Introduction
According to Global Cancer Statistics from 2018,
there were 18.1 million new cancer cases and 9.6 million deaths
attributed to cancer (1). Cancer
ranks as the second leading cause of death globally, following
ischemic heart disease (2).
Current research on cancer diagnosis, treatment and prognosis
primarily focuses on genetic and epigenetic factors, such as
microRNAs (miRNAs). The first miRNA was discovered in nematodes,
leading to further exploration of similar endogenous miRNAs across
species using RNA interference (3). miRNAs not only translate proteins
but also regulate gene transcription, influencing processes such as
cell differentiation and organism development (3,4).
Initially transcribed by RNA polymerase II in the nucleus, primary
miRNAs are processed into precursor miRNAs by the Drosha
enzyme-Dgcr8 complex. After translocating to the cytoplasm, these
precursor miRNAs are cleaved into ~22-nucleotide-long
double-stranded miRNAs by the enzyme Dicer (5). The double strands are then unwound,
and the mature miRNA strand forms an RNA-induced silencing complex,
which binds to the 3'-untranslated region (3'-UTR) of target mRNA
to degrade or inhibit its translation, thereby negatively
regulating gene expression (6).
To date, >1,000 miRNAs have been identified in humans (7). Dysregulation of miRNA, such as
miRNA-122, is associated with various diseases, particularly cancer
(8). miRNA-122 constitutes ~72%
of the total miRNAs found in the liver (9). Its expression is altered in multiple
diseases. For example, elevated levels of miRNA-122 are associated
with bronchiolitis. which may progress to asthma (10). miRNA-122 is also implicated in
promoting diabetic retinopathy (11). Additionally, high levels of
miRNA-122 suppress the release of inflammatory factors in
osteoarthritis, suggesting potential therapeutic applications
(12). Numerous studies have
reported abnormal expression of miRNA-122 in various types of
cancer, where it modulates tumor development by targeting specific
genes (8,13). However, the exact role of
miRNA-122 in cancer remains elusive.
The present review aimed to summarize how miRNA-122
influences various aspects of tumor cell behavior, including
proliferation, migration, metastasis, invasion, angiogenesis and
apoptosis, the role of miRNA-122 in modulating responses to
radiotherapy and chemotherapy in tumor cells, as well as strategies
for its systemic delivery and potential utility as a biomarker.
miRNA-122 and cancer
miRNAs are classified as oncogenic or
tumor-suppressive based on their effects (14). miRNA-122 is derived from a single
genomic locus on human chromosome 18 (15). miRNA-122 regulates tumor cell
processes such as proliferation, angiogenesis, invasion, migration
and apoptosis by targeting downstream genes. Due to its ability to
target a diverse array of downstream genes, including both
oncogenes and tumor suppressors, miRNA-122 exerts varied roles in
cancer development, serving as either an oncogene or tumor
suppressor, and may exhibit dual roles in certain types of cancer
(16-34). Table
I summarizes the role of miRNA-122 in various types of
cancer.
 | Table IRole of miRNA-122 in different types
of cancer. |
Table I
Role of miRNA-122 in different types
of cancer.
| Cancer | Upstream
regulator | Expression | Site | Target | Biological
function | Role | (Refs.) |
|---|
| Bladder | - | Downregulated | Tissue and
cell | VEGFC | Inhibit tumor
proliferation, angiogenesis, migration and invasion | Tumor
suppressor | (8) |
| - | Downregulated | Tissue | CREB1 | Inhibit cell
proliferation and invasion | Tumor
suppressor | (16) |
| Non-small cell
carcinoma | - | Not detected | Cell | IGF1R | Inhibit tumor
proliferation, invasion, and migration | Tumor
suppressor | (17) |
| - | Downregulated | A549/GR Cell | Prx II | Inhibit tumor
progression | Tumor
suppressor | (18) |
| Bile duct | lncRNA UCA1 | Downregulated | Tissue and
cell | CLIC1 | Inhibit cell
invasion and migration | Tumor
suppressor | (19) |
| Nasopharyngeal
carcinoma | - | Downregulated | Tissue and
cell | TRIM29 | Inhibit tumor
proliferation, migration and invasion | Tumor
suppressor | (20) |
| lncRNA DRAIC | Not detected | Not detected | SATB1 | Inhibit cell
proliferation, migration and invasion | Tumor
suppressor | (21) |
| Prostate | - | Downregulated | Tissue and
cell | ROCK2 | Inhibit cell
proliferation | Tumor
suppressor | (22) |
| Breast | - | Upregulated | Radiation-resistant
breast cancer cells | ZNF611 | Promote radiation
resistance in breast cancer cells | Oncogene | (23) |
| lncRNA RPPH1 | Upregulated | Tissue | PKM2 and IGF1R | Inhibit cell
proliferation | Tumor
suppressor | (24) |
| Liver | - | Downregulated | Tissue | LMNB2 | Inhibit tumor
proliferation, migration and invasion | Tumor
suppressor | (25) |
| Colorectal | - | Upregulated | Colorectal cancer
liver metastatic tissue | CAT1 | Promote colorectal
cancer metastasis to the liver | Oncogene | (26) |
| - | Downregulated |
Oxaliplatin-resistant cells | XIAP | Promote colorectal
cancer cell sensitivity to oxaliplatin | Tumor
suppressor | (27) |
| Esophageal | - | Not detected | Not detected | KIF22 | Inhibit cell
proliferation and migration; promote apoptosis | Tumor
suppressor | (28) |
| Glioma | - | Downregulated | Tissue and
cell | RUNX2 | Inhibit cell
proliferation; promote apoptosis | Tumor
suppressor | (29) |
| CircRNA PTN | Downregulated | Cell | SOX6 | Inhibit glioma cell
proliferation; promote apoptosis | Tumor
suppressor | (30) |
| Renal cell
carcinoma | - | Upregulated | Tissue | Dicer | Promote cell
migration, invasion and metastasis | Oncogene | (31) |
| - | Upregulated | Tissue and
cell | FOXO3 | Promote cell
proliferation, migration and invasion | Oncogene | (32) |
| Osteosarcoma | - | Not detected | Cell | CCNG1, Bcl-w and
ADAM10 | Inhibit cell
proliferation, migration and invasion | Tumor
suppressor | (33) |
| Cervical | - | Not detected | Not detected | RAD21 | Inhibit cell
proliferation; promote apoptosis | Tumor
suppressor | (34) |
Non-small cell carcinoma (NSCLC)
Lung cancer includes small cell carcinoma and NSCLC,
with NSCLC being the predominant form, constituting 80-85% of cases
and associated with 2-year relative survival rate of ~42% (35). NSCLC cells do not express
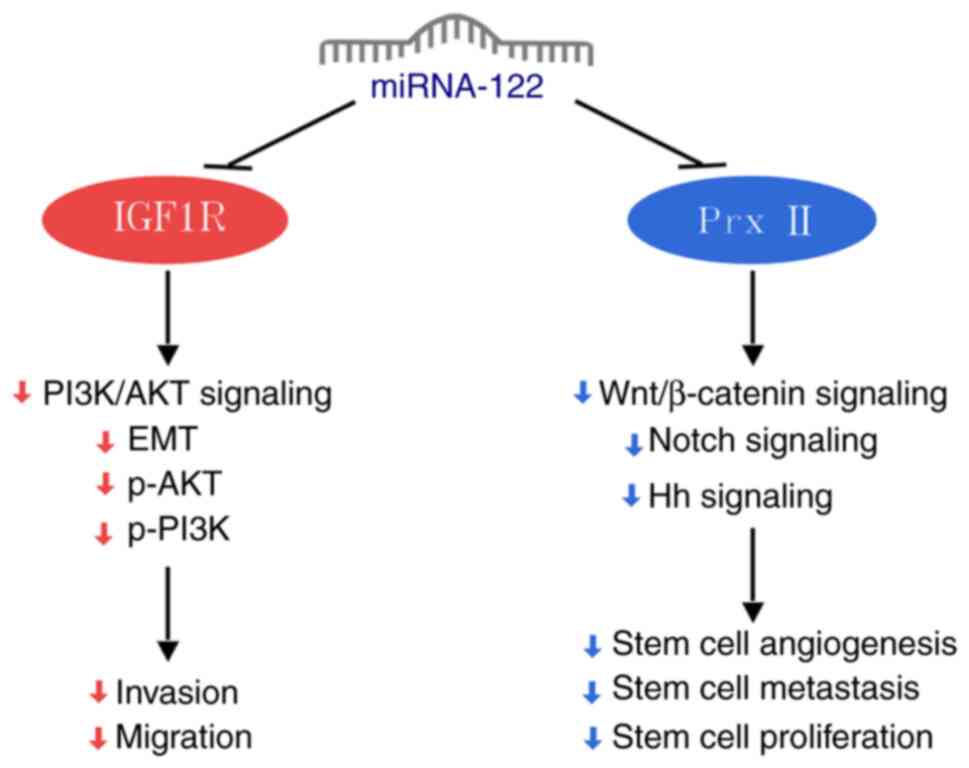
endogenous miRNA-122. miRNA-122 inhibits the PI3K/AKT signaling
pathway through suppression of its target gene insulin-like growth
factor 1 receptor (IGF1R). This suppression blocks PI3K and AKT
phosphorylation, enhances E-cadherin expression and decreases
N-cadherin and vimentin expression. This disruption impedes
epithelial-mesenchymal transition (EMT), thereby inhibiting
migration and invasion of NSCLC cells (17).
