Introduction
Acute myeloid leukemia (AML) is a hematological
malignancy of myeloid cells and is the most common type of leukemia
in adults (1). AML is
characterized mainly by the clonal proliferation of myeloid
precursors and genetic abnormalities of cell components, such as
chromosomal alterations and genetic mutations (2,3),
and predominantly exhibits single lineage dysplasia; however,
blasts from patients are heterogeneous for the antigens expressed
(4,5). Leukemia is associated with genomic
instability and clonal heterogeneity; the heterogeneity includes
differences in tumor cell morphology, cell surface markers and
functional characteristics (6).
The etiology of most AML cases is currently unclear, but a number
of risk factors exist (7).
Moreover, the morbidity of AML has been reported to be increasing
annually, the mortality rate remains high and treatment efficacy is
poor. Notably, the global 5-year survival rate of AML is <50%
(8,9). Therefore, it is necessary to clarify
the molecular pathogenesis of AML and to identify potential
therapeutic strategies.
Circular RNAs (circRNAs) are novel and extensive
noncoding RNAs that regulate key functions in different types of
cancer. The heterogeneity of circRNAs in tissues contributes to
cell transformation and the development of numerous diseases,
promotes the occurrence of tumors, and is a molecular biomarker and
intervention target for the clinical diagnosis, treatment and
prognosis of various types of cancer, including colorectal cancer,
AML, hepatocellular carcinoma and gastric carcinoma (10-12). circRNA_0016624 and circHIPK3 have
been shown to mediate the related mechanisms of osteoblasts and
osteoporotic cells, and serve as new therapeutic targets for
osteoporosis (13). Researchers
studying preeclampsia have successfully identified several circRNAs
that may be used as promising biomarkers for the prediction and
diagnosis of this disease (14).
Furthermore, previous studies have shown that circRNA imbalance
occurs in a variety of tumors. In gastric cancer, circPVT1
(15), circLARP4 (16), hsa-circ-0000096 (17) and circ-100269 (18) have been shown to promote tumor
growth. Among the different circRNAs involved in cancer, circPVT1
has been reported to exhibit important carcinogenic features in
multiple types of cancer. Notably, circPVT1 can promote the
development of osteosarcoma by regulating the microRNA
(miRNA/miR)-205-5p/c-FLIP axis (19). Abnormal expression of circPVT1 has
also been shown to participate in the chemoresistance of lung
adenocarcinoma by regulating the miR-145-5p/ABCC1 axis (20). Another study reported that
circPVT1 is highly expressed in breast cancer tissues and cells,
and inhibition of circPVT1 expression can significantly inhibit the
proliferation and epithelial-mesenchymal transition (EMT) of breast
cancer cells, and promote cell apoptosis (21). In addition, circPVT1 has been
suggested to be carcinogenic in AML (22). However, there are few reports
(23,24) on the specific molecular mechanisms
underlying the effects of circPVT1 on AML progression. Therefore,
the present study aimed to further explore the function of
circPVT1, in order to provide potential molecular targets for the
treatment of AML.
A number of studies have shown that the abnormal
expression or mutation of the muscle cell enhancer factor 2 (MEF2)
family (MEF2A, MEF2B, MEF2C and MEF2D) is inextricably linked with
cancer, including large B-cell lymphoma (25) leukemia (26), and the solid tumors liver cancer
(27) and colorectal cancer
(28). MEF2A, as a transcription
factor, can regulate the expression of various genes, thereby
affecting the cancer process. A previous study reported that MEF2A
binds to the lncHCP5 promoter region, promotes lncHCP5 expression
and affects the progression of gastric cancer (29). MEF2A can also promote colorectal
cancer progression by upregulating the expression of ZEB2 and
CTNNB1 (30). Another study
reported that MEF2A and MEF2D could regulate pancreatic cancer
progression by controlling transcription of FNIP1 and FNIP2
(31). These findings suggested
that MEF2A may serve an important regulatory role in cancer
progression. In addition, our research group analyzed the promoter
binding site of circPVT1 and found that the transcription factor
MEF2A can bind the promoter region of circPVT1 with a high
affinity. However, the mechanism by which MEF2A acts as a
transcription factor for circPVT1 in AML progression is not yet
clear; thus, the present study may provide an opportunity to
develop new AML therapeutic strategies involving MEF2A as a
transcription factor for circPVT1.
A number of studies have shown that circRNAs
participate in the competitive endogenous RNA (ceRNA) mechanism, in
which RNAs with miRNA response elements regulate each other, and
exert their biological functions via means of sponging miRNAs
(32,33). Our previous study confirmed the
low expression of miR-455-3p in AML (34). Therefore, it was hypothesized that
circPVT1 may be involved in the ceRNA mechanism as a miR-455-3p
sponge and serve a role in the progression of AML. miR-455-3p is an
important tumor suppressor that serves an important role in
numerous tumors, and reducing its expression can increase
pancreatic cancer cell migration (35) and promote the malignant
progression of colorectal cancer (36). By contrast, antagonism of
miR-455-3p has been shown to inhibit chemoresistance and
invasiveness in esophageal squamous cell carcinoma (37), and a similar effect has been
observed in non-small cell lung cancer cells (38), triple-negative breast cancer cells
(39) and A375 melanoma cells
(40). Therefore, miR-455-3p may
be closely related to human diseases; however, few studies
(34) have investigated the
potential regulatory mechanisms of miR-455-3p in AML. One aim of
the current study was to identify the molecular mechanism of
miR-455-3p in AML.
The present study explored the influence of MEF2A, a
transcription factor of circPVT1, on the development of AML, and
assessed the molecular mechanism underlying the involvement of
miR-455-3p in the pathogenesis of AML.
Materials and methods
Collection of clinical data and patient
samples
Blood plasma samples from 42 patients with AML and
42 healthy controls (25 men and 17 women; mean age, 44.54 years;
range, 19-76 years) were collected from The First Affiliated
Hospital of Kunming Medical University (Kunming, China) between
April 2021 and March 2022. The AML group consisted of 22 men and 20
women, with a mean age of 46.62 years (range, 16-80 years). The
criteria for the selection of patients in this study included: i)
Male or female patients with a body weight ≥45 kg; ii) no bleeding
tendency; iii) no obvious abnormalities of heart, liver and kidney
function; iv) expected survival, ≥3 months; v) women of
childbearing age must have had a pregnancy test (serum or urine)
within 7 days of enrollment, with a negative result, and be willing
to use an appropriate method of contraception during the trial.
Patients with other serious diseases or who started treatment
within 3 months before admission were excluded, and those in the
healthy control group had normal physiological function according
to whole-body physiological examinations conducted at the
aforementioned hospital. The diagnosis of AML was made according to
the 2016 World Health Organization criteria (41). The basic information for all
patients is shown in Table I. The
present study was approved by the Ethics Committee of The First
Affiliated Hospital of Kunming Medical University (approval no.
L-10), and all individuals provided written informed consent for
participation.
 | Table IClinical and biological
characteristics of patients with AML. |
Table I
Clinical and biological
characteristics of patients with AML.
| Case | Age, years | Sex | WHO
classification | Fusion gene | Mutation | Cytogenetics |
|---|
| 1 | 40 | Male | AML | | FLT-ITD | Normal |
| 2 | 58 | Female | B-ALL | | | Normal |
| 3 | 43 | Female | AML | | TP53 | Normal |
| 4 | 46 | Female | AML | AML1-ETO | |
t(8;21)(q22;q22.1) |
| 5 | 72 | Female | AML | | | Normal |
| 6 | 51 | Female | AML | | NPM1 | Normal |
| 7 | 25 | Male | AML | | | Normal |
| 8 | 66 | Male | AML | | | Normal |
| 9 | 51 | Female | AML | | RUNX1 | Normal |
| 10 | 51 | Male | AML | | | Normal |
| 11 | 77 | Male | AML | | | Normal |
| 12 | 23 | Male | APL | PML-RARA | |
t(15;17)(q22;q12) |
| 13 | 44 | Female | AML | AML1-ETO | |
t(8;21)(q22;q22.1) |
| 14 | 70 | Female | AML | | | Normal |
| 15 | 50 | Male | AML | | TP53 | Normal |
| 16 | 52 | Female | AML | | | Normal |
| 17 | 41 | Male | AML | | | Normal |
| 18 | 78 | Male | APL | | ASLX1 | Normal |
| 19 | 50 | Female | APL | PML-RARA | |
t(15;17)(q22;q12) |
| 20 | 47 | Male | AML | | | Normal |
| 21 | 23 | Male | ALL | MLL-AF4 | | Normal |
| 22 | 52 | Female | ALL | BCR-ABL 210 | |
t(9;22)(q34.1;q11.2) |
| 23 | 45 | Male | APL | PML-RARA | NPM1 |
t(15;17)(q22;q12) |
| 24 | 48 | Male | AML | | ASLX1 | Normal |
| 25 | 57 | Male | APL | PML-RARA | |
t(15;17)(q22;q12) |
| 26 | 53 | Male | AML | | | Normal |
| 27 | 36 | Male | APL | PML-RARA | |
t(15;17)(q22;q12) |
| 28 | 50 | Female | AML | | NPM1 | Normal |
| 29 | 19 | Male | AML | | | Normal |
| 30 | 39 | Male | B-ALL | | | Normal |
| 31 | 35 | Female | T-ALL | | | Normal |
| 32 | 22 | Female | AML | | | Normal |
| 33 | 23 | Male | T-ALL | | NPM1 | Normal |
| 34 | 45 | Female | AML | | | Normal |
| 35 | 51 | Female | AML | | | Normal |
| 36 | 80 | Female | AML | | | Normal |
| 37 | 16 | Male | AML | | | Normal |
| 38 | 25 | Male | AML | | | Normal |
| 39 | 57 | Female | AML | PML-RARA | |
t(15;17)(q22;q12) |
| 40 | 49 | Female | AML | | TP53 | Normal |
| 41 | 34 | Male | AML | | | Normal |
| 42 | 64 | Female | AML | | | Normal |
Cell culture
The following cell lines were used in the present
study: A normal bone marrow stromal cell line (HS-5) and human AML
cell lines (U937, THP-1, HL-60 and NB4), which were purchased from
Shenzhen Haodi Huatuo Biotechnology Co., Ltd. All cells were
cultured in DMEM (MilliporeSigma) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(MilliporeSigma), and were incubated at 37°C in a 5% CO2
incubator. The cells were subcultured when the cell density reached
90%. The cell lines used in the present study were authenticated by
short tandem repeat profiling.
CpG island region in the gene promoter of
circPVT1
The circPVT1 promoter region was identified by
searching the UCSC database (http://genome.ucsc.edu/). Specifically, GRCh38/hg38
was used as the human reference genome. CircPVT1 (hsa_circ_0085536)
was located at chromosome chr8:128806778-128903244, and the
sequence 2,000 bp upstream of its transcription start site was
selected as the promoter region. MethPrimer (http://www.urogene.org/methprimer/) (42) was used to analyze the CpG island
regions in the circPVT1 promoter sequence. Specifically, the
minimum CpG island length was set at 200 bp, the CpG percentage
threshold was 50%, and the expected CpG island ratio threshold was
0.6.
Gene promoter and transcription
factor-binding sites of circPVT1
The circPVT1 promoter sequence was identified as
aforementioned. JASPAR (https://jaspar.genereg.net/) was used to analyze the
transcription factors to which the circPVT1 promoter sequence might
bind. The relative profile score threshold was set to 80%.
