Ovarian cancer (OC) is one of the three most
prevalent and lethal malignancies affecting the female reproductive
organs, alongside endometrial and cervical cancers (1). Its incidence has been increasing
worldwide, and it now has the second highest yearly incidence rate
among cancers of the female reproductive system (2,3).
Epithelial OC (EOC), which constitutes 85 to 90% of all ovarian
tumors, is the most common subtype (4). The histological subtypes of EOC vary
based on the tissue origin, as detailed in Table I. Malignant ovarian germ cell
tumors and sex cord-stromal tumors, however, are relatively rare
(4). OC is characterized by a
combination of direct spread, intraabdominal seeding and lymphatic
metastasis, with peritoneal metastases in the advanced stages of
the disease being associated with high mortality rates and a poor
patient prognosis (5). The
primary treatments for patients with advanced-stage OC currently
include surgical tumor removal and platinum-based combination
chemotherapy (6). Despite
advancements in treatment, the prognosis of patients with
advanced-stage disease remains poor, and OC continues to have the
highest mortality rate among all malignant gynecological
malignancies (7). OC often
remains asymptomatic in the early stages due to the covert growth
of the ovary, which is the reason why numerous younger women do not
experience symptoms from their ovarian tumors. The absence of
reliable biomarkers for the early detection of OC often results in
the disease progressing to a more difficult-to-treat late
stage.
At present, an increasing number of studies have
indicated that epigenetic modifications play a pivotal role in
tumor growth etiology, and variations in the epigenetic status are
emerging as promising non-invasive biomarkers for the early
diagnosis and monitoring of OC (8-10).
DNA methylation, the most extensively studied and most
well-characterized epigenetic modification, regulates gene
expression by adding methyl groups to the promoter region of DNA
(11,12). DNA methylation is a complex
epigenetic modification mediated by a complex network of enzymes,
cofactors, and regulatory proteins in a process that involves a
variety of channels and receptors that facilitate the interaction
between DNA methyltransferases and their targets. These include
chromatin remodeling complexes, histone modifiers and transcription
factors. In turn, matrix proteins provide the structural framework
for enzymes and cofactors involved in methylation and thus play a
key role in the process of DNA methylation (10,13). Unlike normal cells, tumor cells
often display abnormal DNA methylation levels in specific regions
of tumor-suppressor gene and/or oncogene promoters (14,15). This disruption of key biological
processes, including cell proliferation, cell cycle regulation and
apoptosis, due to the abnormal DNA methylation patterns of certain
genes, has been found to be associated with the development of OC
(16,17). Recent studies have also suggested
that DNA methylation plays a role in OC cell metastasis (11,18). The current understanding posits
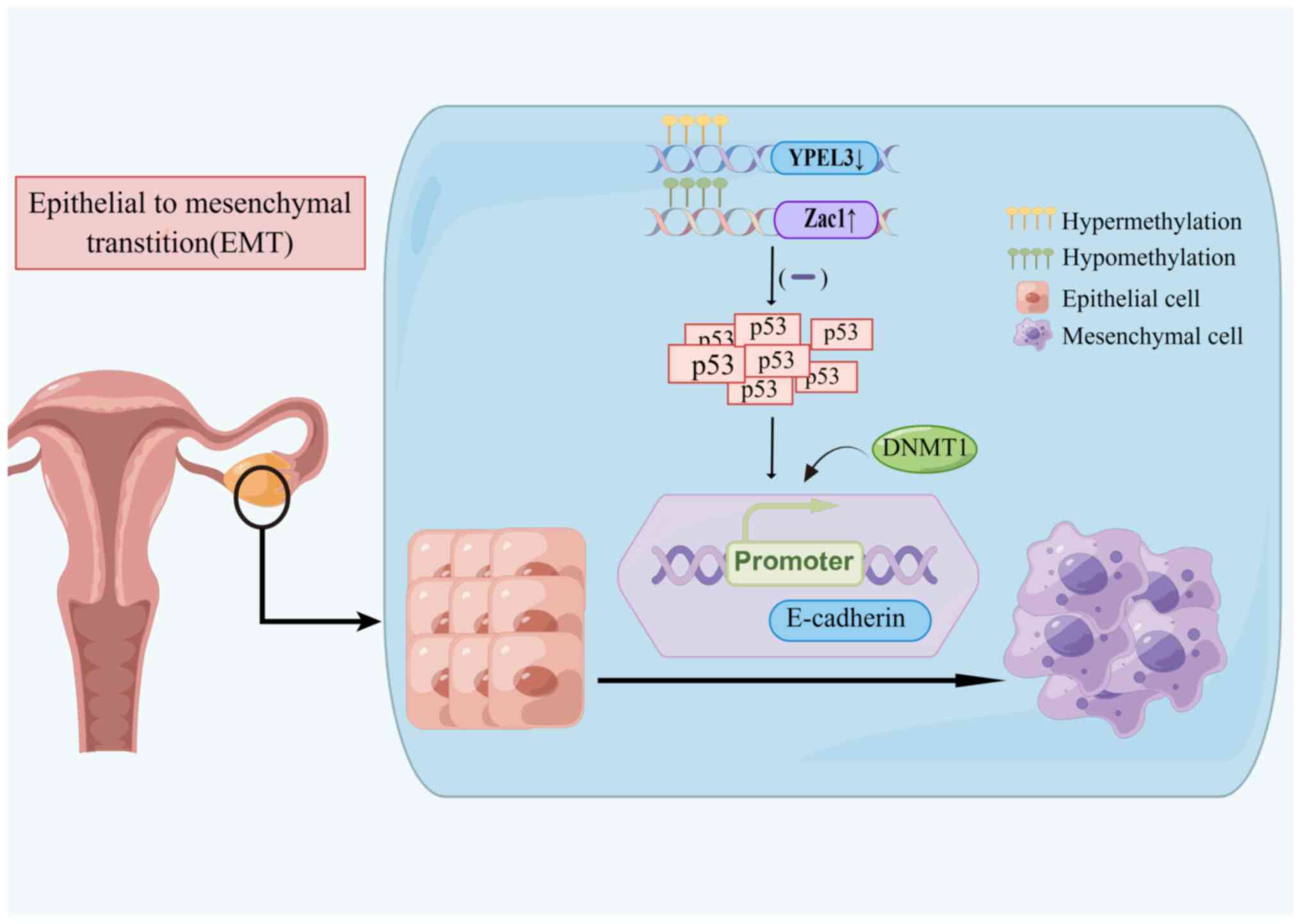
that DNA methylation markers are crucial in the prevention,
diagnosis and treatment of OC, and DNA methylation-related drugs
have also exhibited efficacy in reducing or eliminating resistance
to chemotherapy and molecular targeting in patients with OC
(4,8,19).
High-grade serous OC (HGSOC), the most prevalent subtype of OC, has
the highest recurrence rate and the worst prognosis. It is widely
acknowledged that the primary challenge in treating HGSOC is the
acquired resistance to platinum-based drug therapy (20,21). The study by Feng et al
(22) proposed that NCALD and
LAMA3 could serve as novel markers for determining the sensitivity
to chemotherapy in patients with HGSOC, and that hypermethylation
and the low expression of NCALD and LAMA3 are linked to a poor
progression-free survival. It is thus suggested that the
methylation of gene promoter regions plays a crucial role in
platinum resistance in patients with OC. The present review focuses
on the roles of DNA methylation variations in tumor suppressor
genes, oncogenes, signaling pathway genes and microRNAs
(miRNAs/miRs) involved in the development of OC. Given the
challenges posed by drug resistance and relapse mechanisms, which
significantly affect the management and prognosis of this disease,
the latest findings on the role of DNA methylation in the
screening, diagnosis and the treatment of OC are also summarized.
The present review comprehensively discusses the current evidence
for the role of DNA methylation in both oncogenic and tumor
suppressor pathways implicated in OC, in order to identify
promising biomarkers or therapeutic targets.
Since the 1990s, studies have often been conducted
on BRCA1 and BRCA2 due to their connection to OC. These genes play
an essential role in maintaining human health by regulating
cellular replication, repairing DNA damage, promoting normal cell
development and suppressing tumors (23,24). Since BRCA1 and BRCA2 have
complementary roles in protecting against cancer, they are often
discussed together. Mutations in BRCA1 and BRCA2, which are crucial
genes in the homologous recombination mechanism for the repair of
DNA double-strand breaks, have been shown to be associated with an
increased risk of developing cancer. Women with a hereditary
BRCA1/2 mutation have a higher chance of developing both breast
cancer and OC (25). Unlike
sporadic breast cancer, BRCA-associated breast cancer is more
likely to occur on the side of the body or as a second primary
tumor. By the age of 70 years, women with the BRCA1/2 mutation have
a 10-59% increased chance of developing OC (26). Therefore, it is critical to
conduct additional cancer screening at the time of OC or breast
cancer diagnosis and treatment. The increased likelihood of
developing primary OC, an earlier onset, larger tumor spread and a
more aggressive disease course in BRCA1/2 mutant carriers deserves
special attention. Individuals with BRCA1/2 mutations, particularly
those with recurrent OC, have an improved prognosis and a longer
survival time following surgery compared with individuals with
primary and recurrent OC without BRCA1/2 mutations (23,27). This phenomenon was also observed
in the study by Yang et al (23), in which patients had longer
overall survival and progression-free survival times. However, the
underlying mechanisms of this difference have yet to be
demonstrated, and future studies are required investigate whether
it is linked to DNA methylation as a result of BRCA1/2
mutations.
Clinical trials using poly-ADP ribose polymerase
(PARP) inhibitors for the treatment of individuals with BRCA gene
mutations are currently underway, since an in vitro study
demonstrated that cells with mutations in the BRCA1 or BRCA2 genes
are particularly sensitive to PARP (32). Additionally, a recent study
revealed that females with BRCA methylation were more likely to
benefit from treatment with PARP inhibitors, regardless of whether
they carried BRCA mutations or not (33). However, further clinical trials
are required to confirm the feasibility of the treatment instead.
Cisplatin resistance has been linked to the disruption of the
BRCA/Fanconi anemia (FA) pathway, which occurs when the FA gene (FA
complementation group F) is methylated and silenced (34). Patients with HGSOC commonly
exhibit a defective BRCA/FA pathway, rendering the tumor
susceptible to DNA cross-linking agents and PARP inhibitors
(35). The BRCA1 and BRCA2 genes,
are where the majority of BRCA/FA pathway-inactivating mutations
are found, particularly in HGSOC (36). Although the DNA methylation of FA
complementation group N is rarely observed in HGSOC, it has been
shown to be associated with inactivation in some cases of sporadic
OC (37). However, targeting the
BRCA/FA pathway is expected to overcome OC resistance to
cisplatin.
Different subtypes of OC have been found to be
associated with distinct patterns of BRCA methylation. HGSOC has
been found to have a higher prevalence of BRCA1 hypermethylation
compared with other types of EOC (38). The pathophysiology of OC has been
linked to the hypermethylation of the BRCA promoter (39). Additionally, Soslow et al
(40) revealed that BRCA
methylation was associated with the presence of solid, pseudo
endometrioid and transitional cell carcinoma-like morphology. The
combined effect of BRCA1 and BRCA2 hypermethylation in the
development of OC supports the use of immune checkpoint inhibitors
in clinical trials (41).
Furthermore, the methylation of DNA in the upstream region of BRCA1
transcriptional start sites has been observed to positively
influence the prognosis of patients with HGSOC (42). Bilateral ovarian cancers are
associated with an increased BRCA1 methylation compared with
unilateral cancers, and the methylation status can serve as a
predictor of the survival of patients with sporadic EOC. The
co-expression of DNA methyltransferase (DNMT)1 and 3a, DNMT1 and
3b, or DNMT3a and 3b contributes to the hypermethylation of the
BRCA1 promoter, as previously described by Bai et al
(43). Additionally, Pradjatmo
(44) revealed that BRCA2
methylation was present in the majority of patients with OC, and
that BRCA2 protein expression levels were associated with overall
survival, regardless of the methylation status of BRCA2. This
finding may guide the development of therapeutic approaches aimed
at preventing or reversing BRCA gene methylation. These results
highlight the significance of BRCA methylation in the etiology,
progression and prognosis of OC, and lay the foundation for future
therapeutic advancements.
The aforementioned evidence suggests that
individuals with BRCA1/2 gene mutations, who also exhibit elevated
levels of BRCA gene methylation, leading to decreased BRCA1/2
expression, are at a higher risk of developing OC. The methylation
testing of the BRCA1/2 gene could potentially play a crucial role
in preventing OC. Additionally, individuals with BRCA mutations who
have been diagnosed with OC may benefit from treatment that
specifically targets BRCA. However, the current findings on the
clinical implications of BRCA methylation in OC are complex and
conflicting. Therefore, further substantiated data are warranted in
order to study and validate the impact of DNA methylation of the
BRCA genes in OC.
Both the wild-type and mutant forms of the p53 gene
play prominent roles as tumor suppressors in humans. Wild-type p53
is essential for controlling cell division and growth, inducing the
apoptosis of malignant cells, and blocking carcinogenesis. By
contrast, the mutation of P53 transforms the p53 gene, which is
normally a tumor suppressor, into an oncogene that actively
promotes cancer development at the cellular level. Point mutations,
inactivation and deletions of the p53 gene convert the wild-type to
the mutant type, promoting carcinogenesis and cancer progression
(45). Studies have revealed a
strong association between the methylation of the promoter region
of the p53 gene and the onset of several types of cancer, including
breast cancer (46), lung cancer
(47), prostate cancer (48) and OC (49). A previous study comparing the p53
methylation status in normal and malignant ovarian tissues using
methylation-specific PCR determined that the p53 promoter area
methylation was unique to OC tissue specimens (50). These results suggest the potential
use of p53 promoter area methylation as a screening tool for OC,
and indicate that epigenetic modifications play a critical role in
OC carcinogenesis.
