Introduction
Lung cancer is the most prevalent malignant tumor
among all types of cancer and a common cause of cancer-associated
mortality (18.7% of all cancers globally). The incidence of lung
cancer is considerably higher in males (32.1%) than in females
(16.2% of age standardized rate globally), with smoking being a key
contributing factor (1). Lung
cancer is categorized into small and non-small cell lung cancer
(NSCLC). NSCLC accounts for up to 85% of diagnosed lung cancer
cases (2,3). Despite potent treatments,
advanced-stage NSCLC poses a notable risk of recurrence, with
30-50% of patients experiencing disease progression following
initial treatment (4). NSCLC
cells aggressively proliferate, invade surrounding tissue, and
cross the basement membrane, migrating to other organs in the body
through the vascular or lymphatic systems (5) and leading to poor treatment
outcomes, with 5-year survival rates for metastatic NSCLC remaining
below 5% (6). Advances in
molecular biology tools and biological processes have provided a
comprehensive understanding of the fundamental biology of tumors
(7). Lung cancer progression is
associated with angiogenesis, and extensive angiogenesis is
associated with invasion and poor prognosis. Hence, anti-angiogenic
drugs present potential clinical efficacy in treating patients with
lung cancer, prompting research into associated anti-angiogenic
strategies (8,9).
Cells exhibiting cancer stem cell (CSC) properties
serve a role in tumor initiation, perpetuation and advancement
(10). CSCs are distinguished
based on their differentiation and self-renewal capability
(11) and considerably contribute
to resistance against chemotherapy and radiotherapy (12,13). During cancer progression, CSCs can
lead to tumor recurrence, which involves epithelial-mesenchymal
transition (EMT). EMT represents phenotypical changes in cells
transitioning from the epithelial to mesenchymal type, with high
N-cadherin and vimentin expression in breast, lung, colon and head
and neck carcinoma. EMT involves cell phenotype plasticity,
contributing to intratumor heterogeneity (14,15). The potential association between
EMT and CSCs is the key to drug resistance and cancer cell
plasticity, which contributes to the development of cancer cells
into malignant tumors (16,17).
Marine-derived peptide 06 (MP06), a 22-amino acid
peptide derived from the green sea alga Bryopsis plumosa,
leads to decreased proliferation in NSCLC while minimally affecting
normal lung fibroblasts: Our previous study reported low metastatic
potential of MP06 against lung cancer cells and zebrafish models
(18). MP06 decreased the
phosphorylation of ERK in A549 and H460 cells followed by
downregulation of the ERK pathway (18). The small size (~50 amino acids)
and high solubility of therapeutic potential peptides offer
optimized pharmacokinetics, enhanced uptake in target tissue and
more rapid removal from non-target tissues compared with other
existing therapeutic agents such as antibodies and small molecule,
making them well-suited for anticancer therapy (19). Numerous sources exhibiting
endogenous antiangiogenic properties that regulate tumor growth and
angiogenesis have been reported (20,21). The anti-angiogenic activities
emphasize the potential of MP06 as an effective cancer therapeutic,
in addition to its previously known anti-cancer properties
(18).
Aquaporins (AQPs), a family of transmembrane water
channel proteins, are widely distributed in various types of tissue
and control water movements in extra- and intracellular fluid
passages (22). AQPs serve key
roles in physiological functions, including urine concentration,
lactation and formation of tears, sweat and saliva (23-25). Additionally, increased and ectopic
expression of certain types of AQP is associated with pathological
manifestation and poor prognosis in several types of cancer
(25). AQP3 is widely expressed
in the normal respiratory tract and maintains water homeostasis.
AQP3 inhibition increases sensitivity of prostate cancer cells to
cryotherapy (26). Notably, AQP3
levels are associated with lung cancer progression, specifically
maintenance of water homeostasis and differentiation of lung
carcinoma (27). AQP3 expression
is positively associated with angiogenesis in patients with NSCLC
(28). Furthermore, suppressing
AQP3 expression can inhibit cell proliferation and angiogenesis in
human NSCLC xenografts (29).
Recently, compared with non-neoplastic lung tissue, a notably high
expression of AQP3 was found in lung adenocarcinoma samples
(30). AQP3 expression affects
lung cancer cell properties including proliferation, migration,
metastasis and angiogenic potential (27-29). Therefore, the present study
investigated the anticancer and anti-angiogenesis potential of MP06
in association with AQP3 expression in lung cancer cells and a
zebrafish model. The findings may provide a novel potential
therapeutic target for treating lung cancer metastasis and
angiogenesis.
Materials and methods
Peptide synthesis
MP06 (LAV ISW KCQ EWN SLW KKR KRK T-NH2) and
FITC-MP06 (with FITC tagged at the N-terminal) peptides (>95%
purity) were synthesized by DANDI Cure Co. (Republic of Korea)
through a solid-phase synthesis method. The molecular masses and
purity of the peptides were analyzed using high-performance liquid
chromatography as previously described (18). The synthesized peptides were
prepared as a 10 mM stock solution in distilled water. For
treatment, an aliquot of peptide stock solution was diluted
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum and 1% antibiotics
(penicillin/streptomycin; both HyClone; Cytiva). The 3D structure
of MP06 was predicted using PEP-FOLD3
(bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD3) and PyMOL
3.0 software (pymol.org).
Cell culture
The human H1299 lung cancer cell and MRC5 lung
normal fibroblast cell lines (Korean Cell Line Bank; cat. no. 25803
and 10171) were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum and 1% antibiotics. Human umbilical vein
endothelial cells (HUVECs; American Type Cell Culture, CRL-1730)
were incubated in endothelial basal medium-2 (EBM; cat. no.
CC-3156) containing endothelial cell growth medium supplements
(cat. no. CC-4176; both Lonza). Cells were cultured at 37°C in a
humidified 5% CO2 incubator. HUVECs were used between
passages 3 and 4.
Cytotoxicity assay and transfection
To investigate cytotoxicity, H1299 and human
umbilical vein endothelial cells (HUVEC) were seeded at
5×103 cells/well into a 96-well plate and treated with
MP06 peptide at 5, 10 and 20 μM at 37°C for 24 h.
Subsequently, the medium was replaced with 100 μl fresh
RPMI-1640 and EBM containing 10 μl Cell Counting Kit-8
(Dojindo Molecular). After 3 h, the absorbance was measured using
the Spectramax i3x (Molecular Devices) at 450 nm. To suppress AQP3
expression, H1299 cells were transfected with 10 pmol small
interfering (si)RNA)-AQP3 (cat. no. sc-29713; Santa Cruz
Biotechnology) and negative siRNA control (cat. no. sc-37007) by
Lipofectamine RNAi MAX (Invitrogen; Thermo Fisher Scientific, Inc.)
at 37°C for 48 h according to the manufacturer's instructions.
H1299 cells were cultured at 37°C in a humidified 5% CO2
incubator for at least 48 h after transfection.
Reverse transcription (RT) PCR
To evaluate the expression of genes, total RNA from
H1299 cells and HUVECs was isolated by TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The purity and quality of RNA were
determined using a UV spectrophotometer. First-strand cDNA was
synthesized using the cDNA synthesis kit (iNtRON Biotechnology).
PCR was performed using AccuPower PCR preMix, Bioneer, S. Korea).
Amplification with specific primers (Table I) was conducted as follows:
Initial denaturation at 94°C for 5 min, followed by 30 cycles of
denaturation at 94°C for 5 min, annealing at 56°C for 1 min and
extension at 72°C for 1 min and final extension at 72°C for 3 min.
