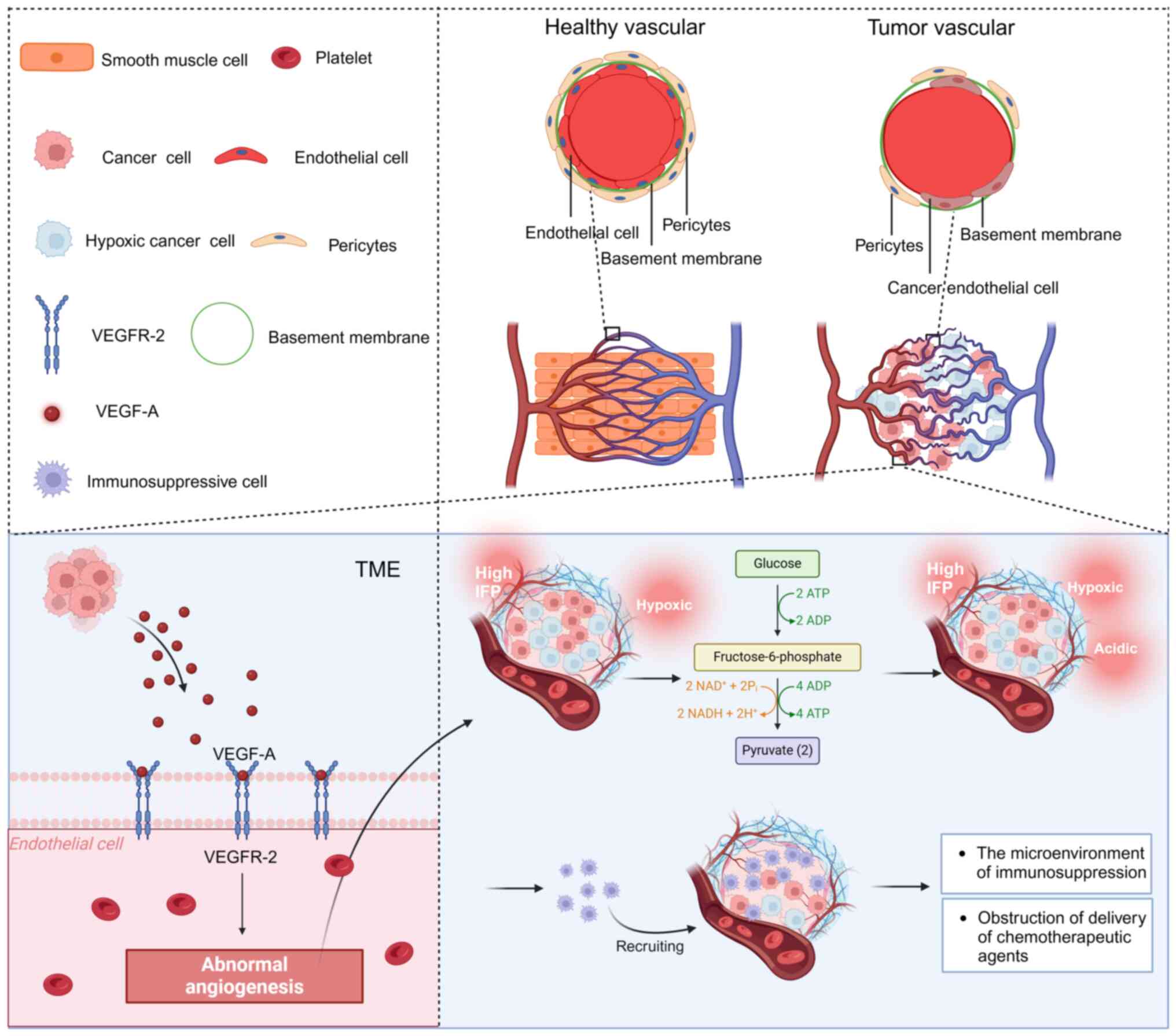
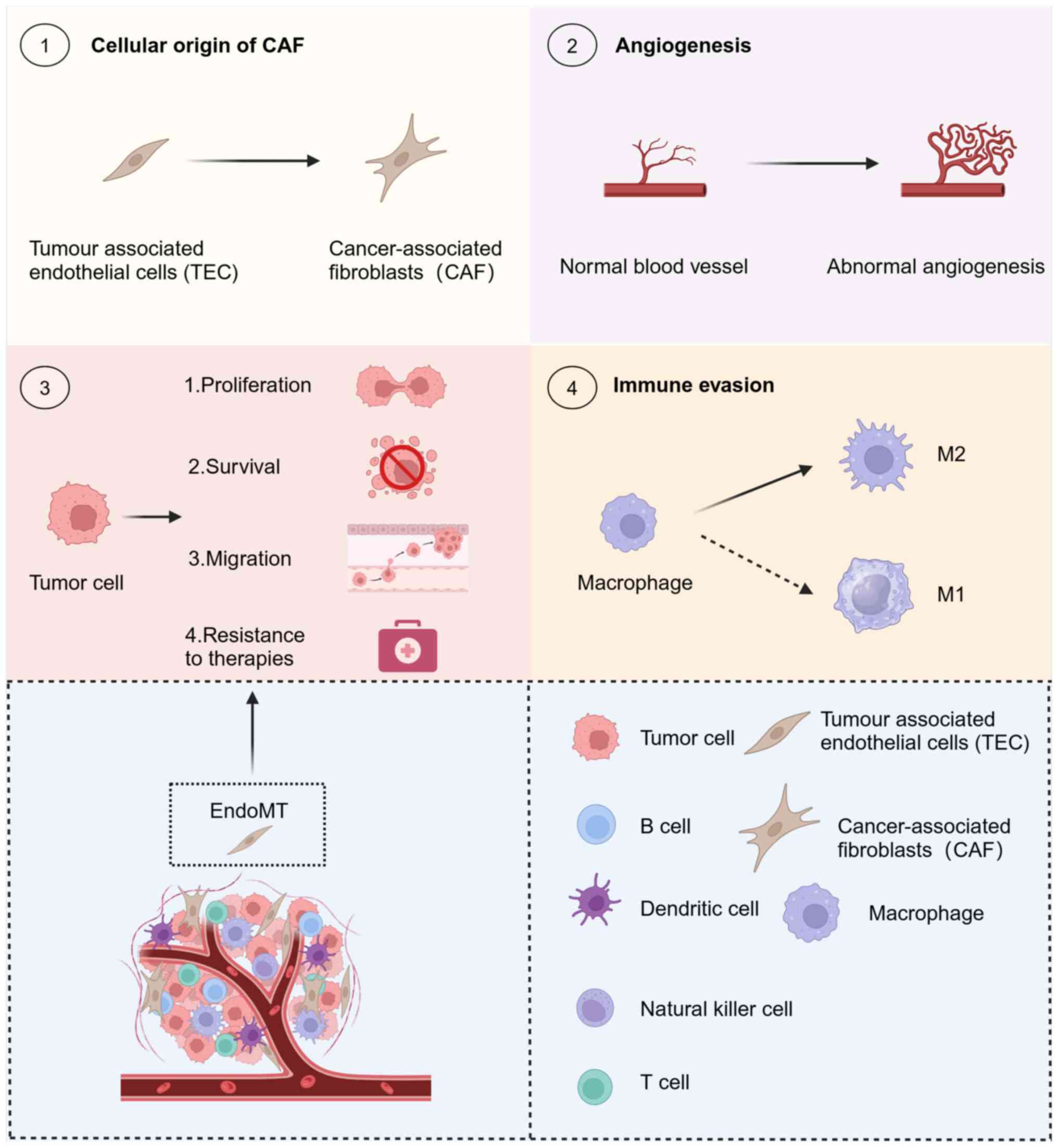
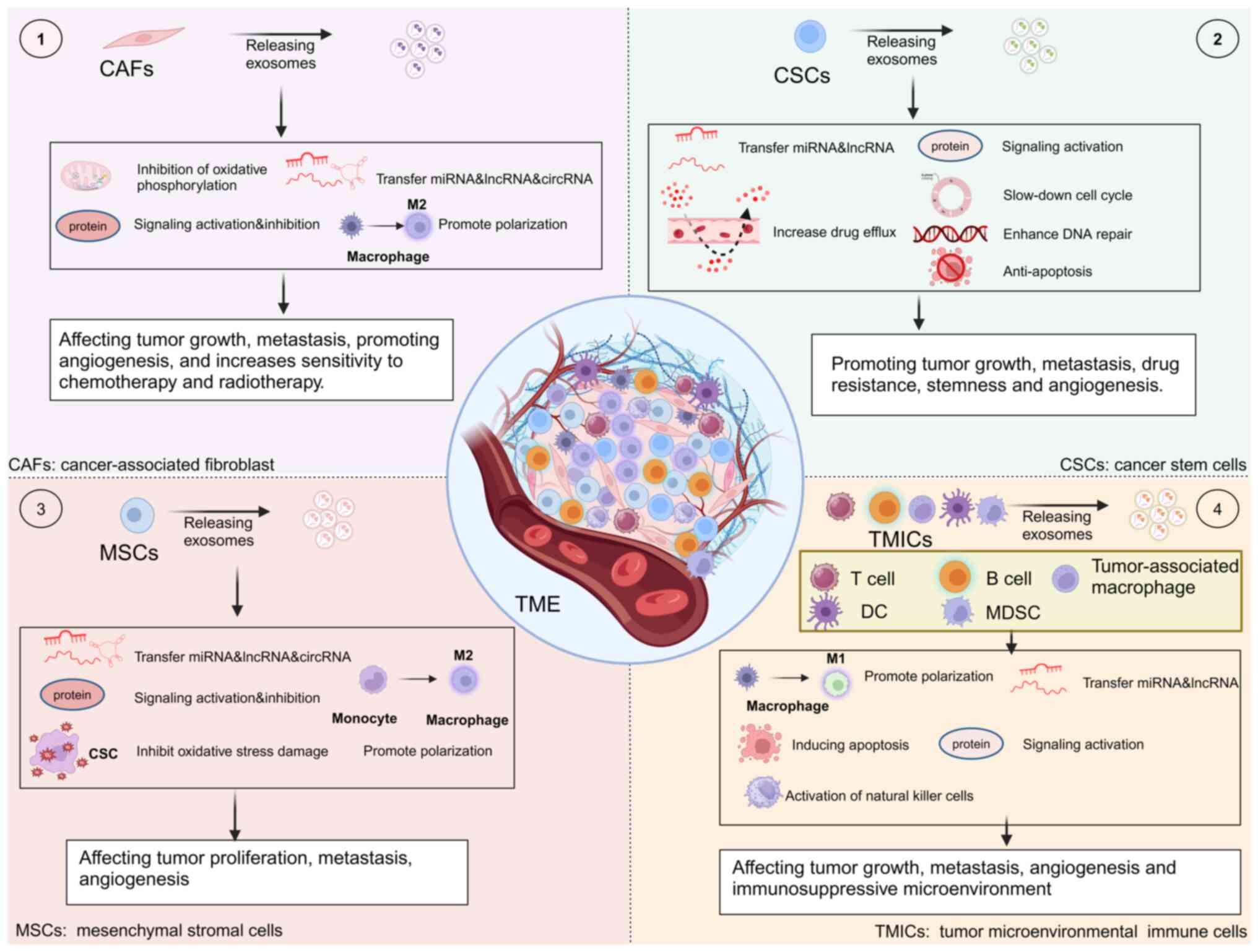
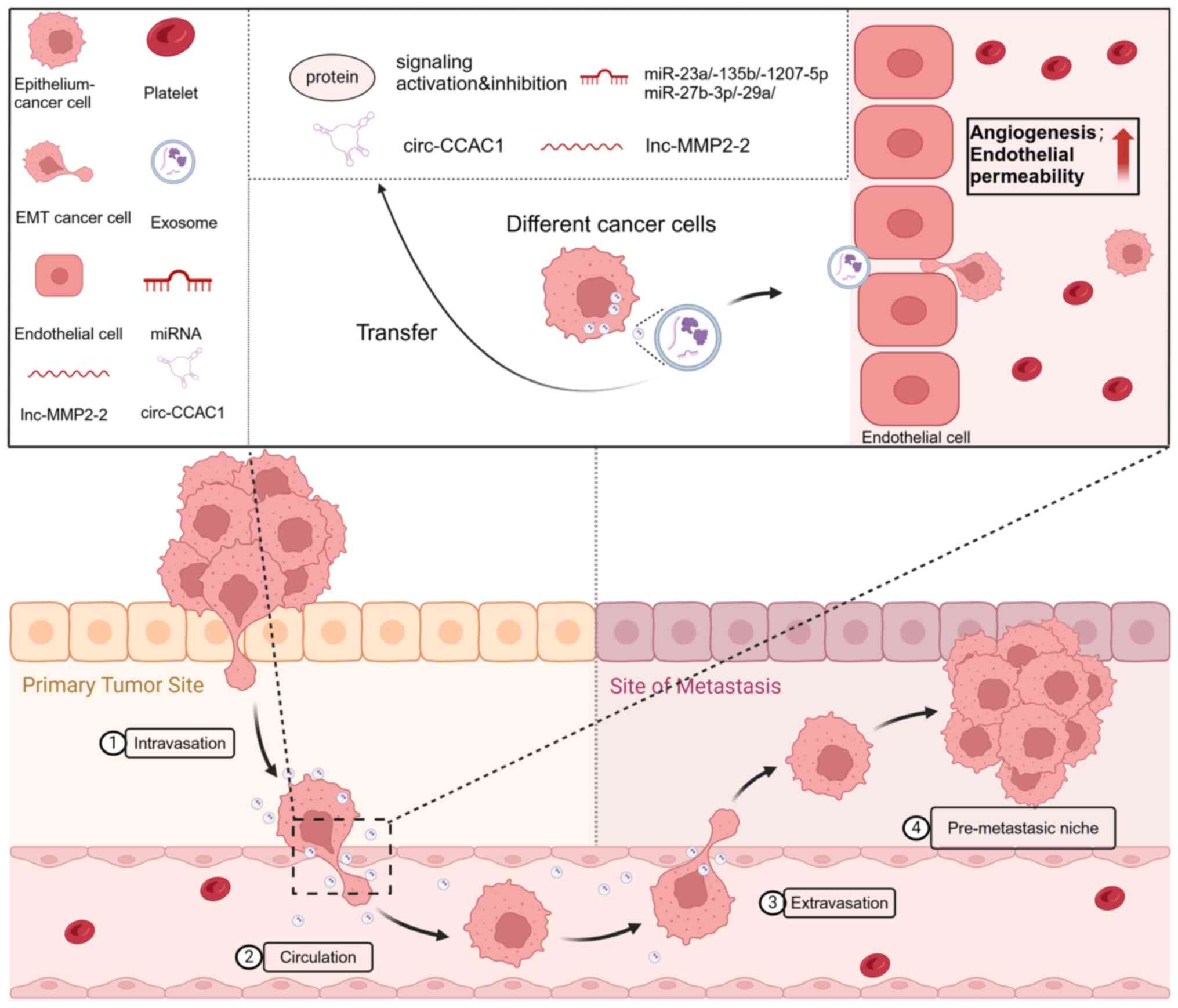
|
1
|
Kamrani A, Hosseinzadeh R, Shomali N,
Heris JA, Shahabi P, Mohammadinasab R, Sadeghvand S, Ghahremanzadeh
K, Sadeghi M and Akbari M: New immunotherapeutic approaches for
cancer treatment. Pathol Res Pract. 248:1546322023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ragusa M, Barbagallo C, Cirnigliaro M,
Battaglia R, Brex D, Caponnetto A, Barbagallo D, Di Pietro C and
Purrello M: Asymmetric RNA distribution among cells and their
secreted exosomes: Biomedical meaning and considerations on
diagnostic applications. Front Mol Biosci. 4:662027. View Article : Google Scholar
|
|
3
|
Betz C, Lenard A, Belting HG and Affolter
M: Cell behaviors and dynamics during angiogenesis. Development.
143:2249–2260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szekanecz Z and Koch AE: Mechanisms of
Disease: Angiogenesis in inflammatory diseases. Nat Clin Pract
Rheumatol. 3:635–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nachmany I, Bogoch Y, Friedlander-Malik G,
Amar O, Bondar E, Zohar N, Hantisteanu S, Fainaru O, Lubezky N,
Klausner JM and Pencovich N: The transcriptional profile of
circulating myeloid derived suppressor cells correlates with tumor
development and progression in mouse. Genes Immun. 20:589–598.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang FT, Sun W, Zhang JT and Fan YZ:
