Integrins are important cell surface adhesion
receptors that bind to both extracellular matrix (ECM) ligands and
cell surface ligands (1). As
pivotal signaling molecules, integrins mediate cell migration and
adhesion (2,3). Previous reports have shown that
integrins are highly expressed in a variety of cancer types
including lung cancer, hepatocellular carcinoma, pancreatic cancer
and head and neck squamous cell carcinomas, and serve crucial roles
in nearly every stage of cancer progression, including the
initiation of primary tumor formation, subsequent growth and the
metastatic cascade (4-8). Integrin-mediated sensing, stiffening
and remodeling of the tumor stroma are key steps in supporting
tumor cell invasion, acquiring cancer stem cell (CSC)
characteristics and developing drug resistance during cancer
progression (9,10). In addition, integrins have emerged
as attractive targets for both predicting cancer prognosis and
devising therapeutic strategies (11). Several integrin inhibitors, which
disrupt the interaction between integrins and their respective
ligands, hold notable therapeutic promise (12).
Exosomes are extracellular vesicles (EVs) secreted
by cells, which serve a crucial role in regulating intercellular
transport. Specifically, exosomes influence the state of both
adjacent and distant cells by delivering nucleic acids, proteins
and lipids (13). Tumor-derived
exosomes (TDEs) are particularly important in angiogenesis, immune
system regulation and the remodeling of surrounding tissues,
thereby supporting tumor progression and metastasis to organs
(14). As intercellular
messengers, exosomes reflect the physiological state of various
tumor cells, thus serving as biomarkers for clinical diagnosis and
evaluation (15). Integrins have
been identified as important components of exosomes. Accumulating
evidence indicates that exosomal integrins assist in exosome
homing, signal transduction and the phenotypic transformation of
recipient cells (16-18). Research on exosomes and integrins
has been steadily advancing. However, there remains a lack of
comprehensive reviews summarizing the role of exosomal integrins in
tumor development. Exploring the role of exosomal integrins in
tumors will enhance the understanding of tumor pathogenesis and
help to identify new diagnostic and therapeutic strategies
(10). In the present review, the
roles of exosomal integrins in tumor migration, tumorigenesis
[including epithelial-mesenchymal transition (EMT)], angiogenesis,
formation of the pre-metastatic niche (PMN) and the development of
tumor drug resistance were summarized. Additionally, the potential
of exosomal integrins in tumor prediction and treatment were
examined, which highlighted their prospects in improving the
efficiency of these aspects.
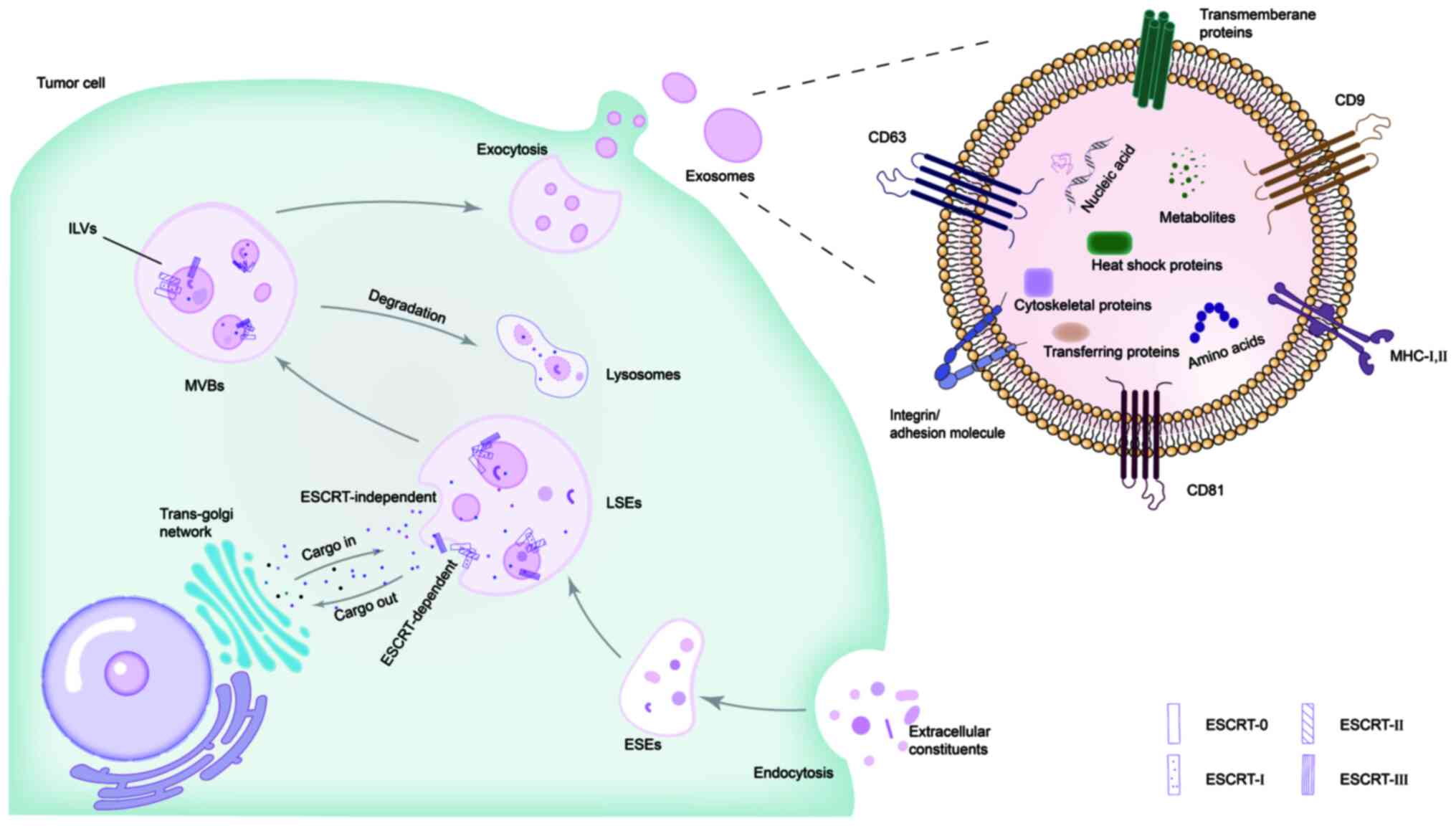
Cells release various types of EVs, including
exosomes, microvesicles and apoptotic bodies. Exosomes are the
smallest entities among EVs, with a diameter ranging 40-160 nm
(average, ~100 nm), are secreted by almost all cell types and have
been found in all biological fluids (19). The contents of exosomes vary and
reflect the composition of the donor cells (20,21). Exosomes contain adhesion
molecules, tetraspanins, major histocompatibility complex
molecules, transmembrane proteins, cytosolic (such as heat shock
proteins, cytoskeletal proteins and transporters) and nuclear
proteins, lipids, nucleic acids (including DNA, mRNA and non-coding
RNAs), amino acids and metabolites (such as bioactive lipids,
glycosidases and nucleotides) (Fig.
1) (22,23).
The formation and secretion of exosomes is a complex
process, which is typically divided into two pathways: The
endosomal sorting complex required for transport (ESCRT)-dependent
pathway and the ESCRT-independent pathway (24). The ESCRT system is a molecular
machine that completes endosomal membrane invagination to form
multivesicular bodies (MVBs) in eukaryotic cells. The
ESCRT-dependent pathway involves a series of complexes such as
ESCRT-0, -I, -II and -III (25).
ESCRT-0 is responsible for the recognition of mono-ubiquitinated
proteins. Then, ESCRT-I and -II bind to ESCRT-0 to induce endosomal
membrane budding. Finally, ESCRT-Ⅲ aggregates at the bud neck to
pinch off the membrane, thereby releasing intraluminal vesicles
(ILVs) into the lumen to form the MVBs (24,26). This process includes three steps:
i) Extracellular substances, such as lipids, proteins and
metabolites, enter cells through the initial plasma membrane
invagination to form early-sorting endosomes (ESEs); ii) ESEs
mature into late-sorting endosomes and then form MVBs. MVBs contain
ILVs regulated by the ESCRT complex, which are the precursors to
exosomes; and iii) MVBs either fuse with the plasma membrane to
release exosomes or merge with autophagosomes and are degraded in
the lysosomes (Fig. 1) (23,27). Studies have shown that, after the
components of the ESCRT complex are depleted, exosome production is
not completely blocked and a small number of exosomes are still
formed (28,29). This suggests that exosomes can
form in a manner independent of the ESCRT pathway. In the
ESCRT-independent mechanism, the release of exosomes is dependent
on sphingomyelinase. This involves the hydrolysis process of
sphingomyelins into ceramides via neutral sphingomyelinase, which
promotes inward budding of vesicles (30).
Exosomes carry a variety of bioactive molecules that
have key roles in physiological and pathological processes through
precise and dynamic intercellular communication. These processes
include immune responses and infections, metabolic diseases,
cardiovascular diseases, neurodegenerative diseases and cancer
(31,32). Particularly, exosomes have gained
attention in the field of cancer biology. Exosomes alter the fate
of both the recipient and exosome-releasing cells through autocrine
and paracrine signaling pathways (33). TDEs affect tumor growth,
metastasis and drug resistance by interacting with tumor and
stromal cells (34). Tumor
stromal cells are primarily cancer-associated fibroblasts (CAFs)
and immune cells (35). Exosomes
have been recognized as crucial mediators in regulating the
extracellular communication and metabolic reprogramming between
CAFs and cancer cells (36,37). Furthermore, TDEs remodel the
distant microenvironment at metastatic sites via blood and
lymphatic circulation (38).
Accumulating evidence indicates that TDEs are associated with
angiogenesis and ECM remodeling in the tumor microenvironment (TME)
(39-41).