The amplified PCR gene products were analyzed by 1% agarose gel
electrophoresis containing redsafe (iNtRON Biotechnology) and
imaged under UV light. β-actin was used as a reference gene.
 | Table IPrimer sequences for reverse
transcription PCR. |
Table I
Primer sequences for reverse
transcription PCR.
| Primer | Sequence, 5′→
3′) |
|---|
| SOX2-F |
CAAGATGCACAACTCGGAGA |
| SOX2-R |
TTCATGTGCGCGTAACTGTC |
| OCT4-F |
TGGGATATACACAGGCCGAT |
| OCT4-R |
GTGACAGAGACAGGGGGAAA |
| KLF4-F |
CCCACCTTCTTCACCCCTAGA |
| KLF4-R |
CCCAGTCACAGTGGTAAGGTT |
| CD44-F |
TCATACCAGCCATCCAATGC |
| CD44-R |
CGTGTGTGGGTAATGAGAGG |
| β-actin-F |
CTTCGCGGGCGACGAT |
| β-actin-R |
CCACATAGGAATCCTTCTGA |
| N-cad-F |
ACTTGCCAGAAAACTCCAGG |
| N-cad-R |
TGGTGTATGGGGTTGATCCT |
| E-cad-F |
TGGATAGAGAACGCATTGCC |
| E-cad-R |
AAAATCCAAGCCCGTGGTG |
| ZEB1-F |
CGGCGCAATAACGTTACAAA |
| ZEB1-R |
AAAGGTGTAACTGCACAGGG |
| Vimentin-F |
GAGAACTTTGCCGTTGAAGC |
| Vimentin-R |
TCTGCTGGTATATGAGTGCTG |
| Snail-F |
GGGACTGTGAGTAATGGCTG |
| Snail-R |
CCCACTCCTCTATGACACCA |
| VEGF-F |
ATCGAGACCCTGGTGGACA |
| VEGF-R |
CCTCGGCTTGTCACATCTGC |
| AQP3-F |
CCCTTATCGTGTGTGTGCTG |
| AQP3-R |
TCAGCTGGTACACGAAGACA |
Western blotting
H1299 and HUVEC cells were lysed by vortexing with
RIPA lysis buffer containing protease and phosphatase inhibitor
cocktails (Sigma-Aldrich; Merck KGaA). The supernatant was
centrifuged at 13,000 × g at 4°C for 30 min and protein
concentration was measured using the Bradford assay (Bio-Rad
Laboratories, Inc.). Equivalent amounts (30 μg) of protein
were boiled for 5 min and separated on 10% SDS-PAGE and transferred
to PVDF membranes. After blocking the membranes with a solution of
tris-buffered saline (TBS) containing bovine serum albumin (BSA,
1%, Sigma-Aldrich; Merck KGaA; cat. no. A3294) at room temperature
for 1 h and incubated overnight at 4°C with primary antibodies (all
1:1,000) against SOX2 (cat. no. sc-365823), Octamer binding
transcription factor (OCT)3/4 (cat. no. sc-5279), Kruppel-like
factor (KLF)4 (cat. no. sc-365144), CD44 (cat. no. sc-7297),
N-cadherin (cat. no. sc-59987), E-cadherin (cat. no. sc-8426),
Zinc-finger E-box-binding homeobox (ZEB)1 (cat. no. sc-515797),
vimentin (cat. no. sc-6260), Snai1 (cat. no. sc-271977), AQP3 (cat.
no. sc-518001) and β-actin (cat. no. sc-47778; all Santa Cruz
Biotechnology) and VEGF (Bioswamp; cat. no. PAB30976). After 1 h
incubation at room temperature with HRP-conjugated secondary
antibodies (1:10,000; cat. nos. sc-2748 and rabbit sc-2357, Santa
Cruz Biotechnology), membranes were rinsed with Tris-buffered
saline and visualized using a western blotting substrate (Thermo
Fisher Scientific, Inc.; cat. no. A38555).
Immunofluorescence
H1299 cells (5×104) were seeded on cover
glass in cell culture plates. The cells were fixed using 4%
paraformaldehyde for 30 min at room temperature, washed and blocked
with phosphate-buffered saline (PBS) containing 1% BSA
(Sigma-Aldrich; Merck KGaA) at room temperature for 40 min. Cells
incubated with anti-SOX2 (cat. nosc-365823; 1:500) and
anti-vimentin (cat. no. sc-6260; 1:500) in a solution of PBS at 4°C
overnight. Then, the cover glass was washed with PBS and incubated
with Alexa Fluor 488-conjugated antibody (Invitrogen; Thermo Fisher
Scientific, Inc.; cat. no. A21202; 1:1,000) for 1 h at room
temperature. Then cells were mounted with aqueous mounting
containing DAPI at room temperature for 5 min (Vectashield Mounting
Medium with DAPI H-1,200; Vector Laboratories). Cell images were
acquired using a Zeiss LSM510 Meta fluorescence microscope at 40X
magnification with ZEN 3.1 software. (Carl Zeiss GmbH).
Tumor sphere forming assay
Stem cell-permissive medium was prepared with
DMEM-F12 (Cat. No. 11320-033; Invitrogen) supplemented with 20
ng/ml epidermal growth factor (E9644; Sigma-Aldrich), 20 ng/ml
basic fibroblast growth factor (13256-029; Invitrogen) and B27
serum-free supplement (Gibco; Thermo Fisher Scientific, Inc).
AggreWell 400(STEMCELL, #34415) or 800(STEMCELL, #34425) microwell
plates were pretreated and washed with anti-adhesion
solution(STEMCELL, #07010) for 5 min at 37°C. Then, H1299 cells
were seeded at 1×105 cells/well and centrifuged at 100 ×
g for 3 min at room temperature to capture cells inside the
microwells with stem cell-permissive medium. Cells were incubated
at 37°C with 5% CO2 for 7-10 days. The formed H1299
spheroids were imaged using an inverted phase contrast microscope
(Olympus Corporation; CKX53 light microscope; magnification,
×100).
Wound healing assay
For wound healing assay, H1299 (1×105)
cells were seeded on a 6-well plate. When cells reached 80%
confluence, a wound was introduced across the diameter of each well
using a 200-μl pipette tip. Images were captured by inverted
phase contrast light microscopy, after 12 and 24 h in serum-free
RPMI-1640 media with MP06 peptide. The healing area was quantified
using ImageJ 1.54g software (National Institutes of Health).
Invasion and migration assay
The migration and invasion assay was conducted using
a Transwell chamber (8-μm pores; BD Biosciences) in a
24-well plate. H1299 (2×104) cells were seeded in the
upper chambers with 200 μl serum-free RPMI-1640 medium with
or without MP06 peptide at 37°C in a humidified 5% CO2
incubator for 24 h. The lower chamber contained 500 μl
RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin.
For the invasion assay, a Transwell chamber was coated with diluted
Matrigel (Corning, Inc.) for 30 min at 37°C. The migratory and
invasive cells from the upper chamber were fixed with 4%
paraformaldehyde for 20 min at 37°C and stained with crystal violet
for 5 min at room temperature. The upper surface of Transwell
membrane was wiped using a cotton swab to remove non-migratory and
-invasive cells. Cells were then imaged using an inverted light
microscope (Olympus CKX53; magnification, ×40).
Tube formation assay
HUVECs (1×104) were seeded to 80%
confluency for final passage at passage 2 into a 96-well plate.
Each well was coated with 50 μl Geltrex matrix (Gibco;
Thermo Fisher Scientific, Inc.) and allowed to solidify at 37°C for
30 min. H1299 culture media was collected, supernatant was
centrifuged at 13,000 × g for 10 min at 4°C. Conditioned media (CM)
were produced using mixed fresh EBM media/H1299 cultured media with
MP06 or siAQP3 ratios (75:25, 50:50). The HUVECs were incubated at
37°C with 5% CO2 for 24 h and stained with Calcein-AM
(Invitrogen) at 37°C for 5 min. Angiogenesis was observed using an
inverted light microscope at 100X magnification.
Zebrafish vascular tube formation
Zebrafish (Danio rerio) were provided by
Professor C-H. Kim (Chungnam National University) and maintained as
described in a previous study (31). Wild-type and transgenic
(kdrl:eGFP) embryos were obtained by breeding males and
females (2:2) in a 14/10-h light/dark cycle at 28.5°C with a
recirculating water system. For anti-angiogenesis assay, fertilized
zebrafish embryos were transferred to a 24-well plate (10
specimens/well) at the 70% epiboly stage. Embryos were exposed to 2
μM MP06 peptide by dissolving in egg water (60 μg/ml
sea salt in distilled water). The zebrafish embryos were incubated
for 24 h at 28.5°C. Embryos were anesthetized and mounted in 3%
methylcellulose (Sigma-Aldrich; Merck KGaA) and then photographed
using a fluorescence microscope at 40X magnification. (Leica DM6 B;
Leica GmbH).
Gene expression profiling and
Kaplan-Meier plotter
The AQP3 of expression profile of lung
adenocarcinoma in tumor and normal tissue was analyzed from Gene
Expression Profiling Interactive Analysis web server
(gepia.cancer-pku.cn/detail. php?gene=AQP3, accessed April 24,
2024). This web server extracts data from TCGA data portal and
Genotype-Tissue Expression (GTEx) database of normal tissue.
Kaplan-Meier survival was analyzed using Gene Expression database
of lung normal and tumor tissues 2 on expression of AQP3
(gent2.appex.kr/gent2/, accessed March 24, 2024).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All experiments were conducted in triplicate. Comparisons
were performed using two-tailed unpaired Student's t test or
one-way ANOVA and Tukey's post hoc test for multiple comparisons.