Long-term exposure of NSCLC cells to gefitinib leads
to emergence of gefitinib-resistant A549/GR cells. As a target of
miRNA-122, peroxiredoxin II (Prx II) inhibition suppresses the
self-renewal and EMT of A549/GR stem cells, which is characterized
by an increase in E-cadherin and decrease in vimentin expression
following miRNA-122 knockout. This process involves inhibition of
Hedgehog, Notch and Wnt/β-catenin signaling pathways following Prx
II targeting by miRNA-122. Knockout of miRNA-122 reverses these
effects (Fig. 1) (18).
Nasopharyngeal carcinoma (NPC)
NPC arises from the nasopharyngeal crypt,
originating from mucosal epithelium of the nasopharynx, and is
characterized by high malignancy (36). NPC cells exhibit significantly
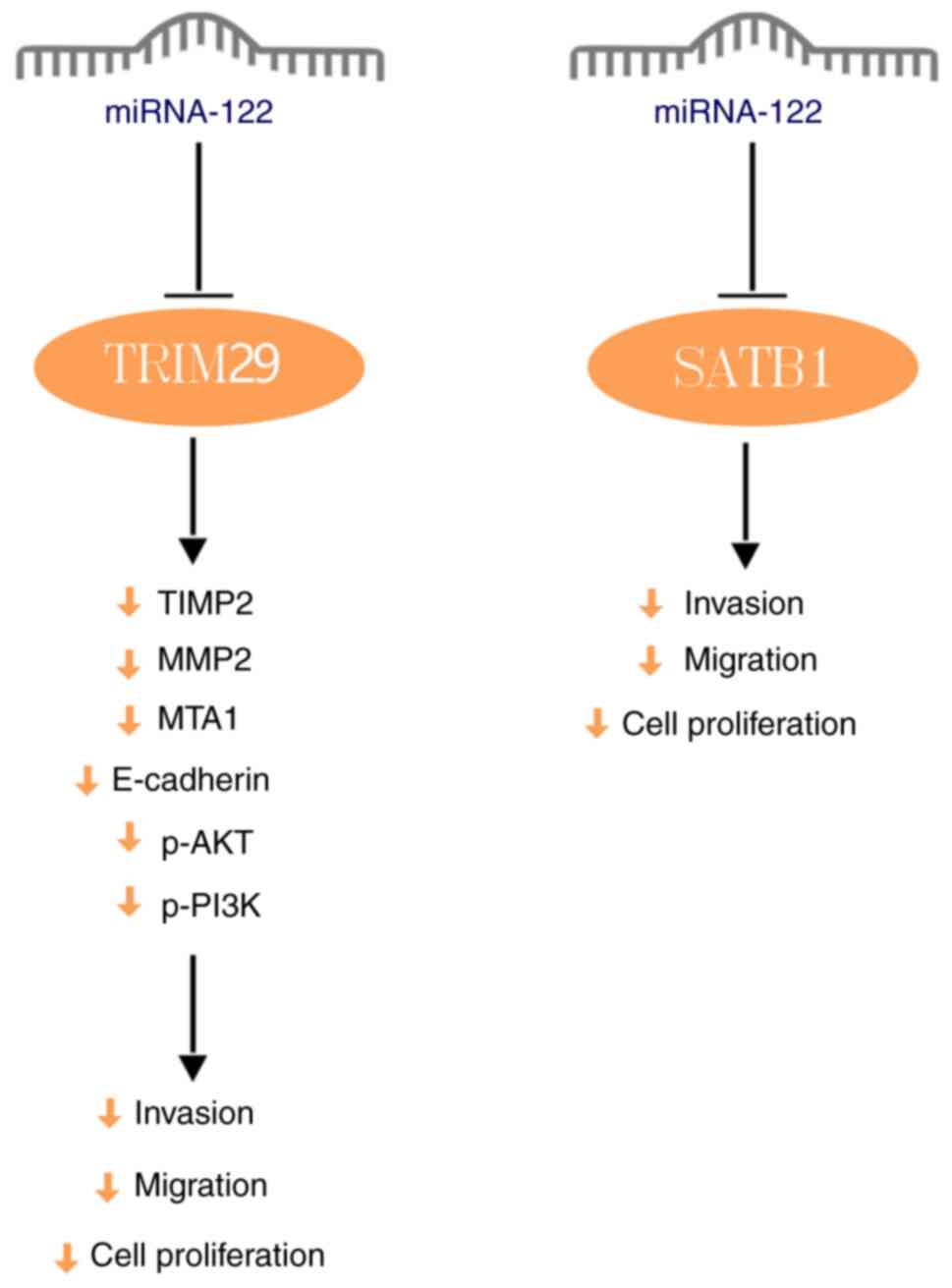
decreased expression of miRNA-122. miRNA-122 suppresses PI3K and
AKT phosphorylation, inhibits the PI3K/AKT signaling pathway and
decreases the expression of E-cadherin, metastasis-associated gene
1, MMP2 and tissue inhibitor of metalloproteinase 2 by targeting
tripartite motif-containing protein 29. This inhibition suppresses
the proliferation, migration and invasion capabilities of NPC cells
(20). Moreover, the long
non-coding RNA (lncRNA) DRAIC (downregulated RNA in cancer)
upregulates special AT-rich binding protein 1 (SATB1) expression by
binding to miRNA-122, thereby alters the configuration of the
miRNA-122 binding site on SATB1 and suppressing miRNA-122
expression. This promotes the proliferation, migration and invasion
of NPC cells; these effects are reversed by miRNA-122
overexpression (Fig. 2) (21).
Prostate cancer
Prostate cancer is a prevalent malignancy in male
patients, accounting for ~26% of newly diagnosed cancer cases
(35). Tumor cells obtain their
energy supply through relatively low-yield glycolysis, which does
not involve oxygen or mitochondria (37). Pyruvate kinase M2 (PKM2) serves as
a key rate-limiting enzyme in glycolysis, driving tumor cell
proliferation (38). In
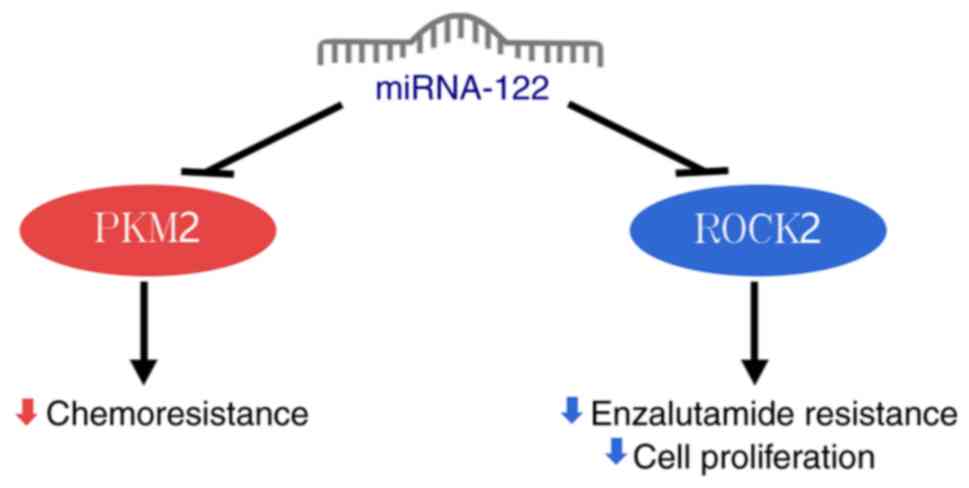
docetaxel-resistant prostate cancer cells, miRNA-122 expression is
notably decreased. This leads to increased PKM2 expression,
enhancing glycolysis, promoting proliferation and reducing
apoptosis in these resistant cells. The mechanism involves
miRNA-122 targeting and inhibiting PKM2 to suppress prostate cancer
progression (39).
miRNA-122 inhibits the proliferation of prostate
cancer cells by downregulating Rho-associated protein kinase 2
(ROCK2) expression (22). ROCK2,
a member of the Rho family, promotes invasion and metastasis in
prostate cancer (40).
Additionally, silencing ROCK2 expression counteracts enzalutamide
resistance in enzalutamide-resistant prostate cancer cells, leading
to inhibition of cancer cell proliferation (Fig. 3) (41).
Bile duct cancer (BDC)
BDC is a malignant tumor of the epithelial cells of
the bile ducts; it is insidious and the 5-year survival rate drops
to 2% if distant metastasis occurs (42). Expression of miRNA-122 is notably
decreased in BDC compared with normal bile duct tissue.
Overexpressed miRNA-122 in BDC cells significantly inhibits tumor
cell proliferation and invasion while promoting apoptosis. However,
the specific mechanism by which miRNA-122 inhibits BDC progression
has not been fully elucidated (43). Wu et al (44) demonstrated that overexpression of
miRNA-122 in BDC cells upregulates P53 expression, thereby
suppressing tumor cell proliferation and invasion while promoting
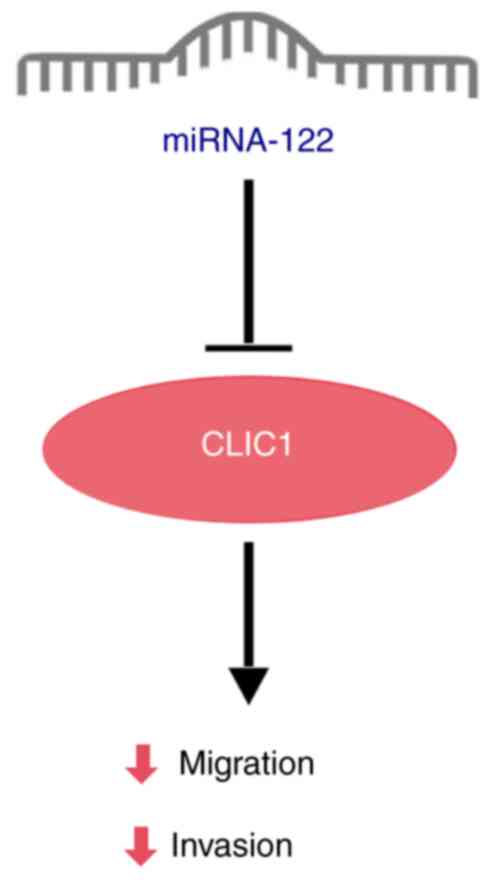
apoptosis. Additionally, miRNA-122 also inhibits BDC cell migration
and invasion by targeting the downstream target gene chloride
intracellular channel 1 (CLIC1). lncRNA urothelial cancer
associated 1 (UCA1) regulates CLIC1 expression through sponging
miRNA-122 to promote BDC metastasis (Fig. 4) (19).
