Liver cancer ranks among the most common
malignancies and is the third leading cause of cancer-related death
worldwide (1). The incidence and
mortality of liver cancer are high, with >900,000 newly
diagnosed cases and >800,000 deaths each year. Common types
include hepatocellular carcinoma (HCC), cholangiocarcinoma (CC),
and mixed HCC/CC (2). HCC
accounts for 75-85% of primary liver cancers (3). While the etiology of HCC is
well-established, the pathogenesis leading to its development
remains unclear.
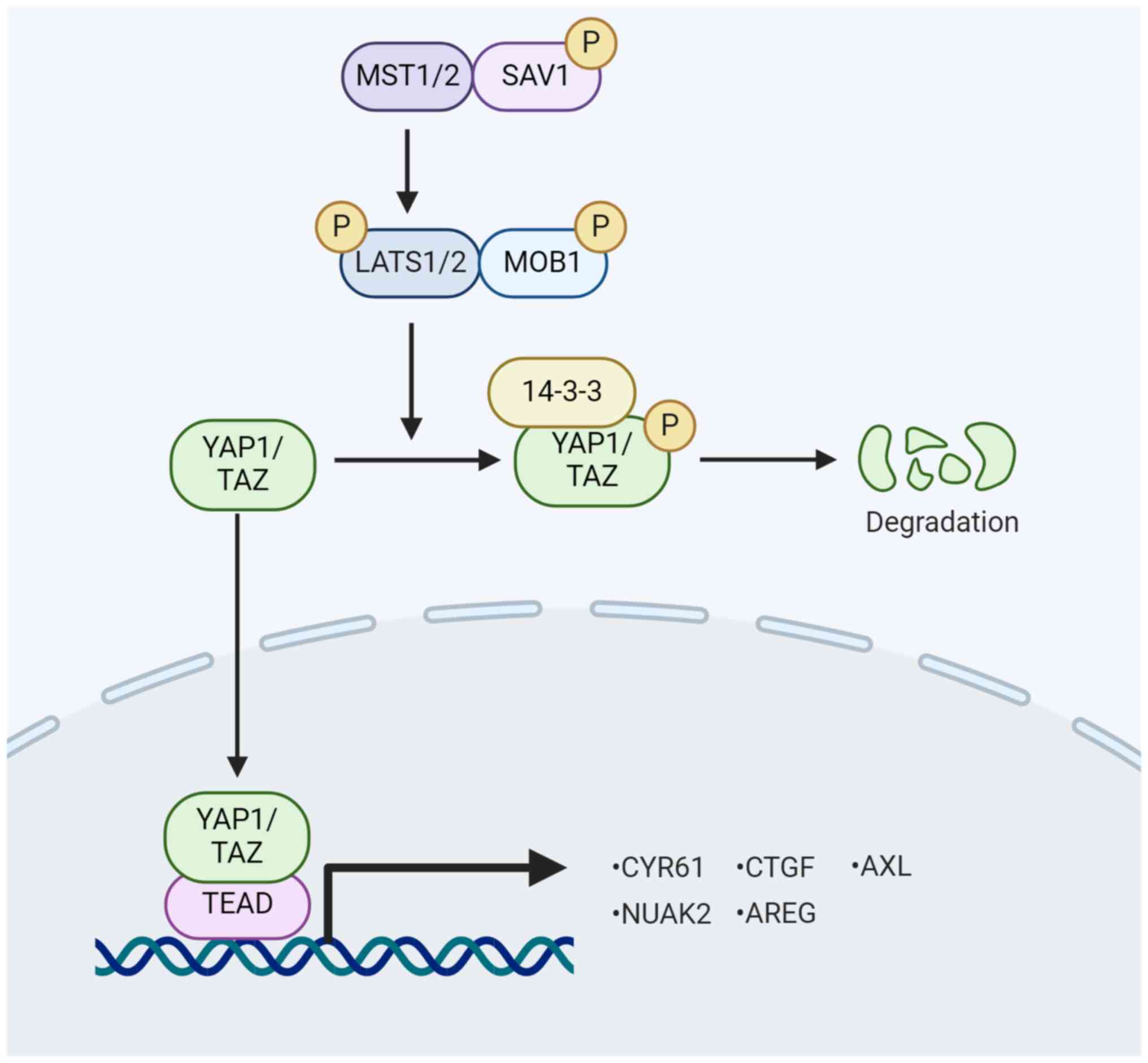
Yes-associated protein 1 (YAP1) serves as a
downstream effector of the Hippo pathway, undergoing
phosphorylation and inactivation via the Hippo signaling cascade.
Inhibition of the Hippo signaling pathway reduces YAP1
phosphorylation, promoting its nuclear localization. Within the
nucleus, YAP1 binds to multiple transcription factors and activates
multiple genes involved in cell proliferation, survival and
invasion. The present study explores the role of the Hippo/YAP1
signaling pathway and the regulatory mechanism of YAP1 in HCC
development. In addition, it reviews the impact of small-molecule
compounds on YAP1 regulation in HCC. This research underscores
YAP1's pivotal role in HCC and its regulatory mechanisms, providing
new insight into HCC progression. These findings highlight YAP1 as
a critical oncogenic driver in liver carcinogenesis, emphasizing
the potential clinical utility of developing drugs targeting YAP1
and its downstream signaling targets for HCC treatment.
Inhibition of the Hippo signaling pathway leads to
YAP1 and TAZ dephosphorylation, allowing their translocation into
the nucleus. In this location, they interact with various
transcription factors, including TEA domain transcription factor
(TEAD), SMAD and RUNX family transcription factor, thereby
promoting gene expression that facilitates cell proliferation and
inhibits apoptosis (10,11). Notably, targets such as cellular
communication network factor 1 (CCN1), CCN2 and others have been
identified for YAP1 and TAZ (12). In addition, YAP1 and TAZ sense
extracellular mechanical stimuli, such as extracellular matrix
(ECM) hardness, and integrate and convert them into intracellular
molecular signals that influence cell proliferation and migration
(13).
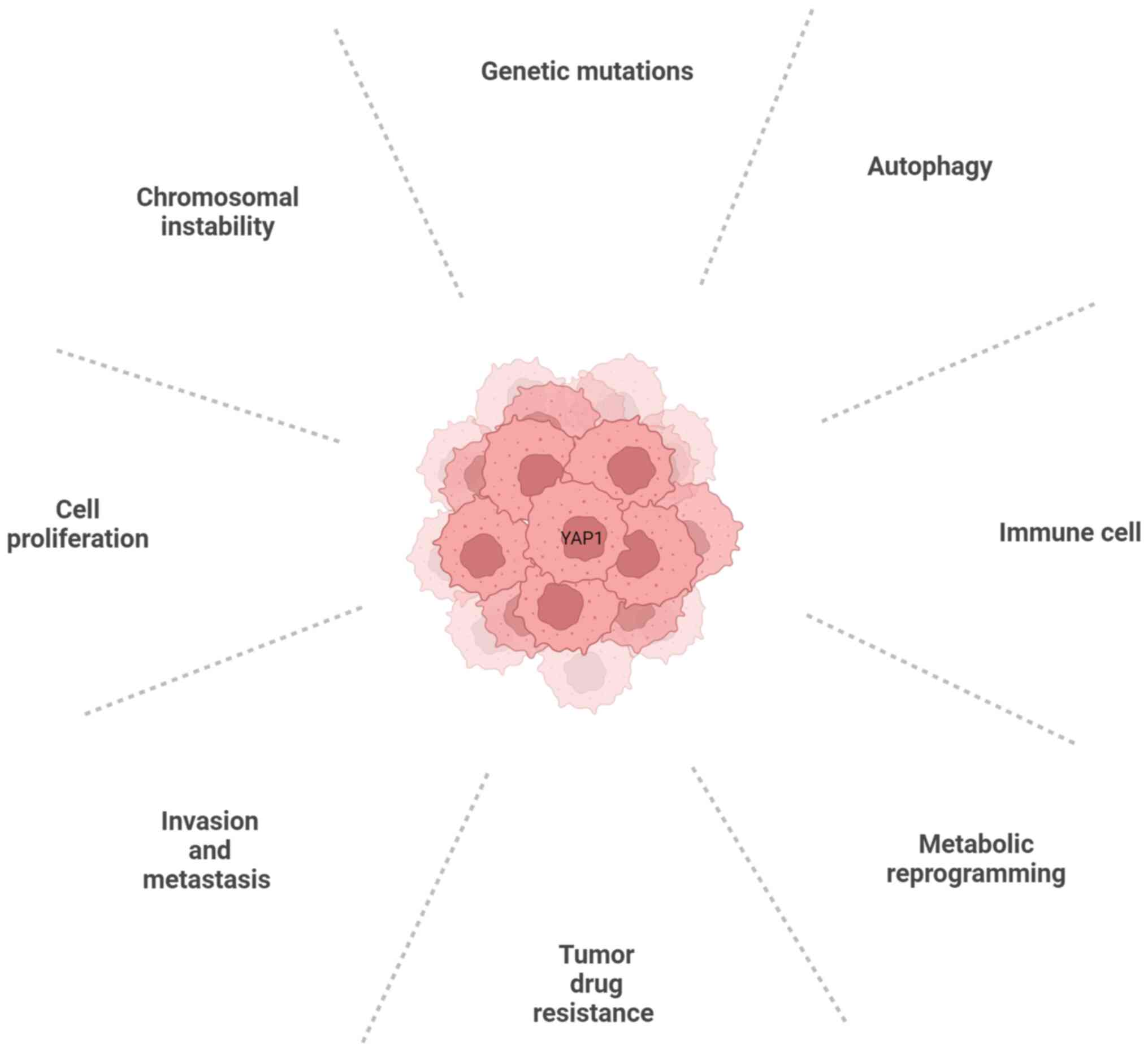
The Hippo/YAP signaling pathway controls organ size
during development and mediates the expansion of tissue-specific
progenitor cells during tissue regeneration and normal cell
proliferation (14). Increasing
evidence indicates that YAP1/TAZ, Hippo kinase or other molecules
are abnormally expressed in various cancers, including HCC
(2,15,16). The regulatory effects of the Hippo
signaling pathway on HCC are primarily reflected in the regulation
of cell proliferation, invasion and metastasis, tumor drug
resistance, metabolic reprogramming, immunomodulatory effects and
autophagy. The connection between YAP1 and the hallmarks of cancer
is depicted in Fig. 2.
YAP1 promotes sorafenib resistance by upregulating
survivin expression in HCC cells (62). Silencing YAP1 and
insulin-like growth factor 2 mRNA binding protein 3 restores
transforming growth factor-β (TGF-β) signaling, suppresses
pluripotent genes and tumorigenesis and eliminates chemotherapy
resistance in tumor-initiating stem-like cells (TICs) (63). Dual downregulation of TAZ/YAP1
reduces chemotherapy resistance and tumorigenicity (64,65). Mex-3 RNA binding family member A
(MEX3A), an RNA-binding protein, has been implicated in cancer
development (66). MEX3A may
promote HCC progression and hinder sorafenib sensitivity by
inactivating the Hippo signaling pathway (67). Claudin 6 (CLDN6) is highly
expressed in a variety of cancers (68). Overexpression of CLDN6 increases
YAP1 and TAZ abundance, making sorafenib treatment less effective
(69).
Tumor-associated macrophages (TAM) are the most
abundant immune-associated stromal cells in the tumor
microenvironment. TAM exhibit great phenotypic heterogeneity and
have various functions, such as promoting tumor growth, metastasis
and angiogenesis (81). Previous
studies have shown that M2-type macrophages in HCC induce cell
proliferation and angiogenesis (82). In addition, M2-type
macrophage-derived extracellular vesicles promote T-cell exhaustion
in HCC by activating the YAP1/β-catenin pathway (83). The interaction between ETS variant
transcription factor 4 and YAP1 promotes the growth of HCC,
resulting in an increase in macrophages and a decrease in T-cell
and natural killer-cell infiltration in the tumor (84). Nogo-B promotes HCC progression by
enhancing YAP1/TAZ-mediated polarization of M2-type
TAM (85). IL-6 secreted by
YAP1-activated HCC cells may induce TAM recruitment (86). The activation of YAP1 is essential
for the recruitment of M2-type macrophages by TICs in the liver and
TAM protect TIC from immune clearance (87). RACGAP1 promotes HCC development
through immunosuppression mediated by YAP1activation (88). In patients with HCC, YAP1
overexpression in peripheral blood T cells is associated with an
increased percentage of T-regulatory cells in peripheral blood
mononuclear cells, indicating a poor prognosis (31).
CIN can induce polyploidy and aneuploidy of cells,
which are hallmarks of cancer. The increase in CIN, polyploid and
aneuploid is closely related to the occurrence and development of
HCC (95). Studies have shown
that YAP1 induces forkhead box (FOX)M1 to drive the expression of
CIN-related genes and promote the development of HCC (96). YAP1 promotes diploid-polyploid
transformation and polyploid cell growth through the protein kinase
B (Akt)-S-phase kinase associated protein 2 (Skp2) axis. YAP1
strongly induces acetyltransferase p300-mediated acetylation of the
E3 ligase Skp2 through Akt signaling. Acetylated Skp2 is localized
only to the cytoplasm, leading to excessive accumulation of the
cyclin-dependent kinase inhibitor p27, resulting in mitotic arrest
and cell polyploidy. In addition, the pro-apoptotic factor FOXO1/3
is over-degraded by acetylated Skp2, leading to polyploid cell
division, genomic instability and tumorigenesis (97). Leucine rich pentatricopeptide
repeat containing inhibits genomic instability and HCC by
maintaining Yap1-p27-mediated cell ploidy and p62-histone
deacetylase 6-controlled autophagy maturation (98).