Cell transfection
NB4 or HL-60 cells were seeded at a density of
2×105 cells/well, and were transfected with 50 nM
miR-455-3p mimic, miR-455-3p inhibitor, small interfering RNA
(siRNA/si)-PVT1, si-MCL1 and 2 μg pcDNA3.1-MEF2A vector
(oe-MEF2A) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Negative control (NC) mimic, NC
inhibitor, NC-small interfering siRNA (NC-si) and pcDNA3.1 empty
vector (NC-oe) were used as NCs. The cells were cultured at 37°C
for 48 h. All oligonucleotides (Table SI) were designed and synthesized
by Shanghai GenePharma Co., Ltd. The transfection efficiency was
measured 48 h after transfection.
Binding site query
The biological information database StarBase
(http://starbase.sysu.edu.cn/) was used
to analyze the binding sites of circPVT1 and miR-455-3p or
miR-455-3p and MCL1.
Dual-luciferase reporter gene assay
The circPVT1 and MCL1 3′UTRs containing the
miR-455-3p binding site were cloned and inserted into the
pGL3-Basic vector (Promega Corporation) to construct the
PVT1-wild-type (WT) vector and the MCL1-WT vector, respectively.
The 3′UTRs of circPVT1 and MCL1 containing mutant (MUT) binding
sequences of miR-455-3p were also amplified and inserted into the
pGL3-Basic vector downstream of the firefly luciferase gene to
generate the PVT1-MUT vector and the MCL1-MUT vector. Subsequently,
293 T cells (Thermo Fisher Scientific, Inc.) were seeded in 24-well
plates at a density of 1.0×105/ml per well.
Subsequently, the WT or MUT vectors were cotransfected with the
miR-455-3p mimic/inhibitor or NC mimic/inhibitor into 293T cells
using Lipofectamine 2000. A total of 48 h after transfection,
luciferase activity was measured using the dual-luciferase reporter
gene assay system (Beyotime Institute of Biotechnology). In
addition, pGL3-Basic-circPVT1 and pGL3-Basic-MEF2A vectors were
transfected into NB4 cells using Lipofectamine 2000, followed by
assessment using the aforementioned luciferase assay kit at 48 h
post-transfection. Firefly luciferase activity was normalized to
Renilla luciferase activity.
Reverse transcription-quantitative PCR
(RT-qPCR)
A TRIzol® RNA extraction kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to extract total RNA from
AML cells or the blood plasma of patients with AML. First-strand
cDNA was subsequently assembled using total RNA as a template with
the PrimeScript RT Reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol, and the cDNA obtained was used as a
template for qPCR amplification. qPCR was performed according to
the instructions of the SYBR Green qPCR kit (Thermo Fisher
Scientific, Inc.), and the qPCR thermocycling conditions were 94°C
for 2 min; followed by 45 cycles at 94°C for 15 sec and 60°C for 30
sec. GAPDH, β-actin and U6 were used as internal controls for
circRNAs, mRNAs and miRNAs, respectively. Moreover, sterilized
deionized water was used as a negative control for PCR in place of
a nucleic acid template. The expression levels of genes were
calculated using the 2−ΔΔCq method (43). The primer sequences are displayed
in Table II.
 | Table IIPCR primer sequences. |
Table II
PCR primer sequences.
| Gene | Sequence,
5′-3′ |
|---|
| circPVT1 | F:
5′-CCGACTCTTCCTGGTGAAGC-3′ |
| R:
5′-TGCTCGCAGCTCGTCG-3′ |
| β-actin | F:
5′-CATGTACGTTGCTATCCAGGC-3′ |
| R:
5′-CTCCTTAATGTCACGCACGAT-3′ |
| miR-455-3p | F:
5′-CGGCAGTCCATGGGCAT-3′ |
| R:
5′-AGTGCAGGGTCCGAGGTATT-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ |
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
| MCL1 | F:
5′-CGCCAAGGACACAAAGCCAATG-3′ |
| R:
5′-AGCCAGCAGCACATTCCTGATG-3′ |
| GAPDH | F:
5′-GGAGCGAGATCCCTCCAAAAT-3′ |
| R:
5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Agarose gel electrophoresis
Agarose powder was dissolved in TBE buffer in a
flask to prepare 2.5% agarose gel. After the agarose powder was
completely dissolved, ethidium bromide was added and then poured
into the gel-making plate. The PCR product of circPVT1 was then
mixed with the loading buffer and applied to the gel, followed by
electrophoresis; finally, the results were observed using a gel
imaging system (Tanon Science & Technology Co., Ltd.).
Argonaute 2-RNA immunoprecipitation
(Ago2-RIP) assay
NB4 cells were transfected with a NC mimic or
inhibitor, and miR-455-3p mimic or inhibitor for 24 h, and a RIP
experiment was performed using the Magna RIP RNA-Binding Protein
Immunoprecipitation Kit (cat. no. 17-700; MilliporeSigma) according
to previously described methods (44). RT-qPCR was performed as
aforementioned to assess circPVT1 and MCL1 levels in Ago2 or IgG
(negative control) immunoprecipitates.
Stability verification
RNase R (10 U/μl; Guangzhou Geneseed Biotech.
Co., Ltd.) was added to the experimental samples (2.5 μg
total RNA extracted from NB4 cells) at 37°C for 30 min. RT-qPCR was
subsequently performed as aforementioned to verify the stability of
circPVT1.
Cell Counting Kit (CCK)-8 analysis of
cell viability
NB4 and HL-60 cell viability was assessed using a
CCK-8 kit (Beyotime Institute of Biotechnology). The cells were
added to 96-well plates at a concentration of 3×103
cells/well and an equal amount of medium was added to each well.
Subsequently, 10 μl CCK-8 solution was added to each well
and the plates were incubated at 37°C and 5% CO2 for 2
h. The absorbance was measured at a wavelength of 450 nm using a
microplate reader. As a blank control, CCK-8 solution was added to
cell-free medium and the absorbance was measured at 450 nm.
Detection of apoptosis by flow
cytometry
The cells from each group were collected and washed
twice with precooled PBS. According to the manufacturer's protocol,
the percentage of apoptotic cells was detected using an
Annexin-V-FITC/PI apoptosis kit (Absin Bioscience, Inc.). Cells
that were not stained with FITC or PI were used as negative
controls. Cells were analyzed using a flow cytometer (FACSCalibur;
BD Biosciences) and FlowJo-V10 software (FlowJo, LLC) was used to
process the experimental results.
Western blotting
Total proteins were extracted from NB4 and HL-60
cells using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.), and the protein concentration was determined
using a BCA kit (Beijing Solarbio Science & Technology Co.,
Ltd.). The proteins (40 μg/lane) were subsequently separated
by SDS-PAGE on 10% gels and were transferred to PVDF membranes. The
membranes were blocked with 5% skim milk for 2 h at room
temperature, and were then incubated with primary antibodies
(Abcam) against MEF2A (1:1,000; cat. no. ab76063), caspase-3
(1:2,000; cat. no. ab184787), Bax (1:1,000; cat. no. ab32503),
Bcl-2 (1:2,000; cat. no. ab182858), E-cadherin (1:1,000; cat. no.
ab231303), N-cadherin (1:1,000; cat. no. ab245117), vimentin
(1:1,000; cat. no. ab92547), fibronectin (1:1,000; cat. no.
ab268020), and MCL1 (1:500; cat. no. ab243136) overnight at 4°C.
GAPDH (1:1,000; cat. no. ab181602) was used as the internal
reference protein. The membrane was subsequently incubated with an
HRP-conjugated secondary antibody (1:2,000; cat. no. ab288151;
Abcam) for 1 h at room temperature. The protein bands were
visualized with an enhanced chemiluminescence detection kit (BD
Biosciences), and ImageJ 1.8.0.345 software (National Institutes of
Health) was used for protein band gray value analysis. In addition,
nuclear and cytoplasmic proteins were isolated from NB4 and HL-60
cells using a Nuclear and Cytoplasmic Protein Extraction Kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. H3 (1:1,000; cat. no. ab1791; Abcam)
was used as the internal reference for nuclear proteins and western
blotting was performed as aforementioned.
Statistical analysis
The cell experiments were repeated three times. All
experimental data are presented as the mean ± standard deviation.
GraphPad Prism 7 (Dotmatics) was used to analyze and plot the data.
For normally distributed and homogeneous variance variables,
unpaired Student's t-test was used for comparisons between two
groups, and one-way ANOVA was used for comparisons between multiple
groups, followed by Tukey's multiple comparisons test. The
Mann-Whitney U test was used for nonparametric data. The
correlations between circPVT1 and miR-455-3p, miR-455-3p and MCL1,
or circPVT1 and MCL1 were assessed by Pearson correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MEF2A is a transcription factor for
circPVT1
To explore the role of MEF2A as a transcription
factor of circPVT1 in the malignant development of AML, the
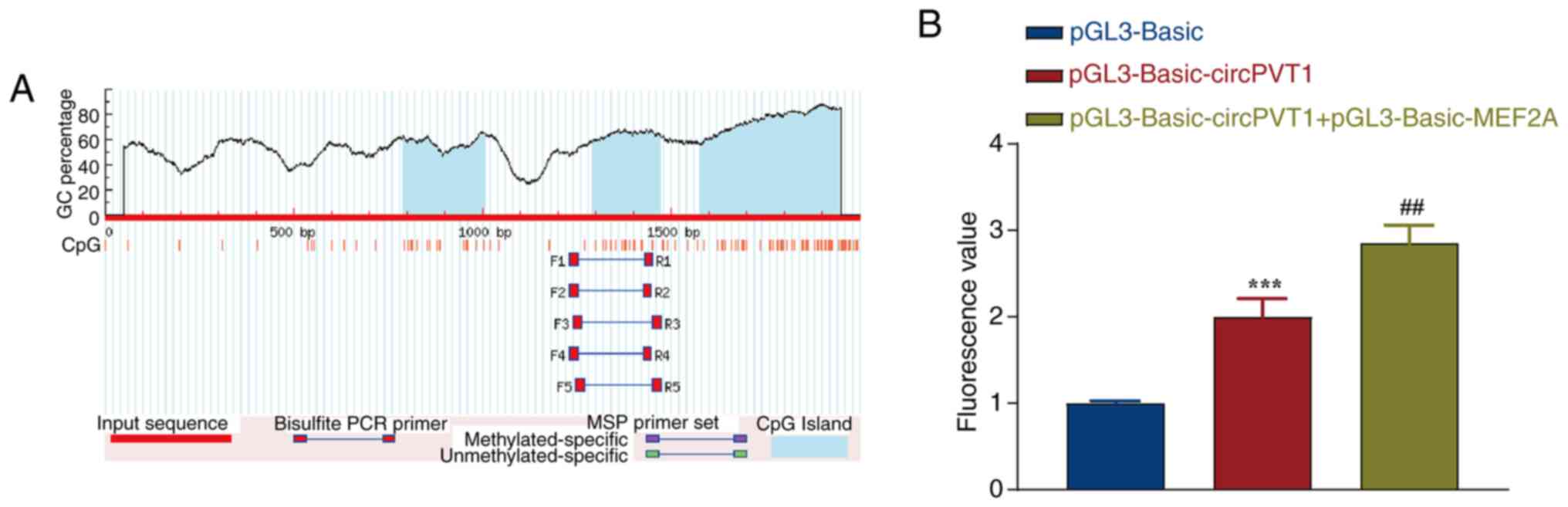
promoter region of circPVT1 was first analyzed. As shown in
Fig. 1A, the promoter region of
circPVT1 was enriched in CpG islands, forming an obvious CG
enrichment phenomenon, and three CpG islands were identified via
Meth Primer analysis. The sizes of CpG islands 1, 2 and 3 were 217,
183 and 368 bp, respectively. CpG islands often appear in the
promoter region and can affect the affinity of the promoter for
transcription factors. The binding sites of the circPVT1 promoter
were then analyzed, and it was revealed that 34 sequences could
bind to the circPVT1 promoter (Table III). It was revealed that the
transcription factor MEF2A can bind to the circPVT1 promoter region
with high affinity. Subsequently, the pGL3-Basic-circPVT1 and
pGL3-Basic-MEF2A eukaryotic expression vectors were cotransfected
into NB4 cells. The empty vector pGL3-Basic was used as a control,
and the luciferase activity was detected after transfection for 24
h. Compared with the pGL3-Basic-circPVT1 group, the cells
cotransfected with pGL3-Basic-circPVT1 and pGL3-Basic-MEF2A group
showed a significant increase in luciferase activity (Fig. 1B). These results indicated that
the MEF2A gene can significantly increase circPVT1 promoter
activity.