Bladder cancer
In 2018, 549,393 patients were diagnosed with
bladder cancer worldwide and 199,922 died from the disease
(1). Angiogenesis is key for
tumor development, making its inhibition a potential treatment
strategy. Vascular endothelial growth factor (VEGF) is associated
with tumor progression (45).
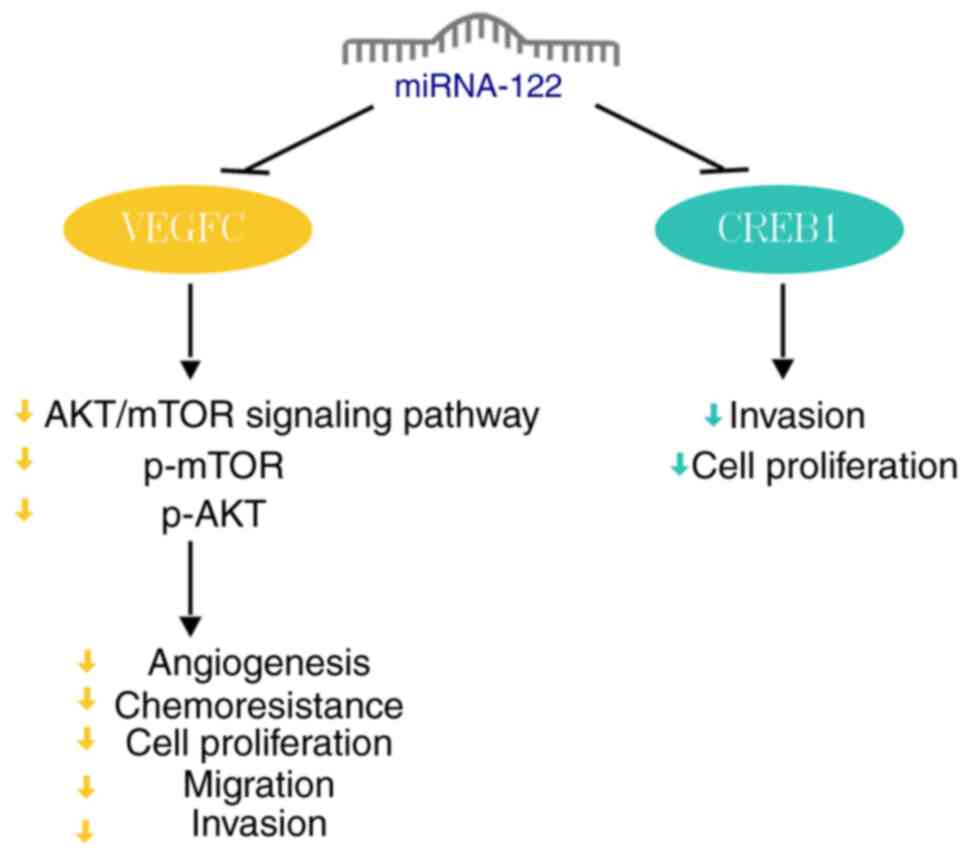
miRNA-122 expression is downregulated in human bladder cancer
tissue. By binding to the 3'-UTR of VEGFC, miRNA-122 significantly
decreases VEGFC expression, inhibiting AKT and mTOR
phosphorylation. This inhibition ultimately suppresses
angiogenesis, invasion, migration and proliferation of bladder
cancer cells (8). Furthermore,
miRNA-122 overexpression enhances sensitivity of bladder cancer
cells to cisplatin and promotes cancer cell apoptosis (8).
Prior research (16) has established that the
cAMP-response element-binding protein (CREB) 1 serves as a
downstream target of miRNA-122. miRNA-122 directly inhibits CREB1
to suppress proliferation and invasion in bladder cancer cells
(Fig. 5) (16).
Breast cancer
Breast cancer is the most common cancer in female
patients and the leading cause of cancer mortality in female
patients worldwide (46). lncRNA
ribonuclease P RNA component H1 (RPPH1) is highly expressed in
breast cancer tissue. lncRNA RPPH1 promotes the expression of
downstream genes, such as PKM2 and IGF1R, by sponging miRNA-122,
which further promotes proliferation of breast cancer cells; by
contrast, miRNA-122 overexpression reverses this process (24). miRNA-122 overexpression enhances
sensitivity of breast cancer cells to radiotherapy, thereby
inhibiting tumor cell survival (23). Radioresistant breast cancer cells
show significantly increased miRNA-122 expression, whereas
miRNA-122 knockout decreases the survival of these cells. This
mechanism may involve miRNA-122 oncogenic potential through
targeting zinc finger proteins 611 (ZNF611) in radioresistant
breast cancer cells (23).
Therefore, in primary breast cancer cells (before radiation therapy
and without radioresistance), elevated miRNA-122 expression
inhibits cancer progression and serves as a tumor suppressor.
Conversely, in radiotherapy-resistant breast cancer cells, elevated
miRNA-122 expression can promote radiotherapy resistance,
demonstrating its dual role as both a tumor suppressor and oncogene
depending on the cellular context (Fig. 6).
Liver cancer
Hepatocellular carcinoma (HCC), the predominant form
of liver cancer, is the fifth most common cause of cancer deaths In
the United States (47).
miRNA-122 disrupts mesenchymal cytoskeleton, upregulates E-cadherin
and α-catenin expression and downregulates vimentin and fibronectin
expression by binding to the 3'-UTR of Ras homologous gene family
member A (RhoA); this triggers mesenchymal-epithelial transition,
reverses EMT and thus inhibits the invasion and migration of HCC
cells (48).
In a study on adriamycin resistance in HCC (49), overexpression of miRNA-122
suppressed expression of ATP-binding cassette superfamily member 2
and multidrug resistance-associated protein 1. This overexpression
enhances sensitivity of HCC cells to chemotherapeutic drugs and
inhibits proliferation.
Polyploidy is a balanced amplification of the genome
and is common in the liver. Hepatocytes become polyploid mostly due
to failure of cytoplasmic division. Approximately 30% of
hepatocytes in the human liver are polyploid. Liver polyploidy
prevents gene mutations in hepatocytes and decreases the formation
of liver tumors (50). miRNA-122
directly targets cytoplasmic cleavage genes cut-like homeobox
protein-1, RhoA, microtubule-associated protein RP/EB family member
1, IQ-containing GTPase-activating protein 1, neural precursor
cell-expressed developmentally down-regulated protein 4-like and
solute carrier family 25 member 34, thereby resulting in
cytoplasmic cleavage failure to increase hepatic polyploidization,
thus suppressing liver tumorigenesis (51). Other studies have shown that
frequent ploidy reduction in polyploid hepatocytes results in
predisposition to liver tumor formation, which may imply that
notable reduction in hepatic polyploidy contributes to cancer
development, as this leads to genetic mutations (Fig. 7) (52,53).