YAP1, a key downstream effector of the Hippo
pathway, is regulated at both the transcriptional and
post-translational levels. Transcription factor CP2 (TFCP2) has
been identified as an oncogenic protein in HCC, acting as a YAP1
cofactor to stimulate YAP1-dependent liver malignancies (99). Serotonin (5-HT) promotes the
proliferation and metastasis of HCC (100). High levels of 5-HT and
YAP1/vestigial like family member 4 ratios in patients with HCC are
closely associated with HCC progression and poor prognosis
(101). The elevated expression
of 5-HT and YAP1 may synergistically promote HCC progression
(100). Ablation of stearoyl CoA
desaturase 2 (Scd2) suppresses YAP1 and prevents liver
tumorigenesis (102). In HCC,
knockdown of aldo-keto reductase 1C3 reduces YAP1 nuclear
translocation, inhibits SLC7A11 expression and induces ferroptosis
(103).
Studies have shown that YAP1/TAZ activity can be
regulated through various post-translational mechanisms, including
acetylation, methylation, phosphorylation, O-GlcNAacylation and
ubiquitination. Lysine acetyltransferase 6A, a histone
acetyltransferase, is involved in drug resistance by inducing YAP1
(104). YAP1 acetylation occurs
on specific and highly conserved C-terminal lysine residues and is
mediated by the nuclear acetyltransferases CREB binding protein and
p300 (105). The nuclear
deacetylase sirtuin 1 (SIRT1) is responsible for YAP1 deacetylation
(105). In HCC cells, high
levels of p300 promote the binding of YAP1 to the melanoma cell
adhesion molecule promoter, thereby promoting tumor development
(106). SIRT1 is a deacetylase
responsible for YAP1 deacetylation. In HCC, SIRT1-induced
deacetylation of YAP1 in HCC contributes to tumor progression
(107). Downregulation of SIRT1
blocks cisplatin-induced YAP2 nuclear translocation and enhances
cisplatin sensitivity (108).
Histone lysine methyltransferase SET domain
containing 1A (SETD1A) is a member of the histone methyltransferase
family. SETD1A enhances YAP1 activation and induces drug resistance
in HCC (109). Loss of spectrin
β, non-erythrocytic 1 inhibits hepatocyte autophagy through
SETD7-mediated YAP1 methylation, promoting the initiation and
development of HCC (110).
Overexpression of Menin and YAP1 in human HCC specimens is
associated with poor prognosis, suggesting H3K4me3 as a potential
therapeutic target for HCC (111). Ten-eleven translocation 1
physically interacts with TEAD to cause regional DNA demethylation,
histone H3K27 acetylation and chromatin opening of YAP1 target
genes, promoting transcriptional activation (112).
Highly expressed lipolysis-stimulated lipoprotein
receptor binds to YAP1 via the PPPY motif, increases YAP1
phosphorylation and inhibits the growth of HCC (113). Overexpression of estrogen
receptor α enhances the phosphorylation of YAP1 and reduces its
nuclear translocation, inhibiting the growth of HCC (114).
Increased O-GlcNAcylation has been observed in the
progression of liver tumors. O-GlcNAcylation is catalyzed by
O-linked β-N-acetylglucosamine transferase, which transfers
O-GlcNAc to the hydroxyl group of the serine or threonine residue
of the target protein. O-GlcNAcylation induces the transforming
phenotype of HCC cells in a YAP1-dependent manner (115).
YAP1 post-translational modification, which
facilitates ubiquitination and apoptosis, is a favorable prognostic
factor in HCC (116).
Ubiquitin-specific protease 46 (USP46) interacts with MST1, causing
YAP1 inactivation and inhibiting HCC proliferation and metastasis
(117). USP10 promotes HCC
proliferation by deubiquitinating and stabilizing YAP1/TAZ
(118). USP19 reduces K11 and
K48-linked multi-ubiquitination of YAP1 at the K76 and K90 sites,
stabilizing YAP1 and promoting HCC cell proliferation (119). Ubiquitin ligase RNF219-mediated
degradation of α-catenin promoted epigenetic modification of the
YAP1/β-catenin complex-dependent lectin galactoside-binding soluble
3 promoter, facilitating HCC metastasis (120). Tribbles homolog 2 promotes YAP1
transcriptional coactivator stabilization by interacting with
β-transducin repeat containing E3 ubiquitin protein ligase
(β-TrCP), which was important for HCC cell survival (121). Chaperonin containing TCP1
subunit 3 extends the half-life of YAP1 and TFCP2 by blocking
ubiquitination caused by poly(rC) binding protein 2 via β-TrCP
(122). Overexpression of E3
ubiquitin ligase F-box and WD repeat domain-containing 7 reduces
YAP1 expression, thereby inhibiting HCC proliferation (123). These studies suggested that YAP1
is regulated extensively at the post-translational level.
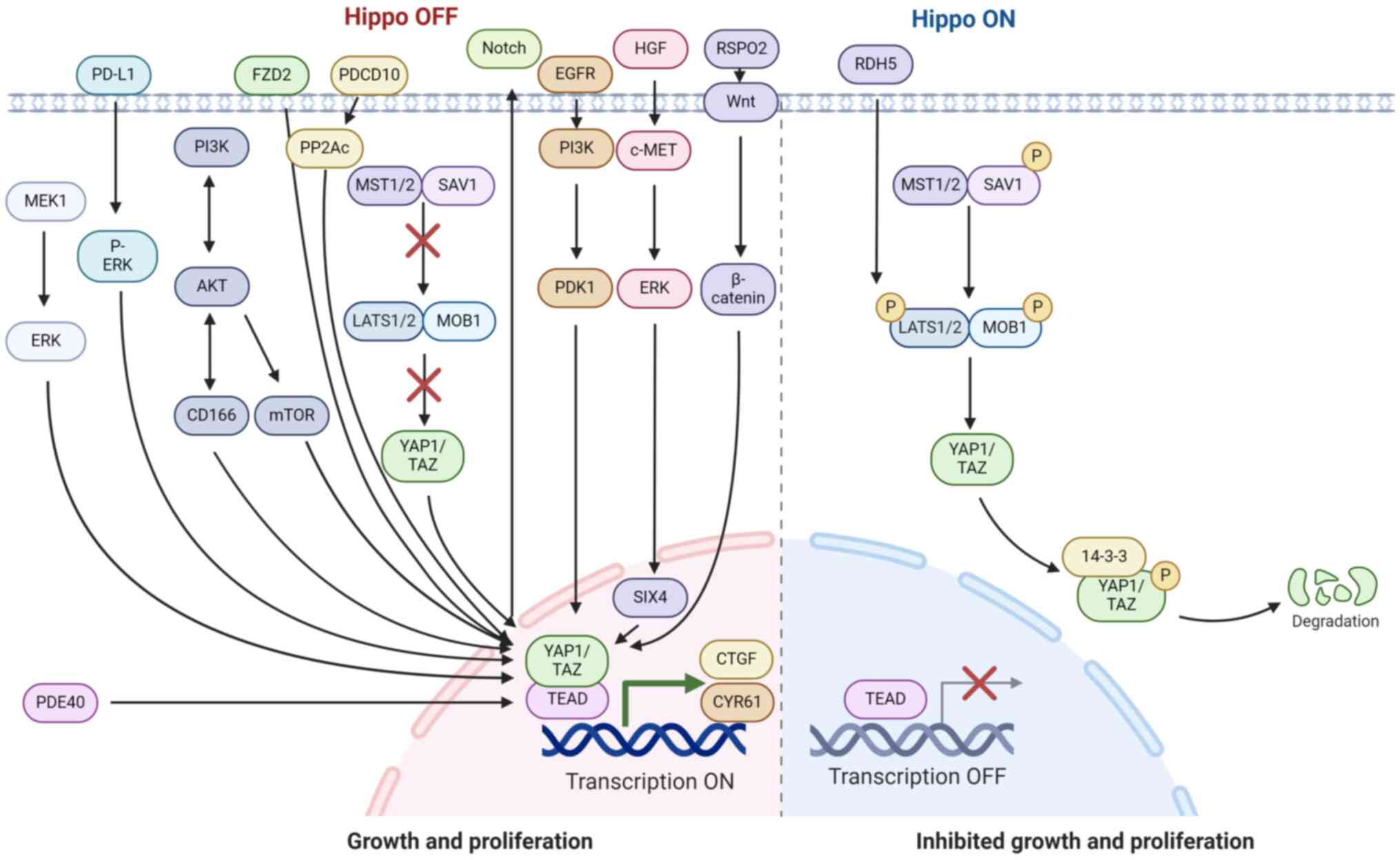
Numerous signaling pathways, including Wnt,
mitogen-activated protein kinase (MAPK),
phosphatidylinositol-3-kinase (PI3K) and Notch, have been shown to
regulate YAP1/TAZ activity in HCC through crosstalk with the Hippo
signaling pathway (2). The
crosstalk of Hippo signaling pathways with other signaling pathways
to regulate YAP1 is illustrated in Fig. 3. Activation of Wnt/β-catenin
signaling inhibits HCC formation by disrupting the positive
feedback loop between YAP1/TAZ and Notch signaling (124). Overexpression of R-spondin 2
activates both typical Wnt/β-catenin and Hippo/YAP1 signaling,
promoting HCC formation (125).
Various mutations in β-catenin combined with YAP1 drive HB
development (126). The
upregulation of YAP1 and β-catenin expression is observed in HCC
(127,128). The role of MYC and β-catenin in
liver tumorigenesis are dependent on Yap1/WW domain containing
transcription regulator 1 activity (129). Frizzled 10 (FZD10) enhances the
self-renewal, tumorigenicity and metastasis of liver cancer stem
cells (CSCs) by activating β-catenin and YAP1 (130). Tribbles homolog 2 is a direct
target of Wnt/T-cell factor in HCC, promoting the stabilization and
nuclear localization of YAP1 and contributing to the development of
fibrosis-associated HCC (131).
The MAPK signaling pathway has a key role in
numerous human diseases, including cancer. It is activated in
numerous tumors, with various components identified as oncogenes
(132). A previous study by our
group found that tumor cells promoted phosphorylated (p-)ERK and
expression of YAP1 through cell surface PD-1/programmed death
ligand-1 (PD-L1) interactions. Therefore, blocking the binding of
PD-1 and PD-L1 inhibited the p-ERK/YAP1 pathway and reduced tumor
cell proliferation (133).
Studies have also shown that MAPK kinase 1 interacts with YAP1 to
promote YAP1 expression, which supports the proliferation and
phenotypic transformation of hepatoma cells (134). In lenvatinib-resistant HCC
cells, the ERK/YAP1 signaling pathway mediates the upregulation of
cyclin-dependent kinase 6 (135).
Simultaneous activation of PI3K and YAP1 pathways
often occurs in HCC and their combined inhibition is unfavorable to
HCC growth (136). PI3K/AKT
signaling regulates CD166 (also known as Alcam), playing an
anti-apoptotic role in HCC via YAP1 (137). Standard CD44 (CD44S) positively
regulates the expression of YAP1 and its target genes in HCC cells
through the PI3K/AKT pathway (138). The mTOR complex 1 (mTORC1)
pathway is a major oncogenic pathway acting downstream of PI3K and
AKT (139). The mTORC1/AT-rich
interaction domain 1A axis promotes carcinogenic chromatin
remodeling and YAP1-dependent transcription, thus promoting HCC
development (140).
In addition, transcriptional regulator YAP1 has been
found to upregulate Notch ligand Jagged-1 (Jag-1), thereby
activating Notch signaling in HCC cells and mouse hepatocytes. In
human HCC and colorectal tumor samples, the activity of
YAP1-dependent Jag-1 and Notch was observed to correlate with
patient survival time. These results show that YAP1 and Notch
inhibitors can be used as therapeutics for gastrointestinal cancers
(147).
The ECM is a major component of tumors, playing a
vital role in mechanical support, microenvironment regulation and
as a source of signaling molecules (148). The Hippo pathway effector
molecules YAP1 and TAZ function as nuclear sensors for mechanical
signals in response to ECM signals. The ECM proteoglycan Agrin
promotes tumorigenesis by activating YAP1 (149). Collagens, a key component of
ECM, play a significant role as well. Collagen I-discoidin domain
receptor 1 signaling inhibits the Hippo pathway by promoting the
recruitment of protein phosphatase 2 scaffold subunit Aα to MST1,
which activates YAP1 and enhances the stem cell properties of HCC
(150).
The EGFR signaling pathway and the Hippo signaling
pathway play important roles in the carcinogenesis of HCC (151). Studies have shown that the
EGFR/PI3K-phosphoinositide-dependent kinase 1 pathway activates
YAP1 signaling in HCC. In addition, activated EGFR signaling can
also promote the growth of HCC cells in a YAP1-independent manner
(151).
TNF receptor II (TNFR2) is required for
TNF-α-induced YAP1 activation during malignant transformation of
hepatic progenitor cells (HPC) and liver tumorigenesis. In HPC-like
cells that drive HCC, the TNFR2/heterogeneous nuclear
ribonucleoprotein K/YAP1 signal is activated and associated with
poorer prognosis (152).
The cyclic adenosine monophosphate (cAMP) signaling
pathway plays a crucial role in cancer development (153). Phosphodiesterase 4D, a major
component of cAMP hydrolysis in many cell types, was observed to
form a complex with YAP1 to promote HCC progression (154).
The hepatocyte growth factor/cellular-mesenchymal
epithelial transition factor (c-MET) signaling pathway is important
for promoting HCC growth, angiogenesis and metastasis (155). Overexpression of SIX homeobox 4
promoted the expression of YAP1 and c-MET, thereby enhancing the
invasion and metastasis of HCC (156).
The upstream components of the Hippo pathway appear
to be human tumor suppressors. Studies have shown that
overexpression of MST1 promotes the phosphorylation of YAP1
(Ser127), inhibits cell proliferation and induces cell apoptosis
(157). Combined Mst1/2
deficiency results in loss of inhibitory Ser127 phosphorylation of
YAP1, massive overgrowth and HCC (158). Studies have shown that
serine/threonine protein kinase 25 enhances YAP1 activation through
the regulation of MST1/2 (159).