 | Table IIIPrediction results of transcription
factor binding sites. |
Table III
Prediction results of transcription
factor binding sites.
| Matrix ID | Name | Score | Relative score | Sequence ID | Start | End | Strand | Predicted
sequence |
|---|
| MA0693.2 | MA0693.2.VDR | 11.801543 |
0.9999999973578093 |
NC_000008.11:128902835-128904835 | 317 | 324 | + | TGAGTTCA |
| MA0909.3 |
MA0909.3.Hoxd13 | 14.179089 |
0.9940158898993562 |
NC_000008.11:128902835-128904835 | 1,749 | 1,758 | − | GGCAATAAAA |
| MA0909.3 |
MA0909.3.Hoxd13 | 14.126415 |
0.9930206731735303 |
NC_000008.11:128902835-128904835 | 1,640 | 1,649 | + | AACAATAAAA |
| MA0136.1 | MA0136.1.ELF5 | 11.374968 |
0.992970134254861 |
NC_000008.11:128902835-128904835 | 1,147 | 1,155 | − | TATTTCCTT |
| MA0693.3 | MA0693.3.Vdr | 13.322502 |
0.990863401631873 |
NC_000008.11:128902835-128904835 | 316 | 326 | + | CTGAGTTCAAA |
| MA0909.1 |
MA0909.1.HOXD13 | 14.127036 |
0.9777348702657696 |
NC_000008.11:128902835-128904835 | 1,748 | 1,757 | − | GCAATAAAAA |
| MA0769.2 | MA0769.2.TCF7 | 13.55147 |
0.9764263544920703 |
NC_000008.11:128902835-128904835 | 1,708 | 1,718 | + | TTCTTTGAAGT |
| MA0769.2 | MA0769.2.TCF7 | 12.878226 |
0.9621331874851317 |
NC_000008.11:128902835-128904835 | 1,389 | 1,399 | + | ATCTTTGAACT |
| MA0909.2 |
MA0909.2.HOXD13 | 14.661904 |
0.9550868989404843 |
NC_000008.11:128902835-128904835 | 1,748 | 1,758 | − | GGCAATAAAAA |
| MA0136.3 | MA0136.3.Elf5 | 13.925515 |
0.9502244196272698 |
NC_000008.11:128902835-128904835 | 169 | 180 | + | AGAAGGAAGGAA |
| MA0909.3 |
MA0909.3.Hoxd13 | 11.67624 |
0.9467267523321995 |
NC_000008.11:128902835-128904835 | 1,961 | 1,970 | + | GCCAATAACA |
| MA0693.2 | MA0693.2.VDR | 9.756103 |
0.9416772888554328 |
NC_000008.11:128902835-128904835 | 1,608 | 1,615 | + | TGAGTTTA |
| MA0505.2 | MA0505.2.Nr5A2 | 15.631966 |
0.9398028693468796 |
NC_000008.11:128902835-128904835 | 1,845 | 1,857 | + | TTGACCTTGAGCA |
| MA0136.3 | MA0136.3.Elf5 | 13.174745 |
0.9358547814540202 |
NC_000008.11:128902835-128904835 | 173 | 184 | + | GGAAGGAAGGGA |
| MA0602.1 |
MA0602.1.Arid5a | 12.836242 |
0.9334683286303311 |
NC_000008.11:128902835-128904835 | 682 | 695 | + | TTAATATTTAAAAT |
| MA0507.1 |
MA0507.1.POU2F2 | 14.1472435 |
0.9318346396422754 |
NC_000008.11:128902835-128904835 | 1,989 | 2,001 | + | TGATTTTGCATGT |
| MA0136.3 | MA0136.3.Elf5 | 12.702013 |
0.9268067684029953 |
NC_000008.11:128902835-128904835 | 212 | 223 | + | TGAAGGAAGGAA |
| MA0602.1 |
MA0602.1.Arid5a | 12.536406 |
0.9263717295874732 |
NC_000008.11:128902835-128904835 | 233 | 246 | + | TTAATATTGTTAAC |
| MA0602.1 |
MA0602.1.Arid5a | 12.319698 |
0.9212426473424906 |
NC_000008.11:128902835-128904835 | 915 | 928 | − | TCAATATTTCTATT |
| MA0602.1 |
MA0602.1.Arid5a | 12.213568 |
0.9187307211047131 |
NC_000008.11:128902835-128904835 | 919 | 932 | + | GAAATATTGAGACT |
| MA0660.1 | MA0660.1.MEF2B | 13.488233 |
0.9172942646213134 |
NC_000008.11:128902835-128904835 | 1,928 | 1,939 | + | ACTACAAATAGC |
| MA0052.4 | MA0052.4.MEF2A | 14.876074 |
0.9166135549477946 |
NC_000008.11:128902835-128904835 | 908 | 922 | + |
GGCAAAAAATAGAAA |
| MA0773.1 | MA0773.1.MEF2D | 12.476424 |
0.9156625804509553 |
NC_000008.11:128902835-128904835 | 1,928 | 1,939 | + | ACTACAAATAGC |
| MA0136.1 | MA0136.1.ELF5 | 8.871671 |
0.9111586439845092 |
NC_000008.11:128902835-128904835 | 171 | 179 | − | TCCTTCCTT |
| MA0136.1 | MA0136.1.ELF5 | 8.871671 |
0.9111586439845092 |
NC_000008.11:128902835-128904835 | 214 | 222 | − | TCCTTCCTT |
| MA0136.1 | MA0136.1.ELF5 | 8.865436 |
0.9109548708370724 |
NC_000008.11:128902835-128904835 | 1,116 | 1,124 | − | CAGTTCCTT |
| MA0052.1 | MA0052.1.MEF2A | 12.713359 |
0.9095717752732639 |
NC_000008.11:128902835-128904835 | 1,929 | 1,938 | − | CTATTTGTAG |
| MA0773.1 | MA0773.1.MEF2D | 11.798319 |
0.9081350993833815 |
NC_000008.11:128902835-128904835 | 909 | 920 | + | GCAAAAAATAGA |
| MA0052.2 | MA0052.2.MEF2A | 13.31947 |
0.9071642295567492 |
NC_000008.11:128902835-128904835 | 1,099 | 1,113 | − |
CACCAAAAATAACAC |
| MA0136.1 | MA0136.1.ELF5 | 8.708229 |
0.9058171258167399 |
NC_000008.11:128902835-128904835 | 960 | 968 | − | AATTTCCTA |
| MA0052.3 | MA0052.3.MEF2A | 12.794866 |
0.9046434712727364 |
NC_000008.11:128902835-128904835 | 909 | 920 | + | GCAAAAAATAGA |
| MA0769.2 | MA0769.2.TCF7 | 10.165019 |
0.9045309633699953 |
NC_000008.11:128902835-128904835 | 1,129 | 1,139 | − | AGCTTTGATTG |
| MA0136.1 | MA0136.1.ELF5 | 8.656569 |
0.9041287820729873 |
NC_000008.11:128902835-128904835 | 218 | 226 | − | TCTTTCCTT |
| MA0909.1 |
MA0909.1.HOXD13 | 10.085875 |
0.9031867671283802 |
NC_000008.11:128902835-128904835 | 1,641 | 1,650 | + | ACAATAAAAG |
Abnormal expression of circPVT1 in
AML
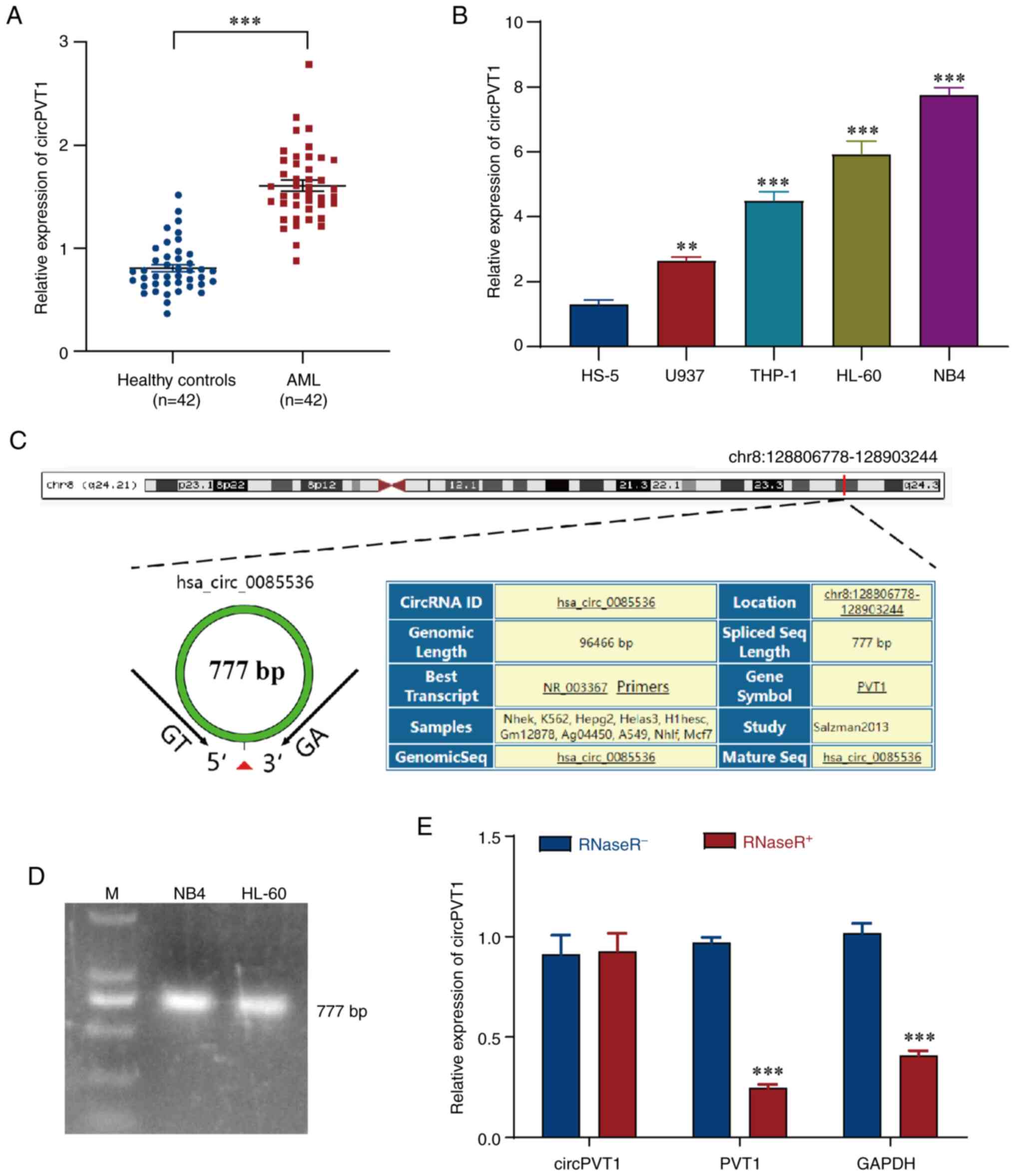
Evaluation by RT-qPCR revealed that the expression
levels of circPVT1 were markedly elevated in the AML group compared
with those in the healthy control group (Fig. 2A). These findings suggested that
circPVT1 may be highly expressed in the blood plasma of patients
with AML. Moreover, the expression levels of circPVT1 were
evaluated in different AML cell lines and it was demonstrated that
circPVT1 expression was greater in AML cell lines (U937, THP-1,
HL-60 and NB4) compared with that in HS-5 cells; this effect was
particularly pronounced in NB4 and HL-60 cells (Fig. 2B). Therefore, NB4 and HL-60 cells
were selected for subsequent experiments. In addition, data
analysis on circPVT1 we performed using the UCSC Genome Browser and
it was revealed that circPVT1 was circularized from the PVT1 gene
and located on chr8:128806778-128903244 (Fig. 2C). The length of the spliced
mature sequence of circPVT1 was 777 bp according to agarose gel
electrophoresis (Fig. 2D).