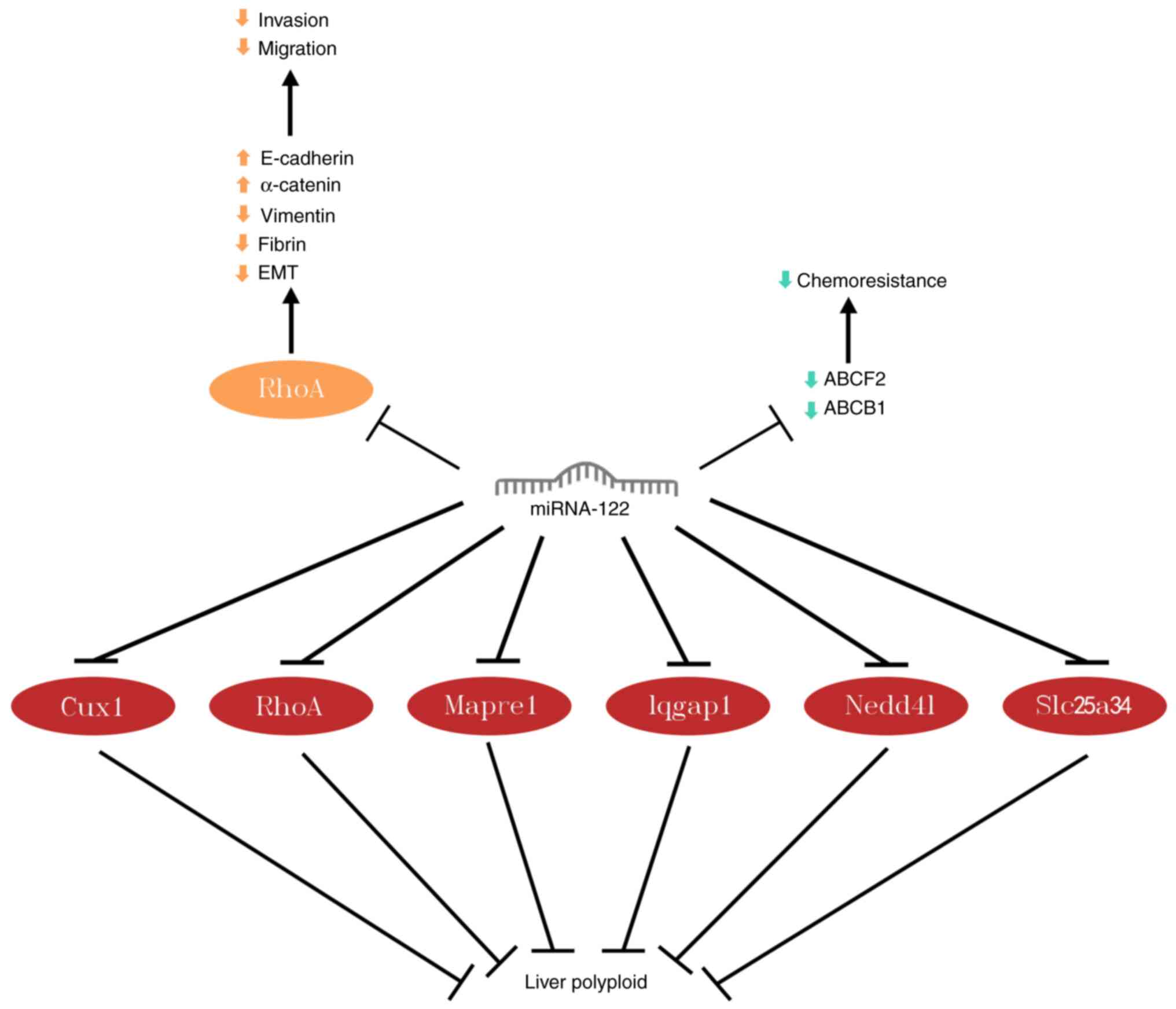 | Figure 7miRNA-122 downregulates ABCF2 and
ABCB1 expression to inhibit adriamycin resistance in hepatocellular
carcinoma. miRNA-122 targeting of RhoA inhibits migration and
invasion of hepatocellular carcinoma cells; targeting Cux1, RhoA,
Mapre1, Iqgap1, Nedd4l, and Slc25a34 promotes liver
polyploidization. miRNA, microRNA; ABCF2, adenosine triphosphate
binding cassette superfamily member 2; ABCB1, multidrug
resistance-associated protein 1; Cux1, cut-like homeobox protein-1;
Mapre1, microtubule-associated protein RP/EB family member 1;
Iqgap1, IQ-containing GTPase-activating protein 1; Nedd41, neural
precursor cell-expressed developmentally down-regulated protein
4-like; Slc25a34, solute carrier family 25 member 34; EMT,
Epithelial mesenchymal transition. |
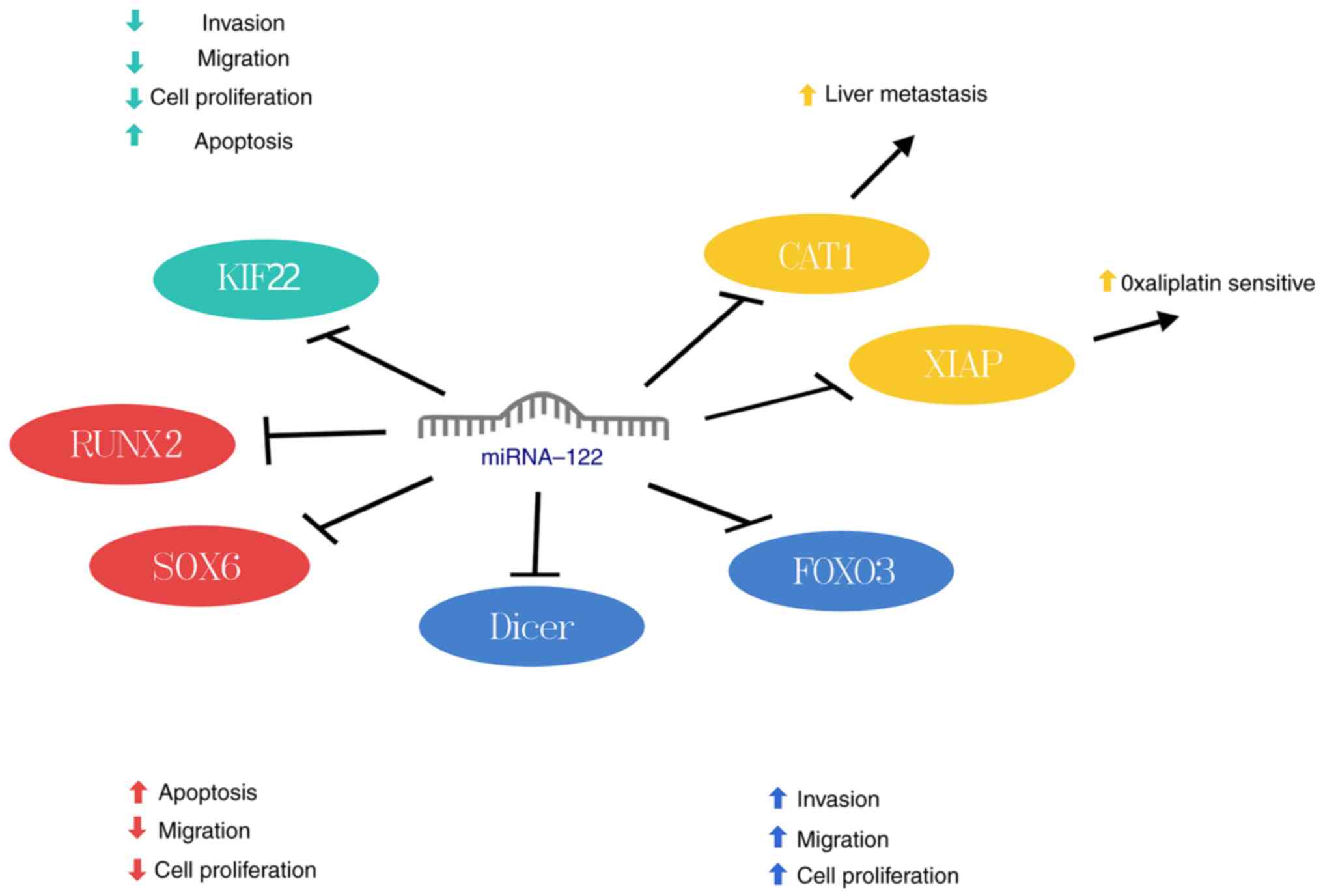
Colorectal cancer (CRC)
CRC accounted for 9.4% of all new cases of cancer in
2020 (54). The liver is the most
important target organ for hematogenous metastasis of CRC. Liver
metastasis is the main cause of death due to CRC (54). Studies have found elevated
miRNA-122 expression in liver cells of metastatic CRC, contrasting
high expression was not detected in CRC cells. miRNA-122 correlates
negatively with cationic amino acid transporter protein 1 (CAT1)
expression and can enhance liver migration by targeting CAT1
(26). In oxaliplatin-resistant
CRC cell lines, miRNA-122 expression is reduced while X-linked
inhibitor of apoptosis protein (XIAP) expression is increased.
miRNA-122 downregulates XIAP to restore oxaliplatin sensitivity in
resistant cells, thereby suppressing CRC progression (27). Thus, elevated miRNA-122 levels in
untreated CRC cells may indicate potential liver metastasis,
suggesting an oncogenic role. Conversely, decreased miRNA-122
expression in oxaliplatin-treated CRC cells may indicate drug
resistance, highlighting its role as a tumor suppressor. In
summary, miRNA-122 exhibits dual roles as both a tumor suppressor
and an oncogene in CRC (Fig.
8).
Esophageal cancer
Due to changes in dietary habits and genetic
factors, esophageal cancer is a significant health challenge
worldwide, with overall 5-year survival rate of ~10% and a
propensity for early metastasis (55). KIF22, a kinesin-like DNA-binding
protein, can promote cancer progression. Kinesin superfamily
protein 22 (KIF22) is prominently expressed in esophageal squamous
carcinoma tissue and cells, correlating significantly with poor
prognosis (28); miRNA-122
negatively regulates KIF22, downregulates the expression of cyclin
G1 (CCNG1), Cyclin dependent kinase 2, N-cadherin and vimentin and
upregulates p21, p27 and E-cadherin expression; this induces S
phase arrest and apoptosis of esophageal squamous carcinoma cells
and inhibits EMT (28) (Fig. 8). Additionally, the response
elements of miRNA-122 and miRNA-143 mediated by adenoviral vectors
cause tumor necrosis factor-associated apoptosis-inducing ligand
(TRAIL) to be highly expressed in esophageal cancer, but not in
normal cells; this selectively induces apoptosis in esophageal
cancer cells and protects against the toxicity of TRAIL to the
liver (56). This may be an
effective approach to treat esophageal cancer and prevent liver
toxicity.