GraphPad Prism 10.3.1 (Dotmatics) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
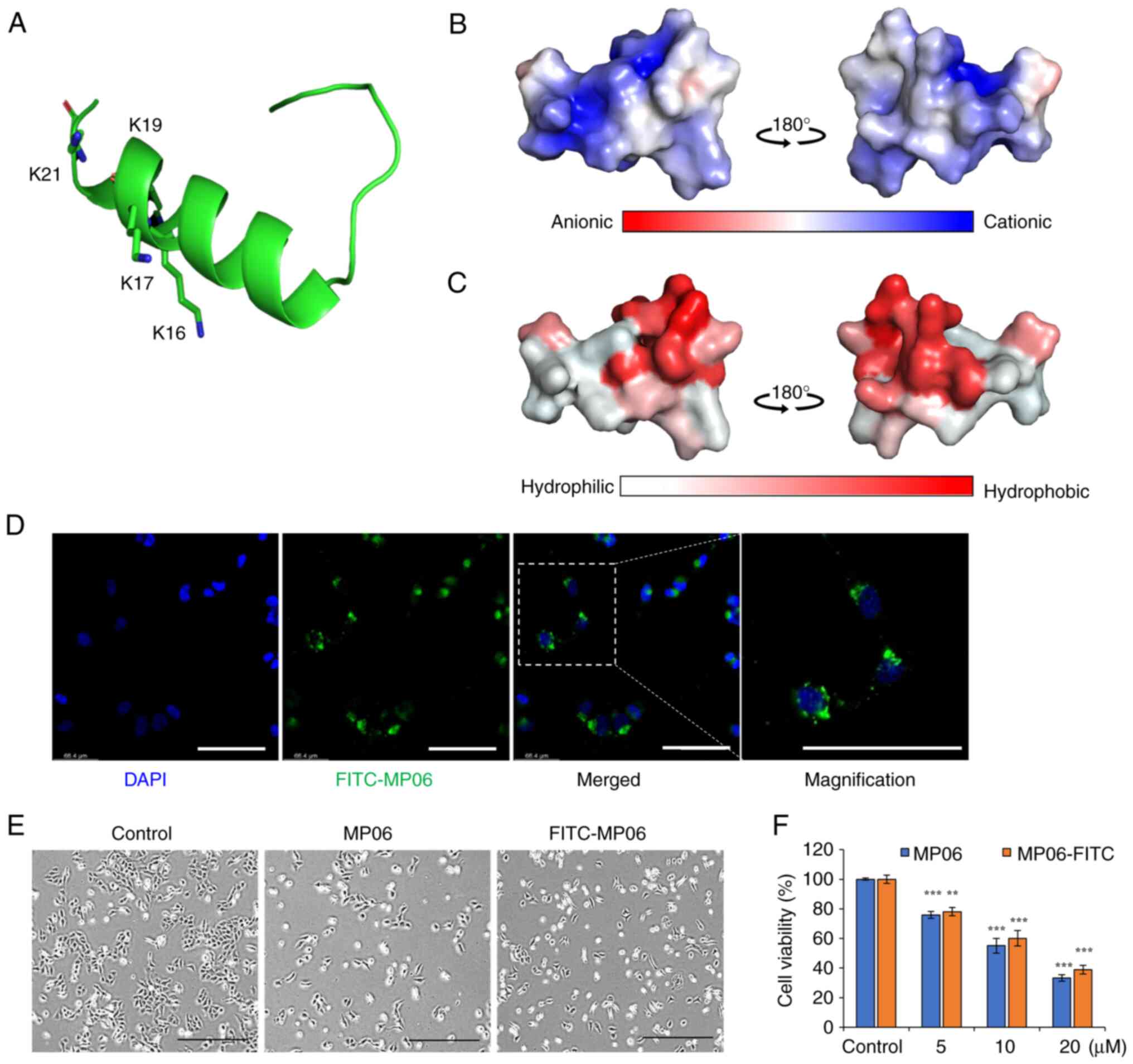
Structure of MP06 and cell viability
MP06 tertiary structure was predicted using
PEP-FOLD3 modeling (32). MP06
contained an N-terminal α-helix, unstructured C-terminal region and
several basic amino acids that make its net charge positive
(Fig. 1A and B). The charged
amino acids were concentrated on one side of the helix, whereas
hydrophobic amino acids formed the other side (Fig. 1C). FITC-MP06 was synthesized to
examine its cellular effects and distribution (Fig. S1). FITC-MP06 accumulation was
observed in the cytoplasm of H1299 cells (Fig. 1D). Morphological changes from
spindle shape to a cobblestone-like shape were observed in cells
treated with 20 μM MP06 or FITC-MP06 when compared to the
untreated control cells (Fig.
1E). There was a dose-dependent decrease in viability in cells
treated with MP06 or FITC-MP06, with a decrease in the cell
viability of up to ~38% of the cells treated with MP06 (Fig. 1F). Collectively, these results
indicated that MP06 and FITC-MP06 efficiently infiltrated cellular
membranes, were localized in cytoplasm, and increased cytotoxicity
in lung cancer cells.
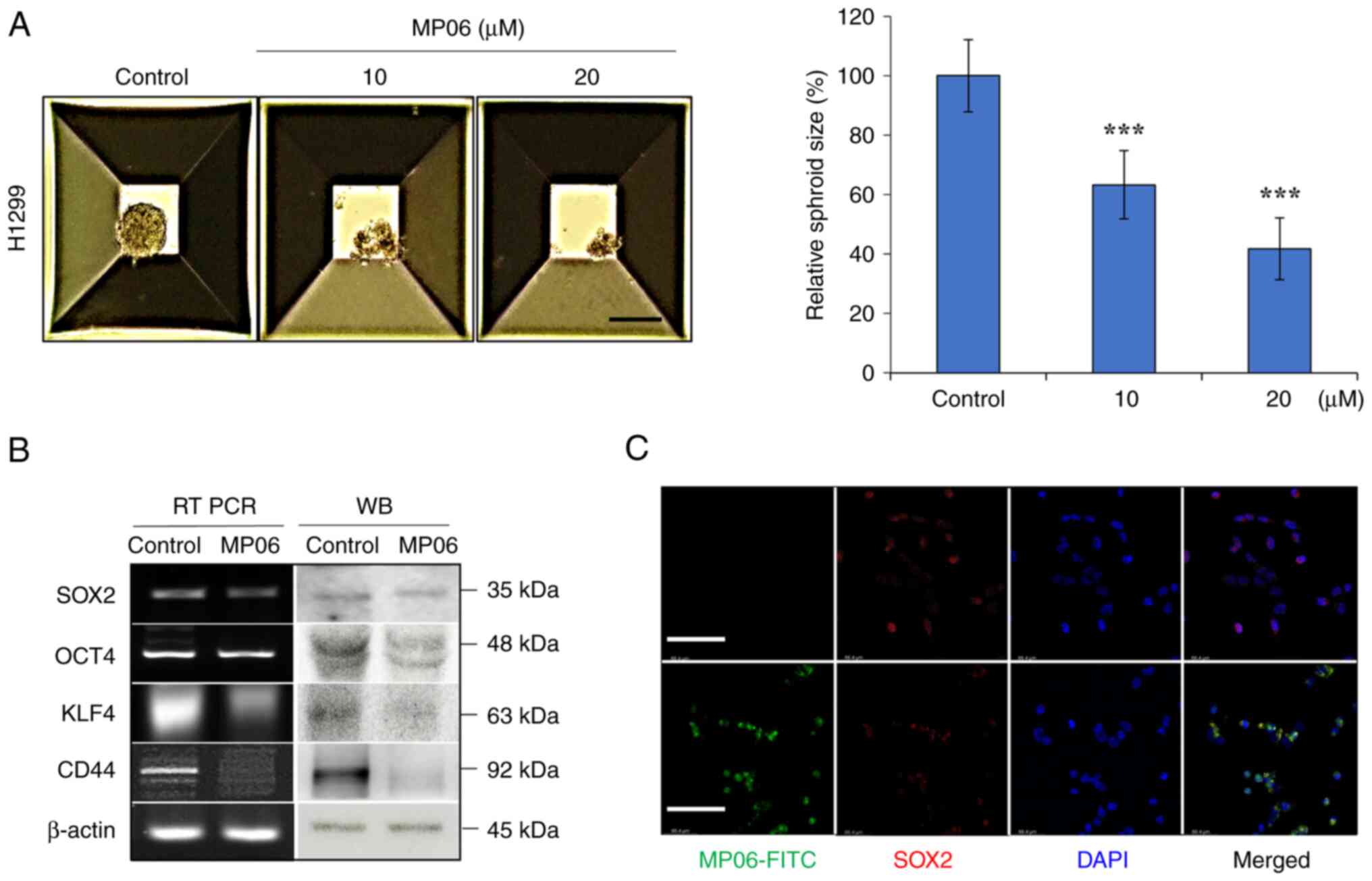
MP06 regulates stemness of lung cancer
cells
CSC transcription factors (such as SOX2, OCT4, KLF4
and CD44) are important marker of cancer and normal stem cells
(35,36). Following treatment with MP06,
spheroid size significantly decreased compared with control H1299
cells (Fig. 2A). MP06 decreased
expression of stemness markers (SOX2, OCT4, KLF4 and CD44), which
indicated decreased self-renewal potential (Fig. 2B) Suppressed SOX2 expression was
detected by immunofluorescence in FITC-MP06-treated H1299 cells
(Fig. 2C). The results showed
that MP06 partly regulated suppression of self-renewal activity in
H1299 cells.