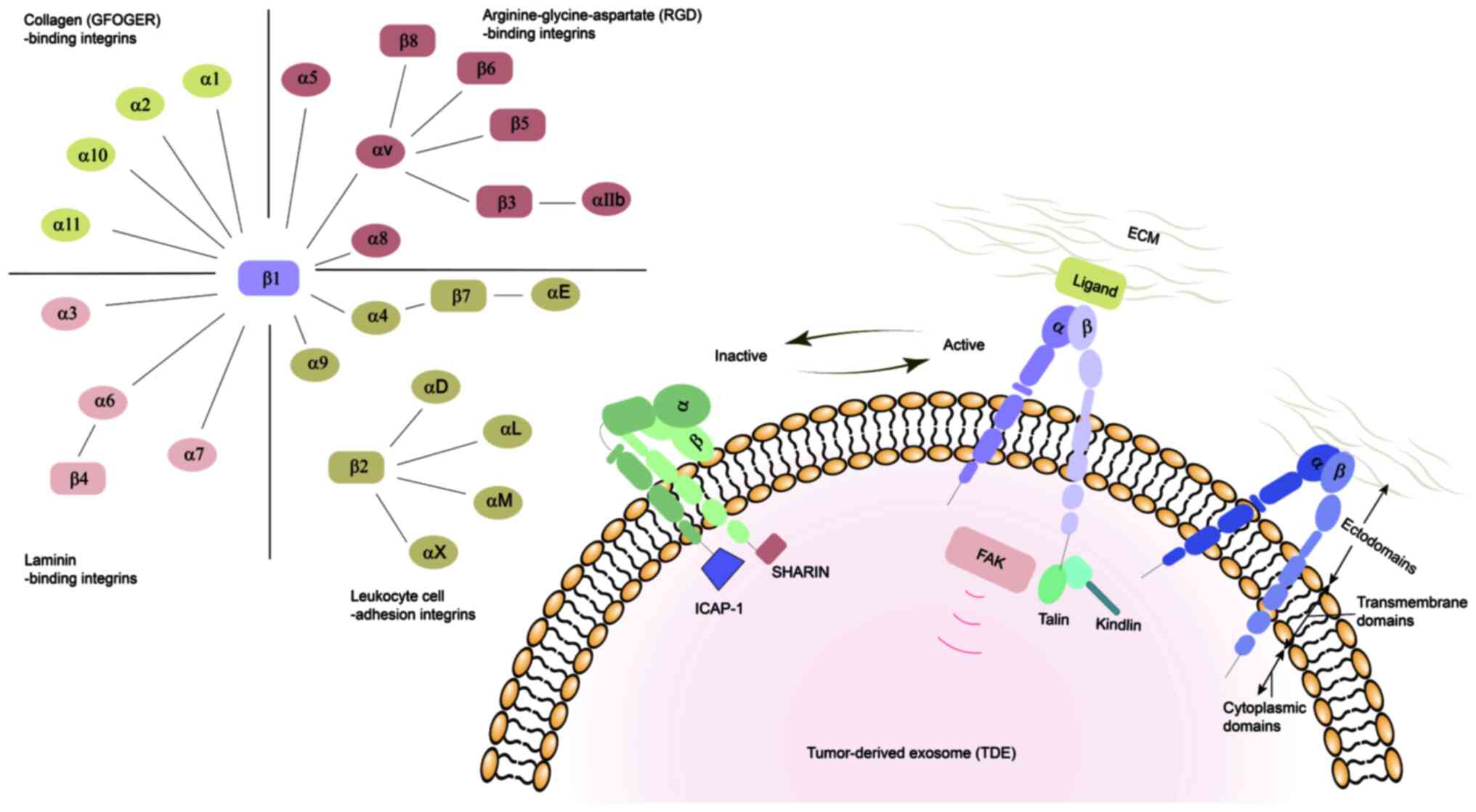
Integrins are cell adhesion molecules that serve as
membrane protein complexes, linking the ECM to the cytoskeleton,
and regulate various cell behaviors, such as adhesion,
proliferation and apoptosis, by triggering signal transduction
through their extracellular connections to the cytoskeleton. This
regulation provides the impetus and direction for cell migration
and invasion (42,43). Integrins are ubiquitous in
mammals, chickens, zebrafish and lower eukaryotes (44). In mammals, integrins are composed
of α and β subunits bound by non-covalent bonds. To date, 18 α
subunits and 8 β subunits have been identified, forming 24 αβ
heterodimeric integrins with different properties and tissue
distributions based on the various combinations. Both the α and β
subunits are type I transmembrane proteins, each containing
ectodomains, transmembrane domains and cytoplasmic domains
(45,46). The integrin ectodomains are
responsible for interacting with integrin ligands of the ECM
(47). Due to the different
characteristics of the integrin-ligand combinations, integrins can
be clustered into four classes: Arginine-glycine-aspartate
(RGD)-binding integrins (including αvβ1, αvβ3, αvβ5, αvβ6, αvβ8,
α5β1, α8β1 and αIIbβ3) (48),
leukocyte cell-adhesion integrins (including α4β1, α9β1, αLβ2,
αMβ2, αXβ2, αDβ2, α4β7 and αEβ7) (49), collagen-binding integrins (which
recognize the GFOGER binding site, including α1β1, α2β1, α10β1 and
α11β1) (50) and laminin-binding
integrins (including α3β1, α6β1, α6β4 and α7β1) (Fig. 2) (51,52).
Integrin-mediated transmembrane signals can exert
their role through conformational transition. These transitions
strictly regulate the transformation of integrins from a
low-affinity state to a high-affinity state (53). Proteins such as talin, kindlin and
tetraspanin trigger integrins to adopt an active open conformation,
while integrin cytoplasmic domain-associated protein-1 and SHARPIN
stabilize and inactivate integrins (54). Integrin-mediated transmembrane
signals are bidirectional. During 'outside-in' signaling, integrins
exposed to exosomes recognize and bind to their specific ligands on
the surface of the target cells, generating intracellular signals.
These signals control a series of physiological functions,
including regulating cell polarity, altering the cytoskeleton
structure, as well as cell survival and proliferation (55). The affinity of cells to
extracellular ligands is regulated by 'inside-out' signaling. As
this affinity increases, the interaction between integrins and the
ECM becomes strong enough to induce cells to migrate and the ECM to
remodel (Fig. 2) (56).
Integrins are expressed on the surface of tumor
cells. The expression of integrins αvβ3, αvβ5, α5β1, α6β4, α4β1 and
αvβ6 on tumor cells may have an important role in the progression
of lung, breast, prostate, pancreatic and colorectal cancers
(4,57). The abnormal expression of
integrins is involved in almost every stage of cancer development,
from the formation of primary tumors to the establishment of
metastatic niches (9). During
tumor cell survival and proliferation, integrin β1 promotes the
survival of cancer cells by activating different cell signaling
proteins and enhances cell proliferation by phosphorylating focal
adhesion kinase (FAK) (58).
Additionally, integrin αvβ3 enhances the migration and invasion of
non-small cell lung cancer (NSCLC) cells by triggering the FAK
signaling pathway (59). A
specific integrin subtype, αvβ6, is expressed in some types of
malignant tumors, such as prostate, breast, colorectal and lung
cancers, but not in normal epithelial cells or benign tumors
(60). Furthermore, αvβ6 confers
an invasive/metastatic phenotype to early colorectal cancer cells,
promoting tumor metastasis and reducing patient survival (61). Integrins regulate the adhesion of
tumor cells and the expression level of integrin is proportional to
the adhesion (62). In the early
stage of tumorigenesis, the expression of integrins is reduced,
which is conducive to the growth and spread of tumors. Then, the
tumor cells enter the blood circulation (63). Thereafter, the expression of
integrin is increased, which is beneficial to the adhesion of tumor
cells to the vascular endothelium. Specifically, integrins are the
main adhesion molecule in the angiogenesis stage of tumorigenesis.
Furthermore, integrins are expressed in the cavity and luminal
surface of vascular endothelial cells, mediating endothelial cell
migration and capillary lumen formation (64).
Exosomal integrins are a subset of integrins. Thus,
cell membrane and exosomal integrins share similarities in that
both are involved in cell-to-cell or cell-to-exosome communication.
Currently, the understanding of the communication mechanism of
exosomal integrins is still in its infancy. However, integrin
function is considered to be regulated by talin in exosomes, as in
other cellular systems (54). In
addition to certain similarities in the communication mechanism,
there are notable differences in the location and function of cell
membrane and exosomal integrins (64,65). As aforementioned, cell membrane
integrins mediate cell-ECM adhesion. In the occurrence, development
and metastasis of tumors, this adhesion directly affects the
physiological processes of tumor cell migration, proliferation and
survival. By contrast, exosomal integrins are embedded in the
membrane of exosomes, which are small EVs that promote
intercellular communication. These exosomal integrins serve a
crucial role in targeting specific cells or tissues to achieve
long-term signal transduction in processes such as cancer
metastasis and immune regulation (16,65). Exosomal integrins also serve an
important role in directing the tissue distribution of exosomes,
thereby supporting long-range cellular interactions (16,66). In addition, exosomal integrins are
involved in multiple steps of tumor formation as they enhance cell
adhesion and migration, participate in PMN formation, and they
modulate angiogenesis (54). It
has been reported that exosomal integrins also confer drug
resistance to tumors and impair drug efficacy (67).
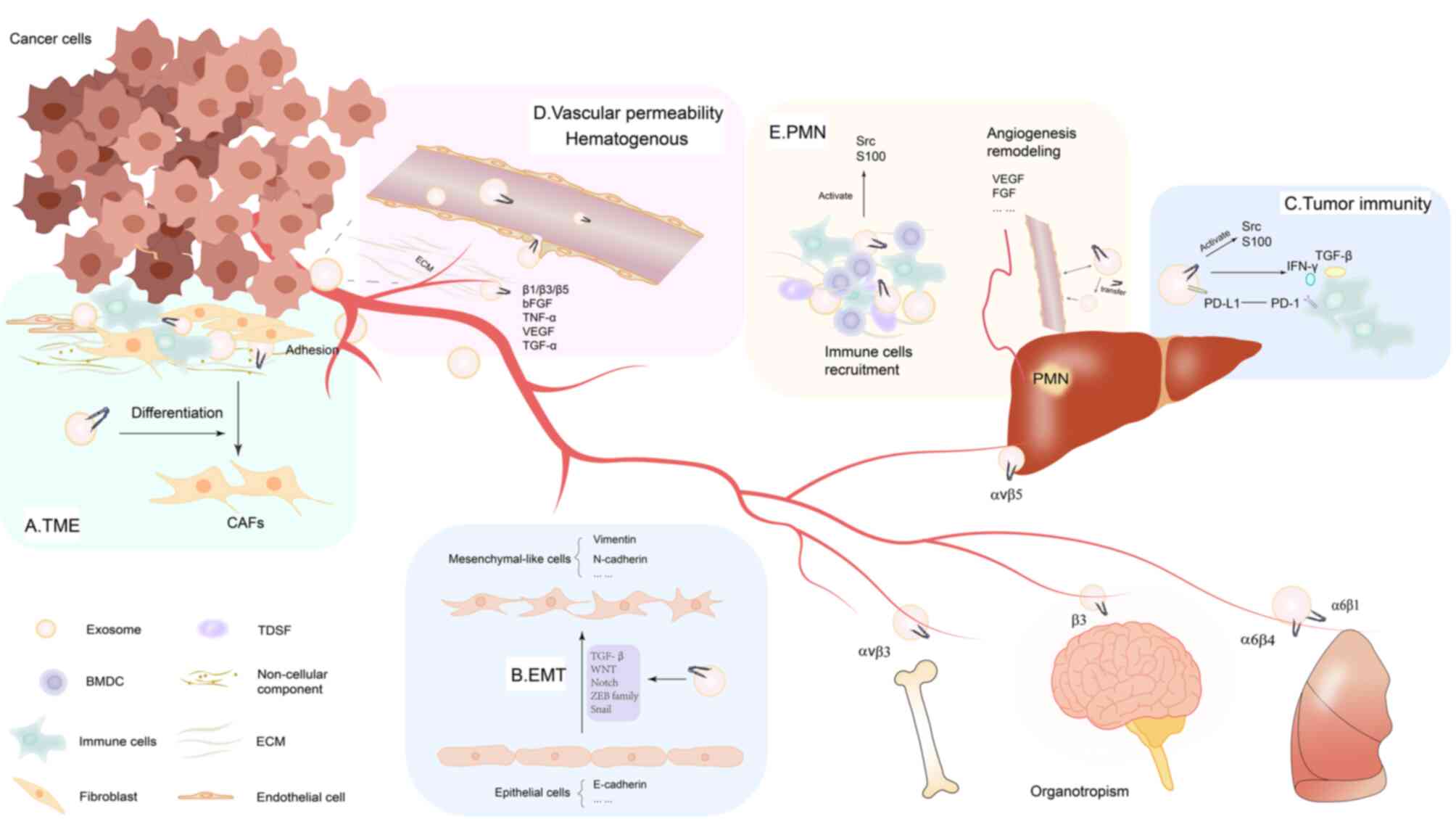
The TME is comprised of primary tumor lesions and
their surrounding cellular and non-cellular components (68). Key features of the TME include low
oxygen and nutritional levels, as well as an acidic environment.