Cancer-associated fibroblast regulation of tumor neo-angiogenesis
as a therapeutic target in cancer. Oncol Lett. 17:3055–3065.
2019.PubMed/NCBI
|
|
7
|
Gasparics A, Kokeny G, Fintha A, Bencs R,
Mozes MM, Agoston EI, Buday A, Ivics Z, Hamar P, Gyorffy B, et al:
Alterations in SCAI expression during cell plasticity, fibrosis and
cancer. Pathol Oncol Res. 24:641–651. 2018. View Article : Google Scholar
|
|
8
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar
|
|
9
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Zhang L, Montgomery KC, Jiang L,
Lyon CJ and Hu TY: Advanced technologies for molecular diagnosis of
cancer: State of pre-clinical tumor-derived exosome liquid
biopsies. Mater Today Bio. 18:1005382022. View Article : Google Scholar
|
|
11
|
Liao J, Liu R, Yin L and Pu Y: Expression
profiling of exosomal miRNAs derived from human esophageal cancer
cells by solexa High-Throughput sequencing. Int J Mol Sci.
15:15530–15551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saunderson SC, Dunn AC, Crocker PR and
McLellan AD: CD169 mediates the capture of exosomes in spleen and
lymph node. Blood. 123:208–216. 2014. View Article : Google Scholar :
|
|
13
|
Andersen JS and Mann M: Organellar
proteomics: Turning inventories into insights. EMBO Rep. 7:874–879.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darband SG, Mirza-Aghazadeh-Attari M,
Kaviani M, Mihanfar A, Sadighparvar S, Yousefi B and Majidinia M:
Exosomes: Natural nanoparticles as bio shuttles for RNAi delivery.
