Colorectal cancer (CRC) is one of the most prevalent
malignancies of the digestive system, ranking third in terms of
global cancer incidence and second in global cancer mortality rates
(1). Of all the patients with
CRC, ~20% are initially diagnosed with metastatic CRC (mCRC) and
50% will develop metastases at a later stage (2). Distant metastasis is a leading cause
of death among patients with CRC, with liver metastasis being the
most common route for distant spread in advanced stages. At the
time of diagnosis, 20-25% of patients with CRC already exhibit
liver metastasis, while up to 50% may develop metachronous liver
metastasis following excision of the primary tumour. Early
detection of liver metastases from CRC and subsequent curative
resections can result in a 5-year survival rate ranging from
30-57%. By contrast, patients who are not eligible for resection
face a 5-year survival rate of <5% (3-5).
Of all the patients with CRC, ~90% cannot undergo curative liver
resection at the time their liver metastasis is diagnosed (6). Therefore, early detection of
CRC-related liver metastases and targeted interventions are crucial
for improving patient prognosis; however, there is currently no
efficient method for the early prediction of these liver metastases
in clinical practice.
In the past century, numerous hypotheses have been
proposed regarding the molecular mechanisms underlying the
metastasis of malignant tumours, with Paget's 'seed and soil'
theory being the most prominent (7). This theory suggests that tumour
metastasis results from the interaction between the 'seed' (tumour
cells) and the 'soil' (tumour microenvironment). Additionally,
epithelial-mesenchymal transition (EMT) is recognized as a critical
factor in facilitating the initiation of metastasis (8-10).
EMT is a biological process wherein epithelial cells undergo
transformation into mesenchymal cells under specific conditions,
significantly influencing distant tumour metastasis (8).
The treatment of liver metastases in patients with
CRC poses significant challenges and remains a leading cause of
mortality. The prognosis for individuals diagnosed with colorectal
liver metastasis (CRLM) is generally poor, with survival outcomes
closely associated with various clinical and pathological factors,
including the type of pathology, tumour grade and extent of tumour
spread and invasion (11).
Specific determinants, such as tumour size, proximity to the anal
margin and the number of metastatic liver lesions also serve
critical roles in the prediction of patient prognosis (12). The primary treatment options for
CRLMs include surgical resection and chemotherapy. However, only
~10-20% of these patients are deemed suitable for surgical
intervention at the time of diagnosis (13). For patients who undergo surgery,
the 5-year survival rate after surgery varies considerably, ranging
from 20-50%. This variability is influenced by factors such as age,
comorbid health conditions and tumour characteristics (14). Conversely, patients who are not
candidates for surgery face a poorer prognosis (15). Despite advancements in
chemotherapy regimens, the effectiveness of these treatments is
frequently compromised by the development of side effects and
resistance, particularly concerning therapies based on fluorouracil
and platinum compounds (16).
This situation highlights the urgent necessity for the development
of more precise and effective treatment strategies aimed at