α2β1 integrin binds to the collagen ECM, inhibits MST1 kinase
phosphorylation and activates YAP1 to promote cancer (160). Knockdown of MST1 or
overexpression of YAP1 reverses tripartite motif containing 21
knockdown-induced HCC growth and chemotherapy-sensitive impairment
(161). SIRT7 inhibits MST1
transcription by binding to its promoter and inducing H3K18
deacetylation of the promoter region. High SIRT7 expression is
associated with increased YAP1 expression and nuclear localization
(162). Striatin 3 inhibits the
Hippo pathway, promoting YAP1 nuclear translocation (163). As an inhibitor of YAP1, LATS1 is
decreased via RNA interference-mediated downregulation of YAP1
(164). Inhibition of
LATS2-mediated dephosphorylation increases the YAP1/TEAD2
association, leading to YAP1/TEAD2 transcriptional activation, as
well as upregulated invasion of HCC cells (165). DND microRNA-mediated repression
inhibitor 1 promotes LATS2 and phosphorylated YAP1 levels,
inhibiting the EMT of HCC (166). TGF-β1 increases the
phosphorylation of LATS1 and YAP1, inhibiting HCC growth (167). Overexpression of WW and C2
domain containing 2 inhibits the invasion and metastasis of HCC
cells by activating LATS1/2 and phosphorylating YAP1 (168). Loss of PDZ and LIM domain
protein 1 leads to dephosphorylation of LATS1 and activation of
YAP1, promoting HCC metastasis (169). α-actinin 1 reduced LATS1 and
YAP1 phosphorylation and promoted HCC cell proliferation through
interaction with MOB1 (170).
Highly expressed LIM domain only 3 inhibits the Hippo signaling
pathway by interacting with LATS1, promoting the invasion and
metastasis of HCC cells (171).
The diacylglycerol lipase α/2-arachidonoylglycerol axis
significantly inhibits LATS1 and YAP1 phosphorylation, promotes
YAP1 nuclear translocation and activity, and induces HCC resistance
(172). SAV1 is required for the
activation of MST1 and subsequent LATS1/2, and SAV1 knockout leads
to the development of HCC (173). Studies have shown that low
levels of YAP1 phosphorylation can still be observed in the case of
SAV1 knockout. Furthermore, almost all liver cancers caused by
specific SAV1 knockout in the liver were mixed liver cancers
(174). Angiomotin (AMOT),
another regulator of YAP1 in the Hippo pathway, forms a typical
Hippo core complex with MST and LATS (175). Studies have shown that AMOT acts
as a YAP1 stimulator at high glucose levels and as a YAP1 inhibitor
at normal glucose levels (176).
LIM domain protein Ajuba regulates YAP1 signaling and is associated
with tumorigenesis. Depletion of Ajuba led to increased YAP1
expression in HCC cells, promoting their growth (177). Neurofibromin 2 (NF2) is a tumor
suppressor gene. NF2 induces LATS1/2 kinase, which inhibits
YAP1/TAZ (178). In the absence
of Nf2, Amot promotes nuclear entry and transcriptional
activity of Yap1 and is required for liver tumorigenesis
(179). Together, these studies
suggest that the Hippo-YAP1 signaling pathway is involved in the
development of HCC. The dysregulation of Hippo signaling pathway
components is frequently observed in HCC.
Although the complete range of downstream targets of
YAP1 has yet to be fully elucidated, numerous identified targets
are linked to cell growth and survival. In addition, TEAD is
essential for the oncogenic function of YAP1/TAZ, thus disrupting
the interaction between TEAD and YAP1/TAZ can inhibit YAP1
activity. Silencing TEAD4 in the TEAD gene persistently inhibited
tumor growth in HB cell lines and decreased the expression of YAP1
target genes (180). TEAD4 was
found to mitigate TGF-β signaling and HCC progression independently
of YAP1 (181). Overexpression
of hepatocyte nuclear factor 4α significantly impaired the
proliferation of YAP1-TEAD-induced HCC cells (182). Targeting downstream effectors of
YAP1 could be a potential strategy to inhibit its oncogenic
properties. The AXL receptor tyrosine kinase (AXL), a downstream
target of YAP1, is involved in cell invasion and metastasis
(183). It has been demonstrated
that RNA interference-mediated downregulation of AXL expression
reduces the proliferation and invasion capabilities of
YAP1-expressing HCC cell lines (184). CTGF, a multifunctional signal
regulator, promotes cancer occurrence, progression and metastasis
by regulating cell proliferation, migration, invasion and drug
resistance (185).
Sphingosine-1-phosphate (S1P) has been shown to stimulate cell
proliferation through YAP1 activation and upregulation of CTGF
expression mediated by S1P receptor 2 (186). Overexpression of TNF-α-induced
protein 8 (TNFAIP8) increases the nuclear localization and
stability of YAP1, upregulates CTGF and promotes HCC progression
(187). Furthermore, the loss of
CYR61 enhances TGF-β-or YAP1-mediated growth and migration of HCC
cells (188). Amphiregulin
(AREG), another downstream target of YAP1, has been shown to play a
crucial role in inhibiting HCC by inactivating YAP1 (189). NUAK family SNF1-like kinase 2
(NUAK2), also known as sucrose nonfermenting-like kinase, is a
member of the AMPK protein kinase family and a direct downstream
target of YAP1 (190).
Pharmacological inactivation of NUAK2 inhibits YAP1-dependent
cancer cell proliferation and liver overgrowth (191). These findings suggest that
targeting the downstream effectors of YAP1, such as AXL, CTGF,
TNFAIP8, CYR61, AREG and NUAK2, may be an effective strategy for
inhibiting YAP1-mediated oncogenesis.
Given the critical role of YAP1 in cancer
development, the existing small molecule compounds targeting YAP1
and their mechanisms of action were summarized (Table I). A previous study by our group
demonstrated that YAP1 blocked the immunosuppressive
microenvironment, thereby enhancing the efficacy of HCC
chemotherapy. For instance, cisplatin promotes PD-L1 expression and
induces immune tolerance through YAP1 in the HCC microenvironment
(192). Verteporfin, a
photodynamic drug approved for treating macular degeneration, has
shown promising preclinical antitumor effects by inhibiting the
YAP1/TAZ pathway (193). In
addition, verteporfin exhibited antitumor effects in both
intrahepatic and extrahepatic CCA and enhanced tumor growth
inhibition when combined with anti-PD-1 (194). Verteporfin significantly
improved the efficacy of transcatheter arterial chemoembolization
in the treatment of transplanted HCC by inhibiting the Hippo/YAP1
signaling pathway (195).
Statins, widely used for treating dyslipidemia and preventing
cardiovascular diseases, were found to be associated with a reduced
incidence of HCC (196). Statins
treatment resulted in the extrusion of YAP1 protein from the
nucleus to the cytoplasm (197).
The synergistic effect of fibroblast growth factor receptor 4 and
EZH2 inhibitors induced the apoptosis of HCC cells by inhibiting
YAP1 (198). The compound
12-O-tetradecanoylphorbol-13-acetate, a known carcinogen in rodent
skin (199), inhibited YAP1 and
HCC cells through AMOT (200).
Talazoparib, a potent poly(ADP-ribose) polymerase 1 inhibitor used
for treating patients with breast cancer with BRCA1 DNA repair
associated (BRCA1) or BRCA2 mutations (201), induced the expression of the
tumor suppressor long non-coding RNA polo-like kinase 4, which
inhibited HCC cell viability and growth by inactivating YAP1 and
inducing cell senescence (202).
Dichloroacetate, used for treating mitochondrial genetic diseases
and lactic acid poisoning (203), reduced the stemness of HCC cells
by promoting the cytoplasmic translocation of YAP1 (204). Fingolimod, a Food and Drug
Administration-approved immunomodulator for multiple sclerosis
(205), inhibited HCC
proliferation by downregulating YAP1 expression (206). Metformin, a first-line treatment
for type 2 diabetes, exhibited promising anti-tumor effects by
directly inhibiting LATS1/2, activating MST1/2 and phosphorylating
YAP1, thereby inhibiting HCC progression (207). Furthermore, metformin increased
the sensitivity to chemotherapeutic agents by inhibiting YAP1 in
HCC (208). Tadalafil, a
phosphodiesterase type 5 (PDE5) inhibitor used for treating
pulmonary hypertension and erectile dysfunction (209), reduced YAP1/TAZ levels by
targeting the PDE5/PKG/Hippo/YAP1/TAZ axis in HCC (210). Vincristine sulfate, a common
chemotherapy drug, inhibited YAP1 transcriptional activity and cell
proliferation when the tumor supernatant was briefly treated with
the drug in vitro (211).
Various studies have shown that natural products
play an important role in HCC. Artemisinin and its derivative
dihydroartemisinin (DHA) have been found to inhibit HCC (212). Artemisinin inhibits the growth,
migration and invasion of HCC by targeting cell bioenergetics and
the Hippo/YAP1 signaling pathway (213). Our research group has been
studying the anti-HCC mechanism of DHA and found that DHA reduced
lipid droplet deposition by YAP1, thereby enhancing the anti-PD-1
effect (214). In addition, DHA
inhibited the Warburg effect in HCC via the YAP1/solute carrier
family 2 member 1 pathway (215). The present study also showed
that DHA increased FXR expression decreased YAP1, and inhibited BA
metabolism (216). A previous
study by our group found that YAP1 was positively correlated with
IL-18, and DHA was effective against HCC by inhibiting both YAP1
and IL-18 (217). Furthermore,
DHA disrupted the tumor immunosuppressive microenvironment by
inhibiting YAP1 expression, enhancing the efficacy of anti-PD-1
(218). Another study by our
group found that DHA increased the abundance of Akkermansia
muciniphila by downregulating YAP1, which increased the
efficacy of anti-PD-1 (219).
These studies confirmed that DHA inhibited HCC progression by
inhibiting YAP1. Decursin, a component of Korean Dang-gui
(Angelica gigas Nakai) root, significantly inhibited
HCC-cell proliferation by upregulating the phosphorylation of LATS1
and β-TrCP and promoting the degradation of YAP1 (220). Myricetin activated LATS1/2
kinase, which directly phosphorylates YAP1 on serine residues,
thereby inhibiting HCC cell proliferation (221). Evodiamine significantly
inhibited YAP1 expression by upregulating LATS1 phosphorylation,
leading to inhibited proliferation and induced apoptosis of HCC
cells (222). Wogonin, an
ingredient extracted from the Scutellaria baicalensis Georgi
root, effectively induced cell cycle arrest and promoted apoptosis
in HCC cells by activating MOB1-LATS1 and inhibiting YAP1 and TAZ
(223). A study reported that
WZ35, a derivative of curcumin, significantly inhibits HCC cell
growth by downregulating YAP1-controlled autophagy (92). Ginsenoside CK, an intestinal
microbial metabolite of panaxadiol saponins, inhibited HCC
proliferation and growth by blocking YAP1/TEAD2 interaction
(224). Apigenin decreased
YAP1expression by regulating autophagy-related genes, reducing HCC
cell migration and invasion (225). Evodiamine, isolated from the
Evodia rutaecarpa fruit, is an effective anti-cancer agent
that reduces YAP1 levels (226).
4-acetylantrocamol LT3, a new ubiquinone from the mycelium of
Antrodia cinna-momea (Polyporaceae), inhibited HepG2 cell
growth by targeting the YAP1/TAZ, mTOR and Wnt/β-catenin signaling
pathways (227). Tanshinone IIA,
an ingredient of Salvia miltiorrhiza, inhibited HCC
proliferation by downregulating YAP1 expression in a TGF-β
signaling pathway-dependent manner (228). Chinese propolis, a resin-like
substance collected by Apis mellifera from various tree
buds, inhibited HepG2 cell proliferation and promoted apoptosis by
inactivating the Hippo/YAP1 and PI3K/AKT pathways (229). Salvianolic acid B, an active
ingredient of Salvia miltiorrhiza, inhibited HCC by
upregulating MST1 protein expression, degrading YAP1 in the
cytoplasm and inhibiting the expression of downstream genes in the
Hippo pathway (230).
Ovatodiolide, an active ingredient of Anisomeles indica (L.)
Kuntze (Labiatae), significantly reduced YAP1 expression and
inhibited the CSC phenotype and associated disease progression
(231). Corosolic acid, an
extract of Actinidia chinensi, inhibited tumor progression
by relocating YAP1 from the nucleus (232). Inhibition of YAP1 by actinomycin
D enhanced the efficacy of corosolic acid in HCC treatment
(233). Ligustilide, the main
ingredient of Angelica sinensis and Ligusticum
chuanxiongs (234),
antagonized macrophage recruitment and M2 polarization induced by
HCC cells by inhibiting YAP1/IL-6-induced IL-6R/STAT3 signaling
activation (235). Myricetin, a
flavonoid compound found in a wide variety of natural plants
(236) and Luteolin, a flavonoid
contained in a variety of fruits, vegetables and herbs (237), both showed potential in HCC
treatment. Luteolin inhibited the biological effects of matrix
stiffness induction and C-X-C motif chemokine receptor type
4-mediated YAP1 signaling pathway in HCC (238). Hydrogen sulfide-releasing
oleanolic acid (HS-OA) decreased the expression of YAP1 and its
downstream targets CTGF and CYR61, promoting cell apoptosis
(239). Daphnane diterpenoids,
specifically 12-O-debenzoyl-yuanhuacine, prepared from dried flower
buds of the Daphne genkwa plant, effectively inhibited the
binding of YAP1 and TEAD1 (240).
In recent years, our research group has conducted
in-depth and comprehensive studies on the relationship between YAP1
and HCC. This included the role of YAP1 in lipid metabolism
(214), glucose metabolism
(215) and BA metabolism
(216) of HCC, as well as its
relationship with autophagy and immune cells, particularly T cells.
Much of our research has focused on HCC cells. However, the
occurrence of HCC is influenced not only by hepatocellular lesion
but also by the entire HCC microenvironment. Therefore, we should
broaden our focus on YAP1 to gain new insights. For instance, in
2019, a study published in Science reported that differences in
YAP1/TAZ expression between HCC tissues and adjacent tissues could
determine the prognosis of HCC (49). Therefore, it is important to
consider the level of YAP1 expression in non-tumor cells within the
HCC microenvironment. Drug development should not only focus on the
effect of drugs on tumor cells but also consider their impact on
non-tumor cells. Our subsequent study reflects this approach. When
investigating the therapeutic effects of DHA on HCC in mice, DHA's
effects on YAP1 were examined in both HCC cells and adjacent
tissues (218). In the absence
of YAP1 in hepatocytes and biliary epithelial cells, YAP1 is
expressed in non-parenchymal cells (NPCs) in a
cholestasis-independent manner. YAP1expression was detected in both
Kupffer cells and endothelial cell subgroups. Serum secretion of
pro-inflammatory chemokines and cytokines increased in YAP1KO
animals, suggesting that YAP1 activation in NPCs may promote
inflammation through TEAD-dependent transcriptional regulation of
secretory factors (241).