J Control Rel. 289:158–170. 2018. View Article : Google Scholar
|
|
15
|
Wang S, Wang J, Wei W and Ma G: Exosomes:
The indispensable messenger in tumor pathogenesis and the rising
star in antitumor applications. Adv Biosyst. 3:e19000082019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Wu D, Ma X, Wang J, Hou W and
Zhang W: Exosomes as drug carriers for cancer therapy and
challenges regarding exosome uptake. Biomed Pharmacother.
128:1102372020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rashed M, Bayraktar EK, Helal G, Abd-Ellah
M, Amero P, Chavez-Reyes A and Rodriguez-Aguayo C: Exosomes: From
garbage bins to promising therapeutic targets. Int J Mol Sci.
18:5382017. View Article : Google Scholar
|
|
18
|
Mannavola F, D'Oronzo S, Cives M, Stucci
LS, Ranieri G, Silvestris F and Tucci M: Extracellular vesicles and
epigenetic modifications are hallmarks of melanoma progression. Int
J Mol Sci. 21:522019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He C, Zheng S, Luo Y and Wang B: Exosome
theranostics: Biology and translational medicine. Theranostics.
8:237–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Kim SY, Tu W, Kim J, Xu A, Yang
YM, Matsuda M, Reolizo L, Tsuchiya T, Billet S, et al:
Extracellular vesicles in fatty liver promote a metastatic tumor
microenvironment. Cell Metab. 35:1209–1226.e13. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu H, Sun T, An J, Wen L, Liu F, Bu Z, Cui
Y and Feng J: Potential roles of exosomes in Parkinson's Disease:
From pathogenesis, diagnosis, and treatment to prognosis. Front
Cell Dev Biol. 8:862020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farooqi AA, Desai NN, Qureshi MZ,
Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V and
Daglia M: Exosome biogenesis, bioactivities and functions as new
delivery systems of natural compounds. Biotechnol Adv. 36:328–334.
2018. View Article : Google Scholar
|
|
23
|
Doyle L and Wang M: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das CK, Jena BC, Banerjee I, Das S, Parekh
A, Bhutia SK and Mandal M: Exosome as a novel shuttle for delivery
of therapeutics across biological barriers. Mol Pharm. 16:24–40.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhang M and Zhou F: Biological
functions and clinical applications of exosomal long non-coding
RNAs in cancer. J Cell Mol Med. 24:11656–11666. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tai Y, Chen KC, Hsieh JT and Shen TL:
Exosomes in cancer development and clinical applications. Cancer
Sci. 109:2364–2374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan X, Li X, Dong L, Liu T, Zhang M, Zhang
L, Zhang X, Huang L, Shi W, Sun H, et al: Tumour vasculature at
single-cell resolution. Nature. 632:429–436. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shashni B, Nishikawa Y and Nagasaki Y:
Management of tumor growth and angiogenesis in triple-negative
breast cancer by using redox nanoparticles. Biomaterials.
269:1206452021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Qu X, Cao B, Yang T, Bao Q, Yue H,
Zhang L, Zhang G, Wang L, Qiu P, et al: Selectively suppressing
tumor angiogenesis for targeted breast cancer therapy by
genetically engineered phage. Adv Mater. 32:e20012602020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schaaf MB, Garg AD and Agostinis P:
Defining the role of the tumor vasculature in antitumor immunity
and immunotherapy. Cell Death Dis. 9:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arneth B: Tumor microenvironment. Medicina
(Kaunas). 56:152019. View Article : Google Scholar
|
|
33
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blankenstein T, Coulie PG, Gilboa E and
Jaffee EM: The determinants of tumour immunogenicity. Nat Rev
Cancer. 12:307–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ntellas P, Mavroeidis L, Gkoura S, Gazouli
I, Amylidi AL, Papadaki A, Zarkavelis G, Mauri D, Karpathiou G,
Kolettas E, et al: Old Player-new tricks: Non angiogenic effects of
the VEGF/VEGFR pathway in cancer. Cancers (Basel). 12:31452020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chouaib S, Noman MZ, Kosmatopoulos K and
Curran MA: Hypoxic stress: Obstacles and opportunities for
innovative immunotherapy of cancer. Oncogene. 36:439–445. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reardon DA: Update on the use of
angiogenesis inhibitors in adult patients with brain tumors. Clin
Adv Hematol Oncol. 12:293–303. 2014.PubMed/NCBI
|
|
42
|
Winkler F, Kozin SV, Tong RT, Chae SS,
Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E,
et al: Kinetics of vascular normalization by VEGFR2 blockade
governs brain tumor response to radiation. Cancer Cell. 6:553–563.
2004.PubMed/NCBI
|
|
43
|
Casazza A, Laoui D, Wenes M, Rizzolio S,
Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JoA,
Tamagnone L and Mazzone M: Impeding macrophage entry into hypoxic
tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis
and restores antitumor immunity. Cancer Cell. 24:695–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rivera Lee B, Meyronet D, Hervieu V,
Frederick, Mitchell J, Bergsland E and Bergers G: Intratumoral
myeloid cells regulate responsiveness and resistance to
antiangiogenic therapy. Cell Reports. 11:577–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chauhan VP, Stylianopoulos T, Martin JD,
Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D and Jain RK:
Normalization of tumour blood vessels improves the delivery of
nanomedicines in a size-dependent manner. Nat Nanotechnol.
7:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stylianopoulos T and Jain RK: Combining
two strategies to improve perfusion and drug delivery in solid
tumors. Proc Natl Acad Sci USA. 110:18632–18637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weiss SA, Han SW, Lui K, Tchack J, Shapiro
R, Berman R, Zhong J, Krogsgaard M, Osman I and Darvishian F:
Immunologic heterogeneity of tumor-infiltrating lymphocyte
composition in primary melanoma. Hum Pathol. 57:16–125. 2016.
View Article : Google Scholar
|
|
48
|
Park JS, Kim IK, Han S, Park I, Kim C, Bae
J, Oh SJ, Lee S, Kim JH, Woo DC, et al: Normalization of tumor
vessels by Tie2 activation and Ang2 inhibition enhances drug
delivery and produces a favorable tumor microenvironment. Cancer
Cell. 30:953–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maes H, Olmeda D, Soengas MS and Agostinis
P: Vesicular trafficking mechanisms in endothelial cells as
modulators of the tumor vasculature and targets of antiangiogenic
therapies. FEBS J. 283:25–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kofler NM, Shawber CJ, Kangsamaksin T,
Reed HO, Galatioto J and Kitajewski J: Notch signaling in
developmental and tumor angiogenesis. Genes Cancer. 2:1106–1116.
2011. View Article : Google Scholar
|
|
51
|
Maes H, Kuchnio A, Peric A, Moens S, Nys
K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, et
al: Tumor vessel normalization by chloroquine independent of
autophagy. Cancer Cell. 26:190–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang X, De Vera ME, Buchser WJ, de Vivar
Chavez AR, Loughran P, Stolz DB, Basse P, Wang T, Van Houten B, Zeh
HJ III and Lotze MT: Inhibiting systemic autophagy during
interleukin 2 immunotherapy promotes long-term tumor regression.
Cancer Res. 72:2791–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Goel S, Wong AH and Jain RK: Vascular
normalization as a therapeutic strategy for malignant and
nonmalignant disease. Cold Spring Harb Perspect Med. 2:a0064862012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guerrouahen BS, Pasquier J, Kaoud NA,
Maleki M, Beauchamp MC, Yasmeen A, Ghiabi P, Lis R, Vidal F, Saleh
A, et al: Akt-activated endothelium constitutes the niche for
residual disease and resistance to bevacizumab in ovarian cancer.
Mol Cancer Ther. 13:3123–3136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McMillin DW, Negri JM and Mitsiades CS:
The role of tumour-stromal interactions in modifying drug response:
Challenges and opportunities. Nat Rev Drug Discov. 12:217–228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jayson GC, Hicklin DJ and Ellis LM:
Antiangiogenic therapy-evolving view based on clinical trial
results. Nat Rev Clin Oncol. 9:297–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao Y, Arbiser J, D'Amato RJ, D'Amore PA,
Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, et al:
Forty-year journey of angiogenesis translational research. Sci
Transl Med. 3:114rv32011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tang T, Huang X, Zhang G, Hong Z, Bai X
and Liang T: Advantages of targeting the tumor immune
microenvironment over blocking immune checkpoint in cancer
immunotherapy. Signal Transduct Target Ther. 6:722021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Song Y, Fu Y, Xie Q, Zhu B, Wang J and
Zhang B: Anti-angiogenic agents in combination with immune
checkpoint inhibitors: A promising strategy for cancer treatment.