improving the long-term survival prospects for patients with CRLMs.
The present review aimed to summarise the mechanisms underlying the
pathogenetic development of CRLM, examine current diagnostic
strategies and explore clinical therapeutic approaches, with a
particular emphasis on integrating emerging therapies alongside
conventional treatments.
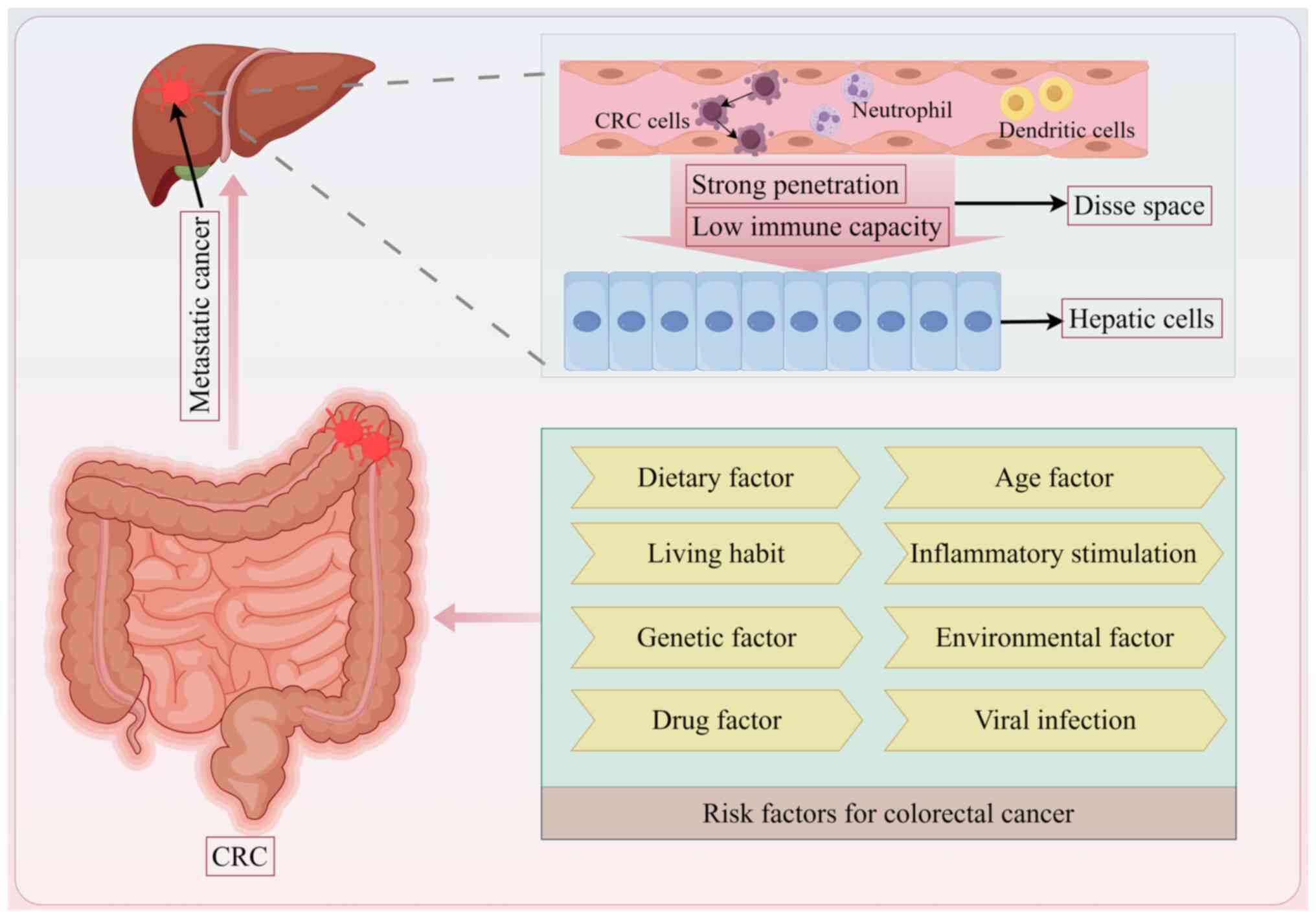
Cancer cells can often be found in specific organs
when cancer cells encounter an organ environment that allows them
to proliferate, leading to metastasis (17). The liver is favoured by various
types of cancer, including cancers of the colon, stomach, breast
and lungs, which often spread to the liver (18). The liver is an organ with both an
active metabolism and immune function, and fosters certain
conditions that can promote the proliferation of cancer cells
(19). Colon cancer, for example,
has been reported to commonly metastasize to the liver (20), largely due to the structure of the
liver as it receives blood from both the portal vein and the
hepatic artery, which provides cancer cells an easier path to enter
the liver (21). Additionally,
the proximity of the colon to the liver, particularly from areas
such as the hepatic flexure, can make it easier for cancer cells to
directly invade the liver from nearby tumours (22). Moreover, factors such as the high
permeability of hepatic sinusoidal endothelial cells combined with
slow blood flow within the liver may further promote tumour
colonization (23). In addition,
the liver has immune properties that allow it to tolerate foreign
cells and this immunosuppressive environment may contribute to
cancer growth (24,25). Certain chemokines produced by
liver cells can help promote the spread of cancers, such as colon
cancer, to the liver (26). The
C-X-C motif chemokine ligand 12/C-X-X motif chemokine receptor
(CXCR) 4/CXCR7 pathway has been associated with CRC recurrence and
its metastasis to the liver (27). Furthermore, extracellular vesicles
released by CRC cells may enhance liver metastasis through the
activation of hepatic stellate cells while modulating the
surrounding tumour microenvironment (28). In conclusion, the liver provides a
suitable environment or 'soil' for the spread and growth of CRC
cells (Fig. 1).
EMT is a biological process in which epithelial
cells change and take on features of mesenchymal cells. This can
occur at different times, such as when embryos are developing,
tissues are forming, healing is occurring or when there is fibrosis
in tissues (29). In cancer, EMT
has been linked to the early stages of tumour growth, spread and
resistance to treatments (30).
As cells undergo EMT, they start losing epithelial cell features,
causing them to be less adherent to other cells and the surrounding
matrix. The key to this shift is a decrease in the expression level
of E-cadherin, an epithelial marker, whereas the expression levels
of N-cadherin and vimentin, markers of mesenchymal cells, increase
(31). Consequently, the ability
of the cells to move or migrate strengthens, allowing them to
invade neighbouring tissues and blood or lymphatic vessels, thereby
travelling to distant organs (31). Therefore, targeting the EMT may
prevent tumours recurring after surgery. However, directly
targeting EMT for drug development poses significant challenges due
to the complexity and heterogeneity of the involved pathways.