The specificity of the p53 gene results in a complex
dichotomy in its role in the pathogenesis of OC. The only studies
thus far have shown that p53 is hypermethylated and downregulated
in the development of OC. However, there is a lack of studies
examining the role of p53 in the epigenetic development of OC,
particularly in the context of DNA methylation, despite the
association of p53 mutations with an increased risk of developing
OC. This presents a key opportunity for further research. Given its
potential utility in clinical screening and prognostication of OC,
the extensive investigation of the p53 gene methylation is
warranted.
RASSF1A is a potential Ras effector that regulates
cellular proliferation and apoptosis in response to extrinsic
signals. Its upregulation leads to the decreased proliferation of
human cancer cells, indicating its crucial role as a tumor
suppressor gene (55). Numerous
studies have demonstrated the epigenetic inactivation of the
RASSF1A isoform in various types of cancer, such as lung cancer
(56), breast cancer (57) and OC (58). Therefore, the methylation of
RASSF1A could serve as a valuable prognostic marker for patients
with cancer, and may play a critical role in the early detection of
cancer (59).
According to recent research, RASSF1A methylation is
increased in OC compared with normal ovarian tissues (60). Furthermore, the methylation
frequency of RASSF1A has been found to be higher in patients with
HGSOC (61). Additionally, the
methylation frequency of RASSF1A was higher in OC than in low
malignant potential tumors, which exhibited higher methylation
levels of RASSF1A than benign ovarian epithelial adenomas (62). Therefore, RASSF1A promoter
hypermethylation and RASSF1A protein levels may serve as reliable
and sensitive tools for the diagnosis and monitoring of patients
with ovarian malignancies. Furthermore, cationic conjugated
polymer-based fluorescence resonance energy transfer techniques for
the detection of the RASSF1A methylation status in EOC may be
useful for diagnosis and screening. Combining the techniques with
the detection of cancer antigen 125 levels may improve the
sensitivity of the diagnosis of EOC (63).
RASSF1A promoter methylation has been reported to be
markedly associated with EOC in a previous study on OC in
Vietnamese women (64). However,
a meta-analysis found that the levels of this modification were not
substantially linked to the clinicopathological characteristics or
the survival outcomes of patients with OC (65). This discrepancy may be due to
differences in sample size or individual variations in experimental
results. Nevertheless, a recent study examined EOC cells and
mesenchymal-like OC stromal progenitor cells to determine their
methylation status at RASSF1A promoters (66). The frequency of RASSF1A promoter
methylation was found to be considerably higher in tumor-derived OC
stromal progenitor cells (OCSPCs) than in epithelial-like OCSPCs,
and it was shown to be associated with the clinicopathological
characteristics and survival outcomes of patients. That study
demonstrated the potential therapeutic value of RASSF1A promoter
methylation in OCSPCs generated from EOC tissues (66). Since OCSPCs with a reduced
expression of tumor suppressor genes in the ovarian tumor
microenvironment can promote tumorigenesis and can be reversed by
the DNA demethylation of genes, reversing the DNA demethylation of
tumor suppressor genes in OCSPCs may represent a potential
therapeutic strategy for OC (67). Reyes et al (68) conducted a study on advanced-stage
HGSOC and a retrospective, nested, case-control study of patients
with recurrent HGSOC. They found that patients with OC in different
states had different frequencies of DNA methylation of RASSF1A, and
methylation was associated with several differentially expressed
genes that could be potential biomarkers and/or therapeutic targets
for HGSOC (68).
In patients with advanced-stage EOC receiving
neoadjuvant therapy, the methylation status of the RASSF1 promoter
has been demonstrated to exhibit a marked association with the
response to chemotherapy. Specifically, by studying aberrant DNA
methylation in 68 normal ovarian tissues, and 29 benign, 100
malignant and 10 junctional ovarian tumor tissues, Feng et
al (69) revealed that
patients with EOC with RASSF1A promoter methylation had markedly
poorer response rates to cisplatin-based neoadjuvant therapy
compared with patients without a methylation status. RASSF1A
promoter methylation is a key predictive factor for the prognosis
of patients with HGSOC (70); the
study by Giannopoulou et al (71) found that RASSF1A promoter
methylation was significantly associated with the OC grade, and
that prognosis tended to be worse for patients with OC in whom
RASSF1A promoter methylation was detected in the tumor and in
adjacent tissues. Furthermore, the identification of aberrant
RASSF1A promoter methylation in cell-free circulating tumor DNA
from low-volume plasma samples of patients with EOC has shown
potential as a prognostic marker for the disease (72). These findings have significant
implications for EOC research, including the development of
improved diagnostic methods and targeted therapy approaches. In
summary, RASSF1A is hypermethylated and downregulated in the
development of OC, and the methylation status of RASSF1A has the
potential to serve as a biomarker for early identification and
diagnosis of OC, as well as for predicting the treatment response
and overall clinical outcomes.
In addition to the tumor suppressor genes described
above that have undergone substantial research, a large number of
other tumor suppressor genes have been linked to the development of
OC. Among the principal molecular determinants that exert a
profound influence on OC are chromodomain helicase DNA binding 5
(CHD5), fructose-1,6-biphosphatase (FBP1), aldehyde dehydrogenase
1-A2 (ALDH1A1), pluripotency-associated transcription factor
forkhead box (FOX)D3, insulin-like growth factor binding protein-3
(IGFBP-3), zinc finger protein 671 (ZNF671), secreted protein
acidic and rich in cysteine (SPARC) and
O6-methylguanine-DNA methyltransferase (MGMT). All the
aforementioned tumor suppressor genes can be accessed from Table II.
CHD5, also known as DNA binding 5, is a member of
the subclass of chromatin remodeling Swi/Snf proteins and is
currently considered to be a tumor suppressor (73). The frequency of abnormal DNA
methylation of the CHD5 gene promoter is inversely related to the
prognosis of patients with cancer and has been observed in several
malignancies (74-78). Despite the limited number of
studies, CHD5 promoter methylation has been shown to be associated
with OC, suggesting its potential clinical applications in OC
metastasis, treatment and prognosis. The downregulation of FBP1, a
tumor suppressor and the rate-limiting enzyme in gluconeogenesis
(79), has been observed in
several malignancies. The DNA methylation of the FBP1 promoter in
patients with OC leads to a decreased expression of FBP1, which is
associated with advanced-stage disease, high malignancy, low
survival, high recurrence rates, and a poor prognosis (80). Compared to the normal ovarian
surface epithelium, OC cells have a significantly lower expression
of ALDH1A2, another rate-limiting enzyme involved in cellular
retinoid production (81). High
ALDH1A2 promoter methylation levels in OC cells promote cell
proliferation, enhance invasive activity and are associated with a
poor prognosis (82). Together,
CHD5, FBP1, and ALDH1A2 have exhibited promise as biomarkers,
therapeutic targets and prognostic indicators in the study of OC
and its clinical management.
FOXD3 is essential for development, cellular
homeostasis and the control of lineage specification (83). The reduced expression of FOXD3 due
to the hypermethylation of its promoter has been linked to the
development of malignant tumors (84,85). The study by Luo et al
(86) revealed that FOXD3
promoter methylation was increased and its expression was decreased
in OC tissues. The inhibition of tumor cell growth, as well as the
effects on tumor cell proliferation and migration, suggest that
FOXD3 promoter methylation may serve as a prognostic marker for OC
(86). IGFBP-3 is a member of the
IGFBP family, which largely governs the mitogenic and
anti-apoptotic effects of insulin-like growth factor, a protein
whose transcription is regulated by p53 and which possesses
anti-proliferative, pro-apoptotic and invasion-inhibiting
activities (87). Wiley et
al (88) discovered that
changes in IGFBP-3 promoter methylation significantly affected the
survival of patients with EOC. This link was found to be
particularly strong in individuals with early-stage OC.
Furthermore, in patients with EOC who lacked p53 overexpression,
elevated DNA methylation levels of the IGFBP-3 promoter were found
to be substantially associated with OC progression (89). ZNF671, a member of the KRAB-ZFP
family that contains C2H2-type zinc fingers and a Krüppel
associated box domain, regulates key functions in cell
differentiation, proliferation, apoptosis and tumor suppression
(90,91). The low expression of ZNF671 is
strongly associated with OC cell motility and invasion, and it is
one of the most heavily methylated genes in patients with early
recurrence. The ZNF671 DNA methylation status following
platinum-based adjuvant chemotherapy may be a potent indicator of
serous OC recurrence (92). In
summary, promoter hypermethylation affects the low expression of
FOXD3, IGFBP-3 and ZNF671, which contributes to the onset and
progression of OC. Patients with early-stage OC may benefit from
using these genes as prognostic indicators. If other members of
this family are found to be involved in the formation of OC, which
is yet to be determined, scholars can explore additional gene
targets that can aid in the clinical diagnosis and therapy of
OC.
To regulate cell adhesion, differentiation,
proliferation, migration, tissue remodeling, morphogenesis and
angiogenesis, SPARC (also known as osteonectin or BM-40) is
expressed in various types of cells (93). Previous studies have linked the
hypermethylation of the SPARC gene promoter to a worse prognosis
and earlier diagnosis in several types of cancer, including OC
(93-95). The SPARC promoter is methylated in
primary OC and that SPARC protein levels decrease as the disease
advances from low to high grade (95-97). Based on these results, it appears
that SPARC promoter methylation plays a critical role in OC
carcinogenesis and survival, and SPARC may serve as a novel
biomarker for OC.
Comparative research has been conducted on the role
of MGMT, a DNA repair gene that is hypermethylated in the majority
of malignancies (98). The
frequency of MGMT gene promoter methylation varies across OC
samples, with EOC having the highest frequency and benign ovarian
tissue having the lowest (99).
The role of MGMT promoter methylation in the onset of OC is
undeniable, despite the lack of clarity regarding the link between
MGMT gene expression and DNA methylation.
DNA hypermethylation and the low expression of the
aforementioned genes is strongly associated with OC etiology and
has significant implications for the treatment and prognosis of
patients with OC. Targeting these genes could greatly benefit the
early screening, diagnosis and therapy of patients with OC. It is
crucial to gather convincing data to identify their precise role in
OC and uncover their latent potential in managing this malignancy,
considering the large number of genes present in the human genome
and the need to discover more tumor suppressor genes.
The atypical expression of oncogenes, which can be
caused by epigenetic alterations, has been found to be associated
with tumor development and a poor prognosis. The significance of
oncogenes in the pathogenesis of OC is demonstrated by the
identification of ~568 oncogenes, of which ~34 are associated with
the risk of developing OC. Common epigenetic abnormalities include
alterations in DNA methylation, RNA interference, histone
modifications and gene mutations (100). The present review focuses on
five oncogenes: Homeobox A9 (HOXA9), chromobox protein homolog 8
(CBX8), solute carrier family 6, member 12 (SLC6A12), anterior
gradient 2 (AGR2), and gamma-aminobutyric acid (GABA) A receptor
subunit (GABRP), due to their known association with OC and
abnormal DNA methylation (Table
III).
The DNA-binding transcription factor, HOXA9,
controls gene expression and plays a role in morphogenesis and
differentiation (101). The
methylation of the HOXA9 promoter has been extensively studied in
relation to the development of OC (102). Widschwendter et al
(102) observed that HOXA9
promoter methylation in normal endometrium increased the incidence
of OC by 12.3-fold across all stages and 14.8-fold in early-stage
OC, independent of age, menstrual cycle and cancer histology. Wu
et al (103) revealed
that HOXA9 promoter hypermethylation was more common in older women
and was associated with a higher frequency of methylation in the
early stages of OC by examining 52 primary OCs and their in
vitro models. Montavon et al (104) found that the HOXA9 promoter was
differentially methylated in primary HGSOC and rarely methylated in
benign ovarian surface epithelium (OSE), suggesting that the
combination of HOXA9 promoter methylation status could distinguish
HGSOC from benign OSE with a sensitivity of 100% when pre-operative
CA125 levels were also included. Studies have also demonstrated
that HOXA9 promoter methylation is highly tumor specific and has
great promise as a diagnostic serum biomarker for the early
screening of OC (95,105). The diagnostic utility of
promoter methylated HOXA9 in circulating tumor specific DNA in
patients with OC was examined by Faaborg et al (106), who deviated from the standard
practice of studying DNA methylation by analyzing both the sense
and antisense strands of the HOXA9. They discovered that compared
to single-stranded assays, OC diagnostics could benefit from
simultaneous testing against both DNA strands, leading to a 59.5%
increase in sensitivity (106).
In addition, HOXA9 promoter methylation was previously found to be
involved in the progression from one grade of OC to another. For
example, in patients with endometriosis-associated OC, lower levels
of HOXA9 promoter methylation were significantly associated with a
higher tumor grade. This suggests that the HOXA9 promoter
methylation pattern is an indicative factor for progression toward
high-grade plasmacytoma (96).
Furthermore, the promoter hypermethylation of HOXA9 can be used as
a diagnostic marker and can also be used to forecast prognosis of
patients. Patients with platinum-resistant BRCA-mutated OC treated
with PARP inhibitors have been shown to have a poor prognosis if
their HOXA9 promoter is highly methylated (107). This suggests that HOXA9 promoter
hypermethylation and the low expression of HOXA9 in OC can be a
valuable predictive biomarker and can inform clinical
decision-making in platinum-resistant BRCA. Overall, these results
highlight the potential of HOXA9 methylation as a diagnostic marker
for OC, with applications in risk prediction and prognosis
forecasting.
CBX8 is a fundamental CBX protein and maintains
pluripotency and self-renewal during developmental program
controls, cell destiny determinations and the regulation of
embryonic stem cells. Cell cycle progression, senescence and
differentiation are all influenced by CBX8, and the hypomethylation
of its promotor leads to an increase in its expression (108). CBX8 has been shown to play a
role in the development of hepatocellular carcinoma, renal cancer
and colorectal cancer, among others (109,110). The DNA hypomethylation of CBX8
leads to an enhanced expression, which acts as a potential
diagnostic and prognostic biomarker for patients with OC and is
associated with a poor prognosis (109). Furthermore, SLC6A12, a
betaine/GABA transporter (111),
is overexpressed in OC metastases, negatively affecting patient
survival due to its promoter hypomethylation. Since SLC6A12
promoter methylation facilitates cancer cell invasion during the
development of OC, it is widely recognized as a prognostic marker
for the chances of survival of patients (111).