Cancer cells become increasingly invasive in such conditions,
affecting tumor development and metastasis (69). TDEs, carrying molecules such as
oncoproteins, lipids and various types of RNA (such as microRNA,
mRNA and long non-coding RNA), induce changes in the TME phenotype
(70). The cellular components of
the TME include stromal cells (such as CAFs, mesenchymal stromal
cells and pericytes) and immune cells [such as T and B lymphocytes,
natural killer cells and tumor-associated macrophages (TAMs)]
(71,72). CAFs serve a notable role in
altering tumor mechanisms and are considered the most effective
cells for the deposition and remodeling of the TME (73). TDEs induce the differentiation of
fibroblasts (36). Integrin
β4-overexpressing triple-negative breast cancer cells transfer
integrin β4 protein to CAFs via exosomes, promoting cancer
progression (74). In the lung
metastasis niche of hepatocellular carcinoma (HCC), highly
metastatic HCC cells secrete exosomal microRNA (miR)-1247-3p,
activating the β1-integrin-NF-κB signaling pathway and transforming
fibroblasts into CAF (75). In
addition, macrophages are abundant in the TME, including both M1
and M2 polarized macrophages. TAMs are considered to promote tumor
invasion (76,77). Exosomal integrin αvβ3 secreted by
M2-like macrophages triggers the FAK signaling pathways in
recipient cells and confers migration and invasion capabilities to
NSCLC cells (59). The
non-cellular components of the TME mainly include the ECM,
including collagen and fibronectin, in which the ECM provides a
biological scaffold for mechanical support (77). Integrin signal transduction drives
intracellular signaling pathways through the interaction between
cells and the ECM. Integrins are critical for cell anchorage to the
ECM. Fibronectin matrix assembly is an integrin-dependent process.
Integrin α5β1 induce initial fibronectin fibrillogenesis by
transmitting cytoskeleton-generated tension to extracellular
fibronectin molecules (78).
Integrins α1β1, α2β1, α10β1 and α11β1 can bind to collagen
(79). In the basement membrane,
α3β1, α6β1 and α6β4 integrins promote epithelial cell adhesion by
recognizing the COOH-terminal globular domain of the laminin α
subunit (80). Integrin αvβ3 and
VEGF have synergistic signaling outputs during endothelial cell
activation and angiogenesis, induced by the interaction of VEGF and
ECM molecules (81). Based on the
essential role of integrins in cells adhesion to the ECM, it could
be suggested that tumor exosomal integrins serve a notable role in
the TME by facilitating adhesion to adjacent cells, exosomes and
the ECM. Abnormal adhesion functions can lead to diseases,
including cancer progression (82). In summary, integrins, including
exosomal integrins, affect the TME by regulating cell signaling
pathways and interactions with surrounding matrices (Fig. 3A).
It has been shown that integrins serve an important
role in regulating EMT and cancer stemness (89). Notably, integrins and TGF-β
effectively synergistically induce the aberrant expression of EMT
transcription factors, such as zinc finger E-box-binding homeobox 1
and snail2 (92,93). In a previous study, exosomes
loaded with integrin β-like 1 (ITGBL1) from primary colorectal
cancer (CRC) cells can convert fibroblasts in distal organs into
CAFs by activating the TNFα-induced protein 3-mediated NF-κB
signaling pathway. In this study, ITGBL1 overexpression enhanced
the secretion of IL-6 and IL-8 from fibroblasts or stellate cells,
thereby promoting the stemness and EMT of CRC cells (94). Additionally, CAF-derived IL-32
binds to integrin β3, thereby activating intracellular p38 MAPK
signaling in breast cancer cells. This signaling increases the
expression of EMT markers (including fibronectin, N-cadherin and
vimentin) and promotes tumor cell invasion (95). These factors drive tumor cells to
undergo EMT by various signaling pathways such as TGF-β, PI3K/AKT,
MAPK and NF-κB (Fig. 3B)
(52,96).
Previous studies have shown that TDEs have a pivotal
role in regulating the TME by inducing immune suppressor cells and
enabling cancer cells to evade immune effector cells (90,97). The antitumor ability of the human
immune system is inhibited by TDEs through inflammatory signaling
pathways (71). Exosomes secreted
by cancer and immune cells deliver protein cargo similar to that of
the primary tumor cells to specific tissues of the homing niche and
alter the gene expression and molecular structure of the homing
niche. Integrins regulate the tissue-specific homing pattern of
exosomes (16). TDEs initiate
immunosuppressive mechanisms at metastatic niches by triggering
immune suppressor cells such as myeloid-derived suppressor cells,
regulatory T cells (Tregs), tumor-associated neutrophils and TAMs
(98).
Integrins on tumor cells are involved in the
suppression of antitumor immunity throughout various stages of
tumor formation and metastasis (99,100). Interferon (IFN)-γ-producing
immune cells, mediated by α4β7, are recruited to CRC tissues where
they exert an effective antitumor immune response (101). Immunologic targeting of integrin
β4 significantly inhibited local tumor growth and metastases in
both 4T1 mammary tumors and SCC7 head and neck squamous carcinoma
models (102). Integrin αvβ3
regulates IFN-induced PD-L1 expression. In a mouse model, silencing
αvβ3 expression reduced IFN-induced STAT1 phosphorylation,
decreased PD-L1 expression and inhibited tumor growth (103). In addition, overexpression of
integrin α2 increased the phosphorylation level of STAT3 in tumor
cells to initiate PD-L1 transcription and thus upregulate PD-L1
expression (104). Integrin also
regulates the activation of transforming growth factor-β (TGF-β) in
immune cells, which may be another mechanism of tumor immune
escape. In mouse melanoma and breast cancer models, Tregs (which
express integrin αvβ8) are the predominant cell type that activate
TGF-β produced by cancer cells (105). The activated TGF-β then protects
the tumor from T cell attack by binding to and releasing αvβ8 on
tumor cells or latent immune cells, thereby preventing T cell
penetration into the tumor (106). The innate nature of integrins
from cells to exosomes has implications for tumor immunity. Based
on the inherent nature of exosomal integrins derived from tumor
cell integrins, it is reasonable to speculate that exosomal
integrins have similar tumor immune capabilities as tumor cell
integrins. Indeed, chronic inflammation is known to contribute to
cancer metastasis (107). Thus,
integrin-guided preferential distribution of TDEs determines which
specific organs may encounter TDE-mediated initiation of
inflammation (108). Exosomal
integrins not only target ECM proteins in distant tissues but are
also delivered to the target cells themselves where they activate
Src kinase signaling, leading to induction of the proinflammatory
S-100 gene (16). The S100
proteins S100A8 and S100A9 are important mediators of various
processes during chronic inflammation. The S100A8/9 proteins
stimulate infiltration of inflammatory lesions by activated myeloid
cells and are involved in leukocyte adhesion and migration
(109). A tumor-bearing mouse
model demonstrated that S100A8/A9 proteins participate in the
activation and accumulation of MDSC cells during the induction of
cancer T cell tolerance (110).
S100A8/A9 proteins activate the NF-κB pathway in a positive
feedback manner and ensure that the protein expression levels of
S100A8/A9 are sufficient to maintain the immunosuppressive function
of MDSC in the inflammatory tumor microenvironment (111). Therefore, the role of exosomal
integrins in tumor immunosuppression underscores the potential of
integrin-targeted immunotherapy (Fig.
3C).
Tumor metastasis refers to the process by which
tumor cells intravasate into blood vessels and lymphatic vessels
from the primary tumor site. Tumor angiogenesis is a complex
biological process involving several key steps, including local
damage to the basement membrane in the tissue, endothelial cell
migration activated by angiogenic factors and endothelial cell
proliferation and stability. VEGF, fibroblast growth factor (FGF)
and other angiogenic signals are key factors regulating the
angiogenic process (112).
Furthermore, exosomes derived from various human tumor cell lines
or plasma are effective inducers of angiogenesis, especially under
hypoxic conditions, by modulating endothelial cell properties to
promote angiogenesis (113).
Exosomes expressing tetraspanins can promote tumor growth by
increasing angiogenesis. For instance, TDEs enriched with
tetraspanin 8 and integrin α4 enhanced endothelial cell
proliferation and angiogenesis in rat pancreatic cancer by
upregulating angiogenesis-related genes through
endothelial-exosomal interactions (114). With improved understanding of
exosome heterogeneity, it is appealing to focus on the role of
exosomal integrins in influencing endothelial metabolism and
angiogenesis. The effect of exosomal integrins on the angiogenic
potential of endothelial cells has also been demonstrated in
prostate cancer (PrCa) progression. PrCa exosomes promote
angiogenesis by transferring exosomal integrin αvβ6 to endothelial
cells that do not typically express epithelial-specific integrin
αvβ6. Exosomal integrin αvβ6 uptake is associated with an
upregulation of the pro-angiogenic survivin levels and a
downregulation of the angiogenic inhibitory phosphorylated STAT1 in
endothelial cells (115). The
increased expression of integrin αvβ3 during angiogenesis in lung,
colon, pancreatic and breast cancer also suggests that integrins
are involved in tumor angiogenesis (62).
In the TME, EMT endows endothelial cells and cancer
cells with invasive capabilities, allowing cancer cells to traverse
the matrix with the assistance of TAM-derived VEGFA. This leads to
an abnormal increase in vascular permeability and the simultaneous
intravasation of tumor cells (116). β1 integrins can affect vascular
permeability, especially during inflammation, as the recruitment of
circulating cells to inflamed tissues involves recognition of cell
adhesion molecules (117).
Specifically, integrin β1 can affect the ability of circulating
cells to block, adhere and extravasate at sites of injury and
vascular permeability (118).
