Multidimensional analysis has indicated that a
special tumor microenvironment (TME) exists in liver cancer tissues
(1). The study of treatment
protocols for tumors in modern medicine from the perspective of
cancer biology no longer considers cancer cells as the central
link, but evolved from the study of cancer cells to the study of
the TME in which cancer cells are located, categorizing cancer
cells in the stromal cell network (2). The molecular mechanisms involved in
tumor development can be elucidated by analyzing the
characteristics of different cell types in the TME (3): Tumor-associated macrophages (TAMs),
CD4+ cells, cytotoxic T lymphocytes (CD8+
cells), regulatory T cells (Tregs), dendritic cells, natural killer
cells (NK cells), tumor-associated endothelial cells,
cancer-associated fibroblasts (CAFs) and myeloid-derived
immunosuppressive cells (MDSCs) (4). Kupffer cells (KCs) and
monocyte-derived hepatic macrophages are among the innate
immune-responsive cells that are widely present in the human body
(5). Macrophages exert
immunomodulatory effects through phagocytosis, exogenous antigen
presentation, and cytokine and growth factor secretion (6).
Macrophage polarization is a process that occurs in
response to changes in microenvironmental stimuli and signals.
Functionally polarized subpopulations acquire substantially
different functional attributes after the action of different
stimuli (7). Lipopolysaccharides
(LPS) and interferon-γ direct macrophage polarization towards the
M1 phenotype by activating the Janus kinase (JAK)/STAT, toll-like
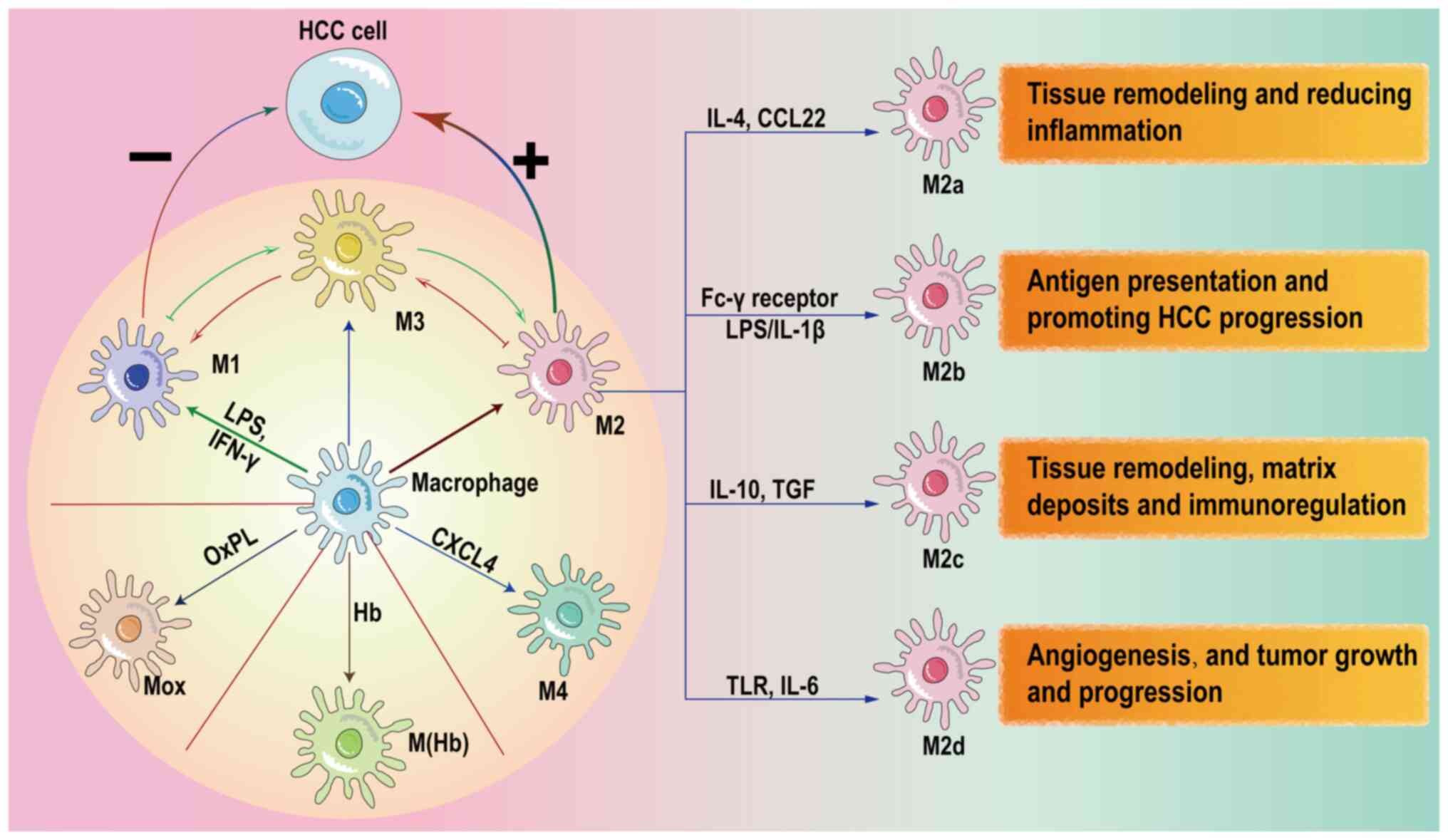
receptor 4 (TLR4)/NF-κB and NOTCH signaling pathways (8). The types of macrophage polarization
are classified into two groups according to their functions
(classically activated M1-polarized macrophages and alternatively
activated M2-polarized macrophages), with different expression
levels of proteins on the cell surface (9). Proinflammatory cytokines, including
IL-1β, IL-6, IL-12, IL-18, IFN-γ, inducible nitric oxide synthase
(iNOS) and TNF-α, are highly expressed in M1-polarized macrophages.
These cytokines also function as tumor suppressors in
hepatocellular carcinoma (HCC) (10). By contrast, M2 macrophages secrete
anti-inflammatory cytokines such as IL-4, TGF-β and IL-10 (11). M2 macrophages exert complex
immunomodulatory functions depending on these cytokines, which
possess cancer-promoting and inflammation-inhibiting effects by
triggering immunosuppression (12). M2 polarization is induced by
factors that activate the TGF-β/Smads and peroxisome
proliferator-activated receptor γ (PPARγ) signaling pathways, such
as TGF-β and growth differentiation factor 3 (13). M2 macrophages induced by diverse
stimulatory factors are further subdivided into four types: M2a,
M2b, M2c and M2d (14).
The M4 macrophage phenotype is induced by chemokine
C-X-C motif ligand (CXCL)4 in atherosclerosis, and characterized by
MMP7, CD68 and S100 calcium binding protein A8 expression (15). Hemoglobin and oxidized
phospholipids polarize M0 macrophages to hemoglobin-stimulated
macrophages and Mox macrophages (16). Mantovani et al (17) hypothesized that regulatory M3
macrophages are a bridge for the mutual transformation of M1 and M2
macrophages. Fig. 1 shows a
summary of the directions of macrophage polarization.
Hypoxic conditions in the HCC TME inhibit the
xanthine oxidoreductase (XOR)/isocitrate dehydrogenase (NAD (+)) 3
catalytic subunit α (IDH3α) axis of TAMs and increase the ratio of
M2 TAMs to total TAMs (18).
Hematopoietic stem cells (HSCs) are induced to differentiate into
CAFs in HCC (4). The expression
levels of IL-6, which is associated with the polarization of the M0
cell phenotype to the M2 phenotype, are increased in
CAFs-conditioned medium compared with HSCs. In addition, high
levels of CXCL12 expression by CAFs regulate the expression of
plasminogen activator (PA) inhibitor-1 (PAI-1) in polarized M2 TAMs
by specifically binding to C-X-C chemokine receptor type 4 (CXCR4)
on the macrophage surface (19).
Angiopoietin (Ang)-like protein 8 (ANGPTL8) is expressed in HCC
cells and ANGPTL8 expression is positively associated with the
degree of malignancy of HCC. The interaction of ANGPTL8 with the
leukocyte Ig-like receptor subfamily B/paired Ig-like receptor
results in the inhibition of the M1 polarization and promotes the
polarization towards the M2 phenotype of TAMs (20). A related study using HCC as a
model has indicated that iron restriction due to HCC activates TAM
polarization towards the M2 phenotype. TAMs in HCC exhibit
upregulated expression levels of the apolipoprotein C1 (APOC1)
gene. APOC1 inhibition activates apoptosis in TAMs, reverses the M2
phenotype to the M1 phenotype and alters the phenotypic proportion
of TAMs in HCC (21).
Metabolism-related RNA expression in HCC is an important regulator
of the activation of TAM polarization. Long non-coding RNAs
(lncRNAs/lncs), microRNAs (miRNAs/miRs) and circular RNAs
(circRNAs/circs) are regulated by gene expression to alter the
polarization of TAMs (22,23).
lncRNA is a type of RNA that is >200 nucleotides long and does
not encode proteins (24). ZNNT1
is a lncRNA, and its high expression is associated with the
infiltration of M2-polarized macrophages, promoting HCC cell
proliferation, migration and invasion, and inhibiting HCC cell
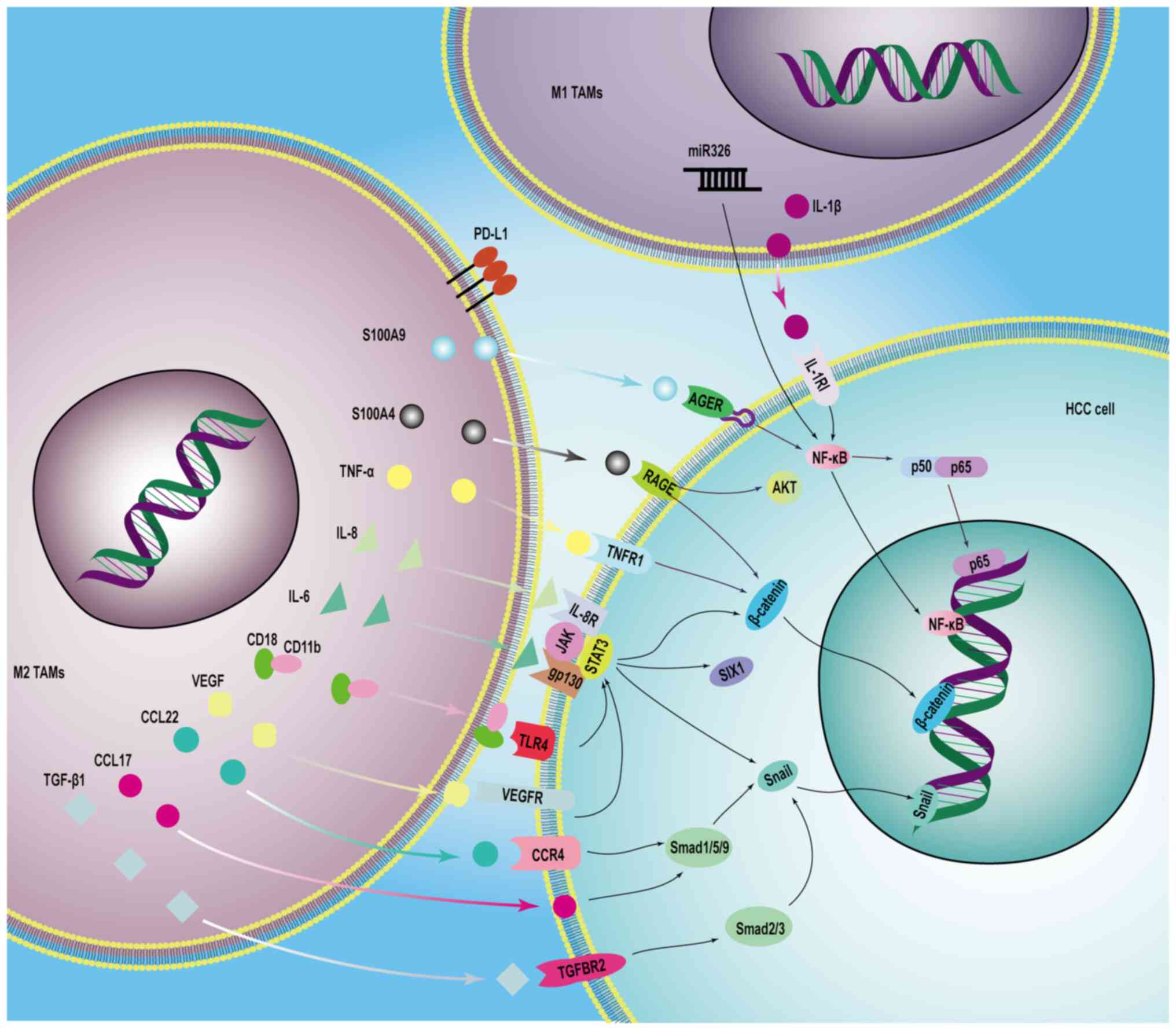
apoptosis (25). S100A9 secreted
by M2 macrophages upregulates ZNNT1 expression in HCC cells through
the activation of advanced glycosylation end-product specific
receptor (AGER)/NF-κB signaling, a process that is related to the
regulation of ZNNT1 transcript stability by m6A
modification (26). MEG3 lncRNA
is widely distributed in a range of solid tumors. Its silencing
promotes drug resistance in various malignant tumors (27,28). Human antigen R (HuR), also known
as embryonic lethal anomalous visual-like 1, is an RNA-binding
protein (29). Chemokine (C-C
motif) ligand (CCL)5, also known as RANTES, participates in
inflammatory and immune responses as a chemokine. HuR is a target
RNA of MEG3 and inhibits CCL5 expression in bone marrow-derived
macrophages by suppressing CCL5 transcription and promoting M2
macrophage polarization. MEG3 inhibits HuR expression in M1 and M2
macrophages, leading to a change in the direction of macrophage
polarization (30,31). Thus, MEG3 regulates HuR at the
post-transcriptional level in HCC, being a potential therapeutic
target for liver disease (32).
Furthermore, lncRNA MEG3 is linked to miR-145-5p in HCC cells, and
MEG3 overexpression leads to a decrease in miR-145-5p mRNA
expression. High miR-145-5p expression is positively associated
with M2 macrophage polarization. DAB adaptor protein 2 (DAB2) is a
downstream target of miR-145-5p, and DAB2 upregulation is a
hallmark of M1 polarization. MEG3 inhibits M2 macrophage
polarization through the upregulation of DAB2 (33). Insulin like growth factor 2 mRNA
binding protein 3 (IGF2BP3) is highly expressed in human solid
tumors and promotes the differentiation, proliferation, invasion
and metastasis of HCC cells (34). When highly expressed, it binds to
the mRNAs of CCL5 or TGF-β1 in HCC cells, promotes the secretion of
cytokines, and induces macrophage M2 polarization. IGF2BP3
knockdown combined with anti-CD47 therapy slows down tumor growth
(35). A combination of in
vivo and in vitro experiments revealed the role of
IGF2BP3 as a specific migration factor in HCC (36). Evidence indicates that circRNAs
absorb miRNAs via the 'sponge adsorption' effect and interact with
RNA-binding proteins. circ_0010882 is an upstream regulatory factor
of miR-382. circ_0010882 silencing inhibits M2 macrophage
polarization by targeting miR-382 (37). miR-21-5p in HCC cell exosomes
affects SP1/X-box binding protein 1 (XBP1) protein expression and
promotes M2 polarization in TAMs (38).
The upregulation of tripartite motif containing 65
(TRIM65) is associated with the high grading of HCC tumors
(39). The JAK1/STAT1 signaling
pathway is activated and the expression of pro-inflammatory M1
markers is increased in TAMs after culturing with conditioned
medium from the supernatant of Hep1-6 cells with TRIM65 knockdown.
This suggests that TRIM65 could serve as a unique tumor
immunotherapeutic target (40).
Although in vivo and in vitro experiments have
validated the therapeutic efficacy of targeting TRIM65 in the
treatment of HCC, there is no evidence that has associated the
effect of TRIM65 knockdown on HCC with its distribution in
macrophages (41). Zinc-fingers
and homeoboxes 2 is a regulator of a number of liver-enriched genes
and it maintains macrophage polarization by regulating interferon
regulatory factor (IRF)1 transcription, which could therefore be a
potential target for macrophage-based cancer immunotherapy
(42). CCL16 is a factor with
strong chemotactic properties. C-C chemokine receptor type 1 (CCR1)
is a receptor that positively regulates the extent of M2 TAM
infiltration in HCC. CCL16 produced by hepatocytes in the normal
liver binds to CCR1 expressed by human KCs. Cancer cells bind to
the CCR1 receptor on macrophages and release CCL16 to increase the
number of CD68+ CCR1+ cells, which induces M2
polarization in TAMs (43).
Auxiliary proteins are involved in IL-4-induced M2 polarization.
Major vault protein (MVP) is the main component in vault
nanoparticles. Its expression is increased in HCC compared with
that in the normal liver (44).
IL-4 activates the dimerization of IL-4 receptor (IL-4R) α and γ
chains by binding to the macrophage surface receptor IL-4R.
Activation of the JAK1/STAT6 signaling pathway increases the M2
polarized subtype of TAMs (45).
MVP interacts with JAK1 and recruits STAT6 to form the ternary
complex JAK1/MVP/STAT6, which enhances IL-4-induced STAT6 activity.
The degree of infiltration of M2 TAMs is increased in HCC with high
MVP expression (46). xCT
(encoded by SLC7A11) is a heavy chain subunit of the system x that
provides a nutrient environment for tumor development by reducing
glutathione biosynthesis and antioxidant defense in tumor cells.
xCT activates the suppressor of cytokine signaling 3/STAT6/PPARγ
signaling axis and regulates the pro-tumorigenic M2-like phenotypic
shift in the TME caused by IL-4 (47). Therefore, xCT knockdown in
macrophages inhibits the pro-tumorigenic phenotypic activation of
IL-4. In addition to IL-4, STAT6 mediates M2 cell polarization via
different mechanisms of activation (48). Myeloid differentiation factor 88
(MyD88) is a ligand for TLR and activates a pro-inflammatory
cascade, while it promotes CCL9/CCL15 secretion in HSCs, which
enhances M2 polarization in macrophages via the activation of the
STAT6/PPARβ signaling pathway in HCC cells (49). Specific deletion of MyD88 in
myofibroblasts reduces the secretion of CCL9, a macrophage
inflammatory protein, in nonalcoholic fatty liver
disease-associated HCC (50).
STAT3, another component of the STAT family, is also involved in
the regulation of TAM polarization in HCC (51). The proteasome subunit α (PSMA)
family of proteins mediates the synthesis of the 20S proteasome
core complex and is present in exosomes secreted by metastatic HCC
tissues (52). PSMA5 is a member
of the PSMA family of proteins, and differences in its expression
are critical for tumor growth and development. PSMA5 is activated
after the activation of JAK2 and JAK1 in TAMs (53). M2 macrophages receive exosomes
from HCC tissues (54).
Formimidoyltransferase cyclodeaminase (FTCD) mediates enzymatic
reactions and cellular biofilm ligation in tumor cells. As a
downstream target of hypoxia-inducible factor-1α (HIF-1α),
FTCD-stimulated macrophages are polarized toward the M1 type, and
M1 TAMs secrete pyruvate kinase M2 (PKM2) to inhibit HCC cell
proliferation (55). Macrophage
glucose metabolism is also a factor inducing M2 polarization in
TAMs in the TME. XOR mediates the regulation of α-ketoglutarate
(α-KG) synthesis and mediates the activation of an M1 promoter in
macrophages (NLR family containing pyrin structural domain 3),
leading to increased IL-1β expression (56). Tumor cells suppress XOR mRNA and
protein expression in macrophages. In macrophages, XOR binds to the
active structural domain of IDH3α. IDH3 is a regulatory enzyme in
the tricarboxylic acid cycle and is involved in the catalytic
synthesis of α-KG (57). XOR
knockdown in macrophages mediates the regulation of IDH3 activity,
leading to an increase in α-KG, and activation of
IDH3α/α-KG/jumonji domain containing 3 signaling, thus enhancing M2
polarization (18) There are
numerous factors that activate macrophage polarization in HCC.
