Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors of the digestive system in China, with new cases
(517,100) and deaths (240,000) rank among the top five of all types
of cancer and show an increasing trend in 2022 (1,2).
Revealing the molecular mechanisms underlying CRC development is
important for increasing the effectiveness of CRC treatment and
improving the prognosis.
Long non-coding RNAs (lncRNAs) are RNA transcripts
that >200 nucleotides in length that have notable spatiotemporal
and temporal expression specificity and are widely involved in gene
regulation (3). Previous studies
have shown that lncRNAs regulate CRC progression. For example, the
lncRNA PVT1 promotes CRC proliferation and metastasis via the
microRNA (miR)-24-3p/neuropilin 1 axis (4) and LINC00955 acts as a molecular
scaffold for tripartite motif containing 25 and Sp1 transcription
factor, leading to G0/G1 cell cycle arrest in CRC cells (5). In addition, lncRNAs constitute a
potential class of biomarkers for CRC screening, diagnosis and
prognosis. Glycolysis- and necrosis-associated lncRNAs are used for
clinical prognostic evaluation (6,7).
lncRNAs play important roles in CRC progression.
lncRNAs have many functions in cancer. lncRNAs may
play contradictory roles by activating different signaling
pathways. For example, lncRNA H19 acts as an oncogene that
activates epithelial-mesenchymal transition (EMT) to promote
proliferation, migration and invasion of skin squamous cell
carcinoma cells (8). However,
lncRNA H19 can inhibit osteosarcoma by regulating the expression of
small nucleolar RNA SNORA7A (9).
High levels of lncRNA ABHD11-AS1 expression in pancreatic cancer
(PC) tissue are associated with distant metastasis, TNM stage and
tumor differentiation (10).
However, another study revealed that ABHD11-AS1 expression is
downregulated in patients with PC with positive lymphatic
metastasis (11). These findings
reflect the heterogeneity of lncRNAs.
The aim of the present study was to investigate the
role and mechanism of lncRNA ABHD11-AS1 in CRC to reveal how
ABHD11-AS1 regulates CRC cell proliferation, migration, and
invasion through interaction with EGFR, and to explore the
regulatory effect of resveratrol on ABHD11-AS1 transcription, with
the goal of evaluating its potential in CRC treatment.
Materials and methods
Human tissue samples
All the participants signed informed consent
(approval no. KYJJ-2022-240). The inclusion criteria were as
follows: i) Patients who were confirmed to have CRC by
post-operative pathological analysis; ii) medical records were
complete. The exclusion criterion were patients with two or more
concurrent primary malignancies. The study was approved by the
Ethical Review Committee of Hunan Cancer Hospital. A total of 66
surgical specimens (39 males and 27 females; median age, 66.5 years
and an age range of 27-82 years) and their adjacent non-tumor (≥5
cm distal to tumor margins) samples (n=39) from patients with CRC
at Hunan Cancer Hospital, Changsha, China, between June 2007 and
September 2011 were collected, fixed (10% neutral buffered formalin
and fix for 24 h at room temperature.) and paraffin-embedded.
Cell lines and culture
The human normal colon epithelial cell line NCM460
(cat. no. CL0393) and four colon cancer cell lines, HCT116 (cat.
no. CL0125), HT29 (cat. no. CL0163), SW480 (cat. no. CL0303) and
SW620 (cat. no. CL0305), were obtained from Hunan Fenghui
Biotechnology. All cells were identified by STR. The culture
conditions were as previously described (12). NCM460 and HCT116 cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (ZETA LIFE, Inc.). HT29, SW480 and SW620
cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS. ABHD11-AS1 overexpression plasmid
and three ABHD11-AS1 short hairpin (sh)RNAs (sh-ABHD11-AS1-1,
sh-ABHD11-AS1-2 and sh-ABHD11-AS1-3) were obtained from Shanghai
GeneChem Co., Ltd. (Table SI).
HCT116 and SW620 cells were transfected with 2 μg plasmid
(ABHD11-AS1: GV658 vector, pcDNA3.1-C
MV-3flag-EF1A-zsGreen-sv40-puromycin; the negative vector: GV658
vector, pcDNA3.1; shRNA: GV102 vector; the negative control
sequence was 5′-TTCTCCGAACGTGTCACGT-3′.) (Shanghai GeneChem Co.,
Ltd.) using Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 6-8 h before changing the
medium. The subsequent experiments were carried out 48 h after
transfection. The effects of overexpression and knockdown were
detected by quantitative (q)PCR.
CCK-8 assay
CCK-8 assay were performed as described previously
(13). Briefly, cell
proliferation was assessed using the Cell Counting Kit (ZETA LIFE,
Inc.; cat. no. K009-500) in accordance with the manufacturer's
protocol. Cells in the logarithmic growth phase) was seeded in
quintuplicate at a density of 5,000 cells per well onto 96-well
plates with 100 μl of medium per well and cultured in
humidified air with 5% CO2 at 37°C. Complete medium
containing 10% CCK-8 reagent was added at time points of 0, 24, 48,
and 72 h, respectively, and the OD value was detected at 450 nm
after continued incubation for 2 h. The proliferation curves were
plotted using Prism 8.0 software.
Colony formation assay
Colony formation assays were performed as described
previously (13). Briefly,
transfected cells (2,000 cells/dish) were seeded onto 6 cm dishes
and incubated in humidified air with 5% CO2 at 37°C for
2 weeks, then stained with 0.1% crystal violet. Images of visible
colonies were captured using a FUSION SOLO S (VILBER), and colonies
containing >50 cells were counted and quantified using ImageJ
software.
Drug treatment
The working concentrations of resveratrol (RSV)
(cat. no. SC0276-10 mM, Beyotime Biotechnology, Jiangsu, China)
were as we measured the IC50 values. In brief, after treatment with
RSV the cells were incubated in humidified air with 5%
CO2 at 37°C for 48 h and used for subsequent
experiments. For NSC228155 (cat. no. S8312, Selleck, Inc.), an EGFR
activator, cells were treated with 100 μM NSC228155 in
humidified air with 5% CO2 at 37°C for 12 h and then
used for subsequent experiments. For chrysophanol (cat. no. S2406,
Selleck, Inc.), an EGFR inhibitor, cells were treated with 10
μM chrysophanol in humidified air with 5% CO2 at
37°C for 48 h and then used for subsequent experiments.
Immunofluorescence
Cells seeded in crawls were fixed with 4%
paraformaldehyde for 15 min, followed by blocking with 5% BSA for 1
h, and then incubated with anti-EGFR antibody (1:50, A11577,
ABclonal) at 4°C overnight, after three washes with 0.5 M PBS,
cells were incubated with appropriate fluorescent secondary
antibodies for 1 h at 37°C. The nucleus was stained with 10
μM DAPI (cat. no. AI-50, ZETA LIFE, Inc.) for 10 min at room
temperature and cells were photographed under a confocal microscope
(Carl Zeiss AG, Germany).
Transwell migration and invasion and
wound healing assays
The pore size of the Transwell plates was 8
μm (cat. no. 3422, Costar, Inc.). A total of 200 μl
RPMI-1640 medium containing transfected cells (2×104
cells/well) was added to the upper chambers and 600 μl
RPMI-1640 medium containing 20% FBS was added to the lower
chambers. For the invasion assay, Matrigel was used to coat the
upper chambers for 30 min at 37°C (50 μl/well, cat. no.
356230, BD Pharmingen; BD Biosciences). After incubation for 48 h
in humidified air with 5% CO2 at 37°C, the invading
cells were fixed with 4% paraformaldehyde at room temperature for
20 min and removed from the upper chamber. Fixed cells were stained
with 0.1% crystal violet for 15 min at room temperature. Images of
the crystal violet-stained cells were captured and the cells were
counted using a light microscope.
For wound healing assay, HCT116 and SW620 cells were
inoculated into 6-well plates. When cells reached 90-95%
confluence, the monolayer was scraped with a sterile 10 μl
pipette tip and dislodged cells were removed with PBS. The cells
were cultured in serum-free medium at 37°C for 48 h, and images
were acquired using a Zeiss light microscope. The wound area at the
same location was subsequently measured by ImageJ (V1.54d, National
Institutes of Health). The cell migration rate was calculated as
follows: Cell migration rate (%)=(initial wound area-wound area
after 48 h)/initial wound area ×100%.
RNA extraction and reverse transcription
(RT)-qPCR
RNA extraction was performed as described previously
(13). Briefly, total cellular
RNA was extracted using TRIzol (cat. no. 15596-018; Invitrogen;
Thermo Fisher Scientific, Inc.). RT was performed using the
iScript™ cDNA Synthesis kit (cat. no. 1708891, Bio-Rad
Laboratories, Inc.) according to the manufacturer's instructions.
RT-qPCR assays were performed on a Roche Light Cycler®
96 instrument (LightCycler® 480 II, Roche, Inc.) using a
SYBR Green Pro Taq HS qPCR kit (cat. no. AG11701, Hunan
Aikerui Bioengineering Co., Ltd.). PCR was performed in triplicate.
Thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec followed by 40 cycles of 95°C (5 sec) and 60°C (30
sec). The data were analyzed by the 2−∆∆Cq method
(14). U6 was used as an internal
reference for the detection of ABHD11-AS1 RNA levels and nuclear
ABHD11-AS1. GAPDH was used as an internal reference for cytoplasmic
ABHD11-AS1. The primer sequences are listed in Table SII.
Western blotting
Total proteins were lysed using RIPA buffer
containing a protease inhibitor cocktail (Beyotime Biotechnology).
After quantifying the protein concentration using the BCA) method,
protein (30 μg/lane) were separated by SDS-PAGE on a 10% gel
and transferred to a PVDF membrane. After blocking with 5%
QuickBlock™ Blocking Buffer (cat. no. P0252, Beyotime Institute of
Biotechnology) at room temperature for 1 h, the membranes were
incubated overnight at 4°C with primary antibodies (all 1:1,000;
see Table SIII). The bound
antibodies were detected with horseradish peroxidase-conjugated
secondary antibodies (all 1:10,000; Table SIII) for 90 min of incubation at
room temperature, and visualized using Pierce™ ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc.). The levels of target
proteins were quantified by densitometric scanning using ImageJ
software (V1.54d, National Institutes of Health).
RIP
RIP assays were performed using the Magna RIP kit
(cat. no. 17-701, MilliporeSigma). Briefly, HCT116 and SW620 cells
were lysed with complete RIP lysis buffer (containing inhibitor and
RNase), followed by centrifugation at 11,000 × g and 4°C for 10
min. A total of 10 μl mixture as input was removed, the
remaining lysate was mixed with the appropriate antibody
(anti-EGFR, 1:20, A11577, ABclonal, 5 ug) and prewashed A/G
magnetic beads, and the mixture was incubated overnight at 4°C. The
precipitate was washed with RIP wash buffer, resuspended in 150
μl proteinase K buffer and incubated at 55°C for 30 min. The
products were used for RNA purification by RT-qPCR.
In situ hybridization (ISH)
ISH was performed as previously described (15). ABHD11-AS1 transcript levels were
assessed in CRC tissues using an ABHD11-AS1-specific probe
(5′-DIG-AAGUCUUGUCUGGAAGAGGUGUCUCACUC-3′, Sangon Biotech Co.,
Ltd.). The samples were scored based on staining intensity and the
number of positive cells, as described previously (15).
Isolation of cytoplasmic and nuclear
fractions
Cytoplasmic and nuclear lysate from HCT116, HT29,
SW480 and SW620 cells was separated using a PARIS kit (cat. no.
AM1921, Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. RNA was extracted from cytoplasmic
and nuclear lysates and analyzed by RT-qPCR. The nuclear control
transcript was U6; cytoplasmic control transcript was GAPDH.
RNA pull-down assay
RNA pull-down was conducted using Ribo™ RNAmax-T7
Biotin Labeling Transcription kit (cat. no. C11002-1, Guangzhou
RiboBio Co., Ltd.). Briefly, PCR primers for the T7 promoter were
designed and amplified by PCR (cat. no. AG12210, ApexHF HS DNA
Polymerase Premix-CL, Hunan ACCURATE BIOLOGY Co., Ltd.) to obtain
DNA templates (sense forward, 5′-TAATACGACTCACTATAGGGCTAGC-3′ and
reverse, 5′-CCCCGAGTACCCTTGGC-3′; antisense forward,
5′-TAATACGACTCACTATAGGGCTAGC-3′ and reverse,
5′-CCCTAAGTCCCAGCCCTTGA-3′). Cells were lysed with 200 μl
lysis buffer for 30 min on ice, followed by centrifugation at
11,000 g for 15 min at 4°C. Then, 40 μl streptavidin
magnetic beads and 10 μl T7 biotin-labeled RNA probe were
mixed at 4°C for 6-8 h to mix the RNA and magnetic beads well. The
prepared protein solution lysate was then added to the above RNA
magnetic beads and incubated overnight at 4°C with gentle vortexing
to fully bind the RNA to the target protein. PCR reaction was
subjected to an initial denaturation at 94°C for 1 min, followed by
35 cycles of denaturation at 98°C for 10 sec, and extension at 68°C
for 30 sec. Purified RNA products were obtained by in vitro
transcription. ABHD11-AS1 intercalating proteins were captured
using 40 μl M-280 streptavidin magnetic beads (cat. no.
11205D, Invitrogen; Thermo Fisher Scientific, Inc.) and target
proteins were subsequently identified by western blotting
(anti-EGFR, 1:1,000, A11577, ABclonal; anti-GAPDH, 1:1,000,
10494-1-AP, Proteintech Group, Inc.).
Bioinformatics analysis
Gene Expression Profiling Interactive Analysis
(GEPIA2) (http://gepia2.cancer-pku.cn/#index) database was used
to predict the expression of ABHD11-AS1 in colon adenocarcinoma
(COAD) and rectal adenocarcinoma (READ) (16). The Cancer Genome Atlas (TCGA;
tcga-xena-hub.s3.us-east-1.amazonaws.com/download/survival%2FCOAD_survival.txt)
database was used to obtain prognostic information for patients
with ABHD11-AS1 and CRC. RPISeq (pridb.gdcb.iastate.edu/RPISeq/)
database was used to predict the RNA-binding proteins of ABHD11-AS1
(17).
Statistical analysis
For paired clinical data, matched samples t-test was
used. For unpaired data, unpaired Student's t-test was performed.
One-way ANOVA followed by Tukey's post hoc test or two-way ANOVA
and Sidak's multiple comparison test were used for comparisons
between multiple groups. Survival analysis was performed by
Kaplan-Meier curves, and the log-rank test was used to determine
significance. All the experiments were repeated ≥3 times. All data
are expressed as the mean ± SD and analyzed with GraphPad Prism 8.0
(Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
Downregulation of ABHD11-AS1 transcripts
in CRC indicates poor prognosis
To investigate ABHD11-AS1 expression in CRC, GEPIA
database was used. ABHD11-AS1 expression was downregulated in COAD
tissue (Fig. 1A). ABHD11-AS1 was
not significantly downregulated in READ tissues. Compared with
those in NCM460 cells, ABHD11-AS1 transcript levels were
significantly decreased in CRC cell lines, with those in HCT116
cells decreasing the most and those in SW620 cells decreasing the
least (Fig. 1B). Therefore, these
cell lines were used for subsequent experiments. Nuclear and
cytoplasmic samples were isolated from CRC cell lines; ABHD11-AS1
was localized mainly in the cytoplasm (Fig. S1A-D). ISH of CRC samples revealed
that ABHD11-AS1 transcript levels were significantly higher in
cancerous (n=66) than in paracancerous tissues (n=39; Fig. 1C-E). By analyzing the
clinicopathological characteristics of 66 patients, low ABHD11-AS1
expression was demonstrated to be associated with tumor progression
and distant metastasis (Table I).
Low ABHD11-AS1 transcript levels were associated with shorter
overall survival in patients with CRC in TCGA cohort (Fig. 1F). Together, these findings
indicated that downregulated ABHD11-AS1 transcripts are related to
poor prognosis in CRC.