Integrin α4β1 serves a role in the homing of circulating progenitor
cells to tumor neovascularization that expresses vascular cell
adhesion protein 1 and cellular fibronectin (119). Additionally, integrin αvβ3 is
required for angiogenesis induced by basic FGF or TNF-α, while αvβ5
is required for angiogenesis induced by VEGF, TGF-α or phorbol
ester (120).
Hematogenous dissemination is often the primary
mechanism of distant metastasis, leading to the implantation of
tumor cells into distant organs through extravasation, ultimately
forming micro and macro tumor metastases (121). Although primary tumors can shed
millions of cells into the blood vessels every day, a very small
number of circulating tumor cells (CTCs) eventually reach distant
organs (122). The permeability
of blood vessels increases the possibility of tumor cells and TDEs
entering the blood. Millions of exosomes are secreted by primary
tumors into the blood vessels every day, and are then transferred
to specific organs via the vascular pathway by exosomal integrins
(16) (Fig. 3D).
The combined systemic effects of tumor-secreted
factors and tumor-shed extracellular vesicles induces a receptive
tissue microenvironment from a distance. The formation of the
microenvironment is initiated with local changes such as the
induction of vascular permeability, remodeling of stroma and
extracellular matrix, followed by systemic effects on the immune
system. These microenvironments are termed PMNs. The presence of a
PMN means that metastasis to specific organs is not random but
predictable (123). Clinical
cases have revealed that metastatic organ patterns follow
particular rules, with certain tumors preferentially metastasizing
and colonizing specific organs. Breast and prostate cancers
preferentially metastasize to bone (124). However, colorectal and
pancreatic cancers preferentially colonize the liver and lung
(125). The mechanisms directing
tumor cells to specific distant organs have long puzzled
researchers, and the precise mechanisms remain largely unknown.
However, Hoshino et al (16) confirmed that the key reason lies
in exosomal integrins. The role of exosomes and exosomal integrins
in tumor growth and metastasis has been emphasized. Exosomal
integrins can be localized to specific organs. After target cells
at the metastatic site ingest these exosomes, the PMN is
established by activating Src phosphorylation and pro-inflammatory
S-100 expression.
Studies have shown that PMN formation is a
chronological event that precedes the arrival and colonization of
tumor cells, effectively initiating the target site of metastasis.
Soluble molecules secreted by the primary tumor have a crucial role
in the formation of the PMN, promoting metastasis and even
determining organ-specific sites of the metastasis (126,127). Hoshino et al (16) injected FM1-43 dye-labeled exosome
isolated from organotropic human breast and pancreatic cancer cell
lines, which predominantly metastasize to the lungs, into naive
animals. Tumor FM1-43-labeled exosomes were then detected in
pre-metastatic cells by electron microscopy, suggesting that tumor
exosome uptake occurs at future metastatic sites. Tumor-derived
secreted factors (TDSFs) and soluble molecular components,
including EVs, secreted by primary tumors induce the mobilization
and recruitment of multiple cell populations to secondary organ
sites (123). Primary TDEs can
promote the formation of the TME at secondary sites and guide bone
marrow-derived dendritic cells (BMDCs) to form a pre-metastatic
microenvironment. For instance, bone marrow-derived hematopoietic
progenitor cells expressing VEGFR1 are mobilized and recruited to
the PMN of the lungs (128).
TDEs promote the formation of the PMN by inducing vascular
remodeling, preparing for the arrival of CTCs, promoting the
development of inflammation and recruiting BMDC (129). Exosomes from metastatic melanoma
increase the metastasis of primary tumors by educating bone marrow
progenitor cells via upregulation of the mesenchymal to epithelial
transition factor receptor. In addition, melanoma-derived exosomes
promote vascular leakage at the pre-metastatic site and reprogram
bone marrow progenitor cells to adopt pro-angiogenic phenotypes
(130). Macrophage inhibitory
factor in pancreatic cancer cell exosomes induces the release of
TGF-β, which in turn promotes the production of fibronectin. The
deposition of fibronectin promotes the colonization of bone
marrow-derived macrophages and neutrophils in liver metastasis
(131).
Through quantitative mass spectrometry and western
blotting analysis, it has been demonstrated that integrin α6,
combined with integrins β4 and β1, is abundantly present in
lung-tropic exosomes (16).
Conversely, integrins β5 and αv are present in liver-derived
exosomes and integrin β3 is predominant in brain-derived exosomes.
Infrared imaging showed that integrins β4 and β5 are responsible
for the specific uptake of exosomes by the liver and lung,
respectively (16,132). In another study, integrin αvβ6
was encapsulated in exosomes isolated from PC3 and RWPE PrCa cell
lines and was effectively transferred from donor cells to
αvβ6-negative recipient cells, colonizing on their surfaces
(133). PrCa is prone to distant
metastasis, with bone metastasis being particularly significant and
a primary cause of death in patients with PrCa (134). Among various integrins, αvβ3 has
gained notable attention for its role in promoting bone metastasis
through multiple regulatory mechanisms (135). Extracellular or membrane ligands
(such as small integrin-binding ligand N-linked glycoproteins,
connective tissue growth factor, cellular chemokines and ion
channel proteins) combine with or activate αvβ3 to mediate PrCa
bone metastasis. Moreover, the FAK, PI3K, ERK and αvβ3/RUNX2/RANKL
intercellular signaling pathway is stimulated by αVβ3 and has been
reported to be related to PrCa metastasis (136). The circulating exosomal integrin
β3 level is associated with the survival rate and intracranial
control after whole-brain radiotherapy in patients with brain
metastasis from lung cancer, supporting the suggestion that
exosomal integrin β3 mediates the pattern of brain metastasis
(137). Additionally, integrins
α3 and β1 are more abundant in urinary exosomes from patients with
metastatic PrCa compared with those from individuals with benign
prostatic hyperplasia or non-metastatic PrCa (138). In conclusion, exosomal integrins
can be identified as determinants of metastatic organotropism
(Fig. 3E).
Exosomes have a notable influence on drug
resistance, which is induced through a variety of mechanisms.
During chemotherapy, cancer cells cannot remove exosomes containing
unfavorable biomolecules. However, drug-resistant cancer cells can
load chemotherapy drugs into exosomes and expel them from tumor
cells directly (139,140). Another mechanism involves
exosomes carrying a drug-resistant phenotype from drug-resistant
cancer cells to drug-sensitive cancer cells (141). Additionally, exosomes can
regulate the transfer of functional proteins and/or miRNAs,
contributing to drug resistance (142). The contents of exosomes can also
cause immunosuppression, leading to drug resistance in HER2+ breast
cancer (143,144). Therefore, the roles of exosomes
and integrins in drug resistance across different cancer types are
reviewed here (Table I).
In drug-resistant human ovarian cancer, cisplatin
(CDDP) is encapsulated in exosomes and released within the
secretory pathway (145). The
ATP-binding cassette (ABC) transporter superfamily, including ABCB1
[also known as P-glycoprotein (P-gp)], ABCC1 (also known as
multidrug resistance protein 1) and ABCG2 (also known as breast
cancer resistance protein), function as multidrug resistance efflux
transporters. These transporters, located on the exosomal membrane,
actively pump anticancer drugs out of cells, resulting in
chemoresistance (146). P-gp, a
key anticancer pump transporter, plays a notable role in drug
resistance by retaining drug concentrations in tumor cells below
the therapeutic levels after chemotherapy, rendering the treatment
ineffective. This process may be mediated by exosomal integrin,
which facilitate the intercellular transfer of P-gp from
multidrug-resistant cells to drug-sensitive cells (147). Suppression of αvβ6 downregulated
the levels of MDR1 gene mRNA and P-gp. In particular, β6
shRNA-mediated silencing of the αvβ6 gene markedly decreased drug
efflux ability (148). In
addition, the delivery mechanisms of exosomes carrying nucleic
acids and proteins are closely related to tumor drug resistance.
For instance, the content of exosomes from paclitaxel-resistant
ovarian cancer cells, namely miR-1246, induces chemoresistance by
inhibiting 3′UTR caveolin-1, which directly suppresses the increase
of p-gp expression (149).
The effect of adhesion crosstalk between tumor cells
and stromal cells on the development of tumor drug resistance has
been studied in detail. Cancer cells adhere to the ECM or stromal
cells and can avoid being killed by radiotherapy and chemotherapy,
which is known as cell adhesion-mediated drug resistance (CAM-DR)
(150). CAM-DR is determined by
the integrin-ECM interaction. For instance, integrin β1 is
considered essential for radiotherapy resistance in human head and
neck cancer (151) and mediates
cell adhesion to the ECM. The role of integrins in tumor drug
resistance is primarily related to integrin-mediated signaling
pathways (152). For instance,
the integrin β1/Src/AKT signaling pathway serves a key role in
acquiring resistance to epidermal growth factor receptor-targeted
anticancer drugs, such as gefitinib and erlotinib, in lung cancer
(153). In human glioblastoma,
resistance to temozolomide is mainly due to integrin α5β1
downregulating the p53 pathway (154). In breast cancer cells, integrin
αvβ3 and its mediated FAK/PI3K/AKT signaling pathway are involved
in CDDP resistance (155).
Additionally, integrin α6 serves a key role in cancer drug
resistance by regulating the MAPK/ERK and PI3K/AKT signaling
pathways (156). Finally,
integrin β4 and vinculin in exosomes are associated with taxane
resistance in PrCa (157).
Exosomes are natural nano-biological delivery
systems with properties of stability, biocompatibility (endogenous
origin) and the ability to cross various physiological barriers.
These features make exosomes promising carriers for delivering
several drugs and biomacromolecules for cancer therapy (158). For instance, loading exosomes
with paclitaxel has indicated the potential for delivering multiple
chemotherapeutics to treat drug-resistant cancer (159). In cancer treatment, exosomes
protect the integrity of nucleic acids and shield proteins from
various enzymes and the immune system, making them excellent
carriers for delivering these macromolecules in therapy. Kobayashi
et al (160) demonstrated
that loading miR-199a-3p into exosomes inhibited c-Met expression
and reduced ovarian cancer cell proliferation, invasiveness and
dissemination. Similarly, exosomes loaded with signal regulatory
protein α (SIRPα) block the CD47 receptor on tumor cells more
effectively when compared with ferritin-SIRPα, indicating that
these exosomes have antitumor applications (161). In addition, since TDEs carry
specific integrins on their surface, they ensure targeted delivery
to specific organs and tissues. Exosome targeting was also
accomplished by genetic modification of exosome donor cells
(162). This characteristic
enhances the accuracy and efficiency of delivering therapeutic
contents through exosomes and exosomal integrins compared with
other biological carriers (163). However, to meet the demands of
large-scale clinical applications, the loading capacity and methods
for exosomes require optimization.
