Ga]Ga-DOTA.SA.FAPi
PET/CT-guided [177Lu]Lu-DOTA. SA.FAPi radionuclide
therapy in an end-stage breast cancer patient: New frontier in
targeted radionuclide therapy. Eur J Nucl Med Mol Imaging.
48:942–944. 2021. View Article : Google Scholar
|
|
195
|
Assadi M, Rekabpour SJ, Jafari E, Divband
G, Nikkholgh B, Amini H, Kamali H, Ebrahimi S, Shakibazad N, Jokar
N, et al: Feasibility and therapeutic potential of 177Lu-fibroblast
activation protein inhibitor-46 for patients with relapsed or
refractory cancers: A preliminary study. Clin Nucl Med.
46:e523–e530. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Baum RP, Schuchardt C, Singh A,
Chantadisai M, Robiller FC, Zhang J, Mueller D, Eismant A, Almaguel
F, Zboralski D, et al: Feasibility, biodistribution, and
preliminary dosimetry in peptide-targeted radionuclide therapy of
diverse adenocarcinomas using 177Lu-FAP-2286:
First-in-humans results. J Nucl Med. 63:415–423. 2022. View Article : Google Scholar :
|
|
197
|
Vallet SD and Ricard-Blum S: Lysyl
oxidases: from enzyme activity to extracellular matrix cross-links.
Essays Biochem. 63:349–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Granzow M, Schierwagen R, Klein S,
Kowallick B, Huss S, Linhart M, Mazar IG, Görtzen J, Vogt A,
Schildberg FA, et al: Angiotensin-II type 1 receptor-mediated Janus
kinase 2 activation induces liver fibrosis. Hepatology. 60:334–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Takiguchi T, Takahashi-Yanaga F, Ishikane
S, Tetsuo F, Hosoda H, Arioka M, Kitazono T and Sasaguri T:
Angiotensin II promotes primary tumor growth and metastasis
formation of murine TNBC 4T1 cells through the fibroblasts around
cancer cells. Eur J Pharmacol. 909:1744152021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang
W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, et al:
Total neoadjuvant therapy with FOLFIRINOX in combination with
losartan followed by chemoradiotherapy for locally advanced
pancreatic cancer: A phase 2 clinical trial. JAMA Oncol.
5:1020–1027. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Wong KM, Horton KJ, Coveler AL, Hingorani
SR and Harris WP: Targeting the tumor stroma: The biology and
clinical development of pegylated recombinant human hyaluronidase
(PEGPH20). Curr Oncol Rep. 19:472017. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kudo D, Suto A and Hakamada K: The
development of a novel therapeutic strategy to target hyaluronan in
the extracellular matrix of pancreatic ductal adenocarcinoma. Int J
Mol Sci. 18:6002017. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Ramanathan RK, McDonough SL, Philip PA,
Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields
AF, Behl D, et al: Phase IB/II randomized study of FOLFIRINOX plus
pegylated recombinant human hyaluronidase versus FOLFIRINOX alone
in patients with metastatic pancreatic adenocarcinoma: SWOG S1313.
J Clin Oncol. 37:1062–1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Van Cutsem E, Tempero MA, Sigal D, Oh DY,
Fazio N, Macarulla T, Hitre E, Hammel P, Hendifar AE, Bates SE, et
al: Randomized phase III trial of pegvorhyaluronidase alfa with
nab-paclitaxel plus gemcitabine for patients with hyaluronan-high
metastatic pancreatic adenocarcinoma. J Clin Oncol. 38:3185–3194.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Sakers A, De Siqueira MK, Seale P and
Villanueva CJ: Adipose-tissue plasticity in health and disease.
Cell. 185:419–446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Koudelka S and Turánek J: Liposomal
paclitaxel formulations. J Control Release. 163:322–334. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Shahriari K, Shen F, Worrede-Mahdi A, Liu
Q, Gong Y, Garcia FU and Fatatis A: Cooperation among heterogeneous
prostate cancer cells in the bone metastatic niche. Oncogene.
36:2846–2856. 2017. View Article : Google Scholar :
|
|
208
|
Nakamura Y, Kinoshita J, Yamaguchi T, Aoki
T, Saito H, Hamabe-Horiike T, Harada S, Nomura S, Inaki N and
Fushida S: Crosstalk between cancer-associated fibroblasts and
immune cells in peritoneal metastasis: Inhibition in the migration
of M2 macrophages and mast cells by Tranilast. Gastric Cancer.