Finally, RNase R, a 3′ exonuclease that has no effect on circRNAs
(45), was added to the
experimental samples to verify the stability of circPVT1. The
RT-qPCR results revealed that RNase R had no significant effect on
the expression levels of circPVT1. However, linear PVT1 was
digested by RNase R (Fig. 2E).
These results demonstrated that circPVT1 has greater stability.
MEF2A affects the viability and apoptosis
of AML cells via circPVT1
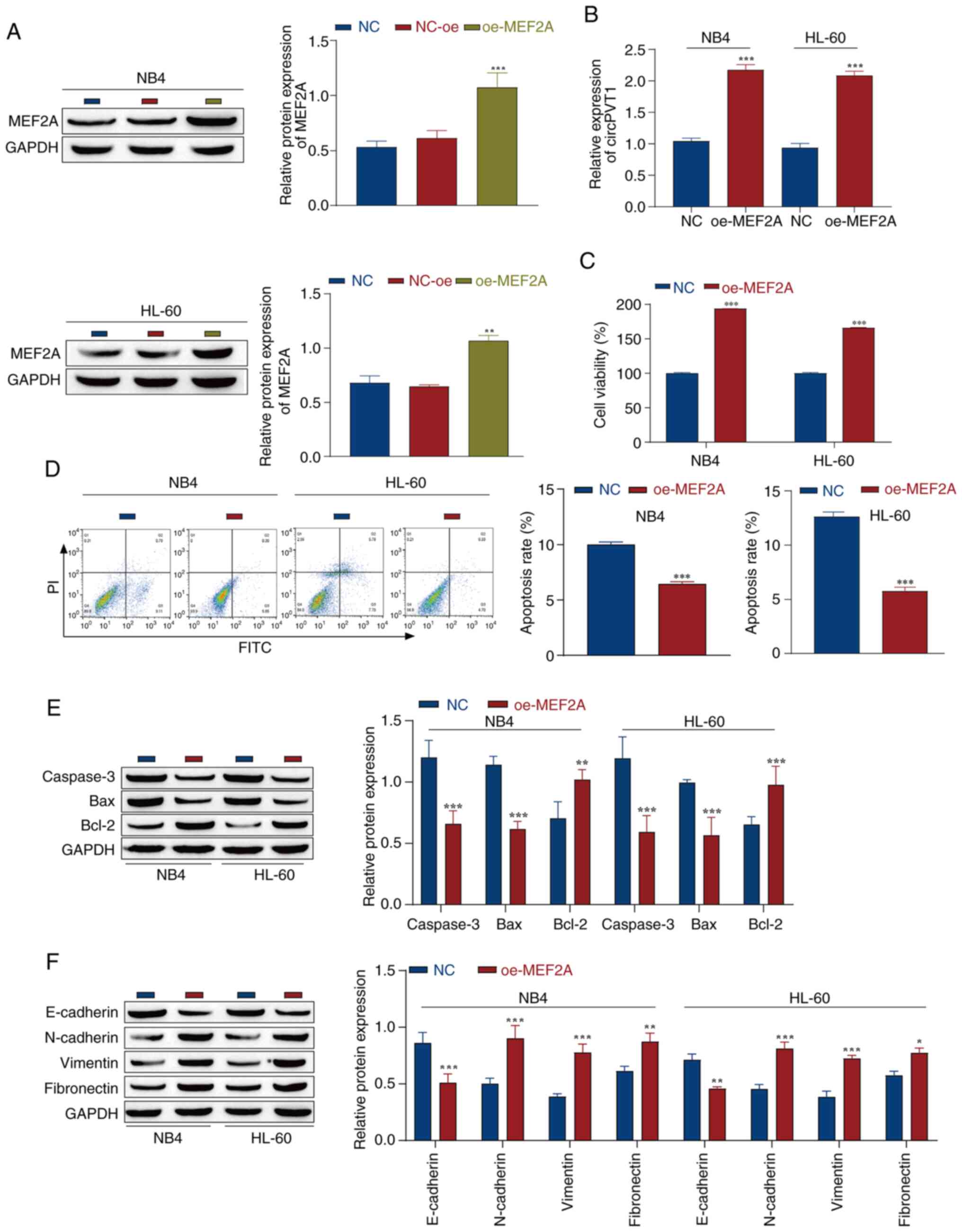
To investigate the effect of MEF2A on AML cells, NB4
and HL-60 cells were transfected with the MEF2A overexpression
vector (oe-MEF2A), with NC-oe as the control. The results of
western blotting revealed that the expression levels of MEF2A
showed a clear upward trend in cells post-transfection with
oe-MEF2A, indicating successful overexpression of MEF2A (Fig. 3A). After successful transfection,
the results of RT-qPCR revealed that, compared with in the NC
group, the overexpression of MEF2A promoted the expression of
circPVT1 in the cells (Fig. 3B).
Furthermore, the results of the CCK-8 assay revealed that
overexpression of MEF2A significantly increased the viability of
NB4 and HL-60 cells (Fig. 3C).
Next, the apoptosis of NB4 and HL-60 cells after oe-MEF2A
transfection was detected by flow cytometry. Compared with that in
the NC group, apoptosis was significantly inhibited after MEF2A
overexpression (Fig. 3D).
Moreover, western blotting was used to detect the expression of
apoptosis-related proteins, and MEF2A overexpression inhibited the
expression of the proapoptotic proteins caspase-3 and Bax, and
promoted the expression levels of the antiapoptotic protein Bcl-2
in AML cells (Fig. 3E), which was
consistent with the results of flow cytometry. In summary, MEF2A
overexpression markedly inhibited the apoptosis of AML cells. In
addition, EMT serves a key role in tumor progression by allowing
cells to metastasize and invade (46). Western blotting was used to detect
the expression levels of EMT marker proteins. It was revealed that
the expression levels of the epithelial marker E-cadherin were
downregulated, whereas the expression levels of the mesenchymal
markers N-cadherin, vimentin and fibronectin were upregulated after
MEF2A was overexpressed in NB4 and HL-60 cell lines, indicating
that MEF2A overexpression promoted the EMT process in NB4 and HL-60
cells (Fig. 3F). Taken together,
these findings suggested that MEF2A may promote the malignant
progression of AML cells through circPVT1.
CircPVT1 targets and regulates miR-455-3p
expression
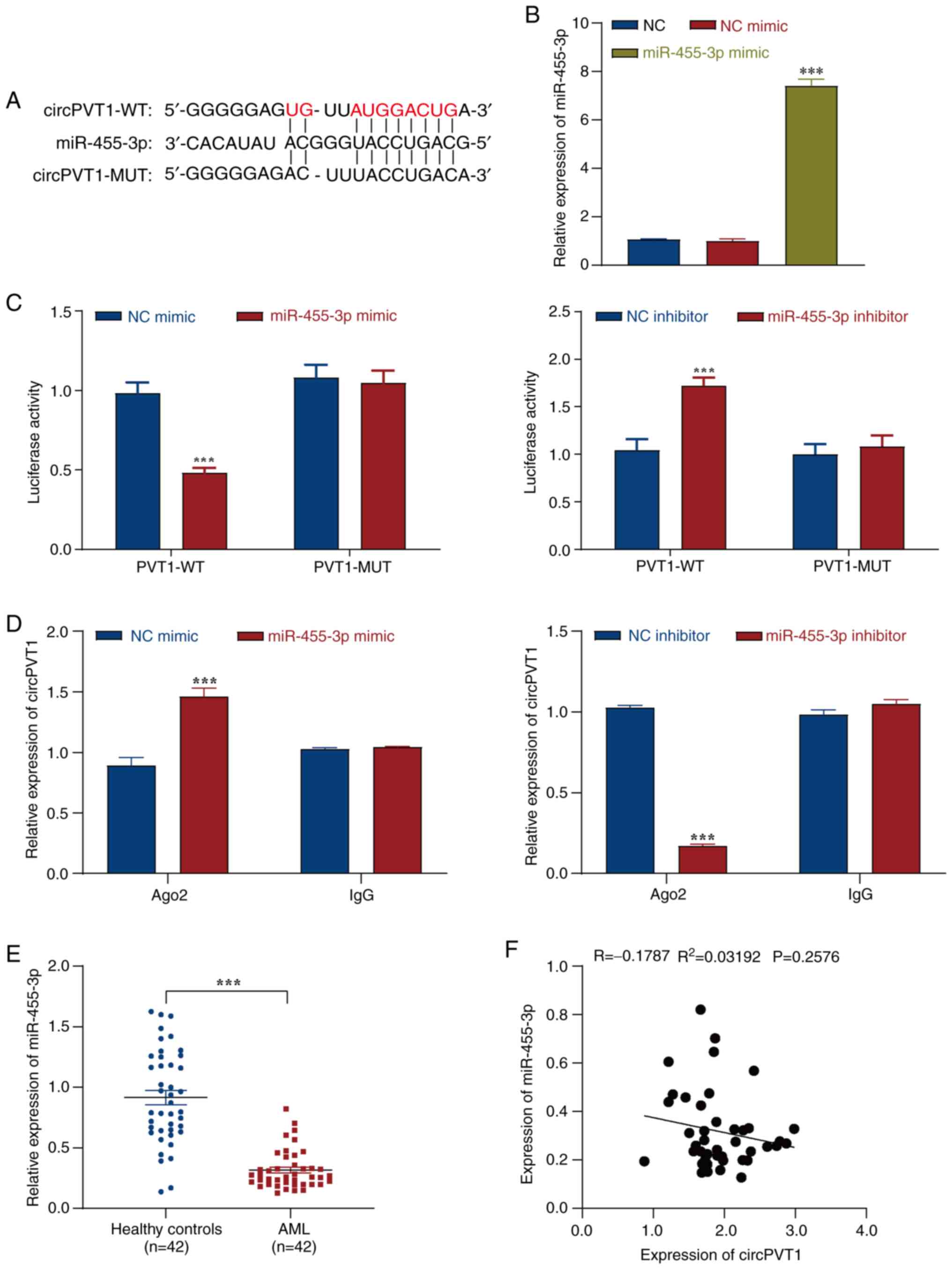
To explore the role of circPVT1 in AML, the
biological information database StarBase was used to analyze
circPVT1 target genes, and it was revealed that circPVT1 could bind
to miR-455-3p (Fig. 4A). RT-qPCR
was used to detect the transfection efficiency of the miR-455-3p
mimic. The results showed that transfection with the miR-455-3p
mimic significantly increased the expression levels of miR-455-3p
in cells compared with those in the NC mimic group, indicating
successful transfection (Fig.