Table I shows the classification
of polarization factors based on whether they also promote
macrophage differentiation in other tumor tissues.
Primary liver cancer includes HCC, intrahepatic
cholangiocarcinoma (iCCA) and combined HCC-CCA (cHCC-CCA).
IgGFc-binding protein+ secreted phosphoprotein 1
(SPP1)+ TAMs are mainly observed in primary HCC and are
commonly distributed in areas of HCC and iCCA, and the poorly
differentiated areas in cHCC-CCA. The necroptotic apoptotic
microenvironment is associated with HCC differentiation, and
IgGFc-binding protein+ SPP1+ TAMs promote HCC
to gradually acquire CCA features, which are important for
intrahepatic biliary metastasis of HCC cells (58).
CSCs in the TME are a type of self-renewing
undifferentiated cells with tumorigenic and stem cell-like
characteristics (59). CSCs are
essential for tumor development and migration (60). Stemness markers, including NANOG,
OCT4, SOX2, MYC oncogene and krüppel-like factor 4 (KLF4), are
essential transcription factors in the pluripotent stemness of CSCs
(61). M2 TAMs act as a 'HCC
stemness regulatory complex' by secreting VEGFA, integrin subunit
β3 binding protein and ADAM metallopeptidase domain 9 ligands
(62,63). The CXCL12-integrin subunit β1 and
VEGFA-integrin subunit αV pairs in M2 TAMs are involved in the
initiation of tumor cells and the stem-like properties of OV6 CSCs,
which are important assistants in the stemness of HCC (64).
TAMs produce IL-6, which promotes the expansion of
these CSCs and tumorigenesis (65). Exploiting the interaction between
TAMs and CSCs in HCC represents a novel approach for the
development of tumor suppressors. S100 calcium binding protein A4
(S100A4) and collagen I enhance HCC stem cell properties by relying
on the receptor for advanced glycation endproducts/β-catenin
signaling pathway. S100A4+ macrophages are a subtype of
M2 macrophages in HCC tissues (66). The stemness markers Oct-4, Nanog,
CD133, SOX2 and CD44 are upregulated in HuH7 liver cancer cells
after stimulation by S100A4. In vivo experiments suggest
that S100A4 knockdown in a mouse model of HCC decreases the level
of CSC markers (67). S100A9,
also belonging to the S100 family, is a common factor expressed in
exosomes of TAMs and CSCs. S100A9 stimulates CCL2 and tumor
stemness-related gene production via the AGER/NF-κB signaling
pathway in HepG2 and MHCC-97H cells. In addition to the effect of
S100A9 expressed by TAMs in malignancy development, S100A9 in the
TME serves a role in the recruitment and polarization of TAMs
(68).
A sharp drop in the size of subcutaneous tumors in a
tumor mouse model was observed after intracardiac injection of HCC
cells co-cultured with M2 TAMs. Furthermore, co-culture of HCC
cells with M2 TAMs resulted in a decrease in the expression levels
of characteristic stem cell surface markers of HCC cells, including
CD133, master transcription factors Oct-4 and SOX-2, N-cadherin,
and vimentin. The in vitro approach revealed that TNF-α
secreted by M2 TAMs activated the Wnt/β-catenin signaling pathways,
leading to epithelial-to-mesenchymal transition (EMT) and the
stemness features of HCC (69).
The properties of HCC cells depend on the
stimulation of various components in the TME, especially cytokines,
chemotactic cytokines and exosomes. IL-6 is a critical cytokine for
HCC cell proliferation (75).
There are numerous cells in the TME secreting the inflammatory
factor IL-6 (76). By contrast,
the scavenger receptor cysteine-rich type 1 protein M130 activates
CD163+ TAMs, a type of M2 TAMs expressing IL-6. This
effect in the HCC TME involves the IL-6/STAT3 signaling pathway
activated by M2 TAMs and their exosomes (77).
Autophagy protects the viability of cells and
maintains the steady state of cells (78). Macrophage autophagy has the dual
function of inhibiting and promoting tumor growth in the TME. The
PI3K/Akt/mTOR signaling pathway is a classical pathway in
autophagy. M2 TAMs are involved in the activation of this pathway
in HCC cells, resulting in the inhibition of M2 TAM autophagy
(79). miRNA-210 is an autophagy
agonist acting on the downregulation of PI3K/Akt/mTOR signaling in
macrophages, as a consequence of which, M2 TAMs increase the
expression levels of IL-10 and TGF-β1, promoting HCC cell
proliferation (80).
The proliferation of HCC cells is also associated
with the cell cycle. M2 TAMs induced by CAFs regulate the HCC cell
cycle directly by inducing PAI-1 expression. PAI-1 downregulates
FAS and Fas ligand, resulting in the decrease of the antiapoptotic
abilities of liver cancer cells and in the promotion of HCC
(19). Extracellular ubiquitin
(eUb) regulates cell apoptosis with the assistance of TAMs. eUb is
not a regulator of TAM activation and polarization. The absence of
TAMs affects the effect of eUb in regulating HCC cell apoptosis.
After inhibition by the pro-inflammatory factor TNF-α released by
M1 TAMs, HCC cells receive the signal from eUb. and activate the
Akt/mTOR signaling pathway and suppress apoptosis (81). In addition, large tumor suppressor
kinase 1 activates the Hippo signaling pathway in HCC cells. The
Hippo signaling pathway is critical for cell apoptosis and
mitochondrial damage (82).
Arsenate induces M2-type polarization of macrophages by inducing
miR-15b expression to inhibit HCC cell apoptosis activated by LAST1
(83). Upregulation of the
EZH2-associated lncRNA HEIH is associated with poor prognosis in
patients with HCC. HEIH promotes macrophage M2 polarization by
targeting the miR-98-5p/STAT3 axis, and promotes tumor growth and
metastasis induced by M2 TAMs (84). Lysosomal acid lipase (LAL)
inhibition leads to the accumulation of cellular lipid content and
reduces the expression levels of CD36 and ATP binding cassette
subfamily a member 1 in macrophages. LAL inhibition slows down
monocyte cholesterol metabolism, which reduces the number of M2
TAMs and decreases the HCC tumor growth-promoting axis in which M2
TAMs are involved (85).
M1 TAMs polarized by the signal NORCH-recombination
signal binding protein for immunoglobulin κJ region (RBPJ) release
exosomes with high RBPJ expression. Hsa-cir-004658 in
RBPJ+/+exosome secreted by M1 TAMs is involved in the
miR499b-5p/junctional adhesion molecule 3 signaling pathway and
promotes the apoptosis of liver cancer cells, while it inhibits the
increase in the number of HCC cells (86). Under the stimulation of hepatoma
cells, macrophages increase the expression of the classical M1 TAM
chemotactic cytokine interleukin-8 (CXCL8) (87). CXCL8 facilitates HCC cell
proliferation by changing the percentage of miRNA-17, which is a
potent proliferative molecule in the miRNA clusters (88). TAM cell glycolysis in HCC is
involved in the proliferative ability of HCC (89). The glycolytic enzymes
6-phosphofructo-2-kinase, hexokinase 2, triose-phosphate isomerase,
PKM2, glucose transporter type 1 and lactate dehydrogenase A
negatively regulate the apoptosis of H22 tumor cells. Dectin3, a
C-type lectin-like receptor, increases HCC cell proliferation by
activating cell glycolysis (90,91). HCC cells undergo circ
fucosyltransferase 8 (FUT8) m6A modification mediated by METTL14,
which promotes the translocation of circFUT8 to the cytoplasm and
the recognition of the specific protein YTH N6-methyladenosine RNA
binding protein C1, and positively regulates the tumor growth
cycle. M1 TAMs competitively bind cytoplasmic circFUT8 through the
exosomal miR-628-5p to inhibit HCC progression (92).
According to several studies, the polarized
orientation of TAMs in the TME is closely associated with the
invasive and metastatic ability of HCC cells through the regulation
of multiple signaling pathways (5,43,45,93). M2 TAMs activate the ability of HCC
cells to invade and migrate (5).
M1 type macrophages antagonize the action of M2 type macrophages by
inhibiting HCC progression (9).
EMT is an important process in cancer progression, and the
prediction of cancer cell metastasis based on the expression of EMT
markers is a well-established and effective method (11).
M2 TAM secretion of the cytokines CXCL12, CXCL16,
epidermal growth factor (EGF), CCL18 and CCL22 enhances the
invasion of HCC cells (94,95). The level of macrophage-derived
chemokine (MDC)/CCL22 secretion is increased in M2 TAMs (96). CCL22 selectively interacts with
C-C motif chemokine receptor 4, and is involved in the direct
activation of EMT in HCC cells (95). In a previous study, the further
away the colony-stimulating factor (CSF)-1 receptor (CSF-1R) was
from the peri-carcinoma tissue, the less expressed it was. The main
drawback of the study was that it did not specifically investigate
the mechanisms associated with CSF-1 affecting intrahepatic
metastasis of tumors (97). High
CSF-1R expression in peritumoral HCC tissues results in an increase
in macrophage infiltration in HCC through CSF-1, affecting the
intrahepatic metastasis of tumors. Specifically, CSF-1 mediates the
upregulation of AIF1 in M0 macrophages, induces M2 macrophage
polarization and increases the secretion of the cytokine CXCL16,
thereby leading to the migration of Hepa1-6 cells (98). M2 TAMs secrete IL-8, activate the
JAK2/STAT3/Snail signaling pathway, upregulate the expression of
the mesenchymal marker N-calmodulin and are involved in the
induction of EMT in the HepG2 cell line (99). TGF-β-treated M2 TAMs upregulate
the expression of T cell immunoglobulin and mucin structural
domain-containing protein-3, activate the NF-κB signaling pathway,
upregulate the expression of the classical cytokines IL-6, colony
stimulating factor 2 (CSF2) and IL-10, and promote tumor migration
(100,101). GSK3β is upregulated in
macrophages, which in turn activates the NF-κB pathway-mediated
direction of M2 TAM polarization, and upregulates the secretion of
STAT1, CCL5, IL-6 and CSF2 in macrophages, promoting HCC metastasis
and invasion (102). The
activation of the STAT3 signaling pathway in HCC cells mediated by
the secretion of IL-6 by M2 TAMs is necessary for HCC invasion and
metastasis (103).
Clinicopathological analysis has revealed that CD163-positive
macrophages aggregate around STAT3-positive HCC cells. M2 TAMs
activate the STAT3 tyrosine phosphorylation (p-STAT3) signaling
pathway through IL-6 upregulation and participate in HCC cell
migration and invasion (104,105). The expression of the homologous
heterotrimeric transcription factor sine oculis homeobox homolog 1
(SIX1) is increased in tumor tissue TAMs (106). Co-culture of macrophages with
high SIX1 expression with HCC cells increases the levels of
p-STAT3, HCC cell migration and invasion, and the extent of EMT.
Specifically, in HCC, macrophages alter the transcriptional level
of the p65 gene in the nucleus, increase IL-6 secretion, activate
the STAT3 signaling pathway in HCC cells, and lead to an increase
in MMP-9 expression through upregulation of SIX1 expression,
regulating the migration, invasion and EMT of HCC cells (107). A study has demonstrated that
MMP-9 degrades the extracellular matrix (ECM) at an early tumor
stage and is a classical pro-metastatic invasive factor for HCC
cells (108). Furthermore, M2
TAM exosomes participate in HCC metastasis by activating the
TLR4/STAT3 signaling pathway (109,110).
Exosomes work as a messaging link for TAMs to
regulate the extent of the invasive migration of HCC cells
(111). The ratio of CD11b/CD18
is increased in M2 macrophage exosomes. CD11b/CD18 upregulates MMP9
expression in HCC cells, and mediates the metastatic invasion of
HepG2 and Huh7 cells (112).
Integrins are proteins that regulate the function of HCC cells.
Upregulation of integrins in HCC regulates tumor cell
proliferation, metastasis, invasion and survival (113). S100A4 secretion by M2 TAMs
regulates ECM remodeling in the TME via the ERK signaling pathway
(65). The heterogeneous nuclear
ribonucleoprotein A1 in M2 TAMs is a component of
miR-23a-3p-containing exosomes, and the formed exosomes mainly act
on the targets PTEN and tight junction protein 1 in HCC, leading to
the secretion of CSF2, VEGF, granulocyte-colony stimulating factor,
monocyte chemoattractant protein-1 (MCP-1) and IL-4, accelerating
the EMT process in HCC cells (114).
By contrast, miR326 expression is abundant in the
exosomes of M1 TAMs and is involved in the tumor-suppressive
effects of M1 TAM exosomes. Specifically, HCC cells receive
exosomes from M1 macrophages, and miR326 in the exosomes decreases
the expression levels of twist family BHLH transcription factor 1
in HCC cells, hindering the metastatic invasion of HCC (115). lnc-Ma301 expression is higher in
M1 TAMs than in M2 TAMs, but lower in HCC tissues. lnc-Ma301 is an
inhibitor of the invasive migration of HCC cells in mouse HCC lung
metastasis. lnc-Ma301 regulates the Akt/Erk1 signaling pathway
downstream proteins by silencing caprin1, and inhibits the
migration and invasion HCC cells, as well as the EMT (116). M2 TAMs secrete the cytokines
IL-6, TNF-α, CSF2 and intercellular cell adhesion molecule-1 to
mediate the Akt/proline-rich Akt substrate of 40 kDa signaling
pathway and activate EMT in interstitial cells of Cajal. Akt
inhibitor VII has a reversal effect on the aforementioned
cytokines, upregulating EMT markers (117). This mechanism provides a
potential approach for Akt-targeted therapy, which might turn into
a main method to cure HCC. Fig. 2
shows the mechanistic process of the crosstalk of HCC cells and
TAMs.
The angiogenesis of new blood vessels is necessary
to supply oxygen and nutrition, and remove carbon dioxide and other
metabolic waste during the proliferation and migration of tumor
cells. The formation of new blood vessels and recruitment of
pre-existing vessels are two main causes of vascular hyperplasia in
the tumor (118). There is a
positive relationship between the increase in macrophage numbers
and high micro-vessel density. The recruitment of TAMs stimulated
by hypoxia is associated with a wide range of roles in the
formation of new blood vessels. After stimulation by hypoxia, TAMs
secrete vascular endothelial growth factor, which binds to
receptors, and promote the movement and direct migration of
endothelial cells (114).
Subsequently, TAMs, regulated by HIF-1α in the hypoxic
lactate-enriched TME, express multiple genes for the fine-tuning of
the polarized M2 macrophage phenotype and secrete several
proangiogenic factors to activate pro-angiogenic signaling pathways
in HCC (119). M2 TAMs produce
miR-23a-3p-containing exosomes that are taken up by HUVECs, leading
to their migration to form new blood vessels (114).
M2d macrophages are the main pro-angiogenic
phenotype in HCC. They form numerous new blood vessels through the
secretion of several vasoactive substances, such as VEGF-A, EGF,
placenta growth factor (PlGF), TGF-β, TNF-α, IL-1β, IL-8, CCL2,
CXCL8, CXCL12, MMP2, MMP9, MMP12, thymidine phosphorylase (TP),
cathepsins and Pas (120,121).
HCC TAM infiltration into tissues inhibits the antiangiogenic
factors endostatin and angiostatin, while it upregulates the
expression of angiogenic factors. VEGFs serve a dominant role in
promoting tumor angiogenesis (122,123). The VEGF family is comprises five
members: VEGF-A, VEGF-B, VEGF-C, VEGF-D and PlGF. Increased
lncRNA-cyclooxygenase-2 (COX2), H3-AS1 and MALAT1 levels are
present in malignant liver tumors (124). These lncRNAs are the messengers
for the increase in the proportion of M2-like TAMs, and the density
and growth of tumor vessels (125). TAMs activate the STAT3 signaling
pathway to stimulate VEGF expression in liver cancer cells through
the regulation of B7-H3 expression (126). VEGFs are involved in tumor
angiogenesis after being secreted, and bind to the cognate
receptors VEGFR-1, VEGFR-2, VEGFR-3 and recombinant neuropilin
protein (127). The proteolytic
modification of the ECM, contributing to the degradation of the
vascular basement membrane, relies on MMPs, TP, cathepsins and PA,
which promote liver fibrosis and angiogenesis (128). Urokinase-type PA and TP found in
the TAM tumor-promoting secretions increase the perivascular space
(129).
In addition to the classical angiogenic factor VEGF,
the TME contains other angiogenetic factors, including the S100
protein family, semaphorin (SEMA) family, COX-2, SPP1, secreted
protein acidic and rich in cysteine, chitinase-like proteins found
mainly in cartilage and connective tissues [chitinase 3-like-2 and
chitinase 3-like-1 (YKL-40)] and Tie-2. These cytokines mainly
target TAMs, increase their infiltration in HCC and are involved in
the increase of proangiogenic factor expression (130-132). For example, S100 calcium binding
protein, frequently identified at the site of tissue injury, is
associated with the recruitment of inflammatory cells, regulating
the inflammatory microenvironment and remodeling vessels (133). In addition, inhibition of the
SEMA3A/neuropilin 1 signaling pathway inhibits the high level of
TAM infiltration response to the hypoxic microenvironment in HCC
(134). Furthermore, the
increase in the number of TAMs results in increased YKL-40 protein
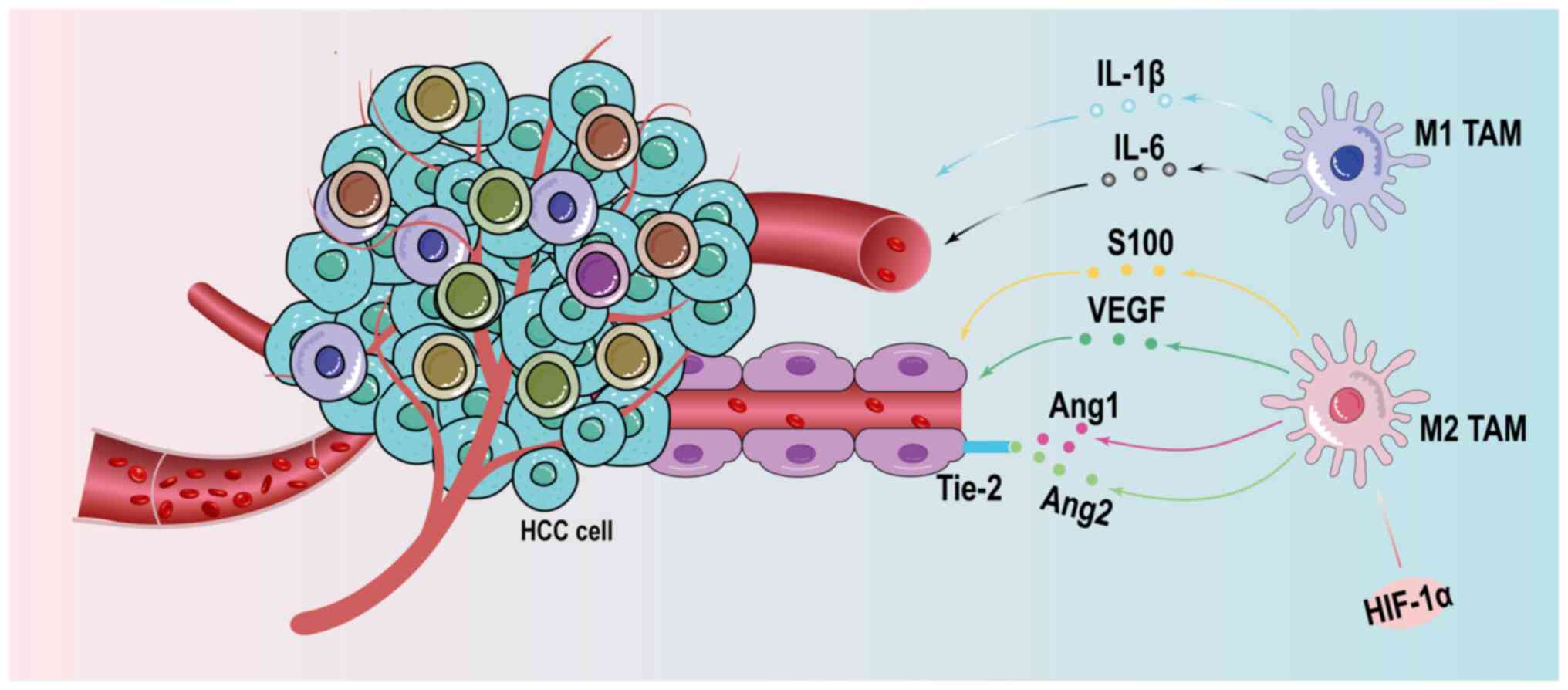
expression in HCC precancerous tissue sections (135). Tie-2-expressing TAMs, a special
subtype of TAMs, are involved in the sustenance of the tumor
vasculature by regulating the Ang-2/Ang-1 ratio, thus exerting
pro-angiogenic effects (136).