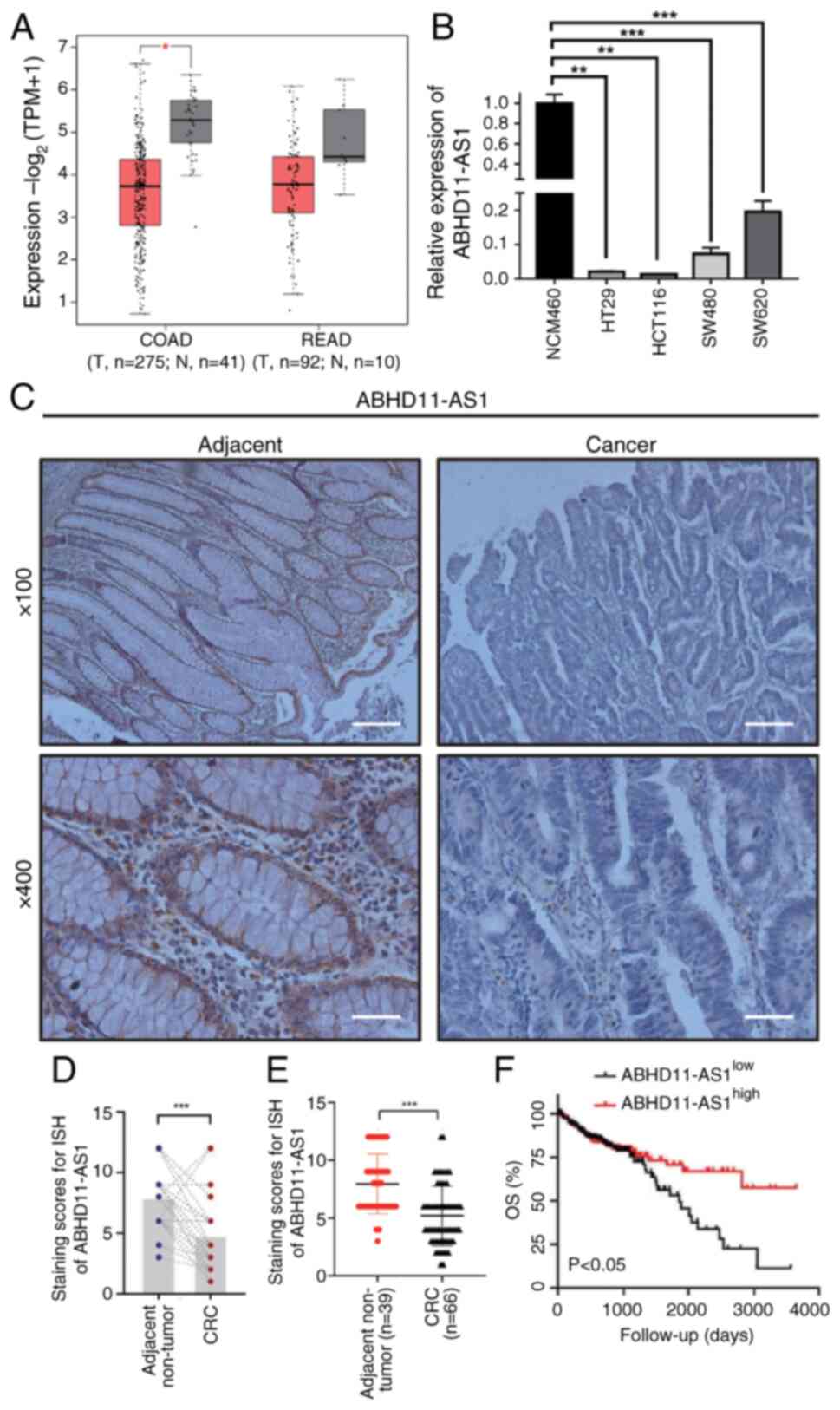 | Figure 1Downregulation of ABHD11-AS1
transcripts is associated with poor prognosis in patients with CRC.
(A) ABHD11-AS1 transcript levels in patients with COAD and READ
retrieved from the Gene Expression Profiling Interactive Analysis
database. (B) Relative levels of ABHD11-AS1 transcripts were
determined by reverse transcription-quantitative PCR (n=3). (C)
Representative ISH of paraneoplastic and cancerous tissue. ISH
staining scores for ABHD11-AS1 in in (D) 39 paired CRC tissues and
adjacent paracancerous tissues. (E) ISH staining scores for
ABHD11-AS1 in 66 CRC tissues and 39 adjacent paracancerous tissues.
(F) Low levels of ABHD11-AS1 transcripts are associated with worse
overall survival in patients with CRC in TCGA cohort.
Magnification, ×100, scale bar=100 μm; magnification, ×400,
scale bar, 20 μm. *P<0.05,
**P<0.01, ***P<0.001. CRC, colorectal
cancer; COAD, colon adenocarcinoma; READ, rectal adenocarcinoma;
ISH, in situ hybridization; T, tumor; N, normal; OS, overall
survival; TCGA, The Cancer Genome Atlas; TPM, transcripts per
million. |
 | Table IAssociation between clinical
parameters with ABHD11-AS1 in patients with colorectal cancer. |
Table I
Association between clinical
parameters with ABHD11-AS1 in patients with colorectal cancer.
| Variable | n | High
ABHD11-AS1 | Low ABHD11-AS1 | P-value |
|---|
| Age, years | | | | |
| <60 | 40 | 14 | 26 | 0.939 |
| ≥60 | 26 | 8 | 18 | |
| Sex | | | | |
| Male | 39 | 12 | 27 | 0.913 |
| Female | 27 | 7 | 20 | |
| Histology | | | | |
|
Well-differentiated | 16 | 3 | 13 | 0.855 |
| Moderately
differentiated | 35 | 12 | 23 | |
| Poorly
differentiated | 15 | 5 | 10 | |
| TNM stage | | | | |
| I | 17 | 6 | 11 | 0.035 |
| II | 25 | 9 | 16 | |
| III | 13 | 5 | 8 | |
| IV | 11 | 4 | 7 | |
| Metastasis | | | | |
| Yes | 43 | 9 | 34 | 0.015 |
| No | 23 | 10 | 13 | |
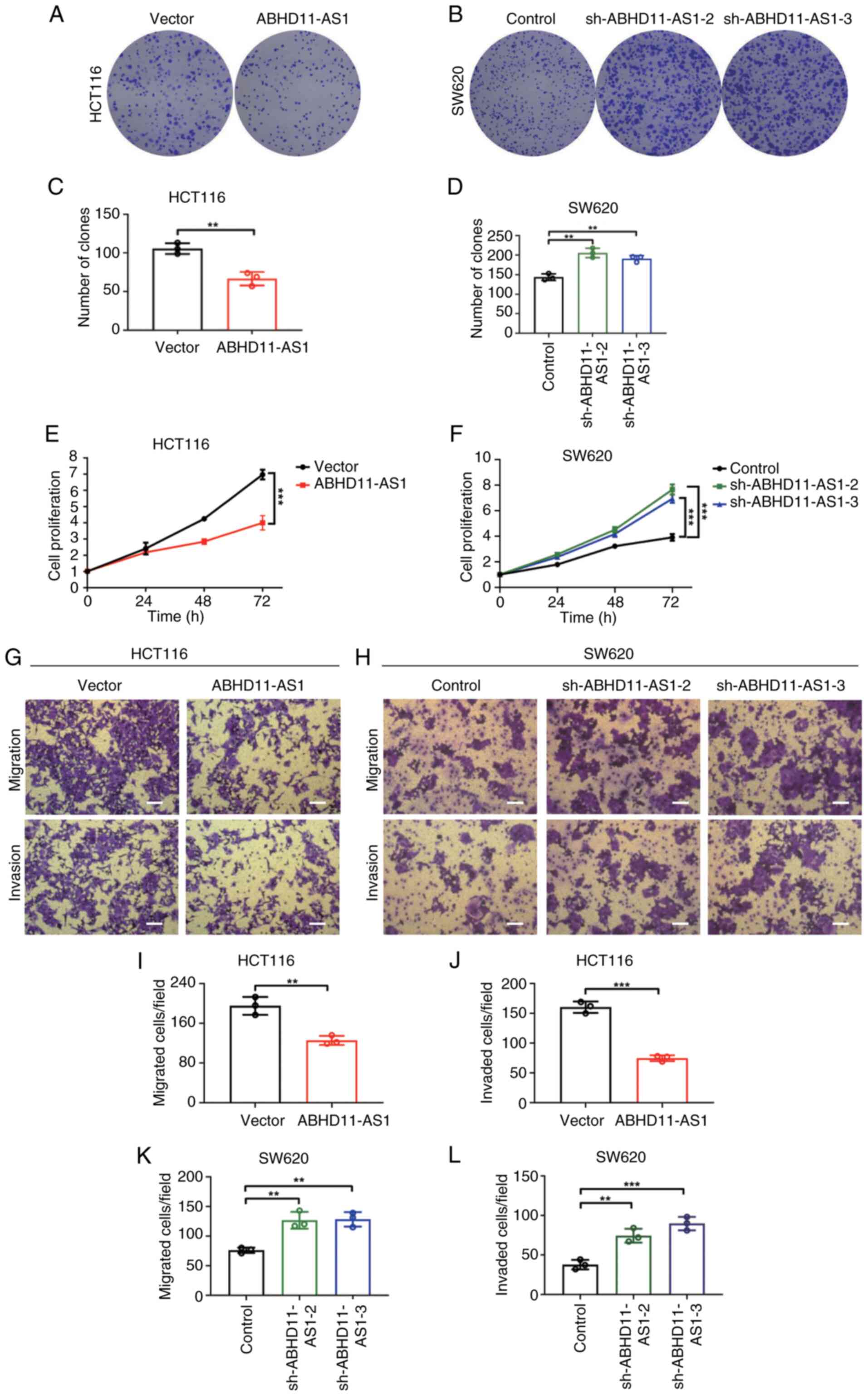
ABHD11-AS1 inhibits malignant behaviors
of CRC cells in vitro
Given the dysregulated expression of ABHD11-AS1 in
CRC, HCT116 and SW620 cells were transfected with plasmids carrying
ABHD11-AS1 or ABHD11-AS1 shRNA to test the biological function of
ABHD11-AS1. ABHD11-AS1 was successfully overexpressed in HCT116
cells, while transfection with sh-ABHD11-AS1-1, sh-ABHD11-AS1-2 or
sh-ABHD11-AS1-3 significantly decreased ABHD11-AS1 expression in
SW620 cells by ~99% (Fig. S1E and
F). Colony formation and CCK-8 assays revealed that ABHD11-AS1
overexpression inhibited the proliferation of HCT116 cells and
ABHD11-AS1 knockdown promoted proliferation of SW620 cells
(Fig. 2A-F). Transwell assay
revealed that ABHD11-AS1 overexpression significantly inhibited
migration and invasion of HCT116 cells, whereas ABHD11-AS1
knockdown significantly promoted migration and invasion capacities
of SW620 cells (Fig. 2G-L).