The diagnosis of cancer is often invasive. However,
non-invasive diagnostic methods in clinical oncology have emerged
as feasible alternatives with the study of exosomes (164,165). Exosomes, which represent their
source cells, contain biological information and are steadily
secreted into body fluids, making them ideal specimens for liquid
biopsy. More specifically, cancer biomarkers can be determined
according to the characteristics of TDE contents (such as the
proteins and nucleic acids) in blood, ascites or urine, as these
exosomes contain information related to cancer progression
(166). A study has shown that
integrin β4 and vinculin levels in exosomes isolated from PC3 cells
can serve as effective biomarkers for diagnosing PrCa associated
with taxane resistance (157).
The presence of integrin α2β1 in exosomes from tumor metastatic
cells, but not in exosomes from non-cancerous WI-38 lung
fibroblasts or epithelial MCF10A cells, indicates that this
integrin can be regarded as a biomarker of metastasis (16).
Integrins have are promising, yet challenging,
targets for the treatment of cancer. The specific expression of
integrins in TDEs allows them to be used for disease monitoring,
predicting patient survival and potentially distinguishing between
cancer types and stages (167-169). For instance, integrin αvβ6,
which is not expressed in normal adult epithelial cells but is
present in cancer cells, can be utilized for the diagnosis and
treatment of certain cancers such as ovarian, pancreatic,
esophageal, bile duct, oral and cervical cancers (170). In mouse models, heavy lead
peptides combined with αvβ6 have been used for non-invasive
imaging, highlighting the potential of αvβ6 as a promising
biomarker (171). Additionally,
a patent states that the monoclonal antibody, 10D5, which
specifically binds to β6, can be used to treat cancer (172). Invasive diagnostic techniques
for multiple brain metastasis are often impractical in clinical
settings, making it attractive to evaluate circulating EVs and
related integrins as biomarkers. Experts isolated and quantified
exosomal integrins of 75 patients with lung cancer with brain
metastasis and analyzed the association of exosomal integrins with
clinical factors, survival and intracranial or extracranial
failure. Accordingly, it was proposed that integrin β3 may serve as
a potential biomarker for the development of brain metastasis
(137). Furthermore, the
differential expression of exosomal integrins α6, αv and β1 is
associated with the tumor stage of various epithelial cancers such
as colon, lung, ovarian and prostate cancers (169).
Integrins are cell adhesion and signaling proteins
present on the surface of various cell subsets, making them
potential therapeutic targets. Numerous integrins involved in tumor
progression have been studied as attractive therapeutic targets for
cancer therapy (173,174). A study has shown that integrin
antagonists, which currently include monoclonal antibodies, RGD
peptide analogues and non-RGD antagonists, inhibit tumor growth by
affecting tumor cells and tumor-associated host cells. For
instance, integrin αvβ3 and αvβ5 inhibitors, such as cilengitide,
have demonstrated this capability (132). Cilengitide can reduce the
expression levels of integrin genes and inhibit the proliferation
of tumor cells (175). In
addition, cilengitide can activate αvβ3 and αvβ5 integrins of the
FAK/Paxillin/AKT signaling pathway to combat chemotherapy
resistance in glioblastoma (176). At present and to the best of our
knowledge, there are seven drugs targeting integrins on the market:
Abciximab, eptifibatide, tirofiban, natalizumab, vidolizumab,
lifitegrast and carotegrast. However, drugs specifically targeting
exosomal integrins have not yet been officially introduced to the
market (52). The application of
exosomes and integrins in cancer treatment and diagnosis is
summarized in Table II.
The effectiveness of bodily fluid biopsy relies on
advanced techniques for the isolation and characterization of
exosomal integrins. Despite significant efforts in developing
liquid biopsy methods and tumor biomarkers in oncology, only a few
have progressed to the clinical stage. This is because clinical
studies using liquid biopsy are often reliant on integrin content
and heterogeneity, thereby rendering them more expensive and
time-consuming compared with other common clinical testing
techniques (177). To promote
exosomal integrins as biomarkers to a broader population, more
portable, efficient and accurate characterization techniques are
needed (178). Standardization
in the selection, separation, characterization, storage, management
and quality control of exosomal integrins is crucial for their
clinical application in tumor diagnosis and treatment. Most
existing studies use tumor cell integrins as markers, but these are
often affected by the complexity of tumors. Using specific
integrins from TDEs in the humoral circulation as markers could
reduce this interference.
Over the past 30 years, research on exosomal
integrins as therapeutic targets has gained significant attention.
However, most drugs have failed in phase III clinical trials,
highlighting the challenges in translating experimental findings
into clinical therapies (179).
The pharmacodynamics and complex physiology of integrins contribute
to the ongoing problems of toxicity and poor efficacy in drugs that
have reached the market (180).
Current treatment strategies mainly focus on interfering with
integrin-ligand interactions. However, the class-specific nature of
integrin-targeted therapy presents another challenge. Since the
same integrin subunit can form different integrin heterodimers, the
accuracy of targeted therapies is affected. Additionally, other
molecules can interfere with targeted drugs. For instance,
abciximab binds to both glycoprotein IIb/IIIa and integrin αvβ3
with a similar affinity, suggesting it may act as an antagonist of
both GPIIb/IIIa and αvβ3 (181).
Such complexities complicate the use of preclinical experimental
data in developing effective therapies.
The heterodimeric structure of integrins enables
them to specifically recognize amino-acid motifs. The binding of
ligands to integrins can affect the allosteric states of integrins,
thereby altering the movement of EVs. This suggests that exosomal
integrins may act as sensors of the molecular environment. To
effectively utilize exosomal integrins in cancer therapy, it is
essential to fully understand their mechanisms of action in tumor
development. Additionally, combining targeted drugs that act on
ligands and integrins or their downstream effectors may offer a
promising approach. This direction could lead to the development of
new therapeutic strategies.
Not applicable.
RX and XZ made significant contributions to the
conception and design of the manuscript. YS, LS, and SW were
responsible for the acquisition, analysis and interpretation of
data. RX and YS undertook the editing, drafting and writing of the
manuscript. Data authentication is not applicable. All authors have
read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present work was supported by the Research Fund of Anhui
Institute of Translational Medicine (grant no. 2023zhyx-C90) and
the Basic and Clinical Cooperative Research and Promotion Program
of Anhui Medical University (grant no. 2023xkjT046).
|
1
|
Moreno-Layseca P, Icha J, Hamidi H and
Ivaska J: Integrin trafficking in cells and tissues. Nat Cell Biol.
21:122–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calderwood DA, Campbell ID and Critchley
DR: Talins and kindlins: Partners in integrin-mediated adhesion.
Nat Rev Mol Cell Biol. 14:503–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grigoryeva ES, Tashireva LA, Savelieva OE,
Zavyalova MV, Popova NO, Kuznetsov GA, Andryuhova ES and Perelmuter
VM: The association of integrins β3, β4, and αVβ5 on exosomes, CTCs
and tumor cells with localization of distant metastasis in breast
cancer patients. Int J Mol Sci. 24:29292023. View Article : Google Scholar
|
|
4
|
Liu F, Wu Q, Dong Z and Liu K: Integrins
in cancer: Emerging mechanisms and therapeutic opportunities.
Pharmacol Ther. 247:1084582023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mammadova-Bach E, Zigrino P, Brucker C,
Bourdon C, Freund M, De Arcangelis A, Abrams SI, Orend G, Gachet C
and Mangin PH: Platelet integrin α6β1 controls lung metastasis
through direct binding to cancer cell-derived ADAM9. JCI Insight.
1:e882452016. View Article : Google Scholar
|
|
6
|
Sun F, Wang J, Sun Q, Li F, Gao H, Xu L,
Zhang J, Sun X, Tian Y, Zhao Q, et al: Interleukin-8 promotes
integrin β3 upregulation and cell invasion through PI3K/Akt pathway
in hepatocellular carcinoma. J Exp Clin Cancer Res. 38:4492019.
View Article : Google Scholar
|
|
7
|
Reader CS, Vallath S, Steele CW, Haider S,
Brentnall A, Desai A, Moore KM, Jamieson NB, Chang D, Bailey P, et
al: The integrin αvβ6 drives pancreatic cancer through diverse
mechanisms and represents an effective target for therapy. J
Pathol. 249:332–342. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gopal S, Veracini L, Grall D, Butori C,
Schaub S, Audebert S, Camoin L, Baudelet E, Radwanska A,
Beghelli-de la Forest Divonne S, et al: Fibronectin-guided
migration of carcinoma collectives. Nat Commun. 8:141052017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamidi H and Ivaska J: Every step of the
way: Integrins in cancer progression and metastasis. Nat Rev
Cancer. 18:533–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raab-Westphal S, Marshall JF and Goodman
SL: Integrins as therapeutic targets: Successes and cancers.
Cancers (Basel). 9:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamidi H, Pietilä M and Ivaska J: The
complexity of integrins in cancer and new scopes for therapeutic
targeting. Br J Cancer. 115:1017–1023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slack RJ, Macdonald SJF, Roper JA, Jenkins
RG and Hatley RJD: Emerging therapeutic opportunities for integrin
inhibitors. Nat Rev Drug Discov. 21:60–78. 2022. View Article : Google Scholar
|
|
13
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Zhang Y, Gong H, Luo S and Cui Y:
The role of exosomes and their applications in cancer. Int J Mol
Sci. 22:122042021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalappurakkal JM, Anilkumar AA, Patra C,
van Zanten TS, Sheetz MP and Mayor S: Integrin mechano-chemical
signaling generates plasma membrane nanodomains that promote cell
spreading. Cell. 177:1738–1756.e23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu H, Bowler N, Harshyne LA, Craig Hooper
D, Krishn SR, Kurtoglu S, Fedele C, Liu Q, Tang HY, Kossenkov AV,
et al: Exosomal αvβ6 integrin is required for monocyte M2
polarization in prostate cancer. Matrix Biol. 70:20–35. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Welsh JA, Goberdhan DCI, O'Driscoll L,
Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks
TAP, Erdbrügger U, et al: Minimal information for studies of
extracellular vesicles (MISEV2023): From basic to advanced
approaches. J Extracell Vesicles. 13:e124042024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Février B and Raposo G: Exosomes:
Endosomal-derived vesicles shipping extracellular messages. Curr
Opin Cell Biol. 16:415–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doyle L and Wang M: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mashouri L, Yousefi H, Aref AR, Ahadi AM,
Molaei F and Alahari SK: Exosomes: Composition, biogenesis, and
mechanisms in cancer metastasis and drug resistance. Mol Cancer.
18:752019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hurley JH: ESCRT complexes and the
biogenesis of multivesicular bodies. Curr Opin Cell Biol. 20:4–11.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mathieu M, Martin-Jaular L, Lavieu G and
Théry C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tricarico C, Clancy J and D'Souza-Schorey
C: Biology and biogenesis of shed microvesicles. Small GTPases.
