A recent statistical survey indicated that there
were almost 20 million new cases of cancer worldwide in 2022, with
9.7 million deaths attributed to cancer (1). Cancer has therefore emerged as a
prominent factor contributing to human mortality. In recent
decades, novel approaches for the prevention and treatment of
cancer, including antibody-drug conjugates and immunotherapy
(2-4), have been discovered. Although these
therapeutic techniques have provided new opportunities for tumor
treatment, their effectiveness is hindered by the resistance of
tumor cells to drugs and the intricate structure of the tumor
microenvironment (TME), which provides challenges in treating
tumors (5,6). The irregularity of vascular networks
within the TME can impede the transport of chemotherapeutic agents.
Additionally, immunosuppressive cells within the TME have the
capability to dampen immune responses, thereby attenuating the
efficacy of immunotherapy. Exploring novel adjuvant therapy drugs
is intended to improve the effectiveness of current treatments,
ease patient distress and even achieve a cancer cure.
A recent study found that a lack of microbiota
within the TME inhibits the production of type I interferon (IFN-I)
by monocytes, thereby inducing M2 polarization of macrophages
(7). This results in the
formation of an immunosuppressive TME, which enables tumor cells to
avoid being detected and destroyed by the immune system. Disruption
of the microbiota has an impact on the advancement of tumors, but
the restoration of imbalances in gut microbial composition through
fecal microbiota transplantation (FMT) has been proposed as a
promising approach for tumor treatment (8). The microbiota also promotes cancer
by inducing chronic inflammation. When Ackermannia
mucinophila is lost in the gut, the intestinal barrier function
is disrupted, resulting in liver inflammation and fibrosis
(9). Prolonged inflammation and
fibrosis can gradually lead to aberrant hepatocyte proliferation
and injury, potentially culminating in the development of
hepatocellular carcinoma. Tumor progression and the microbiome are
strongly interconnected through a number of pathways. For instance
methylobacterium contributes to the development of gastric cancer
by decreasing the production of TGF-β and CD8+
tissue-resident memory T-cells (10). Candida albicans promotes
the secretion of interleukin (IL)-7, which promotes the release of
IL-22 from RORγt+ (group 3) innate lymphoid cells (ILC3s) by
activating the aryl hydrocarbon receptor (AHR) and STAT3 (11). IL-22 can promote tumor cell
metastasis and the formation of intratumoral blood vessels
(12,13). In summary, there have been an
increasing number of studies that specifically investigating the
relationship between the microbiome and cancer.
Microbes colonize numerous parts of the body
(including tumors) and influence host tumorigenesis and tumor
progression (14-17). Microbial metabolites, which are
compounds produced during the growth and metabolic processes of
microorganisms, have also been associated with the development of
cancer (18). Gut bacteria
convert primary bile acids into secondary bile acids, and elevated
concentrations of secondary bile acids facilitate the proliferation
of tumors by impairing the function of natural killer T-cells
(19). In addition, some
microbial metabolites are directly carcinogenic. For instance,
cytotoxin-associated gene A produced by Helicobacter pylori
induces BRCAness (the tumor exhibits characteristics associated
with mutations in the BRCA1 or BRCA2 genes), promotes DNA
double-strand breaks and induces bacteria-associated gastric cancer
(20). In addition to causing
cancer, some microbial metabolites may have anticancer effects.
Reuterin, which is produced by Lactobacillus reuteri,
selectively oxidizes proteins and inhibits ribosomal biogenesis and
protein translation to restrict the proliferation and survival of
colon cancer cells (21). In
summary, microbial metabolites play a role in the development and
advancement of tumors through several processes. Targeting these
metabolites may therefore offer additional advantages in treating
patients, such as enhanced therapeutic efficacy, reduced adverse
effects and overcoming drug resistance.
Microbial metabolites can potentially serve as
supplementary therapeutic agents to enhance current clinical
techniques. Gut microbial metabolism produces butyric acid, which
regulates the T-cell receptor signaling pathway to stimulate the
generation of cytokines that possess antitumor effects (22). Butyric acid notably improves the
antitumor capacity of immune cells, leading to improved
effectiveness of anti-programmed cell death protein 1 (PD-1)
immunotherapy. Microbial metabolites have a reciprocal impact on
clinical therapy. The metabolism of Fusobacterium nucleatum
results in the production of succinic acid, which hampers the
synthesis of IFN-β and restricts entry of CD8+ T-cells
into the TME. As a result, the immune response against the tumor is
suppressed (23). Therefore,
microbial metabolites may have a dual impact, exerting both
beneficial and harmful impacts on tumor treatment. To fully
understand the dual impact, it is crucial to not only clarify its
exact mechanism of action but also to consider the specific
environment in which it produces its effects. Varying amounts of
short-chain fatty acids (SCFAs) yield diverse outcomes in the
therapy of tumors. In non-alcoholic fatty liver disease, high
levels of SCFAs (when the concentration exceeds the threshold of
host tolerance) leads to the progression of hepatocellular
carcinoma (24). However, when
present in normal amounts, SCFAs markedly hinder the advancement of
colorectal cancer (25). Thus,
the effect of microbial metabolites on tumors is not absolute, and
different microbial metabolites have different effects at different
concentrations.
The present review explores the functions and
mechanisms of various microbial metabolites in the development of
tumors, the advancement of tumors and the immune response to
tumors. The impact of microbial metabolites on chemotherapy and
immunotherapy are also investigated, providing a detailed
explanation of the underlying mechanisms. The present review also
evaluates the potential of microbial metabolites as supplementary
therapeutic agents for cancer and suggests future research areas
for microbial metabolites in the field of oncology.
Tumors are not only composed of a simple set of
tumor cells but also infiltrate and host a diverse set of host
cells, cytokines and extracellular matrices (26-28). Consequently, throughout the
process of tumor formation, tumor cells frequently utilize various
tactics to influence both themselves and the neighboring cells.
This fosters a favorable environment for the proliferation and
metastasis of tumor cells. Research has indicated that tumor cells
increase the concentration of lactate at the tumor location by
employing glycolysis. The buildup of lactate is frequently linked
to a reduction in the immune response against the tumor within the
TME (29). Meanwhile, these
lactates also serve as signaling molecules in both autocrine and
paracrine mechanisms within the tumor, activating G protein-coupled
receptor (GPR) 81 (30).
Activation of GPR81 promotes angiogenesis and immune evasion within
the TME. In pancreatic cancer, CD73 mediates activation of the
p38/STAT1 axis by inducing extracellular adenosine accumulation,
leading to the upregulation of C-C motif chemokine ligand (CCL) 5
transcription, which attracts regulatory T cells into the TME
(31). Therefore, tumor cells
exert an influence on the advancement of tumors by altering the
programming of the TME. As the tumor advances, microbial
metabolites are generated by microorganisms in the tumor and stored
in the TME. These microbial metabolites will function as ligands
for specific receptors or as cytokines to regulate the activity of
proteins and modify the TME. Research has shown that in mice with
colon cancer, microbial metabolism leads to high levels of
intestinal ammonia, which induces metabolic reprogramming of T
cells, increasing T cell exhaustion and decreasing its
proliferation (32).
Administering streptomycin to animals with breast cancer results in
a reduction in the abundance of lactobacilli in the intestinal
tract. The proportion of CD4+ and CD8+ T
cells that produce IFN-γ is decreased in the TME. Nevertheless,
following the administration of lactobacilli, there is an
augmentation in the lactic acid content at the location of the
tumor, resulting in the disappearance of this phenomenon (33).
Therefore, microbial metabolites can facilitate the
advancement of tumors by altering the TME. Byproducts of the
microbial community in the gut and the microorganisms associated
with tumors have a role in the advancement of tumors and
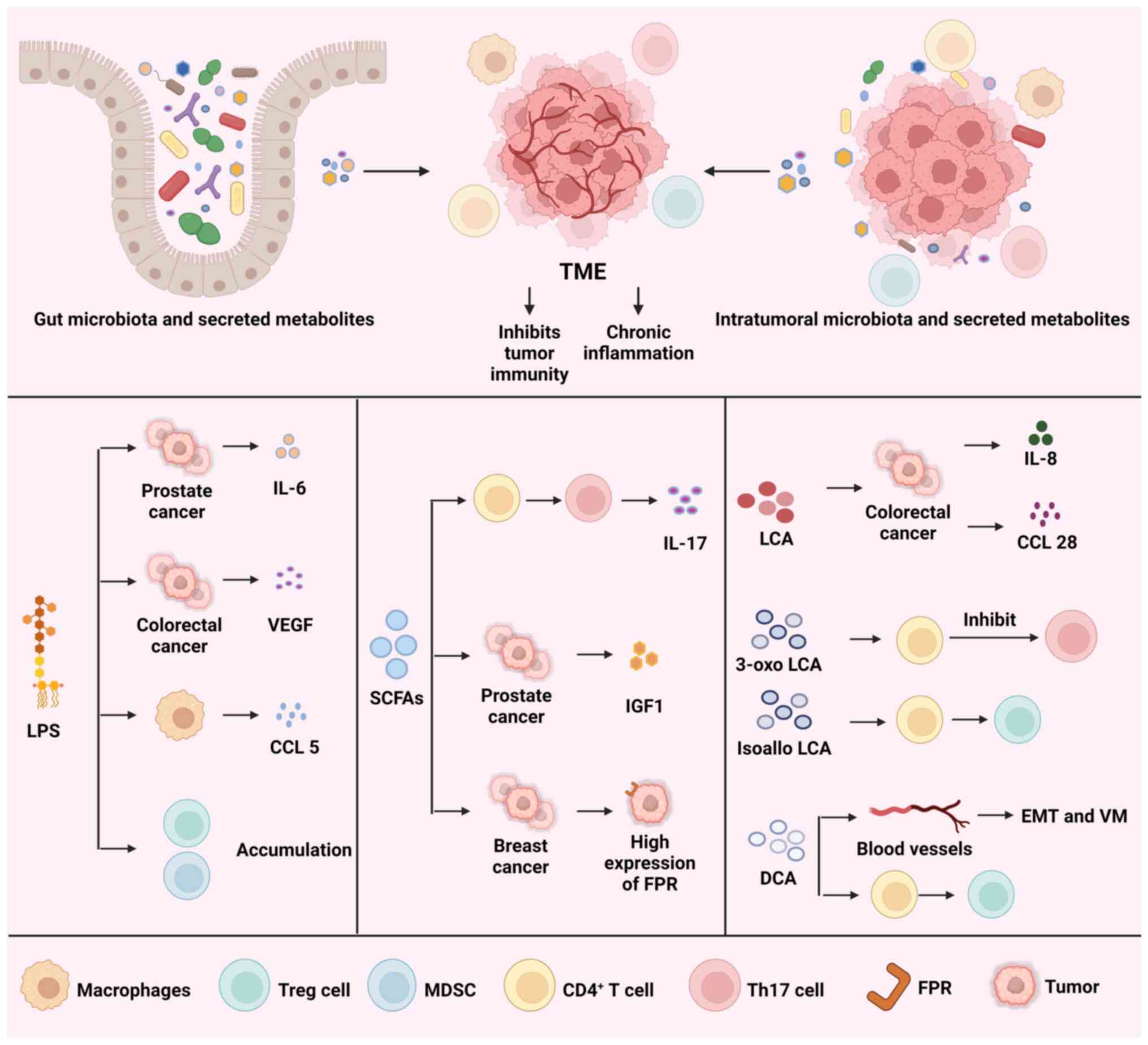
long-lasting inflammation. Fig. 1
illustrates how gut microbiota, as well as intratumoral microbiota,
and their metabolites collectively reprogram the TME. Additionally,
Fig. 1 outlines the mechanisms by
which lipopolysaccharides (LPSs), SCFAs and secondary bile acids
inhibit tumor immunity and induce chronic inflammation.