Front Immunol. 11:19562020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ciciola P, Cascetta P, Bianco C, Formisano
L and Bianco R: Combining immune checkpoint inhibitors with
anti-angiogenic agents. J Clin Med. 9:6752020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer. 18:602019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Neves KB, Montezano AC, Lang NN and Touyz
RM: Vascular toxicity associated with anti-angiogenic drugs. Clin
Sci (Lond). 134:2503–2520. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hilmi M, Neuzillet C, Calderaro J, Lafdil
F, Pawlotsky JM and Rousseau B: Angiogenesis and immune checkpoint
inhibitors as therapies for hepatocellular carcinoma: Current
knowledge and future research directions. J Immunother Cancer.
7:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xiao L, Yan K, Yang Y, Chen N, Li Y, Deng
X, Wang L, Liu Y, Mu L, Li R, et al: Anti-vascular endothelial
growth factor treatment induces blood flow recovery through
vascular remodeling in high-fat diet induced diabetic mice.
Microvasc Re. 105:70–76. 2016. View Article : Google Scholar
|
|
69
|
Broekman F, Giovannetti E and Peters GJ:
Tyrosine kinase inhibitors: Multi-targeted or single-targeted? Mol
Cancer Ther. 2:80–93. 2011.
|
|
70
|
Hutzen B, Bid HK, Houghton PJ, Pierson CR,
Powell K, Bratasz A, Raffel C and Studebaker AW: Treatment of
medulloblastoma with oncolytic measles viruses expressing the
angiogenesis inhibitors endostatin and angiostatin. BMC Cancer.
14:2062014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mohajeri A, Pilehvar-Soltanahmadi Y,
Pourhassan-Moghaddam M, Abdolalizadeh J, Karimi P and Zarghami N:
Cloning and expression of recombinant human endostatin in periplasm
of escherichia coli expression system. Adv Pharm Bull. 6:187–194.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Matejuk A, Collet G, Nadim M, Grillon C
and Kieda C: MicroRNAs and tumor vasculature normalization: Impact
on Anti-Tumor immune response. Arch Immunol Ther Exp (Warsz).
61:285–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yin R, Guo L, Zhang W and Zheng J: The
pleiotropic effects of miRNAs on tumor angiogenesis. J Cell
Biochem. 116:1807–1815. 2015. View Article : Google Scholar
|
|
74
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Karaa ZS, Iacovoni JS, Bastide A,
Lacazette E, Touriol C and Prats H: The VEGF IRESes are
differentially susceptible to translation inhibition by miR-16.
RNA. 15:249–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Azhar M, Runyan RB, Gard C, Sanford LP,
Miller ML, Andringa A, Pawlowski S, Rajan S and Doetschman T:
Ligand-specific function of transforming growth factor beta in
epithelial-mesenchymal transition in heart development. Dev Dyn.
238:431–442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Piera-Velazquez S and Jimenez SA:
Endothelial to mesenchymal transition: Role in physiology and in
the pathogenesis of human diseases. Physiol Rev. 99:1281–1324.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Piera-Velazquez S, Li Z and Jimenez SA:
Role of endothelial-mesenchymal transition (EndoMT) in the
pathogenesis of fibrotic disorders. Am J Pathol. 179:1074–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Medici D and Olsen BR: The role of
endothelial-mesenchymal transition in heterotopic ossification. J
Bone Miner Res. 27:1619–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
van Meeteren LA and ten Dijke P:
Regulation of endothelial cell plasticity by TGF-β. Cell Tissue
Res. 347:177–186. 2011. View Article : Google Scholar
|
|
82
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Heldin CH and Moustakas A: Role of Smads
in TGFβ signaling. Cell Tissue Res. 347:21–36. 2011. View Article : Google Scholar
|
|
84
|
Yeon JH, Jeong HE, Seo H, Cho S, Kim K, Na
D Chung S, Park J, Choi N and Kang JY: Cancer-derived exosomes
trigger endothelial to mesenchymal transition followed by the
induction of cancer-associated fibroblasts. Acta Biomater.
76:146–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yamada NO, Heishima K, Akao Y and Senda T:
Extracellular vesicles containing MicroRNA-92a-3p facilitate
partial Endothelial-mesenchymal transition and angiogenesis in
endothelial cells. Int J Mol Sci. 20:44062019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kim J, Lee C, Kim I, Ro J, Kim J, Min Y,
Park J, Sunkara V, Park YS, Michael I, et al: Three-dimensional
human liver-chip emulating premetastatic niche formation by breast
cancer-derived extracellular vesicles. ACS Nano. 14:14971–14988.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yin Z and Wang L:
Endothelial-to-mesenchymal transition in tumour progression and its
potential roles in tumour therapy. Ann Med. 55:1058–1069. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yin Z, Dong C, Jiang K, Xu Z, Li R, Guo K,
Shao S and Wang L: Heterogeneity of cancer-associated fibroblasts
and roles in the progression, prognosis, and therapy of
hepatocellular carcinoma. J Hematol Oncol. 12:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kobayashi H, Enomoto A, Woods SL, Burt AD,
Takahashi M and Worthley DL: Cancer-associated fibroblasts in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 16:282–295.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar
|
|
92
|
Huang M, Liu T, Ma P, Mitteer RA, Zhang Z,
Kim HJ, Yeo E, Zhang D, Cai P, Li C, et al: c-Met-mediated
endothelial plasticity drives aberrant vascularization and
chemoresistance in glioblastoma. J Clin Invest. 126:1801–1814.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nie L, Lyros O, Medda R, Jovanovic N,
Schmidt JL, Otterson MF, Johnson CP, Behmaram B, Shaker R and
Rafiee P: Endothelial-mesenchymal transition in normal human
esophageal endothelial cells cocultured with esophageal
adenocarcinoma cells: Role of IL-1β and TGF-β2. Am J Physiol Cell
Physiol. 307:C859–C877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu T, Ma W, Xu H, Huang M, Zhang D, He Z,
Zhang L, Brem S, O'Rourke DM, Gong Y, et al: PDGF-mediated
mesenchymal transformation renders endothelial resistance to
anti-VEGF treatment in glioblastoma. Nat Commun. 9:34392018.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu K, Pan Q, Jia LQ, Dai Z, Ke AW, Zeng
HY, Tang ZY, Fan J and Zhou J: MiR-302c inhibits tumor growth of
hepatocellular carcinoma by suppressing the endothelial-mesenchymal
transition of endothelial cells. Sci Rep. 4:55242014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ghiabi P, Jiang J, Pasquier J, Maleki M,
Abu-Kaoud N, Halabi N, Guerrouahen BS, Rafii S and Rafii A: Breast
cancer cells promote a notch-dependent mesenchymal phenotype in
endothelial cells participating to a pro-tumoral niche. J Transl
Med. 13:272015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yoshimatsu Y, Wakabayashi I, Kimuro S,
Takahashi N, Takahashi K, Kobayashi M, Maishi N, Podyma-Inoue KA,
Hida K, Miyazono K and Watabe T: TNF-α enhances TGF-β-induced
endothelial-to-mesenchymal transition via TGF-β signal
augmentation. Cancer Sci. 111:2385–2399. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Smeda M, Kieronska A, Adamski MG,
Proniewski B, Sternak M, Mohaissen T, Przyborowski K, Derszniak K,
Kaczor D, Stojak M, et al: Nitric oxide deficiency and
endothelial-mesenchymal transition of pulmonary endothelium in the
progression of 4T1 metastatic breast cancer in mice. Breast Cancer
Res. 20:862018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Krizbai IA, Gasparics A, Nagyoszi P,
Fazakas C, Molnar J, Wilhelm I, Bencs R, Rosivall L and Sebe A:
Endothelial-mesenchymal transition of brain endothelial cells:
Possible role during metastatic extravasation. PLoS One.
10:e01196552015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Choi SH, Kim AR, Nam JK, Kim JM, Kim JY,
Seo HR, Lee HJ, Cho J and Lee YJ: Tumour-vasculature development
via endothelial-to-mesenchymal transition after radiotherapy
controls CD44v6+ cancer cell and macrophage polarization. Nat
Commun. 9:51082018. View Article : Google Scholar :
|
|
103
|
Ribas A: Adaptive immune resistance: How
cancer protects from immune attack. Cancer Discovery. 5:915–919.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Landsberg J, Kohlmeyer J, Renn M, Bald T,
Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M and Tüting
T: Melanomas resist T-cell therapy through inflammation-induced
reversible dedifferentiation. Nature. 490:412–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Knutson KL, Lu H, Stone B, Reiman JM,
Behrens MD, Prosperi CM, Gad EA, Smorlesi A and Disis ML:
Immunoediting of cancers may lead to epithelial to mesenchymal
transition. J Immunol. 177:1526–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, et al: Immune-induced epithelial to mesenchymal
transition in vivo generates breast cancer stem cells. Cancer Res.
69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fan C, Chen LL, Hsu TA, Chen CC, Chua KV,
LiC P and Huang TS: Endothelial-mesenchymal transition harnesses
HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal
adenocarcinoma. J Hematol Oncol. 12:1382019. View Article : Google Scholar
|
|
109
|
Liu X, Hoft DF and Peng G: Tumor
microenvironment metabolites directing T cell differentiation and
function. Trends Immunol. 43:132–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Riegler J, Gill H, Ogasawara A, Hedehus M,
Javinal V, Oeh J, Ferl GZ, Marik J, Williams S, Sampath D, et al:
VCAM-1 density and tumor perfusion predict T-cell infiltration and
treatment response in preclinical models. Neoplasia. 21:1036–1050.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Borriello L, Seeger RC, Asgharzadeh S and
DeClerck YA: More than the genes, the tumor microenvironment in
neuroblastoma. Cancer Lett. 380:304–314. 2016. View Article : Google Scholar
|
|
113
|
Marques P, Grossman AB and Korbonits M:
The tumour microenvironment of pituitary neuroendocrine tumours.