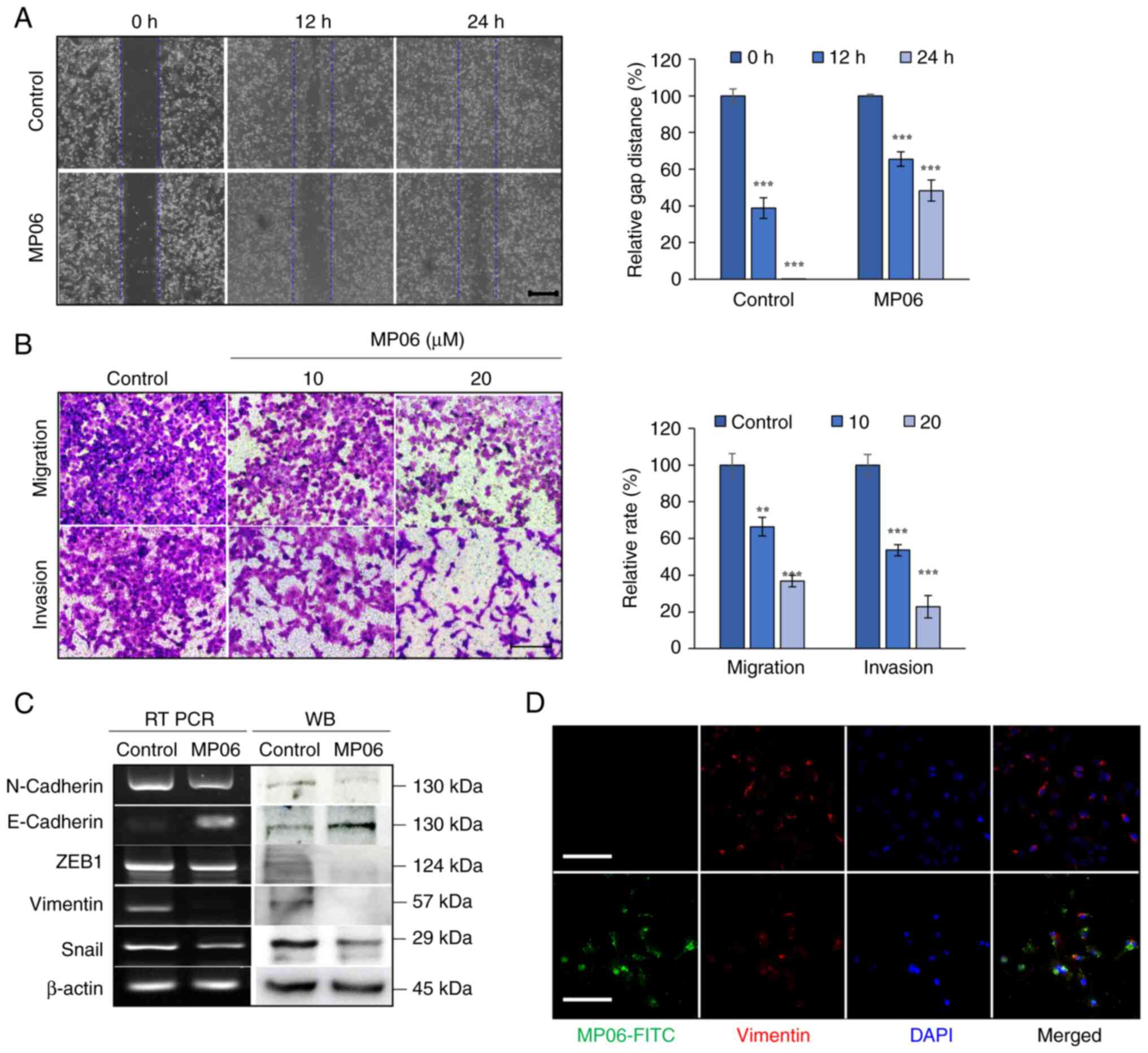
MP06 is involved in reducing EMT
To investigate the metastatic function of MP06 on
H1299 cells, EMT-associated properties were investigated by wound
healing, migration and invasion assay. A significant decrease in
the wound gap closure was observed after MP06 treatment (Fig. 3A). MP06-treated cells exhibited a
significant decrease in migration and invasion abilities (Fig. 3B). MP06 notably decreased the
cellular levels of important EMT markers (such as N-cadherin, ZEB1,
vimentin and Snail) that regulate the migration of mesenchymal
cells. E-cadherin, a representative marker of epithelial cells,
expression increased (Fig. 3C).
Reduced expression of vimentin was confirmed by immunofluorescence
staining in FITC-MP06-treated H1299 cells (Fig. 3D). Collectively, these results
suggested the involvement of MP06 in migration and invasion via
decreased EMT marker expression in H1299 cells.
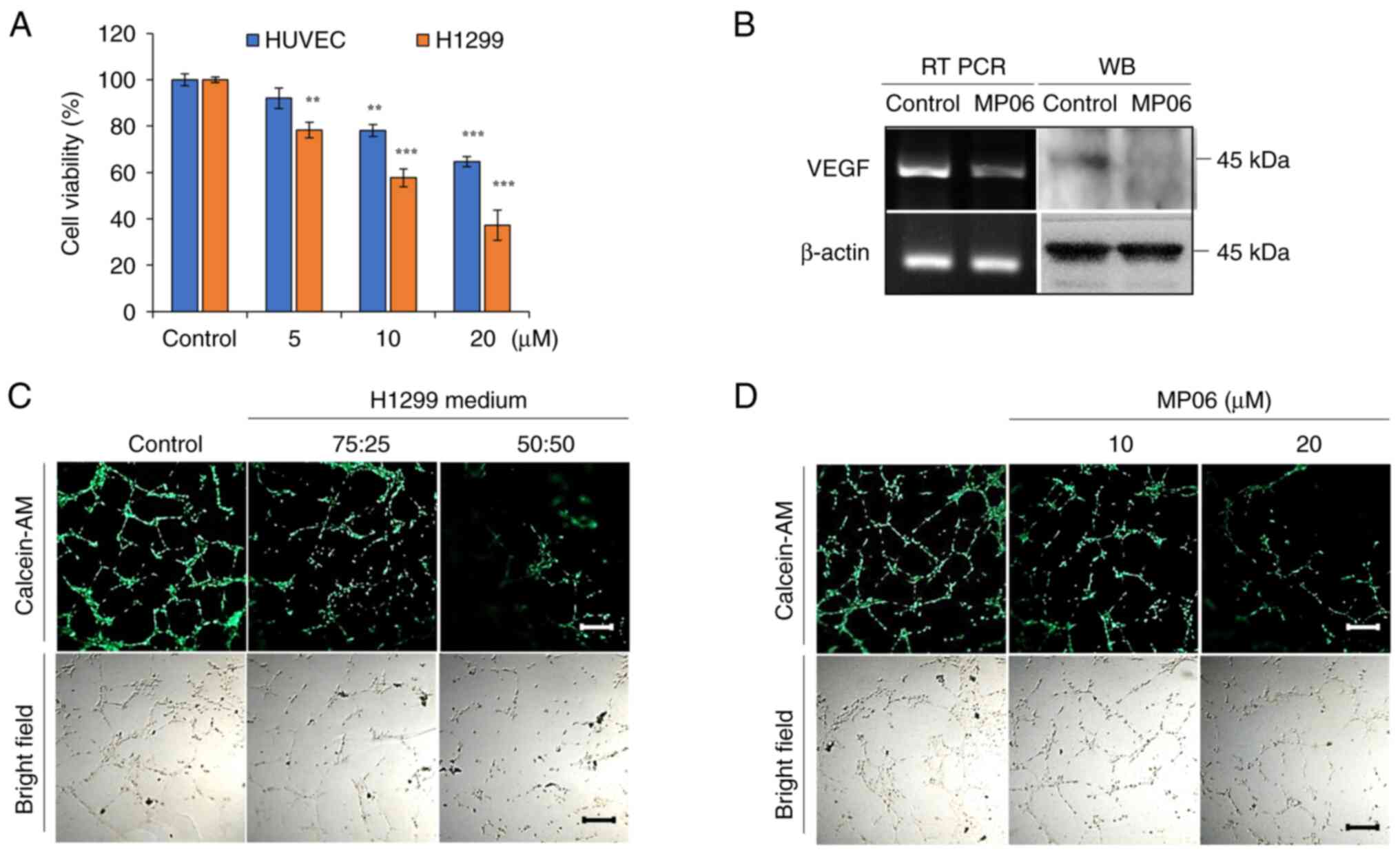
MP06 inhibits angiogenesis by
downregulating VEGF
Effective angiogenesis is key for the growth of the
primary tumor and the cancer cells' ability to spread to other
locations in the body. To investigate the angiogenic ability of
MP06, HUVECs, which can form tube-like structures depending on the
VEGF content in the supernatant (38), were used. MP06-induced
cytotoxicity in HUVECs was significantly lower than that in H1299
cells (Fig. 4A). MP06 notably
decreased VEGF expression in H1299 cells (Fig. 4B). MP06-CM treatment decreased
tube formation compared with that in the control H1299 culture
media, implying that MP06 suppressed angiogenesis activity at a
high rate with MP06-CM (Fig. 4C).