LPS is an essential component of the cell wall in
Gram-negative bacteria (GNB). An examination conducted in 2020 on
bacterial populations present in pan-cancerous tumors demonstrated
that LPS has a crucial role in promoting inflammation in different
types of cancer (34). Using
polymyxin B to clear GNB from the intestines or using TAK-242 to
block LPS activation of Toll-like receptor 4 (TLR4) can both
alleviate the immunosuppressive microenvironment of colorectal
cancer and facilitate T-cell infiltration into the tumor (35). Thus, LPS has the potential to
trigger long-term inflammation, leading to the advancement of
cancer. Long-term inflammation caused by LPS results in T-cell
exhaustion, an increase in the expression of the PD-1/programmed
death-ligand 1 (PD-L1) axis and the buildup of myeloid-derived
suppressor cells (MDSCs) and regulatory T cells (Tregs). This leads
to the development of a microenvironment that suppresses the immune
system and promotes the proliferation and advancement of tumors
(36).
Furthermore, LPS promotes the secretion of various
cytokines that aid in tumor cell proliferation and metastasis.
Activation of the NF-κB signaling pathway by LPS results in the
secretion of IL-6, which activates STAT3 in an autocrine manner.,
promoting prostate cancer progression in mice (37). LPS also stimulates TLR4 to induce
the expression of vascular endothelial growth factor (VEGF) C,
which promotes tumor cell metastasis and lymphangiogenesis
(38). Macrophages, which are
integral components of the TME, exert a marked influence on the
evasion of the immune system by tumors. Research has shown that
stimulation of the TLR4 signaling pathway by LPS leads to an
increase in the production of CCL5 by macrophages. Consequently,
this obstructs the ability of T cells to destroy colorectal cancer
cells and enables the cancer cells to avoid detection by the immune
system. In addition, CCL5 regulates the process of removing
ubiquitin molecules from PD-L1 and maintaining its stability
(39).
When considering the relationship between secondary
bile acids and different tumor types, including colorectal cancer
and liver cancer, it is important to investigate therapeutic
approaches involving secondary bile acids to advance the prevention
and treatment of these tumors (46,47). The gut microbiome produces bile
salt hydrolases (BSHs) to deoxygenate primary bile acids into
secondary bile acids, primarily deoxycholic acid (DCA) and
lithocholic acid (LCA). These compounds can contribute to tumor
progression by reshaping the TME (48). Unconjugated DCA and LCA buildup in
the intestine and stimulate GPRs in regions where BSH is highly
expressed. As a result of this activation, there is an elevated
production of CCL28, which is regulated by β-catenin, in the tumor.
Activation of the β-catenin/CCL28 axis leads to increased amounts
of Tregs in the TME, which in turn creates an immunosuppressive
microenvironment (49). In
Apcmin/+ mice (a commonly used mouse model for colon
cancer), the accumulation of DCA activates VEGF receptor 2,
promoting vasculogenic mimicry and epithelial-mesenchymal
transition (EMT) (50).
Additionally, secondary bile acids also induce the
expression of cytokines that influence tumor progression. LCA
activates Erk1/2 MAPK and inhibits STAT3 phosphorylation to promote
IL-8 expression in colorectal cancer cells (51). LCA induces the progression and
metastasis of colorectal cancer via promoting IL-8 expression
(52). Furthermore, DCA activates
CD4+ T cells and induces IL-10 secretion to induce Treg
cell differentiation, causing an immunosuppressive microenvironment
(53). Furthermore, the release
of IL-10 stimulates the polarization of tumor-associated
macrophages (TAMs) towards the M2 phenotype, hence facilitating the
proliferation and metastasis of colorectal cancer (54). The two derivatives of LCA are
3-oxo LCA and isoallo LCA. 3-oxo LCA hinders Th17 cell
differentiation by binding to retinoid-related orphan receptor-γt,
whereas isoallo LCA encourages the formation of Tregs by producing
mitochondrial reactive oxygen species (ROS) (55).
Studies suggest that the microbiome can improve
disease control in individuals with tumors (56-58). A study found that FMT improved the
effectiveness of anti-PD-1 therapy in mice with colorectal cancer
(59). Another study demonstrated
that FMT altered the cellular constitution within the TME of
patients with melanoma, helping to surmount resistance to anti-PD-1
therapy (60). The TME is
colonized by a diverse range of microbes, but its complex
composition restricts the manipulation of the microbiota. Microbial
metabolites can be utilized to provide a more accurate control of
the immune response in the TME, offering novel approaches for tumor
research and treatment. For instance, a recent study aimed to
examine the effects of the bacterial-derived metabolite,
desaminotyrosine (DAT), on C57BL/6J mice. The results demonstrated
that the group treated with a combination of DAT and anti-CTLA-4
had a higher immunotherapy response compared with the group that
received only anti-CTLA-4 (61).
The success of immune checkpoint inhibitors (ICIs)
in tumor therapy significantly relies on reactivating specific T
cells and promoting the apoptosis of Tregs present within the TME
(62,63). Therefore, it is highly probable
that microbial metabolites can alter tumor immunity by
reprogramming the TME. Alleviating the persistent inflammatory
response in the TME could potentially facilitate the development of
a beneficial immune microenvironment, and hence enhance the
response of the immune system to the tumor. Moreover, activation of
the immune system is essential for enhancing tumor immunity.
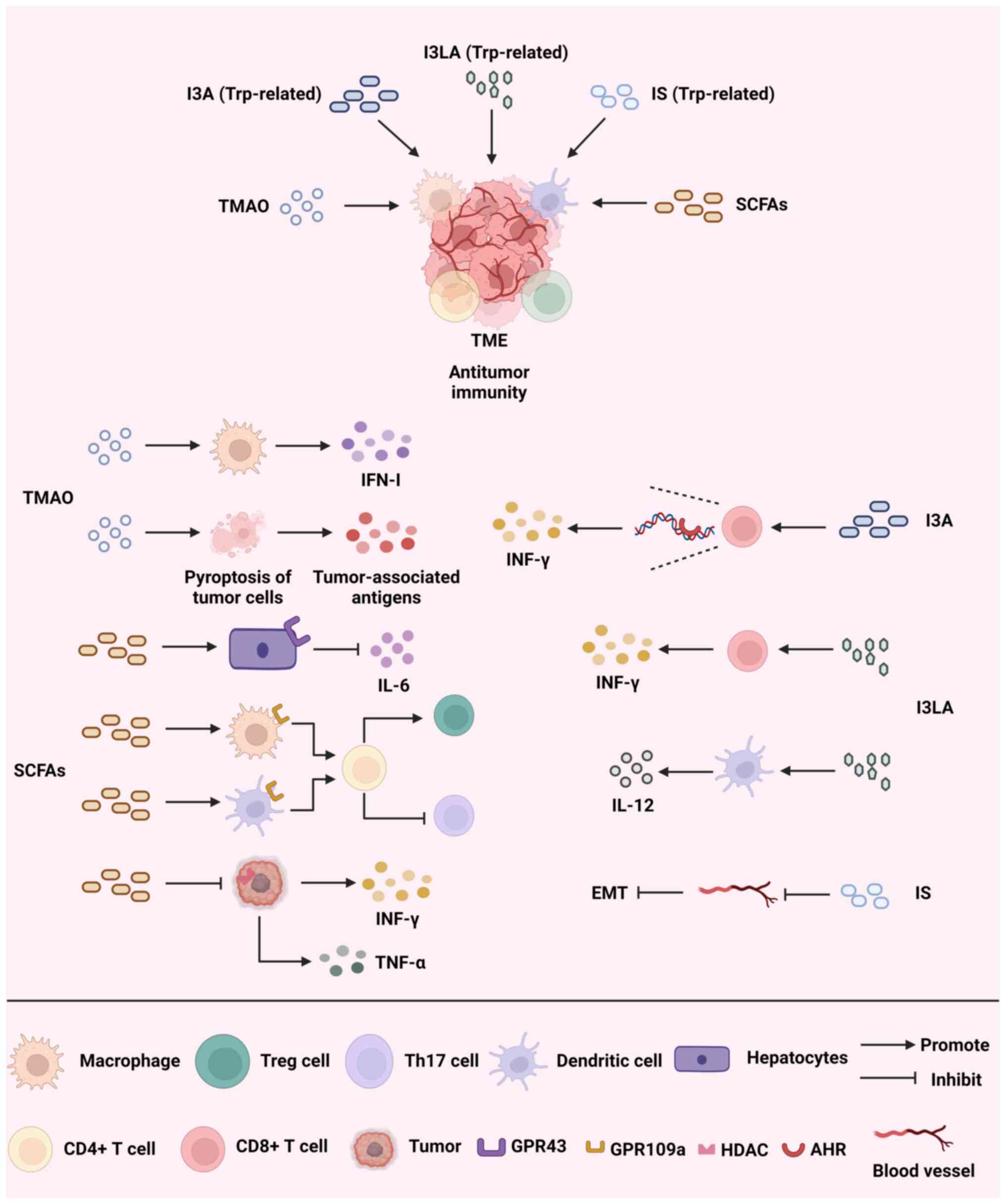
Fig. 2 illustrates how
trimethylamine N-oxide (TMAO), SCFAs and Trp-related metabolites
promote antitumor immunity by reprogramming the TME and outlines
the mechanisms through which they exert their effects.
TMAO has been linked to a range of diseases, such as
cardiovascular disease and liver, pancreatic and colorectal cancer
(64-67). While the exact method by which
TMAO contributes to tumor progression remains unclear, it is known
that elevated levels of amines, as a metabolite, lead to oxidative
stress (68). Oxidative stress
can lead to an increase in intracellular DNA mutations and genomic
instability, which in turn promotes cancer incidence and tumor
progression. Recently, several investigations have established a
connection between TMAO and different cancer types. For instance,
TMAO produced by the gut microbiota during the breakdown of food
has been found to stimulate macrophages, enhance the response of T
cells and decrease the number of tumor cells in pancreatic ductal
adenocarcinoma (PDAC) by increasing the production of IFN-Ⅰ
(66). Due to the connection
between the pancreatic duct and the duodenum, microbial metabolites
from the intestines can reach the pancreatic duct and surrounding
tissues. This could potentially have a role in the development and
treatment of PDAC. Immunotherapy has had little effectiveness in
treating triple-negative breast cancer (TNBC) (69). A study that conducted a
multi-omics analysis of patients with TNBC discovered that TMAO was
linked to a cohort that received immunotherapy and exhibited an
improved immune response. TMAO also triggers tumor cell pyroptosis
by activating the endoplasmic reticulum stress kinase, PERK, while
also boosting CD8+ T cell-driven antitumor immunity in
the host (70). Meanwhile, a
study revealed a correlation between elevated blood levels of TMAO
and colorectal cancer (71).
However, the precise pathophysiology remains uncertain, but it is
likely associated with the stimulation of persistent
gastrointestinal inflammation. Hence, it is crucial to regulate the
concentration of TMAO when employing TAMO for research or cancer
therapy. Several strategies, including the suppression of crucial
metabolic enzymes, dietary regulation and the use of antibiotics,
have been suggested to manipulate the gut microbiome to produce
TMAO (72-74).