Front Neuroendocrinol. 58:1008522020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang L and Yu D: Exosomes in cancer
development, metastasis, and immunity. Biochim Biophys Acta Rev
Cancer. 1871:455–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhao H, Yang L, Baddour J, Achreja A,
Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA,
et al: Tumor microenvironment derived exosomes pleiotropically
modulate cancer cell metabolism. ELife. 5:e102502016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Whiteside TL: Tumor-derived exosomes and
their role in cancer progression. Adv Clin Chem. 174:103–141. 2016.
View Article : Google Scholar
|
|
117
|
Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H,
Li XL, Tao DD, Wu YQ, Gong JP and Qin JC: Exosomal Wnt-induced
dedifferentiation of colorectal cancer cells contributes to
chemotherapy resistance. Oncogene. 38:1951–1965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS,
Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al: CAFs secreted
exosomes promote metastasis and chemotherapy resistance by
enhancing cell stemness and epithelial-mesenchymal transition in
colorectal cancer. Mol Cancer. 18:912019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen X, Liu J, Zhang Q, Liu B, Cheng Y,
Zhang Y, Sun Y, Ge H and Liu Y: Exosome-mediated transfer of
miR-93-5p from cancer-associated fibroblasts confer radioresistance
in colorectal cancer cells by downregulating FOXA1 and upregulating
TGFB3. J Exp Clin Cancer Res. 39:652020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pan S, Deng Y, Fu J, Zhang Y, Zhang Z and
Qin X: N6-methyladenosine upregulates miR-181d-5p in exosomes
derived from cancer-associated fibroblasts to inhibit 5-FU
sensitivity by targeting NCALD in colorectal cancer. Int J Oncol.
60:142022. View Article : Google Scholar :
|
|
121
|
Yuan H, Chen B, Chai R, Gong W, Wan Z,
Zheng B, Hu X, Guo Y, Gao S, Dai Q, et al: Loss of exosomal
micro-RNA-200b-3p from hypoxia cancer-associated fibroblasts
reduces sensitivity to 5-flourouracil in colorectal cancer through
targeting high-mobility group box 3. Front Oncol. 12:9201312022.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jiang Y, Qiu Q, Jing X, Song Z, Zhang Y,
Wang C, Liu K, Ye F, Ji X, Luo F and Zhao R: Cancer-associated
fibroblast-derived exosome miR-181b-3p promotes the occurrence and
development of colorectal cancer by regulating SNX2 expression.
Biochem Biophys Res Commun. 641:177–185. 2023. View Article : Google Scholar
|
|
123
|
Shi W, Liu Y, Qiu X, Yang L and Lin G:
Cancer-associated fibroblasts-derived exosome-mediated transfer of
miR-345-5p promotes the progression of colorectal cancer by
targeting CDKN1A. Carcinogenesis. 44:317–327. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhou L, Li J, Tang Y and Yang M: Exosomal
LncRNA LINC00659 transferred from cancer-associated fibroblasts
promotes colorectal cancer cell progression via miR-342-3p/ANXA2
axis. J Transl Med. 19:82021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Qu Z, Yang KD, Luo BH and Zhang F:
CAFs-secreted exosomal cricN4BP2L2 promoted colorectal cancer
stemness and chemoresistance by interacting with EIF4A3. Exp Cell
Res. 418:1132662022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang X, Li Y, Zou L and Zhu Z: Role of
exosomes in crosstalk between Cancer-associated fibroblasts and
cancer cells. Front Oncol. 9:3561029. View Article : Google Scholar
|
|
127
|
Yan Z, Sheng Z, Zheng Y, Feng R, Xiao Q,
Shi L, Li H, Yin C, Luo H, Hao C, et al: Cancer-associated
fibroblast-derived exosomal miR-18b promotes breast cancer invasion
and metastasis by regulating TCEAL7. Cell Death Dis. 12:11202021.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sun J, Du R, Li X, Liu C, Wang D, He X, Li
G, Zhang K, Wang S, Hao Q, et al: CD63+ cancer-associated
fibroblasts confer CDK4/6 inhibitor resistance to breast cancer
cells by exosomal miR-20. Cancer Lett. 588:2167472024. View Article : Google Scholar
|
|
129
|
Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu
W, Wang D and Lou W: Exosomal miRNA-106b from cancer-associated
fibroblast promotes gemcitabine resistance in pancreatic cancer.
Exp Cell Res. 383:1115432019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhao M, Zhuang A, Fang Y and Chatterjee S:
Cancer-Associated fibroblast-derived exosomal miRNA-320a promotes
macrophage M2 polarization in vitro by regulating PTEN/PI3Kγ
signaling in pancreatic cancer. J Oncol. 2022:95146972022.
View Article : Google Scholar
|
|
131
|
Wang Z, Zhang M, Liu L, Yang Y, Qiu J, Yu
Y and Li J: Prognostic and immunological role of cancer-associated
fibroblasts-derived exosomal protein in esophageal squamous cell
carcinoma. Int Immunopharmacol. 124:1108372023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhao G, Li H, Guo Q, Zhou A, Wang X, Li P
and Zhang S: Exosomal Sonic Hedgehog derived from cancer-associated
fibroblasts promotes proliferation and migration of esophageal
squamous cell carcinoma. Cancer Med. 9:2500–2513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shi Z, Jiang T, Cao B, Sun X and Liu J:
CAF-derived exosomes deliver LINC01410 to promote
epithelial-mesenchymal transition of esophageal squamous cell
carcinoma. Exp Cell Res. 412:1130332022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang F, Yan Y, Yang Y, Hong X, Wang M,
Yang Z, Liu B and Ye L: MiR-210 in exosomes derived from CAFs
promotes non-small cell lung cancer migration and invasion through
PTEN/PI3K/AKT pathway. Cell Signal. 73:1096752020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang T, Zhang P and Li HX: CAFs-Derived
Exosomal miRNA-130a confers Cisplatin resistance of NSCLC cells
through PUM2-dependent packaging. Int J Nanomedicine. 16:561–577.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lu L, Huang J, Mo J, Da X, Li Q, Fan M and
Lu H: Exosomal lncRNA TUG1 from cancer-associated fibroblasts
promotes liver cancer cell migration, invasion, and glycolysis by
regulating the miR-524-5p/SIX1 axis. Cell Mol Biol Lett. 27:172022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhou Y, Tang W, Zhuo H, Zhu D, Rong D, Sun
J and Song J: Cancer-associated fibroblast exosomes promote
chemoresistance to cisplatin in hepatocellular carcinoma through
circZFR targeting signal transducers and activators of
transcription (STAT3)/nuclear factor-kappa B (NF-κB) pathway.
Bioengineered. 13:4786–4797. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhuang J, Lu Q, Shen B, Huang X, Shen L,
Zheng X, Huang R, Yan J and Guo H: TGFβ1 secreted by
cancer-associated fibroblasts induces epithelial-mesenchymal
transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep.
5:119242015. View Article : Google Scholar
|
|
139
|
Wang Y, Li T, Yang L, Zhang X, Wang X, Su
X, Ji C and Wang Z: Cancer-associated fibroblast-released
extracellular vesicles carrying miR-199a-5p induces the progression
of gastric cancer through regulation of FKBP5-mediated AKT1/mTORC1
signaling pathway. Cell Cycle. 21:2590–2601. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Qu X, Liu B, Wang L, Liu L, Zhao W, Liu C,
Ding J, Zhao S, Xu B, Yu H, et al: Loss of cancer-associated
fibroblast-derived exosomal DACT3-AS1 promotes malignant
transformation and ferroptosis-mediated oxaliplatin resistance in
gastric cancer. Drug Resist Updat. 68:1009362023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yugawa K, Yoshizumi T, Mano Y, Itoh S,
Harada N, Ikegami T, Kohashi K, Oda Y and Mori M: Cancer-associated
fibroblasts promote hepatocellular carcinoma progression through
downregulation of exosomal miR-150-3p. Eur J Surg Oncol.
47:384–393. 2021. View Article : Google Scholar
|
|
142
|
Chen X, Ren X, E J, Zhou Y and Bian R:
Exosome-transmitted circ IFNGR2 modulates ovarian cancer metastasis
via miR-378/ST5 Axis. Mol Cell Biol. 43:22–42. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sun Z, Wang L, Dong L and Wang X: Emerging
role of exosome signalling in maintaining cancer stem cell dynamic
equilibrium. J Cell Mol Med. 22:3719–3728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xu J, Liao K and Zhou W: Exosomes regulate
the transformation of cancer cells in cancer stem cell homeostasis.