However, VEGF-A takes part in the functional transformation of the
pro-angiogenic effects of Ang-2 that leads to the degeneration of
blood vessels (137).
C-C motif chemokine receptor 2 (CCR2), the chemokine
receptor of CCL2, is essential for the recruitment of TAMs. The
inhibition of the MCP-1-CCR2 (CCL2-CCR2) axis reduces the TAM
(CCR2+) response and suppresses angiogenesis,
controlling the development of HCC. CCR2+ TAMs, which
are deprived of most of the properties of M2-type macrophages, are
a novel M1-type macrophage. The high level of CCR2+ TAM
infiltration in the boundaries of HCC increases the liver blood
volume and pathogenic neoangiogenesis (138). In addition, M1 TAMs are involved
in the NF-κB, STAT3 and activator protein-1 (AP-1) signaling
pathways in HCC cells through the production of inflammatory
cytokines (IL-1β and IL-6). M1 TAMs activate an angiogenic
response, which is one of the antitumoral proinflammatory effects
(139). Fig. 3 shows the effect of TAMs on
angiogenesis in HCC. Table II
shows the key differences in the molecular mechanisms by which TAMs
directly vs. indirectly regulate HCC progression.
TAMs inhibit effector T cell activation and
proliferation through bidirectional effects. Mitochondrial
autophagy regulates cellular energy metabolism by changing the
mitochondrial DNA copy number (5). Autophagy maintains the basic
cellular function of keeping oxidative phosphorylation nutrient
levels stable, which is essential for tumor maintenance and
progression (140).
Mitochondrial function and β-oxidation production are the main
energy sources for the pro-tumorigenic effects of Tregs or M2 TAMs.
Upregulation of arginase 1 and IL-10 in M2 TAMs enhances
mitochondrial autophagy due to polyamines, which in turn enhances
the metabolic energy supply of the cell and promotes the formation
of an immune-suppressive HCC microenvironment, leading to T cell
depletion and NK cell inactivation, thus inhibiting the progression
of HCC (141). Liu et al
(142) investigated a novel
immunotherapeutic mechanism. It was based on a reductive reactive
nanoplatform consisting of poly (disulfide amide) and a lipid-poly
(ethylene glycol) shell, which repolarizes TAMs to M1 TAMs via IFNγ
and sialic acid binding Ig like lectin 15 (Siglec15) small
interfering RNA. Siglec15 silencing in the repolarized TAMs
enhanced CXCL9 secretion, increased T cell proliferation and
infiltration, and created a tumor immunosuppressive
microenvironment. Resiquimod (R848) is a novel TLR7/8 agonist,
which repolarizes M2 TAMs to M1 TAMs via the TLR7 MyD88-dependent
signaling pathway. Cell microparticles (MPs) are extracellular
vesicles and AFP is a classical tumor marker for HCC widely used in
HCC vaccine-related investigations. Targeting R848 using MPs as
vectors has been used for reprogramming of TAMs after the
recognition of AFP in M2 TAMs. The use of R848@
M2pep-MPsAFP to improve the immunosuppressive
microenvironment is a novel approach for tumor immunotherapy
(143).
On the one hand, TAMs positively inhibit cellular
immunity by mediating the secretion of multiple cytokines that act
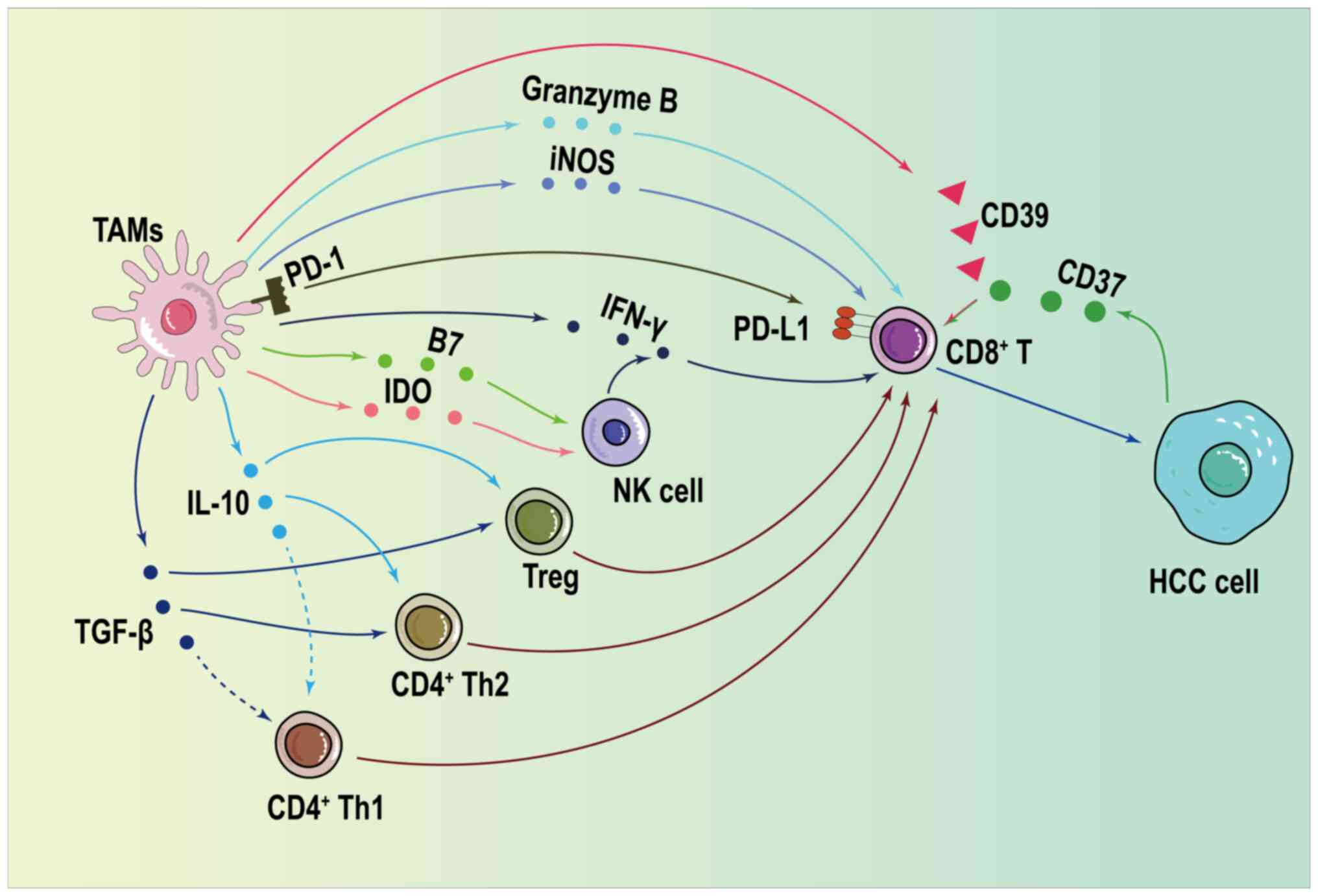
directly on CD8+ cells (144). Immune checkpoint ligand
programmed death ligand 1 (PD-L1) is the main acting protein that
inhibits CD4+ and CD8+ cell infiltration
(145). The binding of
programmed cell death protein-1 (PD-1) to PD-L1 leads to
CD8+ cell depletion, promoting the expression of
regulatory molecules such as B7-H4 and Ig inhibitor of the V
structural domain of T cell activation in T cells, which
effectively attenuates antitumor effects due to CD8+
cell infiltration (146). IL-10
in the TME induces high PD-L1 and PD-1 expression in monocytes
(147). Oncoprotein induced
transcript 3 is upregulated in M2 TAMs and involved in the
activation of the NF-κB/PD-L1 axis (148). Calcyclin binding protein
(CacyBP) competes for the E3 ubiquitin ligase binding site on MyD88
in HCC cells, preventing the degradation of MyD88 via the classical
E3 ubiquitin ligase siah E3 ubiquitin protein ligase 1, leading to
increased C-X3-C motif chemokine ligand 1 (CX3CL1) expression. The
activation of C-X3-C motif chemokine receptor 1/CX3CL1 signaling is
critical for the infiltration of TAMs in HCC tissues. The
inhibition of CacyBP has become an emerging idea to maintain the
therapeutic effect of anti-PD-1 antibodies in HCC (149). In addition, mucosal-associated
invariant T (MAIT) cells are MR1-restricted, innate-like T cells.
There is mutual crosstalk of CD163 TAMs and MAIT cells via cell
contact and a PD-L1-dependent mechanism. PD-L1 blockade and
depletion of CSF-1R TAMs were involved in the functional inhibition
of MAIT cells in an HCC mouse model (150).
TGF-β1, IL-10 and prostaglandin E2 (PGE2) block Th1
cell differentiation, and CD4+ and CD8+ cell
infiltration to exert an antitumor effect (151). Th1 cells assist CD8+
cells to participate in antitumor cellular immunity (152). In vivo and in
vitro experiments have demonstrated that M2 TAMs depleted the
already activated CD8 T cells, reduced the secretion of the related
inflammatory factors IFN-γ and granzyme B in T cells, and activated
the immune escape and immunosuppression in HCC (153). Adenosine in the HCC TME produced
by the ATP-adenosine pathway is a CD8+ cell exhaustion
promoter that interferes with CD8+ cell proliferation.
CD39, secreted by M2 TAMs, is the key enzyme for the activation of
the ATP-adenosine pathway (154). CD39 together with CD73 secreted
by HCC cells increases the proportion of ATP converted to adenosine
and is involved in CD8 T cell activity inhibition (155). Activation of the
PGE2/prostaglandin E receptor 2 and PGE2/prostaglandin E receptor 4
signaling pathways modulates PD-1 expression during CD8+
cell infiltration, leading to immune tolerance in the TME (156). T cells interact with tumor
antigens through the T cell receptor ζ, which is associated with
L-arginine (157). M1 TAMs
express iNOS and M2 TAMs express arginase 1, which metabolizes
arginine to nitric oxide and urea (158).
On the other hand, TAMs exert antitumor effects on
T cells through the interaction with other relevant cells in the
TME. In addition to the immunosuppressive effect of PD-L1 secreted
directly by TAMs, these cells also activate the mitotic spindle
positioning/IQ motif containing GTPase activating protein 1/STAT3
axis in HCC cells, upregulate the levels of PD-L1 secretion and
promote the immune escape of HCC (159). Regulatory T cells can be divided
into naturally occurring natural regulatory T cells (nTregs) and
adaptive regulatory T cells. TAMs upregulate IL-10 and TGF-β
expression, and promote the expression of the chemokines thymic and
activating regulatory chemokine (CCL17), CCL18, CCL5, macrophage
inflammatory protein 3α (CCL20) and small inducible cytokine
subfamily A (Cys-Cys) to recruit T helper 2 cells, induced Tregs
and nTregs (160). Tregs migrate
toward tumors and antagonize CD8+ cell-mediated
antitumor cellular immune functions (161). High protein expression levels of
TGF-β, PGE2, indoleamine 2,3-dioxygenase and B7 in TAMs reduces the
migration, proliferation and apoptotic ability of NK cells, reduces
IFN-γ and TNF-α expression in the TME, and reduces the cytotoxicity
and killing effect of NK cells on tumor cells (162). In addition, CAFs recruit TAMs
from the peripheral blood through the macrophage migration
inhibitory factor/CD74 axis and induce differentiation of TAMs into
CXCL2+ TAMs via the activation of SMAD3. This was
demonstrated in vivo and in vitro, and the binding
was strong enough to explain the activation of TAMs in relation to
CAFs (19). Activated TAMs
possess the ability to recruit B cells via the CXCL12/CXCR4
signaling pathway and enhance cytotoxic T cell depletion through
the expression of PD-L1 regulated by CXCR4 (84). Fig.
4 shows how TAMs enhance immune protection against HCC by
regulating the function of other immune cells.
TAMs are an important type of innate immune cells
in HCC. Studies have identified an inextricable relationship among
polarization, recruitment of TAMs and tumor development (163-167). Therapies targeting TAMs
effectively inhibit the invasion of HCC and the proliferation of
tumor cells. Targeted TAM therapy is mainly carried out as follows:
i) Controlling the number of TAMs in HCC, preventing the generation
of the HCC TME by reducing the infiltration of TAMs and their
polarized phenotypes; ii) restoring the tumor-resistant effects of
TAMs by altering the function of TAMs in the HCC TME, activating
the antitumor mechanism and the synthesis of its products in TAMs;
and iii) inhibition of immune escape in HCC. It has been confirmed
that HCC uses the 'do not kill me' mechanism to evade the
phagocytosis of tumor cells by TAMs. Therefore, tumor cells can be
eliminated by cutting off the signaling pathway of HCC that
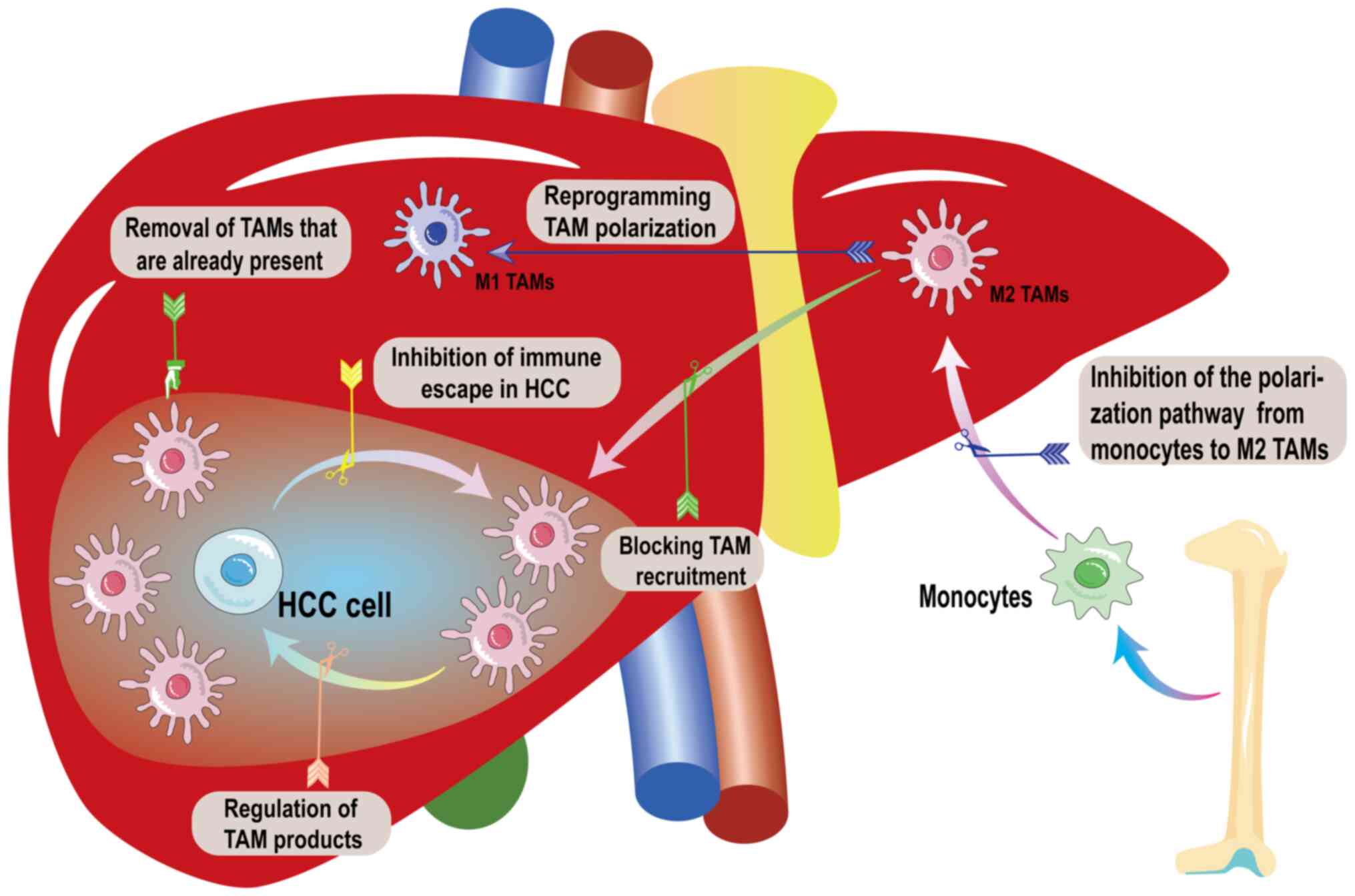
interferes with the phagocytosis of TAMs (3,47,119,168-171). Fig. 5 shows the direction of treatment
options targeting TAMs in HCC.
The control of HCC by the specific depletion of
TAMs is the most direct immunotherapeutic approach to target TAMs.
Sorafenib is a common therapeutic agent used to treat HCC (172). CSF-1, stromal cell-derived
factor 1α (SDF-1α) and VEGF are important chemokines for the
proliferation of macrophages. Studies have indicated that plasma
CSF-1 expression is increased after sorafenib treatment. CSF-1
activates macrophages, promoting macrophage-tumor interactions,
ultimately leading to tumor progression. By contrast, sorafenib in
combination with chlorolipids or zoledronic acid inhibits
macrophage infiltration (173,174). Tumor progression and tumor
angiogenesis are inhibited after the depletion of macrophages by
chlorolipids or zoledronic acid. Furthermore, the combination of
these drugs controls lung metastasis of HCC (175). In vivo experiments were
only performed using mice, and the in vitro experiments were
performed using RAW264.7 mouse macrophages, while human macrophages
such as THP-1 cells were not used; thus, the results are less
representative than those using human macrophages (176). Microtubule depolymerization is
specifically induced by 2'-carboxy-D-arabinitol 1-phosphate (CA1P)
through the inhibition of the p150-AKT-GSK3β signaling pathway
(177). Downregulation of Akt
phosphorylation activates GSK-3β, which in turn inhibits the
Wnt/β-catenin signaling pathway in HepG2 cells (178). This signaling is abnormally
activated during HCC development; thus, CA1P induces apoptosis in
HCC cells in vitro and in vivo. In addition, CA1P
induces apoptosis in tumor cells and TAMs via the same pathway and
alters the TME by disrupting the crosstalk between tumor cells and
tumor-associated immune cells. The effectiveness of CA1P in the
treatment of HCC and the effect of CA1P on macrophages are
mentioned in a study by Mao et al (179); however, to the best of our
knowledge, the relationship between the two has not been confirmed
in any particular study. Since TAMs have multiple polarized
subtypes in HCC, their widespread clearance leads to an imbalance
in immune regulation. Current therapeutic regimens targeting the
removal of TAMs fail to specifically target M2 TAMs, resulting in
the unavoidable removal of antitumor M1 TAMs (180).