AGR2, a protein disulfide isomerase localized to the
endoplasmic reticulum or secreted into the extracellular space, has
been linked to cancer progression in patients with similar tumors
(112-114). According to the study by Sung
et al (115), which used
a mouse model of human OC metastasis, the CpG site in the promoter
region of AGR2 is hypermethylated in metastatic tumor tissue, which
typically results in AGR2 overexpression. AGR2 overexpression was
found to increase SK-OV-3 cell migration and invasion (115). This suggests that AGR2 promoter
hypomethylation may contribute to OC cell metastasis and
invasion.
There are other studies on gene mutations in OC
subtypes, with previous studies finding that HGSOC has prevalent
TP53 mutations, mucinous OC has frequent KRAS mutations, and
mutations in AT-rich interaction domain 1A and
phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3)CA are more
common in clear-cell OC and endometrioid OC (35). However, studies exploring
epigenetic modifications, particularly DNA methylation, in OC
subtypes are limited. A genome-wide DNA methylation analysis
revealed that HGSOC exhibited higher levels of overall DNA
hypermethylation compared to low-grade EOC (116). DNA methylation profiles could
potentially be utilized to predict and classify the characteristics
of aggressive and highor low-grade EOC. HGSOC, which typically
displays a higher overall DNA methylation, is often associated with
varying degrees of platinum resistance, leading to differing
recurrence intervals following initial paclitaxel/platinum-based
therapy (116). The data
reviewed above suggest that oncogene promoter hypomethylation is a
common mechanism leading to oncogene overexpression in patients
with OC. Previous studies support the hypothesis that oncogenes
contribute to OC cell metastasis and have significant clinical
implications for the diagnosis and prognosis of patients with OC
(102-106). However, the possible role of
aberrant oncogene promoter methylation in the therapy and acquired
drug resistance of patients with OC is poorly studied, making it a
promising area for further research.
Wnts are a class of glycoproteins that primarily
exert their effects through autocrine or paracrine secretion. Upon
secretion, they interact with surface receptors, triggering a
cascade of downstream protein phosphorylation and dephosphorylation
events that ultimately result in the accumulation of β-catenin.
Adhesive bands are formed when β-catenin interacts with E-cadherin
at cell junctions. Free β-catenin can enter the nucleus to
influence gene expression. The aberrant expression or activation of
β-catenin can lead to the development of cancer (117). Several types of cancer,
including lung cancer (118),
breast cancer (119) and OC
(120), have been shown to share
a common feature: The oncogenic activation of the Wnt/β-catenin
signaling pathway. Various component alterations (Table IV) in the Wnt/β-catenin signaling
pathway and their significance in OC are discussed below.
Secreted frizzled-related proteins (SFRPs) play a
crucial role in cancer progression and prognosis by functioning as
critical inhibitors of the Wnt/β-catenin signaling pathway. The
SFRP (SFRP1, 2, 3, 4 and 5) genes are heavily methylated, leading
to transcriptional silencing (121). This downregulation of SFRP
expression is a common occurrence in cancer. In OC, the SFRP1 gene
is inactivated due to promoter methylation and participates in the
Wnt/β-catenin signaling pathway (122). Promoter hypermethylation also
contributes to the inactivation of SFRP5, disrupting Wnt/β-catenin
signaling and promoting ovarian carcinogenesis (123). Patients with SFRP5 promoter
methylation have a poorer prognosis (124,125). Moreover, SFRP5 hypermethylation
is associated with an increased risk of EOC recurrence and
mortality, suggesting its potential as a prognostic biomarker
(126). SFRP5 expression also
suppresses epithelial-mesenchymal transition (EMT) and increases
the sensitivity of OC cells to chemotherapy (127). Conversely, SFRP5
hypermethylation in OC leads to the oncogenic activation of the
Wnt/β-catenin pathway, resulting in an increased OC progression and
chemoresistance through TWIST-mediated EMT and AKT2 signaling
(127). Curcumin, a targeted
anticancer agent, inhibits the Wnt/β-catenin signaling, thereby
mitigating the effects of SFRP5 hypermethylation. When used in
combination with 5-aza-2'-deoxycytidine, it attenuates the
development of OC (123). Apart
from SFRP5, there is limited research available onSFRP1/2/3/4, and
further investigations are required to understand the potential of
targeting SFRP and inhibiting the Wnt/β-catenin signaling pathway
for OC.
IQ motif containing GTPase activating protein
(IQGAP)2, a member of the IQGAP family, functions as a tumor
suppressor in the majority of cancers by mainly inhibiting
β-catenin nuclear translocation and transcriptional activity. This
inhibition leads to the suppression of Wnt/β-catenin signaling,
which in turn inhibits the EMT, migration and invasion of OC cells.
In OC, the DNA methylation of IQGAP2 is significant and negatively
correlates with mRNA expression. Survival analyses have revealed
that a reduced expression of IQGAP2 is strongly associated with a
poorer progression-free survival of patients with OC (128,129). Another new protein,
transmembrane 88 (TMEM88), is an inhibitor of Wnt signaling and is
found in the cell membrane (130). Promoter hypermethylation causes
a decrease in TMEM88 expression, which enhances OC cell
proliferation and the development of resistance to platinum
treatments (131). The tumor
suppressive functions of IQGAP2 and TMEM88 in OC are mediated
through the regulation of Wnt/β-catenin signaling, resulting in
reduced cell proliferation and invasion. These findings provide
insight into the pathophysiology of OC and suggest potential
therapeutic interventions for this condition. Additionally, IQGAP2
and TMEM88 may serve as useful biomarkers for the diagnosis and
monitoring of OC.
The role of DNA methylation in OC in the regulation
of the Wnt/β-catenin signaling pathway is illustrated in Fig. 2. The aforementioned results
demonstrate that hypermethylation and the low expression of key
genes in the Wnt/β-catenin signaling cascade can significantly
affect OC pathogenesis, particularly in the context of therapy and
platinum resistance in patients with OC. Therefore, it is crucial
to continue studying the Wnt/β-catenin signaling pathway due to its
potential in combating platinum resistance in patients with OC.
There are three closely comparable structural
isoforms of TGF-β, all of which belong to the same family of
cytokines. The TGF-β signaling pathway has been shown to play a
bidirectional role in cancer progression and is essential for
regulating cellular activities, such as cell proliferation,
differentiation, apoptosis and cellular dynamic homeostasis
(132). In the early stages, it
functions primarily as a tumor suppressor, while in advanced
stages, it may function as a tumor promoter (133). As demonstrated in the study by
Matsumura et al (134),
DNMT inhibitors (DNMTis) can enhance TGF-β pathway activity and
reduce the progression of OC. Furthermore, a follow-up study
revealed that TGF-β therapy causes changes in DNA methylation that
persist throughout the EMT phase of OC cells. Notably, blocking
TGF-β from inducing EMT in cancer cells with DNMTi therapy reduced
cancer cell metastasis (135).
These results suggest that DNMTis may be a promising therapeutic
option for OC by regulating the TGF-β signaling pathway and
preventing cancer cell metastasis.
F-box protein 32 (FBXO32), a member of the F-box
protein family, is highly methylated in advanced OC, resulting in
decreased expression. Since FBXO32 is a target gene of SMAD4, its
loss in OC causes a malfunction in the TGF-β/SMAD4 signaling
pathway, accelerating the development of OC (136). However, OC cells can become
desensitized to cisplatin, and their expression of FBXO32 can be
restored by treatment with epigenetic drugs, which also markedly
reduces the growth of platinum-resistant OC cell lines in
vitro and in vivo. Additionally, the methylation status
of FBXO32 can predict the survival of patients with OC (136). ATP binding cassette subfamily A
member 1 (ABCA1), a signaling target of TGF-β, is also expressed as
DNA hypermethylation in OC, and the higher the methylation level of
ABCA1 promotor, the higher the pathological grade and the shorter
the survival of patients with OC (137). Sex-determining region Y-box 2
(SOX2) is a single-exon transcription factor with key roles in
embryonic development and stem cell maintenance (138). Shonibare et al (139) observed an improved lifespan of
tumor-bearing mice following the promoter methylation of SOX2. This
suggests that the promoter methylation of SOX2 can influence the
TGF-β signaling pathway, which in turn affects the survival of
patients with OC and the metastasis of OC cells (139). TGF-β-induced protein (TGFBI),
also known as βig-H3 and keratoepithelin, is a cellular matrix
protein whose promoter hypermethylation is associated with the
silencing of TGFBI. This can induce OC cell death and is
significantly associated with the development of OC (140). Wang et al (141) confirmed this and also found that
the hypermethylation of TGFBI was associated with paclitaxel
resistance in patients with OC. Therefore, they hypothesized that
TGFBI may be a therapeutic target for improving the
chemotherapeutic response in patients with OC (141). Overall, the expression of
FBXO32, ABCA1, SOX2 and TGFBI, which are genes in the TGF-β
signaling system, is suppressed due to hypermethylation, thereby
accelerating the development of OC. The methylation profiles of
these genes (Table V) can be
utilized to predict the prognosis of patients with OC and can also
be targeted for therapeutic purposes against the disease.
The abnormal activation of the PI3K/AKT/mammalian
target of rapamycin (mTOR) signaling pathway is very common
occurrence in the majority of human cancers compared to other major
signaling pathways. The inactivation of the phosphatase and tensin
homolog (PTEN) gene often occurs at an early stage in ovarian
endometrioid and ovarian clear cell carcinomas, and its promoter is
often methylated in 40% of ovarian clear cell adenocarcinomas
(142), which can negatively
regulate the PI3K/AKT/mTOR signaling pathway. Moreover, PTEN also
plays a role as an oncogene (143). Li et al (144) found that the hypomethylated
PIK3R3 promoter was detected in OC cell lines, which may play a
role in the chemoresistance of OC, and can even restore sensitivity
to platinum-based chemotherapeutic agents. Overall, the current
focus of studies on genetic abnormalities in the PI3K/AKT/mTOR
signaling pathway is on genetic mutation abnormalities; however,
epigenetic regulation, particularly DNA methylation, has been less
extensively studied and warrants further investigations. The MAPK
pathway is another currently well-studied pathway that is
aberrantly activated during tumor progression and is present in
>85% of cancers (145). Human
growth factor receptor-bound protein-7 (GRB7), is overexpressed in
a variety of human cancers (146,147). A recent study by Chen et
al (147) found that
miR-193a-3p directly regulated GRB7 and that miR-193a-3p was
downregulated by DNA hypermethylation during the development of OC,
leading to the elevated expression of GRB7 in OC tissues. In
addition, miR-193a-3p enhances the oncogenicity of OC cells by
regulating Erb-B2 receptor tyrosine kinase (ERBB)4, SOS Ras/Rho
guanine nucleotide exchange factor 2 and KRAS in the MAPK/ERK
signaling pathway (147).
Therefore, miR-193a-3p and GRB7 are promising as targets in OC
therapy and deserve further exploration. The experimental study of
Sung et al (148) found
that the CpG site of the GABRP promoter was hypomethylated in the
metastatic tissues of mice with tumor xenografts, leading to the
overexpression of GABRP, and the promotion of cell migration and
invasion through the activation of the MAPK/ERK pathway. This
suggests that GABRP enhances the invasive phenotype of OC cells and
that the DNA methylation status of the GABRP-963 CpG locus may help
predict the metastatic potential of patients with OC (148). The use of animal models well
reflects the physio-pathological mechanisms in the human body and
helps to assist the target therapy of human diseases; however,
ultimately, research has to be returned to the human body for
validation, in order to provide more realistic and accurate results
for clinical treatment.
Although there are more studies on signaling
pathways in OC, the focus of the studies is on the mutation or
inactivation of key genes, and studies on epigenetic modifications
are limited, which has some limitations. Therefore, further
experimental studies are required for validation, and they are
expected to promote the advancement of OC as a disease in
chemotherapy-resistant treatment and prognostic assessment.