25:515–526. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Suetsugu A, Osawa Y, Nagaki M, Saji S,
Moriwaki H, Bouvet M and Hoffman RM: Imaging the recruitment of
cancer-associated fibroblasts by liver-metastatic colon cancer. J
Cell Biochem. 112:949–953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra
A, Joseph J, Berry JE, McGee S, Lee E, Sun H, et al: Recruitment of
mesenchymal stem cells into prostate tumours promotes metastasis.
Nat Commun. 4:17952013. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Kidd S, Spaeth E, Watson K, Burks J, Lu H,
Klopp A, Andreeff M and Marini FC: Origins of the tumor
microenvironment: Quantitative assessment of adipose-derived and
bone marrow-derived stroma. PLoS One. 7:e305632012. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Tang H, Chu Y, Huang Z, Cai J and Wang Z:
The metastatic phenotype shift toward myofibroblast of
adipose-derived mesenchymal stem cells promotes ovarian cancer
progression. Carcinogenesis. 41:182–193. 2020. View Article : Google Scholar
|
|
213
|
Cho JA, Park H, Lim EH and Lee KW:
Exosomes from breast cancer cells can convert adipose
tissue-derived mesenchymal stem cells into myofibroblast-like
cells. Int J Oncol. 40:130–138. 2012.
|
|
214
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Zhang J, Sun D, Fu Q, Cao Q, Zhang H and
Zhang K: Bone mesenchymal stem cells differentiate into
myofibroblasts in the tumor microenvironment. Oncol Lett.
12:644–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Bochet L, Lehuédé C, Dauvillier S, Wang
YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le
Gonidec S, et al: Adipocyte-derived fibroblasts promote tumor
progression and contribute to the desmoplastic reaction in breast
cancer. Cancer Res. 73:5657–5668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Jotzu C, Alt E, Welte G, Li J, Hennessy
BT, Devarajan E, Krishnappa S, Pinilla S, Droll L and Song YH:
Adipose tissue derived stem cells differentiate into
carcinoma-associated fibroblast-like cells under the influence of
tumor derived factors. Cell Oncol (Dordr). 34:55–67. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Baroni S, Romero-Cordoba S, Plantamura I,
Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini
A, Daidone MG and Iorio MV: Exosome-mediated delivery of miR-9
induces cancer-associated fibroblast-like properties in human
breast fibroblasts. Cell Death Dis. 7:e23122016. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Arina A, Idel C, Hyjek EM, Alegre ML, Wang
Y, Bindokas VP, Weichselbaum RR and Schreiber H: Tumor-associated
fibroblasts predominantly come from local and not circulating
precursors. Proc Natl Acad Sci USA. 113:7551–7556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Vicent S, Sayles LC, Vaka D, Khatri P,
Gevaert O, Chen R, Zheng Y, Gillespie AK, Clarke N, Xu Y, et al:
Cross-species functional analysis of cancer-associated fibroblasts
identifies a critical role for CLCF1 and IL-6 in non-small cell
lung cancer in vivo. Cancer Res. 72:5744–5756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Nair N, Calle AS, Zahra MH, Prieto-Vila M,
Oo AKK, Hurley L, Vaidyanath A, Seno A, Masuda J, Iwasaki Y, et al:
A cancer stem cell model as the point of origin of
cancer-associated fibroblasts in tumor microenvironment. Sci Rep.
7:68382017. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Sandoval P, Jiménez-Heffernan JA,
Rynne-Vidal Á, Pérez-Lozano ML, Gilsanz Á, Ruiz-Carpio V, Reyes R,
García-Bordas J, Stamatakis K, Dotor J, et al: Carcinoma-associated
fibroblasts derive from mesothelial cells via
mesothelial-to-mesenchymal transition in peritoneal metastasis. J
Pathol. 231:517–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Xin Y, Li K, Yang M and Tan Y: Fluid shear
stress induces EMT of circulating tumor cells via JNK signaling in
favor of their survival during hematogenous dissemination. Int J
Mol Sci. 21:81152020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Kan T, Wang W, Ip PP, Zhou S, Wong AS,
Wang X and Yang M: Single-cell EMT-related transcriptional analysis
revealed intra-cluster heterogeneity of tumor cell clusters in
epithelial ovarian cancer ascites. Oncogene. 39:4227–4240. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Yoshimura Y, Suzuki D and Miyahara K:
Measurement accuracy of fat and iron deposits in the liver using
1H-MRS (HISTO). Nihon Hoshasen Gijutsu Gakkai Zasshi.