CCL2 is a feature of inflammatory monocytes, and
its only known receptor CCR2 is expressed in hepatic macrophages.
CCL2-CCR2 signaling is involved in the infiltration of TAMs and the
polarization of M2 TAMs. HCC can be treated at a biomacromolecular
level by targeting CCR2 signaling blockade. A natural product
called 747 has been found to act as a CCR2 antagonist. It reduces
macrophage infiltration by activating the CD8 T cell population
(181). Genipin, also derived
from herbal origins, is a natural cyclic enol ether terpene
glycoside commonly used in clinical practice for the treatment of
inflammatory diseases and heat-associated HCC. Genipin directly
binds to PPARγ and activates PPAR signaling in postoperative
hepatic macrophages in mice (182,183). Activated PPARγ triggers the
degradation of p65/RelA by acting as an E3 ligase (184). p65/RelA is a transcription
factor for CCL2, and its inhibition results in the blockade of the
CCL2-CCR2 signaling pathway. These effects in turn inhibit the
recruitment of the hepatic macrophage population, as well as
macrophage pro-inflammatory cytokine and chemokine expression, and
postoperative HCC recurrence, resulting in a reduction in
postoperative patient survival (5). Cenicriviroc (CVC) is a novel oral
dual CCR2/CCR5 antagonist. CVC reduces macrophage infiltration in
the liver of patients with autoimmune liver disease. CVC treatment
blocks the release of LPS-induced macrophage cytokines. The oral
administration of genipin after CVC treatment in an experimental
mouse model of HCC reduced the risk of lung metastasis of HCC
cells. In addition, CVC prevents pro-inflammatory signaling and
macrophage activation in the liver, blocking the TAM polarization
pathway in the treatment of HCC (185). A total of 10 CCL2 inhibitors
(MK0812, PF04634817, PF04136309, INCB3344, cenicriviroc,
JNJ2714149, CCR2-ra-[R], CAS-445479-97-0, RS504393 and RS102895)
are commercially available at present and are used in clinical HCC
therapy (186). Pharmacological
experiments have confirmed that MK0812 inhibits tumor metastasis in
humanized mice, with the lowest IC50 (1.73 nM) being the
most potent human CCR2 inhibitor. Oral administration of MK0812 in
human CCR2B knock-in mice did not result in weight loss or other
serious side effects (187). The
cytidine analog gemcitabine (GEM) is a first-line chemotherapeutic
agent in the treatment of parenchymal tumors, functioning by
inhibiting DNA replication. However, GEM has some limitations such
as poor delivery and susceptibility to cytidine deaminase-induced
degradation, which hamper its clinical therapeutic application. The
poor delivery of GEM was addressed by constructing GEM-conjugated
polymers (PGEMs) showing high tumor penetration in several
pancreatic ductal adenocarcinoma models. PGEM has a reduced
particle size, being a small and acceptable polymer drug complex. A
study has demonstrated that PGEM induces double-stranded DNA damage
in tumor cells and activates the cyclic GMP-AMP synthase
(cGAS)-interferon gene-stimulating factor (STING) pathway (188). STING is an endoplasmic reticulum
transmembrane protein that activates CCL2 and CCL7 expression,
leading to a marked increase in the percentage of MDSCs and TAMs,
thus regulating tumor innate immunity. PF-6309 is an optimal CCR2
inhibitor for loading PGEM micelles. It was also evident that free
PF-6309 and PGEM/PF-6309 treatments led to a decrease in the
percentage of MDSCs and TAMs, the latter also leading to a decrease
in the percentage of M2-type macrophages and an increase in the
percentage of M1-type macrophages and the M1/M2 ratio, suggesting
that macrophages infiltrating the tumors are polarized from a
tumor-promoting to a tumor-suppressing phenotype (189). Hypoxic conditions in HCC lead to
the upregulation of SDF-1α expression, leading to the recruitment
of M2 macrophages and the promotion of tumor progression (19,190). The expression levels of CXCR4
and VEGF in TAMs, as well as the expression levels of M2 TAM
markers CCL22 and arginase 1 are more than 2-fold higher in TAMs
after sorafenib treatment than in TAMs without sorafenib treatment.
AMD3100 is a classical inhibitor of the SDF-1α receptor (CrXrC
receptor type 4 or CXCR4), which blocks the SDF-1α/CXCR4 axis that
arrests the recruitment of TAMs, prevents polarization to an
immunosuppressive microenvironment after sorafenib treatment,
inhibits tumor growth, reduces lung metastasis and improves
survival. AMD3100 alone or in combination with sorafenib reduces
the expression levels of M2 type markers in TAMs but does not
affect the expression of M1 type markers. This combined therapeutic
approach exerts an anticancer effect not through reprogramming M2
TAMs but by cutting off the recruitment channel of M2 TAMs
(191). CXCL17 is a selective
inhibitor of CXCR4 signaling with low potency. Binding of CXCL17 to
neuropilin-1, a VEGFR2 co-receptor containing glycosaminoglycans,
blocks endogenous CXCL12 binding to CXCR4, thereby inhibiting
ligand binding to NanoLuc/CXCR4 and inhibiting β-arrestin 2
recruitment to CXCR4 (192). The
differentiation of human M2 macrophages results in the upregulation
of the expression levels of P2Y11, a G protein-coupled ATP receptor
that activates the IL-1 receptor in a cyclic AMP-dependent manner,
upregulating the expression levels of CECR7 through increased EGFR
expression, along with the upregulation of CXCR4 expression. The
antagonist NF340 is a specific inhibitor of P2Y11, and this
inhibition controls macrophage infiltration by suppressing CXCR4
expression (193).
Some chemotherapeutic agents, such as Adriamycin
(Dox) and oxaliplatin are effective in tumor immunotherapy
regimens. These drugs applied in combination therapy are effective
in treating the development of HCC (194,195). Manganese (Mn2+) is an
important intracellular ion that activates cGAS-STING signaling.
Cell membrane-derived hybrid nanovesicles (Mn2+/Dox
loaded-hybrid vesicles) have been constructed and applied in
antitumor therapy. Cell-membrane vesicles were derived from M1-like
macrophages and fused with liposomes carrying Mn2+ and
Dox. Finally, the fused vesicles were modified with CXCR4-binding
peptide to target macrophages highly expressing CXCR4. Furthermore,
Mn2+-mediated activation of cGAS leading to an increased
production of cyclic GMP-AMP and activation of STING resulted in
the upregulation of IRF3 transcription. i-type interferon and
cytokines for antitumor immunity were controlled by STING,
upregulating the expression levels of CD80 and CD86, leading to M1
phenotypic polarization (196).
CSF2-derived macrophages preferentially produce IL-6, IL-12 and
TNFα in response to lipopolysaccharide, whereas CSF1-derived
macrophages produce IL-10 and CCL2 but not IL-12. CSF-1R signaling
promotes a tumor-promoting macrophage phenotype, whereas its
inhibition polarizes TAMs to an antitumor phenotype. The inhibition
of CSF1/CSF-1R signaling depletes M2 TAMs to reduce tumor cell
infiltration and reprogram the TME (197). CCAAT-enhancer-binding protein α
(C/EBPα) is known for its ability to inhibit the tumor polarization
of M2 macrophages; however, its role in regulating
immunosuppressive myeloid cells is unclear. Application of
therapeutic agents associated with C/EBPα mediated by small
activating RNA represents a good immunotherapeutic option. The
transcription of the CEBPA gene is specifically regulated by
CEBPA-51. The encapsulation of CEBPA-51 in SMARTICLES liposomal
nanoparticles composed of MTL-CEBPA results in the promotion of
C/EBPα expression. In addition, the upregulation of CEBPA
expression in monocytes after the action of MTL-CEBPA leads to an
increase in the synthesis of PGE2, which is a potent
immunosuppressive mediator, resulting in the rapid downregulation
of genes and proteins involved in MDSC inhibitory activity, as well
as the activation of classical monocytes and granulocytes.
Furthermore, MTL-CEBPA and sorafenib induce the transition from
M2-type polarized TAMs to M1-type polarized TAMs (198).
Focal adhesion kinase (FAK) is a non-receptor
protein tyrosine kinase that promotes M2 polarization of
macrophages through the regulation of multiple signaling pathways,
including the PI3K/Akt/JAK/STAT3 and p38/JNK/ERK signaling
pathways. Phellinus linteus is an anticancer herbal
medicine. Upon binding to FAK, the combination of DBL and hispolon
blocks the action of FAK and inhibits cell proliferation (199).
PLX3397 is a competitive inhibitor targeting CSF-1R
and the affinity for CSF-1R tyrosine kinase is higher than that of
CSF-1R. Bone marrow-derived monocytes are stimulated to polarize
toward an M2-like or M1-like phenotype by either CSF1 or CSF2, the
latter protecting macrophages from PLX3397 treatment; thus, TAMs
from PLX3397-treated tumors show an M1-like phenotype, and PLX3397
inhibits tumor growth without depleting TAM infiltration in
vivo (200).
Blocking the change of already polarized M1 TAMs to
M2 TAMs is a novel line of research. CSF-1 regulates the
repolarization process from M1 macrophages to M2 macrophages
through MAPK/ERK/AP-1 signaling pathway activation. CSF-1 mediates
the high expression of c-Jun, which belongs to the AP-1 family
proteins and is involved in the repolarization process of M1
macrophages with the assistance of NF-κB (201). The triggering receptor expressed
on myeloid cell (TREM) family mainly exerts effects in the
regulation of the inflammatory response through the receptor form.
TREM2 regulates macrophage polarization, and its inhibition leads
to the activation of the PI3K/Akt/NF-κB axis and the increase of
CXCL3 secretion in macrophages. High TREM2 expression is a hallmark
of the repolarizing metabolic reprogramming of M1 macrophages to M2
macrophages (202,203). Echinacoside is a phenyl ethanol
glycoside involved in hepatoprotection, anti-inflammation,
neuroprotection and tumor therapy. A study found that it reduced
TREM2 expression in HCC, while activating PI3K/Akt signaling,
acting as an antitumor agent (204). Oxaliplatin is a commonly used
clinical anticancer drug. TREM2-IN-1 (OPA), a platinum (IV) complex
made from oxaliplatin and artesunate, is a potent TREM2 inhibitor.
OPA is also involved in the cytotoxicity induced by oxidative
damage to nuclear DNA. It serves an antitumor role in both
chemotherapy and immune activation (205). The use of Fc structural domain
effector-enhanced antibodies against TREM2 demonstrated that the
pro-tumor function of TAMs was reversed in the TME after treatment
(206-208). Furthermore, the humanized
anti-TREM2 monoclonal antibody PY314 is already used in a clinical
trial (209).
The use of cellular autophagy to block the
immunoregulation of HCC involving TAMs has received unanimous
confirmation from experts and scholars (163-165,172,173,180). Baicalin inhibits in situ
HCC growth through the reprogramming of macrophages. This process
requires the involvement of RelB and p52, which are highly
expressed in M1-like macrophages compared with M2 TAMs. Baicalin is
an activator of the autophagic degradation of TNF receptor
associated factor (TRAF)2 in TAMs, and TRAF2/TRAF3 has a limiting
effect on intracellular IKKα/RelB/p52 signaling. The indirect
activation of the RelB/p52 signaling pathway by baicalin causes the
expression of the RelB/p52-specific target genes CXCL12 and CCL9,
leading to higher expression of macrophage-secreted IL-6 and TNF-α,
which are cytokines with typical pro-inflammatory effects, and
causes transformation of M1 TAMs to M2 TAMs (210). Activation of the NF-κB signaling
pathway for macrophage autophagy therapy is widely recognized since
it is involved in M1 macrophage polarization. A study has also
identified the role of the tumor vaccine Lmdd-MPFG in targeting
TAMs. The activation of the NF-κB signaling pathway by the
Lmdd-MPFG vaccine induces M1 polarization through macrophage
autophagy (211). Sirtuin 1
(SIRT1) is a deacetylase that regulates transcriptional silencing
and cell viability. Overexpression of SIRT1 in macrophages enhances
the phosphorylation of p65 and IKK, and promotes M1 macrophage
polarization. IKKβ inhibition increases tumor suppressor
polarization of macrophages (212).
lncRNA COX-2 expression is higher in M1 macrophages
than in M2 macrophages. Its inhibition in turn inhibits macrophage
polarization to the M1 type, decreases the ability of M1
macrophages to inhibit HCC cell proliferation and increases the
ability of M2 macrophages to promote proliferation (213).
Targeting multiple receptors is an essential part
of the treatment of HCC, and relevant therapeutic regimens around
TAMs take this into account. Use of TLR inhibitors for the
treatment of HCC has been widely emphasized (210,212,214). The role of TLR in the activation
of the non-specific immunity of the body cannot be ignored. The
non-specific immune system promotes the inflammatory response by
recruiting immune cells such as macrophages and mast cells to
secrete inflammatory factors (215). The most important protein for
mitochondrial fission, dynamin-related protein 1, is involved in
HCC progression by regulating cell survival and metastasis. The
dynamics of mitochondrial fusion and fission are necessary for
mitochondrial DNA (mtDNA) distribution and mitochondrial
homeostasis. Increased mitochondrial fission induces cytoplasmic
mtDNA stress in HCC cells, which in turn promotes CCL2 secretion
via the TLR9-mediated NF-κB signaling pathway, leading to TAM
infiltration in HCC tissues (216). In HCC treatment, motolimod (TLR7
agonist), GS9620 (TLR8 agonist) and R848 (resiquimod; dual TLR7/8
agonist) produce an M1 increase. Certain dextran nanoparticles have
natural macrophage affinity and are effective carriers for
targeting TAMs with drugs. Among them, β-cyclodextrin has a similar
chemical composition to linear dextran, and easily activates the
phagocytic effect of macrophages to exert drug effects (214,217-221). Cyclodextrin nanoparticles
(CDNPs) have a high macrophage affinity and a high drug-carrying
dose. After combining with CDNPs, the so-called nano R484 inhibitor
enhances the reprogramming ability of TAMs (217). In addition, PI3Kγ is a classical
therapeutic target for reprogramming TAMs and is a key signaling
molecule required for macrophage accumulation in inflammation.
PI3Kγ blocks pro-inflammatory responses of stimulating macrophages.
Macrophages deficient in PI3Kγ exhibit a reduced ability to respond
to immunotropism through G protein coupling (222), upregulation of M1
polarization-associated genes and proteins, and downregulation of
M2 polarization-associated cytokine expression. PI3Kγ promotes
immunosuppression through the activation of mTOR/S6Kα/C/EBPβ
signaling and the inhibition of NF-κB (223). Indirect promotion of Th1 cells
and cytotoxicity reduce immune responses (224). A pan-PI3K inhibitor has recently
been identified as an enhancer of pro-inflammatory cytokine
transcription in TAMs (3). The
metabolism of substances in TAMs serves an integral role in the
polarization of cellular phenotypes, and the inhibition of certain
pathways of macrophage substance metabolism activates the
reprogramming of M2 TAMs (225).
Macrophages are a relevant source of active insulin-like growth
factor (IGF-1). IGF-1 is a key growth factor for macrophages to
drive the growth of HCC cells from macrophages. Sorafenib inhibits
the release of IGF-1 (226).
IGF-binding protein 4 (IGBP-4) binds IGF-1 to inhibit its activity
(227). Low expression of IGBP-4
in polarized macrophages leads to active secretion of IGF-1, which
ultimately promotes the proliferation of HCC cells. Targeted
inhibition of the IGF/IGF-1R signaling axis is a promising approach
to modulate the HCC environment (228). Receptor interacting
serine/threonine kinase 3 (RIPK3) is downregulated in
HCC-associated TAMs. The extent of the distribution of lipid
droplet deposition is increased in TAMs after RIPK3 knockdown.
Reactive oxygen species/caspase 1 signaling mediates the inhibition
of PPAR expression by RIPK3. The lack of RIPK3 in TAMs results in
an increase in the expression levels of PPARα and PPARγ, which
regulate fatty acid metabolism, promote TAM infiltration and M2
polarization, and facilitate the development of HCC. The
upregulation of RIPK3 reverses TAM polarization and attenuates HCC.
The RIPK3 inhibitor GSK872 and fatty acid oxidation blockers are
two factors mediating reprogramming of M2 TAMs (229).
TAM products are protein molecules in TAMs that can
act in the tumor microenvironment to regulate tumor development,
such as TGF-β and Wnt1. TAMs accelerate tumor progression by
releasing tumor growth factors. LPS-induced activation of PI3K/Akt
increases the levels of miR-101, which in turn binds to dual
specificity phosphatase 1 (DUSP1) mRNA and directly represses its
expression (230). DUSP1 is a
negative regulator of the activity of MAPKs. MAPKs are highly
conserved serine/threonine protein kinases that include ERK,
JNK/stress-activated protein kinases and p38 (231). Sorafenib inhibits PI3K/Akt
activation and decreases miR-101 expression, and subsequently
inactivates MAPK via the induction of DUSP1 expression in HCC.
Sorafenib inhibits the release of TGF-β and CD206 in M2 macrophages
(232). TGF-β is a key growth
factor for macrophage-driven HCC cell proliferation and metastasis
(217). Wnt/β-catenin signaling
is a classically conserved signaling pathway, and its activation is
associated with the degree of HCC. M2 macrophages specifically
release Wnt1, which stimulates the activation of β-catenin
signaling in tumor cells, leading to HCC cell proliferation and
malignant transformation in a Wnt1-dependent manner. Bufferin
targets M2 macrophages and inhibits HCC growth by specifically
blocking Wnt1/β-catenin signaling (222). Isoproterenol stimulates the
secretion of microvesicles by TAMs, which deliver miR-142-3p from
macrophages to HCC cells. miR-142-3p is taken up by HCC cells and
directly inhibits their viability, proliferation and invasiveness
in vitro by downregulating Rac family small GTPase 1
(224). Compound kosher
injection (CKI; also known as Yanshu injection) is used in clinical
practice in the treatment of a variety of solid tumors, including
HCC, gastric carcinoma, breast carcinoma, lung carcinoma and
colorectal carcinoma. CKI increases the therapeutic effect of
low-dose sorafenib. It also reduces the M2 TAM levels in the TME
and increases the ratio of M1 TAMs to CD8 T cells by targeting TNF
receptor superfamily member 1A and its downstream NF-κB p65 and
MAPK p38 signaling cascades, reducing tumor recurrence and
triggering an effective antitumor memory response against HCC
(3).