In summary, the Hippo signaling pathway and its
downstream effector YAP1 play crucial roles in the development and
progression of HCC. While many regulatory mechanisms of YAP1 have
been identified, interactions between YAP1and HCC have remained to
be fully elucidated, necessitating further research. In addition,
various factors, such as hepatitis B virus (242,243), hypoxia (244-246) and ECM, can influence YAP1
expression (247),
mechanotransduction (248). The
study of the Hippo-YAP1 signaling pathway in relation to HCC
provides new strategies for understanding the pathogenesis,
diagnosis and treatment of HCC. This research also provides new
directions for the development of YAP1-related drugs, highlighting
both the challenges and opportunities in this area. Further
clarification of the relationship between YAP1 and HCC is
essential.
Not applicable.
XH and ED designed the study; SL, LH, NL, and XS
performed the literature search; SL, LH, NL and XS wrote the
manuscript with contributions from all authors. XH, HY and ED
revised the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The present study was financially supported by the Science and
Technology Program of Hebei (grant no. 223777156D), the Clinical
Medical School Graduate Research Innovation Practice Project (grant
no. 2023KCY06) and the National Natural Science Foundation of China
(grant nos. 81973840 and 81273748).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S and Zhou D: Role of the
transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin
Cell Biol. 61:64–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baecker A, Liu X, La Vecchia C and Zhang
ZF: Worldwide incidence of hepatocellular carcinoma cases
attributable to major risk factors. Eur J Cancer Prev. 27:205–212.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nguyen-Lefebvre AT, Selzner N, Wrana JL
and Bhat M: The hippo pathway: A master regulator of liver
metabolism, regeneration, and disease. FASEB J. 35:e215702021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Justice RW, Zilian O, Woods DF, Noll M and
Bryant PJ: The Drosophila tumor suppressor gene warts encodes a
homolog of human myotonic dystrophy kinase and is required for the
control of cell shape and proliferation. Genes Dev. 9:534–546.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tapon N, Harvey KF, Bell DW, Wahrer DC,
Schiripo TA, Haber D and Hariharan IK: Salvador promotes both cell
cycle exit and apoptosis in Drosophila and is mutated in human
cancer cell lines. Cell. 110:467–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia J, Zhang W, Wang B, Trinko R and Jiang
J: The Drosophila Ste20 family kinase dMST functions as a tumor
suppressor by restricting cell proliferation and promoting
apoptosis. Genes Dev. 17:2514–2519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu M, Hu Y, Lan T, Guan KL, Luo T and Luo
M: The Hippo signalling pathway and its implications in human
health and diseases. Signal Transduct Target Ther. 7:3762022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong AW, Meng Z and Guan KL: The Hippo
pathway in intestinal regeneration and disease. Nat Rev
Gastroenterol Hepatol. 13:324–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao F, Xiao Z, Sun Y and Ma L: SKP2 and
OTUD1 govern non-proteolytic ubiquitination of YAP that promotes
YAP nuclear localization and activity. Cell Stress. 2:233–235.
2018. View Article : Google Scholar
|
|
11
|
Yu W, Qiao Y, Tang X, Ma L, Wang Y, Zhang
X, Weng W, Pan Q, Yu Y, Sun F and Wang J: Tumor suppressor long
non-coding RNA, MT1DP is negatively regulated by YAP and Runx2 to
inhibit FoxA1 in liver cancer cells. Cell Signal. 26:2961–2968.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai D, Ho KC, Hao Y and Yang X: Taxol
resistance in breast cancer cells is mediated by the hippo pathway
component TAZ and its downstream transcriptional targets Cyr61 and
CTGF. Cancer Res. 71:2728–2738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
d'Angelo M, Benedetti E, Tupone MG,
Catanesi M, Castelli V, Antonosante A and Cimini A: The role of
stiffness in cell reprogramming: A potential role for biomaterials
in inducing tissue regeneration. Cells. 8:10362019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song H, Mak KK, Topol L, Yun K, Hu J,
Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al: Mammalian
Mst1 and Mst2 kinases play essential roles in organ size control
and tumor suppression. Proc Natl Acad Sci USA. 107:1431–1436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Wang X and Yang Y: Hepatic Hippo
signaling inhibits development of hepatocellular carcinoma. Clin
Mol Hepatol. 26:742–750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Zhu ZM, Liu CL, He XJ, Feng XB,
Zhang L, Dong JH and Zhang I HY: Yes-associated protein in
hepatocellular carcinoma is associated with tumor differentiation
and patient age at diagnosis, but not markers of HBV infection.
Clin Lab. 62:365–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Shin DJ, Pan H, Lin Z, Dreyfuss JM,
Camargo FD, Miao J and Biddinger SB: YAP suppresses gluconeogenic
gene expression through PGC1α. Hepatology. 66:2029–2041. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YA, Noon LA, Akat KM, Ybanez MD, Lee
TF, Berres ML, Fujiwara N, Goossens N, Chou HI, Parvin-Nejad FP, et
al: Autophagy is a gatekeeper of hepatic differentiation and
carcinogenesis by controlling the degradation of Yap. Nat Commun.
9:49622018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He L, Yuan L, Yu W, Sun Y, Jiang D, Wang
X, Feng X, Wang Z, Xu J, Yang R, et al: A regulation loop between
YAP and NR4A1 balances cell proliferation and apoptosis. Cell Rep.
33:1082842020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang C, Zhang L, He Q, Feng X, Zhu J, Xu
Z, Wang X, Chen F, Li X and Dong J: Differences in yes-associated
protein and mRNA levels in regenerating liver and hepatocellular
carcinoma. Mol Med Rep. 5:410–414. 2012.
|
|
22
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
LaQuaglia MJ, Grijalva JL, Mueller KA,
Perez-Atayde AR, Kim HB, Sadri-Vakili G and Vakili K: YAP
subcellular localization and hippo pathway transcriptome analysis
in pediatric hepatocellular carcinoma. Sci Rep. 6:302382016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fitamant J, Kottakis F, Benhamouche S,
Tian HS, Chuvin N, Parachoniak CA, Nagle JM, Perera RM, Lapouge M,
Deshpande V, et al: YAP inhibition restores hepatocyte
differentiation in advanced HCC, leading to tumor regression. Cell
Rep. 10:1692–1707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu H, Liu Y, Jiang XW, Li WF, Guo G, Gong
JP and Ding X: Clinicopathological and prognostic significance of
yesassociated protein expression in hepatocellular carcinoma and
hepatic cholangiocarcinoma. Tumour Biol. 37:13499–13508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim GJ, Kim H and Park YN: Increased
expression of yes-associated protein 1 in hepatocellular carcinoma
with stemness and combined hepatocellular-cholangiocarcinoma. PLoS
One. 8:e754492013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perra A, Kowalik MA, Ghiso E,
Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore
E, Roncalli M, Giordano S and Columbano A: YAP activation is an
early event and a potential therapeutic target in liver cancer
development. J Hepatol. 61:1088–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marquard S, Thomann S, Weiler SME,
Bissinger M, Lutz T, Sticht C, Tóth M, de la Torre C, Gretz N,
Straub BK, et al: Yes-associated protein (YAP) induces a secretome
phenotype and transcriptionally regulates plasminogen activator
Inhibitor-1 (PAI-1) expression in hepatocarcinogenesis. Cell Commun
Signal. 18:1662020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Wang S, Wang G, Zhang Z, Wu X, Zhang
T, Fu B and Chen G: Yes-associated protein expression is a
predictive marker for recurrence of hepatocellular carcinoma after
liver transplantation. Dig Surg. 31:468–478. 2014. View Article : Google Scholar
|
|
30
|
Gao Y, Gong Y, Liu Y, Xue Y, Zheng K, Guo
Y, Hao L, Peng Q and Shi X: Integrated analysis of transcriptomics
and metabolomics in human hepatocellular carcinoma HepG2215 cells
after YAP1 knockdown. Acta Histochem. 125:1519872023. View Article : Google Scholar
|
|
31
|
Fan Y, Gao Y, Rao J, Wang K, Zhang F and
Zhang C: YAP-1 promotes tregs differentiation in hepatocellular
carcinoma by enhancing TGFBR2 transcription. Cell Physiol Biochem.
41:1189–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huo X, Zhang Q, Liu AM, Tang C, Gong Y,
Bian J, Luk JM, Xu Z and Chen J: Overexpression of yes-associated
protein confers doxorubicin resistance in hepatocellullar
carcinoma. Oncol Rep. 29:840–846. 2013. View Article : Google Scholar
|
|
33
|
Gurda GT, Zhu Q, Bai H, Pan D, Schwarz KB
and Anders RA: The use of yes-associated protein expression in the
diagnosis of persistent neonatal cholestatic liver disease. Hum
Pathol. 45:1057–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee K, Lee KB, Jung HY, Yi NJ, Lee KW, Suh
KS and Jang JJ: The correlation between poor prognosis and
increased yes-associated protein 1 expression in keratin 19
expressing hepatocellular carcinomas and cholangiocarcinomas. BMC
Cancer. 17:4412017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smith JL, Rodríguez TC, Mou H, Kwan SY,
Pratt H, Zhang XO, Cao Y, Liang S, Ozata DM, Yu T, et al: YAP1
withdrawal in hepatoblastoma drives therapeutic differentiation of
tumor cells to functional hepatocyte-like cells. Hepatology.
73:1011–1027. 2021. View Article : Google Scholar
|
|
36
|
Gong W, Han Z, Fang F and Chen L: Yap
expression is closely related to tumor angiogenesis and poor
prognosis in hepatoblastoma. Fetal Pediatr Pathol. 41:929–939.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim MK, Park JY and Kang YN: Tumorigenic
role of YAP in hepatocellular carcinogenesis is involved in SHP2
whose function is different in vitro and in vivo. Pathol Res Pract.
214:1031–1039. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Zhuo S, Zhou Y, Ma L, Sun Z, Wu X,
Wang XW, Gao B and Yang Y: Yap-Sox9 signaling determines hepatocyte
plasticity and lineage-specific hepatocarcinogenesis. J Hepatol.
76:652–664. 2022. View Article : Google Scholar :
|
|
39
|
Wei T, Weiler SME, Tóth M, Sticht C, Lutz
T, Thomann S, De La Torre C, Straub B, Merker S, Ruppert T, et al:
YAP-dependent induction of UHMK1 supports nuclear enrichment of the
oncogene MYBL2 and proliferation in liver cancer cells. Oncogene.
38:5541–5550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeric I, Maurer G, Cavallo AL, Raguz J,
Desideri E, Tarkowski B, Parrini M, Fischer I, Zatloukal K and
Baccarini M: A cell-autonomous tumour suppressor role of RAF1 in
hepatocarcinogenesis. Nat Commun. 7:137812016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Badr EA, El Tantawy El Sayed I, Assar MF,
Ali SA and Ibrahim NS: A pilot study of Livin gene and
yes-associated protein 1 expression in hepatocellular carcinoma
patients. Heliyon. 5:e027982019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He
J, Wang H, Xiao W, Li L, Chu Q, et al: Mutual interaction between
YAP and CREB promotes tumorigenesis in liver cancer. Hepatology.
58:1011–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu G, Wang Y, Li W, Cao Y, Xu J, Hu Z, Hao
Y, Hu L and Sun Y: COX-2 forms regulatory loop with YAP to promote
proliferation and tumorigenesis of hepatocellular carcinoma cells.
Neoplasia. 20:324–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang J, Wang H, Zhang Y, Zhen N, Zhang L,
Qiao Y, Weng W, Liu X, Ma L, Xiao W, et al: Mutual inhibition
between YAP and SRSF1 maintains long non-coding RNA, Malat1-induced
tumourigenesis in liver cancer. Cell Signal. 26:1048–1059. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiao K, Liu Y, Xu Z, Zhang H, Zhang H,
Zhang C, Chang Z, Lu X, Li Z, Luo C, et al: RNA m6A methylation
promotes the formation of vasculogenic mimicry in hepatocellular
carcinoma via Hippo pathway. Angiogenesis. 24:83–96. 2021.
View Article : Google Scholar
|
|
46
|
Sweed D, Abd-Elbary A, Sweed E, Mosbeh A,
Moaz I, Yassein T and Elmashad S: Expression of cyclo-oxygenase-2
and yap/taz in hepatocellular carcinoma in untreated and treated
hepatitis C virus patients. Pol J Pathol. 73:88–98. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Van Haele M, Moya IM, Karaman R, Rens G,
Snoeck J, Govaere O, Nevens F, Verslype C, Topal B, Monbaliu D, et
al: YAP and TAZ heterogeneity in primary liver cancer: An analysis
of its prognostic and diagnostic role. Int J Mol Sci. 20:6382019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang H, Wang J, Zhang S, Jia J, Liu X,
Zhang J, Wang P, Song X, Che L, Liu K, et al: Distinct and
overlapping roles of hippo effectors YAP and TAZ during human and
mouse hepatocarcinogenesis. Cell Mol Gastroenterol Hepatol.
11:1095–1117. 2021. View Article : Google Scholar :
|
|
49
|
Moya IM, Castaldo SA, Van den Mooter L,
Soheily S, Sansores-Garcia L, Jacobs J, Mannaerts I, Xie J,
Verboven E, Hillen H, et al: Peritumoral activation of the Hippo
pathway effectors YAP and TAZ suppresses liver cancer in mice.