Overall, these results indicated ABHD11-AS1 served as a tumor
suppressor gene to inhibit proliferation and migration and invasion
of CRC cells in vitro. Furthermore, wound healing assay
revealed that ABHD11-AS1 overexpression inhibited the wound healing
process in HCT116 cells. Conversely, ABHD11-AS1 knockdown promoted
the wound healing process in SW620 cells (Fig. S2A-D).
To elucidate the function of ABHD11-AS1 in CRC,
HCT116 and SW620 cells were transfected with a plasmid encoding
ABHD11-AS1 shRNA and expressing ABHD11-AS1. RT-qPCR revealed that
compared with control + vector, ABHD11-AS1 expression was
effectively downregulated in sh-ABHD11-AS1-2 and ABHD11-AS1-3
groups. ABHD11-AS1 expression was successfully restored in the
sh-ABHD11-AS1-2 and sh-ABHD11-AS1-3 + ABHD11-AS1 groups (Fig. S3A and B). Transwell assay
revealed that ABHD11-AS1 knockdown promoted migration and invasion
of HCT116 and SW620 cells. Notably, this promotion of migration and
invasion was inhibited when ABHD11-AS1 expression returned to
normal levels (Fig. S3C-H).
Wound healing assay revealed that ABHD11-AS1 knockdown promoted
migration of HCT116 and SW620 cells. Notably, this was inhibited
when ABHD11-AS1 expression returned to normal levels (Fig. S2E-H). CCK-8 assay revealed that
ABHD11-AS1 knockdown promoted the proliferation of HCT116 and SW620
cells. Notably, this promotion of proliferation was inhibited when
ABHD11-AS1 expression returned to normal levels (Fig. S3I and J).
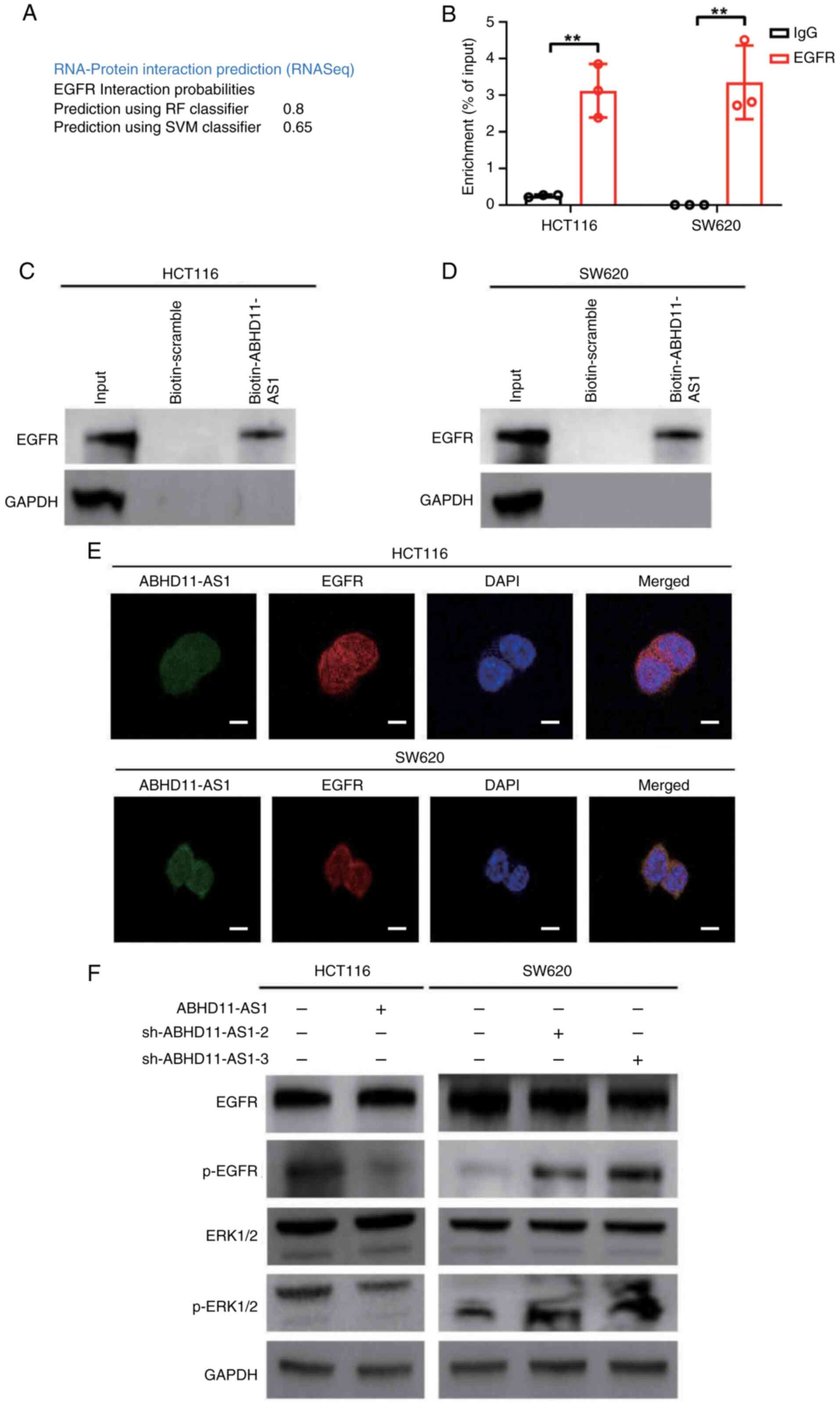
ABHD11-AS1 interacts with EGFR to
attenuate EGFR and ERK signaling in CRC cells
To understand the mechanism underlying the role of
ABHD11-AS1 in the malignant biological behavior of CRC, RPISeq
database was used to predict the RNA-binding proteins of
ABHD11-AS1. ABHD11-AS1 bound to EGFR (random forest classifier,
0.8; support vector machine classifier, 0.65; Fig. 3A). RNA pull-down and RIP assay
confirmed the interactions between ABHD11-AS1 and EGFR (Fig. 3B-D). Furthermore,
immunofluorescence revealed colocalization of ABHD11-AS1 and EGFR
in the cytoplasm (Fig. 3E). These
results indicated that ABHD11-AS1 interacted with EGFR.
To investigate the molecular mechanism, ABHD11-AS1
was overexpressed or knocked down in HCT116 and SW620 cells prior
to detecting proteins associated with the EGFR/ERK signaling
pathway as this pathway is key for tumor progression (18). Western blot analysis revealed that
transfection with ABHD11-AS1 plasmid decreased phosphorylation of
EGFR and ERK1/2 (Figs. 3F and
S4A and B). Conversely,
sh-ABHD11-AS1 plasmid increased the phosphorylation of EGFR and
ERK1/2 (Figs. 3F and S4C and D). These results indicated that
interaction between ABHD11-AS1 and EGFR resulted in attenuation of
ERFR/ERK signaling.
EGFR activation abrogates
ABHD11-AS1-mediated attenuation of proliferative and migratory
behaviors in CRC cells
To confirm that ABHD11-AS1 inhibited proliferative
and migratory behaviors of CRC cells via EGFR, CRC cells with
stably altered ABHD11-AS1 expression were treated with the EGFR
agonist NSC228155 or inhibitor chrysophanol. Compared with control,
NSC228155 increased EGFR and ERK1/2 phosphorylation but had no
effect on total EGFR or ERK1/2 protein expression (Figs. 4A and S4E and F). NSC228155 almost completely
restored ABHD11-AS1-attenuated EGFR and ERK1/2 phosphorylation
(Figs. 4A and S4E and F). Similarly, chrysophanol
attenuated EGFR and ERK1/2 phosphorylation but not total protein
expression (Figs. 4B and S4G and H). Chrysophanol almost
completely abrogated sh-ABHD11-AS1-induced increases in EGFR and
ERK1/2 phosphorylation (Figs. 4B
and S4G and H).