SCFAs are important metabolites generated by the
intestinal microbiota during the digestive process, mainly
consisting of acetic acid, propionic acid and butyric acid. SCFAs
promote the metabolism of intestinal epithelial cells and enhance
the intestinal barrier function (75). Certain research suggests that
increasing the levels of butyric acid in the stomach through pectin
supplementation can promote the infiltration of T cells in the TME
and improve the efficacy of ICIs in treating colorectal cancer
(76). SCFAs have shown strong
antitumor effects in multiple types of cancer, particularly
colorectal cancer (77). The
ability to act through different routes is made possible by the
diversity of SCFAs and their derivatives. The antitumor actions of
SCFAs are achieved through their binding to many GPRs, such as
GPR41, GPR43 and GPR109a. Through the gut-hepatic axis, acetic acid
binds to GPR43 in the liver, limiting IL-6 release and obstructing
the JAK1/STAT3 signaling pathway to stop liver cancer from
progressing (78). Moreover, a
study found that the combination of propionic acid and GPR43 also
inhibited the proliferation of liver cancer cells (79). In addition, propionic acid can
inhibit the Hippo/Yap and MAPK signaling pathways by binding to
GPR41 and GPR43, ultimately inhibiting the metastasis of breast
cancer cells (80). Butyric acid
acts on GPR109a in colon macrophages and dendritic cells, inducing
the differentiation of Tregs and CD4+ T cells and
inhibiting the development of colitis and colorectal cancer
(81). Moreover, a study has
shown that GPR43 deletion accelerates the development of colorectal
cancer by exhausting CD8+ T cells and hyperactivating
dendritic cells (82). In
summary, SCFAs stimulate the proliferation and specialization of
immune cells by attaching to GPRs. SCFAs also hinder the
advancement and metastasis of tumors by attaching to GPRs on tumor
cells. Additionally, SCFAs suppress long-term inflammation by
activating GPR signaling, which prompts the development of
Tregs.
Tumor progression has been closely linked to
epigenetic reprogramming. Tumor cells frequently exhibit elevated
levels of histone deacetylases (HDACs), which lead to increased
histone deacetylation. This alteration affects the control of gene
expression and facilitates the advancement and metastasis of tumors
(83). Additionally, HDACs
suppress both the immune response and inflammatory events in immune
cells (84). HDAC inhibitors
enhance the antitumor therapeutic efficacy of ICIs by modifying the
immunosuppressive function of TAMs and impeding the ingress of
MDSCs into tumors, thereby remodeling the immunosuppressive
microenvironment (85). In a
recent study, inhibition of HDAC by butyric acid caused an
elevation in CD25 expression, INF-γ and tumor necrosis factor-α and
greatly enhanced the antitumor function of cytotoxic T lymphocytes
in pancreatic cancer and melanoma models (86). Propionic acid also inhibits IL-17
and IL-22 secretion by γδ T-cells via inhibition of HDAC, which
could inhibit colitis-associated colorectal cancer development
(87). A study has also
demonstrated that butyric acid inhibits the growth and promotes the
apoptosis of P815 mouse breast cancer cells by inhibiting HDAC, in
addition to the effects of SCFAs on immune cells (88).
The presence of Trp metabolites in organisms is
influenced, either directly or indirectly, by the gastrointestinal
microbiome. Metabolites and enzymes associated with Trp have been
identified as prospective targets for pharmaceutical advancements
in the treatment of various disorders, such as psoriasis, atopic
dermatitis and hepatic fibrosis (89-91). Trp metabolites have been reported
to act selectively on AHR (92).
Indole-3-aldehyde (I3A) activates the AHR of CD8+ T
cells in the melanoma TME, promoting CD8+ T-cell
differentiation and IFN-γ production (93). This enhances ICI-induced antitumor
immunity. However, in PDAC, Trp metabolites bind to the AHR of TAMs
and reduce the frequency of intratumoral CD8+ T cells,
creating an immunosuppressive microenvironment (94). This implies that the effects of
Trp metabolite-mediated AHR activation may vary among tumor types
or be influenced by heterogeneity in the TME.
Epigenetic modifications are of the utmost
importance in the regulation of gene expression and are critical in
the development, progression and treatment of numerous types of
cancer. An examination of the effects that microbial metabolites
have on epigenetic modifications will establish a solid theoretical
basis for the advancement of novel approaches in the field of tumor
immunotherapy. Tumor immunity can be regulated by Trp metabolites
via epigenetic modifications. Indole-3-lactic acid alters chromatin
accessibility to initiate the antitumor activity of CD8+
T cells and directly enhances the secretion of IFN-γ and granzyme B
by tumor-infiltrating CD8+ T cells to kill tumor cells,
and also promotes IL-12a secretion by binding to dendritic cells
(95). IL-12a in turn further
induces the proliferation of CD8+ T cells and promotes
tumor immunity (96).
A modest elevation in oxidative stress and
suppression of EMT can greatly impede the growth and metastasis of
tumor cells. Indoxylsulfate (IS) suppresses the activity of nuclear
factor erythroid 2-related factor 2 and stimulates the production
of inducible nitric oxide synthase, leading to the occurrence of
oxidative and nitrosative stress. Additionally, IS hinders EMT,
resulting in a notable decrease in the metastasis of breast cancer
cells to adjacent tissues (97).
The metabolism of Trp is not only regulated by the gut microbiome
but also by tumor cells (98).
Tumor cells often exhibit heightened activity in Trp metabolic
pathways, such as indoleamine 2,3-dioxygenase and tryptophan
2,3-dioxygenase, which facilitate the conversion of Trp into
kynurenine. This process increases the number of Tregs in the TME
and boosts the expression of PD-1 on CD8+ T cells,
helping tumor cells avoid detection by the immune system (99). An extensive examination of the
regulatory mechanisms of Trp metabolism will provide new targets
and strategies for tumor therapy, given the intricate nature of the
Trp metabolic pathway.
At present, a substantial percentage of individuals
diagnosed with cancer undergo radiotherapy as part of their
treatment. While a number of individuals have experienced positive
outcomes from radiotherapy, it can nevertheless result in various
negative effects. For instance, research has demonstrated that
whole-brain radiotherapy administered to patients with lung cancer
and brain metastases will ultimately result in memory impairment
(100). A retrospective cohort
study found that patients with nasopharyngeal carcinoma had a
higher incidence of oral mucositis during radiotherapy (101). The detrimental consequences of
these side effects might significantly impair the quality of life
of the patient and perhaps result in treatment discontinuation or
decreased patient tolerance. The critical clinical problem of
mitigating harmful effects in patients following radiotherapy is
increasingly recognized. Evidence indicates that microbial
metabolites possess the ability to alleviate detrimental effects on
the host following exposure to ionizing radiation. Increased
concentrations of propionate and Trp metabolites, which show
long-term radioprotection, were found in the intestines of mice
that received high doses of radiation but maintained a normal
lifespan (102). Meanwhile, DCA
can be used to treat radioactive skin injuries, promote wound
healing and reduce epidermal hyperplasia (103). As a result, microbial
metabolites are important in cancer therapy, and an increasing
number of studies are investigating the potential applications of
microbial metabolites to enhance therapeutic outcomes or minimize
side effects in patients with cancer (104-106). The role of microbial metabolites
in immunotherapy and chemotherapy are the focus of this section.
Table I presents clinical trials
regarding LPS, SCFAs and urolithin A (UroA), aimed at investigating
the role of microbial metabolites in cancer treatment. Table II presents experimental and
observational clinical trials of gut microbiota to explore their
impact and mechanisms in cancer treatment.
Chemotherapy is a popular cancer treatment that can
halt cancer from progressing via several methods, but drug
resistance and adverse effects remain a problem. As such, reducing
the adverse effects and medication resistance linked to
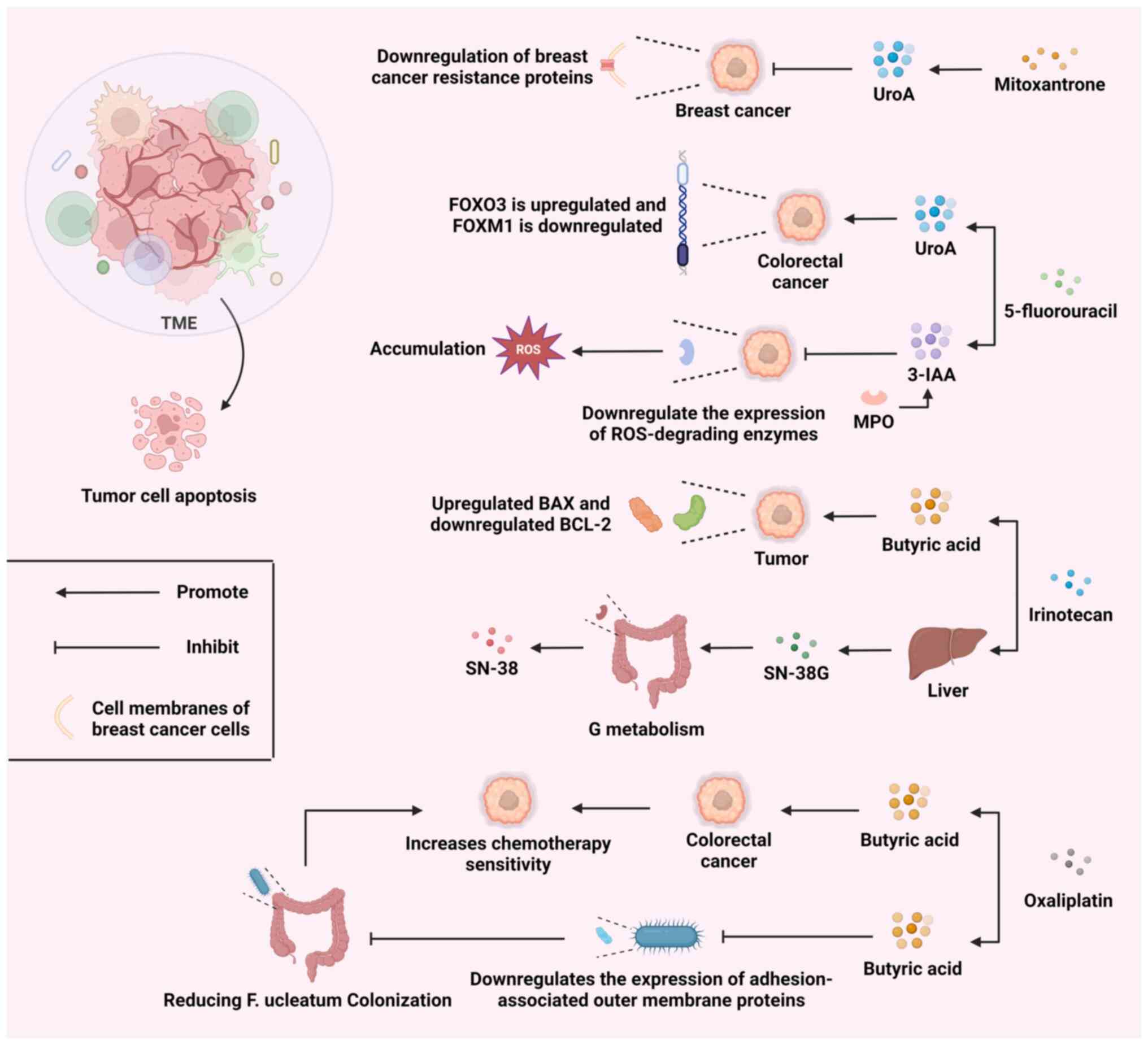
chemotherapy is a major task. As demonstrated in Fig. 3, the combined use of microbial
metabolites and various chemotherapy drugs can regulate the TME
through multiple mechanisms, promoting tumor cell apoptosis.