HCC avoids phagocytosis by TAMs mainly through the
'do not eat me' signaling pathway. Signal-regulated proteins
(SIRPs) have extracellular immunoglobulin-like domains, and SIRPα
and SIRPγ bind to the widely expressed cell-surface transmembrane
glycoprotein CD47 (also known as integrin-associated protein). The
binding of SIRPα to macrophages reduces the production of the
pro-inflammatory cytokine TNF and reduces polarization in the M1
direction. The binding of CD47-SIRPα to macrophages leads to the
inhibition of the 'do not eat me' signaling, negatively regulating
the activation of macrophages and phagocytosis (233). Activation of SIRPα activates the
phosphorylation of tyrosine-based inhibitory motifs of the immune
receptor, preventing the recruitment of Src homologous phosphatase
(SHP)-1 and SHP-2 phosphatases in cells. Two phosphatases are
recruited to prevent myosin-IIa accumulation at phagocytic synapses
in macrophages. The disruption of the CD47-SIRPα axis in
preclinical models leads to enhanced phagocytosis and tumor
reduction, and it is a means of cross-presenting tumor antigens to
T cells (234). Two therapeutic
options are available based on CD47-SIRPα axis inhibition,
anti-CD47 therapy and anti-SIRPα therapy. At present, scholars
generally choose anti-CD47 therapy as the main therapy to restore
macrophage capacity through the use of surface-engineered exosomes
equipped with membrane-bound proteins. Researchers have used
exosome models containing SIRPα variants specifically competing for
SIRPA binding sites on CD47 on the surface of tumor cells. Newly
developed SIRPα variants have an improved affinity for human CD47
compared with wild-type SIRPα. SIRPα exosomes block CD47-SIRPα
interactions, inducing increased phagocytosis of various cancer
cells by bone marrow-derived macrophages (235). Treatment of macrophages with
B6H12 or CD47 monoclonal antibody 400 antibodies, which are two
specific CD47 blocking antibodies, enhances phagocytosis of HCC
cells and reduces tumor size in mice with ectopic tumors.
Furthermore, CD47 inhibition leads to an increase in the migration
of macrophages to HCC in vivo, which is more conducive to
the phagocytosis of macrophages. CD47 blocking antibodies have
great advantages in maintaining the normal physiological functions
of the liver, and the use of the antibodies does not cause direct
in vivo damage to the liver and normal hepatocyte viability
(236). In a recent study, a
high affinity CD47 blocker (modified SIRPα D1 structural domain)
was added to the inactive IgG Fc region, resulting in the
engineered protein evorpacept (also known as ALX148). ALX148 is
recognized by macrophages through the active Fc structural domain
and binds to its Fcγ receptor, and the resulting CD47 inhibitor
targets the CD47/SIRPα signaling pathway, increasing autoimmune
processes in the TME. Evorpacept had a good safety profile both
when administered alone and in combination with the standard
regimen of pembrolizumab and trastuzumab (237). In addition, CD47 expression in
tumor cells is positively regulated by STAT3 phosphorylation
levels. IL-6 reverses the suppressed CD47 expression in HCC cells
after blocking STAT3 and enhances the ability of tumor cells to
evade phagocytosis. Tocilizumab, a humanized monoclonal antibody
directed against the IL-6 receptor, is used to inhibit the
inflammation in rheumatoid arthritis, but it also blocks
TAM-mediated anti-phagocytosis, making it a potential drug for HCC
treatment (238). Histone
deacetylase 6 (HDAC6) is involved in autophagy signaling and
degradation of ubiquitinated proteins. It directly inhibits the
expression of let-7i-5p, which regulates the expression of
thrombospondin-1 (TSP1), which competes with SIRPα. Activation of
the HDAC6/let-7i-5p-TSP1 regulatory axis converts CD47-SIRPα to
CD47-TSP1 interactions between HCC and macrophages, thereby
altering the 'do not eat me' self-protection mechanism of tumors.
Therefore, targeting of the HDAC6/let-7i-5p/TSP1 axis could
represent a novel immunotherapeutic strategy to treat human HCC in
the future (239). B6H12.2 is a
CD47 blocking antibody that enhances CD47-SIRPα expression in CCA,
thus enhancing the phagocytosis of CCA cells highly expressing CD47
(238). Glypican-3 (GPC3) is one
of the most well-characterized HCC-associated antigens. Bispecific
antibodies co-acting with GPC3 and CD47 have excellent antitumor
effects with minimal toxicity. GPC3/CD47 bispecific antibody
readily binds preferentially to bi-antigen-expressing tumor cells,
and efficiently blocks SIRPα/CD47 (240). Anti-SIRPα treatment regimens
have progressed more slowly compared with CD47 antibody therapy
(241). CRISPRed macrophages can
be used for cell-based cancer immunotherapy. The CRISPR-CRISPR
associated protein 9 (Cas9) nuclease system is commonly used to
target modifications of the genome in cells to block gene
expression (242). Cationic
arginine-coated gold nanoparticles (ArgNPs) bind Cas9 proteins, and
CRISPR-Cas9 encapsulated with ArgNPs can knock down SIRPα in
RAW264.7 cells, activating phagocytosis and eliminating cancer
cells (243). In contrast to
therapeutic measures targeting CD47, therapeutic regimens targeting
SIRPα are still missing (244).
In response to a study of tumor-targeted macrophage
therapeutic regimens, current studies have found that the use of
CSF-1R may lead to the development of drug resistance (97,150,197,200). The activation of PI3K is
involved in the resistance of HCC after treatment with the CSF-1R
inhibitor BLZ945. After the treatment with BLZ945, IGF-1 drives the
activation of PI3K signaling (245). Therefore, IGF-1R and PI3K may
serve as biomarkers for predicting drug resistance. Combined
inhibition of IGF-1R and PI3K enhances the efficacy of CSF-1R in
treating tumors by reducing drug resistance. Novel advances in
CSF-1R antagonistic therapy in combination with other receptor
tyrosine kinase inhibitors have been reported (246). The generation of numerous tumors
is inextricably linked to the neogenesis of blood vessels in their
tissues (247). The
complications associated with treatments in patients in whom the
angiogenic pathway is blocked are a difficult problem to resolve.
Monocytes/macrophages expressing the Ang receptor TIE-2 regulate
interstitial angiogenesis in tumors after receiving CXCL12/CXCR4
recruitment messages in combination with Ang (248-250). Targeted therapies are often
applied with TIE-2-expressing monocytes/macrophages (TEMs) as a
therapeutic focus to avoid the development of complications while
determining efficacy. By blocking TEM depletion, targeted
therapies, by avoiding angiogenic mechanisms in tumors, avoid
issues in human physiological functions triggered by extensive
angiogenic inhibition (251).
There are potential risks associated with the regulation of
angiogenesis in tumors, as hypoxia due to TEM inhibition may
activate strong vascular remodeling and maturation in the periphery
of the tumor, leading to a failure of the tumor-suppressive effect
of the drug (252).
The present review focuses on the crosstalk between
tumor cells and macrophages; however, to the best of our knowledge,
there is no in-depth research finding on the mechanism regulating
the crosstalk between macrophages and specific immune cells.
Furthermore, information on the joint crosstalk between multiple
cells is still lacking, and there is no systematic mechanism to
regulate the tumor immune microenvironment. The study of the effect
of HCC on the polarization direction of macrophages revealed the
possibility that some juvenile macrophages may already have shifted
their polarization direction when they are not affected by HCC, a
factor mostly ignored by other studies (3-6,8,13,15). At present, TAMs are roughly
classified into M1 TAMs and M2 TAMs, but more and more polarized
phenotypes of macrophages have been identified, with no clear and
distinct boundary between the macrophage subtypes that have been
investigated. Furthermore, some TAMs have the characteristics of
both the M1 subtype and the M2 subtype of macrophages, suggesting
that the balance of the physiological functions of macrophages in
HCC should be studied in the future. More and more subtypes of TAMs
have been identified, immunotherapy measures targeting macrophages
in HCC are too homogeneous at present, and the proportions of
macrophage subtypes in tumors of different patients with HCC vary
greatly, leading to the conclusion that it is necessary to study
the signature biomarkers of multiple subtypes of TAMs, and use
these biomarkers for the detection of macrophages in tumors as well
as the selection of targeted therapeutic regimens. The TRP ion
pathway is a tumor-related signaling pathway that has received
increasing attention from experts in tumor targeted therapy
(253). The TRP ion pathway is a
classical pain target, and high TRP ion pathway activation is
present in liver cancer. The specific functions and regulatory
factors of the TRP ion pathway have not yet been conclusively
determined (254). Thus, the TRP
ion pathway in liver cancer should be investigated in the future,
potentially representing a novel development direction (255).
Chinese and international scientists have
investigated the role of TAMs in the microenvironment of HCC and
found that TAMs are an important factor in controlling HCC
development. The present review is divided into two parts, focusing
on the role of TAMs in the HCC microenvironment: Direct control of
HCC cells and indirect promotion of HCC development. M2 TAMs
positively regulate the migration, invasion and proliferation of
tumor cells through the secretion of related cytokines, but
inflammatory factors produced by M1 TAMs also contribute to the
formation of blood vessels, leading to the development of
tumors.
Existing scientific studies tend to analyze the
pro-tumor direction of M2 TAMs and study the effect of TAMs on
advanced HCC. Since the polarization of TAMs in the early stages of
a tumor is biased towards the M1 phenotype, there is still a gap in
the study of the pro-tumorigenic role of M1 TAMs and the
repolarization process. After early diagnosis, the inhibition of
the early polarization orientation by M1 TAMs can predictably
target early-stage HCC compared with the treatment of HCC by
inhibiting the pro-tumor effect of M2 TAMs. Early relevant
diagnosis and treatment by enhanced polarization of TAMs towards M1
TAMs can improve the high lethality of advanced HCC. Most studies
macroscopically replace tumor characteristics with only the tumor
cell characteristics, ignoring the characteristics of the immune
cells in HCC. Therefore, more studies on signaling crosstalk
between TAMs and HCC cells should be carried out to investigate the
differences between HCC and other tumor types.
Not applicable.
MinY and HY contributed to the preparation,
creation and description of the work for publication, in particular
writing a first draft (including substantive translation). MinY, HY
and HW contributed to presentation of research ideas and the
development and formation of overall research objectives. CL, XZ
and ZS contributed to the development and design of methods, and
created models. WC and HY contributed to the application of
statistical, mathematical, computer and other forms of techniques
to analyze and integrate research data. XZ, MJ and XX contributed
to implementing research and data/evidence collection. MJ, CL and
XZ provided research materials, reagents, patients, experimental
samples, animals, instruments, computational resources or other
analytical tools. MiaY and MJ carried out metadata management, data
cleansing and data maintenance (including software code needed to
interpret the data) for initial use and subsequent reuse. HW
revised the manuscript. MJ carried out review and revision (both
pre- and post-publication phases). MinY and HW revised the
manuscript. CL and MJ oversaw and led the planning and execution of
research activities. CL and XZ assumed responsibility for the
management and coordination of the planning and execution of
research activities. CL and XZ obtained financial support for this
publication project. Data authentication is not applicable. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Professor Chunhua
Liu, Dr Xiaowei Zhang, Dr Mingchun Jiang, Mrs. Guozheng Xu and Mr.
Changwei Shi [Pharmacy College, Shandong First Medical University
(Shandong Academy of Medical Sciences), Jinan, Shandong, China] for
their guidance and assistance during the writing of the draft. The
authors would like to thank Mr. Yonglin Lu and Mr. Haochen Yang
[School of Clinical Medicine and Basic Medical Science, Shandong
First Medical University (Shandong Academy of Medical Sciences),
Jinan, Shandong, China] for taking care of the team during the
writing process. The authors would like to thank Mr. Jiaju Zhang
(College of Electromechanial Engineering, Qingdao University of
Science and Technology, Qingdao, Shandong, China) for his guidance
and encouragement during the drawing process. The authors would
also like to thank Professor Hongmei Wang (Department of
Pharmacology, School of Medicine, Southeast University, Nanjing,
Jiangsu, China) who contributed to the formulation of the title and
the arrangement of the content at the early stage of writing the
manuscript.
The present study was supported by Projects of Medical and
Health Technology Development Program, Shandong Province (grant no.
202303030702), Shandong Province Medical Health Science and
Technology Development Plan (grant no. 202105010417), Shandong
Provincial Natural Science Foundation (grant no. ZR2021MH151), and
Tai'an Science and Technology Innovation Development Project (grant
nos. 2020NS267, 2022NS149 and 2022NS174).
|
1
|
Jin HR, Wang J, Wang ZJ, Xi MJ, Xia BH,
Deng K and Yang JL: Lipid metabolic reprogramming in tumor
microenvironment: From mechanisms to therapeutics. J Hematol Oncol.
16:1032023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xue R, Zhang Q, Cao Q, Kong R, Xiang X,
Liu H, Feng M, Wang F, Cheng J, Li Z, et al: Liver tumour immune
microenvironment subtypes and neutrophil heterogeneity. Nature.
612:141–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng K, Cai N, Zhu J, Yang X, Liang H and
Zhang W: Tumor-associated macrophages in liver cancer: From
mechanisms to therapy. Cancer Commun (Lond). 42:1112–1140. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilotta MT, Antignani A and Fitzgerald DJ:
Managing the TME to improve the efficacy of cancer therapy. Front
Immunol. 13:9549922022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tacke F: Targeting hepatic macrophages to
treat liver diseases. J Hepatol. 66:1300–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minciuna I, Taru MG, Procopet B and
Stefanescu H: The inter-play between liver sinusoidal endothelial
cells, platelets, and neutrophil extracellular traps in the
development and progression of metabolic dysfunction-associated
steatotic liver disease. J Clin Med. 13:14062024. View Article : Google Scholar
|
|
7
|
McQuiston TJ and Williamson PR:
Paradoxical roles of alveolar macrophages in the host response to
Cryptococcus neoformans. J Infect Chemother. 18:1–9. 2012.
View Article : Google Scholar
|
|
8
|
Yao Z, Bai R, Liu W, Liu Y, Zhou W, Xu Z
and Sheng J: Activation of angiogenin expression in macrophages by
lipopolysaccharide via the TLR4/NF-κB pathway in colitis. Acta
Biochim Biophys Sin (Shanghai). 56:857–865. 2024.PubMed/NCBI
|
|
9
|
Murray PJ: Macrophage Polarization. Annu
Rev Physiol. 79:541–566. 2017. View Article : Google Scholar
|
|
10
|
Mehla K and Singh PK: metabolic regulation
of macrophage polarization in cancer. Trends Cancer. 5:822–834.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms, and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020. View Article : Google Scholar
|
|
12
|
Boutilier AJ and Elsawa SF: Macrophage
polarization states in the tumor microenvironment. Int J Mol Sci.
22:69952021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Li Y, Wang X, Wang P, Essandoh K,
Cui S, Huang W, Mu X, Liu Z, Wang Y, et al: GDF3 protects mice
against sepsis-induced cardiac dysfunction and mortality by
suppression of macrophage pro-inflammatory phenotype. Cells.
9:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Stokes JV, Tan W and Pruett SB: An
optimized flow cytometry panel for classifying macrophage
polarization. J Immunol Methods. 511:1133782022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Domschke G and Gleissner CA: CXCL4-induced
macrophages in human atherosclerosis. Cytokine. 122:1541412019.
View Article : Google Scholar
|
|
16
|
Kadl A, Meher AK, Sharma PR, Lee MY, Doran
AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, et al:
Identification of a novel macrophage phenotype that develops in
response to atherogenic phospholipids via Nrf2. Circ Res.
107:737–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Y, Sun Q, Guan Q, Zhang Z, He Q, He J,
Ji Z, Tian W, Xu X, Liu Y, et al: The XOR-IDH3α axis controls
macrophage polarization in hepatocellular carcinoma. J Hepatol.
79:1172–1184. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Morine Y, Tokuda K, Yamada S,
Saito Y, Nishi M, Ikemoto T and Shimada M: Cancer-associated
fibroblast-induced M2-polarized macrophages promote hepatocellular
carcinoma progression via the plasminogen activator inhibitor-1
pathway. Int J Oncol. 59:592021. View Article : Google Scholar :
|
|
20
|
Gao Y, Yuan Y, Wen S, Chen Y, Zhang Z,
Feng Y, Jiang B, Ma S, Hu R, Fang C, et al: Dual role of ANGPTL8 in
promoting tumor cell proliferation and immune escape during
hepatocarcinogenesis. Oncogenesis. 12:262023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao X, Zheng Z, Liu H, Zhang Y, Kang J,
Kong X, Rong D, Sun G, Sun G, Liu L, et al: Inhibition of APOC1
promotes the transformation of M2 into M1 macrophages via the
ferroptosis pathway and enhances anti-PD1 immunotherapy in
hepatocellular carcinoma based on single-cell RNA sequencing. Redox
Biol. 56:1024632022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu
Y, Zhang Z, Cai S, Xu Y, Li X, et al: Tumor-derived exosomal
miR-934 induces macrophage M2 polarization to promote liver
metastasis of colorectal cancer. J Hematol Oncol. 13:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuo CC, Hanzelmann S, Senturk Cetin N,
Frank S, Zajzon B, Derks JP, Akhade VS, Ahuja G, Kanduri C, Grummt
I, et al: Detection of RNA-DNA binding sites in long noncoding
RNAs. Nucleic Acids Res. 47:e322019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li P, He J, Yang Z, Ge S, Zhang H, Zhong Q
and Fan X: ZNNT1 long noncoding RNA induces autophagy to inhibit
tumorigenesis of uveal melanoma by regulating key autophagy gene
expression. Autophagy. 16:1186–1199. 2020. View Article : Google Scholar :
|
|
25
|
Wang H, Liu Y, Tang A and Zhang X:
Molecular subtypes of clear cell renal carcinoma based on
PCD-related long non-coding RNAs expression: Insights into the
underlying mechanisms and therapeutic strategies. Eur J Med Res.
29:2922024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei H, Li W, Yang M, Fang Q, Nian J, Huang
Y, Wei Q, Huang Z, Liu G, Xu Z, et al: METTL3/16-mediated m(6)A
modification of ZNNT1 promotes hepatocellular carcinoma progression
by activating ZNNT1/osteopontin/S100A9 positive feedback
loop-mediated crosstalk between macrophages and tumour cells. Clin
Immunol. 261:1099242024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Wu W, Jing D, Yang L, Guo H, Wang
L, Zhang W, Pu F and Shao Z: Engineered exosome as targeted lncRNA
MEG3 delivery vehicles for osteosarcoma therapy. J Control Release.
343:107–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Lin Z, Gao Y and Yao T:
Downregulation of long noncoding RNA MEG3 is associated with poor
prognosis and promoter hypermethylation in cervical cancer. J Exp
Clin Cancer Res. 36:52017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu X and Xu L: The RNA-binding protein HuR
in human cancer: A friend or foe? Adv Drug Deliv Rev.
184:1141792022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Majumder M, Chakraborty P, Mohan S,
Mehrotra S and Palanisamy V: HuR as a molecular target for cancer
therapeutics and immune-related disorders. Adv Drug Deliv Rev.
188:1144422022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brauss TF, Winslow S, Lampe S, Scholz A,
Weigert A, Dehne N, von Stedingk K, Schmid T and Brüne B: The
RNA-binding protein HuR inhibits expression of CCL5 and limits
recruitment of macrophages into tumors. Mol Carcinog. 56:2620–2629.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei H, Wu X, Huang L, Long C, Lu Q, Huang
Z, Huang Y, Li W and Pu J: LncRNA MEG3 reduces the ratio of M2/M1
Macrophages Through the HuR/CCL5 axis in hepatocellular carcinoma.
J Hepatocell Carcinoma. 11:543–562. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei Q, Liu G, Huang Z, Huang Y, Huang L,
Huang Z, Wu X, Wei H and Pu J: LncRNA MEG3 inhibits tumor
progression by modulating macrophage phenotypic polarization via
miR-145-5p/DAB2 axis in hepatocellular carcinoma. J Hepatocell
Carcinoma. 10:1019–1035. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su T, Zhang N, Wang T, Zeng J, Li W, Han L
and Yang M: Super Enhancer-Regulated LncRNA LINC01089 induces
alternative splicing of DIAPH3 to drive hepatocellular carcinoma
metastasis. Cancer Res. 83:4080–4094. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng Y, Yin D, Li X, Wang K, Li W, Huang
Y, Liu X, Ren Z, Yang X, Zhang Z, et al: Integration of
transcriptomics and metabolomics reveals a novel gene signature
guided by FN1 associated with immune response in oral squamous cell
carcinoma tumorigenesis. J Cancer Res Clin Oncol. 149:6097–6113.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma L, Jiang J, Si Q, Chen C and Duan Z:
IGF2BP3 enhances the growth of hepatocellular carcinoma tumors by
regulating the properties of macrophages and CD8(+) T cells in the
tumor microenvironment. J Clin Transl Hepatol. 11:1308–1320.