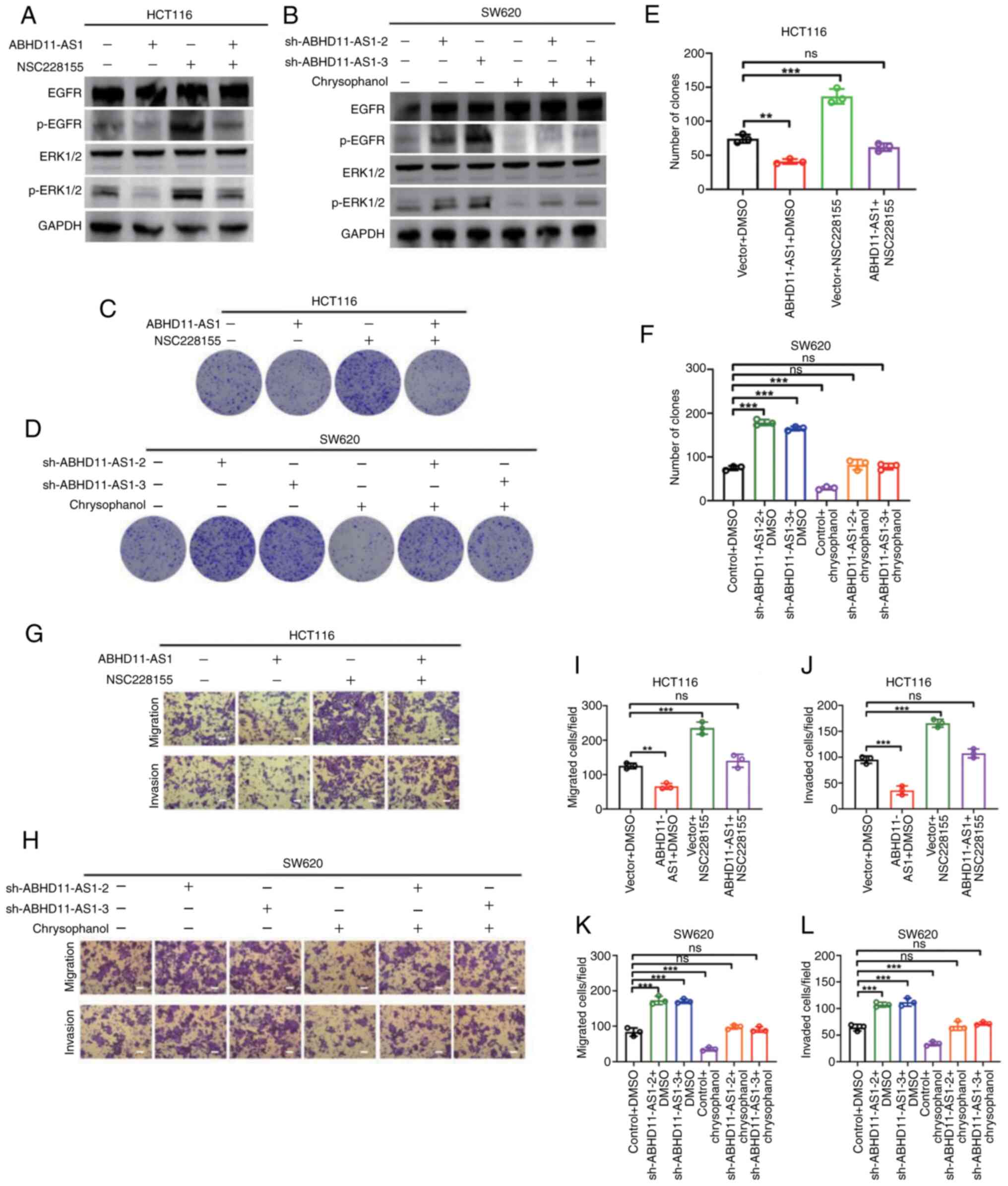 | Figure 4Induction of EGFR overexpression
abrogates ABHD11-AS1-mediated attenuation of proliferative and
migratory behaviors in CRC cells. ABHD11-AS1 was overexpressed or
knocked down in HCT116 and SW620 cells, respectively, and NSC228155
and chrysophanol were administered. (A) Western blot analysis of
EGFR, ERK1/2, and EGFR and ERK1/2 phosphorylation in (A) HCT116
cells. (B) Western blot analysis of EGFR, ERK1/2, and EGFR and (B)
SW620 cells. (C) Effects of NSC228155 on colony formation
capacities in HCT116 cells. (D) Effects of chrysophanol on colony
formation capacities in SW620 cells. (E) Statistical graphs of
colony formation assays in HCT116 cells. (F) Statistical graphs of
colony formation assays in SW620 cells. (G) Effects of NSC228155 on
migration and invasion capacities of HCT116 cells. (H) Effects of
chrysophanol on migration and invasion capacities of SW620 cells.
(I) Statistical graphs of Transwell migration assays in HCT116
cells. (J) Statistical graphs of Transwell invasion assays in
HCT116 cells. (K) Migration assays in SW620 cells. (L) Statistical
graphs of Transwell invasion assays in SW620 cells. Scale bar, 50
μm. **P<0.01, ***P<0.001. CRC,
colorectal cancer; p-, phosphorylated; ns, not significant; sh,
short hairpin. |
It was hypothesized that ABHD11-AS1 inhibits CRC
cell proliferation and invasion by impairing EGFR phosphorylation
and EGFR signaling. Compared with control, NSC228155 promoted the
proliferation, migration and invasion of HCT116 cells in
vitro. NSC228155 almost completely reversed ABHD11-AS1-mediated
inhibition of proliferation, migration and invasion (Figs. 4C, E, G, I and J and S5A). Chrysophanol inhibited
proliferation, migration and invasion of SW620 cells in
vitro (Figs. 4D, F, H, K and
L and S4B). Furthermore,
chrysophanol inhibited proliferation, migration and invasion of
SW620 cells induced by ABHD11-AS1 knockdown. These results
indicated that the ABHD11-AS1/EGFR signaling axis plays a role in
regulating malignant behavior of CRC cells.
Resveratrol enhances ABHD11-AS1
transcript levels to suppress malignant behavior of CRC cells by
downregulating SPT6
The aforementioned results indicated ABHD11-AS1 had
a tumor-suppressive effect on CRC. To explore therapeutic
approaches for CRC that target ABHD11-AS1, resveratrol was
investigated as a potential regulator of ABHD11-AS1 (19,20). RSV can exert antitumor effects by
altering lncRNA transcript levels (21). To investigate the potential
association between RSV and ABHD11-AS1 transcript levels, HCT116
and SW620 cells were treated with RSV.
As RSV has varying degrees of cytotoxicity (22), half-maximal inhibitory
concentration (IC50) values of RSV in HCT116 and SW480 cells were
calculated. IC50 values of RSV were 57.16 in HCT116 and 65.12
μM in SW480 cells (Fig. 5A and
B). CRC cell viability was inhibited by RSV in vitro,
with a mean IC50 value of 60 μM.
RSV significantly increased transcript levels of
ABHD11-AS1 (Fig. 5C and D).
Furthermore, RSV did not affect the subcellular localization of
ABHD11-AS1 (Fig. S1G and H). To
assess whether altered ABHD11-AS1 transcript levels influenced
effects of RSV, RSV-treated HCT116 and SW480 cells were transfected
with plasmids carrying ABHD11-AS1 or ABHD11-AS1 shRNA. Compared
with control, RSV significantly inhibited proliferation, colony
formation, wound healing, migration and invasion of HCT116 and
SW620 cells (Fig. 5E, G-I, K, L and
O). ABHD11-AS1 overexpression further enhanced the inhibitory
effects of RSV on HCT116 cell proliferation, colony formation,
wound healing, migration and invasion. By contrast, ABHD11-AS1
knockdown abolished the inhibitory effects of RSV on SW480 cell
proliferation, colony formation, wound healing, migration and
invasion (Fig. 5F, H, J, M, N and
P). These results indicated that RSV inhibited proliferation,
migration and invasion of HCT116 and SW480 cells by increasing
ABHD11-AS1 transcript levels.
These data suggested that RSV increased ABHD11-AS1
transcript levels. A decrease in SPT6 expression results in
conversion of the H3K36me3 histone mark from protein-coding to
lncRNA-coding, which is associated with increased lncRNA
transcription (23,24). Western blotting results suggested
that RSV could reduce SPT6 protein expression (Fig. 5Q). These results indicated that
RSV may exert antitumor effects by inducing SPT6 protein
deletion.