8:220–232. 2017. View Article : Google Scholar :
|
|
28
|
Stuffers S, Sem Wegner C, Stenmark H and
Brech A: Multivesicular endosome biogenesis in the absence of
ESCRTs. Traffic. 10:925–937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Theos AC, Truschel ST, Tenza D, Hurbain I,
Harper DC, Berson JF, Thomas PC, Raposo G and Marks MS: A novel
pathway for sorting to intralumenal vesicles of multivesicular
endosomes involved in organelle morphogenesis. Dev Cell.
10:343–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang K, Fu W, Deng M, Li X, Wu M and Wang
Y: The sphingolipids change in exosomes from cancer patients and
association between exosome release and sphingolipids level based
on a pseudotargeted lipidomics method. Anal Chim Acta.
1305:3425272024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guay C and Regazzi R: Exosomes as new
players in metabolic organ cross-talk. Diabetes Obes Metab.
19(Suppl 1): S137–S146. 2017. View Article : Google Scholar
|
|
32
|
Quek C and Hill AF: The role of
extracellular vesicles in neurodegenerative diseases. Biochem
Biophys Res Commun. 483:1178–1186. 2017. View Article : Google Scholar
|
|
33
|
Li Y, Chen ZK, Duan X, Zhang HJ, Xiao BL,
Wang KM and Chen G: Targeted inhibition of tumor-derived exosomes
as a novel therapeutic option for cancer. Exp Mol Med.
54:1379–1389. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ciardiello C, Cavallini L, Spinelli C,
Yang J, Reis-Sobreiro M, de Candia P, Minciacchi VR and Di Vizio D:
Focus on extracellular vesicles: New frontiers of cell-to-cell
communication in cancer. Int J Mol Sci. 17:1752016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Whiteside TL: Immune modulation of T-cell
and NK (natural killer) cell activities by TEXs (tumour-derived
exosomes). Biochem Soc Trans. 41:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Webber J, Steadman R, Mason MD, Tabi Z and
Clayton A: Cancer exosomes trigger fibroblast to myofibroblast
differentiation. Cancer Res. 70:9621–9630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rai A, Greening DW, Chen M, Xu R, Ji H and
Simpson RJ: Exosomes derived from human primary and metastatic
colorectal cancer cells contribute to functional heterogeneity of
activated fibroblasts by reprogramming their proteome. Proteomics.
19:e18001482019. View Article : Google Scholar
|
|
38
|
Cueni LN and Detmar M: The lymphatic
system in health and disease. Lymphat Res Biol. 6:109–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang E, Wang X, Gong Z, Yu M, Wu H and
Zhang D: Exosome-mediated metabolic reprogramming: The emerging
role in tumor microenvironment remodeling and its influence on
cancer progression. Signal Transduct Target Ther. 5:2422020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ludwig N and Whiteside TL: Potential roles
of tumor-derived exosomes in angiogenesis. Expert Opin Ther
Targets. 22:409–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu B, Liu DA, Guan L, Myint PK, Chin L,
Dang H, Xu Y, Ren J, Li T, Yu Z, et al: Stiff matrix induces
exosome secretion to promote tumour growth. Nat Cell Biol.
25:415–424. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hynes RO: The emergence of integrins: A
personal and historical perspective. Matrix Biol. 23:333–340. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Campbell ID and Humphries MJ: Integrin
structure, activation, and interactions. Cold Spring Harb Perspect
Biol. 3:a0049942011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Van Der Flier A and Sonnenberg A: Function
and interactions of integrins. Cell Tissue Res. 305:285–298. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng Y and Leftheris K: Insights into
protein-ligand interactions in integrin complexes: Advances in
structure determinations. J Med Chem. 63:5675–5696. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Humphries JD, Byron A and Humphries MJ:
Integrin ligands at a glance. J Cell Sci. 119:3901–3903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun CC, Qu XJ and Gao ZH:
Arginine-glycine-aspartate-binding integrins as therapeutic and
diagnostic targets. Am J Ther. 23:e198–e207. 2016. View Article : Google Scholar
|
|
49
|
Mitroulis I, Alexaki VI, Kourtzelis I,
Ziogas A, Hajishengallis G and Chavakis T: Leukocyte integrins:
Role in leukocyte recruitment and as therapeutic targets in
inflammatory disease. Pharmacol Ther. 147:123–135. 2015. View Article : Google Scholar
|
|
50
|
Zeltz C and Gullberg D: The
integrin-collagen connection-a glue for tissue repair? J Cell Sci.
129:653–664. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aumailley M: The laminin family. Cell
Adhes Migr. 7:48–55. 2013. View Article : Google Scholar
|
|
52
|
Pang X, He X, Qiu Z, Zhang H, Xie R, Liu
Z, Gu Y, Zhao N, Xiang Q and Cui Y: Targeting integrin pathways:
Mechanisms and advances in therapy. Signal Transduct Target Ther.
8:12023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Anthis NJ and Campbell ID: The tail of
integrin activation. Trends Biochem Sci. 36:191–198. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Soe ZY, Park EJ and Shimaoka M: Integrin
regulation in immunological and cancerous cells and exosomes. Int J
Mol Sci. 22:21932021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shattil SJ, Kim C and Ginsberg MH: The
final steps of integrin activation: The end game. Nat Rev Mol Cell
Biol. 11:288–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ginsberg MH, Partridge A and Shattil SJ:
Integrin regulation. Curr Opin Cell Biol. 17:509–516. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ganguly KK, Pal S, Moulik S and Chatterjee
A: Integrins and metastasis. Cell Adhes Migr. 7:251–261. 2013.
View Article : Google Scholar
|
|
58
|
Zhang L, Qu J, Qi Y, Duan Y, Huang YW,
Zhou Z, Li P, Yao J, Huang B, Zhang S and Yu D: EZH2 engages TGFβ
signaling to promote breast cancer bone metastasis via integrin
β1-FAK activation. Nat Commun. 13:25432022. View Article : Google Scholar
|
|
59
|
Huang L, Wang F, Wang X, Su C, Wu S, Yang
C, Luo M, Zhang J and Fu L: M2-like macrophage-derived exosomes
facilitate metastasis in non-small-cell lung cancer by delivering
integrin αVβ3. MedComm (2020). 4:e1912022. View Article : Google Scholar
|
|
60
|
Hazelbag S, Kenter GG, Gorter A, Dreef EJ,
Koopman LA, Violette SM, Weinreb PH and Fleuren GJ: Overexpression
of the alpha v beta 6 integrin in cervical squamous cell carcinoma
is a prognostic factor for decreased survival. J Pathol.
212:316–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cantor DI, Cheruku HR, Nice EC and Baker
MS: Integrin αvβ6 sets the stage for colorectal cancer metastasis.
Cancer Metastasis Rev. 34:715–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li ZH, Zhou Y, Ding YX, Guo QL and Zhao L:
Roles of integrin in tumor development and the target inhibitors.
Chin J Nat Med. 17:241–251. 2019.PubMed/NCBI
|
|
63
|
Lawrence R, Watters M, Davies CR, Pantel K
and Lu YJ: Circulating tumour cells for early detection of
clinically relevant cancer. Nat Rev Clin Oncol. 20:487–500. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Silva R, D'Amico G, Hodivala-Dilke KM and
Reynolds LE: Integrins: The keys to unlocking angiogenesis.
Arterioscler Thromb Vasc Biol. 28:1703–1713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shimaoka M, Kawamoto E, Gaowa A, Okamoto T
and Park EJ: Connexins and integrins in exosomes. Cancers (Basel).
11:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Grigoryeva ES, Savelieva OE, Popova NO,
Cherdyntseva NV and Perelmuter VM: Do tumor exosome integrins alone
determine organotropic metastasis? Mol Biol Rep. 47:8145–8157.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Domenis R, Marino M, Cifù A, Scardino G,
Curcio F and Fabris M: Circulating exosomes express α4β7 integrin
and compete with CD4+ T cells for the binding to Vedolizumab. PLoS
One. 15:e02423422020. View Article : Google Scholar
|
|
68
|
Maman S and Witz IP: A history of
exploring cancer in context. Nat Rev Cancer. 18:359–376. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ma Z, Wang LZ, Cheng JT, Lam WST, Ma X,
Xiang X, Wong AL, Goh BC, Gong Q, Sethi G and Wang L: Targeting
hypoxia-inducible factor-1-mediated metastasis for cancer therapy.
Antioxid Redox Signal. 34:1484–1497. 2021. View Article : Google Scholar
|
|
70
|
Xu R, Rai A, Chen M, Suwakulsiri W,
Greening DW and Simpson RJ: Extracellular vesicles in
cancer-implications for future improvements in cancer care. Nat Rev
Clin Oncol. 15:617–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li K, Chen Y, Li A, Tan C and Liu X:
Exosomes play roles in sequential processes of tumor metastasis.
Int J Cancer. 144:1486–1495. 2019. View Article : Google Scholar
|
|
72
|
Roma-Rodrigues C, Mendes R, Baptista PV
and Fernandes AR: Targeting tumor microenvironment for cancer
therapy. Int J Mol Sci. 20:8402019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sung JS, Kang CW, Kang S, Jang Y, Chae YC,
Kim BG and Cho NH: ITGB4-mediated metabolic reprogramming of
cancer-associated fibroblasts. Oncogene. 39:664–676. 2020.
View Article : Google Scholar
|
|
75
|
Fang T, Lv H, Lv G, Li T, Wang C, Han Q,
Yu L, Su B, Guo L, Huang S, et al: Tumor-derived exosomal
miR-1247-3p induces cancer-associated fibroblast activation to
foster lung metastasis of liver cancer. Nat Commun. 9:1912018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther. 221:1077532021.
View Article : Google Scholar :
|
|
78
|
Pankov R, Cukierman E, Katz BZ, Matsumoto
K, Lin DC, Lin S, Hahn C and Yamada KM: Integrin dynamics and
matrix assembly: Tensin-dependent translocation of alpha(5)beta(1)
integrins promotes early fibronectin fibrillogenesis. J Cell Biol.
148:1075–1090. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bachmann M, Kukkurainen S, Hytönen VP and
Wehrle-Haller B: Cell adhesion by integrins. Physiol Rev.
99:1655–1699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yazlovitskaya EM, Viquez OM, Tu T, De
Arcangelis A, Georges-Labouesse E, Sonnenberg A, Pozzi A and Zent
R: The laminin binding α3 and α6 integrins cooperate to promote
epithelial cell adhesion and growth. Matrix Biol. 77:101–116. 2019.
View Article : Google Scholar
|
|
81
|
Somanath PR, Malinin NL and Byzova TV:
Cooperation between integrin alphavbeta3 and VEGFR2 in
angiogenesis. Angiogenesis. 12:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kanchanawong P and Calderwood DA:
Organization, dynamics and mechanoregulation of integrin-mediated
cell-ECM adhesions. Nat Rev Mol Cell Biol. 24:142–161. 2023.
View Article : Google Scholar
|
|
83
|
Nieto MA, Huang RYJ, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liao TT and Yang MH: Revisiting
epithelial-mesenchymal transition in cancer metastasis: The
connection between epithelial plasticity and stemness. Mol Oncol.