2023.PubMed/NCBI
|
|
37
|
Yang M, Yu T and Han L: Hsa_circ_0010882
facilitates hepatocellular carcinoma progression by modulating
M1/M2 macrophage polarization. J Viral Hepat. 31:189–196. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Z, You L, Hu S, Yu L, Gao Y, Li L and
Zhang S: Hepatocellular carcinoma cell-derived exosomal miR-21-5p
promotes the polarization of tumor-related macrophages (TAMs)
through SP1/XBP1 and affects the progression of hepatocellular
carcinoma. Int Immunopharmacol. 126:1111492024. View Article : Google Scholar
|
|
39
|
Bian Z, Xu C, Wang X, Zhang B, Xiao Y, Liu
L, Zhao S, Huang N, Yang F, Zhang Y, et al: TRIM65/NF2/YAP1
signaling coordinately orchestrates metabolic and immune advantages
in hepatocellular carcinoma. Adv Sci (Weinh). Jul 15–2024.Epub
ahead of print. View Article : Google Scholar
|
|
40
|
Zhu Y, Chen M, Xu D, Li TE, Zhang Z, Li
JH, Wang XY, Yang X, Lu L, Jia HL, et al: The combination of PD-1
blockade with interferon-α has a synergistic effect on
hepatocellular carcinoma. Cell Mol Immunol. 19:726–737. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang M, Wang D, Su N, Lou W, Chen Y, Yang
H, Chen C, Xi F, Chen Y, Deng L and Tang X: TRIM65 knockout
inhibits the development of HCC by polarization tumor-associated
macrophages towards M1 phenotype via JAK1/STAT1 signaling pathway.
Int Immunopharmacol. 128:1114942024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan S, Wang Z, Li N, Guo X, Zhang Y, Ma H,
Peng X, Zhao Y, Li C, Gao L, et al: Transcription factor Zhx2 is a
checkpoint that programs macrophage polarization and antitumor
response. Cell Death Differ. 30:2104–2119. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai Z, Wang Y, Sun N and Zhang C:
Characterizing ligand-receptor interactions and unveiling the
pro-tumorigenic role of CCL16-CCR1 axis in the microenvironment of
hepatocellular carcinoma. Front Immunol. 14:12999532024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu XM, Liao YW, Wang HY, Ji KQ, Li GF and
Zang B: Integrin alphavbeta6 is involved in measles protein-induced
airway immune suppression. Cytokine. 59:59–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu WM, Liu SQ, Zhu KF, Li W, Yang ZJ, Yang
Q, Zhu ZC and Chang J: The ALOX5 inhibitor Zileuton regulates
tumor-associated macrophage M2 polarization by JAK/STAT and
inhibits pancreatic cancer invasion and metastasis. Int
Immunopharmacol. 121:1105052023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu C, Zhu Q, Ma C, Luo C, Nie L, Cai H,
Wang Q, Wang F, Ren H, Yan H, et al: Major vault protein regulates
tumor-associated macrophage polarization through interaction with
signal transducer and activator of transcription 6. Front Immunol.
14:12897952024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang B, Zhu J, Wang Y, Chen W, Fang S, Mao
W, Xu Z, Yang Y, Weng Q, Zhao Z, et al: Targeted xCT-mediated
ferroptosis and protumoral polarization of macrophages is effective
against HCC and enhances the efficacy of the Anti-PD-1/L1 Response.
Adv Sci (Weinh). 10:e22039732023. View Article : Google Scholar
|
|
48
|
Savchenko VL: Regulation of NADPH oxidase
gene expression with PKA and cytokine IL-4 in neurons and
microglia. Neurotox Res. 23:201–213. 2013. View Article : Google Scholar
|
|
49
|
Liu Y, Chen H, Yan X and Zhang J, Deng Z,
Huang M, Gu J and Zhang J: MyD88 in myofibroblasts enhances
nonalcoholic fatty liver disease-related hepatocarcinogenesis via
promoting macrophage M2 polarization. Cell Commun Signal.
22:862024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ravindran C, Cheng YC and Liang SM:
CpG-ODNs induces up-regulated expression of chemokine CCL9 in mouse
macrophages and microglia. Cell Immunol. 260:113–118. 2010.
View Article : Google Scholar
|
|
51
|
Chen S, Wang M, Lu T, Liu Y, Hong W, He X,
Cheng Y, Liu J, Wei Y and Wei X: JMJD6 in tumor-associated
macrophage regulates macrophage polarization and cancer progression
via STAT3/IL-10 axis. Oncogene. 42:2737–2750. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Silswal N, Reis J, Qureshi AA, Papasian C
and Qureshi N: Of mice and men: Proteasome's Role in LPS-Induced
inflammation and tolerance. Shock. 47:445–454. 2017. View Article : Google Scholar
|
|
53
|
Lu F, Zhou J, Chen Q, Zhu J, Zheng X, Fang
N and Qiao L: PSMA5 contributes to progression of lung
adenocarcinoma in association with the JAK/STAT pathway.
Carcinogenesis. 43:624–634. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xie S, Li X, Yan J, Yu H, Chen S and Chen
K: Knockdown of liver cancer cell-secreted exosomal PSMA5 controls
macrophage polarization to restrain cancer progression by blocking
JAK2/STAT3 signaling. Immun Inflamm Dis. 12:e11462024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Y, Tang Y, Jiang H, Zhang X, Chen X,
Guo J, Jin C and Wu M: Exosome-Related FTCD Facilitates M1
macrophage polarization and impacts the prognosis of hepatocellular
carcinoma. Biomolecules. 14:412023. View Article : Google Scholar
|
|
56
|
Liu L, Zhang W, Liu T, Tan Y, Chen C, Zhao
J, Geng H and Ma C: The physiological metabolite α-ketoglutarate
ameliorates osteoarthritis by regulating mitophagy and oxidative
stress. Redox Biol. 62:1026632023. View Article : Google Scholar
|
|
57
|
Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou
X, Zhang J, Zhao S, Zhou BP and Mi J: Metabolic reprogramming of
cancer-associated fibroblasts by IDH3α downregulation. Cell Rep.
10:1335–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang C, Chen C, Hu W, Tao L and Chen J:
Revealing the role of necroptosis microenvironment: FCGBP +
tumor-associated macrophages drive primary liver cancer
differentiation towards cHCC-CCA or iCCA. Apoptosis. 29:460–481.
2024. View Article : Google Scholar
|
|
59
|
Mai Y, Su J, Yang C, Xia C and Fu L: The
strategies to cure cancer patients by eradicating cancer stem-like
cells. Mol Cancer. 22:1712023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao Q, Zong H, Zhu P, Su C, Tang W, Chen
Z and Jin S: Crosstalk between colorectal CSCs and immune cells in
tumorigenesis, and strategies for targeting colorectal CSCs. Exp
Hematol Oncol. 13:62024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu X, Ye Y, Zhu L, Xiao X, Zhou B, Gu Y,
Si H, Liang H, Liu M, Li J, et al: Niche stiffness sustains cancer
stemness via TAZ and NANOG phase separation. Nat Commun.
14:2382023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liang T, Tao T, Wu K, Liu L, Xu W, Zhou D,
Fang H, Ding Q, Huang G and Wu S: Cancer-Associated
fibroblast-induced remodeling of tumor microenvironment in
recurrent bladder cancer. Adv Sci (Weinh). 10:e23032302023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang L, Zhang L, Zhao L, Shao S, Ning Q,
Jing X, Zhang Y, Zhao F, Liu X, Gu S, et al: VEGFA/NRP-1/GAPVD1
axis promotes progression and cancer stemness of triple-negative
breast cancer by enhancing tumor cell-macrophage crosstalk. Int J
Biol Sci. 20:446–463. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang Q, Tsui YM, Zhang VX, Lu AJ, Lee JM,
Lee E, Cheung GC, Li PM, Cheung ET, Chia NH, et al: Reciprocal
interactions between malignant cells and macrophages enhance cancer
stemness and M2 polarization in HBV-associated hepatocellular
carcinoma. Theranostics. 14:892–910. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Qi Y, Zhao T, Li R and Han M:
Macrophage-Secreted S100A4 supports breast cancer metastasis by
remodeling the extracellular matrix in the premetastatic niche.
Biomed Res Int. 2022:98955042022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li Y, Wang J, Song K, Liu S, Zhang H, Wang
F, Ni C, Zhai W, Liang J, Qin Z and Zhang J: S100A4 promotes
hepatocellular carcinogenesis by intensifying fibrosis-associated
cancer cell stemness. Oncoimmunology. 9:17253552020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang A, Lv B, Zhang Y, Yang J, Li J, Li
C, Yu Z and Xia J: Construction of a tumor immune infiltration
macrophage signature for predicting prognosis and immunotherapy
response in liver cancer. Front Mol Biosci. 9:9838402022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wei R, Zhu WW, Yu GY, Wang X, Gao C, Zhou
X, Lin ZF, Shao WQ, Wang SH, Lu M and Qin LX: S100 calcium-binding
protein A9 from tumor-associated macrophage enhances cancer stem
cell-like properties of hepatocellular carcinoma. Int J Cancer.
148:1233–1244. 2021. View Article : Google Scholar
|
|
69
|
Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y,
Huang Y, Qiu Q, Lin J, Huang X, et al: TNF-alpha derived from M2
tumor-associated macrophages promotes epithelial-mesenchymal
transition and cancer stemness through the Wnt/beta-catenin pathway
in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res.
378:41–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen J, Feng W, Sun M, Huang W, Wang G,
Chen X, Yin Y, Chen X, Zhang B, Nie Y, et al: TGF-β1-Induced SOX18
elevation promotes hepatocellular carcinoma progression and
metastasis through transcriptionally upregulating PD-L1 and CXCL12.
Gastroenterology. 167:264–280. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ye S, Huang H, Xiao Y, Han X, Shi F, Luo
W, Chen J, Ye Y, Zhao X, Huang W, et al: Macrophage Dectin-1
mediates Ang II renal injury through neutrophil migration and
TGF-β1 secretion. Cell Mol Life Sci. 80:1842023. View Article : Google Scholar
|
|
72
|
Yao Y, Chen R, Wang G, Zhang Y and Liu F:
Exosomes derived from mesenchymal stem cells reverse EMT via
TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem
Cell Res Ther. 10:2252019. View Article : Google Scholar
|
|
73
|
Liu X, Xu J, Shen B, Xu J and Jiang J:
USP33 promotes pancreatic cancer malignant phenotype through the
regulation of TGFBR2/TGFβ signaling pathway. Cell Death Dis.
14:3622023. View Article : Google Scholar
|
|
74
|
Ye Y, Guo J, Xiao P, Ning J, Zhang R, Liu
P, Yu W, Xu L, Zhao Y and Yu J: Macrophages-induced long noncoding
RNA H19 up-regulation triggers and activates the miR-193b/MAPK1
axis and promotes cell aggressiveness in hepatocellular carcinoma.
Cancer Lett. 469:310–322. 2020. View Article : Google Scholar
|
|
75
|
Chang CH, Chang YT, Tseng TH and Wang CJ:
Mulberry leaf extract inhibit hepatocellular carcinoma cell
proliferation via depressing IL-6 and TNF-α derived from adipocyte.
J Food Drug Anal. 26:1024–1032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang H, Kang B, Ha Y, Lee SH, Kim I, Kim
H, Lee WS, Kim G, Jung S, Rha SY, et al: High serum IL-6 correlates
with reduced clinical benefit of atezolizumab and bevacizumab in
unresectable hepatocellular carcinoma. JHEP Rep. 5:1006722023.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wen JH, Li DY, Liang S, Yang C, Tang JX
and Liu HF: Macrophage autophagy in macrophage polarization,
chronic inflammation and organ fibrosis. Front Immunol.
13:9468322022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ma L, Zhang R, Li D, Qiao T and Guo X:
Fluoride regulates chondrocyte proliferation and autophagy via
PI3K/AKT/mTOR signaling pathway. Chem Biol Interact.
349:1096592021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bi S, Zhang Y, Zhou J, Yao Y, Wang J, Fang
M, Li B, Wu C and Ren C: miR-210 promotes hepatocellular carcinoma
progression by modulating macrophage autophagy through
PI3K/AKT/mTOR signaling. Biochem Biophys Res Commun. 662:47–57.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cai J, Qian X, Qi Q, Han J, Zhu X, Zhang Q
and Xia R: Extracellular ubiquitin inhibits the apoptosis of
hepatoma cells via the involvement of macrophages. Transl Cancer
Res. 9:2855–2864. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
She G, Du JC, Wu W, Pu TT, Zhang Y, Bai
RY, Zhang Y, Pang ZD, Wang HF, Ren YJ, et al: Hippo pathway
activation mediates chemotherapy-induced anti-cancer effect and
cardiomyopathy through causing mitochondrial damage and
dysfunction. Theranostics. 13:560–577. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fang S, Wan X, Zou X, Sun S, Hao X, Liang
C, Zhang Z, Zhang F, Sun B, Li H and Yu B: Arsenic trioxide induces
macrophage autophagy and atheroprotection by regulating
ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell
Death Dis. 12:882021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ai JH, Wen YZ, Dai SJ, Zhang LD, Huang ZJ
and Shi J: Exosomal lncRNA HEIH, an essential communicator for
hepatocellular carcinoma cells and macrophage M2 polarization
through the miR-98-5p/STAT3 axis. J Biochem Mol Toxicol.
38:e236862024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Keawvilai P, Kueanjinda P, Klomsing J and
Palaga T: Coculturing liver cancer cells and monocytes in spheroids
conditions monocytes to adopt tumor-associated macrophage
phenotypes that favor tumor growth via cholesterol metabolism. J
Leukoc Biol. 115:344–357. 2024. View Article : Google Scholar
|
|
86
|
Zhang L, Zhang J, Li P, Li T, Zhou Z and
Wu H: Exosomal hsa_circ_0004658 derived from RBPJ
overexpressed-macrophages inhibits hepatocellular carcinoma
progression via miR-499b-5p/JAM3. Cell Death Dis. 13:322022.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu H, Zhang X, Han D, Cao J and Tian J:
Tumour-associated macrophages mediate the invasion and metastasis
of bladder cancer cells through CXCL8. PeerJ. 8:e87212020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zou S, Chen S, Rao G, Zhang G, Ma M, Peng
B, Du X, Huang W, Lin W, Tian Y and Fu X: Extrachromosomal circular
MiR-17-92 amplicon promotes HCC. Hepatology. 79:79–95. 2024.
View Article : Google Scholar
|
|
89
|
Yu S, Su S, Wang P, Li J, Chen C, Xin H,
Gong Y, Wang H, Ye X, Mao L, et al: Tumor-associated
macrophage-induced circMRCK-alpha encodes a peptide to promote
glycolysis and progression in hepatocellular carcinoma. Cancer
Lett. 591:2168722024. View Article : Google Scholar
|
|
90
|
Qu W, Qiao S, Liu L, Chen Y, Peng C, Hou
Y, Xu Z, Lv M and Wang T: Dectin3 protects against hepatocellular
carcinoma by regulating glycolysis of macrophages. Int
Immunopharmacol. 113(Pt A): 1093842022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ji W, Bai J and Ke Y: Exosomal ZFPM2-AS1
contributes to tumorigenesis, metastasis, stemness, macrophage
polarization, and infiltration in hepatocellular carcinoma through
PKM mediated glycolysis. Environ Toxicol. 38:1332–1346. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang L, Yi X, Xiao X, Zheng Q, Ma L and Li
B: Exosomal miR-628-5p from M1 polarized macrophages hinders m6A
modification of circFUT8 to suppress hepatocellular carcinoma
progression. Cell Mol Biol Lett. 27:1062022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xie Q, Zeng Y, Zhang X and Yu F: The
significance of lipid metabolism reprogramming of tumor-associated
macrophages in hepatocellular carcinoma. Cancer Immunol Immunother.
73:1712024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xie M, Lin Z, Ji X, Luo X, Zhang Z, Sun M,
Chen X, Zhang B, Liang H, Liu D, et al: FGF19/FGFR4-mediated
elevation of ETV4 facilitates hepatocellular carcinoma metastasis
by upregulating PD-L1 and CCL2. J Hepatol. 79:109–125. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and invasiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar
|
|
96
|
Chen J, Zhao D, Zhang L, Zhang J, Xiao Y,
Wu Q, Wang Y and Zhan Q: Tumor-associated macrophage (TAM)-derived
CCL22 induces FAK addiction in esophageal squamous cell carcinoma
(ESCC). Cell Mol Immunol. 19:1054–1066. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jiang Q, Zeng Y, Xu Y, Xiao X, Liu H, Zhou
B, Kong Y, Saw PE and Luo B: Ultrasound molecular imaging as a
potential non-invasive diagnosis to detect the margin of
hepatocarcinoma via CSF-1R Targeting. Front Bioeng Biotechnol.
8:7832020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Pyonteck SM, Akkari L, Schuhmacher AJ,
Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT,
Teijeiro V, et al: CSF-1R inhibition alters macrophage polarization
and blocks glioma progression. Nat Med. 19:1264–1272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Peng S, Chen Y, Gong Y, Li Z, Xie R, Lin
Y, Zou B, Li J and Zeng L: Predictive value of intratumour
inflammatory cytokine mRNA levels of hepatocellular carcinoma
patients and activation of two distinct pathways govern IL-8
induced epithelial-mesenchymal transition in human hepatic cancer
cell lines. Cytokine. 119:81–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yan W, Liu X, Ma H, Zhang H, Song X, Gao
L, Liang X and Ma C: Tim-3 fosters HCC development by enhancing
TGF-β-mediated alternative activation of macrophages. Gut.
64:1593–1604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ganjalikhani Hakemi M, Jafarinia M, Azizi
M, Rezaeepoor M, Isayev O and Bazhin AV: The Role of TIM-3 in
hepatocellular carcinoma: A promising target for immunotherapy?
Front Oncol. 10:6016612020. View Article : Google Scholar
|
|
102
|
Sun G, Liu H, Zhao J, Zhang J, Huang T,
Sun G, Zhao S, Zhang Z, Cao H, Rong D, et al: Macrophage
GSK3β-deficiency inhibits the progression of hepatocellular
carcinoma and enhances the sensitivity of anti-PD1 immunotherapy. J
Immunother Cancer. 10:e0056552022. View Article : Google Scholar
|
|
103
|
Liu D, Luo X, Xie M, Zhang T, Chen X,
Zhang B, Sun M, Wang Y, Feng Y, Ji X, et al: HNRNPC downregulation
inhibits IL-6/STAT3-mediated HCC metastasis by decreasing HIF1A
expression. Cancer Sci. 113:3347–3361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H,
Luo C, Zhou J, Fan J and Zhou S: Tumor-associated neutrophils and
macrophages interaction contributes to intrahepatic
cholangiocarcinoma progression by activating STAT3. J Immunother
Cancer. 9:e0019462021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dong S, Yang Z, Xu P, Zheng W, Zhang B, Fu
F, Mao Z, Yuan J, Chen H and Yu W: Mutually exclusive epigenetic
modification on SIX6 with hypermethylation for precancerous stage
and metastasis emergence tracing. Signal Transduct Target Ther.
7:2082022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu C, Yao Z, Wang J, Zhang W, Yang Y,
Zhang Y, Qu X, Zhu Y, Zou J, Peng S, et al: Macrophage-derived CCL5
facilitates immune escape of colorectal cancer cells via the
p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 27:1765–1781.