The formation of tube-like structures and expression of VEGF in
HUVECs was directly inhibited by MP06-treated EBM (Figs. 4D and S2). These results suggested the
involvement of MP06 in VEGF-mediated tube formation and blockade of
angiogenic responses.
MP06-mediated suppression of angiogenesis
in zebrafish embryos
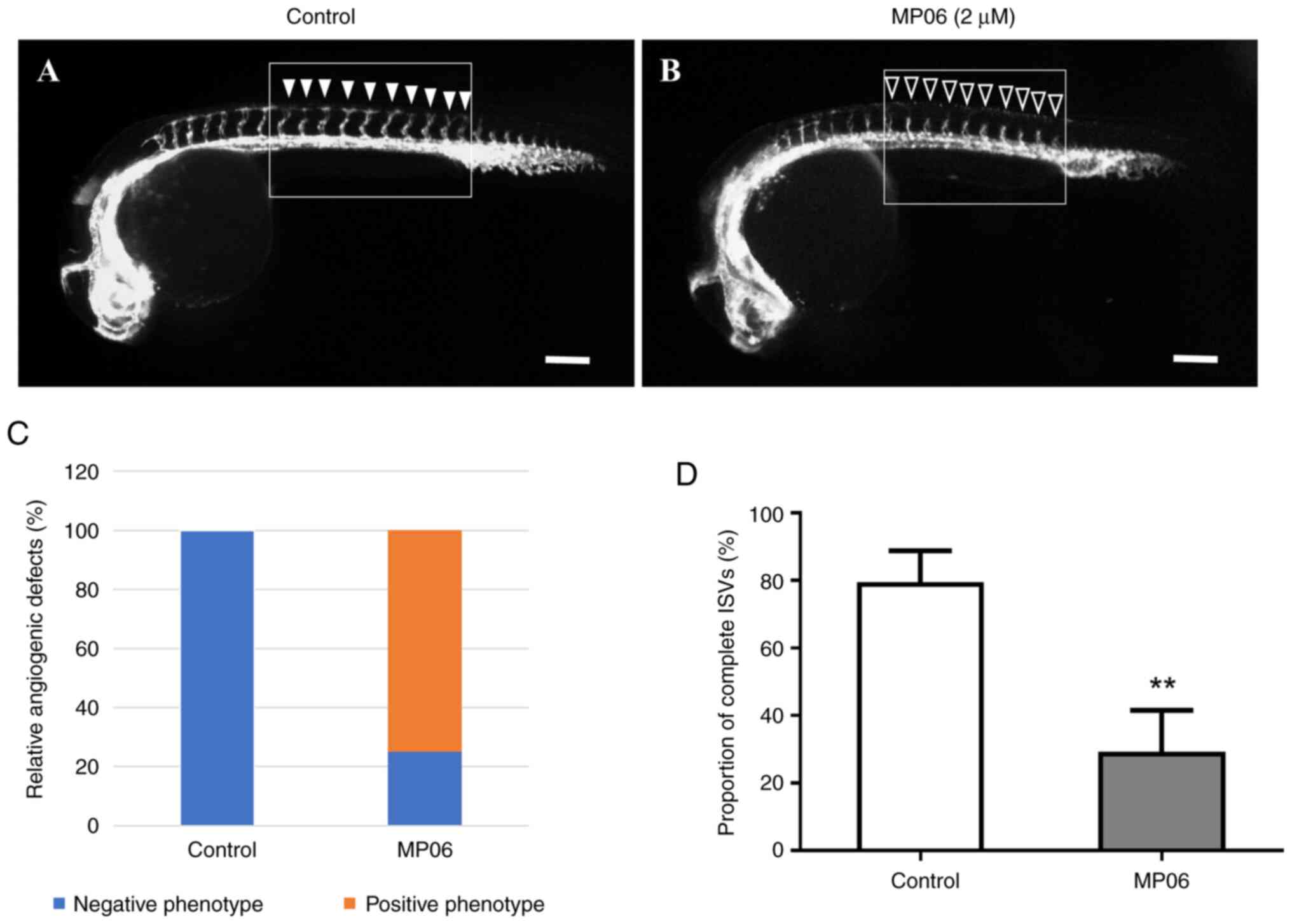
MP06 can be crucial for angiogenesis potential of
zebrafish embryos. A concentration of 2 μM MP06 was used to
observe inhibition of angiogenesis, as concentrations >4
μM resulted in zebrafish embryo death. Changes in vascular
patterning following MP06 treatment were observed during zebrafish
development, particularly in transgenic zebrafish expressing the Tg
(kdrl:eGFP) marker. MP06-treated embryos exhibited reduced growth
of intersegment vessels (ISVs) at the apex compared with untreated
control embryos in lateral view (Fig.
5A and B). In untreated control embryos, robust ISV growth was
observed (filled arrowheads) compared with MP06-treated embryos,
which showed noticeably decreased ISV growth (empty arrowheads) at
the top of the embryo (Fig. S3).
Quantification of the proportion of completed ISV structures in
MP06-treated zebrafish at 30 h post-fertilization revealed a
significant decreased of ~2.7-fold compared with that in untreated
controls, indicating an adverse effect of MP06 treatment on
vascular development (Fig. 5C and
D). These angiogenic events, accompanied by decreased
proliferation and migration in endothelial cell, support the role
of MP06 as a negative regulator of angiogenesis.
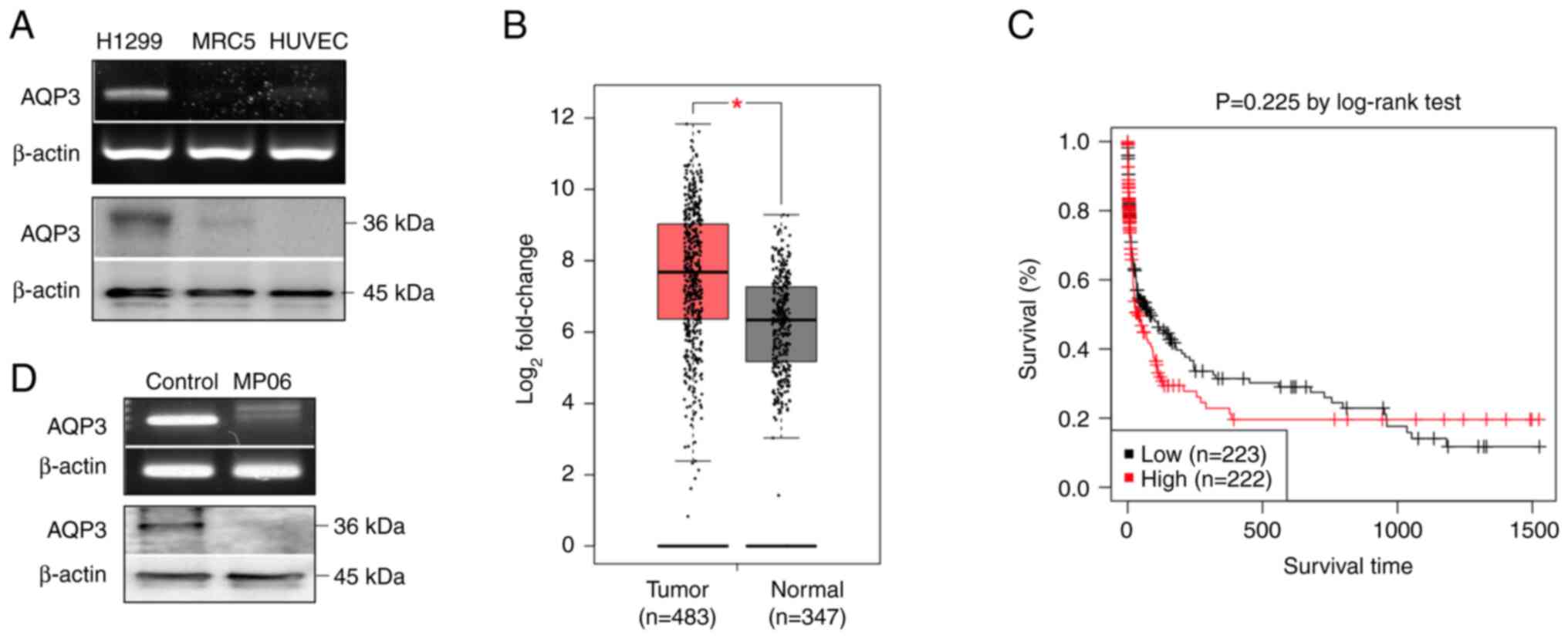
Expression of AQP3 in H1299 cells
Expression levels of AQP3 gene and protein were
higher in H1299 cells than in normal cells (MRC5 cells and HUVECs;
Fig. 6A). Furthermore, it was
confirmed that AQP3 was more highly expressed in lung cancer than
in normal lung tissue using Gene Expression Profiling Interactive
Analysis web server (gepia.cancer-pku.cn/detail.php?gene=AQP3,
accessed April 24, 2024; Fig.