Glioma
Glioma is the most common primary malignant tumors
of the brain. Glioma grows invasively and often involve surrounding
normal brain tissue. RUNX2, part of the RUNX transcription factor
family, regulates gene expression and enhances tumor cell
proliferation by binding to specific DNA sequences (57). Additionally, miRNA-122 is
downregulated in glioma compared with normal tissues (29). By targeting RUNX2, miRNA-122
inhibits proliferation and migration of glioma cells (29). Moreover, miRNA-122 induces cell
cycle arrest, promotes apoptosis, and decreases proliferation in
transglioma cells by targeting SOX6. However, this inhibitory
effect on glioma cells can be reversed by circular RNA
pleiotrophin, which serves as a miRNA-122 sponge (Fig. 8) (30).
Renal cell carcinoma (RCC)
RCC, is a prevalent form of kidney cancer,
constituting for 2 to 3% of all adult malignancies, with 1.8%
mortality rate (1). Fan et
al (31) observed elevated
miRNA-122 expression in RCC cells, correlating with poor prognosis.
miRNA-122 induces EMT by suppressing Dicer, a downstream target
gene, thereby enhancing migration and invasion of RCC cells
(31). Similarly, Nie et
al (32) found increased
miRNA-122 expression in RCC cells, which promotes cell
proliferation, migration and invasion by targeting FOXO3. Thus,
miRNA-122 plays an oncogene role in RCC (Fig. 8).
Other types of cancer
Acute myeloid leukemia (AML) is a blood cancer
characterized by abnormal cell proportions due to impaired
differentiation of hematopoietic stem cells (58). Zhang et al (59) reported that patients with AML with
low miRNA-122 levels in their bone marrow have poorer overall
survival and lower rates of complete remission compared with those
with high miRNA-122 expression (59). Yang et al (60) found that miRNA-122 expression is
significantly reduced in AML bone marrow compared with
non-malignant tissue and high miRNA-122 levels inhibit AML cell
proliferation by affecting cell cycle pathways (60). However, further research is needed
to understand how miRNA-122 suppresses AML progression.
Osteosarcoma, a common bone malignancy in children
and adolescents, is associated with high mortality rates (61). miRNA-122 suppresses proliferation,
migration, and invasion of osteosarcoma cells by decreasing the
expression of CCNG1, Bcl-w and a disintegrin and matrix
metalloproteinase-10 (33).
However, this inhibitory effect is reversed by miRNA-122 sponging
(33). Additionally, Liu et
al (62) noted varied
miRNA-122 expression between different osteosarcoma cell lines;
while miRNA-122 is upregulated in HOS, Saos-2 and U2OS cell lines,
it is downregulated in MG-63 cells. High miRNA-122 levels inhibit
the proliferation, invasion and migration of Saos-2 osteosarcoma
cells, indicating its role as a tumor suppressor despite being
highly expressed in this cell line (62).
Cervical cancer is a common malignant tumor
affecting female patients accounting for about 3.2% of all cancers
(1). According to Yang et
al (34), miRNA-122 targets
RAD21, a component of the cohesin complex, thereby inhibiting the
PI3K/AKT signaling pathway in cervical cancer cells. This
inhibition suppresses cervical cancer cell proliferation and
promotes apoptosis. Elevated levels of miRNA-122 are associated
with improved prognosis in patients with cervical cancer (34).
miRNA-122 as a biomarker
With technological advancements, the quantification
of miRNA-122 has become precise and convenient, highlighting its
potential as a biomarker. Recent studies have indicated that
miRNA-122 may be valuable for diagnosing cancer, predicting
prognosis and assessing treatment response (Table II) (63-67).
 | Table IIApplication of microRNA-122 as a
biomarker. |
Table II
Application of microRNA-122 as a
biomarker.
| Cancer | Expression | Indication | (Refs.) |
|---|
| Acute myeloid
leukemia | Downregulated | Shorter
recurrence-free and overall survival in response to low
expression | (60) |
| Colorectal | Upregulated | High expression
suggests poor prognosis for colorectal cancer with liver
metastasis | (63) |
| Glioma | Downregulated | Glioma diagnosis
and poorer prognosis | (64) |
| Liver cancer | Downregulated | Significant
increase in expression following sorafenib treatment suggests liver
cancer is sensitive to sorafenib | (65) |
| Clear cell renal
cell carcinoma | Upregulated | Diagnosis of clear
cell renal cell carcinoma | (66) |
| Gastric | Downregulated | Low expression is
associated with poorer prognosis | (67) |
Biomarker for diagnosis
The expression levels of miRNA-122 in the urine of
patients with clear cell renal cell carcinoma (ccRCC) show a
significant 13.9-fold elevation; alongside miRNA-1271 and
miRNA-15b, this may be useful in diagnosing ccRCC (66). In prostate cancer, both tissue and
serum levels of miRNA-122 are notably decreased and serum miRNA-122
levels effectively distinguish patients with prostate cancer from
healthy individuals (22).
Additionally, a model combining six miRNAs, including miRNA-122,
has superior accuracy in differentiating patients with oral
squamous cell carcinoma from healthy controls compared with serum
squamous cell carcinoma antigen (68). The aforementioned studies suggest
that miRNA-122 has potential to be used as an additional diagnostic
marker for certain types of cancer.
Biomarker for prognosis
In CRC, miRNA-122 is associated with increased risk
of tumor metastasis (69).
Conversely, miRNA-122 expression is associated with improved
prognosis in HCC (70), AML
(59) and bile duct cancer
(43). Low miRNA-122 expression
also correlates with poor outcomes following radical resection in
liver cancer (71). Yang et
al (20) found that decreased
miRNA-122 expression significantly correlates with advanced tumor
node metastasis stage and distant metastasis in NPC. Therefore,
miRNA-122 shows promise as a useful tool for predicting cancer
prognosis.
Biomarker of therapeutic response
miRNA-122 expression is indicative of treatment
response and aids in tailoring effective treatment plans. Elevated
miRNA-122 levels predict early resistance to transcatheter arterial
chemoembolization in patients with HCC (72). Additionally, patients with HCC who
are responsive to sorafenib exhibit higher miRNA-122 expression
post-chemotherapy compared with non-responsive patients,
underscoring its potential to predict sorafenib efficacy (65).
Application of miRNA-122 in chemotherapy and
radiotherapy
Chemotherapy and radiotherapy are frequently
utilized in cancer treatment; nonetheless, addressing drug
resistance in patients with advanced and recurrent cancer remains a
challenge. Enhancing the sensitivity of patients to chemotherapy
and radiotherapy is key (73).
miRNA-122 targets XIAP to reverse oxaliplatin
resistance in CRC cells (27) and
also sensitizes colon cancer cells to 5-fluorouracil (5-FU) by
targeting PKM2 (74). In prostate
cancer, miRNA-122 enhances cell sensitivity to docetaxel via PKM2
targeting (39). Moreover,
miRNA-122 decreases the expression of drug-resistant P-glycoprotein
and multidrug-resistance proteins by inhibiting small
ubiquitin-like modifier sentrin-specific protease 1, thus
overcoming adriamycin and sorafenib resistance in HCC cells
(75). miRNA-122 directly targets
the Wnt/β-catenin pathway, leading to decreased expression of
multidrug resistance proteins 1 and increased sensitivity of HCC
cells to oxaliplatin (76).
In radiation therapy, miRNA-122 decreases the
expression of stress response regulators such as survivin,
apoptosis inhibitory proteins 1 and 2 and IGF1R. This mechanism
induces DNA double-strand breaks and promotes apoptosis in NSCLC
cells, thereby enhancing inhibition of NSCLC cell proliferation and
invasion (77). Additionally,
miRNA-122 improves the effectiveness of radiation therapy by
decreasing IGF1R expression (78). Thus, adjusting miRNA-122 levels
enhances the efficacy of chemotherapy and radiotherapy in specific
types of cancer.
Systemic delivery strategies for
miRNA-122
Due to the susceptibility of miRNAs to enzymatic
degradation, protection from RNA hydrolases in extracellular serum
is key during systemic delivery to ensure their delivery to target
cells. This necessitates encapsulating miRNA in sealed carriers to
prevent enzymatic hydrolysis during transportation. Therefore,
effective delivery systems are essential for precise delivery of
miRNA to tumor cells (79).
Utilizing exosomal, viral and nanoparticle vectors for miRNA
delivery can address this issue (80).
Exosome vectors
Exosomes are active vesicles secreted by cells to
facilitate intercellular substance exchange and information
transfer. They are commonly utilized as carriers for delivering
miRNA (81). A recent study
demonstrated that exosomes derived from adipose-derived mesenchymal
stem cells effectively deliver miRNA-122 into HCC cells (82). This results in significant
downregulation of CCNG1, disintegrin, MMP10 and IGF1R expression,
enhancing the sensitivity of HCC cells to 5-FU and sorafenib
(82).
Viral vectors
Viral vectors widely used include lentiviruses,
retroviruses, adenoviruses, and adeno-associated viruses (AAVs)
(83,84). In an in vivo mouse
xenograft model, tumor growth of human HCC is notably suppressed by
AAV3 along with miRNA-122 and miRNA-26a lentiviral delivery systems
(85). Additionally, tumor
necrosis factor-associated apoptosis-inducing ligands selectively
expressed in osteosarcoma cells through adenoviral vectors of
miRNA-122 and miRNA-34 promote apoptosis and inhibit cell
proliferation (86). Notably,
linking and cloning miRNA-21 and pre-miRNA-122 sequences into
viral-like particle expression vectors and delivering them into HCC
cells inhibits proliferation, migration and invasion of HCC cells
and promotes apoptosis (87).
While advantages such as easy cell access and high expression of
introduced genes are offered by viral vectors, concerns about
immunogenicity and potential promotion of gene mutagenesis have
prompted a search for alternatives (88).