Stem Cells Int. 2018:48373702018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Li W, Zhang L, Guo B, Deng J, Wu S, Li F,
Wang Y, Lu J and Zhou Y: Exosomal FMR1-AS1 facilitates maintaining
cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc
signaling in female esophageal carcinoma. Mol Cancer. 18:222019.
View Article : Google Scholar
|
|
146
|
Wang L, Yang G, Zhao D, Wang J, Bai Y,
Peng Q, Wang H, Fang R, Chen G, Wang Z, et al: CD103-positive CSC
exosome promotes EMT of clear cell renal cell carcinoma: Role of
remote MiR-19b-3p. Mol Cancer. 18:862019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Cheng Z, Lei Z, Yang P, Si A, Xiang D,
Tang X, Guo G, Zhou J and Hüser N: Exosome-transmitted p120-catenin
suppresses hepatocellular carcinoma progression via STAT3 pathways.
Mol Carcinog. 58:1389–1399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang J, Zheng Y and Zhao M: Exosome-Based
cancer therapy: Implication for targeting cancer stem cells. Front
Pharmacol. 7:5332017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yang Z, Zhao N, Cui J, Wu H, Xiong J and
Peng T: Exosomes derived from cancer stem cells of
gemcitabine-resistant pancreatic cancer cells enhance drug
resistance by delivering miR-210. Cell Oncol. 43:123–136. 2019.
View Article : Google Scholar
|
|
150
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yao H, Liu N, Lin MC and Zheng J: Positive
feedback loop between cancer stem cells and angiogenesis in
hepatocellular carcinoma. Cancer Lett. 379:213–219. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang ZF, Liao F, Wu H and Dai J: Glioma
stem cells-derived exosomal miR-26a promotes angiogenesis of
microvessel endothelial cells in glioma. J Exp Clin Cancer Res.
38:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhang D, Li D, Shen L, Hu D, Tang B, Guo
W, Wang Z, Zhang Z, Wei G and He D: Exosomes derived from
Piwil2-induced cancer stem cells transform fibroblasts into
cancer-associated fibroblasts. Oncol Rep. 43:1125–1132.
2020.PubMed/NCBI
|
|
154
|
Wang L, He J, Hu H, Tu L, Sun Z, Liu Y and
Luo F: Lung CSC-derived exosomal miR-210-3p contributes to a
pro-metastatic phenotype in lung cancer by targeting FGFRL1. J Cell
Mol Med. 24:6324–6339. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Dai W, Jin X, Han L, Huang H, Ji Z, Xu X,
Tang M, Jiang B and Chen W: Exosomal lncRNA DOCK9-AS2 derived from
cancer stem cell-like cells activated Wnt/β-catenin pathway to
aggravate stemness, proliferation, migration, and invasion in
papillary thyroid carcinoma. Cell Death Dis. 11:7432020. View Article : Google Scholar
|
|
156
|
Wu Q, He Y, Liu X, Luo F, Jiang Y, Xiang M
and Zhao R: Cancer stem cell-like cells-derived exosomal CDKN2B-AS1
stabilizes CDKN2B to promote the growth and metastasis of thyroid
cancer via TGF-β1/Smad2/3 signaling. Exp Cell Res. 419:1132682022.
View Article : Google Scholar
|
|
157
|
Wu Q, He Y, Liu X, Luo F, Jiang Y, Xiang M
and Zhao R: Cancer stem cell-like cells-derived exosomal lncRNA
CDKN2B-AS1 promotes biological characteristics in thyroid cancer
via miR-122-5p/P4HA1 axis. Exp Cell Res. 22:19–29. 2023.
|
|
158
|
Li X, Liu D, Chen H, Zeng B, Zhao Q, Zhang
Y, Chen Y, Wang J and Xing HR: Melanoma stem cells promote
metastasis via exosomal miR-1268a inactivation of autophagy. Biol
Res. 55:292022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Han T, Chen L and Li K, Hu Q, Zhang Y, You
X, Han L, Chen T and Li K: Significant CircRNAs in liver cancer
stem cell exosomes: Mediator of malignant propagation in liver
cancer? Mol Cancer. 22:1972023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Deng H, Sun C, Sun Y, Li H, Yang L, Wu D,
Gao Q and Jiang X: Lipid, Protein, and MicroRNA composition within
mesenchymal stem Cell-derived exosomes. Cell Cell Reprogram.
20:178–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Sharma A: Role of stem cell derived
exosomes in tumor biology. Int J Cancer. 142:1086–1092. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yang YP, Nguyen PNN, Ma HI, Ho WJ, Chen
YW, Chien Y, Yarmishyn AA, Huang PI, Lo WL, Wang CY, et al: Tumor
mesenchymal stromal cells regulate cell migration of atypical
teratoid rhabdoid tumor through Exosome-mediated miR155/SMARCA4
pathway. Cancers (Basel). 11:7202019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Figueroa J, Phillips LM, Shahar T, Hossain
A, Gumin J, Kim H, Bean AJ, Calin GA, Fueyo J, Walters ET, et al:
Exosomes from glioma-associated mesenchymal stem cells increase the
tumorigenicity of Glioma Stem-like cells via transfer of miR-1587.
Cancer Res. 77:5808–5819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Toh WS, Lai RC, Zhang B and Lim SK: MSC
exosome works through a protein-based mechanism of action. Biochem
Soc Trans. 46:843–853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Lee C, Mitsialis SA, Aslam M, Vitali SH,
Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A and
Kourembanas S: Exosomes mediate the cytoprotective action of
mesenchymal stromal cells on hypoxia-induced pulmonary
hypertension. Circulation. 126:2601–2611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol.
40:457–470. 2017. View Article : Google Scholar
|
|
167
|
Biswas S, Mandal G, Roy Chowdhury S,
Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X,
Conejo-Garcia JR and Bhattacharyya A: Exosomes produced by
mesenchymal stem cells drive differentiation of myeloid cells into
immunosuppressive M2-Polarized macrophages in breast cancer. J
Immunol. 203:3447–3460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xu H, Zhao G, Zhang Y, Jiang H, Wang W,
Zhao D, Hong J, Yu H and Qi L: Mesenchymal stem cell-derived
exosomal microRNA-133b suppresses glioma progression via
Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res
Ther. 10:3812019. View Article : Google Scholar
|
|
169
|
Xu Z, Zhou X, Wu J, Cui X, Wang M, Wang X
and Gao Z: Mesenchymal stem cell-derived exosomes carrying
microRNA-150 suppresses the proliferation and migration of
osteosarcoma cells via targeting IGF2BP1. Transl Cancer Res.
9:5323–5335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Qi J, Zhang R and Wang Y: Exosomal
miR-21-5p derived from bone marrow mesenchymal stem cells promote
osteosarcoma cell proliferation and invasion by targeting PIK3R1. J
Cell Mol Med. 25:11016–11030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li T, Wan Y, Su Z, Li J, Han M and Zhou C:
Mesenchymal stem Cell-derived exosomal microRNA-3940-5p inhibits
colorectal cancer metastasis by targeting integrin α6. Dig Dis Sci.
66:1916–1927. 2020. View Article : Google Scholar
|
|
172
|
Gu H, Yan C, Wan H, Wu L, Liu J, Zhu Z and
Gao D: Mesenchymal stem cell-derived exosomes block malignant
behaviors of hepatocellular carcinoma stem cells through a lncRNA
C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Human Cell.
34:1812–1829. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lyu ZZ, Li M, Yang MY, Han M and Yang Z:
Exosome-mediated transfer of circRNA563 promoting hepatocellular
carcinoma by targeting the microRNA148a-3p/metal-regulatory
transcription factor-1 pathway. World J Gastroenterol.
29:6060–6075. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yong SB, Chung JY, Song Y, Kim J, Ra S and
Kim YH: Non-viral nano-immunotherapeutics targeting tumor
microenvironmental immune cells. Biomaterials. 219:1194012019.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhang Q, Fan Z, Zhang L, You Q and Wang L:
Strategies for targeting Serine/Threonine protein phosphatases with
small molecules in cancer. J Med Chem. 64:8916–8938. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Li Z, Suo B, Long G, Gao Y, Song J, Zhang
M, Feng B, Shang C and Wang D: Exosomal miRNA-16-5p derived from M1
macrophages enhances T cell-dependent immune response by regulating
PD-L1 in gastric cancer. Front Cell Dev Biol. 8:5726892020.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Jiang H, Zhou L, Shen N, Ning X, Wu D,
Jiang K and Huang X: M1 macrophage-derived exosomes and their key
molecule lncRNA HOTTIP suppress head and neck squamous cell
carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell
Death Dis. 13:1832022. View Article : Google Scholar
|
|
178
|
Li X and Tang M: Exosomes released from M2
macrophages transfer miR-221-3p contributed to EOC progression
through targeting CDKN1B. Cancer Med. 9:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan
J, Zou Y and Chen S: Macrophage-derived exosomal microRNA-501-3p
promotes progression of pancreatic ductal adenocarcinoma through
the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res.