miRNAs are a class of short, non-coding RNAs that
function by simultaneously repressing translation and/or causing
RNA degradation by targeting numerous mRNAs. Previous research has
shown that OC tissues have distinct miRNA expression profiles from
those of normal human ovarian tissues (149,150). DNA methylation at the promoter
of the host gene controls the expression of numerous miRNAs. The
methylation alterations of genes associated with miRNAs in the
development of OC are summarized in Table VI. Cancer cells rely on a
specific type of energy metabolism known as the Warburg effect,
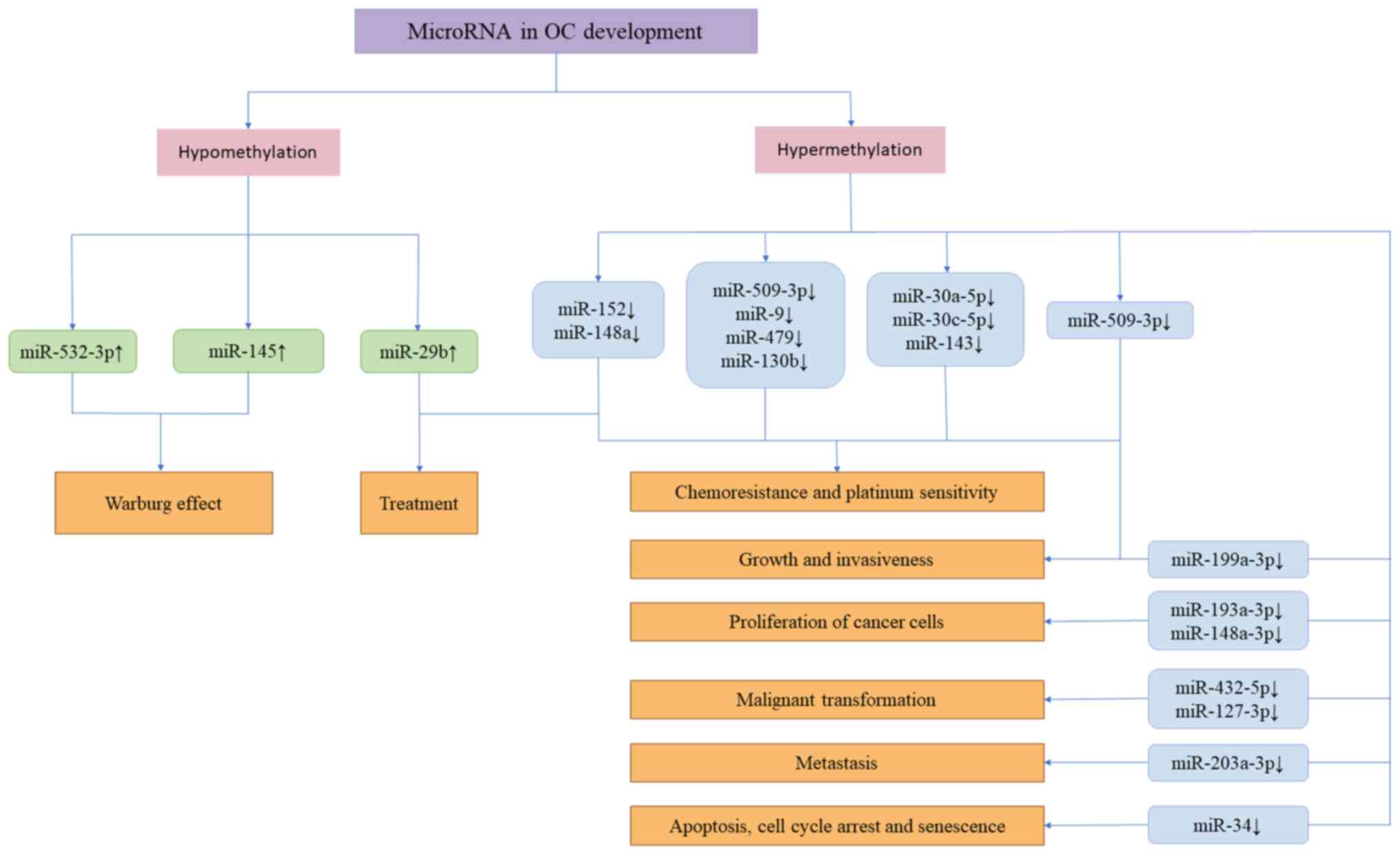
which is partially controlled by miRNAs (151). miR-532-3p and miR-145, which are
overexpressed in OC tissues, have been shown to prevent the Warburg
effect in OC cells (152,153)
and exhibit a negative correlation with DNMT3A expression. More
specifically, miR-145 predominantly operates via the
miR-133b/pyruvate kinase M2 pathway to induce the Warburg effect
(154). The hypomethylation of
the DNMT3A/3B CpG island promoter area enhances miR-29b expression,
and there is an inverse association between miR-29b and DNMT3A/3B
expression levels in OC tissues. The therapeutic targeting of
miR-29b may represent a promising new avenue for the management of
OC (155).
The secretory epithelial cells of the fallopian
tube (FTSECs) play a crucial role in the maturation of HGSOC. The
malignant transformation of FTSECs is more common following
long-term exposure to iron. Chhabra et al (156) found that the expression of
miR-432-5p and miR-127-3p was considerably downregulated during
this malignant transformation. Chronic exposure to iron can affect
miRNA expression by causing epigenetic modifications; however, this
effect can be reversed by treatment with DNA methyltransferase
inhibitors (156). The tumor
suppressor gene, DNMT1/UTF1, is also downregulated by miR-148a-3p,
which has been found to reduce cancer cell proliferation (157). Based on these results, miRNAs
may be useful as diagnostic indicators and therapeutic targets for
treating OC.
miRNAs have also been linked to platinum
resistance, which is a well-known and challenging barrier in the
treatment of OC with chemotherapy. The low expression of miR-509-3p
and a significantly higher frequency of miR-509-3p methylation have
been shown to be associated with a shorter overall survival of OC
cells derived from patients who have undergone primary tumor
cytoreductive surgery and post-operative platinum-based
chemotherapy (158). This
association is primarily mediated by collagen type XI alpha 1,
which increases the phosphorylation and stability of DNMT1
(158). In cisplatin-resistant
OC cells, the overexpression of DNMT1 induces methylation and the
subsequent downregulation of miR-30a-5p and miR-30c-5p, resulting
in cisplatin resistance (159).
The poor prognosis of patients with OC corresponds with the
dysregulation of miR-7, miR-132, miR-335 and miR-148a in
cisplatin-resistant cell lines, where miR-7 tends to exhibit
specific methylation and is associated with a worse prognosis of
patients with OC (160).
Restoring miR-9 expression by demethylating the miR-9-1/3 gene can
desensitize OC cells to paclitaxel (161). The downregulation of miR-9
expression in paclitaxel-resistant EOC cells is related to
resistance to paclitaxel. It has also been found that promoter
hypermethylation in OC tissues reduces the expression of miR-479
and miR-130b, decreasing the sensitivity of cancer cells to
platinum-based therapies (162,163). Recent studies have reported a
newly discovered substance, miR-143, which has been proven to play
a role in the chemotherapy of tumors. DNMT3A is a direct target of
miR-143, and the overexpression of DNMT3A antagonizes the
sensitivity of miR-143 to cisplatin in OC cells, possibly as DNMT3A
leads to the hypermethylation of the miR-143 precursor gene,
resulting in the downregulation of its expression and generating
cisplatin resistance (164).
All these points emphasize the importance of miRNAs
in the therapeutic intervention of OC, specifically in relation to
the reported link between miRNAs and DNA methylation. This notable
finding suggests potential new avenues for the treatment of OC, and
further research is required to determine its therapeutic
implications.
In addition to their roles in diagnosis and
treatment, miRNAs also play crucial roles in the progression of OC.
Previous research has demonstrated that the expression of total
miRNAs can be used to reliably distinguish between normal and
malignant cells, and that miRNAs are abnormally expressed in human
OC compared to normal ovaries (165). Among the miRNAs examined,
miR-141, miR-200a, miR-200b and miR-200c were found to be
significantly overexpressed, while miR-199a, miR-140 and miR-145
were significantly downregulated in OC tissues (166). Furthermore, the overexpression
of miR-21, miR-203 and miR-205 in OC tissues, as opposed to normal
tissues, may be attributed to DNA hypomethylation, which has been
observed following the treatment of OVCAR3 cells with
5-aza-2'-deoxycytidine demethylation (166). Additionally, the
hypermethylation of miRNAs has been found to be associated with a
shorter survival rate of patients with OC, and the expression of
miRNAs, particularly that of miR-203a-3p, has been shown to be
significantly reduced in OC metastatic tumors (167). Knockdown of DNMT increases the
expression of miR-199a-3p, and the level of miR-199a-3p promoter
methylation is also significantly elevated in OC cells (168). The overexpression of miR-199a-3p
leads to a decrease in the expression of discoidin domain receptor
1, which subsequently reduces the migration and invasiveness of OC
cells (168). Moreover, the
miR-34 family has been shown to possess tumor suppressive
properties that mediate apoptosis and promote cellular senescence;
however, its expression in OC cells is significantly reduced,
primarily due to the methylation of miR-34a CpG islands. This
downregulation of miR-34a expression affects the grading and
prognosis of patients with OC (169). Zuberi et al (170) reported that DNA hypermethylation
may be involved in the inactivation of miR-125b, and miR-125b was
shown to be significantly associated with FIGO staging and the
metastasis of OC. The overexpression of ERBB2 or ERBB3 is known to
be associated with cancer development and poor prognosis. He et
al (171) demonstrated that
reactive oxygen species inhibited the expression of miR-199a and
miR-125b by increasing the promoter methylation of the miR-199a and
miR-125b genes through DNMT1. This led to changes in the expression
levels of ERBB2 and/or ERBB3 in OC cells, thereby attenuating the
progression of OC (171).
It has been reported that the overexpression of tet
methylcytosine dioxygenase 3 (TET3) can reverse TGF-β1-induced
EMT-like changes, mainly by demethylating the promoter of the
precursor gene of miR-30d. Thus, there is an association between
TET3 and the grade of differentiation of OC, and TET3 plays a role
in suppressing the progression of OC (172). miR-34a and miR-34b/c are direct
target genes of p53 and have tumor suppressor properties, as they
mediate apoptosis, cell cycle arrest and senescence (169). However, the inactivation of
miR-34 in OC suggests that the CpG methylation of miR-34a and
miR-34-b/c may be of diagnostic value. The mutual exclusivity of
miR-34a methylation and p53 mutations suggests that the
inactivation of miR-34a may substitute for the loss of p53 function
in cancer and induce the proliferation of OC cells (173). Therefore, conducting in-depth
studies on miRNAs may be beneficial for the further elucidation of
the pathogenesis of OC.
The expression levels of DNMTs in various ovarian
tissues have been found to be highly associated with the pathology
and survival outcomes of patients with OC. The 15-spliced protein
product or isoform encoded by DNMT3B is essential for the migration
and invasion of OC (174).
DNMT3B has been shown to methylate retinol binding protein 1, which
has both oncogenic and autophagic effects in OC cells (175). The inhibitory effects of TET3 on
the migration and invasion of OC can be diminished by DNMT3B
binding to the TET3 promoter, resulting in methylation of the
promoter region (176).
Therefore, blocking DNMT3B can reduce the growth, migration and
invasion of OC cells. However, in HGSOC, DNMT3B1 and DNMT3B3 are
overexpressed, and the overexpression of DNMT3B3 leads to the
marginal gene demethylation of OVCAR3 human OC cells, which is
associated with a poor prognosis (177). DNMTis, which are chemically
similar to deoxycytidine, have been shown to block methyl transfer
by inhibiting DNMT activity. Previous studies have confirmed the
potential therapeutic benefits of targeting DNMT in OC, leading to
better clinical outcomes and prognoses (174,178).
Through a process of viral sensing, DNMTis can also
induce the production of endogenous retroviruses, which in turn
triggers an interferon response in OC stem cells. Patients with OC
who exhibit a high expression of endogenous retrovirus have a
better chance of surviving their disease, as it increases the
efficiency with which cytotoxic immune cells kill EOC, and alters
the immune infiltration of tumors (179). It has also been demonstrated
that the use of DNMTis to suppress cadherin 13 (CDH13) promoter
methylation can lead to an increase in CDH13 expression in OC
cells, and a reversal of the malignant phenotype promoted by
hsa_circ_0000119 (180).
Additionally, several studies have demonstrated that combination
therapy with DNMTis and other treatments is more effective than
DNMTi monotherapy in the treatment of OC (181-183). In order to overcome platinum
resistance in patients with HGSOC, consecutive treatment with the
DNMTi, azacytidine, and carboplatin can demethylate and upregulate
immune response-related cells (181). Patients with HGSOC have
exhibited greater benefits from treatment with DNMTis when used in
combination with a histone methyltransferase inhibitor (182). Furthermore, the combined use of
a DNMTi and PARP inhibitor has been found to effectively inhibit
tumor cell proliferation and migration, while promoting apoptosis,
suggesting a potential therapeutic strategy for EOC (183).
Drug-resistant cancer cells have been found to have
DNA hypermethylation aberrations, and DNA hypermethylation,
produced by chemotherapy, has been proposed as a mechanism and
biomarker of drug resistance (174). Patients who have stopped
responding to conventional chemotherapy for OC may regain platinum
sensitivity following treatment with DNMTis. Patients with
recurrent platinum-resistant or poorly responding OC to
immunotherapy have been shown to have improved prognostic outcomes
and a longer survival time when treated with a combination of
DNMTis (184,185). Additionally, in patients with
recurrent platinum-resistant OC, the addition of decitabine (a DNA
hypomethylating drug) has been shown to enhance the clinical
results (186). Patients with OC
are more responsive to platinum therapy and have a better prognosis
when treated with decitabine plus carboplatin (12).
A previous study using a mouse model demonstrated
that DNMTis can also improve survival by increasing immunological
signaling, increasing viral defense gene expression in tumor and
immune cells, and decreasing the frequency of macrophages and
myeloid-derived suppressor cells in the tumor microenvironment
(184). Chemokine-like factor
(CKLF)-like MARVEL transmembrane domain containing 6 (CMTM6) is
overexpressed in OC compared to normal cells due to decreased DNA
methylation. Of note, a higher expression of CMTM6 has been shown
to be associated with higher immune cell infiltration, which, in
turn, can afefct prognosis (187). A recent study demonstrated that
the production of pro-inflammatory cytokines/chemokines in human OC
cell lines was markedly increased following in vitro DNMTi
therapy in combination with the editing of transposable factors
(188). These results suggest
that the therapeutic effects of DNMTis may occur through the
modification of the immune response and the OC
microenvironment.
Although DNMTis are effective in preventing,
treating and determining the prognosis of OC, drug resistance,
adverse effects and a poor treatment response continue to be
obstacles to the widespread implementation of DNMTi therapeutic
regimens. CpG hypermethylation may enhance cancer cell
proliferation and alter the response to DNMTis, as previously
observed by Giri et al (189), which raises doubts about the
usefulness of DNMTis in the treatment of patients with OC. However,
DNMTis have demonstrated efficacy in reducing or eliminating
resistance to chemotherapy and molecular targeting in OC patients.
Therefore, further research is required in order to explore the
application of DNMTis and verify their viability through clinical
studies.