Nanocarriers
Nanocarriers offer advantages such as low toxicity,
minimal immunogenicity, high permeability and non-integration of
genes into the host cell genome, making them essential alternatives
to viral vectors (89). Sendi
et al (90) developed a
galactose-targeted lipid calcium phosphate nanoformulation noted
for its stability and efficient delivery of miRNA-122 to CRC liver
metastatic hepatocytes. This formulation effectively prevents liver
metastasis and prolongs survival in CRC mouse models without
significant toxicity (90).
Additionally, an ultrasound-triggered, phase-transitioning cationic
nanodroplet delivers miRNA-122 to HCC cells, resulting in a
significant increase in miRNA-122 expression and substantial
inhibition of HCC cell proliferation, migration, and invasion
(91). Zeng et al
(92) created
graphene-P-gluoprotein loaded with miR-122-InP@ZnS quantum dot
nanocomposites, demonstrating effective delivery of miRNA-122 to
multidrug-resistant HCC cells and induction of apoptosis (92). Furthermore, Zhang et al
(93) utilized amphiphilic
gemcitabine-oleic acid prodrug nanoparticles for encapsulating
miRNA-122, effectively targeting HCC cells and significantly
inhibiting proliferation in xenograft nude mice (93).
Clinical implications and limitations
Cell proliferation, metastasis and apoptosis are
pivotal in tumor growth and development. The hallmark of cancer is
aberrant cell proliferation, with the regulation of cell
proliferation and survival being crucial in tumor formation
(94). Thus, strategies that
inhibit tumor proliferation and enhance apoptosis are key in cancer
treatment. Additionally, cell metastasis significantly impacts
cancer prognosis, as its occurrence signifies disease progression.
Therefore, inhibiting tumor cell metastasis is a key therapeutic
approach in cancer management (95).
miRNA-122 has garnered notable attention in cancer
and liver disease research (96,97). Upregulation of miRNA-122
expression inhibits self-renewal, EMT and angiogenic capacity of
NSCLC tumor stem cells (18). In
HCC, elevated miRNA-122 levels enhance sensitivity to chemotherapy,
suppress cell proliferation, metastasis and invasion and promote
liver polyploidization (51,76). Conversely, as an oncogene, high
miRNA-122 expression promotes migration and invasion in RCC
(31). miRNA-122 functions by
targeting various genes, exerting either oncogenic or
tumor-suppressive effects. Moreover, miRNA-122 serves as a
biomarker for diagnosing, prognosticating and monitoring response
to treatment, thereby enhancing sensitivity to radiotherapy and
chemotherapy (66,69). Systemic delivery strategies for
miRNA-122 circumvent enzymatic hydrolysis, ensuring precise
delivery to tumor cells and enhancing its stability during cancer
treatment (90). Consequently,
targeting miRNA-122 presents a promising avenue for future cancer
therapies, although challenges remain. Determining specific
diagnostic and prognostic thresholds for miRNA-122 given its varied
roles is a primary challenge. Additionally, understanding
interactions between miRNA-122 downstream target genes and cancer
signaling pathways requires further investigation. Furthermore,
while research on miRNA-122 and cancer treatment shows potential,
its clinical translation remains incomplete, necessitating
large-scale controlled experiments and clinical trials to elucidate
its full mechanistic role in cancer.
Conclusion
The present review outlines the multifaceted role of
miRNA-122 in various types of cancer. Dysregulation of miRNA-122 is
observed in different types of cancer, influencing pathways key to
tumor cell behavior such as proliferation, angiogenesis,
differentiation, metastasis and apoptosis. Mechanistically,
miRNA-122 modulates complex signaling pathways by targeting various
genes, although certain lncRNAs and circ RNAs may serve as upstream
regulators to regulate miRNA-122 expression. This complexity
underscores miRNA-122 as a promising therapeutic target for cancer
treatment. However, it lacks a universal or primary pathway across
different types of cancer. Furthermore, miRNA-122 shows potential
as a biomarker for cancer diagnosis, prognosis and treatment
response, enhancing sensitivity to radiotherapy and chemotherapy in
specific types of cancer. The systemic delivery of miRNA-122 holds
promise in advancing cancer therapy. These insights suggest new
avenues for research in cancer diagnosis and treatment.
Availability of data and materials
Not applicable.
Authors' contributions
JZ wrote the manuscript and constructed figures and
tables. XD and RD revised the manuscript. ZC performed the
literature review. LW conceptualized the study. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Taizhou Hailing District
Science and Technology Development Program Project (grant no.
HLKF-2019-4) and Guangxi University of Traditional Chinese Medicine
Gui School of Chinese Medicine Inheritance Innovation Team Grant
(grant no. 2022B004).
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Britton C, Laing R and Devaney E: Small
RNAs in parasitic nematodes-forms and functions. Parasitology.
147:855–864. 2020. View Article : Google Scholar
|
|
4
|
Morales-Martínez M and Vega MI: Role of
MicroRNA-7 (MiR-7) in cancer physiopathology. Int J Mol Sci.
23:90912022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hebbar S, Panzade G, Vashisht AA,
Wohlschlegel JA, Veksler-Lublinsky I and Zinovyeva AY: Functional
identification of microRNA-centered complexes in C: elegans. Sci
Rep. 12:71332022. View Article : Google Scholar
|
|
6
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48(D1): D127–D131. 2020. View Article : Google Scholar :
|
|
8
|
Wang Y, Xing QF, Liu XQ, Guo ZJ, Li CY and
Sun G: MiR-122 targets VEGFC in bladder cancer to inhibit tumor
growth and angiogenesis. Am J Transl Res. 8:3056–3066.
2016.PubMed/NCBI
|
|
9
|
Chun KH: Molecular Targets and signaling
pathways of microRNA-122 in hepatocellular carcinoma.
Pharmaceutics. 14:13802022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collison AM, Sokulsky LA, Kepreotes E,
Pereira de Siqueira A, Morten M, Edwards MR, Walton RP, Bartlett
NW, Yang M, Nguyen TH, et al: miR-122 promotes virus-induced lung
disease by targeting SOCS1. JCI Insight. 6:e1279332021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Zheng H, Zhou X, Zhang J and Shao
G: miR-122 promotes diabetic retinopathy through targeting TIMP3.
Anim Cells Syst (Seoul). 24:275–281. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scott KM, Cohen DJ, Boyan BD and Schwartz
Z: miR-122 and the WNT/β-catenin pathway inhibit effects of both
interleukin-1β and tumor necrosis factor-α in articular
chondrocytes in vitro. J Cell Biochem. 123:1053–1063. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li A, Wu J, Zhai A, Qian J, Qian J, Wang
X, Qaria MA, Zhang Q, Li Y, Fang Y, Kao W, et al: HBV triggers
APOBEC2 expression through miR-122 regulation and affects the
proliferation of liver cancer cells. Int J Oncol. 55:1137–1148.
2019.PubMed/NCBI
|
|
14
|
Smolarz B, Durczyński A, Romanowicz H,
Szyłło K and Hogendorf P: miRNAs in cancer (review of literature).
Int J Mol Sci. 23:28052022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Song JW, Lin JY, Miao R and Zhong
JC: Roles of MicroRNA-122 in cardiovascular fibrosis and related
diseases. Cardiovasc Toxicol. 20:463–473. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo L, Yin M and Wang Y: CREB1, a direct
target of miR-122, promotes cell proliferation and invasion in
bladder cancer. Oncol Lett. 16:3842–3848. 2018.PubMed/NCBI
|
|
17
|
Qin H, Sha J, Jiang C, Gao X, Qu L, Yan H,
Xu T, Jiang Q and Gao H: miR-122 inhibits metastasis and
epithelial-mesenchymal transition of non-small-cell lung cancer
cells. Onco Targets Ther. 8:3175–3184. 2015.PubMed/NCBI
|
|
18
|
Chandimali N, Huynh DL, Zhang JJ, Lee JC,
Yu DY, Jeong DK and Kwon T: MicroRNA-122 negatively associates with
peroxiredoxin-II expression in human gefitinib-resistant lung
cancer stem cells. Cancer Gene Ther. 26:292–304. 2019. View Article : Google Scholar :
|
|
19
|
Kong L, Wu Q, Zhao L, Ye J, Li N and Yang
H: Upregulated lncRNA-UCA1 contributes to metastasis of bile duct
carcinoma through regulation of miR-122/CLIC1 and activation of the
ERK/MAPK signaling pathway. Cell Cycle. 18:1212–1228. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Li Q and Guo L: MicroRNA-122 acts
as tumor suppressor by targeting TRIM29 and blocking the activity
of PI3K/AKT signaling in nasopharyngeal carcinoma in vitro. Mol Med
Rep. 17:8244–8252. 2018.PubMed/NCBI
|
|
21
|
Liao B, Wang Z, Zhu Y, Wang M and Liu Y:
Long noncoding RNA DRAIC acts as a microRNA-122 sponge to
facilitate nasopharyngeal carcinoma cell proliferation, migration
and invasion via regulating SATB1. Artif Cells Nanomed Biotechnol.
47:3585–3597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu H, Hou T, Ju W, Xing Y, Zhang X and
Yang J: MicroRNA-122 downregulates Rho-associated protein kinase 2
expression and inhibits the proliferation of prostate carcinoma
cells. Mol Med Rep. 19:3882–3888. 2019.PubMed/NCBI
|
|
23
|
Perez-Añorve IX, Gonzalez-De la Rosa CH,
Soto-Reyes E, Beltran-Anaya FO, Del Moral-Hernandez O,
Salgado-Albarran M, Angeles-Zaragoza O, Gonzalez-Barrios JA,
Landero-Huerta DA and Chavez-Saldaña M: New insights into
radioresistance in breast cancer identify a dual function of
miR-122 as a tumor suppressor and oncomiR. Mol Oncol. 13:1249–1267.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y and Tang L: Inhibition of breast
cancer cell proliferation and tumorigenesis by long non-coding RNA
RPPH1 down-regulation of miR-122 expression. Cancer Cell Int.