6B). Gene expression profiling revealed that AQP3 was markedly
upregulated in lung adenocarcinoma tissue. Additionally, the
Kaplan-Meier survival analysis using Gene Expression database of
normal and tumor tissues 2 (gent2.appex.kr/gent2/, accessed March
24, 2024) revealed an association between AQP3 and lung cancer. The
Kaplan-Meier graph confirmed that the survival rate of patients
with lung cancer and high AQP3 expression was low (Fig. 6C). H1299 cells treated with MP06
showed decreased levels of AQP3 expression (Fig. 6D). These results indicated that
high AQP3 expression was associated with malignancy and decreased
survival in lung cancer.
Regulation of stemness, EMT and
angiogenesis by downregulating AQP3
AQP3 gene was commonly overexpressed in H1299 cells
compared with normal cells (Fig.
6A). AQP3 was knocked down to determine its effects on
stemness, EMT and angiogenesis-associated changes in H1299 cells.
Expression of the marker proteins SOX2, OCT4, KLF4 and CD44 was
decreased following AQP3 knockdown, which was consistent with the
decreased spheroid size (Fig. 7A and
B). AQP3 knockdown decreased expression of EMT markers such as
N-cadherin, ZEB1 and Snail, accompanied by decreased
migration/invasion of H1299 cells (Fig. 7C and D). siAQP3-CM decreased tube
formation compared with that in control H1299 culture medium,
suggesting that AQP3 knockdown suppressed angiogenesis activity in
siAQP3-CM-cultured cells (Fig.
7E). VEGF secretion regulates CSC and EMT phenomena through
autocrine action (36).
Consistent with VEGF expression in MP06-treated H1299 cells, VEGF
expression consistently decreased in H1299 cells treated with
siRNA-AQP3 (Fig. 7F).
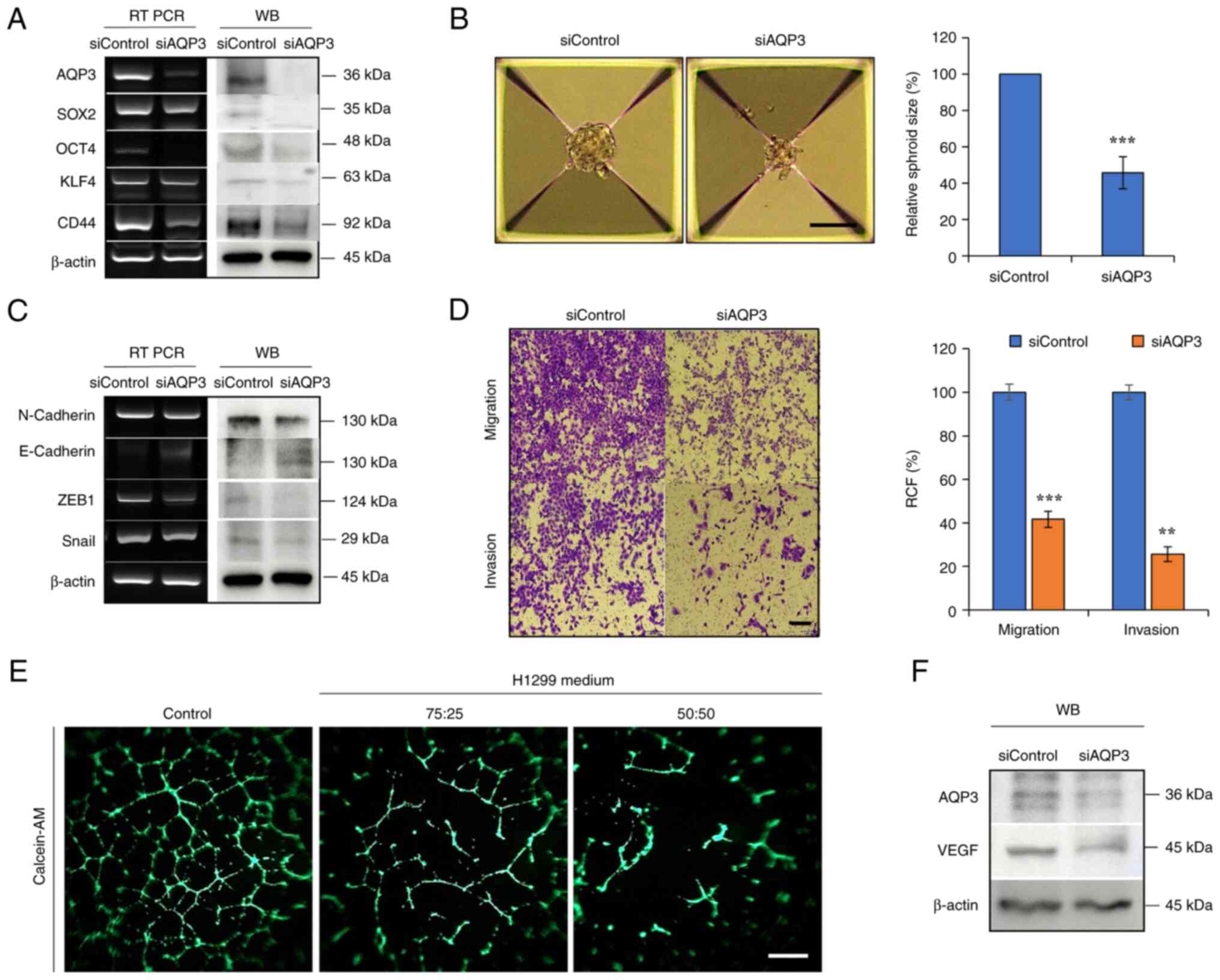 | Figure 7Regulation of stemness, EMT and
angiogenesis by downregulating AQP3. (A) Expression of AQP3 and
cancer stem cell markers in AQP3-knockdown H1299 cells. (B)
Sphere-forming ability in AQP3-knockdown H1299 cells. Scale bar,
100 μm. (C) Expression of AQP3 and EMT markers in
AQP3-knockdown H1299 cells. (D) Migratory and invasive ability of
AQP3-knockdown H1299 cells. Scale bar, 200 μm. (E)
Angiogenesis activity suppressed by conditioned medium (fresh EBM
media/H1299 cultured media with siAQP3 ratios (75:25, 50:50). (F)
VEGF expression. **P<0.01, ***P<0.001.
EMT, epithelial-mesenchymal transition; AQP, aquaporin; RT, reverse
transcription; WB, western blotting; si, small interfering; OCT,
octamer binding transcription factor; KLF, Kruppel-like factor;
VEGF, vascular endothelial growth factor; RCF, relative colony
forming; ZEB, Zinc-finger E-box-binding homeobox. |
Altogether, the in vitro and in vivo
data suggested that MP06 was partially associated with cancer
stemness and EMT in H1299 cells and that suppressing AQP3
expression to target VEGF may be an effective anti-angiogenic
therapy.