Discussion
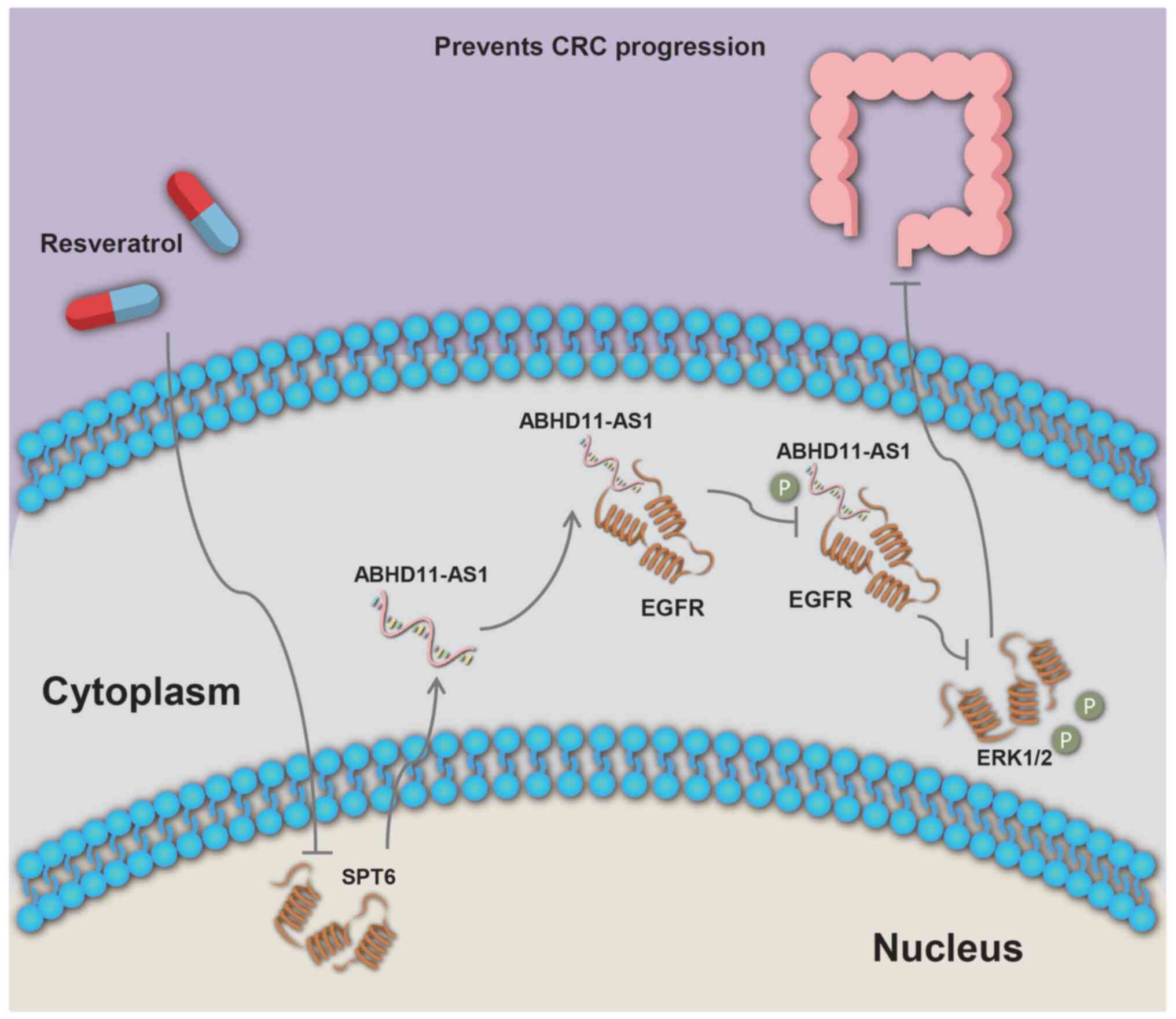
CRC is one of the three most common types of cancer
in the world (2). A total of ~60%
of patients with CRC have insidious presentation and the 5-year
survival rate is 30-40% (25);
thus, there is need to develop novel therapeutic targets to promote
early diagnosis and precision treatment of CRC (Fig. 6).
lncRNAs serve roles in regulating expression of
genes that are involved in tumor progression (26). Recent studies have shown that
CCAT1, H19 and SNHG6, which are notably overexpressed in peripheral
blood or cancer tissue of patients with CRC, are potential
biomarkers for diagnosis and prognostic assessment of CRC (27-29). In addition, autophagy-,
pyroptosis- and EMT-associated lncRNAs may serve as biomarkers for
diagnosis and prognosis of CRC (30-32).
Dysregulated expression of lncRNAs contributes to
tumor progression. lncRNAs serve opposite roles in different types
of cancer. For example, H19 is highly expressed in skin squamous
cell carcinoma tissue and cell lines and promotes cancer cell
migration and invasion by inhibiting p53 expression (33). A study on osteosarcoma revealed
that lncRNA H19 inhibits SNORA7A expression to suppress
osteosarcoma progression (9).
lncRNA TINCR has been widely reported to be an oncogene that
promotes the progression of numerous types of tumors (including
cervical and colon cancer and hepatocellular carcinomas) (34-36). However, TINCR can act as a tumor
suppressor gene and inhibit the proliferation and metastasis of
laryngeal squamous cell carcinoma cells by regulating the
miR-210/anti-proliferation factor 2 axis (37). PVT1 was the first reported
tumor-associated lncRNA with pro-oncogenic functions in various
types of tumor (38,39). Promoter of PVT1 functions as a
tumor suppressor DNA element because MYC is located on the same TAD
structure as PVT1 (40). SNHG1 is
associated with growth, metastasis, ferroptosis and chemoresistance
in various types of tumor (41,42). There are various perspectives on
the role of SNHG1 in gastric cancer. One study reported that SNHG1
serves as a sponge for miR-195-5p, targeting Yes-associated protein
1 to promote the proliferation of gastric cancer cells (43). Another study demonstrated that
SNHG1 inhibits invasion of gastric cancer cells by regulating the
suppressor of cytokine signaling 2/JAK2/STAT pathway (44). Here, ABHD11-AS1 expression was
downregulated in TCGA COAD and READ datasets compared with normal
dataset. The present experiments confirmed that ABHD11-AS1 has an
antitumor function. Although other studies (45,46) have demonstrated that ABHD11-AS1
expression is elevated in TCGA COAD and READ data relative to TCGA
and Genotype-Tissue Expression (GTEx) normal data, this may be
attributed to differences in the cited sources, as GTEx encompasses
54 distinct tissue organ sites (such as brain and whole blood).
ABHD11-AS1 interacts with and upregulates cyclin D1
in endometrial cancer (47). The
ABHD11-AS1/miR-1231/cyclin E1 axis may regulate the proliferation
of pancreatic cancer cells (48).
Notably, knockdown of ABHD11-AS1 and EGFR inhibits proliferation of
cervical cancer SiHa and Hela cells by downregulating cyclin D1
(49). To the best of our
knowledge, there is a lack of evidence indicating a direct impact
of ABHD11-AS1 on expression of cyclin superfamily proteins in CRC
cells and associations between ABHD11-AS1 and cyclin-dependent
kinase, cyclin-dependent kinase inhibitor (CKI) and
cyclin-dependent kinase activating kinase (CAK) superfamily
proteins; however, it is hypothesized that ABHD11-AS1 may influence
the cell cycle in CRC (50).
RSV is a polyphenolic antioxidant with a wide range
of functions, including anti-inflammatory and antioxidant effects
(51). It also inhibits tumor
growth and prevents tumorigenesis. RSV inhibits the proliferation
and invasion of colon cancer cells and promotes apoptosis by
modulating EMT, activating the BMP/Smad signaling and reactive
oxygen species-mediated mitochondrial apoptosis pathway (20,52). Notably, RSV may exert antitumor
effects by altering lncRNA expression. For example, RSV modulates
the expression of the lncRNA ZFAF1, thereby enhancing the efficacy
of paclitaxel in non-small cell lung cancer (53). The antitumor effect of RSV in CRC
was first reported to involve the inhibition of cancer cell
invasion and metastasis via downregulation of lncRNA MALAT1
(54). These findings suggest
that RSV may have potential as a therapeutic agent for CRC.
Combination of RSV and BIBR1532 (a telomerase inhibitor) exerts an
antiproliferative effect by downregulating expression of lncRNAs,
including CCAT1, CRNDE, HOTAIR, PCAT1, PVT1 and SNHG16 (55). Here, RSV enhanced ABHD11-AS1
transcript levels to suppress the malignant behavior of CRC cells,
which may be mediated by SPT6 deletion.
SPT6 is a conserved histone chaperone and
transcription elongation factor that serves a key role in the
transcription elongation process (56). Recent studies have also
demonstrated its impact on lncRNA transcription. The
phosphorylation of RNA polymerase II, coupled with loss of SPT6
expression, may promote transcription of lncRNAs (23,57,58). Decreased SPT6 expression leads to
the conversion of the H3K36me3 histone mark from protein- to
lncRNA-coding, potentially increasing lncRNA transcription
(23). In RSV-treated HCT116 and
SW620 cells, SPT6 was downregulated. Silencing SPT6 has been shown
to inhibit the growth and metastasis of human-derived colon cancer
cell xenograft tumors (59).
These results suggest that RSV may target ABHD11-AS1 via SPT6 to
exert an antitumor effect.
To investigate potential downstream molecules of
ABHD11-AS1, the present study conducted bioinformatics analysis and
identified EGFR as one of its interacting proteins. Previous
studies have shown that ABHD11-AS1 regulates expression and
phosphorylation of EGFR (49,60,61). Furthermore, RNA pull-down and RIP
assays revealed formation of lncRNA-protein complexes between
ABHD11-AS1 and EGFR. Induction of EGFR overexpression impaired the
tumor-suppressive effect of ABHD11-AS1 in CRC cells. EGFR is a key
target for the clinical treatment of CRC, as it is generally highly
expressed in patients with CRC (62). The present results indicated that
downregulation of ABHD11-AS1 expression may be a causative factor
for EGFR phosphorylation upregulation in CRC.