74:148–153. 2018.In Japanese. View Article : Google Scholar
|
|
227
|
Ringuette Goulet C, Bernard G, Tremblay S,
Chabaud S, Bolduc S and Pouliot F: Exosomes induce fibroblast
differentiation into cancer-associated fibroblasts through TGFbeta
signaling. Mol Cancer Res. 16:1196–1204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Zhang J, Fu L, Yasuda-Yoshihara N,
Yonemura A, Wei F, Bu L, Hu X, Akiyama T, Kitamura F, Yasuda T, et
al: IL-1β derived from mixed-polarized macrophages activates
fibroblasts and synergistically forms a cancer-promoting
microenvironment. Gastric Cancer. 26:187–202. 2023. View Article : Google Scholar
|
|
229
|
Wei LY, Lee JJ, Yeh CY, Yang CJ, Kok SH,
Ko JY, Tsai FC and Chia JS: Reciprocal activation of
cancer-associated fibroblasts and oral squamous carcinoma cells
through CXCL1. Oral Oncol. 88:115–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Goulet CR, Champagne A, Bernard G, Vandal
D, Chabaud S, Pouliot F and Bolduc S: Cancer-associated fibroblasts
induce epithelial-mesenchymal transition of bladder cancer cells
through paracrine IL-6 signalling. BMC Cancer. 19:1372019.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Scognamiglio I, Cocca L, Puoti I, Palma F,
Ingenito F, Quintavalle C, Affinito A, Roscigno G, Nuzzo S,
Chianese RV, et al: Exosomal microRNAs synergistically trigger
stromal fibroblasts in breast cancer. Mol Ther Nucleic Acids.
28:17–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Qin X, Lu M, Li G, Zhou Y and Liu Z:
Downregulation of tumor-derived exosomal miR-34c induces
cancer-associated fibroblast activation to promote
cholangiocarcinoma progress. Cancer Cell Int. 21:3732021.
View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Zhou X, Yan T, Huang C, Xu Z, Wang L,
Jiang E, Wang H, Chen Y, Liu K, Shao Z and Shang Z: Melanoma
cell-secreted exosomal miR-155-5p induce proangiogenic switch of
cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling
pathway. J Exp Clin Cancer Res. 37:2422018. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Ye B, Duan Y, Zhou M, Wang Y, Lai Q, Yue
K, Cao J, Wu Y, Wang X and Jing C: Hypoxic tumor-derived exosomal
miR-21 induces cancer-associated fibroblast activation to promote
head and neck squamous cell carcinoma metastasis. Cell Signal.
108:1107252023. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Xu Y, Kuai R, Chu YM, Zhou L, Zhang HQ and
Li J: Hypoxia facilitates the proliferation of colorectal cancer
cells by inducing cancer-associated fibroblast-derived IL6.
Neoplasma. 68:1015–1022. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Butti R, Nimma R, Kundu G, Bulbule A,
Kumar TVS, Gunasekaran VP, Tomar D, Kumar D, Mane A, Gill SS, et
al: Tumor-derived osteopontin drives the resident fibroblast to
myofibroblast differentiation through Twist1 to promote breast
cancer progression. Oncogene. 40:2002–2017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G and Sahai E: Mechanotransduction and YAP-dependent
matrix remodelling is required for the generation and maintenance
of cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Foster CT, Gualdrini F and Treisman R:
Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in
cancer-associated fibroblasts is indirect and mediated by
cytoskeletal dynamics. Genes Dev. 31:2361–2375. 2017. View Article : Google Scholar
|
|
239
|
Cadamuro M, Nardo G, Indraccolo S,
Dall'olmo L, Sambado L, Moserle L, Franceschet I, Colledan M,
Massani M, Stecca T, et al: Platelet-derived growth factor-D and
Rho GTPases regulate recruitment of cancer-associated fibroblasts
in cholangiocarcinoma. Hepatology. 58:1042–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Pietras K, Pahler J, Bergers G and Hanahan
D: Functions of paracrine PDGF signaling in the proangiogenic tumor
stroma revealed by pharmacological targeting. PLoS Med. 5:e192008.
View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Scherz-Shouval R, Santagata S, Mendillo
ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M,
Stemmer SM, Whitesell L and Lindquist S: The reprogramming of tumor
stroma by HSF1 is a potent enabler of malignancy. Cell.
158:564–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Ferrari N, Ranftl R, Chicherova I, Slaven
ND, Moeendarbary E, Farrugia AJ, Lam M, Semiannikova M, Westergaard
MCW, Tchou J, et al: Dickkopf-3 links HSF1 and YAP/TAZ signalling
to control aggressive behaviours in cancer-associated fibroblasts.