UroA is a product of gut microbial metabolism. UroA
has been reported to downregulate the expression of breast cancer
resistance protein (a drug efflux transporter protein), which would
contribute to the sustained efficacy of mitoxantrone in cancer
cells (107). Meanwhile, in a
recent study, UroA and its structural analog, UAS03, were combined
with the chemotherapeutic drug, 5-fluorouracil (5FU), and
investigated for their anticancer effects. The results showed that
UroA/UAS03 re-sensitized 5FU-resistant colorectal cancer to
chemotherapy by modulating the FOXO3/FOXM1 axis (108). These findings indicate that the
use of UroA in combination with low-dose chemotherapeutic drugs can
provide similar antitumor effects as high-dose chemotherapeutic
drugs, potentially minimizing the negative side effects associated
with chemotherapy. Furthermore, UroA can suppress the growth of
gastric cancer cells, hinder their capacity to invade and
metastasize and stimulate their programmed cell death (109). This indicates that UroA may
emerge as a novel adjuvant cancer therapy medication. However,
further investigations are necessary to confirm the safety and
effectiveness of UroA, as well as to identify the ideal therapeutic
concentration and clinical protocol.
Butyrate is a SCFA produced by gut microbiota,
typically generated as a byproduct during the fermentation of
dietary fibers in the colon. Butyrate has demonstrated potential
efficacy as an adjuvant treatment drug in chemotherapy by
modulating cellular metabolism, reducing oxidative stress and
preserving liver function through various mechanisms (110-112). The coadministration of butyrate
and irinotecan induces programmed cell death in colorectal cancer
cells by modulating the BAX/BCL-2 ratio, while also augmenting the
responsiveness of cancer cells to irinotecan (113). Meanwhile, butyrate and
oxaliplatin can synergistically inhibit the proliferation, invasion
and metastasis of colorectal cancer cells and promote the apoptosis
of these cells (114).
Furthermore, the intestinal bacterium, F. nucleatum,
promotes resistance to chemotherapy in colorectal cancer by
modulating autophagy (115).
Butyrate downregulates the expression of adhesion-associated outer
membrane proteins and inhibits F. nucleatum growth,
enrichment and adhesion in colorectal tissues, thereby reducing
F. nucleatum colonization and mitigating F.
nucleatum-induced chemoresistance (116). Gut microbiota can produce
indole-3-acetic acid (3-IAA) by metabolizing tryptophan from the
diet. Neutrophil-derived myeloperoxidase (MPO) is essential for the
joint action of 3-IAA and chemotherapy. MPO catalyzes the oxidation
of 3-IAA. When combined with chemotherapeutic drugs, the oxidative
products of 3-IAA can reduce the production of enzymes that degrade
ROS. This leads to an increase in ROS levels and a decrease in
tumor cell autophagy, ultimately inhibiting the development of
tumor cells (117). Therefore,
appropriate intervention in the nutrition of patients with cancer
during treatment may have a positive impact on chemotherapy.
Microbial metabolites can both increase and impair
the efficacy of chemotherapy. A study has demonstrated that
Lactobacillus residing in tumors can lead to resistance to
radiotherapy and chemotherapy. This resistance is achieved by
modifying the metabolism of the tumor and the signaling pathways
related to lactate (118).
Furthermore, the metabolites produced by microbes as they
metabolize chemotherapy drugs can lead to undesirable responses. A
randomized controlled trial conducted in 2021 revealed that
bacterial glucuronidase can break down the inactive metabolite of
irinotecan, SN-38G, into its active form, SN-38. This process
subsequently leads to damage in the intestinal mucosa (119).
ICIs have been used with great success in cancer
treatment, but many adverse effects (such as gastrointestinal
reactions) that occur during treatment have limited their wider
application (120).
Nevertheless, the integration of ICIs with microbial metabolites
could potentially resolve this issue. I3A was found to have a
protective impact on enteritis generated by ICIs in mice.
Additionally, I3A protected enteritis induced by ICIs in mice that
received FMT from I3A-treated mice via modifying the composition
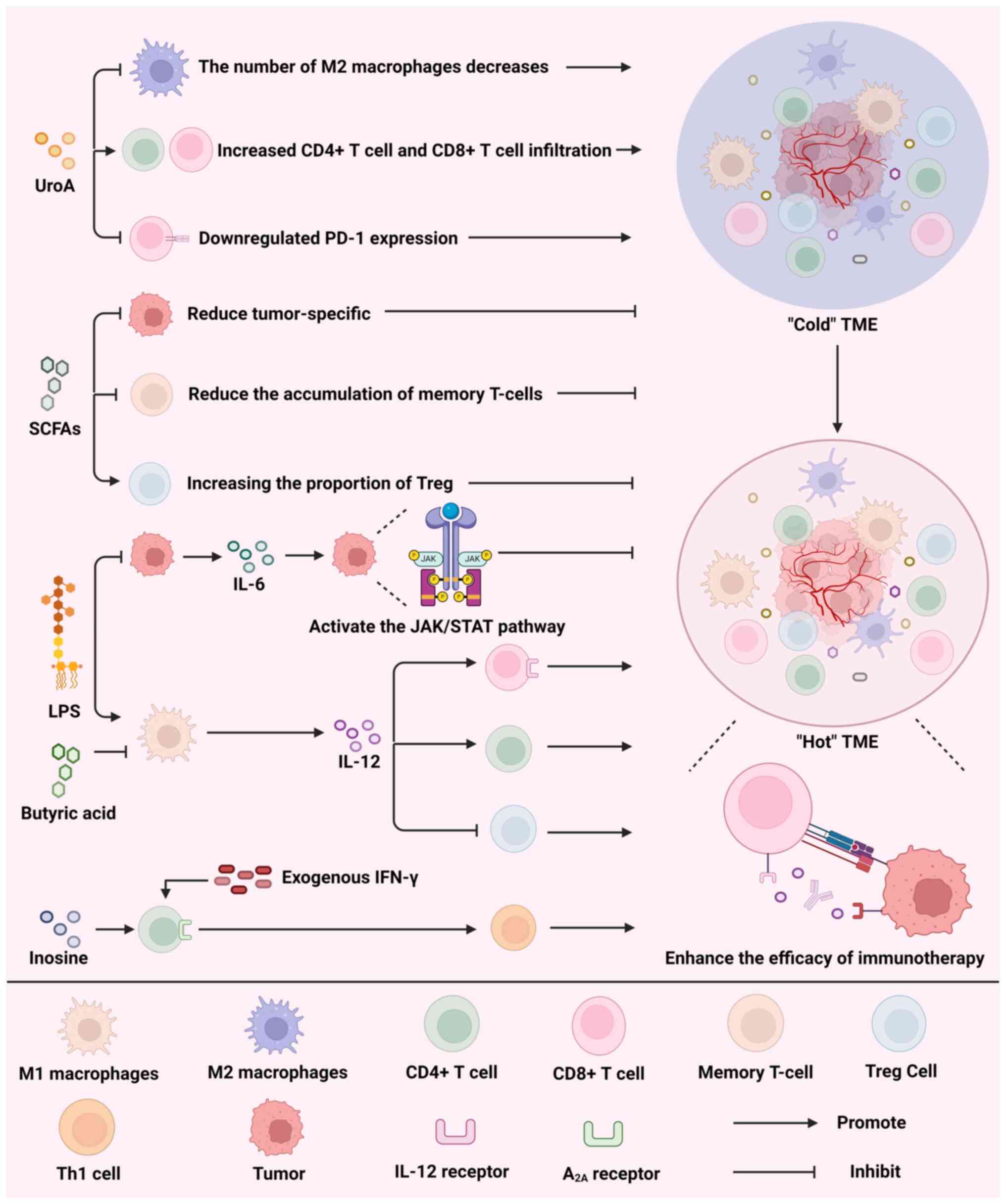
and function of the gut microbiome (121). The TME consists of a complex
network of various cytokines, immune cells and cancer cells.
Fig. 4 illustrates that UroA,
SCFAs, LPS and inosine act on components of the TME to activate the
immune system and promote the transition from a 'cold' TME (TME
lacking immune cell infiltration and activity) to a 'hot' TME,
thereby enhancing the efficacy of immunotherapy.
At present, a considerable proportion of individuals
with cancer do not demonstrate a strong reaction to ICI therapy due
to cancer cells employing various strategies to avoid detection by
the immune system (122-125). Therefore, activation of immune
cells within the microenvironment has become a crucial research
avenue to enhance the efficacy of ICIs. In a recent study, UroA
alone led to a notable decrease in M2-like macrophages and an
elevation in CD4+ and CD8+ T-cell
infiltration within the TME, as well as downregulation of PD-1
expression and a rise in the overall survival rate of mice with
cancer (126). Moreover, the
reciprocal impacts of microbial metabolites can potentially lead to
a reduction in the effectiveness of ICIs. Despite their anticancer
properties, SCFAs hinder the effectiveness of anti-CTLA-4
antibodies in treating melanoma. This is due to their ability to
decrease the presence of tumor-specific and memory T cells, while
simultaneously boosting the proportion of Tregs (127).
Prior research has demonstrated that cytokines exert
influence on the proliferation, progression and therapeutic
interventions of cancer by regulating the function of both immune
cells and tumor cells. Tumors with excessively active cytokine
signaling pathways frequently experience increased tumor
proliferation and invasion, significantly reducing the
effectiveness of ICIs (128-130). Targeted binding against
cytokines and their receptors may also leading to new strategies
for tumor therapy (131-133). Microbial metabolites may
indirectly influence the efficacy of ICIs by modulating cytokines.
LPS upregulates IL-6 levels, which leads to tumor cell
proliferation by activating JAK/STAT signaling (134). Furthermore, elevated levels of
IL-6 in the TME not only diminishes the effectiveness of ICIs but
also intensifies the negative side effects caused by ICI treatment
(135). In addition, IL-12 can
reprogram the TME, directly bind to the IL-12 receptor on
CD8+ T cells and activate these cells, while promoting
the infiltration of CD4+ T cells and downregulating the
number of Tregs, which ultimately significantly improves the
efficacy of ICIs (136,137). A variety of microbial
metabolites modulate IL-12 secretion. Research has shown that LPS
induces IL-12 secretion, which is inhibited by butyrate (138).
In summary, microbial metabolites can modify the
immunological status of the TME, converting it from a 'cold' to a
'hot' environment, and control the relative amounts of different
cytokines, all of which will increase the effectiveness of ICIs.
Microbial metabolites also have the potential to influence cytokine
receptors in addition to immune cells and cytokines. It has been
found that inosine binds to A2A in the presence of
exogenous IFN-γ to promote the differentiation of Th1 cells and
significantly increase the expression of the IL-12 receptor and
IFN-γ in Th1 cells, which may effectively improve the efficacy of
ICIs (139).
The present review examined the relationship between
microbial metabolites and cancer based on previous studies,
specifically focusing on two aspects: Tumor therapy and tumor
progression. Microbial metabolites exert dual impacts on tumors.
Microbial metabolites impact tumor development by reprogramming the
TME, which involves altering the makeup of immune cells and the
expression of cytokines. Microbial metabolites also enhance tumor
cell proliferation, invasion and metastasis by influencing a number
of signaling pathways, resulting in increased tumor cell growth.