Science. 366:1029–1034. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu YP, Pan LL and Kong CC: Stathmin 1
promotes the progression of liver cancer through interacting with
YAP1. Eur Rev Med Pharmacol Sci. 24:7335–7344. 2020.PubMed/NCBI
|
|
51
|
Miao HL, Pan ZJ, Lei CJ, Wen JY, Li MY,
Liu ZK, Qiu ZD, Lin MZ, Chen NP and Chen M: Knockdown of GPC3
inhibits the proliferation of Huh7 hepatocellular carcinoma cells
through down-regulation of YAP. J Cell Biochem. 114:625–631. 2013.
View Article : Google Scholar
|
|
52
|
Yang XM, Cao XY, He P, Li J, Feng MX,
Zhang YL, Zhang XL, Wang YH, Yang Q, Zhu L, et al: Overexpression
of Rac GTPase activating protein 1 contributes to proliferation of
cancer cells by reducing Hippo signaling to promote cytokinesis.
Gastroenterology. 155:1233–1249.e22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miao X and Zhang N: Role of RBM3 in the
regulation of cell proliferation in hepatocellular carcinoma. Exp
Mol Pathol. 117:1045462020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yuan T, Zhou T, Qian M, Du J, Liu Y, Wang
J, Li Y, Fan G, Yan F, Dai X, et al: SDHA/B reduction promotes
hepatocellular carcinoma by facilitating the deNEDDylation of
cullin1 and stabilizing YAP/TAZ. Hepatology. 78:103–119. 2023.
View Article : Google Scholar
|
|
55
|
Zhang H, Liu H and Bi H: MicroRNA-345
inhibits hepatocellular carcinoma metastasis by inhibiting YAP1.
Oncol Rep. 38:843–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang S, Li H, Wang G, Zhang T, Fu B, Ma M,
Quan Z and Chen G: Yes-associated protein (YAP) expression is
involved in epithelial-mesenchymal transition in hepatocellular
carcinoma. Clin Transl Oncol. 18:172–177. 2016. View Article : Google Scholar
|
|
57
|
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu
S, Zhu P, Wang W and Zhou H: Yap promotes hepatocellular carcinoma
metastasis and mobilization via governing
cofilin/F-actin/lamellipodium axis by regulation of
JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 14:59–71. 2018.
View Article : Google Scholar
|
|
58
|
Wu D, Liu G, Liu Y, Saiyin H, Wang C, Wei
Z, Zen W, Liu D, Chen Q, Zhao Z, et al: Zinc finger protein 191
inhibits hepatocellular carcinoma metastasis through discs large
1-mediated yes-associated protein inactivation. Hepatology.
64:1148–1162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Weiler SME, Lutz T, Bissinger M, Sticht C,
Knaub M, Gretz N, Schirmacher P and Breuhahn K: TAZ target gene
ITGAV regulates invasion and feeds back positively on YAP and TAZ
in liver cancer cells. Cancer Lett. 473:164–175. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tang Y, Thiess L, Weiler SME, Tóth M, Rose
F, Merker S, Ruppert T, Schirmacher P and Breuhahn K: α-Catenin
interaction with YAP/FoxM1/TEAD-induced CEP55 supports liver cancer
cell migration. Cell Commun Signal. 21:1622023. View Article : Google Scholar
|
|
61
|
Fan Y, Du Z, Ding Q, Zhang J, Op Den
Winkel M, Gerbes AL, Liu M and Steib CJ: SEPT6 drives
hepatocellular carcinoma cell proliferation, migration and invasion
via the Hippo/YAP signaling pathway. Int J Oncol. 58:132021.
View Article : Google Scholar
|
|
62
|
Sun T, Mao W, Peng H, Wang Q and Jiao L:
YAP promotes sorafenib resistance in hepatocellular carcinoma by
upregulating survivin. Cell Oncol (Dordr). 44:689–699. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen CL, Tsukamoto H, Liu JC, Kashiwabara
C, Feldman D, Sher L, Dooley S, French SW, Mishra L, Petrovic L, et
al: Reciprocal regulation by TLR4 and TGF-β in tumor-initiating
stem-like cells. J Clin Invest. 123:2832–2849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hayashi H, Higashi T, Yokoyama N, Kaida T,
Sakamoto K, Fukushima Y, Ishimoto T, Kuroki H, Nitta H, Hashimoto
D, et al: An imbalance in TAZ and YAP expression in hepatocellular
carcinoma confers cancer stem cell-like behaviors contributing to
disease progression. Cancer Res. 75:4985–4997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gao R, Kalathur RKR, Coto-Llerena M, Ercan
C, Buechel D, Shuang S, Piscuoglio S, Dill MT, Camargo FD,
Christofori G and Tang F: YAP/TAZ and ATF4 drive resistance to
Sorafenib in hepatocellular carcinoma by preventing ferroptosis.
EMBO Mol Med. 13:e143512021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang D, Jiao Y, Li Y and Fang X: Clinical
characteristics and prognostic value of MEX3A mRNA in liver cancer.
PeerJ. 8:e82522020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fang S, Zheng L, Chen X, Guo X, Ding Y, Ma
J, Ding J, Chen W, Yang Y, Chen M, et al: MEX3A determines in vivo
hepatocellular carcinoma progression and induces resistance to
sorafenib in a Hippo-dependent way. Hepatol Int. 17:1500–1518.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Du H, Yang X, Fan J and Du X: Claudin 6:
Therapeutic prospects for tumours, and mechanisms of expression and
regulation (Review). Mol Med Rep. 24:6772021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kong FE, Li GM, Tang YQ, Xi SY, Loong JHC,
Li MM, Li HL, Cheng W, Zhu WJ, Mo JQ, et al: Targeting tumor
lineage plasticity in hepatocellular carcinoma using an anti-CLDN6
antibody-drug conjugate. Sci Transl Med. 13:eabb62822021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Di Benedetto G, Parisi S, Russo T and
Passaro F: YAP and TAZ mediators at the crossroad between metabolic
and cellular reprogramming. Metabolites. 11:1542021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu Q, Li J, Zhang W, Xiao C, Zhang S,
Nian C, Li J, Su D, Chen L, Zhao Q, et al: Glycogen accumulation
and phase separation drives liver tumor initiation. Cell.
184:5559–5576.e19. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sun L, Suo C, Zhang T, Shen S, Gu X, Qiu
S, Zhang P, Wei H, Ma W, Yan R, et al: ENO1 promotes liver
carcinogenesis through YAP1-dependent arachidonic acid metabolism.
Nat Chem Biol. 19:1492–1503. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen R, Zhu S, Fan XG, Wang H, Lotze MT,
Zeh HJ III, Billiar TR, Kang R and Tang D: High mobility group
protein B1 controls liver cancer initiation through yes-associated
protein-dependent aerobic glycolysis. Hepatology. 67:1823–1841.
2018. View Article : Google Scholar
|
|
75
|
Athavale D, Song Z, Desert R, Han H, Das
S, Ge X, Komakula SSB, Chen W, Gao S, Lantvit D, et al: Ablation of
high-mobility group box-1 in the liver reduces hepatocellular
carcinoma but causes hyperbilirubinemia in Hippo
signaling-deficient mice. Hepatol Commun. 6:2155–2169. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cox AG, Hwang KL, Brown KK, Evason K,
Beltz S, Tsomides A, O'Connor K, Galli GG, Yimlamai D, Chhangawala
S, et al: Yap reprograms glutamine metabolism to increase
nucleotide biosynthesis and enable liver growth. Nat Cell Biol.
18:886–896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Park YY, Sohn BH, Johnson RL, Kang MH, Kim
SB, Shim JJ, Mangala LS, Kim JH, Yoo JE, Rodriguez-Aguayo C, et al:
Yes-associated protein 1 and transcriptional coactivator with
PDZ-binding motif activate the mammalian target of rapamycin
complex 1 pathway by regulating amino acid transporters in
hepatocellular carcinoma. Hepatology. 63:159–172. 2016. View Article : Google Scholar
|
|
78
|
Anakk S, Bhosale M, Schmidt VA, Johnson
RL, Finegold MJ and Moore DD: Bile acids activate YAP to promote
liver carcinogenesis. Cell Rep. 5:1060–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Delgado ER, Erickson HL, Tao J, Monga SP,
Duncan AW and Anakk S: Scaffolding protein IQGAP1 is dispensable,
but its overexpression promotes hepatocellular carcinoma via YAP1
signaling. Mol Cell Biol. 41:e00596–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li K, Zhang J, Lyu H, Yang J, Wei W, Wang
Y, Luo H, Zhang Y, Jiang X, Yi H, et al: CSN6-SPOP-HMGCS1 axis
promotes hepatocellular carcinoma progression via YAP1 activation.
Adv Sci (Weinh). 2:e23068272024. View Article : Google Scholar
|
|
81
|
Petty AJ and Yang Y: Tumor-associated
macrophages: Implications in cancer immunotherapy. Immunotherapy.
9:289–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Z, Wu T, Zheng B and Chen L:
Individualized precision treatment: Targeting TAM in HCC. Cancer
Lett. 458:86–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pu J, Xu Z, Nian J, Fang Q, Yang M, Huang
Y, Li W, Ge B, Wang J and Wei H: M2 macrophage-derived
extracellular vesicles facilitate CD8+T cell exhaustion in
hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin
pathway. Cell Death Discov. 7:1822021. View Article : Google Scholar
|
|
84
|
Xu X, Wang B, Liu Y, Jing T, Xu G, Zhang
L, Jiao K, Chen Z, Xiang L, Xu C, et al: ETV4 potentiates nuclear
YAP retention and activities to enhance the progression of
hepatocellular carcinoma. Cancer Lett. 537:2156402022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhao X, Wang X, You Y, Wen D, Feng Z, Zhou
Y, Que K, Gong J and Liu Z: Nogo-B fosters HCC progression by
enhancing Yap/Taz-mediated tumor-associated macrophages M2
polarization. Exp Cell Res. 391:1119792020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhou TY, Zhou YL, Qian MJ, Fang YZ, Ye S,
Xin WX, Yang XC and Wu HH: Interleukin-6 induced by YAP in
hepatocellular carcinoma cells recruits tumor-associated
macrophages. J Pharmacol Sci. 138:89–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai
X, Ji X, Ji F, Gong XG, Li L, et al: Single tumor-initiating cells
evade immune clearance by recruiting type II macrophages. Genes
Dev. 31:247–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhou Y, Zheng S, Guo Q, Wei N, Xiao Z and
Song Y: Upregulation of RACGAP1 is correlated with poor prognosis
and immune infiltration in hepatocellular carcinoma. Transl Cancer
Res. 13:847–863. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Desideri E, Castelli S, Dorard C, Toifl S,
Grazi GL, Ciriolo MR and Baccarini M: Impaired degradation of YAP1
and IL6ST by chaperone-mediated autophagy promotes proliferation
and migration of normal and hepatocellular carcinoma cells.
Autophagy. 19:152–162. 2023. View Article : Google Scholar :
|
|
90
|
Lefort S, Joffre C, Kieffer Y, Givel AM,
Bourachot B, Zago G, Bieche I, Dubois T, Meseure D, Vincent-Salomon
A, et al: Inhibition of autophagy as a new means of improving
chemotherapy efficiency in high-LC3B triple-negative breast
cancers. Autophagy. 10:2122–2142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang CZ, Yan GX, Dong DS, Xin H and Liu
ZY: LncRNA-ATB promotes autophagy by activating yes-associated
protein and inducing autophagy-related protein 5 expression in
hepatocellular carcinoma. World J Gastroenterol. 25:5310–5322.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang L, Zhu Z, Han L, Zhao L, Weng J, Yang
H, Wu S, Chen K, Wu L and Chen T: A curcumin derivative, WZ35,
suppresses hepatocellular cancer cell growth via downregulating
YAP-mediated autophagy. Food Funct. 10:3748–3757. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gao Y, Peng Q, Li S, Zheng K, Gong Y, Xue
Y, Liu Y, Lu J, Zhang Y and Shi X: YAP1 suppression inhibits
autophagy and improves the efficacy of anti-PD-1 immunotherapy in
hepatocellular carcinoma. Exp Cell Res. 424:1134862023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yan Z, Guo D, Tao R, Yu X, Zhang J, He Y,
Zhang J, Li J, Zhang S and Guo W: Fluid shear stress induces cell
migration via RhoA-YAP1-autophagy pathway in liver cancer stem
cells. Cell Adh Migr. 16:94–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Carter SL, Eklund AC, Kohane IS, Harris LN
and Szallasi Z: A signature of chromosomal instability inferred
from gene expression profiles predicts clinical outcome in multiple
human cancers. Nat Genet. 38:1043–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Weiler SME, Pinna F, Wolf T, Lutz T,
Geldiyev A, Sticht C, Knaub M, Thomann S, Bissinger M, Wan S, et
al: Induction of chromosome instability by activation of
yes-associated protein and forkhead box M1 in liver cancer.
Gastroenterology. 152:2037–2051.e22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang S, Chen Q, Liu Q, Li Y, Sun X, Hong
L, Ji S, Liu C, Geng J, Zhang W, et al: Hippo signaling suppresses
cell ploidy and tumorigenesis through Skp2. Cancer Cell.
31:669–684.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li W, Dai Y, Shi B, Yue F, Zou J, Xu G,
Jiang X, Wang F, Zhou X and Liu L: LRPPRC sustains Yap-P27-mediated
cell ploidy and P62-HDAC6-mediated autophagy maturation and
suppresses genome instability and hepatocellular carcinomas.