4B). Moreover, a dual-luciferase reporter gene assay was used
to verify the relationship between circPVT1 and miR-455-3p. The
results revealed that the miR-455-3p mimic or the miR-455-3p
inhibitor significantly affected the luciferase activity of the
PVT1-WT vector, whereas there was no significant effect on the
luciferase activity of the PVT1-MUT vector (Fig. 4C). Ago2-RIP assay was further used
to verify the binding relationship between circPVT1 and miR-455-3p,
and the IgG group was used as a negative control. The results
revealed that circPVT1 was enriched by the miR-455-3p mimic,
whereas the miR-455-3p inhibitor did not enrich circPVT1 (Fig. 4D). These results indicated that
circPVT1 may have a targeted binding relationship with miR-455-3p.
In addition, after evaluation by RT-qPCR, it was revealed that the
expression levels of miR-455-3p showed a clear downward trend in
the AML group compared with those in the healthy control group
(Fig. 4E). Pearson correlation
analysis revealed that miR-455-3p and circPVT1 were negatively
correlated; however, the R-value was <-0.3, indicating that the
relationship was weak (Fig. 4F).
Thus, these results identified an interaction between miR-455-3p
and circPVT1, and indicated that circPVT1 may negatively regulate
miR-455-3p.
CircPVT1 promotes the viability and EMT
of AML cells through miR-455-3p
The present study next explored how circPVT1 affects
the viability and EMT of AML cells through miR-455-3p. Cells were
transfected with the miR-455-3p inhibitor and/or si-circPVT1, and
the transfection efficiency was detected by RT-qPCR. The results
revealed that transfection with the miR-455-3p inhibitor
significantly decreased the expression levels of miR-455-3p in
cells, and knockdown of circPVT1 significantly reduced circPVT1
expression levels compared with those in the NC inhibitor or NC-si
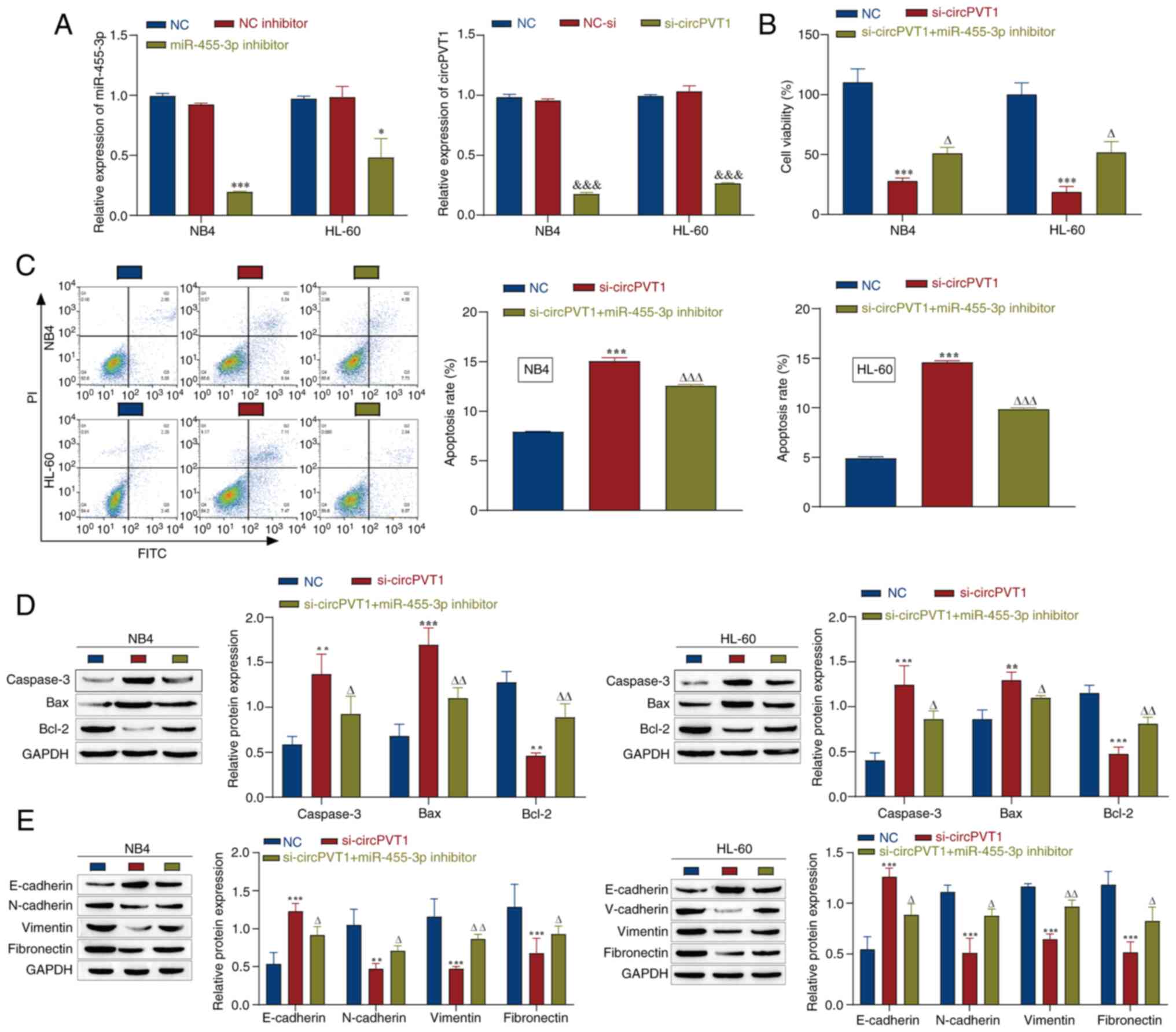
groups, thus indicating successful transfection (Fig. 5A). The NC group in Fig. 5B-E refers to NB4 and HL-60 cells
without any treatment. Next, cell viability was detected by a CCK-8
assay. The results revealed that circPVT1 knockdown significantly
inhibited the viability of NB4 and HL-60 cells, whereas miR-455-3p
inhibitor transfection weakened the effect of circPVT1 knockdown
and promoted cell viability (Fig.
5B). Moreover, flow cytometry revealed that si-circPVT1
promoted apoptosis, whereas the miR-455-3p inhibitor weakened the
effect of si-circPVT1 and inhibited apoptosis (Fig. 5C). Western blot analysis of
apoptosis-related proteins in NB4 and HL-60 cells revealed that
cotransfection with si-circPVT1 and the miR-455-3p inhibitor
reversed the promoting effect of si-PVT1 on caspase-3 and Bax, and
its inhibitory effect on Bcl-2 (Fig.
5D). In addition, the expression of EMT-related proteins was
detected by western blotting, and it was demonstrated that the
expression levels of E-cadherin were increased, whereas those of
N-cadherin, vimentin and fibronectin were decreased after circPVT1
was knocked down. However, after transfection with the miR-455-3p
inhibitor, the opposite results were observed and the effects of
si-circPVT1 were markedly reversed (Fig. 5E). These data suggested that
circPVT1 knockdown may promote apoptosis and inhibit the
progression of EMT, whereas miR-455-3p knockdown could alleviate
the effects of circPVT1 knockdown, thereby inhibiting apoptosis and
promoting the progression of EMT. In summary, miR-455-3p is
associated with the regulatory effects of circPVT1 on AML cell
viability and EMT.
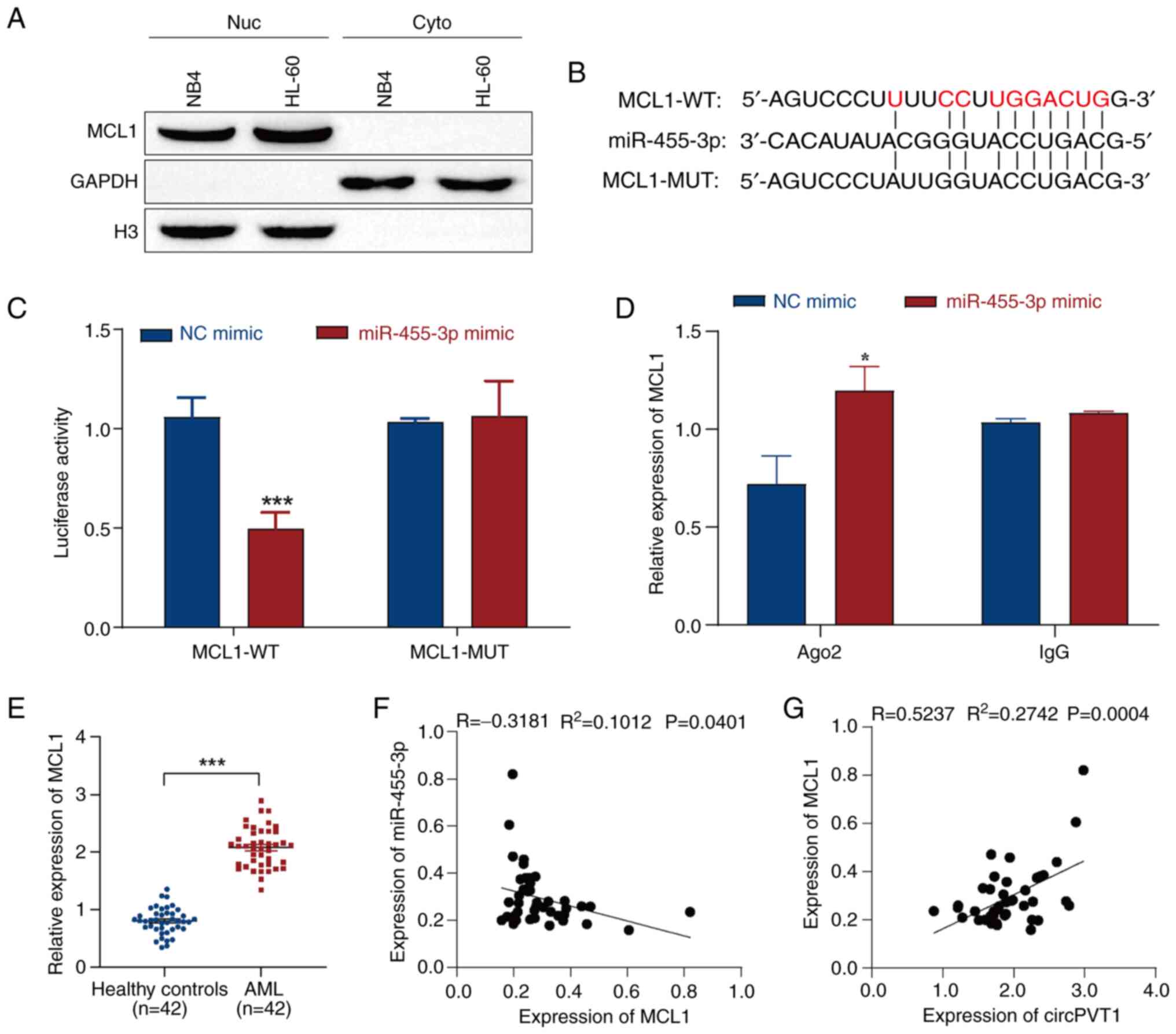
miR-455-3p targets and regulates MCL1
expression
MCL1 has been shown to be upregulated in patients
with AML, and it has been suggested that MCL1 is associated with
poor prognosis in patients with AML and could be used for disease
surveillance (47). In addition,
Lu et al (48) revealed
that miR-181b could improve the drug resistance of AML cells by
decreasing the levels of MCL1. Therefore, it was hypothesized that
MCL1 may be a target of miR-455-3p, and that miR-455-3p may
regulate the progression of AML by targeting MCL1. First, the
expression of MCL1 in the nucleus and cytoplasm of NB4 and HL-60
cells was detected by western blotting. The results indicated that
MCL1 was mainly expressed in the nucleus (Fig. 6A). StarBase predicted that MCL1
would be targeted by miR-455-3p (Fig.