The present review also summarized the impact of microbial
metabolites on current cancer treatments and described the
mechanisms by which microbial metabolites influence treatment
efficacy. In this context, microbial metabolites again exhibit dual
effects. Microbe-derived metabolic products have the potential to
improve the outcomes of immunotherapy, chemotherapy and radiation,
and can also strengthen the immune system in patients with cancer,
lessen the negative effects of drugs and fight drug resistance.
Meanwhile, by promoting the immunosuppressive TME, microbial
metabolites can also lessen the effectiveness of therapies.
There is a limitation in the current review of
microbial metabolites and cancer, as research on gut microbiota
metabolites is primarily focused on colorectal cancer, resulting in
previous reviews being confined to the impact of microbial
metabolites on colorectal cancer. However, in the present review,
studies on colorectal cancer were not only examined but research on
the association between microbial metabolites and other cancer
types such as melanoma, breast cancer, liver cancer and pancreatic
cancer, were also included (140-142). Furthermore, in reviews
concerning microbial metabolites and cancer, researchers tend to
focus more on summarizing the connection between microbial
metabolites and antitumor immunity, while overlooking the negative
impact that microbial metabolites may also have in promoting tumor
progression. In contrast to previous reviews, the present review
supplements the understanding of the influence of microbial
metabolites on tumor progression (143). With the rapid advancement of
immunotherapy, reviews focusing on microbial metabolites have
increasingly emphasized their impact on immunotherapy. However,
considering the high cost of immunotherapy, the majority of
patients with cancer still undergo chemotherapy and radiation
therapy. Therefore, research on microbial metabolites in the fields
of chemotherapy were further explored to assess their potential in
mainstream cancer treatments (144). However, the present review has
limitations. Given that clinical trials investigating microbial
metabolites are currently either recruiting or are in the
experimental stages, clinical trial results were not reviewed to
assess the potential of microbial metabolites as adjunctive cancer
therapeutics.
In recent decades, cancer has come to be seen as a
serious threat to the safety of human life. Nonetheless, cancer
treatment has proven to be difficult due to the intricate and
varied characteristics of the TME. To find a solution, scientists
are continuously experimenting with novel approaches and enhancing
current therapies. As research on the gut microbiome continues,
researchers are beginning to recognize that there is a significant
relationship between the gut microbiome and cancer. Scientists have
found microbial colonization within the TME, attributed to the
rapid progress of next-generation sequencing technologies and
analytical resources. The metabolites of this group of bacteria do
not require the employment of various intricate transporters to
reach the tumor. Instead, they directly release metabolites,
resulting in the heterogeneity of the TME. However, additional
research is necessary to clarify the relationship between microbial
metabolites and cancer. Advancements in this area will provide new
research ideas and therapeutic strategies for the prevention and
control of cancer.
Prior research has discovered microbial metabolites
that impact tumor treatment and advancement by influencing the
immune system of the patient, cancer or immune-related signaling
pathways, protein epigenetic alteration and DNA damage. Notably,
microbial metabolites have dual impacts on both tumor development
and tumor immunology. As a result, developing a more intricate
comprehension of microbial metabolites will facilitate their
utilization as supplementary therapeutic agents, as well as in
conjunction with anticancer drugs, thereby enhancing effectiveness.
Furthermore, recent studies have focused on the influence of
certain microbial metabolites on cancer, with particular emphasis
on metabolites that exhibit notable disparities in metabolomics
analysis. This could potentially neglect the significant
contribution of other metabolites or the combined and opposing
impacts of metabolites.
Future research should aim to gain a comprehensive
understanding of the processes by which microbial metabolites
operate in the TME and explore how these microbial metabolites can
be harnessed in clinical settings to develop more effective cancer
treatments. These studies should specifically investigate the
combined impact of microbial metabolites with current treatments.
Furthermore, it is necessary to investigate the synthesis and
modification of microbial metabolites to optimize their
effectiveness and guarantee their safety. These studies will
therefore extensively investigate the potential of microbial
metabolites as supplementary therapeutic agents and present new
opportunities for cancer treatment.
Not applicable.
YZ, WH, YF and YW performed the literature review
and wrote the manuscript. YZ and JX revised the figures and tables.
YZ, WH and JX revised the manuscript. YZ, WH, TS and JX were
involved in the conception of the study. All authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by National Nature Science Foundation of
China (grant no. 82373113), Shenyang Breast Cancer Clinical Medical
Research Center (grant no. 2020-48-3-1), LiaoNing Revitalization
Talents Program (grant no. XLYC1907160), Beijing Medical Award
Foundation (grant no. YXJL-2020-0941-0752), Wu Jieping Medical
Foundation (grant no. 320.6750.2020-12-21, 320.6750.2020-6-30) and
The Fundamental Research Funds for the Central Universities (grant
nos. 202229 and 202230).
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damelin M, Bankovich A, Bernstein J, Lucas
J, Chen L, Williams S, Park A, Aguilar J, Ernstoff E, Charati M, et
al: A PTK7-targeted antibody-drug conjugate reduces
tumor-initiating cells and induces sustained tumor regressions. Sci
Transl Med. 9:eaag26112017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamada T, Togashi Y, Tay C, Ha D, Sasaki
A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, et al: PD-1+
regulatory T cells amplified by PD-1 blockade promote
hyperprogression of cancer. Proc Natl Acad Sci USA. 116:9999–10008.
2019. View Article : Google Scholar :
|
|
4
|
Srivastava S, Furlan SN, Jaeger-Ruckstuhl
CA, Sarvothama M, Berger C, Smythe KS, Garrison SM, Specht JM, Lee
SM, Amezquita RA, et al: Immunogenic chemotherapy enhances
recruitment of CAR-T cells to lung tumors and improves antitumor
efficacy when combined with checkpoint blockade. Cancer Cell.
39:193–208.e10. 2021. View Article : Google Scholar
|
|
5
|
Zhao Y, Li ZX, Zhu YJ, Fu J, Zhao XF,
Zhang YN, Wang S, Wu JM, Wang KT, Wu R, et al: Single-Cell
transcriptome analysis uncovers intratumoral heterogeneity and
underlying mechanisms for drug resistance in hepatobiliary tumor
organoids. Adv Sci (Weinh). 8:e20038972021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He X, Smith SE, Chen S, Li H, Wu D,
Meneses-Giles PI, Wang Y, Hembree M, Yi K, Zhao X, et al:
Tumor-initiating stem cell shapes its microenvironment into an
immunosuppressive barrier and pro-tumorigenic niche. Cell Rep.
36:1096742021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lam KC, Araya RE, Huang A, Chen Q, Di
Modica M, Rodrigues RR, Lopès A, Johnson SB, Schwarz B, Bohrnsen E,
et al: Microbiota triggers STING-type I IFN-dependent monocyte
reprogramming of the tumor microenvironment. Cell.
184:5338–5356.e21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Routy B, Lenehan JG, Miller WH Jr, Jamal
R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L,
Punčochář M, et al: Fecal microbiota transplantation plus anti-PD-1
immunotherapy in advanced melanoma: A phase I trial. Nat Med.
29:2121–2132. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schneider KM, Mohs A, Gui W, Galvez EJC,
Candels LS, Hoenicke L, Muthukumarasamy U, Holland CH, Elfers C,
Kilic K, et al: Imbalanced gut microbiota fuels hepatocellular
carcinoma development by shaping the hepatic inflammatory
microenvironment. Nat Commun. 13:39642022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng R, Liu S, You W, Huang Y, Hu C, Gao
Y, Jia X, Li G, Xu Z and Chen Y: Gastric microbiome alterations are
associated with decreased CD8+ Tissue-Resident Memory T cells in
the tumor microenvironment of gastric cancer. Cancer Immunol Res.
10:1224–1240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu Y, Shi T, Lu X, Xu Z, Qu J, Zhang Z,
Shi G, Shen S, Hou Y, Chen Y and Wang T: Fungal-induced glycolysis
in macrophages promotes colon cancer by enhancing innate lymphoid
cell secretion of IL-22. EMBO J. 40:e1053202021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Protopsaltis NJ, Liang W, Nudleman E and
Ferrara N: Interleukin-22 promotes tumor angiogenesis.
Angiogenesis. 22:311–323. 2019. View Article : Google Scholar
|
|
13
|
Briukhovetska D, Suarez-Gosalvez J, Voigt
C, Markota A, Giannou AD, Schübel M, Jobst J, Zhang T, Dörr J,
Märkl F, et al: T cell-derived interleukin-22 drives the expression
of CD155 by cancer cells to suppress NK cell function and promote
metastasis. Immunity. 56:143–161.e11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Song X, Wei W, Zhong H, Dai J, Lan
Z, Li F, Yu X, Feng Q, Wang Z, et al: The microbiota continuum
along the female reproductive tract and its relation to
uterine-related diseases. Nat Commun. 8:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flemer B, Warren RD, Barrett MP, Cisek K,
Das A, Jeffery IB, Hurley E, O'Riordain M, Shanahan F and O'Toole
PW: The oral microbiota in colorectal cancer is distinctive and
predictive. Gut. 67:1454–1463. 2018. View Article : Google Scholar
|
|
16
|
Soto-Pantoja DR, Gaber M, Arnone AA,
Bronson SM, Cruz-Diaz N, Wilson AS, Clear KYJ, Ramirez MU, Kucera
GL, Levine EA, et al: Diet alters entero-mammary signaling to
regulate the breast microbiome and tumorigenesis. Cancer Res.
81:3890–3904. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Dwyer DN, Ashley SL, Gurczynski SJ, Xia
M, Wilke C, Falkowski NR, Norman KC, Arnold KB, Huffnagle GB,
Salisbury ML, et al: Lung microbiota contribute to pulmonary
inflammation and disease progression in pulmonary fibrosis. Am J
Respir Crit Care Med. 199:1127–1138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sipos A, Ujlaki G, Mikó E, Maka E, Szabó
J, Uray K, Krasznai Z and Bai P: The role of the microbiome in
ovarian cancer: Mechanistic insights into oncobiosis and to
bacterial metabolite signaling. Mol Med. 27:332021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma C, Han M, Heinrich B, Fu Q, Zhang Q,
Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al: Gut
microbiome-mediated bile acid metabolism regulates liver cancer via
NKT cells. Science. 360:eaan59312018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imai S, Ooki T, Murata-Kamiya N, Komura D,
Tahmina K, Wu W, Takahashi-Kanemitsu A, Knight CT, Kunita A, Suzuki
N, et al: Helicobacter pylori CagA elicits BRCAness to induce
genome instability that may underlie bacterial gastric
carcinogenesis. Cell Host Microbe. 29:941–958.e10. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bell HN, Rebernick RJ, Goyert J, Singhal
R, Kuljanin M, Kerk SA, Huang W, Das NK, Andren A, Solanki S, et
al: Reuterin in the healthy gut microbiome suppresses colorectal
cancer growth through altering redox balance. Cancer Cell.
40:185–200.e6. 2022. View Article : Google Scholar :
|
|
22
|
Zhu X, Li K, Liu G, Wu R, Zhang Y, Wang S,
Xu M, Lu L and Li P: Microbial metabolite butyrate promotes
anti-PD-1 antitumor efficacy by modulating T cell receptor
signaling of cytotoxic CD8 T cell. Gut Microbes. 15:22491432023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang SS, Xie YL, Xiao XY, Kang ZR, Lin
XL, Zhang L, Li CS, Qian Y, Xu PP, Leng XX, et al: Fusobacterium
nucleatum-derived succinic acid induces tumor resistance to
immunotherapy in colorectal cancer. Cell Host Microbe.