17:1092017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XN, Yang H and Yang T: miR-122 inhibits
hepatocarcinoma cell progression by targeting LMNB2. Oncol Res.
28:41–49. 2020. View Article : Google Scholar
|
|
26
|
Iino I, Kikuchi H, Miyazaki S, Hiramatsu
Y, Ohta M, Kamiya K, Kusama Y, Baba S, Setou M and Konno H: Effect
of miR-122 and its target gene cationic amino acid transporter 1 on
colorectal liver metastasis. Cancer Sci. 104:624–630. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua Y, Zhu Y, Zhang J, Zhu Z, Ning Z, Chen
H, Liu L, Chen Z and Meng Z: miR-122 targets X-linked inhibitor of
apoptosis protein to sensitize oxaliplatin-resistant colorectal
cancer cells to oxaliplatin-mediated cytotoxicit. Cell Physiol
Biochem. 51:2148–2159. 2018. View Article : Google Scholar
|
|
28
|
Wang J, Yu PY, Yu JP, Luo JD, Sun ZQ, Sun
F, Kong Z and Wang JL: KIF22 promotes progress of esophageal
squamous cell carcinoma cells and is negatively regulated by
miR-122. Am J Transl Res. 13:4152–4166. 2021.PubMed/NCBI
|
|
29
|
Ding CQ, Deng WS, Yin XF and Ding XD:
MiR-122 inhibits cell proliferation and induces apoptosis by
targeting runt-related transcription factors 2 in human glioma. Eur
Rev Med Pharmacol Sci. 22:4925–4933. 2018.PubMed/NCBI
|
|
30
|
Chen C, Deng L, Nie DK, Jia F, Fu LS, Wan
ZQ and Lan Q: Circular RNA Pleiotrophin promotes carcinogenesis in
glioma via regulation of microRNA-122/SRY-box transcription factor
6 axis. Eur J Cancer Prev. 29:165–173. 2020. View Article : Google Scholar
|
|
31
|
Fan Y, Ma X, Li H, Gao Y, Huang Q, Zhang
Y, Bao X, Du Q, Luo G, Liu K, et al: miR-122 promotes metastasis of
clear-cell renal cell carcinoma by downregulating Dicer. Int J
Cancer. 142:547–560. 2018. View Article : Google Scholar
|
|
32
|
Nie W, Ni D, Ma X, Zhang Y, Gao Y, Peng C
and Zhang X: miR-122 promotes proliferation and invasion of clear
cell renal cell carcinoma by suppressing Forkhead box O3. Int J
Oncol. 54:559–571. 2019.
|
|
33
|
Ma J, Wu Q, Zhang Y, Li J, Yu Y, Pan Q and
Sun F: MicroRNA sponge blocks the tumor-suppressing functions of
microRNA-122 in human hepatoma and osteosarcoma cells. Oncol Rep.
32:2744–2752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Liu Y, Liu W, Li C, Liu Y, Hu W
and Song H: miR-122 inhibits the cervical cancer development by
targeting the oncogene RAD21. Biochem Genet. 60:303–314. 2022.
View Article : Google Scholar
|
|
35
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bossi P, Chan AT, Licitra L, Trama A,
Orlandi E, Hui EP, Halámková J, Mattheis S, Baujat B, Hardillo J,
et al: Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice
guidelines for diagnosis, treatment and follow-up†. Ann
Oncol. 32:452–465. 2021. View Article : Google Scholar
|
|
37
|
Chelakkot C, Chelakkot VS, Shin Y and Song
K: Modulating glycolysis to improve cancer therapy. Int J Mol Sci.
24:26062023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li TE, Wang S, Shen XT, Zhang Z, Chen M,
Wang H, Zhu Y, Xu D, Hu BY, Wei R, et al: PKM2 drives
hepatocellular carcinoma progression by inducing immunosuppressive
microenvironment. Front Immunol. 11:5899972020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Z, Tang G and Yan J: MicroRNA-122
regulates docetaxel resistance of prostate cancer cells by
regulating PKM2. Exp Ther Med. 20:2472020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gong H, Zhou L, Khelfat L, Qiu G, Wang Y,
Mao K and Chen W: Rho-associated protein kinase (ROCK) promotes
proliferation and migration of PC-3 and DU145 prostate cancer cells
by targeting LIM kinase 1 (LIMK1) and matrix metalloproteinase-2
(MMP-2). Med Sci Monit. 25:3090–3099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen X, Yin L, Xu H, Rong J, Feng M, Jiang
D and Bai Y: Knockdown of RhoA expression reverts enzalutamide
resistance via the P38 MAPK pathway in castration-resistant
prostate cancer. Recent Pat Anticancer Drug Discov. 18:92–99. 2023.
View Article : Google Scholar
|
|
42
|
Sato K, Glaser S, Alvaro D, Meng F,
Francis H and Alpini G: Cholangiocarcinoma: Novel therapeutic
targets. Expert Opin Ther Targets. 24:345–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu N, Jiang F, He TL, Zhang JK, Zhao J,
Wang C, Jiang GX, Cao LP, Kang PC, Zhong XY, et al: The roles of
MicroRNA-122 overexpression in inhibiting proliferation and
invasion and stimulating apoptosis of human cholangiocarcinoma
cells. Sci Rep. 5:165662015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu C, Zhang J, Cao X, Yang Q and Xia D:
Effect of Mir-122 on human cholangiocarcinoma proliferation,
invasion, and apoptosis through P53 expression. Med Sci Monit.
22:2685–2690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Haibe Y, Kreidieh M, El Hajj H, Khalifeh
I, Mukherji D, Temraz S and Shamseddine A: Resistance mechanisms to
anti-angiogenic therapies in cancer. Front Oncol. 10:2212020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilkinson L and Gathani T: Understanding
breast cancer as a global health concern. Br J Radiol.
95:202110332022. View Article : Google Scholar :
|
|
47
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar :
|
|
48
|
Wang SC, Lin XL, Li J, Zhang TT, Wang HY,
Shi JW, Yang S, Zhao WT, Xie RY, Wei F, et al: MicroRNA-122
triggers mesenchymal-epithelial transition and suppresses
hepatocellular carcinoma cell motility and invasion by targeting
RhoA. PloS One. 9:e1013302014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yahya SMM, Fathy SA, El-Khayat ZA,
El-Toukhy SE, Hamed AR, Hegazy MGA and Nabih HK: Possible role of
microRNA-122 in Modulating multidrug resistance of hepatocellular
carcinoma. Indian J Clin Biochem. 33:21–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duncan AW: Hepatocyte ploidy modulation in
liver cancer. EMBO Rep. 21:e519222020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsu SH, Delgado ER, Otero PA, Teng KY,
Kutay H, Meehan KM, Moroney JB, Monga JK, Hand NJ, Friedman JR, et
al: MicroRNA-122 regulates polyploidization in the murine liver.
Hepatology. 64:599–615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Matsumoto T, Wakefield L, Peters A, Peto
M, Spellman P and Grompe M: Proliferative polyploid cells give rise
to tumors via ploidy reduction. Nat Commun. 12:6462021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matsumoto T: Implications of polyploidy
and ploidy alterations in hepatocytes in liver injuries and
cancers. Int J Mol Sci. 23:94092022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang F, Long J, Li L, Wu ZX, Da TT, Wang
XQ, Huang C, Jiang YH, Yao XQ, Ma HQ, et al: Single-cell and
spatial transcriptome analysis reveals the cellular heterogeneity
of liver metastatic colorectal cancer. Sci Adv. 9:eadf54642023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang FL and Yu SJ: Esophageal cancer:
Risk factors, genetic association, and treatment. Asian J Surg.
41:210–215. 2018. View Article : Google Scholar
|
|
56
|
Zhou K, Yan Y and Zhao S: Esophageal
cancer-selective expression of TRAIL mediated by MREs of miR-143
and miR-122. Tumour Biology: Tumour Biol. 35:5787–5795. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Samarakkody AS, Shin NY and Cantor AB:
Role of RUNX family transcription factors in DNA damage response.
Mol Cells. 43:99–106. 2020.PubMed/NCBI
|
|
58
|
DiNardo CD, Erba HP, Freeman SD and Wei
AH: Acute myeloid leukaemia. Lancet. 401:2073–2086. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang TJ, Qian Z, Wen XM, Zhou JD, Li XX,
Xu ZJ, Ma JC, Zhang ZH, Lin J and Qian J: Lower expression of bone
marrow miR-122 is an independent risk factor for overall survival
in cytogenetically normal acute myeloid leukemia. Pathol Res Pract.