38:3102019. View Article : Google Scholar
|
|
180
|
Mi X, Xu R, Hong S, Xu T, Zhang W and Liu
M: M2 Macrophage-Derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a
affect cell migration and metastasis in esophageal cancer. Mol Ther
Nucl Acids. 22:779–790. 2020. View Article : Google Scholar
|
|
181
|
Yang Y, Guo Z, Chen W, Wang X, Cao M, Han
X, Zhang K, Teng B, Cao J, Wu W, et al: M2 Macrophage-Derived
exosomes promote angiogenesis and growth of pancreatic ductal
adenocarcinoma by Targeting E2F2. Mol Ther. 29:1226–1238. 2021.
View Article : Google Scholar :
|
|
182
|
Chen S, Lv M, Fang S, Ye W, Gao Y and Xu
Y: Poly(I:C) enhanced anti-cervical cancer immunities induced by
dendritic cells-derived exosomes. Int J Biol Macromol.
113:1182–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Viaud S, Terme M, Flament C, Taieb J,
André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz
T, et al: Dendritic cell-derived exosomes promote natural killer
cell activation and proliferation: A role for NKG2D ligands and
IL-15Ralpha. PLoS One. 4:e49422009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Wang Y, Yin K, Tian J, Xia X, Ma J, Tang
X, Xu H and Wang S: Granulocytic Myeloid-Derived suppressor cells
promote the stemness of colorectal cancer cells through exosomal
S100A9. Adv Sci (Weinh). 6:19012782019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhou JH, Yao ZX, Zheng Z, Yang J, Wang R,
Fu SJ, Pan XF, Liu ZH and Wu K: G-MDSCs-derived Exosomal
miRNA-143-3p promotes proliferation via targeting of ITM2B in lung
cancer. Onco Targets Ther. 13:9701–9719. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Zhou WJ, Zhang J, Xie F, Wu JN, Ye JF,
Wang J, Wu K and Li MQ: CD45RO-CD8+ T cell-derived exosomes
restrict estrogen-driven endometrial cancer development via the
ERβ/miR-765/PLP2/Notch axis. Theranostics. 11:5330–5345. 2021.
View Article : Google Scholar :
|
|
187
|
Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang
Q, Yang Y, Wang L, Cao X and Wang J: Activated T cell exosomes
promote tumor invasion via Fas signaling pathway. J Immunol.
188:5954–5961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Xie Y, Zhang X, Zhao T, Li W and Xiang J:
Natural CD8+25+ regulatory T cell-secreted
exosomes capable of suppressing cytotoxic T lymphocyte-mediated
immunity against B16 melanoma. Biochem Biophys Res Commun.
438:152–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Guyon N, Garnier D, Briand J, Nadaradjane
A, Bougras-Cartron G, Raimbourg J, Campone M, Heymann D, Vallette
FM, Frenel JS and Cartron PF: Anti-PD1 therapy induces
lymphocyte-derived exosomal miRNA-4315 release inhibiting
Bim-mediated apoptosis of tumor cells. Cell Death Dis. 11:10482020.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhang F, Li R, Yang Y, Shi C, Shen Y, Lu
C, Chen Y, Zhou W, Lin A, Yu L, et al: Specific decrease in
B-cell-derived extracellular vesicles enhances
post-chemotherapeutic CD8+ T cell responses. Immunity.
50:738–750.e7. 2019. View Article : Google Scholar
|
|
191
|
Yang Z, Wang W, Zhao L, Wang X, Gimple RC,
Xu L, Wang Y, Rich JN and Zhou S: Plasma cells shape the
mesenchymal identity of ovarian cancers through transfer of
exosome-derived microRNAs. Sci Adv. 7:eabb07372021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Aguilar-Cazares D, Chavez-Dominguez R,
Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON and
Lopez-Gonzalez JS: Contribution of angiogenesis to inflammation and
cancer. Front Oncol. 9:13992019. View Article : Google Scholar
|
|
193
|
Dominiak A, Chełstowska B, Olejarz W and
Nowicka G: Communication in the cancer microenvironment as a target
for therapeutic interventions. Cancers (Basel). 12:12322020.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Stec M, Baj-Krzyworzeka M, Baran J,
Węglarczyk K and Zembala M, Barbasz J, Szczepanik A and Zembala M:
Isolation and characterization of circulating micro(nano)vesicles
in the plasma of colorectal cancer patients and their interactions
with tumor cells. Oncol Rep. 34:2768–2775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Aslan C, Maralbashi S, Salari F, Kahroba
H, Sigaroodi F, Kazemi T and Kharaziha P: Tumor-derived exosomes:
Implication in angiogenesis and antiangiogenesis cancer therapy. J
Cell Physiol. 234:16885–16903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zhao Z, Sun W, Guo Z, Zhang J, Yu H and
Liu B: Mechanisms of lncRNA/microRNA interactions in angiogenesis.
Life Sci. 254:1169002020. View Article : Google Scholar
|
|
197
|
Folkman J, Merler E, Abernathy C and
Williams G: Isolation of a tumor factor responsible for
angiogenesis. J Exp Med. 133:275–288. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Weinstein N, Mendoza L, Gitler I and Klapp
J: A Network model to explore the effect of the Micro-environment
on endothelial cell behavior during angiogenesis. Front Physiol.
8:9602017. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Vavourakis V, Wijeratne PA, Shipley R,
Loizidou M, Stylianopoulos T and Hawkes DJ: A validated multiscale
In-silico model for mechano-sensitive tumour angiogenesis and
growth. PLoS Comput Biol. 13:e10052592017. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Varberg KM, Winfree S, Dunn KW and
Haneline LS: Kinetic analysis of vasculogenesis quantifies dynamics
of vasculogenesis and angiogenesis in vitro. J Vis Exp. 57044:2018.
View Article : Google Scholar
|
|
201
|
Ludwig N and Whiteside TL: Potential roles
of tumor-derived exosomes in angiogenesis. Expert Opin Ther
Targets. 22:409–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kucharzewska P, Christianson HC, Welch JE,
Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain
E, Bengzon J and Belting M: Exosomes reflect the hypoxic status of
glioma cells and mediate hypoxia-dependent activation of vascular
cells during tumor development. Proc Natl Acad Sci USA.
110:7312–7317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Kaur B, Cork SM, Sandberg EM, Devi NS,
Zhang Z, Klenotic PA, Febbraio M, Shim H, Mao H, Tucker-Burden C,
et al: Vasculostatin inhibits intracranial glioma growth and
negatively regulates in vivo angiogenesis through a CD36-dependent
mechanism. Cancer Res. 69:1212–1220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Taverna S, Flugy A, Saieva L, Kohn EC,
Santoro A, Meraviglia S, De Leo G and Alessandro R: Role of
exosomes released by chronic myelogenous leukemia cells in
angiogenesis. Int J Cancer. 130:2033–2043. 2012. View Article : Google Scholar
|
|
205
|
Siemann DW and Horsman MR: Modulation of
the tumor vasculature and oxygenation to improve therapy. Pharmacol
Ther. 153:107–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Hida K, Maishi N, Annan DA and Hida Y:
Contribution of tumor endothelial cells in cancer progression. Int
J Mol Sci. 19:12722018. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y,
Wang C, Zhang Q, Yang S, Cao L, Zhang X, et al: Hypoxic exosomes
facilitate angiogenesis and metastasis in esophageal squamous cell
carcinoma through altering the phenotype and transcriptome of
endothelial cells. Int J Mol Sci. 38:3892019.
|
|
208
|
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC,
Tsai PH, Wu CY and Kuo PL: Hypoxic lung cancer-secreted exosomal
miR-23a increased angiogenesis and vascular permeability by
targeting prolyl hydroxylase and tight junction protein ZO-1.
Oncogene. 36:4929–4942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Sruthi TV, Edatt L, Raji GR, Kunhiraman H,
Shankar SS, Shankar V, Ramachandran V, Poyyakkara A and Kumar SVB:
Horizontal transfer of miR-23a from hypoxic tumor cell colonies can
induce angiogenesis. J Cell Physiol. 233:3498–3514. 2018.
View Article : Google Scholar
|
|
210
|
Gesierich S, Berezovskiy I, Ryschich E and
Zöller M: Systemic induction of the angiogenesis switch by the
tetraspanin D6.1A/CO-029. Cancer Res. 66:7083–7094. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sheldon H, Heikamp E, Turley H, Dragovic
R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RCA, et
al: New mechanism for Notch signaling to endothelium at a distance
by Delta-like 4 incorporation into exosomes. Blood. 116:2385–2394.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Tang MKS, Yue PYK, Ip PP, Huang RL, Lai
HC, Cheung ANY, Tse KY, Ngan HYS and Wong AST: Soluble E-cadherin
promotes tumor angiogenesis and localizes to exosome surface.
Nature Commun. 9:22702018. View Article : Google Scholar
|
|
213
|
Svensson KJ, Kucharzewska P, Christianson
HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W
and Belting M: Hypoxia triggers a proangiogenic pathway involving
cancer cell microvesicles and PAR-2-mediated heparin-binding EGF
signaling in endothelial cells. Proc Natl Acad Sci USA.