31:781–797.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Behary J, Amorim N, Jiang XT, Raposo A,
Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, et al:
Gut microbiota impact on the peripheral immune response in
non-alcoholic fatty liver disease related hepatocellular carcinoma.
Nat Commun. 12:1872021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Høgh RI, Møller SH, Jepsen SD, Mellergaard
M, Lund A, Pejtersen M, Fitzner E, Andresen L and Skov S:
Metabolism of short-chain fatty acid propionate induces surface
expression of NKG2D ligands on cancer cells. FASEB J.
34:15531–15546. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun K, Xu R, Ma F, Yang N, Li Y, Sun X,
Jin P, Kang W, Jia L, Xiong J, et al: scRNA-seq of gastric tumor
shows complex intercellular interaction with an alternative T cell
exhaustion trajectory. Nat Commun. 13:49432022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leader AM, Grout JA, Maier BB, Nabet BY,
Park MD, Tabachnikova A, Chang C, Walker L, Lansky A, Le Berichel
J, et al: Single-cell analysis of human non-small cell lung cancer
lesions refines tumor classification and patient stratification.
Cancer Cell. 39:1594–1609.e12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang J, Lee HY, Zhao X, Han J, Su Y, Sun
Q, Shao J, Ge J, Zhao Y, Bai X, et al: Interleukin-17D regulates
group 3 innate lymphoid cell function through its receptor CD93.
Immunity. 54:673–686.e4. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu L, Jin Y, Zhao X, Tang K, Zhao Y, Tong
L, Yu X, Xiong K, Luo C, Zhu J, et al: Tumor aerobic glycolysis
confers immune evasion through modulating sensitivity to T
cell-mediated bystander killing via TNF-α. Cell Metab.
35:1580–1596.e9. 2023. View Article : Google Scholar
|
|
30
|
Brown TP and Ganapathy V: Lactate/GPR81
signaling and proton motive force in cancer: Role in angiogenesis,
immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther.
206:1074512020. View Article : Google Scholar
|
|
31
|
Tang T, Huang X, Lu M, Zhang G, Han X and
Liang T: Transcriptional control of pancreatic cancer
immunosuppression by metabolic enzyme CD73 in a tumor-autonomous
and -autocrine manner. Nat Commun. 14:33642023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bell HN, Huber AK, Singhal R, Korimerla N,
Rebernick RJ, Kumar R, El-Derany MO, Sajjakulnukit P, Das NK, Kerk
SA, et al: Microenvironmental ammonia enhances T cell exhaustion in
colorectal cancer. Cell Metab. 35:134–149.e6. 2023. View Article : Google Scholar :
|
|
33
|
Shi Q, Wang J, Zhou M, Zheng R, Zhang X
and Liu B: Gut Lactobacillus contribute to the progression of
breast cancer by affecting the antitumor activities of immune cells
in the TME of tumor-bearing mice. Int Immunopharmacol. 124(Pt B):
1110392023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nejman D, Livyatan I, Fuks G, Gavert N,
Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E,
et al: The human tumor microbiome is composed of tumor
type-specific intracellular bacteria. Science. 368:973–980. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song W, Tiruthani K, Wang Y, Shen L, Hu M,
Dorosheva O, Qiu K, Kinghorn KA, Liu R and Huang L: Trapping of
lipopolysaccharide to promote immunotherapy against colorectal
cancer and attenuate liver metastasis. Adv Mater. 30:e18050072018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu CH, Chen Z, Chen K, Liao FT, Chung CE,
Liu X, Lin YC, Keohavong P, Leikauf GD and Di YP:
Lipopolysaccharide-Mediated chronic inflammation promotes tobacco
carcinogen-induced lung cancer and determines the efficacy of
immunotherapy. Cancer Res. 81:144–157. 2021. View Article : Google Scholar :
|
|
37
|
Zhong W, Wu K, Long Z, Zhou X, Zhong C,
Wang S, Lai H, Guo Y, Lv D, Lu J and Mao X: Gut dysbiosis promotes
prostate cancer progression and docetaxel resistance via activating
NF-κB-IL6-STAT3 axis. Microbiome. 10:942022. View Article : Google Scholar
|
|
38
|
Zhu G, Huang Q, Huang Y, Zheng W, Hua J,
Yang S, Zhuang J, Wang J and Ye J: Lipopolysaccharide increases the
release of VEGF-C that enhances cell motility and promotes
lymphangiogenesis and lymphatic metastasis through the
TLR4-NF-κB/JNK pathways in colorectal cancer. Oncotarget.
7:73711–73724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu C, Yao Z, Wang J, Zhang W, Yang Y,
Zhang Y, Qu X, Zhu Y, Zou J, Peng S, et al: Macrophage-derived CCL5
facilitates immune escape of colorectal cancer cells via the
p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 27:1765–1781.
2020. View Article : Google Scholar :
|
|
40
|
Feitelson MA, Arzumanyan A, Medhat A and
Spector I: Short-chain fatty acids in cancer pathogenesis. Cancer
Metastasis Rev. 42:677–698. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brennan CA, Clay SL, Lavoie SL, Bae S,
Lang JK, Fonseca-Pereira D, Rosinski KG, Ou N, Glickman JN and
Garrett WS: Fusobacterium nucleatum drives a pro-inflammatory
intestinal microenvironment through metabolite receptor-dependent
modulation of IL-17 expression. Gut Microbes. 13:19877802021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsushita M, Fujita K, Hayashi T, Kayama
H, Motooka D, Hase H, Jingushi K, Yamamichi G, Yumiba S, Tomiyama
E, et al: Gut microbiota-derived short-chain fatty acids promote
prostate cancer growth via IGF1 signaling. Cancer Res.
81:4014–4026. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meiser J, Schuster A, Pietzke M, Vande
Voorde J, Athineos D, Oizel K, Burgos-Barragan G, Wit N, Dhayade S,
Morton JP, et al: Increased formate overflow is a hallmark of
oxidative cancer. Nat Commun. 9:13682018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hennequart M, Pilley SE, Labuschagne CF,
Coomes J, Mervant L, Driscoll PC, Legrave NM, Lee Y, Kreuzaler P,
Macintyre B, et al: ALDH1L2 regulation of formate,
formyl-methionine, and ROS controls cancer cell migration and
metastasis. Cell Rep. 42:1125622023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ternes D, Tsenkova M, Pozdeev VI, Meyers
M, Koncina E, Atatri S, Schmitz M, Karta J, Schmoetten M, Heinken
A, et al: The gut microbial metabolite formate exacerbates
colorectal cancer progression. Nat Metab. 4:458–475. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim M, Vogtmann E, Ahlquist DA, Devens ME,
Kisiel JB, Taylor WR, White BA, Hale VL, Sung J, Chia N, et al:
Fecal metabolomic signatures in colorectal adenoma patients are
associated with gut microbiota and early events of colorectal
cancer pathogenesis. mBio. 11:e03186–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Petrick JL, Florio AA, Koshiol J, Pfeiffer
RM, Yang B, Yu K, Chen CJ, Yang HI, Lee MH and McGlynn KA:
Prediagnostic concentrations of circulating bile acids and
hepatocellular carcinoma risk: REVEAL-HBV and HCV studies. Int J
Cancer. 147:2743–2753. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Funabashi M, Grove TL, Wang M, Varma Y,
McFadden ME, Brown LC, Guo C, Higginbottom S, Almo SC and Fischbach
MA: A metabolic pathway for bile acid dehydroxylation by the gut
microbiome. Nature. 582:566–570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun L, Zhang Y, Cai J, Rimal B, Rocha ER,
Coleman JP, Zhang C, Nichols RG, Luo Y, Kim B, et al: Bile salt
hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal
cancer. Nat Commun. 14:7552023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Song X, An Y, Chen D, Zhang W, Wu X, Li C,
Wang S, Dong W, Wang B, Liu T, et al: Microbial metabolite
deoxycholic acid promotes vasculogenic mimicry formation in
intestinal carcinogenesis. Cancer Sci. 113:459–477. 2022.
View Article : Google Scholar :
|
|
51
|
Nguyen TT, Lian S, Ung TT, Xia Y, Han JY
and Jung YD: Lithocholic acid stimulates IL-8 expression in human
colorectal cancer cells via activation of Erk1/2 MAPK and
suppression of STAT3 activity. J Cell Biochem. 118:2958–2967. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee YS, Choi I, Ning Y, Kim NY,
Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD,
et al: Interleukin-8 and its receptor CXCR2 in the tumour
microenvironment promote colon cancer growth, progression and
metastasis. Br J Cancer. 106:1833–1841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fang ZZ, Zhang D, Cao YF, Xie C, Lu D, Sun
DX, Tanaka N, Jiang C, Chen Q, Chen Y, et al: Irinotecan
(CPT-11)-induced elevation of bile acids potentiates suppression of
IL-10 expression. Toxicol Appl Pharmacol. 291:21–27. 2016.
View Article : Google Scholar :
|
|
54
|
Liu Q, Yang C, Wang S, Shi D, Wei C, Song
J, Lin X, Dou R, Bai J, Xiang Z, et al: Wnt5a-induced M2
polarization of tumor-associated macrophages via IL-10 promotes
colorectal cancer progression. Cell Commun Signal. 18:512020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hang S, Paik D, Yao L, Kim E, Trinath J,
Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al: Bile acid metabolites
control TH17 and Treg cell differentiation. Nature. 576:143–148.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang N, Yang J, Han W, Han M, Liu X, Jiang
L, Cao H, Jing M, Sun T and Xu J: Identifying distinctive tissue
and fecal microbial signatures and the tumor-promoting effects of
deoxycholic acid on breast cancer. Front Cell Infect Microbiol.
12:10299052022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Riquelme E, Zhang Y, Zhang L, Montiel M,
Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et
al: Tumor microbiome diversity and composition influence pancreatic
cancer outcomes. Cell. 178:795–806.e12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar
|
|
59
|
Huang J, Zheng X, Kang W, Hao H, Mao Y,
Zhang H, Chen Y, Tan Y, He Y, Zhao W and Yin Y: Metagenomic and
metabolomic analyses reveal synergistic effects of fecal microbiota
transplantation and anti-PD-1 therapy on treating colorectal
cancer. Front Immunol. 13:8749222022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Davar D, Dzutsev AK, McCulloch JA,
Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding
Q, Pagliano O, et al: Fecal microbiota transplant overcomes
resistance to anti-PD-1 therapy in melanoma patients. Science.
371:595–602. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Joachim L, Göttert S, Sax A, Steiger K,
Neuhaus K, Heinrich P, Fan K, Orberg ET, Kleigrewe K, Ruland J, et
al: The microbial metabolite desaminotyrosine enhances T-cell
priming and cancer immunotherapy with immune checkpoint inhibitors.