Despite the notable advances made in the treatment
of OC in recent years, the majority of patients with advanced-stage
OC continue to experience recurrence and eventually succumb to
chemoresistance. Tumorigenesis, progression and resistance to
treatment are predominantly mediated by epigenetic regulation,
particularly DNA methylation. The present review aimed to provide
an overview of methylation-specific modifications of genes related
to OC and their clinical applications, thereby emphasizing the
significance of DNA methylation in OC. In general, tumor suppressor
genes, such as BRCA1/2, p53, RASSF1A, CHD5, FBP1, ALDH1A2, FOXD3,
IGFBP-3, ZNF671, SPARC and MGMT are often found to be
underexpressed and hypermethylated in OC tissues (Table II). Conversely, oncogenes, such
as HOXA9, CBX8, SLC6A12, AGR2 and GABRP exhibit a high expression
and DNA hypomethylation (Table
III). In addition to this, the study by Bauerschlag et
al (190) discovered that
the hypomethylation of genes such as growth regulating estrogen
receptor binding 1, TGFB induced factor homeobox 1 and transducer
of ERBB2, and the hypermethylation of genes such as transmembrane
and coiled-coil domains 5, protein tyrosine phosphatase receptor
type N and guanylate cyclase 2C, were associated with longer
survival periods of patients with OC, suggesting potential
prognostic value. The altered DNA methylation of some genes of the
classical pathway can also have an impact on the development of OC
(Tables IV and V). miRNAs play a more intricate role in
the development of OC. Their expression may be downregulated due to
gene hypermethylation, such as the expression of miR-152 and
miR-148a (191), or they may be
overexpressed due to gene hypomethylation, such as miR-21, miR-203
and miR-205 (166) (Table VI). Overall, miRNAs serve as
target genes, and investigating whether they are regulated by DNA
methylation contributes to the development, diagnosis, staging and
treatment resistance of OC, and thus warrants further exploration
of their potential clinical applications. Building on comprehensive
clinical studies exploring the link between DNA methylation and OC,
DNMTis have emerged as a promising therapeutic avenue in clinical
settings. They play a pivotal role in overcoming chemoresistance
and recurrence in OC. Current therapeutic strategies include the
combined use of DNMTis with histone deacetylase inhibitors, DNMTis
with PARP inhibitors, among others. These combinations could
potentially open up new clinical trial opportunities for patients
with advanced malignant ovarian tumors who are unresponsive to
immunotherapy.
Epigenetics, and in particular DNA methylation, is
now providing novel and very promising techniques for the discovery
of specific biomarkers and their subsequent screening. As
previously described by Belsky et al (192), the DNA methylation of related
genes can be used as a biomarker to predict the rate of aging.
Previous studies on DNA methylation in OC have provided critical
evidence for understanding ovarian tumorigenesis (61-64). These findings provide potential
diagnostic biomarkers and therapeutic targets. However, numerous
inconsistencies remain regarding the results of aberrant DNA
methylation within these tumor suppressor genes in OC. When
analyzing the possible reasons for these inconsistencies, the most
significant reason is the sample size. Human samples vary greatly
in terms of genetics, environment, lifestyle and individual
differences. In a number of studies, the sample size is usually too
small due to the huge variation in patients with OC. Future studies
are urgently required to address these controversies by analyzing
large sample sizes. In addition to this, factors such as the lack
of functional studies, differences in the methods of DNA
methylation detection used, and different promoter regions for DNA
methylation detection may also contribute to the discrepancies.
Based on the evidence provided in the present review, targeted DNA
methylation inhibitors have promising applications in the treatment
of OC. Therefore, it is evident that additional studies are
warranted to bridge existing knowledge gaps and reconcile
inconsistencies. In light of the known limitations, future research
directions, including conducting larger multicenter studies, the
development of animal models to determine causality, and the
initiation of clinical trials involving methylating or
demethylating drugs are proposed. In conclusion, the present review
aimed to broaden the understanding of the role of DNA methylation
in OC and determine its potential as a biomarker. This could also
the focus for future research and further in-depth analysis of this
disease. The ultimate goal is to facilitate early diagnosis and
treatment, and to promptly address the pressing clinical issues of
OC recurrence and chemoresistance.
Not applicable.
HD, QG and MF were involved in the writing and
preparation of the original draft of the manuscript. QG, HD, FD,
MF, YC, JZ, TX, JC, JL and LF were involved in the writing,
reviewing and editing of the manuscript. QG, FD, YC, JZ, HD and TX
supervised the study. QG, JC, JL, MF and HD were involved in
project administration. All authors have read and agreed to the
published version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported in part by the National Nature
and Science Foundation of China (grant nos. 82271724, 81873841,
81741024 and 81401244), the Ministry of Science and Technology
(grant no. 2019YFA0802600), the Suzhou City 'Wei Sheng Ren Cai
(GSWS2019029)' program, the General Programs of Jiangsu Commission
of Health (grant no. M2021087), the Nature and Science Foundation
of Jiangsu (grant no. BK20221243) and the Suzhou city Medical and
health technology innovation Project (grant no. SKY2021035).
|
1
|
Lumish MA, Kohn EC and Tew WP: Top
advances of the year: Ovarian cancer. Cancer. 130:837–845.
2024.
|
|
2
|
Tang H, Kulkarni S, Peters C, Eddison J,
Al-Ani M and Madhusudan S: The current status of
DNA-repair-directed precision oncology strategies in epithelial
ovarian cancers. Int J Mol Sci. 24:72932023.
|
|
3
|
Feng J, Xu L, Chen Y, Lin R, Li H and He
H: Trends in incidence and mortality for ovarian cancer in China
from 1990 to 2019 and its forecasted levels in 30 years. J Ovarian
Res. 16:1392023.
|
|
4
|
Bodelon C, Killian JK, Sampson JN,
Anderson WF, Matsuno R, Brinton LA, Lissowska J, Anglesio MS,
Bowtell DDL, Doherty JA, et al: Molecular classification of
epithelial ovarian cancer based on methylation profiling: Evidence
for survival heterogeneity. Clin Cancer Res. 25:5937–5946.
2019.
|
|
5
|
Zhang M, Cheng S, Jin Y, Zhao Y and Wang
Y: Roles of CA125 in diagnosis, prediction, and oncogenesis of
ovarian cancer. Biochim Biophys Acta Rev Cancer.
1875:1885032021.
|
|
6
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.
|
|
7
|
Natanzon Y, Goode EL and Cunningham JM:
Epigenetics in ovarian cancer. Semin Cancer Biol. 51:160–169.
2018.
|
|
8
|
Moufarrij S, Dandapani M, Arthofer E,
Gomez S, Srivastava A, Lopez-Acevedo M, Villagra A and Chiappinelli
KB: Epigenetic therapy for ovarian cancer: Promise and progress.
Clin Epigenetics. 11:72019.
|
|
9
|
Peng S, Zhang X and Wu Y: Potential
applications of DNA methylation testing technology in female tumors
and screening methods. Biochim Biophys Acta Rev Cancer.
1878:1889412023.
|
|
10
|
Antonino M, Nicolò M, Jerome Renee L,
Federico M, Chiara V, Stefano S, Maria S, Salvatore C, Antonio B,
Calvo-Henriquez C, et al: Single-nucleotide polymorphism in chronic
rhinosinusitis: A systematic review. Clin Otolaryngol. 47:14–23.
2022.
|
|
11
|
Matei D and Nephew KP: Epigenetic attire
in ovarian cancer: The emperor's new clothes. Cancer Res.
80:3775–3785. 2020.
|
|
12
|
Matei D, Fang F, Shen C, Schilder J,
Arnold A, Zeng Y, Berry WA, Huang T and Nephew KP: Epigenetic
resensitization to platinum in ovarian cancer. Cancer Res.
72:2197–2205. 2012.
|
|
13
|
Meng H, Cao Y, Qin J, Song X, Zhang Q, Shi
Y and Cao L: DNA methylation, its mediators and genome integrity.
Int J Biol Sci. 11:604–617. 2015.
|
|
14
|
Singh A, Gupta S and Sachan M: Epigenetic
biomarkers in the management of ovarian cancer: Current
prospectives. Front Cell Dev Biol. 7:1822019.
|
|
15
|
Ma L, Li C, Yin H, Huang J, Yu S, Zhao J,
Tang Y, Yu M, Lin J, Ding L and Cui Q: The mechanism of DNA
methylation and miRNA in breast cancer. Int J Mol Sci.
24:93602023.
|
|
16
|
Coughlan AY and Testa G: Exploiting
epigenetic dependencies in ovarian cancer therapy. Int J Cancer.
149:1732–1743. 2021.
|
|
17
|
Xie W, Sun H, Li X, Lin F, Wang Z and Wang
X: Ovarian cancer: Epigenetics, drug resistance, and progression.
Cancer Cell Int. 21:4342021.
|
|
18
|
Klymenko Y and Nephew KP: Epigenetic
crosstalk between the tumor microenvironment and ovarian cancer
cells: A therapeutic road less traveled. Cancers (Basel).
10:2952018.
|
|
19
|
Yang Y, Wu L, Shu X, Lu Y, Shu XO, Cai Q,
Beeghly-Fadiel A, Li B, Ye F, Berchuck A, et al: Genetic data from
nearly 63,000 women of european descent predicts DNA methylation
biomarkers and epithelial ovarian cancer risk. Cancer Res.
79:505–517. 2019.
|
|
20
|
Lo Riso P, Villa CE, Gasparoni G, Vingiani
A, Luongo R, Manfredi A, Jungmann A, Bertolotti A, Borgo F, Garbi
A, et al: A cell-of-origin epigenetic tracer reveals clinically
distinct subtypes of high-grade serous ovarian cancer. Genome Med.
12:942020.
|
|
21
|
Tomar T, Alkema NG, Schreuder L, Meersma
GJ, de Meyer T, van Criekinge W, Klip HG, Fiegl H, van
Nieuwenhuysen E, Vergote I, et al: Methylome analysis of extreme
chemoresponsive patients identifies novel markers of platinum
sensitivity in high-grade serous ovarian cancer. BMC Med.
15:1162017.
|
|
22
|
Feng LY, Yan BB, Huang YZ and Li L:
Abnormal methylation characteristics predict chemoresistance and
poor prognosis in advanced high-grade serous ovarian cancer. Clin
Epigenetics. 13:1412021.
|
|
23
|
Yang D, Khan S, Sun Y, Hess K, Shmulevich
I, Sood AK and Zhang W: Association of BRCA1 and BRCA2 mutations
with survival, chemotherapy sensitivity, and gene mutator phenotype
in patients with ovarian cancer. JAMA. 306:1557–1565. 2011.
|
|
24
|
Chan KY, Ozçelik H, Cheung AN, Ngan HY and
Khoo US: Epigenetic factors controlling the BRCA1 and BRCA2 genes
in sporadic ovarian cancer. Cancer Res. 62:4151–4156. 2002.
|
|
25
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019.
|
|
26
|
Moschetta M, George A, Kaye SB and
Banerjee S: BRCA somatic mutations and epigenetic BRCA
modifications in serous ovarian cancer. Ann Oncol. 27:1449–1455.
2016.
|
|
27
|
Glajzer J, Castillo-Tong DC, Richter R,
Vergote I, Kulbe H, Vanderstichele A, Ruscito I, Trillsch F, Mustea
A, Kreuzinger C, et al: Impact of BRCA mutation status on tumor
dissemination pattern, surgical outcome and patient survival in
primary and recurrent high-grade serous ovarian cancer: A
multicenter retrospective study by the ovarian cancer
therapy-innovative models prolong survival (OCTIPS) consortium. Ann
Surg Oncol. 30:35–45. 2023.
|
|
28
|
Jung Y, Hur S, Liu J, Lee S, Kang BS, Kim
M and Choi YJ: Peripheral blood BRCA1 methylation profiling to
predict familial ovarian cancer. J Gynecol Oncol. 32:e232021.
|
|
29
|
Barrett JE, Jones A, Evans I, Reisel D,
Herzog C, Chindera K, Kristiansen M, Leavy OC, Manchanda R, Bjørge
L, et al: The DNA methylome of cervical cells can predict the
presence of ovarian cancer. Nat Commun. 13:4482022.
|
|
30
|
Wu TI, Huang RL, Su PH, Mao SP, Wu CH and
Lai HC: Ovarian cancer detection by DNA methylation in cervical
scrapings. Clin Epigenetics. 11:1662019.
|
|
31
|
Bartlett TE, Chindera K, McDermott J,
Breeze CE, Cooke WR, Jones A, Reisel D, Karegodar ST, Arora R, Beck
S, et al: Epigenetic reprogramming of fallopian tube fimbriae in
BRCA mutation carriers defines early ovarian cancer evolution. Nat
Commun. 7:116202016.
|
|
32
|
Ibragimova I and Cairns P: Assays for
hypermethylation of the BRCA1 gene promoter in tumor cells to
predict sensitivity to PARP-inhibitor therapy. Methods Mol Biol.
780:277–291. 2011.
|
|
33
|
Sahnane N, Carnevali I, Formenti G,
Casarin J, Facchi S, Bombelli R, Di Lauro E, Memoli D, Salvati A,
Rizzo F, et al: BRCA methylation testing identifies a subset of
ovarian carcinomas without germline variants that can benefit from
PARP inhibitor. Int J Mol Sci. 21:97082020.
|
|
34
|
Taniguchi T, Tischkowitz M, Ameziane N,
Hodgson SV, Mathew CG, Joenje H, Mok SC and D'Andrea AD: Disruption
of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian
tumors. Nat Med. 9:568–574. 2003.
|
|
35
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011.
|
|
36
|
Alsop K, Fereday S, Meldrum C, deFazio A,
Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, et
al: BRCA mutation frequency and patterns of treatment response in
BRCA mutation-positive women with ovarian cancer: A report from the
Australian ovarian cancer study group. J Clin Oncol. 30:2654–2663.
2012.
|
|
37
|
Mikeska T, Alsop K; Australian Ovarian
Cancer Study Group; Mitchell G, Bowtell DD and Dobrovic A: No
evidence for PALB2 methylation in high-grade serous ovarian cancer.
J Ovarian Res. 6:262013.
|
|
38
|
McAlpine JN, Porter H, Köbel M, Nelson BH,
Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, et al:
BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and
presence of immune cell infiltrates in ovarian high-grade serous
carcinoma. Mod Pathol. 25:740–750. 2012.
|
|
39
|
Shariati-Kohbanani M, Zare-Bidaki M,
Taghavi MM, Taghipour Z, Shabanizadeh A, Kennedy D, Dahim H,
Salahshoor MR, Jalili C and Kazemi Arababadi M: DNA methylation and
microRNA patterns are in association with the expression of BRCA1
in ovarian cancer. Cell Mol Biol (Noisy-le-grand). 62:16–23.