214:896–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang J, Yuan Y, Yang X, Hong Z and Yang L:
Decreased expression of microRNA-122 is associated with an
unfavorable prognosis in childhood acute myeloid leukemia and
function analysis indicates a therapeutic potential. Pathol Res
Pract. 213:1166–1172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Y, Wang C, Xia M, Tian Z, Zhou J,
Berger JM, Zhang XH and Xiao H: Engineering small-molecule and
protein drugs for targeting bone tumors. Mol Ther. 32:1219–1237.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu B, Yao S and Zhou J: Micro-RNA 122 and
micro-RNA 96 affected human osteosarcoma biological behavior and
associated with prognosis of patients with osteosarcoma. Biosci
Rep. 40:BSR202015292020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun L, Liu X, Pan B, Hu X, Zhu Y, Su Y,
Guo Z, Zhang G, Xu M, Xu X, et al: Serum exosomal miR-122 as a
potential diagnostic and prognostic biomarker of colorectal cancer
with liver metastasis. J Cancer. 11:630–637. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tang Y, Zhao S, Wang J, Li D, Ren Q and
Tang Y: Plasma miR-122 as a potential diagnostic and prognostic
indicator in human glioma. Neurol Sci. 38:1087–1092. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhan G, Jiang H, Yang R and Yang K:
miR-122 and miR-197 expressions in hepatic carcinoma patients
before and after chemotherapy and their effect on patient
prognosis. Am J Transl Res. 13:6731–6737. 2021.PubMed/NCBI
|
|
66
|
Cochetti G, Cari L, Nocentini G, Maulà V,
Suvieri C, Cagnani R, Rossi De Vermandois JA and Mearini E:
Detection of urinary miRNAs for diagnosis of clear cell renal cell
carcinoma. Sci Rep. 10:212902020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen Q, Ge X, Zhang Y, Xia H, Yuan D, Tang
Q, Chen L, Pang X, Leng W and Bi F: Plasma miR-122 and miR-192 as
potential novel biomarkers for the early detection of distant
metastasis of gastric cancer. Oncol Rep. 31:1863–1870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nakamura K, Hiyake N, Hamada T, Yokoyama
S, Mori K, Yamashiro K, Beppu M, Sagara Y, Sagara Y and Sugiura T:
Circulating microRNA panel as a potential novel biomarker for oral
squamous cell carcinoma diagnosis. Cancers (Basel). 13:4492021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Maierthaler M, Benner A, Hoffmeister M,
Surowy H, Jansen L, Knebel P, Chang-Claude J, Brenner H and
Burwinkel B: Plasma miR-122 and miR-200 family are prognostic
markers in colorectal cancer. Int J Cancer. 140:176–187. 2017.
View Article : Google Scholar
|
|
70
|
Deng P, Li M and Wu Y: The predictive
efficacy of serum exosomal microRNA-122 and microRNA-148a for
hepatocellular carcinoma based on smart healthcare. J Healthc Eng.
2022:59145412022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ha SY, Yu JI, Choi C, Kang SY, Joh JW,
Paik SW, Kim S, Kim M, Park HC and Park CK: Prognostic significance
of miR-122 expression after curative resection in patients with
hepatocellular carcinoma. Sci Rep. 9:147382019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim SS, Nam JS, Cho HJ, Won JH, Kim JW, Ji
JH, Yang MJ, Park JH, Noh CK, Shin SJ, et al: Plasma micoRNA-122 as
a predictive marker for treatment response following transarterial
chemoembolization in patients with hepatocellular carcinoma. J
Gastroenterol Hepatol. 32:199–207. 2017. View Article : Google Scholar
|
|
73
|
Wang S, Liu Y, Feng Y, Zhang J, Swinnen J,
Li Y and Ni Y: A review on curability of cancers: More efforts for
novel therapeutic options are needed. Cancers (Basel). 11:17822019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He J, Xie G, Tong J, Peng Y, Huang H, Li
J, Wang N and Liang H: Overexpression of microRNA-122 re-sensitizes
5-FU-resistant colon cancer cells to 5-FU through the inhibition of
PKM2 in vitro and in vivo. Cell Biochem Biophys. 70:1343–1350.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dai J, Hao Y, Chen X, Yu Q and Wang B:
miR-122/SENP1 axis confers stemness and chemoresistance to liver
cancer through Wnt/β-catenin signaling. Oncol Lett. 26:3902023.
View Article : Google Scholar
|
|
76
|
Cao F and Yin LX: miR-122 enhances
sensitivity of hepatocellular carcinoma to oxaliplatin via
inhibiting MDR1 by targeting Wnt/β-catenin pathway. Exp Mol Pathol.
106:34–43. 2019. View Article : Google Scholar
|
|
77
|
Ma D, Jia H, Qin M, Dai W, Wang T, Liang
E, Dong G, Wang Z, Zhang Z and Feng F: MiR-122 induces
radiosensitization in non-small cell lung cancer cell line. Int J
Mol Sci. 16:22137–22150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu G, Bu S, Wang X and Ge H: MiR-122
radiosensitize hepatocellular carcinoma cells by suppressing cyclin
G1. Int J Radiat Biol. 98:11–17. 2022. View Article : Google Scholar
|
|
79
|
Zhao ZL, Liu C, Wang QZ, Wu HW and Zheng
JW: Engineered exosomes for targeted delivery of miR-187-3p
suppress the viability of hemangioma stem cells by targeting notch
signaling. Ann Transl Med. 10:6212022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu L, Zhou W, Lin L, Chen A, Feng J, Qu X,
Zhang H and Yue J: Delivery of therapeutic oligonucleotides in
nanoscale. Bioact Mater. 7:292–323. 2021.PubMed/NCBI
|
|
81
|
Liang Y, Xu X, Li X, Xiong J, Li B, Duan
L, Wang D and Xia J: Chondrocyte-targeted MicroRNA delivery by
engineered exosomes toward a cell-free osteoarthritis therapy. ACS
Appl Mater Interfaces. 12:36938–36947. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lou G, Song X, Yang F, Wu S, Wang J, Chen
Z and Liu Y: Exosomes derived from miR-122-modified adipose
tissue-derived MSCs increase chemosensitivity of hepatocellular
carcinoma. J Hematol Oncol. 8:1222015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sweef O, Zaabout E, Bakheet A, Halawa M,
Gad I, Akela M, Tousson E, Abdelghany A and Furuta S: Unraveling
therapeutic opportunities and the diagnostic potential of microRNAs
for human lung cancer. Pharmaceutics. 15:20612023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li X, Le Y, Zhang Z, Nian X, Liu B and
Yang X: Viral vector-based gene therapy. Int J Mol Sci.
24:77362023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yin L, Keeler GD, Zhang Y, Hoffman BE,
Ling C, Qing K and Srivastava A: AAV3-miRNA vectors for growth
suppression of human hepatocellular carcinoma cells in vitro and
human liver tumors in a murine xenograft model in vivo. Gene Ther.
28:422–434. 2021. View Article : Google Scholar :
|
|
86
|
Xiao F, Chen J, Lian C, Han P and Zhang C:
Tumor necrosis factor-related apoptosis-inducing ligand induces
cytotoxicity specific to osteosarcoma by microRNA response
elements. Mol Med Rep. 11:739–745. 2015. View Article : Google Scholar
|
|
87
|
Zhang J, Li D, Zhang R, Peng R and Li J:
Delivery of microRNA-21-sponge and pre-microRNA-122 by MS2
virus-like particles to therapeutically target hepatocellular
carcinoma cells. Exp Biol Med (Maywood). 246:2463–2472. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shui M, Chen Z, Chen Y, Yuan Q, Li H, Vong
CT, Farag MA and Wang S: Engineering polyphenol-based carriers for
nucleic acid delivery. Theranostics. 13:3204–3223. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yan Y, Liu XY, Lu A, Wang XY, Jiang LX and
Wang JC: Non-viral vectors for RNA delivery. J Control Release.
342:241–279. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sendi H, Yazdimamaghani M, Hu M,
Sultanpuram N, Wang J, Moody AS, McCabe E, Zhang J, Graboski A, Li
L, et al: Nanoparticle delivery of miR-122 inhibits colorectal
cancer liver metastasis. Cancer Res. 82:105–113. 2022. View Article : Google Scholar :
|
|
91
|
Guo H, Xu M, Cao Z, Li W, Chen L, Xie X,
Wang W and Liu J: Ultrasound-assisted miR-122-loaded polymeric
nanodroplets for hepatocellular carcinoma gene therapy. Mol Pharm.
17:541–553. 2020.
|
|
92
|
Zeng X, Yuan Y, Wang T, Wang H, Hu X, Fu
Z, Zhang G, Liu B and Lu G: Targeted imaging and induction of
apoptosis of drug-resistant hepatoma cells by miR-122-loaded
graphene-InP nanocompounds. J Nanobiotechnology. 15:92017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang HT, Sun J, Yan Y, Cui SH, Wang H,
Wang CH, Qiu C, Chen X, Ding JS, Qian HG, et al: Encapsulated
microRNA by gemcitabine prodrug for cancer treatment. J Control
Release. 316:317–330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang Z, Xu Z, Zhu X and Zhang J: New
insights into molecules and pathways of cancer metabolism and
therapeutic implications. Cancer Commun (Lond). 41:16–36. 2021.
View Article : Google Scholar
|
|
95
|
Novikov NM, Zolotaryova SY, Gautreau AM
and Denisov EV: Mutational drivers of cancer cell migration and
invasion. Br J Cancer. 124:102–114. 2021. View Article : Google Scholar :
|
|
96
|
Hsu KH, Wei CW, Su YR, Chou T, Lin YL,
Yang FC, Tsou AP, Hsu CL, Tseng PH, Chen NJ, et al: Upregulation of
RelB in the miR-122 knockout mice contributes to increased levels
of proinflammatory chemokines/cytokines in the liver and
macrophages. Immunol Lett. 226:22–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nabih HK: The significance of HCV viral
load in the incidence of HCC: A correlation between Mir-122 and
CCL2. J Gastrointest Cancer. 51:412–417. 2020. View Article : Google Scholar
|