EBioMedicine. 97:1048342023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Green BL, Myojin Y, Ma C, Ruf B, Ma L,
Zhang Q, Rosato U, Qi J, Revsine M, Wabitsch S and Bauer K:
Immunosuppressive CD29+ Treg accumulation in the liver in mice on
checkpoint inhibitor therapy. Gut. 73:509–520. 2024.
|
|
63
|
Klement JD, Paschall AV, Redd PS, Ibrahim
ML, Lu C, Yang D, Celis E, Abrams SI, Ozato K and Liu K: An
osteopontin/CD44 immune checkpoint controls CD8+ T cell activation
and tumor immune evasion. J Clin Invest. 128:5549–5560. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Thomas MS and Fernandez ML: Trimethylamine
N-Oxide (TMAO), diet and cardiovascular disease. Curr Atheroscler
Rep. 23:122021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu Y, Rong X, Pan M, Wang T, Yang H, Chen
X, Xiao Z and Zhao C: Integrated analysis reveals the gut microbial
metabolite TMAO promotes inflammatory hepatocellular carcinoma by
upregulating POSTN. Front Cell Dev Biol. 10:8401712022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mirji G, Worth A, Bhat SA, El Sayed M,
Kannan T, Goldman AR, Tang HY, Liu Q, Auslander N, Dang CV, et al:
The microbiome-derived metabolite TMAO drives immune activation and
boosts responses to immune checkpoint blockade in pancreatic
cancer. Sci Immunol. 7:eabn07042022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jalandra R, Dalal N, Yadav AK, Verma D,
Sharma M, Singh R, Khosla A, Kumar A and Solanki PR: Emerging role
of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl
Microbiol Biotechnol. 105:7651–7660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Luo Z, Yu X, Wang C, Zhao H, Wang X and
Guan X: Trimethylamine N-oxide promotes oxidative stress and lipid
accumulation in macrophage foam cells via the Nrf2/ABCA1 pathway. J
Physiol Biochem. 80:67–79. 2024. View Article : Google Scholar
|
|
69
|
Baldominos P, Barbera-Mourelle A, Barreiro
O, Huang Y, Wight A, Cho JW, Zhao X, Estivill G, Adam I, Sanchez X,
et al: Quiescent cancer cells resist T cell attack by forming an
immunosuppressive niche. Cell. 185:1694–1708.e19. 2022. View Article : Google Scholar
|
|
70
|
Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma
D, Jin X, Wu Y, Yan Y, Yang H, et al: The microbial metabolite
trimethylamine N-oxide promotes antitumor immunity in
triple-negative breast cancer. Cell Metab. 34:581–594.e8. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang S, Dai H, Lu Y, Li R, Gao C and Pan
S: Trimethylamine N-Oxide promotes cell proliferation and
angiogenesis in colorectal cancer. J Immunol Res. 2022:70438562022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Roberts AB, Gu X, Buffa JA, Hurd AG, Wang
Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, et al: Development
of a gut microbe-targeted nonlethal therapeutic to inhibit
thrombosis potential. Nat Med. 24:1407–1417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhu W, Gregory JC, Org E, Buffa JA, Gupta
N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al: Gut microbial
metabolite TMAO enhances platelet hyperreactivity and thrombosis
risk. Cell. 165:111–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y,
Ou C and Chen M: Gut microbe-derived metabolite trimethylamine
N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest.
99:346–357. 2019. View Article : Google Scholar
|
|
75
|
Peng L, Li ZR, Green RS, Holzman IR and
Lin J: Butyrate enhances the intestinal barrier by facilitating
tight junction assembly via activation of AMP-activated protein
kinase in Caco-2 cell monolayers. J Nutr. 139:1619–1625. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang SL, Mao YQ, Zhang ZY, Li ZM, Kong
CY, Chen HL, Cai PR, Han B, Ye T and Wang LS: Pectin supplement
significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice
humanized with gut microbiota from patients with colorectal cancer.
Theranostics. 11:4155–4170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yao Y, Cai X, Fei W, Ye Y, Zhao M and
Zheng C: The role of short-chain fatty acids in immunity,
inflammation and metabolism. Crit Rev Food Sci Nutr. 62:1–12. 2022.
View Article : Google Scholar
|
|
78
|
Song Q, Zhang X, Liu W, Wei H, Liang W,
Zhou Y, Ding Y, Ji F, Ho-Kwan Cheung A, Wong N and Yu J:
Bifidobacterium pseudolongum-generated acetate suppresses
non-alcoholic fatty liver disease-associated hepatocellular
carcinoma. J Hepatol. 79:1352–1365. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bindels LB, Porporato P, Dewulf EM, Verrax
J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O,
Muccioli GG, et al: Gut microbiota-derived propionate reduces
cancer cell proliferation in the liver. Br J Cancer. 107:1337–1344.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Thirunavukkarasan M, Wang C, Rao A, Hind
T, Teo YR, Siddiquee AA, Goghari MAI, Kumar AP and Herr DR:
Short-chain fatty acid receptors inhibit invasive phenotypes in
breast cancer cells. PLoS One. 12:e01863342017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Singh N, Gurav A, Sivaprakasam S, Brady E,
Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et
al: Activation of Gpr109a, receptor for niacin and the commensal
metabolite butyrate, suppresses colonic inflammation and
carcinogenesis. Immunity. 40:128–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lavoie S, Chun E, Bae S, Brennan CA,
Gallini Comeau CA, Lang JK, Michaud M, Hoveyda HR, Fraser GL,
Fuller MH, et al: Expression of free fatty acid receptor 2 by
dendritic cells prevents their expression of interleukin 27 and is
required for maintenance of mucosal barrier and immune response
against colorectal tumors in mice. Gastroenterology.
158:1359–1372.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ramaiah MJ, Tangutur AD and Manyam RR:
Epigenetic modulation and understanding of HDAC inhibitors in
cancer therapy. Life Sci. 277:1195042021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shanmugam G, Rakshit S and Sarkar K: HDAC
inhibitors: Targets for tumor therapy, immune modulation and lung
diseases. Transl Oncol. 16:1013122022. View Article : Google Scholar
|
|
85
|
Li X, Su X, Liu R, Pan Y, Fang J, Cao L,
Feng C, Shang Q, Chen Y, Shao C and Shi Y: HDAC inhibition
potentiates antitumor activity of macrophages and enhances
anti-PD-L1-mediated tumor suppression. Oncogene. 40:1836–1850.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Luu M, Riester Z, Baldrich A, Reichardt N,
Yuille S, Busetti A, Klein M, Wempe A, Leister H, Raifer H, et al:
Microbial short-chain fatty acids modulate CD8+ T cell responses
and improve adoptive immunotherapy for cancer. Nat Commun.
12:40772021. View Article : Google Scholar :
|
|
87
|
Dupraz L, Magniez A, Rolhion N, Richard
ML, Da Costa G, Touch S, Mayeur C, Planchais J, Agus A, Danne C, et
al: Gut microbiota-derived short-chain fatty acids regulate IL-17
production by mouse and human intestinal γδ T cells. Cell Rep.
36:1093322021. View Article : Google Scholar
|
|
88
|
Zhang H, Du M, Yang Q and Zhu MJ: Butyrate
suppresses murine mast cell proliferation and cytokine production
through inhibiting histone deacetylase. J Nutr Biochem. 27:299–306.
2016. View Article : Google Scholar
|
|
89
|
Qiao P, Zhang C, Yu J, Shao S, Zhang J,
Fang H, Chen J, Luo Y, Zhi D, Li Q, et al: Quinolinic acid, a
tryptophan metabolite of the skin microbiota, negatively regulates
NLRP3 inflammasome through AhR in psoriasis. J Invest Dermatol.
142:2184–2193.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fang Z, Pan T, Li L, Wang H, Zhu J, Zhang
H, Zhao J, Chen W and Lu W: Bifidobacterium longum mediated
tryptophan metabolism to improve atopic dermatitis via the gut-skin
axis. Gut Microbes. 14:20447232022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sehgal R, Ilha M, Vaittinen M, Kaminska D,
Männistö V, Kärjä V, Tuomainen M, Hanhineva K, Romeo S, Pajukanta
P, et al: Indole-3-Propionic acid, a Gut-Derived tryptophan
metabolite, associates with hepatic fibrosis. Nutrients.
13:35092021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cheng Y, Jin UH, Allred CD, Jayaraman A,
Chapkin RS and Safe S: Aryl hydrocarbon receptor activity of
tryptophan metabolites in young adult mouse colonocytes. Drug Metab
Dispos. 43:1536–1543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bender MJ, McPherson AC, Phelps CM, Pandey
SP, Laughlin CR, Shapira JH, Medina Sanchez L, Rana M, Richie TG,
Mims TS, et al: Dietary tryptophan metabolite released by
intratumoral Lactobacillus reuteri facilitates immune checkpoint
inhibitor treatment. Cell. 186:1846–1862.e26. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hezaveh K, Shinde RS, Klötgen A, Halaby
MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, et
al: Tryptophan-derived microbial metabolites activate the aryl
hydrocarbon receptor in tumor-associated macrophages to suppress
antitumor immunity. Immunity. 55:324–340.e8. 2022. View Article : Google Scholar
|
|
95
|
Zhang Q, Zhao Q, Li T, Lu L, Wang F, Zhang
H, Liu Z, Ma H, Zhu Q, Wang J, et al: Lactobacillus
plantarum-derived indole-3-lactic acid ameliorates colorectal
tumorigenesis via epigenetic regulation of CD8+ T cell immunity.
Cell Metab. 35:943–960.e9. 2023. View Article : Google Scholar
|
|
96
|
Garris CS, Arlauckas SP, Kohler RH, Trefny
MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M,
Gungabeesoon J, et al: Successful Anti-PD-1 cancer immunotherapy
requires T cell-dendritic cell crosstalk involving the cytokines
IFN-γ and IL-12. Immunity. 49:1148–1161.e7. 2018. View Article : Google Scholar
|
|
97
|
Sári Z, Mikó E, Kovács T, Boratkó A,
Ujlaki G, Jankó L, Kiss B, Uray K and Bai P: Indoxylsulfate, a
metabolite of the microbiome, has cytostatic effects in breast
cancer via activation of AHR and PXR receptors and induction of
oxidative stress. Cancers (Basel). 12:29152020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sharma MD, Pacholczyk R, Shi H, Berrong
ZJ, Zakharia Y, Greco A, Chang CS, Eathiraj S, Kennedy E, Cash T,
et al: Inhibition of the BTK-IDO-mTOR axis promotes differentiation
of monocyte-lineage dendritic cells and enhances antitumor T cell
immunity. Immunity. 54:2354–2371.e8. 2021. View Article : Google Scholar
|
|
99
|
Campesato LF, Budhu S, Tchaicha J, Weng
CH, Gigoux M, Cohen IJ, Redmond D, Mangarin L, Pourpe S, Liu C, et
al: Blockade of the AHR restricts a Treg-macrophage suppressive
axis induced by L-Kynurenine. Nat Commun. 11:40112020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu H, Xu X, Wang J, Wang W, Ma C, Sun T
and Shao Q: Clinical study on different doses and fractionated
radiotherapies for multiple brain metastases of non-EGFR mutant
lung adenocarcinoma. Ann Palliat Med. 9:2003–2012. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu Z, Huang L, Wang H, Shi Z, Huang Y,
Liang L, Wang R and Hu K: Predicting nomogram for severe oral
mucositis in patients with nasopharyngeal carcinoma during
intensity-modulated radiation therapy: A retrospective cohort
study. Curr Oncol. 30:219–232. 2022. View Article : Google Scholar
|
|
102
|
Guo H, Chou WC, Lai Y, Liang K, Tam JW,
Brickey WJ, Chen L, Montgomery ND, Li X, Bohannon LM, et al:
Multi-omics analyses of radiation survivors identify
radioprotective microbes and metabolites. Science.