2016.
|
|
40
|
Soslow RA, Han G, Park KJ, Garg K, Olvera
N, Spriggs DR, Kauff ND and Levine DA: Morphologic patterns
associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod
Pathol. 25:625–536. 2012.
|
|
41
|
Kraya AA, Maxwell KN, Eiva MA, Wubbenhorst
B, Pluta J, Feldman M, Nayak A, Powell DJ Jr, Domchek SM,
Vonderheide RH and Nathanson KL: PTEN loss and BRCA1 promoter
hypermethylation negatively predict for immunogenicity in
BRCA-deficient ovarian cancer. JCO Precis Oncol.
6:e21001592022.
|
|
42
|
Ebata T, Yamashita S, Takeshima H, Yoshida
H, Kawata Y, Kino N, Yasugi T, Terao Y, Yonemori K, Kato T and
Ushijima T: DNA methylation of the immediate upstream region of
BRCA1 major transcription start sites is an independent favorable
prognostic factor in patients with high-grade serous ovarian
cancer. Gynecol Oncol. 167:513–518. 2022.
|
|
43
|
Bai X, Fu Y, Xue H, Guo K, Song Z, Yu Z,
Jia T, Yan Y, Zhao L, Mi X, et al: BRCA1 promoter hypermethylation
in sporadic epithelial ovarian carcinoma: Association with low
expression of BRCA1, improved survival and co-expression of DNA
methyltransferases. Oncol Lett. 7:1088–1096. 2014.
|
|
44
|
Pradjatmo H: Methylation status and
expression of BRCA2 in epithelial ovarian cancers in Indonesia.
Asian Pac J Cancer Prev. 16:8599–8604. 2015.
|
|
45
|
Lane DP: p53 and human cancers. Br Med
Bull. 50:582–599. 1994.
|
|
46
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012.
|
|
47
|
Chen YC, Young MJ, Chang HP, Liu CY, Lee
CC, Tseng YL, Wang YC, Chang WC and Hung JJ: Estradiol-mediated
inhibition of DNMT1 decreases p53 expression to induce
M2-macrophage polarization in lung cancer progression. Oncogenesis.
11:252022.
|
|
48
|
Suh SO, Chen Y, Zaman MS, Hirata H,
Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, et
al: MicroRNA-145 is regulated by DNA methylation and p53 gene
mutation in prostate cancer. Carcinogenesis. 32:772–778. 2011.
|
|
49
|
Cunningham JM, Winham SJ, Wang C, Weiglt
B, Fu Z, Armasu SM, McCauley BM, Brand AH, Chiew YE, Elishaev E, et
al: DNA methylation profiles of ovarian clear cell carcinoma.
Cancer Epidemiol Biomarkers Prev. 31:132–141. 2022.
|
|
50
|
Chmelarova M, Krepinska E, Spacek J, Laco
J, Beranek M and Palicka V: Methylation in the p53 promoter in
epithelial ovarian cancer. Clin Transl Oncol. 15:160–163. 2013.
|
|
51
|
Kelley KD, Miller KR, Todd A, Kelley AR,
Tuttle R and Berberich SJ: YPEL3, a p53-regulated gene that induces
cellular senescence. Cancer Res. 70:3566–3575. 2010.
|
|
52
|
Abdollahi A: LOT1 (ZAC1/PLAGL1) and its
family members: Mechanisms and functions. J Cell Physiol.
210:16–25. 2007.
|
|
53
|
Su HC, Wu SC, Yen LC, Chiao LK, Wang JK,
Chiu YL, Ho CL and Huang SM: Gene expression profiling identifies
the role of Zac1 in cervical cancer metastasis. Sci Rep.
10:118372020.
|
|
54
|
Cheng JC, Auersperg N and Leung PC:
Inhibition of p53 represses E-cadherin expression by increasing DNA
methyltransferase-1 and promoter methylation in serous borderline
ovarian tumor cells. Oncogene. 30:3930–3942. 2011.
|
|
55
|
Bin Y, Ding Y, Xiao W and Liao A: RASSF1A:
A promising target for the diagnosis and treatment of cancer. Clin
Chim Acta. 504:98–108. 2020.
|
|
56
|
Wei B, Wu F, Xing W, Sun H, Yan C, Zhao C,
Wang D, Chen X, Chen Y, Li M and Ma J: A panel of DNA methylation
biomarkers for detection and improving diagnostic efficiency of
lung cancer. Sci Rep. 11:167822021.
|
|
57
|
Tang Q, Cheng J, Cao X, Surowy H and
Burwinkel B: Blood-based DNA methylation as biomarker for breast
cancer: A systematic review. Clin Epigenetics. 8:1152016.
|
|
58
|
Shi H, Li Y, Wang X, Lu C, Yang L, Gu C,
Xiong J, Huang Y, Wang S and Lu M: Association between RASSF1A
promoter methylation and ovarian cancer: A meta-analysis. PLoS One.
8:e767872013.
|
|
59
|
Dammann R, Schagdarsurengin U, Strunnikova
M, Rastetter M, Seidel C, Liu L, Tommasi S and Pfeifer GP:
Epigenetic inactivation of the Ras-association domain family 1
(RASSF1A) gene and its function in human carcinogenesis. Histol
Histopathol. 18:665–677. 2003.
|
|
60
|
Terp SK, Stoico MP, Dybkaer K and Pedersen
IS: Early diagnosis of ovarian cancer based on methylation profiles
in peripheral blood cell-free DNA: A systematic review. Clin
Epigenetics. 15:242023.
|
|
61
|
Rezk NA, Mohamed RH, Alnemr AA and Harira
M: Promoter methylation of RASSF1A gene in egyptian patients with
ovarian cancer. Appl Biochem Biotechnol. 185:153–162. 2018.
|
|
62
|
Bhagat R, Chadaga S, Premalata CS, Ramesh
G, Ramesh C, Pallavi VR and Krishnamoorthy L: Aberrant promoter
methylation of the RASSF1A and APC genes in epithelial ovarian
carcinoma development. Cell Oncol (Dordr). 35:473–479. 2012.
|
|
63
|
Xing BL, Li T, Tang ZH, Jiao L, Ge SM,
Qiang X and OuYang J: Cumulative methylation alternations of gene
promoters and protein markers for diagnosis of epithelial ovarian
cancer. Genet Mol Res. 14:4532–4540. 2015.
|
|
64
|
Vo LT, Thuan TB, Thu DM, Uyen NQ, Ha NT
and To TV: Methylation profile of BRCA1, RASSF1A and ER in
Vietnamese women with ovarian cancer. Asian Pac J Cancer Prev.
14:7713–7718. 2013.
|
|
65
|
Wang H, Cui M, Zhang S, He J, Song L and
Chen Y: Relationship between RAS association domain family protein
1A promoter methylation and the clinicopathological characteristics
in patients with ovarian cancer: A systematic meta-analysis.
Gynecol Obstet Invest. 83:349–357. 2018.
|
|
66
|
Ho CM, Yen TL, Chien TY and Huang SH:
Distinct promotor methylation at tumor suppressive genes in ovarian
cancer stromal progenitor cells and ovarian cancer and its clinical
implication. Am J Cancer Res. 12:5325–5341. 2022.
|
|
67
|
Ho CM, Shih DTB, Hsiao CC, Huang SH, Chang
SF and Cheng WF: Gene methylation of human ovarian carcinoma
stromal progenitor cells promotes tumorigenesis. J Transl Med.
13:3672015.
|
|
68
|
Reyes HD, Devor EJ, Warrier A, Newtson AM,
Mattson J, Wagner V, Duncan GN, Leslie KK and Gonzalez-Bosquet J:
Differential DNA methylation in high-grade serous ovarian cancer
(HGSOC) is associated with tumor behavior. Sci Rep.
9:179962019.
|
|
69
|
Feng Q, Deftereos G, Hawes SE, Stern JE,
Willner JB, Swisher EM, Xi L, Drescher C, Urban N and Kiviat N: DNA
hypermethylation, Her-2/neu overexpression and p53 mutations in
ovarian carcinoma. Gynecol Oncol. 111:320–329. 2008.
|
|
70
|
Xie G, Hu C and Huang M: Methylation
status of RASSF1A and clinical efficacy of neoadjuvant therapy in
patients with advanced epithelial ovarian cancer. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 36:631–633. 2011.In Chinese.
|
|
71
|
Giannopoulou L, Chebouti I, Pavlakis K,
Kasimir-Bauer S and Lianidou ES: RASSF1A promoter methylation in
high-grade serous ovarian cancer: A direct comparison study in
primary tumors, adjacent morphologically tumor cell-free tissues
and paired circulating tumor DNA. Oncotarget. 8:21429–21443.
2017.
|
|
72
|
S SK, Swamy SN, Premalatha CS, Pallavi VR
and Gawari R: Aberrant promoter hypermethylation of RASSF1a and
BRCA1 in circulating cell-free tumor DNA serves as a biomarker of
ovarian carcinoma. Asian Pac J Cancer Prev. 20:3001–3005. 2019.
|
|
73
|
Marfella CG and Imbalzano AN: The Chd
family of chromatin remodelers. Mutat Res. 618:30–40. 2007.
|
|
74
|
Zhao R, Yan Q, Lv J, Huang H, Zheng W,
Zhang B and Ma W: CHD5, a tumor suppressor that is epigenetically
silenced in lung cancer. Lung Cancer. 76:324–231. 2012.
|
|
75
|
Xie CR, Li Z, Sun HG, Wang FQ, Sun Y, Zhao
WX, Zhang S, Zhao WX, Wang XM and Yin ZY: Mutual regulation between
CHD5 and EZH2 in hepatocellular carcinoma. Oncotarget.
6:40940–40952. 2015.
|
|
76
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013.
|
|
77
|
Du Z, Li L, Huang X, Jin J, Huang S, Zhang
Q and Tao Q: The epigenetic modifier CHD5 functions as a novel
tumor suppressor for renal cell carcinoma and is predominantly
inactivated by promoter CpG methylation. Oncotarget. 7:21618–21630.
2016.
|
|
78
|
Ma Z, Song J, Liu S, Han L, Chen Y, Wang
Y, Yu C and Hou L: Decreased expression of the CHD5 gene and its
clinicopathological significance in breast cancer: Correlation with
aberrant DNA methylation. Oncol Lett. 12:4021–4026. 2016.
|
|
79
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013.
|
|
80
|
Li H, Qi Z, Niu Y, Yang Y, Li M, Pang Y,
Liu M, Cheng X, Xu M and Wang Z: FBP1 regulates proliferation,
metastasis, and chemoresistance by participating in C-MYC/STAT3
signaling axis in ovarian cancer. Oncogene. 40:5938–5949. 2021.
|
|
81
|
Wang Y, Shao F and Chen L: ALDH1A2
suppresses epithelial ovarian cancer cell proliferation and
migration by downregulating STAT3. Onco Targets Ther. 11:599–608.
2018.
|
|
82
|
Choi JA, Kwon H, Cho H, Chung JY, Hewitt
SM and Kim JH: ALDH1A2 is a candidate tumor suppressor gene in
ovarian cancer. Cancers (Basel). 11:15532019.
|
|
83
|
Zhu L, Zhang S and Jin Y: Foxd3 suppresses
NFAT-mediated differentiation to maintain self-renewal of embryonic
stem cells. EMBO Rep. 15:1286–1296. 2014.
|
|
84
|
He GY, Hu JL, Zhou L, Zhu XH, Xin SN,
Zhang D, Lu GF, Liao WT, Ding YQ and Liang L: The
FOXD3/miR-214/MED19 axis suppresses tumour growth and metastasis in
human colorectal cancer. Br J Cancer. 115:1367–1378. 2016.
|
|
85
|
Cheng AS, Li MS, Kang W, Cheng VY, Chou
JL, Lau SS, Go MY, Lee CC, Ling TK, Ng EK, et al: Helicobacter
pylori causes epigenetic dysregulation of FOXD3 to promote gastric
carcinogenesis. Gastroenterology. 144:122–133.e9. 2013.
|
|
86
|
Luo GF, Chen CY, Wang J, Yue HY, Tian Y,
Yang P, Li YK and Li Y: FOXD3 may be a new cellular target
biomarker as a hypermethylation gene in human ovarian cancer.
Cancer Cell Int. 19:442019.
|
|
87
|
Katsaros D, Yu H, Levesque MA, Danese S,
Genta F, Richiardi G, Fracchioli S, Khosravi MJ, Diamandi A,
Gordini G, et al: IGFBP-3 in epithelial ovarian carcinoma and its
association with clinico-pathological features and patient
survival. Eur J Cancer. 37:478–485. 2001.
|
|
88
|
Wiley A, Katsaros D, Fracchioli S and Yu
H: Methylation of the insulin-like growth factor binding protein-3
gene and prognosis of epithelial ovarian cancer. Int J Gynecol
Cancer. 16:210–218. 2006.
|
|
89
|
Torng PL, Lin CW, Chan MW, Yang HW, Huang
SC and Lin CT: Promoter methylation of IGFBP-3 and p53 expression
in ovarian endometrioid carcinoma. Mol Cancer. 8:1202009.
|
|
90
|
Urrutia R: KRAB-containing zinc-finger
repressor proteins. Genome Biol. 4:2312003.
|
|
91
|
Cheng Y, Geng H, Cheng SH, Liang P, Bai Y,
Li J, Srivastava G, Ng MH, Fukagawa T, Wu X, et al: KRAB zinc
finger protein ZNF382 is a proapoptotic tumor suppressor that
represses multiple oncogenes and is commonly silenced in multiple
carcinomas. Cancer Res. 70:6516–6526. 2010.
|
|
92
|
Mase S, Shinjo K, Totani H, Katsushima K,
Arakawa A, Takahashi S, Lai HC, Lin RI, Chan MWY, Sugiura-Ogasawara
M and Kondo Y: ZNF671 DNA methylation as a molecular predictor for
the early recurrence of serous ovarian cancer. Cancer Sci.