11:792–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liao TT and Yang MH: Hybrid
epithelial/mesenchymal state in cancer metastasis: Clinical
significance and regulatory mechanisms. Cells. 9:6232020.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lu W and Kang Y: Epithelial-mesenchymal
plasticity in cancer progression and metastasis. Dev Cell.
49:361–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tanabe S, Quader S, Cabral H and Ono R:
Interplay of EMT and CSC in cancer and the potential therapeutic
strategies. Front Pharmacol. 11:9042020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Syn N, Wang L, Sethi G, Thiery JP and Goh
BC: Exosome-mediated metastasis: From epithelial-mesenchymal
transition to escape from immunosurveillance. Trends Pharmacol Sci.
37:606–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mamuya FA and Duncan MK: aV integrins and
TGF-β-induced EMT: A circle of regulation. J Cell Mol Med.
16:445–455. 2012. View Article : Google Scholar :
|
|
93
|
Caramel J, Papadogeorgakis E, Hill L,
Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J,
Hutchinson P, Tse G, et al: A switch in the expression of embryonic
EMT-inducers drives the development of malignant melanoma. Cancer
Cell. 24:466–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q,
Jia R, Li R, Sun J, Wang Z, et al: Primary tumors release
ITGBL1-rich extracellular vesicles to promote distal metastatic
tumor growth through fibroblast-niche formation. Nat Commun.
11:12112020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M,
Qin Y, Sun K, Teng Y and Liu M: Cancer-associated fibroblast
(CAF)-derived IL32 promotes breast cancer cell invasion and
metastasis via integrin β3-p38 MAPK signalling. Cancer Lett.
442:320–332. 2019. View Article : Google Scholar
|
|
96
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hao Q, Wu Y, Wu Y, Wang P and Vadgama JV:
Tumor-derived exosomes in tumor-induced immune suppression. Int J
Mol Sci. 23:14612022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao L, Ma X and Yu J: Exosomes and
organ-specific metastasis. Mol Ther Methods Clin Dev. 22:133–147.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen Y, Yang S, Tavormina J, Tampe D,
Zeisberg M, Wang H, Mahadevan KK, Wu CJ, Sugimoto H, Chang CC, et
al: Oncogenic collagen I homotrimers from cancer cells bind to α3β1
integrin and impact tumor microbiome and immunity to promote
pancreatic cancer. Cancer Cell. 40:818–834.e9. 2022. View Article : Google Scholar
|
|
100
|
Genduso S, Freytag V, Schetler D, Kirchner
L, Schiecke A, Maar H, Wicklein D, Gebauer F, Bröker K, Stürken C,
et al: Tumor cell integrin β4 and tumor stroma E-/P-selectin
cooperatively regulate tumor growth in vivo. J Hematol Oncol.
16:232023. View Article : Google Scholar
|
|
101
|
Zhang Y, Xie R, Zhang H, Zheng Y, Lin C,
Yang L, Huang M, Li M, Song F, Lu L, et al: Integrin β7 inhibits
colorectal cancer pathogenesis via maintaining antitumor immunity.
Cancer Immunol Res. 9:967–980. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ruan S, Lin M, Zhu Y, Lum LG, Thakur A,
Jin R, Shao W, Zhang Y, Hu Y, Huang S, et al: Integrin β4-targeted
cancer immunotherapies inhibit tumor growth and decrease
metastasis. Cancer Res. 80:771–783. 2020. View Article : Google Scholar
|
|
103
|
Vannini A, Leoni V, Barboni C, Sanapo M,
Zaghini A, Malatesta P, Campadelli-Fiume G and Gianni T:
αvβ3-integrin regulates PD-L1 expression and is involved in cancer
immune evasion. Proc Natl Acad Sci USA. 116:20141–20150. 2019.
View Article : Google Scholar
|
|
104
|
Ren D, Zhao J, Sun Y, Li D, Meng Z, Wang
B, Fan P, Liu Z, Jin X and Wu H: Overexpressed ITGA2 promotes
malignant tumor aggression by up-regulating PD-L1 expression
through the activation of the STAT3 signaling pathway. J Exp Clin
Cancer Res. 38:4852019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lainé A, Labiad O, Hernandez-Vargas H,
This S, Sanlaville A, Léon S, Dalle S, Sheppard D, Travis MA,
Paidassi H and Marie JC: Regulatory T cells promote cancer
immune-escape through integrin αvβ8-mediated TGF-β activation. Nat
Commun. 12:62282021. View Article : Google Scholar
|
|
106
|
Takasaka N, Seed RI, Cormier A, Bondesson
AJ, Lou J, Elattma A, Ito S, Yanagisawa H, Hashimoto M, Ma R, et
al: Integrin αvβ8-expressing tumor cells evade host immunity by
regulating TGF-β activation in immune cells. JCI Insight.
3:e1225912018. View Article : Google Scholar
|
|
107
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Myint PK, Park EJ, Gaowa A, Kawamoto E and
Shimaoka M: Targeted remodeling of breast cancer and immune cell
homing niches by exosomal integrins. Diagn Pathol. 15:382020.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lukanidin E and Sleeman JP: Building the
niche: The role of the S100 proteins in metastatic growth. Semin
Cancer Biol. 22:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nagaraj S, Gupta K, Pisarev V, Kinarsky L,
Sherman S, Kang L, Herber DL, Schneck J and Gabrilovich DI: Altered
recognition of antigen is a mechanism of CD8+ T cell
tolerance in cancer. Nat Med. 13:828–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ichikawa M, Williams R, Wang L, Vogl T and
Srikrishna G: S100A8/A9 activate key genes and pathways in colon
tumor progression. Mol Cancer Res. 9:133–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar
|
|
113
|
Sharghi-Namini S, Tan E, Ong LLS, Ge R and
Asada HH: Dll4-containing exosomes induce capillary sprout
retraction in a 3D microenvironment. Sci Rep. 4:40312014.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Nazarenko I, Rana S, Baumann A, McAlear J,
Hellwig A, Trendelenburg M, Lochnit G, Preissner KT and Zöller M:
Cell surface tetraspanin Tspan8 contributes to molecular pathways
of exosome-induced endothelial cell activation. Cancer Res.
70:1668–1678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Krishn SR, Singh A, Bowler N, Duffy AN,
Friedman A, Fedele C, Kurtoglu S, Tripathi SK, Wang K, Hawkins A,
et al: Prostate cancer sheds the αvβ3 integrin in vivo through
exosomes. Matrix Biol. 77:41–57. 2019. View Article : Google Scholar
|
|
116
|
Harney AS, Arwert EN, Entenberg D, Wang Y,
Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG and Condeelis JS:
Real-time imaging reveals local, transient vascular permeability
and tumor cell intravasation stimulated by Tie2Hi
macrophage-derived VEGFA. Cancer Discov. 5:932–943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sixt M, Bauer M, Lämmermann T and Fässler
R: Beta1 integrins: Zip codes and signaling relay for blood cells.
Curr Opin Cell Biol. 18:482–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sun L, Guo S, Xie Y and Yao Y: The
characteristics and the multiple functions of integrin β1 in human
cancers. J Transl Med. 21:7872023. View Article : Google Scholar
|
|
119
|
Jin H, Su J, Garmy-Susini B, Kleeman J and
Varner J: Integrin alpha4beta1 promotes monocyte trafficking and
angiogenesis in tumors. Cancer Res. 66:2146–2152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Weis SM: Evaluating integrin function in
models of angiogenesis and vascular permeability. Methods Enzymol.
426:505–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sobierajska K, Ciszewski WM,
Sacewicz-Hofman I and Niewiarowska J: Endothelial cells in the
tumor microenvironment. Adv Exp Med Biol. 1234:71–86. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Labelle M and Hynes RO: The initial hours
of metastasis: The importance of cooperative host-tumor cell
interactions during hematogenous dissemination. Cancer Discov.
2:1091–1099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Peinado H, Lavotshkin S and Lyden D: The
secreted factors responsible for pre-metastatic niche formation:
Old sayings and new thoughts. Semin Cancer Biol. 21:139–146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Coleman RE, Croucher PI, Padhani AR,
Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primer. 6:832020. View Article : Google Scholar
|
|
125
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Peinado H, Zhang H, Matei IR, Costa-Silva
B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang
Y, et al: Pre-metastatic niches: Organ-specific homes for
metastases. Nat Rev Cancer. 17:302–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu Y and Cao X: Characteristics and
significance of the pre-metastatic niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar
|
|
133
|
Fedele C, Singh A, Zerlanko BJ, Iozzo RV
and Languino LR: The αvβ6 integrin is transferred intercellularly
via exosomes. J Biol Chem. 290:4545–4551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang X: Interactions between cancer cells
and bone microenvironment promote bone metastasis in prostate
cancer. Cancer Commun (Lond). 39:762019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Geng X, Chang B and Shan J: Role and
correlation of exosomes and integrins in bone metastasis of
prostate cancer. Andrologia. 54:e145502022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tang L, Xu M, Zhang L, Qu L and Liu X:
Role of αVβ3 in prostate cancer: Metastasis initiator and important
therapeutic target. Onco Targets Ther. 13:7411–7422. 2020.
View Article : Google Scholar :
|
|
137
|
Chen GY, Cheng JCH, Chen YF, Yang JCH and
Hsu FM: Circulating exosomal integrin β3 is associated with
intracranial failure and survival in lung cancer patients receiving
cranial irradiation for brain metastases: A prospective
observational study. Cancers (Basel). 13:3802021. View Article : Google Scholar
|
|
138
|
Bijnsdorp IV, Geldof AA, Lavaei M, Piersma
SR, Van Moorselaar RJA and Jimenez CR: Exosomal ITGA3 interferes
with non-cancerous prostate cell functions and is increased in
urine exosomes of metastatic prostate cancer patients. J Extracell
Vesicles. 2: View Article : Google Scholar : 2013.PubMed/NCBI
|
|
139
|
Dallavalle S, Dobričić V, Lazzarato L,
Gazzano E, Machuqueiro M, Pajeva I, Tsakovska I, Zidar N and
Fruttero R: Improvement of conventional anti-cancer drugs as new
tools against multidrug resistant tumors. Drug Resist Updat.
50:1006822020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sousa D, Lima RT and Vasconcelos MH:
Intercellular transfer of cancer drug resistance traits by
extracellular vesicles. Trends Mol Med. 21:595–608. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma
TF, Zhang J, Chen L, Tang JH and Zhao JH: Exosomes mediate drug
resistance transfer in MCF-7 breast cancer cells and a probable
mechanism is delivery of P-glycoprotein. Tumor Biol.