2020. View Article : Google Scholar :
|
|
108
|
Chang M and Nguyen TT: Strategy for
treatment of infected diabetic foot ulcers. Acc Chem Res.
54:1080–1093. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yao RR, Li JH, Zhang R, Chen RX and Wang
YH: M2-polarized tumor-associated macrophages facilitated migration
and epithelial-mesenchymal transition of HCC cells via the
TLR4/STAT3 signaling pathway. World J Surg Oncol. 16:92018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lin A, Wang G, Zhao H, Zhang Y, Han Q,
Zhang C, Tian Z and Zhang J: TLR4 signaling promotes a
COX-2/PGE(2)/STAT3 positive feedback loop in hepatocellular
carcinoma (HCC) cells. Oncoimmunology. 5:e10743762016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu M, Zhou C, Weng J, Chen Z, Zhou Q, Gao
J, Shi G, Ke A, Ren N, Sun H and Shen Y: Tumor associated
macrophages-derived exosomes facilitate hepatocellular carcinoma
malignance by transferring lncMMPA to tumor cells and activating
glycolysis pathway. J Exp Clin Cancer Res. 41:2532022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan
AWH, Tong JH, Wong J, Chong CCN, Lai PBS, et al: Hepatoma-intrinsic
CCRK inhibition diminishes myeloid-derived suppressor cell
immunosuppression and enhances immune-checkpoint blockade efficacy.
Gut. 67:931–944. 2018. View Article : Google Scholar
|
|
113
|
Wu J, Gao W, Tang Q, Yu Y, You W, Wu Z,
Fan Y, Zhang L, Wu C, Han G, et al: M2 macrophage-derived exosomes
facilitate HCC metastasis by transferring αM
β2 Integrin to Tumor Cells. Hepatology. 73:1365–1380.
2021. View Article : Google Scholar
|
|
114
|
Lu Y, Han G, Zhang Y, Zhang L, Li Z, Wang
Q, Chen Z, Wang X and Wu J: M2 macrophage-secreted exosomes promote
metastasis and increase vascular permeability in hepatocellular
carcinoma. Cell Commun Signal. 21:2992023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bai ZZ, Li HY, Li CH, Sheng CL and Zhao
XN: Retraction Note: M1 Macrophage-Derived Exosomal MicroRNA-326
Suppresses Hepatocellular Carcinoma Cell Progression Via Mediating
NF-κB Signaling Pathway. Nanoscale Res Lett. 17:1222022. View Article : Google Scholar
|
|
116
|
Luo HL, Luo T, Liu JJ, Wu FX, Bai T, Ou C,
Chen J, Li LQ and Zhong JH: Macrophage polarization-associated
lnc-Ma301 interacts with caprin-1 to inhibit hepatocellular
carcinoma metastasis through the Akt/Erk1 pathway. Cancer Cell Int.
21:4222021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sun D, Luo T, Dong P, Zhang N, Chen J and
Zhang S, Dong L, Janssen HLA and Zhang S: M2-polarized
tumor-associated macrophages promote epithelial-mesenchymal
transition via activation of the AKT3/PRAS40 signaling pathway in
intrahepatic cholangiocarcinoma. J Cell Biochem. 121:2828–2838.
2020. View Article : Google Scholar
|
|
118
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dong S, Guo X, Han F, He Z and Wang Y:
Emerging role of natural products in cancer immunotherapy. Acta
Pharm Sin B. 12:1163–1185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pollard JW: Trophic macrophages in
development and disease. Nat Rev Immunol. 9:259–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Palazon A, Tyrakis PA, Macias D, Veliça P,
Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk
I, et al: An HIF-1α/VEGF-A Axis in Cytotoxic T Cells regulates
tumor progression. Cancer Cell. 32:669–683 e5. 2017. View Article : Google Scholar
|
|
122
|
Xu K, Wu CL, Wang ZX, Wang HJ, Yin FJ, Li
WD, Liu CC and Fan HN: VEGF family gene expression as prognostic
biomarkers for Alzheimer's disease and primary liver cancer. Comput
Math Methods Med. 2021:34223932021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Simons M, Gordon E and Claesson-Welsh L:
Mechanisms and regulation of endothelial VEGF receptor signalling.
Nat Rev Mol Cell Biol. 17:611–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Pu J, Li W, Wang A, Zhang Y, Qin Z, Xu Z,
Wang J, Lu Y, Tang Q and Wei H: Long non-coding RNA HOMER3-AS1
drives hepatocellular carcinoma progression via modulating the
behaviors of both tumor cells and macrophages. Cell Death Dis.
12:11032021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Cheng N, Bei Y, Song Y, Zhang W, Xu L,
Zhang W, Yang N, Bai X, Shu Y and Shen P: B7-H3 augments the
pro-angiogenic function of tumor-associated macrophages and acts as
a novel adjuvant target for triple-negative breast cancer therapy.
Biochem Pharmacol. 183:1142982021. View Article : Google Scholar
|
|
127
|
Mabeta P and Steenkamp V: The VEGF/VEGFR
axis revisited: Implications for cancer therapy. Int J Mol Sci.
23:155852022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Grillet B, Pereira RVS, Van Damme J, Abu
El-Asrar A, Proost P and Opdenakker G: Matrix metalloproteinases in
arthritis: Towards precision medicine. Nat Rev Rheumatol.
19:363–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Peacock JW, Takeuchi A, Hayashi N, Liu L,
Tam KJ, Al Nakouzi N, Khazamipour N, Tombe T, Dejima T, Lee KC, et
al: SEMA3C drives cancer growth by transactivating multiple
receptor tyrosine kinases via Plexin B1. EMBO Mol Med. 10:219–238.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Korbecki J, Siminska D,
Gassowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D and
Baranowska-Bosiacka I: Chronic and cycling hypoxia: Drivers of
cancer chronic inflammation through HIF-1 and NF-kappaB Activation:
A review of the molecular mechanisms. Int J Mol Sci. 22:107012021.
View Article : Google Scholar
|
|
132
|
He H, Chen S, Fan Z, Dong Y, Wang Y, Li S,
Sun X, Song Y, Yang J, Cao Q, et al: Multi-dimensional single-cell
characterization revealed suppressive immune microenvironment in
AFP-positive hepatocellular carcinoma. Cell Discov. 9:602023.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhou Y, Zha Y, Yang Y, Ma T, Li H and
Liang J: S100 proteins in cardiovascular diseases. Mol Med.
29:682023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Casazza A, Laoui D, Wenes M, Rizzolio S,
Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA,
Tamagnone L and Mazzone M: Impeding macrophage entry into hypoxic
tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis
and restores antitumor immunity. Cancer Cell. 24:695–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shao R: YKL-40 acts as an angiogenic
factor to promote tumor angiogenesis. Front Physiol. 4:1222013.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Qi M, Zhou Y, Liu J, Ou X, Li M, Long X,
Ye J and Yu G: AngII induces HepG2 cells to activate
epithelial-mesenchymal transition. Exp Ther Med. 16:3471–3477.
2018.PubMed/NCBI
|
|
137
|
Duran CL, Borriello L, Karagiannis GS,
Entenberg D, Oktay MH and Condeelis JS: Targeting Tie2 in the tumor
microenvironment: From angiogenesis to dissemination. Cancers
(Basel). 13:57302021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bartneck M, Schrammen PL, Mockel D,
Govaere O, Liepelt A, Krenkel O, Ergen C, McCain MV, Eulberg D,
Luedde T, et al: The CCR2(+) macrophage subset promotes pathogenic
angiogenesis for tumor vascularization in fibrotic livers. Cell Mol
Gastroenterol Hepatol. 7:371–390. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xiang X, Pathak JL, Wu W, Li J, Huang W,
Wu Q, Xin M, Wu Y, Huang Y, Ge L and Zeng S: Human serum-derived
exosomes modulate macrophage inflammation to promote VCAM1-mediated
angiogenesis and bone regeneration. J Cell Mol Med. 27:1131–1143.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rabinowitz JD and White E: Autophagy and
metabolism. Science. 330:1344–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Liu Q, Yan X, Li R, Yuan Y, Wang J, Zhao
Y, Fu J and Su J: Polyamine Signal through HCC Microenvironment: A
key regulator of mitochondrial preservation and turnover in TAMs.
Int J Mol Sci. 25:9962024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liu X, Zhang Q, Liang Y, Xiong S, Cai Y,
Cao J, Xu Y, Xu X, Wu Y, Lu Q, et al: Nanoparticles (NPs)-mediated
Siglec15 silencing and macrophage repolarization for enhanced
cancer immunotherapy. Acta Pharm Sin B. 13:5048–5059. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhang X, Wei Z, Yong T, Li S, Bie N, Li J,
Li X, Liu H, Xu H, Yan Y, et al: Cell microparticles loaded with
tumor antigen and resiquimod reprogram tumor-associated macrophages
and promote stem-like CD8(+) T cells to boost anti-PD-1 therapy.
Nat Commun. 14:56532023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Fang W, Zhou T, Shi H, Yao M, Zhang D,
Qian H, Zeng Q, Wang Y, Jin F, Chai C and Chen T: Progranulin
induces immune escape in breast cancer via up-regulating PD-L1
expression on tumor-associated macrophages (TAMs) and promoting
CD8(+) T cell exclusion. J Exp Clin Cancer Res. 40:42021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Caraban BM, Matei E, Cozaru GC, Aşchie M,
Deacu M, Enciu M, Bălţătescu GI, Chisoi A, Dobrin N, Petcu L, et
al: PD-L1, CD4+, and CD8+ Tumor-Infiltrating Lymphocytes (TILs)
expression profiles in melanoma tumor microenvironment cells. J
Pers Med. 13:2212023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yuan L, Tatineni J, Mahoney KM and Freeman
GJ: VISTA: A mediator of quiescence and a promising target in
cancer immunotherapy. Trends Immunol. 42:209–227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Shiri AM, Zhang T, Bedke T, Zazara DE,
Zhao L, Lücke J, Sabihi M, Fazio A, Zhang S, Tauriello DVF, et al:
IL-10 dampens antitumor immunity and promotes liver metastasis via
PD-L1 induction. J Hepatol. 80:634–644. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wen J, Yang S, Yan G, Lei J, Liu X, Zhang
N, Zhang J, Deng H, Wu L and Li Y: Increased OIT3 in macrophages
promotes PD-L1 expression and hepatocellular carcinogenesis via
NF-κB signaling. Exp Cell Res. 428:1136512023. View Article : Google Scholar
|
|
149
|
Wang J, Zhang X, Ma X, Chen D, Cai M, Xiao
L, Li J, Huang Z, Huang Y and Lian Y: Blockage of CacyBP inhibits
macrophage recruitment and improves anti-PD-1 therapy in
hepatocellular carcinoma. J Exp Clin Cancer Res. 42:3032023.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ruf B, Bruhns M, Babaei S, Kedei N, Ma L,
Revsine M, Benmebarek MR, Ma C, Heinrich B, Subramanyam V, et al:
Tumor-associated macrophages trigger MAIT cell dysfunction at the
HCC invasive margin. Cell. 186:3686–3705 e32. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Saadey AA, Yousif A, Osborne N, Shahinfar
R, Chen YL, Laster B, Rajeev M, Bauman P, Webb A and Ghoneim HE:
Rebalancing TGFβ1/BMP signals in exhausted T cells unlocks
responsiveness to immune checkpoint blockade therapy. Nat Immunol.
24:280–294. 2023. View Article : Google Scholar
|
|
152
|
Wang J, Zhou J, Zhou Q, Qi Y, Zhang P, Yan
C and Ren X: Dysregulated Th1 cells in lung squamous cell
carcinoma. J Leukoc Biol. 112:1567–1576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Xun X, Zhang C, Wang S, Hu S, Xiang X,
Cheng Q, Li Z, Wang Y and Zhu J: Cyclooxygenase-2 expressed
hepatocellular carcinoma induces cytotoxic T lymphocytes exhaustion
through M2 macrophage polarization. Am J Transl Res. 13:4360–4375.
2021.PubMed/NCBI
|
|
154
|
Vignali PDA, DePeaux K, Watson MJ, Ye C,
Ford BR, Lontos K, McGaa NK, Scharping NE, Menk AV, Robson SC, et
al: Hypoxia drives CD39-dependent suppressor function in exhausted
T cells to limit antitumor immunity. Nat Immunol. 24:267–279. 2023.
View Article : Google Scholar
|
|
155
|
Lu JC, Zhang PF, Huang XY, Guo XJ, Gao C,
Zeng HY, Zheng YM, Wang SW, Cai JB, Sun QM, et al: Amplification of
spatially isolated adenosine pathway by tumor-macrophage
interaction induces anti-PD1 resistance in hepatocellular
carcinoma. J Hematol Oncol. 14:2002021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wang J, Zhang L, Kang D, Yang D and Tang
Y: Activation of PGE2/EP2 and PGE2/EP4 signaling pathways
positively regulate the level of PD-1 in infiltrating CD8(+) T
cells in patients with lung cancer. Oncol Lett. 15:552–558.
2018.
|
|
157
|
Szefel J, Danielak A and Kruszewski WJ:
Metabolic pathways of L-arginine and therapeutic consequences in
tumors. Adv Med Sci. 64:104–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kumari A, Syeda S, Rawat K, Kumari R and
Shrivastava A: Melatonin modulates L-arginine metabolism in
tumor-associated macrophages by targeting arginase 1 in lymphoma.
Naunyn Schmiedebergs Arch Pharmacol. 397:1163–1179. 2024.
View Article : Google Scholar
|
|
159
|
Wang X, Ye X, Chen Y and Lin J: Mechanism
of M2 type macrophage-derived extracellular vesicles regulating
PD-L1 expression via the MISP/IQGAP1 axis in hepatocellular
carcinoma immunotherapy resistance. Int Immunopharmacol. 124(Pt A):
1108482023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Goswami KK, Bose A and Baral R:
Macrophages in tumor: An inflammatory perspective. Clin Immunol.
232:1088752021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Moreno Ayala MA, Campbell TF, Zhang C,
Dahan N, Bockman A, Prakash V, Feng L, Sher T and DuPage M: CXCR3
expression in regulatory T cells drives interactions with type I
dendritic cells in tumors to restrict CD8(+) T cell antitumor
immunity. Immunity. 56:1613–1630 e5. 2023. View Article : Google Scholar
|
|
162
|
Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu
K, Hu X, Bu Z, Peng J, Ren X and Zhang Z: Immune phenotypic linkage
between colorectal cancer and liver metastasis. Cancer Cell.
40:424–437 e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P and
Xu D: Redefining tumor-associated macrophage subpopulations and
functions in the tumor microenvironment. Front Immunol.
11:17312020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wang H, Tian T and Zhang J:
Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC):
From mechanism to therapy and prognosis. Int J Mol Sci.
22:84702021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-Associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Nowak M and Klink M: The role of
tumor-associated macrophages in the progression and chemoresistance
of ovarian cancer. Cells. 9:12992020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Li C, Xu X, Wei S, Jiang P, Xue L and Wang
J: Senior Correspondence; Tumor-associated macrophages: Potential
therapeutic strategies and future prospects in cancer. J Immunother
Cancer. 9:e0013412021. View Article : Google Scholar
|
|
168
|
Zheng H, Peng X, Yang S, Li X, Huang M,
Wei S, Zhang S, He G, Liu J, Fan Q, et al: Targeting
tumor-associated macrophages in hepatocellular carcinoma: biology,
strategy, and immunotherapy. Cell Death Discov. 9:652023.
View Article : Google Scholar
|
|
169
|
Li Z, Wu T, Zheng B and Chen L:
Individualized precision treatment: Targeting TAM in HCC. Cancer
Lett. 458:86–91. 2019. View Article : Google Scholar
|
|
170
|
Argentiero A, Delvecchio A, Fasano R,
Andriano A, Caradonna IC, Memeo R and Desantis V: The complexity of
the tumor microenvironment in hepatocellular carcinoma and emerging
therapeutic developments. J Clin Med. 12:74692023. View Article : Google Scholar
|
|
171
|
Agirre-Lizaso A, Huici-Izagirre M,
Urretabizkaia-Garmendia J, Rodrigues PM, Banales JM and Perugorria
MJ: Targeting the heterogeneous tumour-associated macrophages in
hepatocellular carcinoma. Cancers (Basel). 15:49772023. View Article : Google Scholar
|
|
172
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar
|
|
173
|
Cassetta L, Fragkogianni S, Sims AH,
Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P,
Lin EY, et al: Human tumor-associated macrophage and monocyte
transcriptional landscapes reveal cancer-specific reprogramming,
biomarkers, and therapeutic targets. Cancer Cell. 35:588–602 e10.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wen J, Wang S, Guo R and Liu D: CSF1R
inhibitors are emerging immunotherapeutic drugs for cancer
treatment. Eur J Med Chem. 245(Pt 1): 1148842023. View Article : Google Scholar
|
|
175
|
Tkach M, Thalmensi J, Timperi E, Gueguen
P, Névo N, Grisard E, Sirven P, Cocozza F, Gouronnec A,
Martin-Jaular L, et al: Extracellular vesicles from triple negative
breast cancer promote pro-inflammatory macrophages associated with
better clinical outcome. Proc Natl Acad Sci USA.
119:e21073941192022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang
PY, Xu HX, Kong LQ, Wang L, Wu WZ and Tang ZY: Depletion of
tumor-associated macrophages enhances the effect of sorafenib in
metastatic liver cancer models by antimetastatic and antiangiogenic
effects. Clin Cancer Res. 16:3420–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Jo H, Loison F and Luo HR: Microtubule
dynamics regulates Akt signaling via dynactin p150. Cell Signal.
26:1707–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Liu Y, Ma J, Lu S, He P and Dong W: USP25
promotes hepatocellular carcinoma progression by interacting with
TRIM21 via the Wnt/β-catenin signaling pathway. Chin Med J (Engl).
136:2229–2242. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Mao J, Wang D, Wang Z, Tian W, Li X, Duan
J, Wang Y, Yang H, You L, Cheng Y, et al: Combretastatin A-1
phosphate, a microtubule inhibitor, acts on both hepatocellular
carcinoma cells and tumor-associated macrophages by inhibiting the
Wnt/β-catenin pathway. Cancer Lett. 380:134–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Xiang X, Wang J, Lu D and Xu X: Targeting
tumor-associated macrophages to synergize tumor immunotherapy.
Signal Transduct Target Ther. 6:752021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y,
Wang F, Duan X, Li J, Zhang W and Wang H: A Natural CCR2 antagonist
relieves tumor-associated macrophage-mediated immunosuppression to
produce a therapeutic effect for liver cancer. EBioMedicine.
22:58–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wu J, Chan YT, Lu Y, Feng Z, Yuan H, Xu X,
Xu L, Zhang C, Feng Y, Tan HY and Wang N: Genipin-activating
PPARgamma impedes CCR2-mediated macrophage infiltration into
postoperative liver to suppress recurrence of hepatocellular
carcinoma. Int J Biol Sci. 19:5257–5274. 2023. View Article : Google Scholar :
|
|
183
|
Hong M, Lee S, Clayton J, Yake W and Li J:
Genipin suppression of growth and metastasis in hepatocellular
carcinoma through blocking activation of STAT-3. J Exp Clin Cancer
Res. 39:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Gallage S, Ali A, Barragan Avila JE,
Seymen N, Ramadori P, Joerke V, Zizmare L, Aicher D, Gopalsamy IK,
Fong W, et al: A 5:2 intermittent fasting regimen ameliorates NASH
and fibrosis and blunts HCC development via hepatic PPARα and PCK1.