108:13147–13152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Wu D, Deng S, Li L, Liu T, Zhang T, Li J,
Yu Y and Xu Y: TGF-β1-mediated exosomal lnc-MMP2-2 increases
blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis
to promote non-small cell lung cancer brain metastasis. Cell Death
Dis. 12:7212021. View Article : Google Scholar
|
|
216
|
Dou R, Liu K, Yang C, Zheng J, Shi D, Lin
X, Wei C, Zhang C, Fang Y, Huang S, et al: EMT-cancer cells-derived
exosomal miR-27b-3p promotes circulating tumour cells-mediated
metastasis by modulating vascular permeability in colorectal
cancer. Cell Death Dis. 11:e5952021.
|
|
217
|
Liu K, Dou R, Yang C, Di Z, Shi D, Zhang
C, Song J, Fang Y, Huang S, Xiang Z, et al: Exosome-transmitted
miR-29a induces colorectal cancer metastasis by destroying the
vascular endothelial barrier. Carcinogenesis. 44:356–367. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Xu Y, Leng K, Yao Y, Kang P, Liao G, Han
Y, Shi G, Ji D, Huang P, Zheng W, et al: Circular RNA,
Cholangiocarcinoma-associated circular RNA 1, contributes to
Cholangiocarcinoma progression, induces angiogenesis, and disrupts
vascular endothelial barriers. Hepatology. 73:1419–1435. 2021.
View Article : Google Scholar
|
|
219
|
Li K, Xue W, Lu Z, Wang S, Zheng J, Lu K,
Li M, Zong Y, Xu F, Dai J, et al: Tumor-derived exosomal ADAM17
promotes pre-metastatic niche formation by enhancing vascular
permeability in colorectal cancer. J Exp Clin Cancer Res.
43:592024. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Nazarenko I, Rana S, Baumann A, McAlear J,
Hellwig A, Trendelenburg M, Lochnit G, Preissner KT and Zöller M:
Cell surface tetraspanin Tspan8 contributes to molecular pathways
of exosome-induced endothelial cell activation. Cancer Res.
70:1668–1678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Hood JL, San RS and Wickline SA: Exosomes
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Akoto T and Saini S: Role of exosomes in
prostate cancer metastasis. Int J Mol Sci. 22:35282021. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Valencia K, Luis-Ravelo D, Bovy N, Antón
I, Martínez-Canarias S, Zandueta C, Ormazábal C, Struman I, Tabruyn
S, Rebmann V, et al: miRNA cargo within exosome-like vesicle
transfer influences metastatic bone colonization. Mol Oncol.
8:689–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
You L, Wu W, Wang X, Fang L, Adam V,
Nepovimova E, Wu Q and Kuca K: The role of hypoxia-inducible factor
1 in tumor immune evasion. Med Res Rev. 41:1622–1643. 2021.
View Article : Google Scholar
|
|
226
|
Mu W, Rana S and Zöller M: Host matrix
modulation by tumor exosomes promotes motility and invasiveness.
Neoplasia. 15:875–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F,
Ma P, Jiang H, Wu X, Shu Y and Xu T: Exosomal circSHKBP1 promotes
gastric cancer progression via regulating the miR-582-3p/HUR/VEGF
axis and suppressing HSP90 degradation. Mol Cancer. 19:1122020.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Gomes FG, Sandim V, Almeida VH, Rondon
AMR, Succar BB, Hottz ED, Leal AC, Verçoza BRF, Rodrigues JCF,
Bozza PT, et al: Breast-cancer extracellular vesicles induce
platelet activation and aggregation by tissue factor-independent
and -dependent mechanisms. Thromb Res. 159:24–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Zhang X, Zhang H, Gu J, Zhang J, Shi H,
Qian H, Wang D, Xu W, Pan J and Santos HA: Engineered extracellular
vesicles for cancer therapy. Adv Mater. 33:e20057092021. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Peterson MF, Otoc N, Sethi JK, Gupta A and
Antes TJ: Integrated systems for exosome investigation. Methods.
87:31–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Contreras-Naranjo JC, Wu HJ and Ugaz VM:
Microfluidics for exosome isolation and analysis: Enabling liquid
biopsy for personalized medicine. Lab Chip. 17:3558–3577. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Casadei L, Choudhury A, Sarchet P, Mohana
Sundaram P, Lopez G, Braggio D, Balakirsky G, Pollock R and Prakash
S: Cross-flow microfiltration for isolation selective capture and
release of liposarcoma extracellular vesicles. J Extracell
Vesicles. 10:e120622021. View Article : Google Scholar
|
|
233
|
Huang X, Wu W, Jing D, Yang L, Guo H, Wang
L, Zhang W, Pu F and Shao Z: Engineered exosome as targeted lncRNA
MEG3 delivery vehicles for osteosarcoma therapy. J Control Release.
343:107–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Lu Y, Li L, Lin Z, Li M, Hu X, Zhang Y,
Peng M, Xia H and Han G: Enhancing osteosarcoma killing and CT
imaging using ultrahigh drug loading and NIR-responsive bismuth
Sulfide@ Mesoporous silica nanoparticles. Adv Healthc Mater.
7:e18006022018. View Article : Google Scholar
|
|
235
|
Raghav KP, Wang W, Liu S, Chavez-MacGregor
M, Meng X, Hortobagyi GN, Mills GB, Meric-Bernstam F, Blumenschein
GR and Gonzalez-Angulo AM: cMET and Phospho-cMET protein levels in
breast cancers and survival outcomes. Clin Cancer Res.
18:2269–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Li S, Wu Y, Ding F, Yang J, Li J, Gao X,
Zhang C and Feng J: Engineering macrophage-derived exosomes for
targeted chemotherapy of triple-negative breast cancer. Nanoscale.
12:10854–10862. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Gonçalves MS: Fluorescent labeling of
biomolecules with organic probes. Clin Cancer Res. 109:190–212.
2009.
|
|
238
|
Gray WD, Mitchell AJ and Searles CD: An
accurate, precise method for general labeling of extracellular
vesicles. MethodsX. 2:360–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Takahashi Y, Nishikawa M, Shinotsuka H,
Matsui Y, Ohara S, Imai T and Takakura Y: Visualization and in vivo
tracking of the exosomes of murine melanoma B16-BL6 cells in mice
after intravenous injection. J Biotechnol. 165:77–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Lai CP, Mardini O, Ericsson M, Prabhakar
S, Maguire C, Chen JW, Tannous BA and Breakefield XO: Dynamic
biodistribution of extracellular vesicles in vivo using a
multimodal imaging reporter. ACS Nano. 8:483–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Bose RJC, Uday Kumar S, Zeng Y, Afjei R,
Robinson E, Lau K, Bermudez A, Habte F, Pitteri SJ, Sinclair R, et
al: Tumor cell-derived extracellular vesicle-coated nanocarriers:
An efficient theranostic platform for the cancer-specific delivery
of anti-miR-21 and imaging agents. ACS Nano. 12:10817–10832. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang
D, Dong H and Zhang X: Engineered exosome-mediated near-infrared-II
region V(2)C quantum dot delivery for nucleus-target
low-temperature photothermal therapy. ACS Nano. 13:1499–1510.
2019.PubMed/NCBI
|
|
243
|
Anguela XM and High KA: Entering the
modern era of gene therapy. Annu Rev Med. 70:273–288. 2019.
View Article : Google Scholar
|
|
244
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar :
|
|
245
|
Paunovska K, Loughrey D and Dahlman JE:
Drug delivery systems for RNA therapeutics. Natu Rev Genet.
23:265–280. 2022. View Article : Google Scholar
|
|
246
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Winkle M, El-Daly SM, Fabbri M and Calin
GA: Noncoding RNA therapeutics-challenges and potential solutions.
Nat Rev Drug Discov. 20:629–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Bose RJ, Kumar US, Garcia-Marques F, Zeng
Y, Habte F, McCarthy JR, Pitteri S, Massoud TF and Paulmurugan R:
Engineered cell-derived vesicles displaying targeting peptide and
functionalized with nanocarriers for therapeutic microRNA delivery
to triple-negative breast cancer in mice. Adv Healthc Mater.
11:e21013872022. View Article : Google Scholar :
|
|
249
|
Olejarz W, Kubiak-Tomaszewska G,
Chrzanowska A and Lorenc T: Exosomes in Angiogenesis and
Anti-angiogenic therapy in cancers. Int J Mol Sci. 21:58402020.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Ghafouri-Fard S, Shoorei H, Mohaqiq M and
Taheri M: Non-coding RNAs regulate angiogenic processes. Vascular
Pharmacol. 133-134:1067782020. View Article : Google Scholar
|
|
251
|
Yuan Y, Mei Z, Qu Z, Li G, Yu S, Liu Y,
Liu K, Shen Z, Pu J, Wang Y, et al: Exosomes secreted from
cardiomyocytes suppress the sensitivity of tumor ferroptosis in
ischemic heart failure. Signal Transduct Target Ther. 8:1212023.
View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Caller T, Rotem I, Shaihov-Teper O,
Lendengolts D, Schary Y, Shai R, Glick-Saar E, Dominissini D,
Motiei M, Katzir I, et al: Small extracellular vesicles from
infarcted and failing heart accelerate tumor growth. Circulation.
149:1729–1748. 2024. View Article : Google Scholar : PubMed/NCBI
|