Discussion
Excessive angiogenesis notably contributes to cancer
progression by supplying key nutrients and oxygen to support tumor
growth and metastasis (2,5). Thus, development of novel
antiangiogenic therapeutic agents that exhibit high effectiveness
and few side effects is crucial. Effectiveness of conventional
therapy is often limited by drug resistance and lack of specificity
(3,4). Peptides have emerged as key
therapeutic agents for the study of angiogenesis-dependent disease
because of efficient penetration of cancer cells, high specificity
and low toxicity. Many anti-angiogenic proteins are large and
complex and have limited tissue penetration ability, making their
production at therapeutic volumes costly (38,39). However, peptides have garnered
interest as anti-angiogenic candidates owing to specific
advantages, such as smaller size, easier tissue penetration and
lower production costs compared with proteins and antibodies
(18,19). Anticancer peptides (including
Tebentafusp, Buserelin, Plitidepsin, Triptorelin, and
Dactinomycin), serve key roles in cancer treatment, positioning
them as promising future therapeutics (38,40). MP06 had a hydrophilic/phobic ratio
of 59% and a net charge of +6. Because of this positive charge, it
interacts electrostatically with the negatively charged membrane,
enhancing attachment and activity during membrane permeation. The
cationic amphipathic helical structure of MP06 was consistent with
that of other anticancer peptides, such as GI-15 and A12L/A20L.
Anticancer and antimicrobial peptides exert their activity through
characteristic structural features (33,34). A. The potential role of MP06 was
investigated based on its promising anticancer anti-angiogenic
activities in NSCLCs. MP06 exhibits lower toxicity in normal lung
fibroblasts and HUVECs than in H1299 cells and is soluble in water,
making it a potential candidate for drug development (18). Hemolysis is not observed in horse
erythrocytes treated with MP06 at ≥100 μM, indicating MP06
efficiently penetrates horse erythrocytes without cytotoxic
effects.
CSCs modulate extracellular matrix (ECM) and use
intracellular signaling pathways to maintain homeostatic processes
such as EMT and angiogenesis (41). ECM-mediated changes in the
expression and/or cellular localization of SOX2, OCT4 and KLF4 are
associated with prostate and breast cancer (35,36). The ECM microenvironment can revert
non-tumorigenic cells into CSCs via EMT-associated processes,
thereby increasing cell invasion and metastasis (42,43). Here, MP06 suppressed migration and
invasion, which are key factors in reducing tumorigenicity in H1299
cells. However, whether MP06 regulates cellular migration and
cancer stemness is unclear. Further studies are required to
investigate whether MP06 influences the expression of genes
associated with migration and CSCs.
Angiogenesis results from an imbalance between pro-
and anti-angiogenic endogenous factors that contribute to disease
progression (8). The key factors
include VEGF, fibroblast and platelet-derived growth factor and
angiopoietins, which interact with the ECM. The interaction between
ECM and endothelial cells is crucial for various cellular processes
in many cancers, including NSCLC, gastric, and uterine cancer
(9). The role of VEGF in
angiogenesis makes it a promising target for cancer therapy.
However, the clinical use of VEGF-targeted therapies is hindered by
the potential side effects such as hypertension, proteinuria,
bleeding, and cardiovascular complications in achieving optimal
therapeutic concentrations (44,45). The present study ascertained the
effects of anti-angiogenesis of MP06 and its anticancer properties
using a zebrafish embryo model for screening. MP06 decreased gene
and protein levels of VEGF in H1299 and HUVECs, which was
associated with vessel formation of zebrafish embryos. Suppression
in vascular patterning following MP06 treatment were observed
during zebrafish development. Strategies such as controlled release
of VEGF from ECM scaffolds may improve the efficacy and safety of
anti-angiogenic therapy (8,9).
AQPs regulate vascular formation and proliferation
through VEGF within tumors and offer targets for cancer therapy
(23-25). Cell migration and regulation of
angiogenesis are suppressed following AQP5 knockdown via the
EGFR/ERK signaling pathway (46).
Similarly, downregulation of AQP3 inhibits proliferation via the
hypoxia-inducible factor (HIF)-1a/VEGF and ERK pathways in NSCLC
(47). AQP3, 4 and 5 are
expressed in H1299 cell line, derived from the lymph nodes and has
been widely used to investigate various disease-associated tumor
metastases (29,46). The present study demonstrated that
AQP3 was more highly expressed in lung cancer than in normal
tissues. AQP3 serves pivotal roles in NSCLC progression, migration
and angiogenesis (27,29,48). AQP3 is associated with maintenance
of stemness not only in CSCs but also in normal stem cells
(49,50). AQP3 promotes stem cell-like
properties by regulating AQP3/STAT3/CD133 expression in
hepatocellular carcinoma cells (51). AQP3 serves a key role in the
progression and metastasis of various types of cancer: AQP3 can
upregulate matrix metalloproteinases (MMP1, MMP2 and MMP9) and
induce EMT by activating the PI3K/AKT signaling in gastric cancer
(52,53). A previous study indicated that
MP06 suppresses the ERK signaling pathway, which regulates cancer
cell migration and proliferation in NSCLC (18). The regulation of these signaling
pathways may facilitate cancer treatment by inhibiting
tumor-specific angiogenesis. The present study suggested
downregulation of AQP3 may suppress tumor-specific vascularization.
However, the direct association between MP06 and AQP3 remains
elusive and further studies are required to verify the role of VEGF
in vivo.
The multifaceted approach of targeting angiogenesis
and EMT signaling holds promise for development of effective cancer
therapy with minimal toxicity. Further research on the mechanisms
of action and clinical translation of therapeutic strategies are
warranted to improve cancer treatment outcomes. Collectively, the
present study showed that MP06 may decrease AQP3 expression and
serve as a new target for suppressing angiogenesis in NSCLC.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JHL performed experiments, analyzed data and wrote
the manuscript. HK, SHJ and SJ designed and performed experiments
and reviewed and edited the manuscript. JWH, MY and JHL conceived
the study and reviewed the manuscript. JHL and HK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The use of human-derived primary cell line was
approved by the Public Ethics Committee (approval no.
P01-202410-02-007). All animal studies were approved by the
Institutional Animal Care and Use Committee of the National Marine
Biodiversity Institute of Korea (approval no. MAB-23-03) and
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Abbreviations:
|
AQP
|
aquaporin
|
|
MP06
|
marine-derived peptide 06
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
EMT
|
epithelial-mesenchymal transition
|
|
NSCLC
|
non-small-cell lung cancer
|
|
CSC
|
cancer stem cell
|
|
Oct
|
octamer binding transcription
factor
|
|
KLF4
|
Kruppel-like factor 4
|
|
WB
|
western blotting
|
|
Zeb1
|
zinc-finger E-box-binding homeobox
1
|
|
VEGF
|
vascular endothelial growth factor
|
|
siRNA
|
small interfering RNA
|
|
ISV
|
intersegment vessel
|
|
CM
|
conditioned medium
|
|
ECM
|
extracellular matrix
|
Acknowledgements
Not applicable.
Funding
The present study was supported by Research Program of the
National Marine Biodiversity Institute of Korea (grant no.
MABIK2024M00500) funded by the Ministry of Oceans and
Fisheries.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans M: Lung cancer: Needs assessment,
treatment and therapies. Br J Nurs. 22(Suppl 17): S15–S22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar
|
|
4
|
Hirsch FR, Suda K, Wiens J and Bunn PA Jr:
New and emerging targeted treatments in advanced non-small-cell
lung cancer. Lancet. 388:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu W and Kang Y: Epithelial-mesenchymal
plasticity in cancer progression and metastasis. Dev Cell.
49:361–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsoukalas N, Aravantinou-Fatorou E, Tolia
M, Giaginis C, Galanopoulos M, Kiakou M, Kostakis ID, Dana E,
Vamvakaris I, Korogiannos A, et al: Epithelial-mesenchymal
transition in non-small-cell lung cancer. Anticancer Res.
37:1773–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majeed U, Manochakian R, Zhao Y and Lou Y:
Targeted therapy in advanced non-small cell lung cancer: Current
advances and future trends. J Hematol Oncol. 14:1082021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brock CS and Lee S: Anti-angiogenic
strategies and vascular targeting in the treatment of lung cancer.
Eur Resp J. 19:557–570. 2002. View Article : Google Scholar
|
|
9
|
Tian W, Cao C, Shu L and Wu F:
Anti-angiogenic therapy in the treatment of non-small cell lung
cancer. Onco Targets Ther. 13:12113–12129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prabavathy D, Swarnalatha Y and Ramadoss
N: Lung cancer stem cells-origin, characteristics and therapy. Stem
Cell Investig. 5:62018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leon G, MacDonagh L, Finn SP, Cuffe S and
Barr MP: Cancer stem cells in drug resistant lung cancer: Targeting
cell surface markers and signaling pathways. Pharmacol Ther.