In summary, ABHD11-AS1 inhibited proliferation and
invasion of CRC cells by binding EGFR proteins and suppressing EGFR
phosphorylation. In addition, RSV increased ABHD11-AS1 transcript
levels, which inhibited proliferation and invasion of CRC cells.
The present findings may provide novel biomarkers for CRC prognosis
and new targets for CRC treatment.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ST and SL confirm the authenticity of all the raw
data. ST and SL drafted the manuscript and constructed the figures.
LX, XJ, ZR, QP, MP, WY, XX, LO, MS, JW, HL and YT performed the
literature review. QL, JL and YZ designed the study and revised the
manuscript. All authors have read and approved the final
manuscript. ST conceived the study, conducted formal analysis and
performed experiments. SL conceptualized the study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hunan Cancer Hospital (approval no. KYJJ-2022-240).
All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant nos. 82302987, 82303534, 82203233,
82202966 and 82173142), Natural Science Foundation of Hunan
Province (grant nos. 2023JJ60469, 2023JJ40413, 2023JJ30372,
2023JJ30375, 2022JJ80078 and 2020JJ5336), Key Research and
Development Program of Hunan Province (grant no. 2022SK2051),
Science and Technology Innovation Program of Hunan Province (grant
nos. 2023RC3199, 2023SK4034 and 2023RC1073), Research Project of
Health Commission of Hunan Province (grant nos. 202203034978,
202202055318, 202109031837, 202109032010 and 20201020), Changsha
Science and Technology Board (grant no. kh2201054), Ascend
Foundation of the National Cancer Center (grant no. NCC201909B06)
and Hunan Cancer Hospital Climb Plan (grant nos. ZX2020001-3,
YF2020002, 2023NSFC-A001, 2023NSFC-A002 and 2023NSFC-A004).
References
|
1
|
Hang D, Sun D, Du L, Huang J, Li J, Zhu C,
Wang L, He J, Zhu X, Zhu M, et al: Development and evaluation of a
risk prediction tool for risk-adapted screening of colorectal
cancer in China. Cancer Lett. 597:2170572024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu S, Jiao B, Zhao H, Liang X, Jin F, Liu
X and Hu JF: LncRNAs-circRNAs as rising epigenetic binary
superstars in regulating lipid metabolic reprogramming of cancers.
Adv Sci (Weinh). 11:e23035702024. View Article : Google Scholar
|
|
4
|
Yin H, Gu S, Li G, Yu H, Zhang X and Zuo
Y: Long noncoding RNA PVT1 predicts poor prognosis and promotes the
progression of colorectal cancer through the miR-24-3p/NRP1 axis in
zebrafish xenografts. Neoplasma. 70:500–513. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren G, Li H, Hong D, Hu F, Jin R, Wu S,
Sun W, Jin H, Zhao L, Zhang X, et al: LINC00955 suppresses
colorectal cancer growth by acting as a molecular scaffold of
TRIM25 and Sp1 to Inhibit DNMT3B-mediated methylation of the PHIP
promoter. BMC Cancer. 23:8982023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ZH, Lin YL, Chen SQ and Yang XY:
Identification of necroptosis-related lncRNAs for prognosis
prediction and screening of potential drugs in patients with
colorectal cancer. World J Gastrointest Oncol. 15:1951–1973. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao R, Xu C, Zhang Q, Wang Z, Liu Y, Peng
Y and Li M: Predictive significance of glycolysis-associated lncRNA
profiles in colorectal cancer progression. BMC Med Genomics.
17:1122024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Zhou K, Zhang X, Wu C, Deng D and
Yao Z: [Corrigendum] Roles of the H19/microRNA-675 axis in the
proliferation and epithelial-mesenchymal transition of human
cutaneous squamous cell carcinoma cells. Oncol Rep. 50:1492023.
View Article : Google Scholar
|
|
9
|
Xu A, Huang MF, Zhu D, Gingold JA, Bazer
DA, Chang B, Wang D, Lai CC, Lemischka IR, Zhao R and Lee DF:
LncRNA H19 suppresses osteosarcomagenesis by regulating snoRNAs and
DNA repair protein complexes. Front Genet. 11:6118232021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiao X, Lv SX, Qiao Y, Li QP, Ye B, Wang
CC and Miao L: Long noncoding RNA ABHD11-AS1 predicts the prognosis
of pancreatic cancer patients and serves as a promoter by
activating the PI3K-AKT pathway. Eur Rev Med Pharmacol Sci.
22:8630–8639. 2018.PubMed/NCBI
|
|
11
|
Zhou X, Zhong F, Yan Y, Wu S, Wang H, Liu
J, Li F, Cui D and Xu M: Pancreatic cancer cell-derived exosomes
promote lymphangiogenesis by downregulating ABHD11-AS1 expression.
Cancers (Basel). 14:46122022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia L, Lin J, Peng M, Jiang X, Peng Q, Cui
S, Zhang W, Li S, Wang J, Oyang L, et al: Diallyl disulfide induces
DNA damage and growth inhibition in colorectal cancer cells by
promoting POU2F1 ubiquitination. Int J Biol Sci. 20:1125–1141.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin J, Xia L, Oyang L, Liang J, Tan S, Wu
N, Yi P, Pan Q, Rao S, Han Y, et al: The POU2F1-ALDOA axis promotes
the proliferation and chemoresistance of colon cancer cells by
enhancing glycolysis and the pentose phosphate pathway activity.
Oncogene. 41:1024–1039. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Yang W, Tan S, Yang L, Chen X, Yang R,
Oyang L, Lin J, Xia L, Wu N, Han Y, et al: Exosomal miR-205-5p
enhances angiogenesis and nasopharyngeal carcinoma metastasis by
targeting desmocollin-2. Mol Ther Oncolytics. 24:612–623. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47(W1): W556–W560.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muppirala UK, Honavar VG and Dobbs D:
Predicting RNA-protein interactions using only sequence
information. BMC Bioinformatics. 12:4892011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Chu Y, Zhang H, Qu X, Wang B and
Han Y: FOXD3 suppresses the proliferation of CRC bone metastatic
cells via the Ras/Raf/MEK/ERK signaling pathway. Comb Chem High
Throughput Screen. 27:436–445. 2024. View Article : Google Scholar :
|
|
19
|
Liu Z, Zhang Z, Song G, Wang X, Xing H and
Wang C: Resveratrol alleviates skeletal muscle insulin resistance
by downregulating long noncoding RNA. Int J Endocrinol.
2022:25395192022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vernousfaderani EK, Akhtari N, Rezaei S,
Rezaee Y, Shiranirad S, Mashhadi M, Hashemi A, Khankandi HP and
Behzad S: Resveratrol and colorectal cancer: A molecular approach
to clinical researches. Curr Top Med Chem. 21:2634–2646. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J,
Zhang S, Peng B, Zhang Y and Jiang Y: A novel long noncoding RNA
AK001796 acts as an oncogene and is involved in cell growth
inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol.
285:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Djaldetti M: Immunomodulatory and
chemopreventive effects of resveratrol on the digestive system
cancers. Oncol Res. 32:1389–1399. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nojima T, Tellier M, Foxwell J, Ribeiro de
Almeida C, Tan-Wong SM, Dhir S, Dujardin G, Dhir A, Murphy S and
Proudfoot NJ: Deregulated expression of mammalian lncRNA through
loss of SPT6 induces R-loop formation, replication stress, and
cellular senescence. Mol Cell. 72:970–984.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Begum NA, Stanlie A, Nakata M, Akiyama H
and Honjo T: The histone chaperone Spt6 is required for
activation-induced cytidine deaminase target determination through
H3K4me3 regulation. J Biol Chem. 287:32415–32429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng H, Chen W, Zheng R, Zhang S, Ji JS,
Zou X, Xia C, Sun K, Yang Z, Li H, et al: Changing cancer survival
in China during 2003-15: A pooled analysis of 17 population-based
cancer registries. Lancet Glob Health. 6:e555–e567. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu P, Liu B and Fan Z: Noncoding RNAs in
tumorigenesis and tumor therapy. Fundam Res. 3:692–706. 2023.
View Article : Google Scholar
|
|
27
|
Li B, Zheng L, Ye J, Zhang C, Zhou J,
Huang Q, Guo Y, Wang L, Yu P, Liu S, et al: CREB1 contributes
colorectal cancer cell plasticity by regulating lncRNA CCAT1 and
NF-κB pathways. Sci China Life Sci. 65:1481–1497. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chowdhury PR, Salvamani S, Gunasekaran B,
Peng HB and Ulaganathan V: H19: An oncogenic long non-coding RNA in
colorectal cancer. Yale J Biol Med. 96:495–509. 2023. View Article : Google Scholar :
|
|
29
|
Jurkiewicz M, Szczepaniak A and Zielinska
M: Long non-coding RNAs-SNHG6 emerge as potential marker in
colorectal cancer. Biochim Biophys Acta Rev Cancer.