Cell Metab. 36:1371–1393 e7. 2024. View Article : Google Scholar
|
|
185
|
Ambade A, Lowe P, Kodys K, Catalano D,
Gyongyosi B, Cho Y, Iracheta-Vellve A, Adejumo A, Saha B, Calenda
C, et al: Pharmacological Inhibition of CCR2/5 signaling prevents
and reverses alcohol-induced liver damage, steatosis, and
inflammation in mice. Hepatology. 69:1105–1121. 2019. View Article : Google Scholar
|
|
186
|
Pozzi S and Satchi-Fainaro R: The role of
CCL2/CCR2 axis in cancer and inflammation: The next frontier in
nanomedicine. Adv Drug Deliv Rev. 209:1153182024. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Sugiyama S, Yumimoto K, Fujinuma S and
Nakayama KI: Identification of effective CCR2 inhibitors for cancer
therapy using humanized mice. J Biochem. 175:195–204. 2024.
View Article : Google Scholar
|
|
188
|
Yuan JM, Grouls M, Carmella SG, Wang R,
Heskin A, Jiang Y, Tan YT, Adams-Haduch J, Gao YT, Hecht SS, et al:
Prediagnostic levels of urinary 8-epi-prostaglandin F2α and
prostaglandin E2 metabolite, biomarkers of oxidative damage and
inflammation, and risk of hepatocellular carcinoma. Carcinogenesis.
40:989–997. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wan Z, Huang H, West RE III, Zhang M,
Zhang B, Cai X, Zhang Z, Luo Z, Chen Y, Zhang Y, et al: Overcoming
pancreatic cancer immune resistance by codelivery of CCR2
antagonist using a STING-activating gemcitabine-based nanocarrier.
Mater Today (Kidlington). 62:33–50. 2023. View Article : Google Scholar
|
|
190
|
Sattiraju A, Kang S, Giotti B, Chen Z,
Marallano VJ, Brusco C, Ramakrishnan A, Shen L, Tsankov AM,
Hambardzumyan D, et al: Hypoxic niches attract and sequester
tumor-associated macrophages and cytotoxic T cells and reprogram
them for immunosuppression. Immunity. 56:1825–1843 e6. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Chen Y, Ramjiawan RR, Reiberger T, Ng MR,
Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, et al:
CXCR4 inhibition in tumor microenvironment facilitates
anti-programmed death receptor-1 immunotherapy in sorafenib-treated
hepatocellular carcinoma in mice. Hepatology. 61:1591–1602. 2015.
View Article : Google Scholar
|
|
192
|
White CW, Platt S, Kilpatrick LE, Dale N,
Abhayawardana RS, Dekkers S, Kindon ND, Kellam B, Stocks MJ,
Pfleger KDG and Hill SJ: CXCL17 is an allosteric inhibitor of CXCR4
through a mechanism of action involving glycosaminoglycans. Sci
Signal. 17:eabl37582024. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Klaver D, Gander H, Frena B, Amato M and
Thurnher M: Crosstalk between purinergic receptor P2Y11
and chemokine receptor CXCR7 is regulated by CXCR4 in human
macrophages. Cell Mol Life Sci. 81:1322024. View Article : Google Scholar
|
|
194
|
Ashrafizadeh M, Zarrabi A, Hashemi F,
Zabolian A, Salek i H, Bagher ian M, Azam i N, Bejandi A K,
Hushmandi K, Ang HL, et al: Polychemotherapy with curcumin and
doxorubicin via biological nanoplatforms: enhancing antitumor
activity. Pharmaceutics. 12:10842020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Ma X, Yang S, Zhang T, Wang S, Yang Q,
Xiao Y, Shi X, Xue P, Kang Y, Liu G, et al: Bioresponsive
immune-booster-based prodrug nanogel for cancer immunotherapy. Acta
Pharm Sin B. 12:451–466. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Jang Y, Cho YS, Kim A, Zhou X, Kim Y, Wan
Z, Moon JJ and Park H: CXCR4-Targeted macrophage-derived biomimetic
hybrid vesicle nanoplatform for enhanced cancer therapy through
codelivery of manganese and doxorubicin. ACS Appl Mater Interfaces.
16:17129–17144. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Ries CH, Cannarile MA, Hoves S, Benz J,
Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I,
et al: Targeting tumor-associated macrophages with anti-CSF-1R
antibody reveals a strategy for cancer therapy. Cancer Cell.
25:846–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Hashimoto A, Sarker D, Reebye V, Jarvis S,
Sodergren MH, Kossenkov A, Sanseviero E, Raulf N, Vasara J,
Andrikakou P, et al: Upregulation of C/EBPα inhibits suppressive
activity of myeloid cells and potentiates antitumor response in
mice and patients with cancer. Clin Cancer Res. 27:5961–5978. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Chen F, Gong M, Weng D, Jin Z, Han G, Yang
Z, Han J and Wang J: Phellinus linteus activates Treg cells via FAK
to promote M2 macrophage polarization in hepatocellular carcinoma.
Cancer Immunol Immunother. 73:182024. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Ao JY, Zhu XD, Chai ZT, Cai H, Zhang YY,
Zhang KZ, Kong LQ, Zhang N, Ye BG, Ma DN and Sun HC:
Colony-Stimulating Factor 1 receptor blockade inhibits tumor growth
by altering the polarization of tumor-associated macrophages in
hepatocellular carcinoma. Mol Cancer Ther. 16:1544–1554. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Yang Y, Qin J, Lan L, Li N, Wang C, He P,
Liu F, Ni H and Wang Y: M-CSF cooperating with NFκB induces
macrophage transformation from M1 to M2 by upregulating c-Jun.
Cancer Biol Ther. 15:99–107. 2014. View Article : Google Scholar
|
|
202
|
Fang C, Zhong R, Lu S, Yu G, Liu Z, Yan C,
Gao J, Tang Y, Wang Y, Zhao Q and Feng X: TREM2 promotes macrophage
polarization from M1 to M2 and suppresses osteoarthritis through
the NF-κB/CXCL3 axis. Int J Biol Sci. 20:1992–2007. 2024.
View Article : Google Scholar :
|
|
203
|
Zhu D, Huang M, Shen P, Zhang B, Chen G,
Chen J, Duan L and Duan Y: TREM2 expression promotes liver and
peritoneal M2 macrophage polarization in mice infected with
Schistosoma japonicum. J Cell Mol Med. 27:2261–2269. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Ye Y, Song Y, Zhuang J, Wang G, Ni J and
Xia W: Anticancer effects of echinacoside in hepatocellular
carcinoma mouse model and HepG2 cells. J Cell Physiol.
234:1880–1888. 2019. View Article : Google Scholar
|
|
205
|
Yang T, Zhang S, Yuan H, Wang Y, Cai L,
Chen H and Wang X, Song D and Wang X, Guo Z and Wang X:
Platinum-Based TREM2 inhibitor suppresses tumors by remodeling the
immunosuppressive microenvironment. Angew Chem Int Ed Engl.
62:e2022133372023. View Article : Google Scholar
|
|
206
|
Zhou L, Wang M, Guo H, Hou J, Zhang Y, Li
M, Wu X, Chen X and Wang L: Integrated analysis highlights the
immunosuppressive role of TREM2(+) macrophages in hepatocellular
carcinoma. Front Immunol. 13:8483672022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Tan J, Fan W, Liu T, Zhu B, Liu Y, Wang S,
Wu J, Liu J, Zou F, Wei J, et al: TREM2(+) macrophages suppress
CD8(+) T-cell infiltration after transarterial chemoembolisation in
hepatocellular carcinoma. J Hepatol. 79:126–140. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Esparza-Baquer A, Labiano I, Sharif O,
Agirre-Lizaso A, Oakley F, Rodrigues PM, Zhuravleva E, O'Rourke CJ,
Hijona E, Jimenez-Agüero R, et al: TREM-2 defends the liver against
hepatocellular carcinoma through multifactorial protective
mechanisms. Gut. 70:1345–1361. 2021. View Article : Google Scholar
|
|
209
|
Binnewies M, Pollack JL, Rudolph J, Dash
S, Abushawish M, Lee T, Jahchan NS, Canaday P, Lu E, Norng M, et
al: Targeting TREM2 on tumor-associated macrophages enhances
immunotherapy. Cell Rep. 37:1098442021. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Tan HY, Wang N, Man K, Tsao SW, Che CM and
Feng Y: Autophagy-induced RelB/p52 activation mediates
tumour-associated macrophage repolarisation and suppression of
hepatocellular carcinoma by natural compound baicalin. Cell Death
Dis. 6:e19422015. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Xu G, Feng D, Yao Y, Li P, Sun H, Yang H,
Li C, Jiang R, Sun B and Chen Y: Listeria-based hepatocellular
carcinoma vaccine facilitates anti-PD-1 therapy by regulating
macrophage polarization. Oncogene. 39:1429–1444. 2020. View Article : Google Scholar
|
|
212
|
Zhou B, Yang Y and Li C: SIRT1 inhibits
hepatocellular carcinoma metastasis by promoting M1 macrophage
polarization via NF-κB pathway. Onco Targets Ther. 12:2519–2529.
2019. View Article : Google Scholar :
|
|
213
|
Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, Luo
X, Chen R and Chen T: Long non-coding RNA cox-2 prevents immune
evasion and metastasis of hepatocellular carcinoma by altering
M1/M2 macrophage polarization. J Cell Biochem. 119:2951–2963. 2018.
View Article : Google Scholar
|
|
214
|
Vyas AK, Jindal A, Hissar S, Ramakrishna G
and Trehanpati N: Immune balance in Hepatitis B Infection: Present
and future therapies. Scand J Immunol. 86:4–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Anderson NR, Minutolo NG, Gill S and
Klichinsky M: Macrophage-Based approaches for cancer immunotherapy.
Cancer Res. 81:1201–1208. 2021. View Article : Google Scholar
|
|
216
|
Bao D, Zhao J, Zhou X, Yang Q, Chen Y, Zhu
J, Yuan P, Yang J, Qin T, Wan S and Xing J: Mitochondrial
fission-induced mtDNA stress promotes tumor-associated macrophage
infiltration and HCC progression. Oncogene. 38:5007–5020. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Rodell CB, Arlauckas SP, Cuccarese MF,
Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ and Weissleder R:
TLR7/8-agonist-loaded nanoparticles promote the polarization of
tumour-associated macrophages to enhance cancer immunotherapy. Nat
Biomed Eng. 2:578–588. 2018. View Article : Google Scholar :
|
|
218
|
Powers N, Massena C, Crouse B, Smith M,
Hicks L, Evans JT, Miller S, Pravetoni M and Burkhart D:
Self-Adjuvanting TLR7/8 agonist and fentanyl hapten co-conjugate
achieves enhanced protection against fentanyl challenge. Bioconjug
Chem. 34:1811–1821. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Kim H, Khanna V, Kucaba TA, Zhang W,
Sehgal D, Ferguson DM, Griffith TS and Panyam J: TLR7/8
Agonist-Loaded Nanoparticles Augment NK Cell-Mediated
Antibody-Based Cancer Immunotherapy. Mol Pharm. 17:2109–2124. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Kim H, Griffith TS and Panyam J:
Poly(d,l-lactide-co-glycolide) Nanoparticles as delivery platforms
for TLR7/8 agonist-based cancer vaccine. J Pharmacol Exp Ther.
370:715–724. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Davenne T, Bridgeman A, Rigby RE and
Rehwinkel J: Deoxyguanosine is a TLR7 agonist. Eur J Immunol.
50:56–62. 2020. View Article : Google Scholar :
|
|
222
|
Hirsch E, Katanaev VL, Garlanda C,
Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F
and Wymann MP: Central role for G protein-coupled phosphoinositide
3-kinase gamma in inflammation. Science. 287:1049–1053. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Zhang Y, Long X, Ruan X, Wei Q, Zhang L,
Wo L, Huang D, Lin L, Wang D, Xia L, et al: SIRT2-mediated
deacetylation and deubiquitination of C/EBPbeta prevents
ethanol-induced liver injury. Cell Discov. 7:932021. View Article : Google Scholar
|
|
224
|
Kaneda MM, Messer KS, Ralainirina N, Li H,
Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P,
et al: PI3Kgamma is a molecular switch that controls immune
suppression. Nature. 539:437–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Rao J, Wang H, Ni M, Wang Z, Wang Z, Wei
S, Liu M, Wang P, Qiu J, Zhang L, et al: FSTL1 promotes liver
fibrosis by reprogramming macrophage function through modulating
the intracellular function of PKM2. Gut. 71:2539–2550. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Yang XM, Wang XQ, Hu LP, Feng MX, Zhou YQ,
Li DX, Li J, Miao XC, Zhang YL, Yao LL, et al: Nucleolar HEAT
Repeat Containing 1 up-regulated by the mechanistic target of
rapamycin complex 1 signaling promotes hepatocellular carcinoma
growth by dominating ribosome biogenesis and proteome homeostasis.
Gastroenterology. 165:629–646. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
O'Grady S, Crown J and Duffy MJ: Statins
inhibit proliferation and induce apoptosis in triple-negative
breast cancer cells. Med Oncol. 39:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Sprinzl MF, Puschnik A, Schlitter AM,
Schad A, Ackermann K, Esposito I, Lang H, Galle PR, Weinmann A,
Heikenwälder M and Protzer U: Sorafenib inhibits macrophage-induced
growth of hepatoma cells by interference with insulin-like growth
factor-1 secretion. J Hepatol. 62:863–870. 2015. View Article : Google Scholar
|
|
229
|
Wu L, Zhang X, Zheng L, Zhao H, Yan G,
Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al: RIPK3 Orchestrates
Fatty Acid Metabolism in Tumor-Associated Macrophages and
Hepatocarcinogenesis. Cancer Immunol Res. 8:710–721. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Hu Q, Li Y, Chen H, Liao H, He Y and Zheng
Q: CCDC88A Post-Transcriptionally Regulates VEGF via miR-101 and
subsequently regulates hepatocellular carcinoma. Front Immunol.
13:8593312022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Xin Y, Tang L, Chen J, Chen D, Wen W and
Han F: Inhibition of miR-101-3p protects against sepsis-induced
myocardial injury by inhibiting MAPK and NF-κB pathway activation
via the upregulation of DUSP1. Int J Mol Med. 47:202021. View Article : Google Scholar
|
|
232
|
Delire B and Starkel P: The Ras/MAPK
pathway and hepatocarcinoma: pathogenesis and therapeutic
implications. Eur J Clin Invest. 45:609–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Takizawa H and Manz MG: Macrophage
tolerance: CD47-SIRP-alpha-mediated signals matter. Nat Immunol.
8:1287–1289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
McCracken MN, Cha AC and Weissman IL:
Molecular Pathways: Activating T Cells after Cancer Cell
Phagocytosis from Blockade of CD47 'Don't Eat Me' Signals. Clin
Cancer Res. 21:3597–3601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang
Y and Kim IS: Exosome-SIRPα, a CD47 blockade increases cancer cell
phagocytosis. Biomaterials. 121:121–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Xiao Z, Chung H, Banan B, Manning PT, Ott
KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, et
al: Antibody mediated therapy targeting CD47 inhibits tumor
progression of hepatocellular carcinoma. Cancer Lett. 360:302–309.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Lakhani NJ, Chow LQM, Gainor JF, LoRusso
P, Lee KW, Chung HC, Lee J, Bang YJ, Hodi FS, Kim WS, et al:
Evorpacept alone and in combination with pembrolizumab or
trastuzumab in patients with advanced solid tumours (ASPEN-01): A
first-in-human, open-label, multicentre, phase 1 dose-escalation
and dose-expansion study. Lancet Oncol. 22:1740–1751. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Vaeteewoottacharn K, Kariya R, Pothipan P,
Fujikawa S, Pairojkul C, Waraasawapati S, Kuwahara K, Wongkham C,
Wongkham S and Okada S: Attenuation of CD47-SIRPα signal in
cholangiocarcinoma potentiates tumor-associated macrophage-mediated
phagocytosis and suppresses intrahepatic metastasis. Transl Oncol.
12:217–225. 2019. View Article : Google Scholar
|
|
239
|
Yang HD, Kim HS, Kim SY, Na MJ, Yang G,
Eun JW, Wang HJ, Cheong JY, Park WS and Nam SW: HDAC6 Suppresses
Let-7i-5p to Elicit TSP1/CD47-Mediated anti-tumorigenesis and
phagocytosis of hepatocellular carcinoma. Hepatology. 70:1262–1279.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Du K, Li Y, Liu J, Chen W, Wei Z, Luo Y,
Liu H, Qi Y, Wang F and Sui J: A bispecific antibody targeting GPC3
and CD47 induced enhanced antitumor efficacy against dual
antigen-expressing HCC. Mol Ther. 29:1572–1584. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Kamber RA, Nishiga Y, Morton B, Banuelos
AM, Barkal AA, Vences-Catalán F, Gu M, Fernandez D, Seoane JA, Yao
D, et al: Inter-cellular CRISPR screens reveal regulators of cancer
cell phagocytosis. Nature. 597:549–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Chen M, Mao A, Xu M, Weng Q, Mao J and Ji
J: CRISPR-Cas9 for cancer therapy: Opportunities and challenges.
Cancer Lett. 447:48–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Mout R and Rotello VM: Cytosolic and
nuclear delivery of CRISPR/Cas9-ribonucleoprotein for gene editing
using arginine functionalized gold nanoparticles. Bio Protoc.
7:e25862017. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Ray M, Lee YW, Hardie J, Mout R, Yeşilbag
Tonga G, Farkas ME and Rotello VM: CRISPRed macrophages for
cell-based cancer immunotherapy. Bioconjug Chem. 29:445–450. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Quail DF, Bowman RL, Akkari L, Quick ML,
Schuhmacher AJ, Huse JT, Holland EC, Sutton JC and Joyce JA: The
tumor microenvironment underlies acquired resistance to CSF-1R
inhibition in gliomas. Science. 352:aad30182016. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Patwardhan PP, Surriga O, Beckman MJ, de
Stanchina E, Dematteo RP, Tap WD and Schwartz GK: Sustained
inhibition of receptor tyrosine kinases and macrophage depletion by
PLX3397 and rapamycin as a potential new approach for the treatment
of MPNSTs. Clin Cancer Res. 20:3146–3158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Welford AF, Biziato D, Coffelt SB, Nucera
S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM
and Lewis CE: TIE2-expressing macrophages limit the therapeutic
efficacy of the vascular-disrupting agent combretastatin A4
phosphate in mice. J Clin Invest. 121:1969–1973. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
De Palma M and Naldini L: Angiopoietin-2
TIEs up macrophages in tumor angiogenesis. Clin Cancer Res.
17:5226–5232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
De Palma M, Venneri MA, Galli R, Sergi
Sergi L, Politi LS, Sampaolesi M and Naldini L: Tie2 identifies a
hematopoietic lineage of proangiogenic monocytes required for tumor
vessel formation and a mesenchymal population of pericyte
progenitors. Cancer Cell. 8:211–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
De Palma M, Murdoch C, Venneri MA, Naldini
L and Lewis CE: Tie2-expressing monocytes: Regulation of tumor
angiogenesis and therapeutic implications. Trends Immunol.
28:519–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Quail DF and Joyce JA: Molecular pathways:
Deciphering mechanisms of resistance to macrophage-targeted
therapies. Clin Cancer Res. 23:876–884. 2017. View Article : Google Scholar :
|
|
252
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Koivisto AP, Belvisi MG, Gaudet R and
Szallasi A: Advances in TRP channel drug discovery: From target
validation to clinical studies. Nat Rev Drug Discov. 21:41–59.
2022. View Article : Google Scholar
|
|
254
|
Wang W, Liu P, Zhang Y, Yan L, Zhu MX,
Wang J and Yu Y: Expression and functions of transient receptor
potential channels in liver diseases. Acta Pharm Sin B. 13:445–459.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Liu Y, Lyu Y, Zhu L and Wang H: Role of
TRP channels in liver-related diseases. Int J Mol Sci.
24:125092023. View Article : Google Scholar : PubMed/NCBI
|