6B). Moreover, dual-luciferase reporter gene and Ago2-RIP
assays were used to verify the targeting relationship between
miR-455-3p and MCL1. The results of the dual-luciferase reporter
gene assay revealed that the miR-455-3p mimic reduced the
luciferase activity of the MCL1-WT vector but had no significant
effect on the luciferase activity of the MCL1-MUT vector (Fig. 6C). The Ago2-RIP assay results
revealed that the miR-455-3p mimic significantly enriched MCL1; the
IgG group was used as a negative control (Fig. 6D). These results indicated that
miR-455-3p may interact with MCL1. In addition, after evaluation by
RT-qPCR, the results revealed that the expression levels of MCL1
exhibited a clear upward trend in the plasma of patients with AML
compared with in those from healthy controls (Fig. 6E). The relationship between
miR-455-3p and MCL1 was then analyzed by correlation analysis,
which revealed that miR-455-3p was negatively correlated with MCL1
(Fig. 6F). In addition, MCL1 was
positively correlated with circPVT1 (Fig. 6G). These results suggested that
MCL1 may be a target gene of miR-455-3p and could serve an
important role in circPVT1/miR-455-3p-mediated AML.
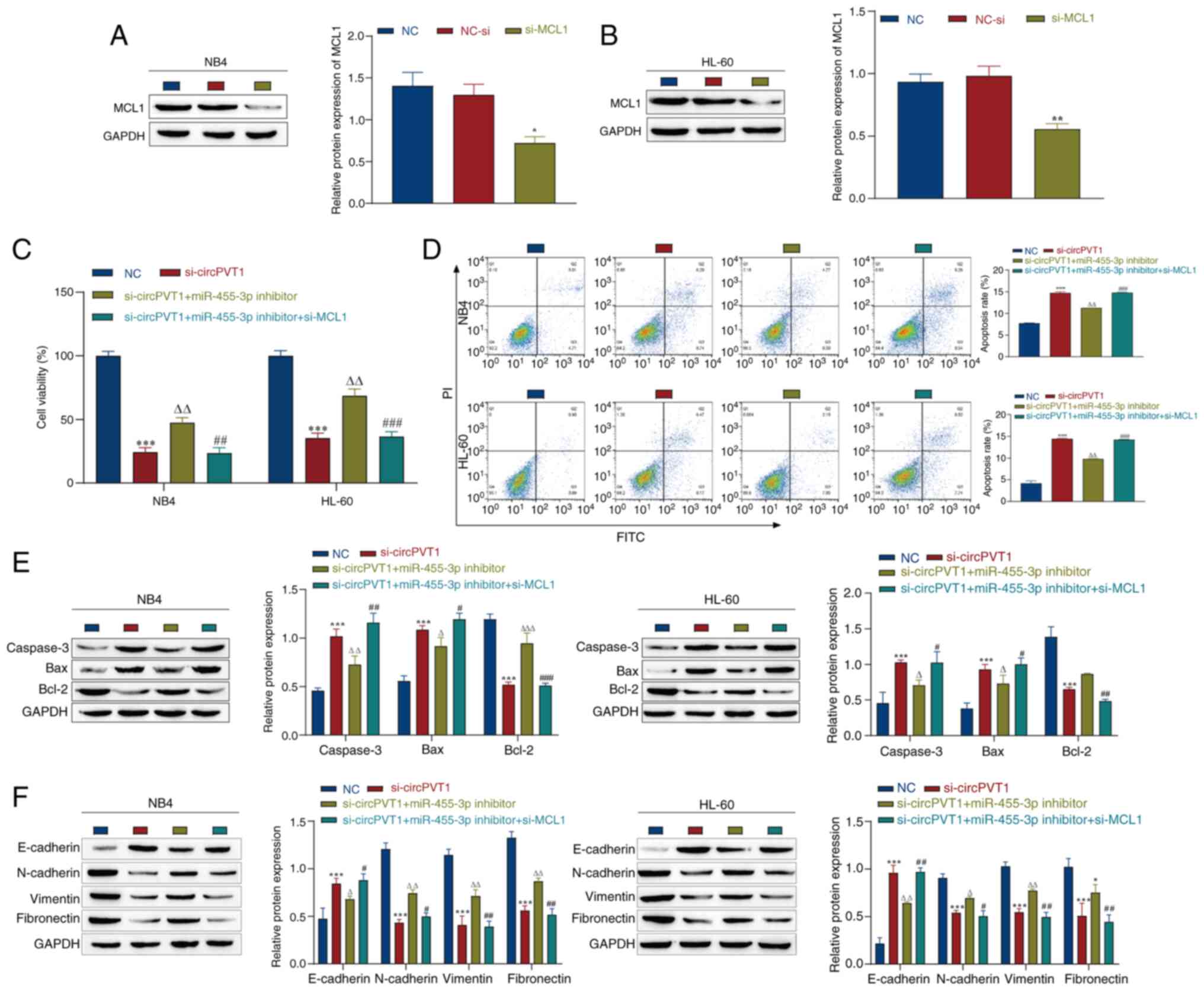
CircPVT1 promotes the viability and EMT
of AML cells through the miR-455-3p/MCL1 axis
The present study further explored how circPVT1
influences the malignant progression of AML through the
miR-455-3p/MCL1 axis. Following the observation that knockdown of
circPVT1 and miR-455-3p affected the viability and EMT of NB4 and
HL-60 cells, the effects of MCL1 knockdown on these cells were
further explored. First, si-MCL1 was transfected into the cells,
and the transfection efficiency was detected by western blotting.
The expression levels of MCL1 in the si-MCL1 group were
significantly lower than those in the NC-si group, indicating
successful transfection (Fig. 7A and
B). The NC group in Fig. 7C-F
refers to NB4 and HL-60 cells without any treatment. The CCK-8
assay was used to assess the effects on cell viability, and it was
revealed that knockdown of MCL1 weakened the effect of the
miR-455-3p inhibitor, and inhibited the viability of NB4 and HL-60
cells (Fig. 7C). Moreover, flow
cytometry was used to detect apoptosis, and the results showed that
the knockdown of MCL1 promoted apoptosis compared with that in the
si-circPVT1 + miR-455-3p inhibitor group (Fig. 7D). Furthermore, western blotting
was used to detect the expression levels of apoptosis-related
proteins. Notably, si-MCL1 partially reversed the effect of the
miR-455-3p inhibitor, and promoted the expression of Caspase-3 and
Bax, and inhibited the expression of Bcl-2 (Fig. 7E). In addition, the expression
levels of EMT-related markers were detected by western blotting.
The results showed that si-MCL1 promoted the expression levels of
E-cadherin, and inhibited the expression levels of N-cadherin,
vimentin and fibronectin compared with those in the si-circPVT1 +
miR-455-3p inhibitor group (Fig.
7F). These data indicated that further knockdown of MCL1 may
weaken the effects of the miR-455-3p inhibitor, promote the
apoptosis of AML cells, and inhibit cell viability and the EMT
process. These findings suggested that circPVT1 could influence the
malignant progression of AML through the miR-455-3p/MCL1 axis.
Discussion
Despite the current advances in the treatment of
AML, including immunotherapy, its treatment remains challenging due
to drug resistance and relapse (49). Therefore, fully understanding the
molecular mechanism of AML occurrence and development, and
exploring new molecular therapeutic strategies are important. In
recent years, increasing evidence has shown that circRNAs have
potential in the diagnosis and treatment of tumors. Notably,
circRNAs are conserved across species, are stably expressed in
exosomes, plasma and saliva, and can promote cancer and
chemotherapy resistance (45,50). Specific patterns of circRNA
expression have been reported to be associated with tumor
development and patient prognosis, indicating that they can serve
as potential diagnostic and prognostic biomarkers (51). Previous studies have reported that
a number of circRNAs, such as circMYBL2 (52), hsa_circ_0004277 (53) and circPVT1 (54), are abnormally expressed in AML.
Alterations in circPVT1 can lead to amplification of the downstream
oncogene MYC, and overexpression of MYC is associated with
prognosis in AML (54). The
present study revealed that circPVT1 expression was significantly
upregulated in patients with AML and in AML cells, which was
consistent with previous findings (23). These findings suggested that
circPVT1 may be a regulator of AML progression. However, few
studies have investigated the mechanism of circPVT1 in AML
(23,24).
MEF2 family members (MEF2A, 2B, 2C and 2D) serve key
regulatory roles in AML progression (55,56). MEF2A is a well-known transcription
factor that regulates the expression of numerous genes. It has
previously been shown that MEF2A can be activated by TGF-β through
the upregulation of MMP10, which affects the ability of TGF-β to
induce breast cancer metastasis (57). In ovarian cancer, MEF2A is thought
to be one of the transcription factors involved in tumorigenesis in
response to norepinephrine (58).
It has also been reported that MEF2A can bind to the promoter
region of lncHCP5, upregulate the expression of lncHCP5 and affect
the progression of gastric cancer (29). In addition, the transcription of
circRNAs can be influenced by transcription factors (59). At present, to the best of our
knowledge, there are no reports on the role of MEF2A transcription
in regulating circPVT1 expression in disease. In the present study,
through bioinformatics analysis, it was revealed that the
transcription factor MEF2A could specifically bind to the circPVT1
promoter region with high affinity, which may significantly
increase circPVT1 promoter activity. In addition, the
overexpression of MEF2A in the AML cell lines NB4 and HL-60
significantly promoted the expression of circPVT1. Western
blotting, CCK-8 assay and flow cytometry revealed that MEF2A
overexpression significantly inhibited the expression of the
proapoptotic proteins caspase-3 and Bax, enhanced the expression of
the antiapoptotic protein Bcl-2, promoted the viability of NB4 and
HL-60 cells, and inhibited their apoptosis. Moreover, EMT serves a
key role in tumor progression, and the expression of E-cadherin is
decreased, whereas the expression levels of N-cadherin, vimentin
and fibronectin are upregulated during EMT (46). The current study revealed that
MEF2A overexpression promoted EMT in AML cells. Therefore, it was
hypothesized that MEF2A may affect the AML process by increasing
circPVT1 expression.