1879:1890562024. View Article : Google Scholar
|
|
30
|
Zhao D, Sun X, Long S and Yao S: An
autophagy-related long non-coding RNA signature for patients with
colorectal cancer. Physiol Int. Jul 5–2021.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen S, Zhu J and Zhi X: A novel
pyroptosis-associated long noncoding RNA signature to predict the
prognosis of patients with colorectal cancer. Int J Gen Med.
14:6111–6123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang S, Fan W and He D: Constructing a
personalized prognostic risk model for colorectal cancer using
machine learning and multi-omics approach based on
epithelial-mesenchymal transition-related genes. J Gene Med.
26:e36602024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Zhou K, Zhang X, Wu C, Deng D and
Yao Z: Roles of the H19/microRNA-675 axis in the proliferation and
epithelial-mesenchymal transition of human cutaneous squamous cell
carcinoma cells. Oncol Rep. 45:392021. View Article : Google Scholar :
|
|
34
|
Ren Z, Liu J, Li J and Yao L: Decreased
lncRNA, TINCR, promotes growth of colorectal carcinoma through
upregulating microRNA-31. Aging (Albany NY). 12:14219–14231. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Wang CX, Feng Q and Zhang T: lncRNA
TINCR promotes the development of cervical cancer via the
miRNA-7/mTOR axis in vitro. Exp Ther Med. 26:4872023. View Article : Google Scholar
|
|
36
|
Shi J, Guo C, Li Y and Ma J: The long
noncoding RNA TINCR promotes self-renewal of human liver cancer
stem cells through autophagy activation. Cell Death Dis.
13:9612022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He G, Pang R, Han J, Jia J, Ding Z, Bi W,
Yu J, Chen L, Zhang J and Sun Y: TINCR inhibits the proliferation
and invasion of laryngeal squamous cell carcinoma by regulating
miR-210/BTG2. BMC Cancer. 21:7532021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li G, Feng J, Huang S and Li Q:
LncRNA-PVT1 inhibits ferroptosis through activating STAT3/GPX4 axis
to promote osteosarcoma progression. Front Biosci (Landmark Ed).
29:2072024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun Z, Li X, Shi Y and Yao Y: LncRNA PVT1
facilitates the growth and metastasis of colorectal cancer by
sponging with miR-3619-5p to regulate TRIM29 expression. Cancer Rep
(Hoboken). 7:e20852024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olivero CE, Martinez-Terroba E, Zimmer J,
Liao C, Tesfaye E, Hooshdaran N, Schofield JA, Bendor J, Fang D,
Simon MD, et al: p53 activates the long noncoding RNA Pvt1b to
inhibit myc and suppress tumorigenesis. Mol Cell. 77:761–774.e8.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou L, Zhang Q, Cheng J, Shen X, Li J,
Chen M, Zhou C and Zhou J: LncRNA SNHG1 upregulates FANCD2 and G6PD
to suppress ferroptosis by sponging miR-199a-5p/3p in
hepatocellular carcinoma. Drug Discov Ther. 17:248–256. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu J, Xu Y, Ye G and Qiu J: LncRNA-SNHG1
promotes paclitaxel resistance of gastric cancer cells through
modulating the miR-216b-5p-hexokianse 2 axis. J Chemother.
35:527–538. 2023. View Article : Google Scholar
|
|
43
|
Cheng F, Wang L, Yi S and Liu G: Long
non-coding RNA SNHG1/microRNA-195-5p/Yes-associated protein axis
affects the proliferation and metastasis of gastric cancer via the
Hippo signaling pathway. Funct Integr Genomics. 22:1043–1055. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang S, Han H, Meng J, Yang W, Lv Y and
Wen X: Long non-coding RNA SNHG1 suppresses cell migration and
invasion and upregulates SOCS2 in human gastric carcinoma. Biochem
Biophys Rep. 27:1010522021.PubMed/NCBI
|
|
45
|
He D, Yue Z, Liu L, Fang X, Chen L and Han
H: Long noncoding RNA ABHD11-AS1 promote cells proliferation and
invasion of colorectal cancer via regulating the miR-1254-WNT11
pathway. J Cell Physiol. 234:12070–12079. 2019. View Article : Google Scholar
|
|
46
|
Luo J, Jiang Y, Wu L, Zhuo D, Zhang S,
Jiang X, Sun Y and Huang Y: Long non-coding RNA ABHD11-AS1 promotes
colorectal cancer progression and invasion through targeting the
integrin subunit alpha 5/focal adhesion kinase/phosphoinositide 3
kinase/Akt signaling pathway. Aging (Albany NY). 13:20179–20191.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Wang LL, Chen S, Zong ZH, Guan X
and Zhao Y: LncRNA ABHD11-AS1 promotes the development of
endometrial carcinoma by targeting cyclin D1. J Cell Mol Med.
22:3955–3964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu B, Wang W, Sun S, Ding H, Lan L, Li X
and Han S: Knockdown of lncRNA ABHD11-AS1 suppresses the
tumorigenesis of pancreatic cancer via sponging miR-1231. Onco
Targets Ther. 13:11347–11358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang T, Tian S, Zhao J, Pei M, Zhao M and
Yang X: LncRNA ABHD11-AS1 activates EGFR signaling to promote
cervical cancer progression by preventing FUS-mediated degradation
of ABHD11 mRNA. Cell Cycle. 22:2538–2551. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang G, Qiu Y, Fan Y and Liu J:
METTL3-deficiency suppresses neural apoptosis to induce protective
effects in cerebral I/R injury via inhibiting RNA m6A
modifications: A pre-clinical and pilot study. Neurochem Res.
49:85–98. 2024. View Article : Google Scholar
|
|
51
|
Yang X, Liu X, Nie Y, Zhan F and Zhu B:
Oxidative stress and ROS-mediated cellular events in RSV infection:
Potential protective roles of antioxidants. Virol J. 20:2242023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fu Y, Ye Y, Zhu G, Xu Y, Sun J, Wu H, Feng
F, Wen Z, Jiang S, Li Y and Zhang Q: Resveratrol induces human
colorectal cancer cell apoptosis by activating the mitochondrial
pathway via increasing reactive oxygen species. Mol Med Rep.
23:1702021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kong F, Xie C, Zhao X, Zong X, Bu L, Zhang
B, Tian H and Ma S: Resveratrol regulates PINK1/Parkin-mediated
mitophagy via the lncRNA ZFAS1-miR-150-5p-PINK1 axis, and enhances
the antitumor activity of paclitaxel against non-small cell lung
cancer. Toxicol Res (Camb). 11:962–974. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J and Li Q: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar
|
|
55
|
Cesmeli S, Goker Bagca B, Caglar HO,
Ozates NP, Gunduz C and Biray Avci C: Combination of resveratrol
and BIBR1532 inhibits proliferation of colon cancer cells by
repressing expression of LncRNAs. Med Oncol. 39:122021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miller CLW, Warner JL and Winston F:
Insights into Spt6: A histone chaperone that functions in
transcription, DNA replication, and genome stability. Trends Genet.
39:858–872. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nojima T and Proudfoot NJ: Mechanisms of
lncRNA biogenesis as revealed by nascent transcriptomics. Nat Rev
Mol Cell Biol. 23:389–406. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shehzada S, Noto T, Saksouk J and
Mochizuki K: A SUMO E3 ligase promotes long non-coding RNA
transcription to regulate small RNA-directed DNA elimination.
Elife. 13:e953372024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Diao C, Guo P, Yang W, Sun Y, Liao Y, Yan
Y, Zhao A, Cai X, Hao J, Hu S, et al: SPT6 recruits SND1 to
co-activate human telomerase reverse transcriptase to promote colon
cancer progression. Mol Oncol. 15:1180–1202. 2021. View Article : Google Scholar :
|
|
60
|
No authors listed. Retraction: lncRNA
ABHD11-AS1, regulated by the EGFR pathway, contributes to the
ovarian cancer tumorigenesis by epigenetically suppressing TIMP2.
Cancer Med. 13:e70982024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lu H, Zhu C, Chen Y, Ruan Y, Fan L, Chen Q
and Wei Q: LncRNA ABHD11-AS1 promotes tumor progression in
papillary thyroid carcinoma by regulating EPS15L1/EGFR signaling
pathway. Clin Transl Oncol. 24:1124–1133. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Richiardone E, Al Roumi R, Lardinois F,
Giolito MV, Ambroise J, Boidot R, Drotleff B, Ghesquière B,
Bellahcène A, Bardelli A, et al: MCT1-dependent lactate recycling
is a metabolic vulnerability in colorectal cancer cells upon
acquired resistance to anti-EGFR targeted therapy. Cancer Lett.
598:2170912024. View Article : Google Scholar : PubMed/NCBI
|