158:71–90. 2016. View Article : Google Scholar
|
|
13
|
Codony-Servat J, Verlicchi A and Rosell R:
Cancer stem cells in small cell lung cancer. Transl Lung Cancer
Res. 5:16–25. 2016.PubMed/NCBI
|
|
14
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krebs AM, Mitschke J, Losada ML,
Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D,
Reichardt W, Bronsert P, et al: The EMT-activator Zeb1 is a key
factor for cell plasticity and promotes metastasis in pancreatic
cancer. Nat Cell Biol. 19:518–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loret N, Denys H, Tummers P and Berx G:
The role of epithelial-to-mesenchymal plasticity in ovarian cancer
progression and therapy resistance. Cancers (Basel). 11:8382019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanabe S, Quader S, Cabral H and Ono R:
Interplay of EMT and CSC in cancer and the potential therapeutic
strategies. Front Pharmacol. 11:9042020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim H, Kim HT, Jung SH, Han JW, Jo S, Kim
IG, Kim RK, Kahm YJ, Choi TI, Kim CH and Lee JH: A novel anticancer
peptide derived from bryopsis plumosa regulates proliferation and
invasion in non-small cell lung cancer cells. Mar Drugs.
21:6072023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosca EV, Koskimaki JE, Rivera CG, Pandey
NB, Tamiz AP and Popel AS: Anti-angiogenic peptides for cancer
therapeutics. Curr Pharm Biotechnol. 12:1101–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karagiannis ED and Popel AS: A systematic
methodology for proteome-wide identification of peptides inhibiting
the proliferation and migration of endothelial cells. Proc Natl
Acad Sci USA. 105:13775–13780. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koskimaki JE, Karagiannis ED, Rosca EV,
Vesuna F, Winnard PT Jr, Raman V, Bhujwalla ZM and Popel AS:
Peptides derived from type IV collagen, CXC chemokines, and
thrombospondin-1 domain-containing proteins inhibit
neovascularization and suppress tumor growth in MDA-MB-231 breast
cancer xenografts. Neoplasia. 11:1285–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishimoto S, Wada K, Usami Y, Tanaka N,
Aikawa T, Okura M, Nakajima A, Kogo M and Kamisaki Y: Differential
expression of aquaporin 5 and aquaporin 3 in squamous cell
carcinoma and adenoid cystic carcinoma. Int J Oncol. 41:67–75.
2012.PubMed/NCBI
|
|
23
|
Agre P: Aquaporin water channels. Biosci
Rep. 24:127–163. 2004. View Article : Google Scholar
|
|
24
|
Delporte C: Aquaporins and gland
secretion. Aquaporins. 969:63–79. 2017. View Article : Google Scholar
|
|
25
|
Papadopoulos MC and Saadoun S: Key roles
of aquaporins in tumor biology. Biochim Biophys Acta.
1848:2576–2583. 2015. View Article : Google Scholar
|
|
26
|
Ismail M, Bokaee S, Morgan R, Davies J,
Harrington KJ and Pandha H: Inhibition of the aquaporin 3 water
channel increases the sensitivity of prostate cancer cells to
cryotherapy. Br J Cancer. 100:1889–1895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu YL, Matsuzaki T, Nakazawa T, Murata
SI, Nakamura N, Kondo T, Iwashina M, Mochizuki K, Yamane T, Takata
K and Katoh R: Expression of aquaporin 3 (AQP3) in normal and
neoplastic lung tissues. Hum Pathol. 38:171–178. 2007. View Article : Google Scholar
|
|
28
|
Li B, Jin L, Zhong K and Du D: Correlation
of aquaporin 3 expression with the clinicopathologic
characteristics of non-small cell lung cancer. Zhongguo Fei Ai Za
Zhi. 15:404–408. 2012.In Chinese. PubMed/NCBI
|
|
29
|
Xia H, Ma YF, Yu CH, Li YJ, Tang J, Li JB,
Zhao YN and Liu Y: Aquaporin 3 knockdown suppresses tumour growth
and angiogenesis in experimental non-small cell lung cancer. Exp
Physiol. 99:974–984. 2014. View Article : Google Scholar
|
|
30
|
Jaskiewicz L, Hejne K, Szostak B,
Osowiecka K, Skowronski MT, Lepiarczyk E, Doboszynska A, Majewska
M, Kordowitzki P and Skowronska A: Expression profiles of AQP3 and
AQP4 in lung adenocarcinoma samples generated via Bronchoscopic
biopsies. J Clin Med. 11:59542022. View Article : Google Scholar :
|
|
31
|
Nusslein-Volhard C and Dahm R: Zebrafish:
A Practical Approach. Oxford University Press; New York, NY: 2002,
View Article : Google Scholar
|
|
32
|
Khater I and Nassar A: Potential antiviral
peptides targeting the SARS-CoV-2 spike protein. BMC Pharmacol
Toxicol. 23:912022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hadianamrei R, Tomeh MA, Brown S, Wang J
and Zhao X: Rationally designed short cationic α-helical peptides
with selective anticancer activity. J Colloid Interface Sci.
607:488–501. 2022. View Article : Google Scholar
|
|
34
|
Huang YB, He LY, Jiang HY and Chen YX:
Role of helicity on the anticancer mechanism of action of
cationic-helical peptides. Int J Mol Sci. 13:6849–6862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee S, Wottrich S and Bonavida B:
Crosstalks between Raf-kinase inhibitor protein and cancer stem
cell transcription factors (Oct4, KLF4, Sox2, Nanog). Tumor Biol.
39:10104283176922532017. View Article : Google Scholar
|
|
36
|
van Schaijik B, Davis PF, Wickremesekera
AC, Tan ST and Itinteang T: Subcellular localisation of the stem
cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: A review.
J Clin Pathol. 71:88–91. 2018. View Article : Google Scholar
|
|
37
|
Fantozzi A, Gruber DC, Pisarsky L, Heck C,
Kunita A, Yilmaz M, Meyer-Schaller N, Cornille K, Hopfer U,
Bentires-Alj M and Christofori G: VEGF-mediated angiogenesis links
EMT-induced cancer stemness to tumor initiation. Cancer Res.
74:1566–1575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hilchie A, Hoskin D and Coombs MR:
Anticancer activities of natural and synthetic peptides. Adv Exp
Med Biol. 1117:131–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shin MK, Jang BY, Bu KB, Lee SH, Han DH,
Oh JW and Sung JS: De novo design of AC-P19M, a novel anticancer
peptide with apoptotic effects on lung cancer cells and
anti-angiogenic activity. Int J Mol Sci. 23:155942022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chinnadurai RK, Khan N, Meghwanshi GK,
Ponne S, Althobiti M and Kumar R: Current research status of
anti-cancer peptides: Mechanism of action, production, and clinical
applications. Biomed Pharmacother. 164:1149962023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Borovski T, Melo FD, Vermeulen L and
Medema JP: Cancer stem cell niche: The place to be. Cancer Res.
71:634–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Al-Ostoot FH, Salah S, Khamees HA and
Khanum SA: Tumor angiogenesis: Current challenges and therapeutic
opportunities. Cancer Treat Res Commun. 28:1004222021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marmé D: The impact of anti-angiogenic
agents on cancer therapy. J Cancer Res Clin Oncol. 129:607–620.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Elkhider A, Wang B, Ouyang X, Al-Azab M,
Walana W, Sun X, Li H, Tang Y, Wei J and Li X: Aquaporin 5 promotes
tumor migration and angiogenesis in non-small cell lung cancer cell
line H1299. Oncol Lett. 19:1665–1672. 2020.PubMed/NCBI
|
|
47
|
Hou SY, Li YP, Wang JH, Yang SL, Wang Y,
Wang Y and Kuang Y: Aquaporin-3 inhibition reduces the growth of
NSCLC cells induced by hypoxia. Cell Physiol Biochem. 38:129–140.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marlar S, Jensen HH, Login FH and Nejsum
LN: Aquaporin-3 in cancer. Int J Mol Sci. 18:21062017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Graziano AC, Avola R, Pannuzzo G and
Cardile V: Aquaporin1 and 3 modification as a result of
chondrogenic differentiation of human mesenchymal stem cell. J Cell
Physiol. 233:2279–2291. 2018. View Article : Google Scholar
|
|
50
|
Zhou Y, Wang Y, Wen J, Zhao H, Dong X,
Zhang Z, Wang S and Shen L: Aquaporin 3 promotes the stem-like
properties of gastric cancer cells via Wnt/GSK-3β/β-catenin
pathway. Oncotarget. 7:16529–16541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Y, Wu G, Fu X, Xu S, Wang T, Zhang Q
and Yang Y: Aquaporin 3 maintains the stemness of CD133+
hepatocellular carcinoma cells by activating STAT3. Cell Death Dis.
10:4652019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu H, Xu Y, Zhang W, Shen L, Yang L and Xu
Z: Aquaporin-3 positively regulates matrix metalloproteinases via
PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J
Exp Clin Cancer Res. 30:862011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen J, Wang T, Zhou YC, Gao F, Zhang ZH,
Xu H, Wang SL and Shen LZ: Aquaporin 3 promotes
epithelial-mesenchymal transition in gastric cancer. J Exp Clin
Cancer Res. 33:382014. View Article : Google Scholar : PubMed/NCBI
|