There is growing evidence that circRNAs serve a
functional role in a variety of biological processes, primarily by
acting as miRNA sponges and ceRNAs (60). Through bioinformatics analysis,
dual-luciferase reporter gene and Ago2-RIP assays, it was revealed
that circPVT1 may have a targeted binding relationship with
miR-455-3p. miR-455, a family of miRNAs closely related to several
biological processes, has been shown to be clearly dysregulated in
various human tumors, and is involved in the occurrence and
progression of multiple malignancies (61-63). miR-455-3p is a member of the
miR-455 family, and is involved in the occurrence and development
of various malignant tumors. A previous study confirmed that
miR-455-3p can promote the invasion and migration of
triple-negative breast cancer by targeting the tumor suppressor
EI24 (39). By contrast,
miR-455-3p can act as a tumor inhibitor by targeting hTERT in
melanoma A375 cells (40). In
addition, transfection of a miR-455-3p mimic has been reported to
inhibit the viability of AML cells (34). These findings suggested that
miR-455-3p serves an important role in AML progression; however,
studies on the regulatory mechanism of miR-455-3p in AML are
limited. The present study demonstrated that the relative
expression of miR-455-3p in the serum of patients with AML was
decreased, and low expression of miR-455-3p was associated with the
malignant progression of AML cells, suggesting that miR-455-3p may
act as a tumor suppressor in AML. The results also revealed that
circPVT1 and miR-455-3p had a negative targeting relationship.
Knocking down circPVT1 significantly inhibited the viability and
EMT of NB4 and HL-60 cells, and promoted their apoptosis, whereas
knocking down miR-455-3p promoted cell viability and EMT, and
inhibited apoptosis. In summary, miR-455-3p may have a unique
amelioratory function in AML by inhibiting cell viability.
Therefore, miR-455-3p may serve as a potential biomarker for the
treatment of AML.
The biological function of circRNAs as ceRNAs mainly
depends on the downstream target mRNAs of miRNAs (29). Through bioinformatics analysis, it
was revealed that MCL1 was a downstream target of miR-455-3p, and
the interaction between miR-455-3p and MCL1 was confirmed by
dual-luciferase reporter gene and Ago2-RIP assays. The current
study revealed that MCL1 was positively correlated with circPVT1
and negatively correlated with miR-455-3p. MCL1 is a member of the
Bcl-2 protein family with significant functions in regulating
cellular metabolism and cancer progression (64). It has been shown that circHIPK3
can promote the proliferation and invasion of prostate cancer cells
by sponging miR-193a-3p and regulating MCL1 expression (65). Li et al (47) reported that MCL1 upregulation was
a common event in Chinese patients with de novo AML, which
indicated that MCL1 may be associated with poor prognosis in
patients with AML and can be used for disease surveillance. In
addition, Lu et al (48)
reported that miR-181b could improve the drug resistance of AML
cells by decreasing the levels of MCL1. The present results
revealed that the expression of MCL1 in the plasma of patients with
AML was significantly upregulated. Furthermore, MCL1 knockdown
weakened the effect of miR-455-3p knockdown, inhibited cell
viability and EMT, and promoted cell apoptosis. To the best of our
knowledge, the present study is the first to demonstrate that
miR-455-3p interacts with MCL1 to influence the progression of AML.
These findings provide a new theoretical basis for the treatment of
AML.
Notably, the present study has limitations. First,
the study only investigated the role of the
MEF2A/circPVT1/miR-455-3p/MCL1 molecular axis in AML progression
through in vitro experiments, and in vivo animal
experiments are needed to further verify the role of this molecular
axis. Second, miR-455-3p is not the only downstream target of
circPVT1, and additional studies are needed to further explore the
role of more miRNAs in AML and to develop more potential molecular
therapeutic targets for AML. In addition, methylation of CpG
islands has an important role in gene regulation. There are often
differences in CpG island methylation between tumor cells and
normal cells, and CpG island methylation is a common mechanism of
gene inactivation in tumor cells, with AML samples often exhibiting
very high methylation levels (66,67). However, whether the expression
levels of circPVT1 are affected by the methylation of CpG islands
in its promoter region, and whether this affects the AML process,
requires further study.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that MEF2A, a
transcription factor for circPVT1, could significantly promote the
malignant progression of AML cells. Notably, circPVT1 knockdown
inhibited the malignant progression of AML cells through the
miR-455-3p/MCL1 axis. The identification of the
circPVT1/miR-455-3p/MCL1 molecular axis may expand understanding of
the underlying mechanisms of AML progression. Therefore, circPVT1
could be considered a diagnostic biomarker or a molecular
therapeutic target for AML, and targeting the
circPVT1/miR-455-3p/MCL1 molecular axis may provide novel
therapeutic strategies for AML.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KW, MS and YL conceptualized the study. KW, MS, YZ
and JY designed the experimental methods. YL, YZ and BN used
software to analyze data. CG and XM validated the data. KW and XM
carried out formal analysis. KW, MS, YZ and JY conducted the
investigation. MS, YZ and JY provided resources. CG, LL and SC were
responsible for acquisition of data. BN and SL were responsible for
interpretation of data. KW, SC and YL were responsible for writing
the original draft. KW, LL and SL reviewed and edited the
manuscript. YL and SL edited the figures. SL, YZ, JY and MS
supervised the study. MS, JY and SL received funding. KW, JY and MS
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Kunming Medical University
(approval no. L-10; approval date, March 8, 2021) and was conducted
in accordance with The Declaration of Helsinki. The participants
provided written informed consent prior to taking part in the
study. The guardian of the 16-year-old patient also provided
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the 'Xingdian Talents'
Support Project of Yunnan Province (grant nos. RLMY20220006 and
RLMY20200020) and the Kunming-Medical Joint Special Project of the
Yunnan Provincial Department of Science and Technology (grant no.
202201AY070001-058).
References
|
1
|
Juliusson G, Lazarevic V, Hörstedt AS,
Hagberg O and Höglund M; Swedish Acute Leukemia Registry Group:
Acute myeloid leukemia in the real world: Why population-based
registries are needed. Blood. 119:3890–3899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El Hussein S, Wang SA, Pemmaraju N, Khoury
JD and Loghavi S: Chronic Myelomonocytic leukemia: Hematopathology
perspective. J Immunother Precis Oncol. 4:142–149. 2021. View Article : Google Scholar
|
|
3
|
Lonetti A, Pession A and Masetti R:
Targeted therapies for pediatric AML: Gaps and perspective. Front
Pediatr. 7:4632019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anguille S, Van Tendeloo VF and Berneman
ZN: Leukemia-associated antigens and their relevance to the
immunotherapy of acute myeloid leukemia. Leukemia. 26:2186–2196.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsykunova G, Reikvam H, Hovland R and
Bruserud Ø: The surface molecule signature of primary human acute
myeloid leukemia (AML) cells is highly associated with NPM1
mutation status. Leukemia. 26:557–559. 2012. View Article : Google Scholar
|
|
6
|
Hope KJ, Jin L and Dick JE: Acute myeloid
leukemia originates from a hierarchy of leukemic stem cell classes
that differ in self-renewal capacity. Nat Immunol. 5:738–743. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song X, Peng Y, Wang X, Chen Y, Jin L,
Yang T, Qian M, Ni W, Tong X and Lan J: Incidence, survival, and
risk factors for adults with acute myeloid leukemia not otherwise
specified and acute myeloid leukemia with recurrent genetic
abnormalities: Analysis of the surveillance, epidemiology, and end
results (SEER) database, 2001-2013. Acta Haematol. 139:115–127.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Zhu Y, Liang Z, Wang X, Meng S, Xu
X, Xu X, Wu J, Ji A, Hu Z, et al: Correction: Up-regulation of p16
by miR-877-3p inhibits proliferation of bladder cancer. Oncotarget.
10:6842019. View Article : Google Scholar :
|
|
9
|
Guerra VA, Dinardo C and Konopleva M:
Venetoclax-based therapies for acute myeloid leukemia. Best Pract
Res Clin Haematol. 32:145–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan B, Qin J, Liu X, He B, Wang X, Pan Y,
Sun H, Xu T, Xu M, Chen X, et al: Identification of serum exosomal
hsa-circ-0004771 as a novel diagnostic biomarker of colorectal
cancer. Front Genet. 10:10962019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jamal M, Song T, Chen B, Faisal M, Hong Z,
Xie T, Wu Y, Pan S, Yin Q, Shao L and Zhang Q: Recent progress on
circular RNA research in acute myeloid leukemia. Front Oncol.
9:11082019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin Y, Long J, He Q, Li Y, Liao Y, He P
and Zhu W: Emerging roles of circRNA in formation and progression
of cancer. J Cancer. 10:5015–5021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi
G, Zhichen W, Zirui W and Shengwang W: The roles of miRNA, lncRNA
and circRNA in the development of osteoporosis. Biol Res.
53:402020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shafabakhsh R, Mirhosseini N, Chaichian S,
Moazzami B, Mahdizadeh Z and Asemi Z: Could circRNA be a new
biomarker for pre-eclampsia? Mol Reprod Dev. 86:1773–1780. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017. View Article : Google Scholar
|
|
16
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424-5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar :
|
|
17
|
Li P, Chen H, Chen S, Mo X, Li T, Xiao B,
Yu R and Guo J: Circular RNA 0000096 affects cell growth and
migration in gastric cancer. Br J Cancer. 116:626–633. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei CB, Tao K, Jiang R, Zhou LD, Zhang QH
and Yuan CS: Quercetin protects mouse liver against
triptolide-induced hepatic injury by restoring Th17/Treg balance
through Tim-3 and TLR4-MyD88-NF-κB pathway. Int Immunopharmacol.
53:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu YP, Wan J, Long F, Tian J and Zhang C:
circPVT1 facilitates invasion and metastasis by regulating
miR-205-5p/c-FLIP axis in osteosarcoma. Cancer Manag Res.
12:1229–1240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng F and Xu R: CircPVT1 contributes to
chemotherapy resistance of lung adenocarcinoma through
miR-145-5p/ABCC1 axis. Biomed Pharmacother. 124:1098282020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Huang K, Shi L, Zhang Q and Zhang
S: CircPVT1 promoted the progression of breast cancer by regulating
MiR-29a-3p-Mediated AGR2-HIF-1α pathway. Cancer Manag Res.
12:11477–11490. 2020. View Article : Google Scholar :
|
|
22
|
Chen T and Chen F: The role of circular
RNA plasmacytoma variant translocation 1 as a biomarker for
prognostication of acute myeloid leukemia. Hematology.
26:1018–1024. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghetti M, Vannini I, Bochicchio MT, Azzali
I, Ledda L, Marconi G, Melloni M, Fabbri F, Rondoni M, Chicchi R,
et al: Uncovering the expression of circPVT1 in the extracellular
vesicles of acute myeloid leukemia patients. Biomed Pharmacother.