Oncogene. 39:3879–3892. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang X, Sun F, Qiao Y, Zheng W, Liu Y,
Chen Y, Wu Q, Liu X, Zhu G, Chen Y, et al: TFCP2 is required for
YAP-dependent transcription to stimulate liver malignancy. Cell
Rep. 21:1227–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu S, Zhai M, Xiao W, Zhou Q, Zhang D,
Gong Y, Deng C, Liu C, Li L and He C: Intra-platelet serotonin and
YAP contributed to poor prognosis of hepatocellular carcinoma. Life
Sci. 270:1191402021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shu B, Zhai M, Miao X, He C, Deng C, Fang
Y, Luo M, Liu L and Liu S: Serotonin and YAP/VGLL4 balance
correlated with progression and poor prognosis of hepatocellular
carcinoma. Sci Rep. 8:97392018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sinha S, Aizawa S, Nakano Y, Rialdi A,
Choi HY, Shrestha R, Pan SQ, Chen Y, Li M, Kapelanski-Lamoureux A,
et al: Hepatic stellate cell stearoyl co-A desaturase activates
leukotriene B4 receptor 2-β-catenin cascade to promote liver
tumorigenesis. Nat Commun. 14:26512023. View Article : Google Scholar
|
|
103
|
Chen J, Zhang J, Tian W, Ge C, Su Y, Li J
and Tian H: AKR1C3 suppresses ferroptosis in hepatocellular
carcinoma through regulation of YAP/SLC7A11 signaling pathway. Mol
Carcinog. 62:833–844. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jin Y, Yang R, Ding J, Zhu F, Zhu C, Xu Q
and Cai J: KAT6A is associated with sorafenib resistance and
contributes to progression of hepatocellular carcinoma by targeting
YAP. Biochem Biophys Res Commun. 585:185–190. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hata S, Hirayama J, Kajiho H, Nakagawa K,
Hata Y, Katada T, Furutani-Seiki M and Nishina H: A novel
acetylation cycle of transcription co-activator yes-associated
protein that is downstream of Hippo pathway is triggered in
response to SN2 alkylating agents. J Biol Chem. 287:22089–22098.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang J, Tang X, Weng W, Qiao Y, Lin J, Liu
W, Liu R, Ma L, Yu W, Yu Y, et al: The membrane protein melanoma
cell adhesion molecule (MCAM) is a novel tumor marker that
stimulates tumorigenesis in hepatocellular carcinoma. Oncogene.
34:5781–5795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang Y, Cui R, Zhang X, Qiao Y, Liu X,
Chang Y, Yu Y, Sun F and Wang J: SIRT1 increases YAP- and
MKK3-dependent p38 phosphorylation in mouse liver and human
hepatocellular carcinoma. Oncotarget. 7:11284–11298. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan
F, Meng S, Wang Y, Yuan Z and Bi W: SIRT1 regulates YAP2-mediated
cell proliferation and chemoresistance in hepatocellular carcinoma.
Oncogene. 33:1468–1474. 2014. View Article : Google Scholar
|
|
109
|
Wu J, Chai H, Li F, Ren Q and Gu Y: SETD1A
augments sorafenib primary resistance via activating YAP in
hepatocellular carcinoma. Life Sci. 260:1184062020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen S, Wu H, Wang Z, Jia M, Guo J, Jin J,
Li X, Meng D, Lin L, He AR, et al: Loss of SPTBN1 suppresses
autophagy Via SETD7-mediated YAP methylation in hepatocellular
carcinoma initiation and development. Cell Mol Gastroenterol
Hepatol. 13:949–973.e7. 2022. View Article : Google Scholar :
|
|
111
|
Xu B, Li SH, Zheng R, Gao SB, Ding LH, Yin
ZY, Lin X, Feng ZJ, Zhang S, Wang XM and Jin GH: Menin promotes
hepatocellular carcinogenesis and epigenetically up-regulates Yap1
transcription. Proc Natl Acad Sci USA. 110:17480–17485. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wu BK, Mei SC, Chen EH, Zheng Y and Pan D:
YAP induces an oncogenic transcriptional program through
TET1-mediated epigenetic remodeling in liver growth and
tumorigenesis. Nat Genet. 54:1202–1213. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dong X, Zhang X, Liu P, Tian Y, Li L and
Gong P: Lipolysis-stimulated lipoprotein receptor impairs
hepatocellular carcinoma and inhibits the oncogenic activity of
YAP1 via PPPY motif. Front Oncol. 12:8964122022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jeon Y, Yoo JE, Rhee H, Kim YJ, Il Kim G,
Chung T, Yoon S, Shin B, Woo HG and Park YN: YAP inactivation in
estrogen receptor alpha-positive hepatocellular carcinoma with less
aggressive behavior. Exp Mol Med. 53:1055–1067. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu
X, Zhu G, Zhao Y, Chen Y, Yu Y, et al: The essential role of YAP
O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat
Commun. 8:152802017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Simile MM, Latte G, Demartis MI, Brozzetti
S, Calvisi DF, Porcu A, Feo CF, Seddaiu MA, Daino L, Berasain C, et
al: Post-translational deregulation of YAP1 is genetically
controlled in rat liver cancer and determines the fate and
stem-like behavior of the human disease. Oncotarget. 7:49194–49216.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Qiu Y, Huang D, Sheng Y, Huang J, Li N,
Zhang S, Hong Z, Yin X and Yan J: Deubiquitinating enzyme USP46
suppresses the progression of hepatocellular carcinoma by
stabilizing MST1. Exp Cell Res. 405:1126462021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhu H, Yan F, Yuan T, Qian M, Zhou T, Dai
X, Cao J, Ying M, Dong X, He Q and Yang B: USP10 promotes
proliferation of hepatocellular carcinoma by deubiquitinating and
stabilizing YAP/TAZ. Cancer Res. 80:2204–2216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tian Z, Xu C, He W, Lin Z, Zhang W, Tao K,
Ding R, Zhang X and Dou K: The deubiquitinating enzyme USP19
facilitates hepatocellular carcinoma progression through
stabilizing YAP. Cancer Lett. 577:2164392023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang S, Xu Y, Xie C, Ren L, Wu G, Yang M,
Wu X, Tang M, Hu Y, Li Z, et al: RNF219/α-catenin/LGALS3 axis
promotes hepatocellular carcinoma bone metastasis and associated
skeletal complications. Adv Sci (Weinh). 8:20019612020. View Article : Google Scholar
|
|
121
|
Wang J, Park JS, Wei Y, Rajurkar M, Cotton
JL, Fan Q, Lewis BC, Ji H and Mao J: TRIB2 acts downstream of
Wnt/TCF in liver cancer cells to regulate YAP and C/EBPα function.
Mol Cell. 51:211–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liu Y, Zhang X, Lin J, Chen Y, Qiao Y, Guo
S, Yang Y, Zhu G, Pan Q, Wang J and Sun F: CCT3 acts upstream of
YAP and TFCP2 as a potential target and tumour biomarker in liver
cancer. Cell Death Dis. 10:6442019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C,
Yao Y and Liu Q: Fbxw7 is an independent prognostic marker and
induces apoptosis and growth arrest by regulating YAP abundance in
hepatocellular carcinoma. Mol Cancer. 13:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim
Y, Dahlman J, Kim H, Park O, Ishitani T, Jho EH, Gao B and Yang Y:
Hippo signaling interactions with Wnt/β-catenin and Notch signaling
repress liver tumorigenesis. J Clin Invest. 127:137–152. 2017.
View Article : Google Scholar
|
|
125
|
Conboy CB, Vélez-Reyes GL, Tschida BR, Hu
H, Kaufmann G, Koes N, Keller B, Alsinet C, Cornellà H, Pinyol R,
et al: R-spondin 2 drives liver tumor development in a
yes-associated protein-dependent manner. Hepatol Commun.
3:1496–1509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Min Q, Molina L, Li J, Adebayo Michael AO,
Russell JO, Preziosi ME, Singh S, Poddar M, Matz-Soja M,
Ranganathan S, et al: β-Catenin and yes-associated protein 1
cooperate in hepatoblastoma pathogenesis. Am J Pathol.
189:1091–1104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lu S, Jiang M, Chen Q, Luo X, Cao Z, Huang
H, Zheng M and Du J: Upregulated YAP promotes oncogenic CTNNB1
expression contributing to molecular pathology of hepatoblastoma.
Pediatr Blood Cancer. 69:e297052022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Tao J, Calvisi DF, Ranganathan S, Cigliano
A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger
S, et al: Activation of β-catenin and Yap1 in human hepatoblastoma
and induction of hepatocarcinogenesis in mice. Gastroenterology.
147:690–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bisso A, Filipuzzi M, Gamarra Figueroa GP,
Brumana G, Biagioni F, Doni M, Ceccotti G, Tanaskovic N, Morelli
MJ, Pendino V, et al: Cooperation between MYC and β-catenin in
liver tumorigenesis requires Yap/Taz. Hepatology. 72:1430–1443.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang J, Yu H, Dong W, Zhang C, Hu M, Ma W,
Jiang X, Li H, Yang P and Xiang D: N6-methyladenosine-mediated
up-regulation of FZD10 regulates liver cancer stem cells'
properties and lenvatinib resistance through WNT/β-catenin and
Hippo signaling pathways. Gastroenterology. 164:990–1005. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xiang D, Zhu X, Zhang Y, Zou J, Li J, Kong
L and Zhang H: Tribbles homolog 2 promotes hepatic fibrosis and
hepatocarcinogenesis through phosphatase 1A-mediated stabilization
of yes-associated protein. Liver Int. 41:1131–1147. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang Q, Feng J and Tang L: Non-coding RNA
related to MAPK signaling pathway in liver cancer. Int J Mol Sci.
23:119082022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li S, Dai M, Wang F, Hao L, Feng C, Jia Y,
Li Y, Kang X, Hu X and Yan H: PD-1/PD-L1 interaction upregulates
YAP1 expression in HepG2 cells through MAPK/ERK pathway. Nat Prod
Commun. 18:1–12. 2023.
|
|
134
|
Li L, Wang J, Zhang Y, Zhang Y, Ma L, Weng
W, Qiao Y, Xiao W, Wang H, Yu W, et al: MEK1 promotes YAP and their
interaction is critical for tumorigenesis in liver cancer. FEBS
Lett. 587:3921–3927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Leung CON, Yang Y, Leung RWH, So KKH, Guo
HJ, Lei MML, Muliawan GK, Gao Y, Yu QQ, Yun JP, et al:
Broad-spectrum kinome profiling identifies CDK6 upregulation as a
driver of lenvatinib resistance in hepatocellular carcinoma. Nat
Commun. 14:66992023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li X, Tao J, Cigliano A, Sini M, Calderaro
J, Azoulay D, Wang C, Liu Y, Jiang L, Evert K, et al: Co-activation
of PIK3CA and Yap promotes development of hepatocellular and
cholangiocellular tumors in mouse and human liver. Oncotarget.
6:10102–10115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ma L, Wang J, Lin J, Pan Q, Yu Y and Sun
F: Cluster of differentiation 166 (CD166) regulated by
phosphatidylinositide 3-Kinase (PI3K)/AKT signaling to exert its
anti-apoptotic role via yes-associated protein (YAP) in liver
cancer. J Biol Chem. 289:6921–6933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fan Z, Xia H, Xu H, Ma J, Zhou S, Hou W,
Tang Q, Gong Q, Nie Y and Bi F: Standard CD44 modulates YAP1
through a positive feedback loop in hepatocellular carcinoma.
Biomed Pharmacother. 103:147–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Cho DC: Targeting the PI3K/Akt/mTOR
pathway in malignancy: Rationale and clinical outlook. BioDrugs.
28:373–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zhang S, Zhou YF, Cao J, Burley SK, Wang
HY and Zheng XFS: mTORC1 promotes ARID1A degradation and oncogenic
chromatin remodeling in hepatocellular carcinoma. Cancer Res.
81:5652–5665. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Niu Q, Ye S, Zhao L, Qian Y and Liu F: The
role of liver cancer stem cells in hepatocellular carcinoma
metastasis. Cancer Biol Ther. 25:23217682024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yoshida J, Ishikawa T, Endo Y, Matsumura
S, Ota T, Mizushima K, Hirai Y, Oka K, Okayama T, Sakamoto N, et
al: Metformin inhibits TGF-β1-induced epithelial-mesenchymal
transition and liver metastasis of pancreatic cancer cells. Oncol
Rep. 44:371–381. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ou H, Chen Z, Xiang L, Fang Y, Xu Y, Liu
Q, Hu Z, Li X, Huang Y and Yang D: Frizzled 2-induced
epithelial-mesenchymal transition correlates with vasculogenic
mimicry, stemness, and Hippo signaling in hepatocellular carcinoma.
Cancer Sci. 110:1169–1182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Sun B, Zhong FJ, Xu C, Li YM, Zhao YR, Cao
MM and Yang LY: Programmed cell death 10 promotes metastasis and
epithelial-mesenchymal transition of hepatocellular carcinoma via
PP2Ac-mediated YAP activation. Cell Death Dis. 12:8492021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hu H, Xu L, Luo SJ, Xiang T, Chen Y, Cao
ZR, Zhang YJ, Mo Z, Wang Y, Meng DF, et al: Retinal dehydrogenase 5
(RHD5) attenuates metastasis via regulating HIPPO/YAP signaling
pathway in hepatocellular carcinoma. Int J Med Sci. 17:1897–1908.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Xu M, Wang J, Xu Z, Li R, Wang P, Shang R,
Cigliano A, Ribback S, Solinas A, Pes GM, et al: SNAI1 promotes the
cholangiocellular phenotype, but not epithelial-mesenchymal
transition, in a murine hepatocellular carcinoma model. Cancer Res.
79:5563–5574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Tschaharganeh DF, Chen X, Latzko P, Malz
M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al:
Yes-associated protein up-regulates Jagged-1 and activates the
Notch pathway in human hepatocellular carcinoma. Gastroenterology.
144:1530–1542.e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Huang J, Zhang L, Wan D, Zhou L, Zheng S,
Lin S and Qiao Y: Extracellular matrix and its therapeutic
potential for cancer treatment. Signal Transduct Target Ther.
6:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chakraborty S, Njah K, Pobbati AV, Lim YB,
Raju A, Lakshmanan M, Tergaonkar V, Lim CT and Hong W: Agrin as a
mechanotransduction signal regulating YAP through the Hippo
pathway. Cell Rep. 18:2464–2479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Xiong YX, Zhang XC, Zhu JH, Zhang YX, Pan
YL, Wu Y, Zhao JP, Liu JJ, Lu YX, Liang HF, et al: Collagen I-DDR1
signaling promotes hepatocellular carcinoma cell stemness via Hippo
signaling repression. Cell Death Differ. 30:1648–1665. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Xia H, Dai X, Yu H, Zhou S, Fan Z, Wei G,
Tang Q, Gong Q and Bi F: EGFR-PI3K-PDK1 pathway regulates YAP
signaling in hepatocellular carcinoma: The mechanism and its
implications in targeted therapy. Cell Death Dis. 9:2692018.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Meng Y, Zhao Q, An L, Jiao S, Li R, Sang
Y, Liao J, Nie P, Wen F, Ju J, et al: A TNFR2-hnRNPK axis promotes
primary liver cancer development via activation of YAP signaling in
hepatic progenitor cells. Cancer Res. 81:3036–3050. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ahmed MB, Alghamdi AAA, Islam SU, Lee JS
and Lee YS: cAMP signaling in cancer: A PKA-CREB and EPAC-centric
approach. Cells. 11:20202022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ren H, Chen Y, Ao Z, Cheng Q, Yang X, Tao
H, Zhao L, Shen A, Li P and Fu Q: PDE4D binds and interacts with
YAP to cooperatively promote HCC progression. Cancer Lett.
541:2157492022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hu CT, Wu JR, Cheng CC and Wu WS: The
therapeutic targeting of HGF/c-Met signaling in hepatocellular
carcinoma: Alternative approaches. Cancers (Basel). 9:582017.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
He Q, Lin Z, Wang Z, Huang W, Tian D, Liu
M and Xia L: SIX4 promotes hepatocellular carcinoma metastasis
through upregulating YAP1 and c-MET. Oncogene. 39:7279–7295. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Xu C, Liu C, Huang W, Tu S and Wan F:
Effect of Mst1 overexpression on the growth of human hepatocellular
carcinoma HepG2 cells and the sensitivity to cisplatin in vitro.
Acta Biochim Biophys Sin (Shanghai). 45:268–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zhou D, Conrad C, Xia F, Park JS, Payer B,
Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J and Bardeesy N: Mst1
and Mst2 maintain hepatocyte quiescence and suppress hepatocellular
carcinoma development through inactivation of the Yap1 oncogene.
Cancer Cell. 16:425–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Jiang J, Zheng Y, Chen F, Dong L and Guo
X: Activation of YAP1 by STK25 contributes to the progression of
hepatocellular carcinoma. Tissue Cell. 76:1017972022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wong KF, Liu AM, Hong W, Xu Z and Luk JM:
Integrin α2β1 inhibits MST1 kinase phosphorylation and activates
yes-associated protein oncogenic signaling in hepatocellular
carcinoma. Oncotarget. 7:77683–77695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shu B, Zhou Y, Lei G, Peng Y, Ding C, Li Z
and He C: TRIM21 is critical in regulating hepatocellular carcinoma
growth and response to therapy by altering the MST1/YAP pathway.
Cancer Sci. 115:1476–1491. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Gu Y, Ding C, Yu T, Liu B, Tang W, Wang Z,
Tang X, Liang G, Peng J, Zhang X and Li Z: SIRT7 promotes Hippo/YAP
activation and cancer cell proliferation in hepatocellular
carcinoma via suppressing MST1. Cancer Sci. 115:1209–1223. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhu J, Tang W, Fang P, Wang C, Gu M, Yang
W, Pan B, Wang B and Guo W: STRN3 promotes tumour growth in
hepatocellular carcinoma by inhibiting the hippo pathway. J Cell
Mol Med. 28:e181472024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wang C, Zhu ZM, Liu CL, He XJ, Zhang HY
and Dong JH: Knockdown of yes-associated protein inhibits
proliferation and downregulates large tumor suppressor 1 expression
in MHCC97H human hepatocellular carcinoma cells. Mol Med Rep.
11:4101–4108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Guo C, Wang X and Liang L: LATS2-mediated
YAP1 phosphorylation is involved in HCC tumorigenesis. Int J Clin
Exp Pathol. 8:1690–1697. 2015.PubMed/NCBI
|
|
166
|
Xu W, Gong F, Zhang T, Chi B and Wang J:
RNA-binding protein Dnd1 inhibits epithelial-mesenchymal transition
and cancer stem cell-related traits on hepatocellular carcinoma
cells. Biotechnol Lett. 39:1359–1367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhang X, Fan Q, Li Y, Yang Z, Yang L, Zong
Z, Wang B, Meng X, Li Q, Liu J and Li H: Transforming growth
factor-beta1 suppresses hepatocellular carcinoma proliferation via
activation of Hippo signaling. Oncotarget. 8:29785–29794. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhang Y, Yan S, Chen J, Gan C, Chen D, Li
Y, Wen J, Kremerskothen J, Chen S, Zhang J and Cao Y: WWC2 is an
independent prognostic factor and prevents invasion via Hippo
signalling in hepatocellular carcinoma. J Cell Mol Med.
21:3718–3729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Huang Z, Zhou JK, Wang K, Chen H, Qin S,
Liu J, Luo M, Chen Y, Jiang J, Zhou L, et al: PDLIM1 inhibits tumor
metastasis through activating Hippo signaling in hepatocellular
carcinoma. Hepatology. 71:1643–1659. 2020. View Article : Google Scholar
|
|
170
|
Chen Q, Zhou XW, Zhang AJ and He K: ACTN1
supports tumor growth by inhibiting Hippo signaling in
hepatocellular carcinoma. J Exp Clin Cancer Res. 40:232021.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Cheng Y, Hou T, Ping J, Chen T and Yin B:
LMO3 promotes hepatocellular carcinoma invasion, metastasis and
anoikis inhibition by directly interacting with LATS1 and
suppressing Hippo signaling. J Exp Clin Cancer Res. 37:2282018.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Yan YC, Meng GX, Yang CC, Yang YF, Tan SY,
Yan LJ, Ding ZN, Ma YL, Dong ZR and Li T: Diacylglycerol lipase
alpha promotes hepatocellular carcinoma progression and induces
lenvatinib resistance by enhancing YAP activity. Cell Death Dis.
14:4042023. View Article : Google Scholar
|
|
173
|
Jeong SH, Kim HB, Kim MC, Lee JM, Lee JH,
Kim JH, Kim JW, Park WY, Kim SY, Kim JB, et al: Hippo-mediated
suppression of IRS2/AKT signaling prevents hepatic steatosis and
liver cancer. J Clin Invest. 128:1010–1025. 2018. View Article : Google Scholar
|
|
174
|
Lee KP, Lee JH, Kim TS, Kim TH, Park HD,
Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM and Lim DS: The
Hippo-Salvador pathway restrains hepatic oval cell proliferation,
liver size, and liver tumorigenesis. Proc Natl Acad Sci USA.
107:8248–8253. 2010. View Article : Google Scholar
|
|
175
|
Adler JJ, Johnson DE, Heller BL, Bringman
LR, Ranahan WP, Conwell MD, Sun Y, Hudmon A and Wells CD: Serum
deprivation inhibits the transcriptional co-activator YAP and cell
growth via phosphorylation of the 130-kDa isoform of Angiomotin by
the LATS1/2 protein kinases. Proc Natl Acad Sci USA.
110:17368–17373. 2013. View Article : Google Scholar
|
|
176
|
Liu Y, Lu Z, Shi Y and Sun F: AMOT is
required for YAP function in high glucose induced liver malignancy.
Biochem Biophys Res Commun. 495:1555–1561. 2018. View Article : Google Scholar
|
|
177
|
Liu M, Jiang K, Lin G, Liu P, Yan Y, Ye T,
Yao G, Barr MP, Liang D, Wang Y, et al: Ajuba inhibits
hepatocellular carcinoma cell growth via targeting of β-catenin and
YAP signaling and is regulated by E3 ligase Hakai through
neddylation. J Exp Clin Cancer Res. 37:1652018. View Article : Google Scholar
|
|
178
|
Wang Y, Zhu Y, Gu Y, Ma M, Wang Y, Qi S,
Zeng Y, Zhu R, Wang X, Yu P, et al: Stabilization of Motin family
proteins in NF2-deficient cells prevents full activation of YAP/TAZ
and rapid tumorigenesis. Cell Rep. 36:1095962021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yi C, Shen Z, Stemmer-Rachamimov A, Dawany
N, Troutman S, Showe LC, Liu Q, Shimono A, Sudol M, Holmgren L, et
al: The p130 isoform of angiomotin is required for Yap-mediated
hepatic epithelial cell proliferation and tumorigenesis. Sci
Signal. 6:ra772013. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zhang J, Liu P, Tao J, Wang P, Zhang Y,
Song X, Che L, Sumazin P, Ribback S, Kiss A, et al: TEA domain
transcription factor 4 is the major mediator of yes-associated
protein oncogenic activity in mouse and human hepatoblastoma. Am J
Pathol. 189:1077–1090. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Luo W, Li Y, Zeng Y, Li Y, Cheng M, Zhang
C, Li F, Wu Y, Huang C, Yang X, et al: Tea domain transcription
factor TEAD4 mitigates TGF-β signaling and hepatocellular carcinoma
progression independently of YAP. J Mol Cell Biol. 15:mjad0102023.
View Article : Google Scholar
|
|
182
|
Cai WY, Lin LY, Hao H, Zhang SM, Ma F,
Hong XX, Zhang H, Liu QF, Ye GD, Sun GB, et al: Yes-associated
protein/TEA domain family member and hepatocyte nuclear factor
4-alpha (HNF4α) repress reciprocally to regulate
hepatocarcinogenesis in rats and mice. Hepatology. 65:1206–1221.
2017. View Article : Google Scholar
|
|
183
|
Zhu C, Wei Y and Wei X: AXL receptor
tyrosine kinase as a promising anti-cancer approach: Functions,
molecular mechanisms and clinical applications. Mol Cancer.
18:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST,
Chen J, Poon RT, Zender L, Lowe SW, Hong W and Luk JM: AXL receptor
kinase is a mediator of YAP-dependent oncogenic functions in
hepatocellular carcinoma. Oncogene. 30:1229–1240. 2011. View Article : Google Scholar
|
|
185
|
Shen YW, Zhou YD, Chen HZ, Luan X and
Zhang WD: Targeting CTGF in cancer: An emerging therapeutic
opportunity. Trends Cancer. 7:511–524. 2021. View Article : Google Scholar
|
|
186
|
Cheng JC, Wang EY, Yi Y, Thakur A, Tsai SH
and Hoodless PA: S1P stimulates proliferation by upregulating CTGF
expression through S1PR2-mediated YAP activation. Mol Cancer Res.
16:1543–1555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Dong Q, Fu L, Zhao Y, Xie C, Li Q and Wang
E: TNFAIP8 interacts with LATS1 and promotes aggressiveness through
regulation of Hippo pathway in hepatocellular carcinoma.
Oncotarget. 8:15689–15703. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Zhang C, Wei W, Tu S, Liang B, Li C, Li Y,
Luo W, Wu Y, Dai X, Wang Y, et al: Upregulation of CYR61 by TGF-β
and YAP signaling exerts a counter-suppression of hepatocellular
carcinoma. J Biol Chem. 21:1072082024. View Article : Google Scholar
|
|
189
|
Ahn EY, Kim JS, Kim GJ and Park YN:
RASSF1A-mediated regulation of AREG via the Hippo pathway in
hepatocellular carcinoma. Mol Cancer Res. 11:748–758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Sun X, Gao L, Chien HY, Li WC and Zhao J:
The regulation and function of the NUAK family. J Mol Endocrinol.
51:R15–R22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Yuan WC, Pepe-Mooney B, Galli GG, Dill MT,
Huang HT, Hao M, Wang Y, Liang H, Calogero RA and Camargo FD: NUAK2
is a critical YAP target in liver cancer. Nat Commun. 9:48342018.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Li S, Ji J, Zhang Z, Peng Q, Hao L, Guo Y,
Zhou W, Cui Q and Shi X: Cisplatin promotes the expression level of
PD-L1 in the microenvironment of hepatocellular carcinoma through
YAP1. Mol Cell Biochem. 475:79–91. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Fu J, McGrath NA, Lee J, Wang X, Brar G
and Xie C: Verteporfin synergizes the efficacy of anti-PD-1 in
cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 21:485–492.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Quan Y, Li Z, Zhu K and Liang J:
Transcatheter arterial chemoembolization combined with Hippo/YAP
inhibition significantly improve the survival of rats with
transplanted hepatocellular carcinoma. Lipids Health Dis.
20:742021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Pinyopornpanish K, Al-Yaman W, Butler RS,
Carey W, McCullough A and Romero-Marrero C: Chemopreventive effect
of statin on hepatocellular carcinoma in patients with nonalcoholic
steatohepatitis cirrhosis. Am J Gastroenterol. 116:2258–2269. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Benhammou JN, Qiao B, Ko A, Sinnett-Smith
J, Pisegna JR and Rozengurt E: Lipophilic statins inhibit YAP
coactivator transcriptional activity in HCC cells through
Rho-mediated modulation of actin cytoskeleton. Am J Physiol
Gastrointest Liver Physiol. 325:G239–G250. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Yang Y, Zhang Y, Cao J, Su Z, Li F, Zhang
P, Zhang B, Liu R, Zhang L, Xie J, et al: FGFR4 and EZH2 inhibitors
synergistically induce hepatocellular carcinoma apoptosis via
repressing YAP signaling. J Exp Clin Cancer Res. 42:962023.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Fukushima K, Takahashi K, Fukushima N,
Honoki K and Tsujiuchi T: Different effects of GPR120 and GPR40 on
cellular functions stimulated by
12-O-tetradecanoylphorbol-13-acetate in melanoma cells. Biochem
Biophys Res Commun. 475:25–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zhu G, Chen Y, Zhang X, Wu Q, Zhao Y, Chen
Y, Sun F, Qiao Y and Wang J: 12-O-Tetradecanoylphorbol-13-acetate
(TPA) is anti-tumorigenic in liver cancer cells via inhibiting YAP
through AMOT. Sci Rep. 7:449402017. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germline BRCA mutation. N Engl J Med. 379:753–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Jia Y, Jin H, Gao L, Yang X, Wang F, Ding
H, Chen A, Tan S, Zhang F, Shao J, et al: A novel lncRNA PLK4
up-regulated by talazoparib represses hepatocellular carcinoma
progression by promoting YAP-mediated cell senescence. J Cell Mol
Med. 24:5304–5316. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Tataranni T and Piccoli C: Dichloroacetate
(DCA) and cancer: An overview towards clinical applications. Oxid
Med Cell Longev. 2019:82010792019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Su D and Lin Z: Dichloroacetate attenuates
the stemness of hepatocellular carcinoma cells via promoting
nucleus-cytoplasm translocation of YAP. Environ Toxicol.