110:1105–1116. 2019.
|
|
93
|
Yan J, Zhang J, Zhang X, Li X, Li L, Li Z,
Chen R, Zhang L, Wu J, Wang X, et al: SPARC is down-regulated by
DNA methylation and functions as a tumor suppressor in T-cell
lymphoma. Exp Cell Res. 364:125–132. 2018.
|
|
94
|
Nagaraju GP and El-Rayes BF: SPARC and DNA
methylation: Possible diagnostic and therapeutic implications in
gastrointestinal cancers. Cancer Lett. 328:10–17. 2013.
|
|
95
|
Singh A, Gupta S and Sachan M: Evaluation
of the diagnostic potential of candidate hypermethylated genes in
epithelial ovarian cancer in North Indian population. Front Mol
Biosci. 8:7190562021.
|
|
96
|
Niskakoski A, Kaur S, Staff S,
Renkonen-Sinisalo L, Lassus H, Järvinen HJ, Mecklin JP, Bützow R
and Peltomäki P: Epigenetic analysis of sporadic and
Lynch-associated ovarian cancers reveals histology-specific
patterns of DNA methylation. Epigenetics. 9:1577–1587. 2014.
|
|
97
|
Socha MJ, Said N, Dai Y, Kwong J,
Ramalingam P, Trieu V, Desai N, Mok SC and Motamed K: Aberrant
promoter methylation of SPARC in ovarian cancer. Neoplasia.
11:126–135. 2009.
|
|
98
|
Gusyatiner O and Hegi ME: Glioma
epigenetics: From subclassification to novel treatment options.
Semin Cancer Biol. 51:50–58. 2018.
|
|
99
|
Shilpa V, Bhagat R, Premalata CS, Pallavi
VR, Ramesh G and Krishnamoorthy L: Relationship between promoter
methylation & tissue expression of MGMT gene in ovarian cancer.
Indian J Med Res. 140:616–623. 2014.
|
|
100
|
Martínez-Jiménez F, Muiños F, Sentís I,
Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, Mularoni L, Pich O,
Bonet J, Kranas H, et al: A compendium of mutational cancer driver
genes. Nat Rev Cancer. 20:555–572. 2020.
|
|
101
|
Eun Kwon H and Taylor HS: The role of HOX
genes in human implantation. Ann N Y Acad Sci. 1034:1–18. 2004.
|
|
102
|
Widschwendter M, Apostolidou S, Jones AA,
Fourkala EO, Arora R, Pearce CL, Frasco MA, Ayhan A, Zikan M,
Cibula D, et al: HOXA methylation in normal endometrium from
premenopausal women is associated with the presence of ovarian
cancer: A proof of principle study. Int J Cancer. 125:2214–2218.
2009.
|
|
103
|
Wu Q, Lothe RA, Ahlquist T, Silins I,
Tropé CG, Micci F, Nesland JM, Suo Z and Lind GE: DNA methylation
profiling of ovarian carcinomas and their in vitro models
identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol
Cancer. 6:452007.
|
|
104
|
Montavon C, Gloss BS, Warton K, Barton CA,
Statham AL, Scurry JP, Tabor B, Nguyen TV, Qu W, Samimi G, et al:
Prognostic and diagnostic significance of DNA methylation patterns
in high grade serous ovarian cancer. Gynecol Oncol. 124:582–588.
2012.
|
|
105
|
Singh A, Gupta S, Badarukhiya JA and
Sachan M: Detection of aberrant methylation of HOXA9 and HIC1
through multiplex MethyLight assay in serum DNA for the early
detection of epithelial ovarian cancer. Int J Cancer.
147:1740–1752. 2020.
|
|
106
|
Faaborg L, Fredslund Andersen R, Waldstrøm
M, Høgdall E, Høgdall C, Adimi P, Jakobsen A and Dahl Steffensen K:
Analysis of HOXA9 methylated ctDNA in ovarian cancer using
sense-antisense measurement. Clin Chim Acta. 522:152–157. 2021.
|
|
107
|
Rusan M, Andersen RF, Jakobsen A and
Steffensen KD: Circulating HOXA9-methylated tumour DNA: A novel
biomarker of response to poly (ADP-ribose) polymerase inhibition in
BRCA-mutated epithelial ovarian cancer. Eur J Cancer. 125:121–129.
2020.
|
|
108
|
van Wijnen AJ, Bagheri L, Badreldin AA,
Larson AN, Dudakovic A, Thaler R, Paradise CR and Wu Z: Biological
functions of chromobox (CBX) proteins in stem cell self-renewal,
lineage-commitment, cancer and development. Bone.
143:1156592021.
|
|
109
|
Lin J, Chen L, Wu D, Lin J, Liu B and Guo
C: Potential diagnostic and prognostic values of CBX8 Expression in
liver hepatocellular carcinoma, kidney renal clear cell carcinoma,
and ovarian cancer: A study based on TCGA data mining. Comput Math
Methods Med. 2022:13728792022.
|
|
110
|
Li Q, Pan Y, Cao Z and Zhao S:
Comprehensive analysis of prognostic value and immune infiltration
of chromobox family members in colorectal cancer. Front Oncol.
10:5826672020.
|
|
111
|
Sung HY, Yang SD, Park AK, Ju W and Ahn
JH: Aberrant hypomethylation of solute carrier family 6 member 12
promoter induces metastasis of ovarian cancer. Yonsei Med J.
58:27–34. 2017.
|
|
112
|
Higa A, Mulot A, Delom F, Bouchecareilh M,
Nguyên DT, Boismenu D, Wise MJ and Chevet E: Role of pro-oncogenic
protein disulfide isomerase (PDI) family member anterior gradient 2
(AGR2) in the control of endoplasmic reticulum homeostasis. J Biol
Chem. 286:44855–44868. 2011.
|
|
113
|
Zhang S, Liu Q, Wei Y, Xiong Y, Gu Y,
Huang Y, Tang F and Ouyang Y: Anterior gradient-2 regulates cell
communication by coordinating cytokine-chemokine signaling and
immune infiltration in breast cancer. Cancer Sci. 114:2238–2253.
2023.
|
|
114
|
He J, Fu Y, Hu J, Chen J and Lou G:
Hypomethylation-mediated AGR2 overexpression facilitates cell
proliferation, migration, and invasion of lung adenocarcinoma.
Cancer Manag Res. 13:5177–5185. 2021.
|
|
115
|
Sung HY, Choi EN, Lyu D, Park AK, Ju W and
Ahn JH: Aberrant hypomethylation-mediated AGR2 overexpression
induces an aggressive phenotype in ovarian cancer cells. Oncol Rep.
32:815–820. 2014.
|
|
116
|
Chan DW, Lam WY, Chen F, Yung MMH, Chan
YS, Chan WS, He F, Liu SS, Chan KKL, Li B and Ngan HYS: Genome-wide
DNA methylome analysis identifies methylation signatures associated
with survival and drug resistance of ovarian cancers. Clin
Epigenetics. 13:1422021.
|
|
117
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017.
|
|
118
|
Li HJ, Ke FY, Lin CC, Lu MY, Kuo YH, Wang
YP, Liang KH, Lin SC, Chang YH, Chen HY, et al: ENO1 promotes lung
cancer metastasis via HGFR and WNT signaling-driven
epithelial-to-mesenchymal transition. Cancer Res. 81:4094–4109.
2021.
|
|
119
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: biological mechanisms, challenges and
opportunities. Mol Cancer. 19:1652020.
|
|
120
|
Hu W, Li M, Chen Y and Gu X: UBE2S
promotes the progression and Olaparib resistance of ovarian cancer
through Wnt/β-catenin signaling pathway. J Ovarian Res.
14:1212021.
|
|
121
|
Deshmukh A, Arfuso F, Newsholme P and
Dharmarajan A: Epigenetic demethylation of sFRPs, with emphasis on
sFRP4 activation, leading to Wnt signalling suppression and histone
modifications in breast, prostate, and ovary cancer stem cells. Int
J Biochem Cell Biol. 109:23–32. 2019.
|
|
122
|
Takada T, Yagi Y, Maekita T, Imura M,
Nakagawa S, Tsao SW, Miyamoto K, Yoshino O, Yasugi T, Taketani Y
and Ushijima T: Methylation-associated silencing of the Wnt
antagonist SFRP1 gene in human ovarian cancers. Cancer Sci.
95:741–744. 2004.
|
|
123
|
Yen HY, Tsao CW, Lin YW, Kuo CC, Tsao CH
and Liu CY: Regulation of carcinogenesis and modulation through
Wnt/β-catenin signaling by curcumin in an ovarian cancer cell line.
Sci Rep. 9:172672019.
|
|
124
|
Ho CM, Lai HC, Huang SH, Chien TY, Lin MC
and Chang SF: Promoter methylation of sFRP5 in patients with
ovarian clear cell adenocarcinoma. Eur J Clin Invest. 40:310–318.
2010.
|
|
125
|
Ho CM, Huang CJ, Huang CY, Wu YY, Chang SF
and Cheng WF: Promoter methylation status of HIN-1 associated with
outcomes of ovarian clear cell adenocarcinoma. Mol Cancer.
11:532012.
|
|
126
|
Lin HW, Fu CF, Chang MC, Lu TP, Lin HP,
Chiang YC, Chen CA and Cheng WF: CDH1, DLEC1 and SFRP5 methylation
panel as a prognostic marker for advanced epithelial ovarian
cancer. Epigenomics. 10:1397–1413. 2018.
|
|
127
|
Su HY, Lai HC, Lin YW, Liu CY, Chen CK,
Chou YC, Lin SP, Lin WC, Lee HY and Yu MH: Epigenetic silencing of
SFRP5 is related to malignant phenotype and chemoresistance of
ovarian cancer through Wnt signaling pathway. Int J Cancer.
127:555–567. 2010.
|
|
128
|
Kumar D, Patel SA, Hassan MK, Mohapatra N,
Pattanaik N and Dixit M: Reduced IQGAP2 expression promotes EMT and
inhibits apoptosis by modulating the MEK-ERK and p38 signaling in
breast cancer irrespective of ER status. Cell Death Dis.
12:3892021.
|
|
129
|
Deng Z, Wang L, Hou H, Zhou J and Li X:
Epigenetic regulation of IQGAP2 promotes ovarian cancer progression
via activating Wnt/β-catenin signaling. Int J Oncol. 48:153–160.
2016.
|
|
130
|
Ge YX, Wang CH, Hu FY, Pan LX, Min J, Niu
KY, Zhang L, Li J and Xu T: New advances of TMEM88 in cancer
initiation and progression, with special emphasis on Wnt signaling
pathway. J Cell Physiol. 233:79–87. 2018.
|
|
131
|
de Leon M, Cardenas H, Vieth E, Emerson R,
Segar M, Liu Y, Nephew K and Matei D: Transmembrane protein 88
(TMEM88) promoter hypomethylation is associated with platinum
resistance in ovarian cancer. Gynecol Oncol. 142:539–547. 2016.
|
|
132
|
Yang L, Pang Y and Moses HL: TGF-beta and
immune cells: An important regulatory axis in the tumor
microenvironment and progression. Trends Immunol. 31:220–227.
2010.
|
|
133
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol.
8:a0218732016.
|
|
134
|
Matsumura N, Huang Z, Mori S, Baba T,
Fujii S, Konishi I, Iversen ES, Berchuck A and Murphy SK:
Epigenetic suppression of the TGF-beta pathway revealed by
transcriptome profiling in ovarian cancer. Genome Res. 21:74–82.
2011.
|
|
135
|
Cardenas H, Vieth E, Lee J, Segar M, Liu
Y, Nephew KP and Matei D: TGF-β induces global changes in DNA
methylation during the epithelial-to-mesenchymal transition in
ovarian cancer cells. Epigenetics. 9:1461–1472. 2014.
|
|
136
|
Chou JL, Su HY, Chen LY, Liao YP,
Hartman-Frey C, Lai YH, Yang HW, Deatherage DE, Kuo CT, Huang YW,
et al: Promoter hypermethylation of FBXO32, a novel TGF-beta/SMAD4
target gene and tumor suppressor, is associated with poor prognosis
in human ovarian cancer. Lab Invest. 90:414–425. 2010.
|
|
137
|
Chou JL, Huang RL, Shay J, Chen LY, Lin
SJ, Yan PS, Chao WT, Lai YH, Lai YL, Chao TK, et al:
Hypermethylation of the TGF-β target, ABCA1 is associated with poor
prognosis in ovarian cancer patients. Clin Epigenetics.
7:12015.
|
|
138
|
Wegner M: All purpose Sox: The many roles
of Sox proteins in gene expression. Int J Biochem Cell Biol.
42:381–390. 2010.
|
|
139
|
Shonibare Z, Monavarian M, O'Connell K,
Altomare D, Shelton A, Mehta S, Jaskula-Sztul R, Phaeton R, Starr
MD, Whitaker R, et al: Reciprocal SOX2 regulation by SMAD1-SMAD3 is
critical for anoikis resistance and metastasis in cancer. Cell Rep.
40:1110662022.
|
|
140
|
Ween MP, Oehler MK and Ricciardelli C:
Transforming growth factor-beta-induced protein (TGFBI)/(βig-H3): A
matrix protein with dual functions in ovarian cancer. Int J Mol
Sci. 13:10461–10477. 2012.
|
|
141
|
Wang N, Zhang H, Yao Q, Wang Y, Dai S and
Yang X: TGFBI promoter hypermethylation correlating with paclitaxel
chemoresistance in ovarian cancer. J Exp Clin Cancer Res.