165:1152352023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheng XF, Hong LL, Fan L, Zhang Y, Chen
KL, Mu J, Shen SY and Zhuang HF: Circular RNA PVT1 regulates cell
proliferation, migration, and apoptosis by stabilizing c-Myc and
downstream target CXCR4 expression in acute myeloid leukemia. Turk
J Haematol. 40:82–91. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryan RJH, Drier Y, Whitton H, Cotton MJ,
Kaur J, Issner R, Gillespie S, Epstein CB, Nardi V, Sohani AR, et
al: Detection of enhancer-associated rearrangements reveals
mechanisms of oncogene dysregulation in B-cell lymphoma. Cancer
Discov. 5:1058–1071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown FC, Still E, Koche RP, Yim CY, Takao
S, Cifani P, Reed C, Gunasekera S, Ficarro SB, Romanienko P, et al:
MEF2C phosphorylation is required for chemotherapy resistance in
acute myeloid leukemia. Cancer Discov. 8:478–497. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma L, Liu J, Liu L, Duan G, Wang Q, Xu Y,
Xia F, Shan J, Shen J, Yang Z, et al: Overexpression of the
transcription factor MEF2D in hepatocellular carcinoma sustains
malignant character by suppressing G2-M transition genes. Cancer
Res. 74:1452–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang J, Sun H, Su L, Liu L, Shan J, Shen
J, Yang Z, Chen J, Zhong X, Ávila MA, et al: Myocyte enhancer
factor 2D promotes colorectal cancer angiogenesis downstream of
hypoxia-inducible factor 1α. Cancer Lett. 400:117–126. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Zhang K, Yang Y, Guo Z, Wang X,
Teng B, Zhao Q, Huang C and Qiu Z: MEF2A-mediated lncRNA HCP5
inhibits gastric cancer progression via MiR-106b-5p/p21 axis. Int J
Biol Sci. 17:623–634. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao Q, Gan Y, Li Y, Fan L, Liu J, Lu P,
Liu J, Chen A, Shu G and Yin G: MEF2A transcriptionally upregulates
the expression of ZEB2 and CTNNB1 in colorectal cancer to promote
tumor progression. Oncogene. 40:3364–3377. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia L, Nie T, Lu F, Huang L, Shi X, Ren D,
Lu J, Li X, Xu T, Cui B, et al: Direct regulation of FNIP1 and
FNIP2 by MEF2 sustains MTORC1 activation and tumor progression in
pancreatic cancer. Autophagy. 20:505–524. 2024. View Article : Google Scholar
|
|
32
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yi J, Wang L, Hu GS, Zhang YY, Du J, Ding
JC, Ji X, Shen HF, Huang HH, Ye F and Liu W: CircPVT1 promotes
ER-positive breast tumorigenesis and drug resistance by targeting
ESR1 and MAVS. EMBO J. 42:e1124082023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie Y, Tan L, Wu K, Li D and Li C:
MiR-455-3p mediates PPARα through UBN2 to promote apoptosis and
autophagy in acute myeloid leukemia cells. Exp Hematol. 128:77–88.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhan T, Zhu Q, Han Z, Tan J, Liu M, Liu W,
Chen W, Chen X, Chen X, Deng J, et al: miR-455-3p functions as a
tumor suppressor by restraining Wnt/β-catenin signaling via TAZ in
pancreatic cancer. Cancer Manag Res. 12:1483–1492. 2020. View Article : Google Scholar :
|
|
36
|
Ni X, Ding Y, Yuan H, Shao J, Yan Y, Guo
R, Luan W and Xu M: Long non-coding RNA ZEB1-AS1 promotes colon
adenocarcinoma malignant progression via miR-455-3p/PAK2 axis. Cell
Prolif. 53:e127232020. View Article : Google Scholar
|
|
37
|
Liu A, Zhu J, Wu G, Cao L, Tan Z, Zhang S,
Jiang L, Wu J, Li M, Song L and Li J: Correction to: Antagonizing
miR-455-3p inhibits chemoresistance and aggressiveness in
esophageal squamous cell carcinoma. Mol Cancer. 20:1522021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao X, Zhao H, Diao C, Wang X, Xie Y, Liu
Y, Han J and Zhang M: miR-455-3p serves as prognostic factor and
regulates the proliferation and migration of non-small cell lung
cancer through targeting HOXB5. Biochem Biophys Res Commun.
495:1074–1080. 2018. View Article : Google Scholar
|
|
39
|
Li Z, Meng Q, Pan A, Wu X, Cui J, Wang Y
and Li L: MicroRNA-455-3p promotes invasion and migration in triple
negative breast cancer by targeting tumor suppressor EI24.
Oncotarget. 8:19455–19466. 2017. View Article : Google Scholar :
|
|
40
|
Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR,
Ding Y, Liang JQ, Li TT and Zhao J: MiR-497-5p, miR-195-5p and
miR-455-3p function as tumor suppressors by targeting hTERT in
melanoma A375 cells. Cancer Manag Res. 10:989–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ryu S, Park HS, Kim SM, Im K, Kim JA,
Hwang SM, Yoon SS and Lee DS: Shifting of erythroleukemia to
myelodysplastic syndrome according to the revised WHO
classification: Biologic and cytogenetic features of shifted
erythroleukemia. Leuk Res. 70:13–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li LC and Dahiya R: MethPrimer: Designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
44
|
Li L, Lv G, Wang B and Kuang L: The role
of lncRNA XIST/miR-211 axis in modulating the proliferation and
apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK
signaling. Biochem Biophys Res Commun. 503:2555–2562. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li S, Liu F, Zheng K, Wang W, Qiu E, Pei
Y, Wang S, Zhang J and Zhang X: CircDOCK1 promotes the
tumorigenesis and cisplatin resistance of osteogenic sarcoma via
the miR-339-3p/IGF1R axis. Mol Cancer. 20:1612021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matoba R, Morizane Y, Shiode Y, Hirano M,
Doi S, Toshima S, Araki R, Hosogi M, Yonezawa T and Shiraga F:
Suppressive effect of AMP-activated protein kinase on the
epithelial-mesenchymal transition in retinal pigment epithelial
cells. PLoS One. 12:e01814812017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li XX, Zhou JD, Wen XM, Zhang TJ, Wu DH,
Deng ZQ, Zhang ZH, Lian XY, He PF, Yao XY, et al: Increased MCL-1
expression predicts poor prognosis and disease recurrence in acute
myeloid leukemia. Onco Targets Ther. 12:3295–3304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu F, Zhang J, Ji M, Li P, Du Y, Wang H,
Zang S, Ma D, Sun X and Ji C: miR-181b increases drug sensitivity
in acute myeloid leukemia via targeting HMGB1 and Mcl-1. Int J
Oncol. 45:383–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu M and Li S: The opportunities and
challenges of using PD-1/PD-L1 inhibitors for leukemia treatment.
Cancer Lett. 593:2169692024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guo X, Gao C, Yang DH and Li S: Exosomal
circular RNAs: A chief culprit in cancer chemotherapy resistance.
Drug Resist Updat. 67:1009372023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Q and Li S: Exosomal circRNAs: Novel
biomarkers and therapeutic targets for urinary tumors. Cancer Lett.
588:2167592024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun YM, Wang WT, Zeng ZC, Chen TQ, Han C,
Pan Q, Huang W, Fang K, Sun LY, Zhou YF, et al: circMYBL2, a
circRNA from MYBL2, regulates FLT3 translation by recruiting PTBP1
to promote FLT3-ITD AML progression. Blood. 134:1533–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li W, Zhong C, Jiao J, Li P, Cui B, Ji C
and Ma D: Characterization of hsa_circ_0004277 as a new biomarker
for acute myeloid leukemia via circular RNA profile and
bioinformatics analysis. Int J Mol Sci. 18:5972017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu J, Han Q, Gu Y, Ma J, Mcgrath M, Qiao
F, Chen B, Song C and Ge Z: Circular RNA PVT1 expression and its
roles in acute lymphoblastic leukemia. Epigenomics. 10:723–732.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tarumoto Y, Lin S, Wang J, Milazzo JP, Xu
Y, Lu B, Yang Z, Wei Y, Polyanskaya S, Wunderlich M, et al:
Salt-inducible kinase inhibition suppresses acute myeloid leukemia
progression in vivo. Blood. 135:56–70. 2020. View Article : Google Scholar :
|
|
56
|
Zhao L, Zhang P, Galbo PM, Zhou X, Aryal
S, Qiu S, Zhang H, Zhou Y, Li C, Zheng D, et al: Transcription
factor MEF2D is required for the maintenance of MLL-rearranged
acute myeloid leukemia. Blood Adv. 5:4727–4740. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ishikawa F, Miyoshi H, Nose K and
Shibanuma M: Transcriptional induction of MMP-10 by TGF-beta,
mediated by activation of MEF2A and downregulation of class IIa
HDACs. Oncogene. 29:909–919. 2010. View Article : Google Scholar
|
|
58
|
Gjyshi A, Dash S, Cen L, Cheng CH, Zhang
C, Yoder SJ, Teer JK, Armaiz-Pena GN and Monteiro ANA: Early
transcriptional response of human ovarian and fallopian tube
surface epithelial cells to norepinephrine. Sci Rep. 8:82912018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang S, Li X, Zheng H, Si X, Li B, Wei G,
Li C, Chen Y, Chen Y, Liao W, et al: Loss of
super-enhancer-regulated circRNA Nfix induces cardiac regeneration
after myocardial infarction in adult mice. Circulation.
139:2857–2876. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang X, Han F, Hu X, Li G, Wu H, Can C,
Wei Y, Liu J, Wang R, Jia W, et al: EIF4A3-induced Circ_0001187
facilitates AML suppression through promoting ubiquitin-proteasomal
degradation of METTL3 and decreasing m6A modification level
mediated by miR-499a-5p/RNF113A pathway. Biomark Res. 11:592023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sand M, Skrygan M, Sand D, Georgas D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Expression of
microRNAs in basal cell carcinoma. Br J Dermatol. 167:847–855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang Z, Hou C, Meng F, Zhao X, Zhang Z,
Huang G, Chen W, Fu M and Liao W: MiR-455-3p regulates early
chondrogenic differentiation via inhibiting Runx2. FEBS Lett.
589:3671–3678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Boisen MK, Dehlendorff C, Linnemann D,
Nielsen BS, Larsen JS, Osterlind K, Nielsen SE, Tarpgaard LS,
Qvortrup C, Pfeiffer P, et al: Tissue microRNAs as predictors of
outcome in patients with metastatic colorectal cancer treated with
first line capecitabine and oxaliplatin with or without
bevacizumab. PLoS One. 9:e1094302014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Williams MM, Lee L, Hicks DJ, Joly MM,
Elion D, Rahman B, Mckernan C, Sanchez V, Balko JM, Stricker T, et
al: Key Survival factor, Mcl-1, correlates with sensitivity to
combined Bcl-2/Bcl-xL blockade. Mol Cancer Res. 15:259–268. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen D, Lu X, Yang F and Xing N: Circular
RNA circHIPK3 promotes cell proliferation and invasion of prostate
cancer by sponging miR-193a-3p and regulating MCL1 expression.
Cancer Manag Res. 11:1415–1423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Strathdee G, Sim A, Soutar R, Holyoake TL
and Brown R: HOXA5 is targeted by cell-type-specific CpG island
methylation in normal cells and during the development of acute
myeloid leukaemia. Carcinogenesis. 28:299–309. 2007. View Article : Google Scholar
|
|
67
|
Zhou H, Zhang Q, Huang W, He C, Zhou C,
Zhou J and Ning Y: Epigenetic silencing of ZCCHC10 by the lncRNA
SNHG1 promotes progression and venetoclax resistance of acute
myeloid leukemia. Int J Oncol. 62:642023. View Article : Google Scholar : PubMed/NCBI
|