36:975–983. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Kappos L, Antel J, Comi G, Montalban X,
O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G and Radue EW;
FTY720 D2201 Study Group: Oral fingolimod (FTY720) for relapsing
multiple sclerosis. N Engl J Med. 355:1124–1140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Du J, Qian M, Yuan T, Zhang B, Chen X, An
N, He Q, Yang B, Ye S and Zhu H: Fingolimod exerts in vitro
anticancer activity against hepatocellular carcinoma cell lines via
YAP/TAZ suppression. Acta Pharm. 72:427–436. 2022. View Article : Google Scholar
|
|
207
|
Zhao D, Xia L, Geng W, Xu D, Zhong C,
Zhang J and Xia Q: Metformin suppresses interleukin-22 induced
hepatocellular carcinoma by upregulating Hippo signaling pathway. J
Gastroenterol Hepatol. 36:3469–3476. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Tian Y, Tang B, Wang C, Sun D, Zhang R,
Luo N, Han Z, Liang R, Gao Z and Wang L: Metformin mediates
resensitivity to 5-fluorouracil in hepatocellular carcinoma via the
suppression of YAP. Oncotarget. 7:46230–46241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Serafini P, Meckel K, Kelso M, Noonan K,
Califano J, Koch W, Dolcetti L, Bronte V and Borrello I:
Phosphodiesterase-5 inhibition augments endogenous antitumor
immunity by reducing myeloid-derived suppressor cell function. J
Exp Med. 203:2691–2702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kong D, Jiang Y, Miao X, Wu Z, Liu H and
Gong W: Tadalafil enhances the therapeutic efficacy of BET
inhibitors in hepatocellular carcinoma through activating Hippo
pathway. Biochim Biophys Acta Mol Basis Dis. 1867:1662672021.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Zhong Y, Qi H, Li X, An M, Shi Q and Qi J:
Tumor supernatant derived from hepatocellular carcinoma cells
treated with vincristine sulfate have therapeutic activity. Eur J
Pharm Sci. 155:1055572020. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Wu L, Pang Y, Qin G, Xi G, Wu S, Wang X
and Chen T: Farnesylthiosalicylic acid sensitizes hepatocarcinoma
cells to artemisinin derivatives. PLoS One. 12:e01718402017.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Li Y, Lu J, Chen Q, Han S, Shao H, Chen P,
Jin Q, Yang M, Shangguan F, Fei M, et al: Artemisinin suppresses
hepatocellular carcinoma cell growth, migration and invasion by
targeting cellular bioenergetics and Hippo-YAP signaling. Arch
Toxicol. 93:3367–3383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Hao L, Guo Y, Peng Q, Zhang Z, Ji J, Liu
Y, Xue Y, Li C, Zheng K and Shi X: Dihydroartemisinin reduced lipid
droplet deposition by YAP1 to promote the anti-PD-1 effect in
hepatocellular carcinoma. Phytomedicine. 96:1539132022. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Peng Q, Hao L, Guo Y, Zhang Z, Ji J, Xue
Y, Liu Y, Li C, Lu J and Shi X: Dihydroartemisinin inhibited the
Warburg effect through YAP1/SLC2A1 pathway in hepatocellular
carcinoma. J Nat Med. 77:28–40. 2023. View Article : Google Scholar
|
|
216
|
Guo Y, Peng Q, Hao L, Ji J, Zhang Z, Xue
Y, Liu Y, Gao Y, Li C and Shi X: Dihydroartemisinin promoted FXR
expression independent of YAP1 in hepatocellular carcinoma. FASEB
J. 36:e223612022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Gong Y, Peng Q, Gao Y, Yang J, Lu J, Zhang
Y, Yang Y, Liang H, Yue Y and Shi X: Dihydroartemisinin inhibited
interleukin-18 expression by decreasing YAP1 in hepatocellular
carcinoma cells. Acta Histochem. 125:1520402023. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Peng Q, Li S, Shi X, Guo Y, Hao L, Zhang
Z, Ji J, Zhao Y, Li C, Xue Y and Liu Y: Dihydroartemisinin broke
the tumor immunosuppressive microenvironment by inhibiting YAP1
expression to enhance anti-PD-1 efficacy. Phytother Res.
37:1740–1753. 2023. View Article : Google Scholar
|
|
219
|
Zhang Z, Shi X, Ji J, Guo Y, Peng Q, Hao
L, Xue Y, Liu Y, Li C, Lu J and Yu K: Dihydroartemisinin increased
the abundance of Akkermansia muciniphila by YAP1 depression that
sensitizes hepatocellular carcinoma to anti-PD-1 immunotherapy.
Front Med. 17:729–746. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Li J, Wang H and Wang L, Tan R, Zhu M,
Zhong X, Zhang Y, Chen B and Wang L: Decursin inhibits the growth
of HepG2 hepatocellular carcinoma cells via Hippo/YAP signaling
pathway. Phytother Res. 32:2456–2465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Li M, Chen J, Yu X, Xu S, Li D, Zheng Q
and Yin Y: Myricetin suppresses the propagation of hepatocellular
carcinoma via down-regulating expression of YAP. Cells. 8:3582019.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Zhao S, Xu K, Jiang R, Li DY, Guo XX, Zhou
P, Tang JF, Li LS, Zeng D, Hu L, et al: Evodiamine inhibits
proliferation and promotes apoptosis of hepatocellular carcinoma
cells via the Hippo-yes-associated protein signaling pathway. Life
Sci. 251:1174242020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wu K, Teng M, Zhou W, Lu F, Zhou Y, Zeng
J, Yang J, Liu X, Zhang Y, Ding Y and Shen W: Wogonin induces cell
cycle arrest and apoptosis of hepatocellular carcinoma cells by
activating Hippo signaling. Anticancer Agents Med Chem.
22:1551–1560. 2022. View Article : Google Scholar
|
|
224
|
Zhang J, Tong Y, Lu X, Dong F, Ma X, Yin
S, He Y, Liu Y, Liu Q and Fan D: A derivant of ginsenoside CK and
its inhibitory effect on hepatocellular carcinoma. Life Sci.
304:1206982022. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Zeng J, Xie H, Zhang ZL, Li ZX, Shi L, Wu
KY, Zhou Y, Tian Z, Zhang Y, Zhou W and Shen WG: Apigenin regulates
the migration, invasion, and autophagy of hepatocellular carcinoma
cells by downregulating YAP. Neoplasma. 69:292–302. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Yun UJ, Bae SJ, Song YR and Kim YW: A
critical YAP in malignancy of HCC is regulated by evodiamine. Int J
Mol Sci. 23:18552022. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Chen YL, Yen IC, Lin KT, Lai FY and Lee
SY: 4-Acetylantrocamol LT3, a new ubiquinone from Antrodia
cinnamomea, inhibits hepatocellular carcinoma HepG2 cell growth by
targeting YAP/TAZ, mTOR, and WNT/β-catenin signaling. Am J Chin
Med. 48:1243–1261. 2020. View Article : Google Scholar
|
|
228
|
Ma L, Jiang H, Xu X, Zhang C, Niu Y, Wang
Z, Tao Y, Li Y, Cai F, Zhang X, et al: Tanshinone IIA mediates
SMAD7-YAP interaction to inhibit liver cancer growth by
inactivating the transforming growth factor beta signaling pathway.
Aging (Albany NY). 11:9719–9737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Liu H, Li J, Yuan W, Hao S, Wang M, Wang F
and Xuan H: Bioactive components and mechanisms of poplar propolis
in inhibiting proliferation of human hepatocellular carcinoma HepG2
cells. Biomed Pharmacother. 144:1123642021. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Xu W, Shi Z, Yu X, Xu Y, Chen Y, He Y,
Gong Y, Huang C, Tan C and Yang Y: Salvianolic acid B exerts an
anti-hepatocellular carcinoma effect by regulating the Hippo/YAP
pathway and promoting pSmad3L to pSmad3C simultaneously. Eur J
Pharmacol. 939:1754232023. View Article : Google Scholar
|
|
231
|
Chang HL, Chen HA, Bamodu OA, Lee KF,
Tzeng YM, Lee WH and Tsai JT: Ovatodiolide suppresses
yes-associated protein 1-modulated cancer stem cell phenotypes in
highly malignant hepatocellular carcinoma and sensitizes cancer
cells to chemotherapy in vitro. Toxicol In Vitro. 51:74–82. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Jia M, Xiong Y, Li M and Mao Q: Corosolic
acid inhibits cancer progress through inactivating YAP in
hepatocellular carcinoma. Oncol Res. 28:371–383. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Xu Y, Zhao Y, Guan Y, Guan Y, Zhang X,
Chen Y, Wu Q, Zhu G, Chen Y, Sun F, et al: Blocking inhibition to
YAP by ActinomycinD enhances anti-tumor efficacy of corosolic acid
in treating liver cancer. Cell Signal. 29:209–217. 2017. View Article : Google Scholar
|
|
234
|
Wu Q, Liu J, Mao Z, Tian L, Wang N, Wang
G, Wang Y and Seto S: Ligustilide attenuates ischemic stroke injury
by promoting Drp1-mediated mitochondrial fission via activation of
AMPK. Phytomedicine. 95:1538842022. View Article : Google Scholar
|
|
235
|
Yang J and Xing Z: Ligustilide counteracts
carcinogenesis and hepatocellular carcinoma cell-evoked macrophage
M2 polarization by regulating yes-associated protein-mediated
interleukin-6 secretion. Exp Biol Med (Maywood). 246:1928–1937.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Song X, Tan L, Wang M, Ren C, Guo C, Yang
B, Ren Y, Cao Z, Li Y and Pei J: Myricetin: A review of the most
recent research. Biomed Pharmacother. 134:1110172021. View Article : Google Scholar
|
|
237
|
Kou JJ, Shi JZ, He YY, Hao JJ, Zhang HY,
Luo DM, Song JK, Yan Y, Xie XM, Du GH and Pang XB: Luteolin
alleviates cognitive impairment in Alzheimer's disease mouse model
via inhibiting endoplasmic reticulum stress-dependent
neuroinflammation. Acta Pharmacol Sin. 43:840–849. 2022. View Article : Google Scholar
|
|
238
|
Yang N, Chen T, Wang L, Liu R, Niu Y, Sun
L, Yao B, Wang Y, Yang W, Liu Q, et al: CXCR4 mediates matrix
stiffness-induced downregulation of UBTD1 driving hepatocellular
carcinoma progression via YAP signaling pathway. Theranostics.
10:5790–5801. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Xu G, Wang J, Wu F, Wang N, Zhou W, Wang
Q, Pan W, Ao G and Yang J: YAP and 14-3-3γ are involved in
HS-OA-induced growth inhibition of hepatocellular carcinoma cells:
A novel mechanism for hydrogen sulfide releasing oleanolic acid.
Oncotarget. 7:52150–52165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Wang X, Wang Y, Liu Z, Zhao H, Yao GD, Liu
Q and Song SJ: New daphnane diterpenoidal 1,3,4-oxdiazole
derivatives as potential anti-hepatoma agents: Synthesis,
biological evaluation and molecular modeling studies. Bioorg Chem.
145:1072082024. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Liu K, Wehling L, Wan S, Weiler SME, Tóth
M, Ibberson D, Marhenke S, Ali A, Lam M, Guo T, et al: Dynamic YAP
expression in the non-parenchymal liver cell compartment controls
heterologous cell communication. Cell Mol Life Sci. 81:1152024.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Gao Y, Feng J, Yang G, Zhang S, Liu Y, Bu
Y, Sun M, Zhao M, Chen F, Zhang W, et al: Hepatitis B virus X
protein-elevated MSL2 modulates hepatitis B virus covalently closed
circular DNA by inducing degradation of APOBEC3B to enhance
hepatocarcinogenesis. Hepatology. 66:1413–1429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Wang Y, Fang R, Cui M, Zhang W, Bai X,
Wang H, Liu B, Zhang X and Ye L: The oncoprotein HBXIP up-regulates
YAP through activation of transcription factor c-Myb to promote
growth of liver cancer. Cancer Lett. 385:234–242. 2017. View Article : Google Scholar
|
|
244
|
Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng
X, Zong Z, Sun X, Hua X and Li H: Yes-associated protein (YAP)
binds to HIF-1α and sustains HIF-1α protein stability to promote
hepatocellular carcinoma cell glycolysis under hypoxic stress. J
Exp Clin Cancer Res. 37:2162018. View Article : Google Scholar
|
|
245
|
Liu Y, Ren H, Zhou Y, Shang L, Zhang Y,
Yang F and Shi X: The hypoxia conditioned mesenchymal stem cells
promote hepatocellular carcinoma progression through YAP mediated
lipogenesis reprogramming. J Exp Clin Cancer Res. 38:2282019.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Yan B, Li T, Shen L, Zhou Z, Liu X, Wang X
and Sun X: Simultaneous knockdown of YAP and TAZ increases
apoptosis of hepatocellular carcinoma cells under hypoxic
condition. Biochem Biophys Res Commun. 515:275–281. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Fan W, Adebowale K, Váncza L, Li Y, Rabbi
MF, Kunimoto K, Chen D, Mozes G, Chiu DK, Li Y, et al: Matrix
viscoelasticity promotes liver cancer progression in the
pre-cirrhotic liver. Nature. 626:635–642. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Weiler SME, Bissinger M, Rose F, von
Bubnoff F, Lutz T, Ori A, Schirmacher P and Breuhahn K:
SEPTIN10-mediated crosstalk between cytoskeletal networks controls
mechanotransduction and oncogenic YAP/TAZ signaling. Cancer Lett.
584:2166372024. View Article : Google Scholar : PubMed/NCBI
|