370:eaay90972020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang Y, Yan T, Mo W, Song B, Zhang Y,
Geng F, Hu Z, Yu D and Zhang S: Altered bile acid metabolism in
skin tissues in response to ionizing radiation: deoxycholic acid
(DCA) as a novel treatment for radiogenic skin injury. Int J Radiat
Biol. 100:87–98. 2024. View Article : Google Scholar
|
|
104
|
Han JX, Tao ZH, Wang JL, Zhang L, Yu CY,
Kang ZR, Xie Y, Li J, Lu S, Cui Y, et al: Microbiota-derived
tryptophan catabolites mediate the chemopreventive effects of
statins on colorectal cancer. Nat Microbiol. 8:919–933. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Deng B, Yang B, Chen J, Wang S, Zhang W,
Guo Y, Han Y, Li H, Dang Y, Yuan Y, et al: Gallic acid induces
T-helper-1-like Treg cells and strengthens immune checkpoint
blockade efficacy. J Immunother Cancer. 10:e0040372022. View Article : Google Scholar :
|
|
106
|
Li K, Xiao Y, Bian J, Han L, He C, El-Omar
E, Gong L and Wang M: Ameliorative effects of gut microbial
metabolite urolithin a on pancreatic diseases. Nutrients.
14:25492022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
González-Sarrías A, Miguel V, Merino G,
Lucas R, Morales JC, Tomás-Barberán F, Alvarez AI and Espín JC: The
gut microbiota ellagic acid-derived metabolite urolithin A and its
sulfate conjugate are substrates for the drug efflux transporter
breast cancer resistance protein (ABCG2/BCRP). J Agric Food Chem.
61:4352–4359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ghosh S, Singh R, Vanwinkle ZM, Guo H,
Vemula PK, Goel A, Haribabu B and Jala VR: Microbial metabolite
restricts 5-fluorouracil-resistant colonic tumor progression by
sensitizing drug transporters via regulation of FOXO3-FOXM1 axis.
Theranostics. 12:5574–5595. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Y, Jiang L, Su P, Yu T, Ma Z, Liu Y
and Yu J: Urolithin A suppresses tumor progression and induces
autophagy in gastric cancer via the PI3K/Akt/mTOR pathway. Drug Dev
Res. 84:172–184. 2023. View Article : Google Scholar
|
|
110
|
Blouin JM, Penot G, Collinet M, Nacfer M,
Forest C, Laurent-Puig P, Coumoul X, Barouki R, Benelli C and
Bortoli S: Butyrate elicits a metabolic switch in human colon
cancer cells by targeting the pyruvate dehydrogenase complex. Int J
Cancer. 128:2591–2601. 2011. View Article : Google Scholar
|
|
111
|
Yuksel B, Deveci Ozkan A, Aydın D and
Betts Z: Evaluation of the antioxidative and genotoxic effects of
sodium butyrate on breast cancer cells. Saudi J Biol Sci.
29:1394–1401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhao ZH, Wang ZX, Zhou D, Han Y, Ma F, Hu
Z, Xin FZ, Liu XL, Ren TY, Zhang F, et al: Sodium butyrate
supplementation inhibits hepatic steatosis by stimulating liver
kinase B1 and insulin-induced gene. Cell Mol Gastroenterol Hepatol.
12:857–871. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Encarnação JC, Pires AS, Amaral RA,
Gonçalves TJ, Laranjo M, Casalta-Lopes JE, Gonçalves AC,
Sarmento-Ribeiro AB, Abrantes AM and Botelho MF: Butyrate, a
dietary fiber derivative that improves irinotecan effect in colon
cancer cells. J Nutr Biochem. 56:183–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shuwen H, Yangyanqiu W, Jian C, Boyang H,
Gong C and Jing Z: Synergistic effect of sodium butyrate and
oxaliplatin on colorectal cancer. Transl Oncol. 27:1015982023.
View Article : Google Scholar
|
|
115
|
Yu T, Guo F, Yu Y, Sun T, Ma D, Han J,
Qian Y, Kryczek I, Sun D, Nagarsheth N, et al: Fusobacterium
nucleatum promotes chemoresistance to colorectal cancer by
modulating autophagy. Cell. 170:548–563.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen L, Zhao R, Kang Z, Cao Z, Liu N, Shen
J, Wang C, Pan F, Zhou X, Liu Z, et al: Delivery of short chain
fatty acid butyrate to overcome Fusobacterium nucleatum-induced
chemoresistance. J Control Release. 363:43–56. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tintelnot J, Xu Y, Lesker TR, Schönlein M,
Konczalla L, Giannou A D, Pelcza r P, Kylies D, Puelles VG,
Bielecka AA, et al: Microbiota-derived 3-IAA influences
chemotherapy efficacy in pancreatic cancer. Nature. 615:168–174.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Colbert LE, El Alam MB, Wang R, Karpinets
T, Lo D, Lynn EJ, Harris TA, Elnaggar JH, Yoshida-Court K, Tomasic
K, et al: Tumor-resident Lactobacillus iners confer chemoradiation
resistance through lactate-induced metabolic rewiring. Cancer Cell.
41:1945–1962.e11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chang TK, Yin TC, Su WC, Tsai HL, Huang
CW, Chen YC, Li CC, Chen PJ, Ma CJ, Chuang KH, et al: A Pilot Study
of Silymarin as Supplementation to reduce toxicities in metastatic
colorectal cancer patients treated with first-line FOLFIRI Plus
Bevacizumab. Oncol Res. 28:801–809. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang W, Chang L, Guo Q, Chen J, Yu W and
Zhang W: Programmed cell death protein-1 inhibitors in the
treatment of digestive system tumors in Chinese population: An
observational study of effectiveness and safety. Ann Palliat Med.
10:9015–9024. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Renga G, Nunzi E, Pariano M, Puccetti M,
Bellet MM, Pieraccini G, D'Onofrio F, Santarelli I, Stincardini C,
Aversa F, et al: Optimizing therapeutic outcomes of immune
checkpoint blockade by a microbial tryptophan metabolite. J
Immunother Cancer. 10:e0037252022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lu C, Liu Z, Klement JD, Yang D, Merting
AD, Poschel D, Albers T, Waller JL, Shi H and Liu K: WDR5-H3K4me3
epigenetic axis regulates OPN expression to compensate PD-L1
function to promote pancreatic cancer immune escape. J Immunother
Cancer. 9:e0026242021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang J, Ge J, Wang Y, Xiong F, Guo J,
Jiang X, Zhang L, Deng X, Gong Z, Zhang S, et al: EBV miRNAs BART11
and BART17-3p promote immune escape through the enhancer-mediated
transcription of PD-L1. Nat Commun. 13:8662022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lainé A, Labiad O, Hernandez-Vargas H,
This S, Sanlaville A, Léon S, Dalle S, Sheppard D, Travis MA,
Paidassi H and Marie JC: Regulatory T cells promote cancer
immune-escape through integrin αvβ8-mediated TGF-β activation. Nat
Commun. 12:62282021. View Article : Google Scholar
|
|
125
|
Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li
K, Nishiyama A, Arai S, Yano S and Wang W: EGFR-TKI resistance
promotes immune escape in lung cancer via increased PD-L1
expression. Mol Cancer. 18:1652019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mehra S, Garrido VT, Dosch AR, Lamichhane
P, Srinivasan S, Singh SP, Zhou Z, De Castro Silva I, Joshi C, Ban
Y, et al: Remodeling of stromal immune microenvironment by
urolithin a improves survival with immune checkpoint blockade in
pancreatic cancer. Cancer Res Commun. 3:1224–1236. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Coutzac C, Jouniaux JM, Paci A, Schmidt J,
Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix
L, et al: Systemic short chain fatty acids limit antitumor effect
of CTLA-4 blockade in hosts with cancer. Nat Commun. 11:21682020.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong
T, Chen H and Wang C: Immunotherapy: Reshape the tumor immune
microenvironment. Front Immunol. 13:8441422022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: new findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Korbecki J, Kojder K, Simińska D,
Bohatyrewicz R, Gutowska I, Chlubek D and Baranowska-Bosiacka I: CC
Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer
Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4.
Int J Mol Sci. 21:84122020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hennessy M, Wahba A, Felix K, Cabrera M,
Segura MG, Kundra V, Ravoori MK, Stewart J, Kleinerman ES, Jensen
VB, et al: Bempegaldesleukin (BEMPEG; NKTR-214) efficacy as a
single agent and in combination with checkpoint-inhibitor therapy
in mouse models of osteosarcoma. Int J Cancer. 148:1928–1937. 2021.
View Article : Google Scholar
|
|
132
|
Rosen DB, Kvarnhammar AM, Laufer B, Knappe
T, Karlsson JJ, Hong E, Lee YC, Thakar D, Zúñiga LA, Bang K, et al:
TransCon IL-2 β/γ: A novel long-acting prodrug with sustained
release of an IL-2Rβ/γ-selective IL-2 variant with improved
pharmacokinetics and potent activation of cytotoxic immune cells
for the treatment of cancer. J Immunother Cancer. 10:e0049912022.
View Article : Google Scholar
|
|
133
|
Naing A, Papadopoulos KP, Autio KA, Ott
PA, Patel MR, Wong DJ, Falchook GS, Pant S, Whiteside M, Rasco DR,
et al: Safety, antitumor activity, and immune activation of
pegylated recombinant human interleukin-10 (AM0010) in patients
with advanced solid tumors. J Clin Oncol. 34:3562–3569. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Taniguchi Y, Kurokawa Y, Hagi T, Takahashi
T, Miyazaki Y, Tanaka K, Makino T, Yamasaki M, Nakajima K, Mori M
and Doki Y: Methylprednisolone inhibits tumor growth and peritoneal
seeding induced by surgical stress and post-operative
complications. Ann Surg Oncol. 26:2831–2838. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hailemichael Y, Johnson DH, Abdel-Wahab N,
Foo WC, Bentebibel SE, Daher M, Haymaker C, Wani K, Saberian C,
Ogata D, et al: Interleukin-6 blockade abrogates immunotherapy
toxicity and promotes tumor immunity. Cancer Cell. 40:509–523.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Xue D, Moon B, Liao J, Guo J, Zou Z, Han
Y, Cao S, Wang Y, Fu YX and Peng H: A tumor-specific pro-IL-12
activates preexisting cytotoxic T cells to control established
tumors. Sci Immunol. 7:eabi68992022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Agliardi G, Liuzzi AR, Hotblack A, De Feo
D, Núñez N, Stowe CL, Friebel E, Nannini F, Rindlisbacher L,
Roberts TA, et al: Intratumoral IL-12 delivery empowers CAR-T cell
immunotherapy in a pre-clinical model of glioblastoma. Nat Commun.
12:4442021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chang PV, Hao L, Offermanns S and
Medzhitov R: The microbial metabolite butyrate regulates intestinal
macrophage function via histone deacetylase inhibition. Proc Natl
Acad Sci USA. 111:2247–2252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Mager LF, Burkhard R, Pett N, Cooke NCA,
Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, et al:
Microbiome-derived inosine modulates response to checkpoint
inhibitor immunotherapy. Science. 369:1481–1489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
O'Keefe SJ: Diet, microorganisms and their
metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol.
13:691–706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Niekamp P and Kim CH: Microbial metabolite
dysbiosis and colorectal cancer. Gut Liver. 17:190–203. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wu X, Wu Y, He L, Wu L, Wang X and Liu Z:
Effects of the intestinal microbial metabolite butyrate on the
development of colorectal cancer. J Cancer. 9:2510–2517. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kaźmierczak-Siedlecka K, Marano L, Merola
E, Roviello F and Połom K: Sodium butyrate in both prevention and
supportive treatment of colorectal cancer. Front Cell Infect
Microbiol. 12:10238062022. View Article : Google Scholar
|
|
144
|
Zhao H, Wang D, Zhang Z, Xian J and Bai X:
Effect of gut microbiota-derived metabolites on immune checkpoint
inhibitor therapy: Enemy or friend? Molecules. 27:47992022.
View Article : Google Scholar : PubMed/NCBI
|