Nat Commun. 10:1302019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Guo Z, Zhang H, Fu Y, Kuang J, Zhao B,
Zhang L, Lin J, Lin S, Wu D and Xie G: Cancer-associated
fibroblasts induce growth and radioresistance of breast cancer
cells through paracrine IL-6. Cell Death Discov. 9:62023.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Çermik TF, Ergül N, Yılmaz B and
Mercanoğlu G: Tumor imaging with 68Ga-DOTA-FAPI-04 PET/CT:
Comparison with 18F-FDG PET/CT in 22 different cancer types. Clin
Nucl Med. 47:e333–e339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Pang Y, Zhao L, Meng T, Xu W, Lin Q, Wu H,
Zhang J, Chen X, Sun L and Chen H: PET imaging of fibroblast
activation protein in various types of cancer using
68Ga-FAP-2286: comparison with 18F-FDG and
68Ga-FAPI-46 in a single-center, prospective study. J
Nucl Med. 64:386–394. 2023. View Article : Google Scholar :
|
|
246
|
Hosein AN, Huang H, Wang Z, Parmar K, Du
W, Huang J, Maitra A, Olson E, Verma U and Brekken RA: Cellular
heterogeneity during mouse pancreatic ductal adenocarcinoma
progression at single-cell resolution. JCI Insight. 5:e1292122019.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Neuzillet C, Tijeras-Raballand A, Ragulan
C, Cros J, Patil Y, Martinet M, Erkan M, Kleeff J, Wilson J, Apte
M, et al: Inter- and intra-tumoural heterogeneity in
cancer-associated fibroblasts of human pancreatic ductal
adenocarcinoma. J Pathol. 248:51–65. 2019. View Article : Google Scholar :
|
|
248
|
Lin W, Noel P, Borazanci EH, Lee J, Amini
A, Han IW, Heo JS, Jameson GS, Fraser C, Steinbach M, et al:
Single-cell transcriptome analysis of tumor and stromal
compartments of pancreatic ductal adenocarcinoma primary tumors and
metastatic lesions. Genome Med. 12:802020. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Sebastian A, Hum NR, Martin KA, Gilmore
SF, Peran I, Byers SW, Wheeler EK, Coleman MA and Loots GG:
Single-cell transcriptomic analysis of tumor-derived fibroblasts
and normal tissue-resident fibroblasts reveals fibroblast
heterogeneity in breast cancer. Cancers (Basel). 12:13072020.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Li H, Courtois ET, Sengupta D, Tan Y, Chen
KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, et al: Reference
component analysis of single-cell transcriptomes elucidates
cellular heterogeneity in human colorectal tumors. Nat Genet.
49:708–718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Peng Z, Ye M, Ding H, Feng Z and Hu K:
Spatial transcriptomics atlas reveals the crosstalk between
cancer-associated fibroblasts and tumor microenvironment components
in colorectal cancer. J Transl Med. 20:3022022. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Lambrechts D, Wauters E, Boeckx B, Aibar
S, Nittner D, Burton O, Bassez A, Decaluwé H, Pircher A, Van den
Eynde K, et al: Phenotype molding of stromal cells in the lung
tumor microenvironment. Nat Med. 24:1277–1289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Hornburg M, Desbois M, Lu S, Guan Y, Lo
AA, Kaufman S, Elrod A, Lotstein A, DesRochers TM, Munoz-Rodriguez
JL, et al: Single-cell dissection of cellular components and
interactions shaping the tumor immune phenotypes in ovarian cancer.
Cancer Cell. 39:928–944.e6. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Izar B, Tirosh I, Stover EH, Wakiro I,
Cuoco MS, Alter I, Rodman C, Leeson R, Su MJ, Shah P, et al: A
single-cell landscape of high-grade serous ovarian cancer. Nat Med.
26:1271–1279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge
C, Liu Y, Hao Y, Zhang D, Shi G, et al: Single-cell transcriptomic
architecture and intercellular crosstalk of human intrahepatic
cholangiocarcinoma. J Hepatol. 73:1118–1130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Davidson S, Efremova M, Riedel A, Mahata
B, Pramanik J, Huuhtanen J, Kar G, Vento-Tormo R, Hagai T, Chen X,
et al: Single-cell RNA sequencing reveals a dynamic stromal niche
that supports tumor growth. Cell Rep. 31:1076282020. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Chen Z, Zhou L, Liu L, Hou Y, Xiong M,
Yang Y, Hu J and Chen K: Single-cell RNA sequencing highlights the
role of inflammatory cancer-associated fibroblasts in bladder
urothelial carcinoma. Nat Commun. 11:50772020. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Kürten CHL, Kulkarni A, Cillo AR, Santos
PM, Roble AK, Onkar S, Reeder C, Lang S, Chen X, Duvvuri U, et al:
Investigating immune and non-immune cell interactions in head and
neck tumors by single-cell RNA sequencing. Nat Commun. 12:73382021.
View Article : Google Scholar : PubMed/NCBI
|