35:10773–10779. 2014. View Article : Google Scholar
|
|
142
|
Wang B, Zhang Y, Ye M, Wu J, Ma L and Chen
H: Cisplatin-resistant MDA-MB-231 cell-derived exosomes increase
the resistance of recipient cells in an exosomal
miR-423-5p-dependent manner. Curr Drug Metab. 20:804–814. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Dong X, Bai X, Ni J, Zhang H, Duan W,
Graham P and Li Y: Exosomes and breast cancer drug resistance. Cell
Death Dis. 11:9872020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Martinez VG, O'Neill S, Salimu J, Breslin
S, Clayton A, Crown J and O'Driscoll L: Resistance to HER2-targeted
anti-cancer drugs is associated with immune evasion in cancer cells
and their derived extracellular vesicles. Oncoimmunology.
6:e13625302017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Safaei R, Larson BJ, Cheng TC, Gibson MA,
Otani S, Naerdemann W and Howell SB: Abnormal lysosomal trafficking
and enhanced exosomal export of cisplatin in drug-resistant human
ovarian carcinoma cells. Mol Cancer Ther. 4:1595–1604. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Goler-Baron V and Assaraf YG: Structure
and function of ABCG2-rich extracellular vesicles mediating
multidrug resistance. PLoS One. 6:e160072011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Mollazadeh S, Sahebkar A, Hadizadeh F,
Behravan J and Arabzadeh S: Structural and functional aspects of
P-glycoprotein and its inhibitors. Life Sci. 214:118–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhang YH, Gao ZF, Dong GH, Li X, Wu Y, Li
G, Wang AL, Li HL and Yin DL: Suppression of αvβ6 downregulates
P-glycoprotein and sensitizes multidrug-resistant breast cancer
cells to anticancer drugs. Neoplasma. 67:379–388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Lopes-Rodrigues V, Seca H, Sousa D, Sousa
E, Lima RT and Vasconcelos MH: The network of P-glycoprotein and
microRNAs interactions. Int J Cancer. 135:253–263. 2014. View Article : Google Scholar
|
|
150
|
Damiano JS, Cress AE, Hazlehurst LA, Shtil
AA and Dalton WS: Cell adhesion mediated drug resistance (CAM-DR):
Role of integrins and resistance to apoptosis in human myeloma cell
lines. Blood. 93:1658–1667. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Seguin L, Desgrosellier JS, Weis SM and
Cheresh DA: Integrins and cancer: Regulators of cancer stemness,
metastasis, and drug resistance. Trends Cell Biol. 25:234–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Cooper J and Giancotti FG: Integrin
signaling in cancer: Mechanotransduction, stemness, epithelial
plasticity, and therapeutic resistance. Cancer Cell. 35:347–367.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kanda R, Kawahara A, Watari K, Murakami Y,
Sonoda K, Maeda M, Fujita H, Kage M, Uramoto H, Costa C, et al:
Erlotinib resistance in lung cancer cells mediated by integrin
β1/Src/Akt-driven bypass signaling. Cancer Res. 73:6243–6253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Janouskova H, Maglott A, Leger DY, Bossert
C, Noulet F, Guerin E, Guenot D, Pinel S, Chastagner P, Plenat F,
et al: Integrin α5β1 plays a critical role in resistance to
temozolomide by interfering with the p53 pathway in high-grade
glioma. Cancer Res. 72:3463–3470. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Luo J, Yao JF, Deng XF, Zheng XD, Jia M,
Wang YQ, Huang Y and Zhu JH: 14, 15-EET induces breast cancer cell
EMT and cisplatin resistance by up-regulating integrin αvβ3 and
activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res.
37:232018. View Article : Google Scholar
|
|
156
|
Khademi R, Malekzadeh H, Bahrami S, Saki
N, Khademi R and Villa-Diaz LG: Regulation and functions of
α6-integrin (CD49f) in cancer biology. Cancers (Basel).
15:34662023. View Article : Google Scholar
|
|
157
|
Kawakami K, Fujita Y, Kato T, Mizutani K,
Kameyama K, Tsumoto H, Miura Y, Deguchi T and Ito M: Integrin β4
and vinculin contained in exosomes are potential markers for
progression of prostate cancer associated with taxane-resistance.
Int J Oncol. 47:384–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen
CA and Zhou QA: Exosomes-nature's lipid nanoparticles, a rising
star in drug delivery and diagnostics. ACS Nano. 16:17802–17846.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar
|
|
160
|
Kobayashi M, Sawada K, Miyamoto M, Shimizu
A, Yamamoto M, Kinose Y, Nakamura K, Kawano M, Kodama M, Hashimoto
K and Kimura T: Exploring the potential of engineered exosomes as
delivery systems for tumor-suppressor microRNA replacement therapy
in ovarian cancer. Biochem Biophys Res Commun. 527:153–161. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cho E, Nam GH, Hong Y, Kim YK, Kim DH,
Yang Y and Kim IS: Comparison of exosomes and ferritin protein
nanocages for the delivery of membrane protein therapeutics. J
Controlled Release. 279:326–335. 2018. View Article : Google Scholar
|
|
162
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJA: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Gaurav I, Thakur A, Iyaswamy A, Wang X,
Chen X and Yang Z: Factors affecting extracellular vesicles based
drug delivery systems. Molecules. 26:15442021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Li MY, Liu LZ and Dong M: Progress on
pivotal role and application of exosome in lung cancer
carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer.
20:222021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhao L, Wang H, Fu J, Wu X, Liang XY, Liu
XY, Wu X, Cao LL, Xu ZY and Dong M: Microfluidic-based exosome
isolation and highly sensitive aptamer exosome membrane protein
detection for lung cancer diagnosis. Biosens Bioelectron.
214:1144872022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhang L and Yu D: Exosomes in cancer
development, metastasis, and immunity. Biochim Biophys Acta Rev
Cancer. 1871:455–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yang H, Xu Z, Peng Y, Wang J and Xiang Y:
Integrin β4 as a potential diagnostic and therapeutic tumor marker.
Biomolecules. 11:11972021. View Article : Google Scholar
|
|
168
|
Valdembri D and Serini G: The roles of
integrins in cancer. Fac Rev. 10:452021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Hurwitz SN and Meckes DG Jr: Extracellular
vesicle integrins distinguish unique cancers. Proteomes. 7:142019.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang Z, Wang Z, Liu T, Tang J, Liu Y, Gou
T, Chen K, Wang L, Zhang J, Yang Y and Zhang H: Exploring the role
of ITGB6: Fibrosis, cancer, and other diseases. Apoptosis.
29:570–585. 2024. View Article : Google Scholar
|
|
171
|
Bandyopadhyay A and Raghavan S: Defining
the role of integrin αvβ6 in cancer. Curr Drug Targets. 10:645–652.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Hausner SH, DiCara D, Marik J, Marshall JF
and Sutcliffe JL: Use of a peptide derived from foot-and-mouth
disease virus for the noninvasive imaging of human cancer:
Generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in
vivo imaging of integrin alphavbeta6 expression with positron
emission tomography. Cancer Res. 67:7833–7840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Su L, Chen Y, Huang C, Wu S, Wang X, Zhao
X, Xu Q, Sun R, Kong X, Jiang X, et al: Targeting Src reactivates
pyroptosis to reverse chemoresistance in lung and pancreatic cancer
models. Sci Transl Med. 15:eabl78952023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Chen JR, Zhao JT and Xie ZZ:
Integrin-mediated cancer progression as a specific target in
clinical therapy. Biomed Pharmacother. 155:1137452022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhang L, Gülses A, Purcz N, Weimer J,
Wiltfang J and Açil Y: A comparative assessment of the effects of
integrin inhibitor cilengitide on primary culture of head and neck
squamous cell carcinoma (HNSCC) and HNSCC cell lines. Clin Transl
Oncol. 21:1052–1060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Yu Q, Xiao W, Sun S, Sohrabi A, Liang J
and Seidlits SK: Extracellular matrix proteins confer cell
adhesion-mediated drug resistance through integrin αv in
glioblastoma cells. Front Cell Dev Biol. 9:6165802021. View Article : Google Scholar
|
|
177
|
Ignatiadis M, Sledge GW and Jeffrey SS:
Liquid biopsy enters the clinic-implementation issues and future
challenges. Nat Rev Clin Oncol. 18:297–312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Yu W, Hurley J, Roberts D, Chakrabortty
SK, Enderle D, Noerholm M, Breakefield XO and Skog JK:
Exosome-based liquid biopsies in cancer: Opportunities and
challenges. Ann Oncol. 32:466–477. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Bergonzini C, Kroese K, Zweemer AJM and
Danen EHJ: Targeting integrins for cancer therapy-disappointments
and opportunities. Front Cell Dev Biol. 10:8638502022. View Article : Google Scholar
|
|
180
|
Cox D: How not to discover a
drug-integrins. Expert Opin Drug Discov. 16:197–211. 2021.
View Article : Google Scholar
|
|
181
|
Tam SH, Sassoli PM, Jordan RE and Nakada
MT: Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent
affinity and functional blockade of glycoprotein IIb/IIIa and
alpha(v)beta3 integrins. Circulation. 98:1085–1091. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Corcoran C, Rani S, O'Brien K, O'Neill A,
Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J and
O'Driscoll L: Docetaxel-resistance in prostate cancer: Evaluating
associated phenotypic changes and potential for resistance transfer
via exosomes. PLoS One. 7:e509992012. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu
Y and Feng J: Exosomes: Decreased sensitivity of lung cancer A549
cells to cisplatin. PLoS One. 9:e895342014. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hazlehurst LA, Argilagos RF and Dalton WS:
Beta1 integrin mediated adhesion increases Bim protein degradation
and contributes to drug resistance in leukaemia cells. Br J
Haematol. 136:269–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C,
Liu Z, Zhong G and Lin J: A nanodrug consisting of doxorubicin and
exosome derived from mesenchymal stem cells for osteosarcoma
treatment in vitro. Int J Nanomedicine. 14:8603–8610. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Tian Y, Li S, Song J, Ji T, Zhu M,
Anderson GJ, Wei J and Nie G: A doxorubicin delivery platform using
engineered natural membrane vesicle exosomes for targeted tumor
therapy. Biomaterials. 35:2383–2390. 2014. View Article : Google Scholar
|
|
187
|
Chen K and Chen X: Integrin targeted
delivery of chemotherapeutics. Theranostics. 1:189–200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Albelda SM, Mette SA, Elder DE, Stewart R,
Damjanovich L, Herlyn M and Buck CA: Integrin distribution in
malignant melanoma: Association of the beta 3 subunit with tumor
progression. Cancer Res. 50:6757–6764. 1990.PubMed/NCBI
|
|
189
|
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo
JK and Choi C: Biodistribution of exosomes and engineering
strategies for targeted delivery of therapeutic exosomes. Tissue
Eng Regen Med. 18:499–511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Gingras MC, Roussel E, Bruner JM, Branch
CD and Moser RP: Comparison of cell adhesion molecule expression
between glioblastoma multiforme and autologous normal brain tissue.
J Neuroimmunol. 57:143–153. 1995. View Article : Google Scholar : PubMed/NCBI
|