31:62012.
|
|
142
|
Ho CM, Lin MC, Huang SH, Huang CJ, Lai HC,
Chien TY and Chang SF: PTEN promoter methylation and LOH of
10q22-23 locus in PTEN expression of ovarian clear cell
adenocarcinomas. Gynecol Oncol. 112:307–313. 2009.
|
|
143
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019.
|
|
144
|
Li M, Balch C, Montgomery JS, Jeong M,
Chung JH, Yan P, Huang TH, Kim S and Nephew KP: Integrated analysis
of DNA methylation and gene expression reveals specific signaling
pathways associated with platinum resistance in ovarian cancer. BMC
Med Genomics. 2:342009.
|
|
145
|
Li GN, Zhao XJ, Wang Z, Luo MS, Shi SN,
Yan DM, Li HY, Liu JH, Yang Y, Tan JH, et al: Elaiophylin triggers
paraptosis and preferentially kills ovarian cancer drug-resistant
cells by inducing MAPK hyperactivation. Signal Transduct Target
Ther. 7:3172022.
|
|
146
|
Walch A, Specht K, Braselmann H, Stein H,
Siewert JR, Hopt U, Höfler H and Werner M: Coamplification and
coexpression of GRB7 and ERBB2 is found in high grade
intraepithelial neoplasia and in invasive Barrett's carcinoma. Int
J Cancer. 112:747–753. 2004.
|
|
147
|
Chen K, Liu MX, Mak CS, Yung MM, Leung TH,
Xu D, Ngu SF, Chan KK, Yang H, Ngan HY and Chan DW:
Methylation-associated silencing of miR-193a-3p promotes ovarian
cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways.
Theranostics. 8:423–436. 2018.
|
|
148
|
Sung HY, Yang SD, Ju W and Ahn JH:
Aberrant epigenetic regulation of GABRP associates with aggressive
phenotype of ovarian cancer. Exp Mol Med. 49:e3352017.
|
|
149
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531.
2004.
|
|
150
|
Kuhlmann JD, Rasch J, Wimberger P and
Kasimir-Bauer S: microRNA and the pathogenesis of ovarian cancer-a
new horizon for molecular diagnostics and treatment? Clin Chem Lab
Med. 50:601–615. 2012.
|
|
151
|
Cairns RA: Drivers of the Warburg
phenotype. Cancer J. 21:56–61. 2015.
|
|
152
|
Zhou Y, Zheng X, Lu J, Chen W, Li X and
Zhao L: Ginsenoside 20(S)-Rg3 inhibits the Warburg effect via
modulating DNMT3A/MiR-532-3p/HK2 pathway in ovarian cancer cells.
Cell Physiol Biochem. 45:2548–2559. 2018.
|
|
153
|
Zhang S, Pei M, Li Z, Li H, Liu Y and Li
J: Double-negative feedback interaction between DNA
methyltransferase 3A and microRNA-145 in the Warburg effect of
ovarian cancer cells. Cancer Sci. 109:2734–2745. 2018.
|
|
154
|
Li J, Zhang S, Zou Y, Wu L, Pei M and
Jiang Y: miR-145 promotes miR-133b expression through c-myc and
DNMT3A-mediated methylation in ovarian cancer cells. J Cell
Physiol. 235:4291–4301. 2020.
|
|
155
|
Teng Y, Zuo X, Hou M, Zhang Y, Li C, Luo W
and Li X: A double-negative feedback interaction between
MicroRNA-29b and DNMT3A/3B contributes to ovarian cancer
progression. Cell Physiol Biochem. 39:2341–2352. 2016.
|
|
156
|
Chhabra R, Rockfield S, Guergues J, Nadeau
OW, Hill R, Stevens SM Jr and Nanjundan M: Global miRNA/proteomic
analyses identify miRNAs at 14q32 and 3p21, which contribute to
features of chronic iron-exposed fallopian tube epithelial cells.
Sci Rep. 11:62702021.
|
|
157
|
Chen Q, Wang Y, Dang H and Wu X:
MicroRNA-148a-3p inhibits the proliferation of cervical cancer
cells by regulating the expression levels of DNMT1 and UTF1. Oncol
Lett. 22:6172021.
|
|
158
|
Wu YH, Huang YF, Wu PY, Chang TH, Huang SC
and Chou CY: The downregulation of miR-509-3p expression by
collagen type XI alpha 1-regulated hypermethylation facilitates
cancer progression and chemoresistance via the DNA
methyltransferase 1/Small ubiquitin-like modifier-3 axis in ovarian
cancer cells. Res Sq. rs.3.rs–2592453. 2023.
|
|
159
|
Han X, Zhen S, Ye Z, Lu J, Wang L, Li P,
Li J, Zheng X, Li H, Chen W, et al: A feedback loop between
miR-30a/c-5p and DNMT1 mediates cisplatin resistance in ovarian
cancer cells. Cell Physiol Biochem. 41:973–986. 2017.
|
|
160
|
Vera O, Jimenez J, Pernia O,
Rodriguez-Antolin C, Rodriguez C, Sanchez Cabo F, Soto J, Rosas R,
Lopez-Magallon S, Esteban Rodriguez I, et al: DNA methylation of
miR-7 is a mechanism involved in platinum response through MAFG
overexpression in cancer cells. Theranostics. 7:4118–4134.
2017.
|
|
161
|
Li X, Pan Q, Wan X, Mao Y, Lu W, Xie X and
Cheng X: Methylation-associated Has-miR-9 deregulation in
paclitaxel-resistant epithelial ovarian carcinoma. BMC Cancer.
15:5092015.
|
|
162
|
Yang C, Cai J, Wang Q, Tang H, Cao J, Wu L
and Wang Z: Epigenetic silencing of miR-130b in ovarian cancer
promotes the development of multidrug resistance by targeting
colony-stimulating factor 1. Gynecol Oncol. 124:325–334. 2012.
|
|
163
|
Xu S, Fu GB, Tao Z, OuYang J, Kong F,
Jiang BH, Wan X and Chen K: MiR-497 decreases cisplatin resistance
in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015.
|
|
164
|
Han X, Liu D, Zhou Y, Wang L, Hou H, Chen
H, Zhang L, Chen W, Li X and Zhao L: The negative feedback between
miR-143 and DNMT3A regulates cisplatin resistance in ovarian
cancer. Cell Biol Int. 45:227–237. 2021.
|
|
165
|
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang
J and Wu X: Ovarian cancer cell-secreted exosomal miR-205 promotes
metastasis by inducing angiogenesis. Theranostics. 9:8206–8220.
2019.
|
|
166
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007.
|
|
167
|
Loginov VI, Pronina IV, Filippova EA,
Burdennyy AM, Lukina SS, Kazubskaya TP, Uroshlev LA, Fridman MV,
Brovkina OI, Apanovich NV, et al: Aberrant methylation of 20 miRNA
genes specifically involved in various steps of ovarian carcinoma
spread: From primary tumors to peritoneal macroscopic metastases.
Int J Mol Sci. 23:13002022.
|
|
168
|
Deng Y, Zhao F, Hui L, Li X, Zhang D, Lin
W, Chen Z and Ning Y: Suppressing miR-199a-3p by promoter
methylation contributes to tumor aggressiveness and cisplatin
resistance of ovarian cancer through promoting DDR1 expression. J
Ovarian Res. 10:502017.
|
|
169
|
Schmid G, Notaro S, Reimer D, Abdel-Azim
S, Duggan-Peer M, Holly J, Fiegl H, Rössler J, Wiedemair A, Concin
N, et al: Expression and promotor hypermethylation of miR-34a in
the various histological subtypes of ovarian cancer. BMC Cancer.
16:1022016.
|
|
170
|
Zuberi M, Khan I, Mir R, Gandhi G, Ray PC
and Saxena A: Utility of serum miR-125b as a diagnostic and
prognostic indicator and its alliance with a panel of tumor
suppressor genes in epithelial ovarian cancer. PLoS One.
11:e01539022016.
|
|
171
|
He J, Xu Q, Jing Y, Agani F, Qian X,
Carpenter R, Li Q, Wang XR, Peiper SS, Lu Z, et al: Reactive oxygen
species regulate ERBB2 and ERBB3 expression via miR-199a/125b and
DNA methylation. EMBO Rep. 13:1116–1122. 2012.
|
|
172
|
Ye Z, Li J, Han X, Hou H, Chen H, Zheng X,
Lu J, Wang L, Chen W, Li X and Zhao L: TET3 inhibits TGF-β1-induced
epithelial-mesenchymal transition by demethylating miR-30d
precursor gene in ovarian cancer cells. J Exp Clin Cancer Res.
35:722016.
|
|
173
|
Vogt M, Munding J, Grüner M, Liffers ST,
Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A and Hermeking H:
Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG
methylation in colorectal, pancreatic, mammary, ovarian,
urothelial, and renal cell carcinomas and soft tissue sarcomas.
Virchows Arch. 458:313–322. 2011.
|
|
174
|
Cai Y, Tsai HC, Yen RC, Zhang YW, Kong X,
Wang W, Xia L and Baylin SB: Critical threshold levels of DNA
methyltransferase 1 are required to maintain DNA methylation across
the genome in human cancer cells. Genome Res. 27:533–544. 2017.
|
|
175
|
Li H, Lei Y, Li S, Li F and Lei J:
MicroRNA-20a-5p inhibits the autophagy and cisplatin resistance in
ovarian cancer via regulating DNMT3B-mediated DNA methylation of
RBP1. Reprod Toxicol. 109:93–100. 2022.
|
|
176
|
Ye Z, Jiang Y and Wu J: DNMT3B attenuated
the inhibition of TET3 on epithelial-mesenchymal transition in
TGF-β1-induced ovarian cancer by methylating the TET3 promoter.
Reprod Biol. 22:1007012022.
|
|
177
|
Del Castillo Falconi VM, Díaz-Chávez J,
Torres-Arciga K, Luna-Maldonado F, Gudiño-Gomez AA, Pedroza-Torres
A, Castro-Hernández C, Cantú de León D and Herrera LA: Expression
of DNA methyltransferase 3B isoforms is associated with DNA
satellite 2 hypomethylation and clinical prognosis in advanced
high-grade serous ovarian carcinoma. Int J Mol Sci.
23:127592022.
|
|
178
|
Lyko F and Brown R: DNA methyltransferase
inhibitors and the development of epigenetic cancer therapies. J
Natl Cancer Inst. 97:1498–1506. 2005.
|
|
179
|
Natoli M, Gallon J, Lu H, Amgheib A,
Pinato DJ, Mauri FA, Marafioti T, Akarca AU, Ullmo I, Ip J, et al:
Transcriptional analysis of multiple ovarian cancer cohorts reveals
prognostic and immunomodulatory consequences of ERV expression. J
Immunother Cancer. 9:e0015192021.
|
|
180
|
Ma G, Li Y, Meng F, Sui C, Wang Y and
Cheng D: Hsa_ circ_0000119 promoted ovarian cancer development via
enhancing the methylation of CDH13 by sponging miR-142-5p. J
Biochem Mol Toxicol. 37:e232642023.
|
|
181
|
Wong-Brown MW, van der Westhuizen A and
Bowden NA: Sequential azacitidine and carboplatin induces immune
activation in platinum-resistant high-grade serous ovarian cancer
cell lines and primes for checkpoint inhibitor immunotherapy. BMC
Cancer. 22:1002022.
|
|
182
|
Liu M, Thomas SL, DeWitt AK, Zhou W, Madaj
ZB, Ohtani H, Baylin SB, Liang G and Jones PA: Dual inhibition of
DNA and histone methyltransferases increases viral mimicry in
ovarian cancer cells. Cancer Res. 78:5754–5766. 2018.
|
|
183
|
Shim JI, Ryu JY, Jeong SY, Cho YJ, Choi
JJ, Hwang JR, Choi JY, Sa JK and Lee JW: Combination effect of poly
(ADP-ribose) polymerase inhibitor and DNA demethylating agents for
treatment of epithelial ovarian cancer. Gynecol Oncol. 165:270–280.
2022.
|
|
184
|
McDonald JI, Diab N, Arthofer E, Hadley M,
Kanholm T, Rentia U, Gomez S, Yu A, Grundy EE, Cox O, et al:
Epigenetic therapies in ovarian cancer alter repetitive element
expression in a TP53-dependent manner. Cancer Res. 81:5176–5189.
2021.
|
|
185
|
Steele N, Finn P, Brown R and Plumb JA:
Combined inhibition of DNA methylation and histone acetylation
enhances gene re-expression and drug sensitivity in vivo. Br J
Cancer. 100:758–763. 2009.
|
|
186
|
Fang F, Balch C, Schilder J, Breen T,
Zhang S, Shen C, Li L, Kulesavage C, Snyder AJ, Nephew KP and Matei
DE: A phase 1 and pharmacodynamic study of decitabine in
combination with carboplatin in patients with recurrent,
platinum-resistant, epithelial ovarian cancer. Cancer.
116:4043–4053. 2010.
|
|
187
|
Yin B, Ding J, Hu H, Yang M, Huang B, Dong
W, Li F and Han L: Overexpressed CMTM6 improves prognosis and
associated with immune infiltrates of ovarian cancer. Front Mol
Biosci. 9:7690322022.
|
|
188
|
Gomez S, Cox OL, Walker RR III, Rentia U,
Hadley M, Arthofer E, Diab N, Grundy EE, Kanholm T, McDonald JI, et
al: Inhibiting DNA methylation and RNA editing upregulates
immunogenic RNA to transform the tumor microenvironment and prolong
survival in ovarian cancer. J Immunother Cancer.
10:e0049742022.
|
|
189
|
Giri AK and Aittokallio T: DNMT inhibitors
increase methylation in the cancer genome. Front Pharmacol.
10:3852019.
|
|
190
|
Bauerschlag DO, Ammerpohl O, Bräutigam K,
Schem C, Lin Q, Weigel MT, Hilpert F, Arnold N, Maass N,
Meinhold-Heerlein I and Wagner W: Progression-free survival in
ovarian cancer is reflected in epigenetic DNA methylation profiles.
Oncology. 80:12–20. 2011.
|
|
191
|
Khajehnoori S, Zarei F, Mazaheri M and
Dehghani-Firoozabadi A: Epidrug modulated expression of MiR-152 and
MiR-148a reverse cisplatin resistance in ovarian cancer cells: An
experimental in-vitro study. Iran J Pharm Res. 19:509–519.
2020.
|
|
192
|
Belsky DW, Caspi A, Corcoran DL, Sugden K,
Poulton R, Arseneault L, Baccarelli A, Chamarti K, Gao X, Hannon E,
et al: DunedinPACE, a DNA methylation biomarker of the pace of
aging. Elife. 11:e734202022.
|