Nevertheless, EMT inhibitors together with chemotherapy may have
potential for use in patients with cancer to help prevent drug
resistance, particularly in metastatic CRC. Using such inhibitors
after tumour removal could reduce the chances of tumour recurrence
(32,33).
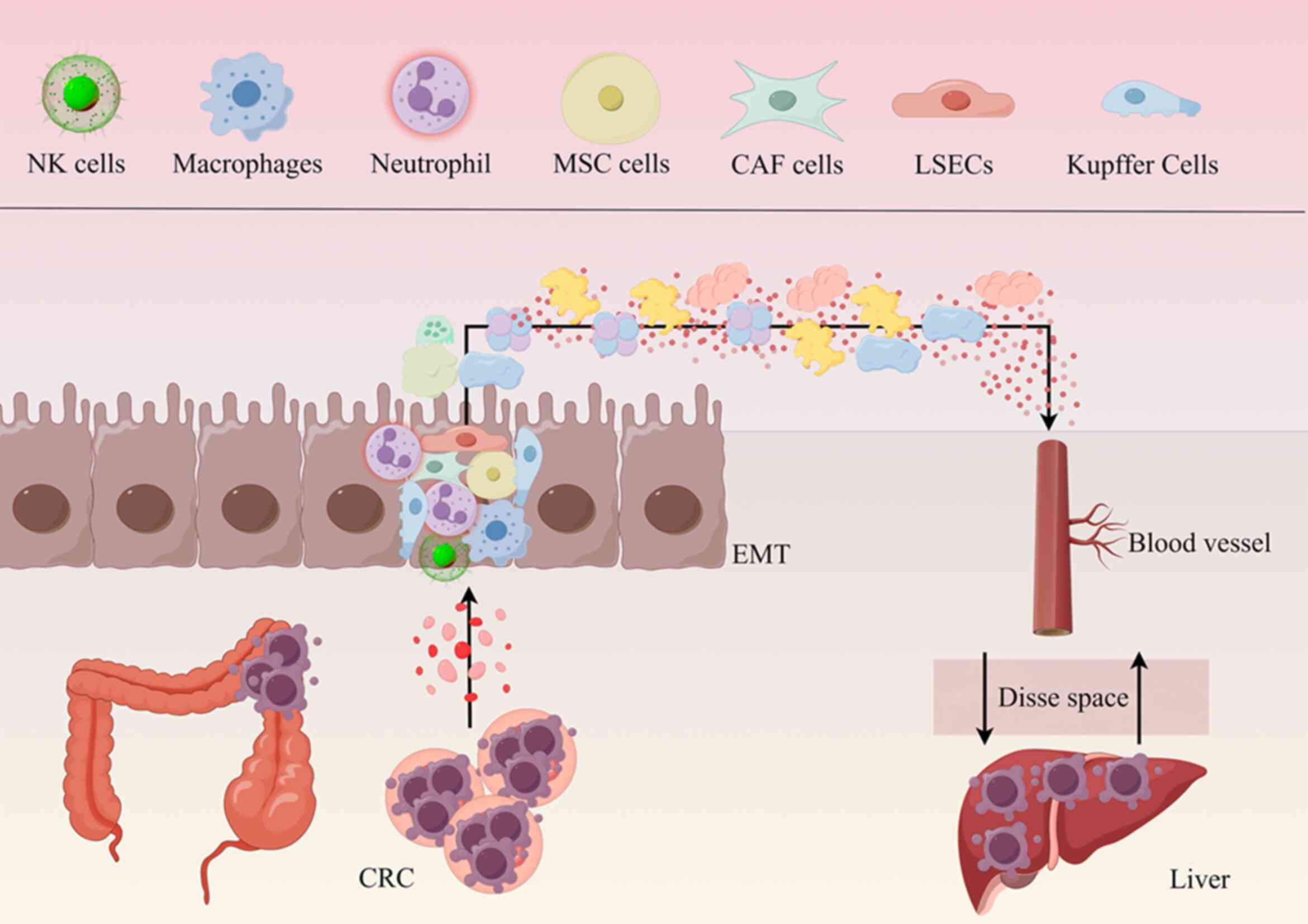
Upon entering the liver, metastatic cells interact
with native liver cells, thereby establishing a unique niche
(46). This specialized
microenvironment comprises various cell types, including
parenchymal hepatocytes, liver sinusoidal endothelial cells
(LSECs), Kupffer cells, hepatic stellate cells (HSCs) and natural
killer (NK) cells (47). These
resident intrahepatic cells have specific functions and can
interact with tumour cells, affecting the metastasis and
progression of tumour cells. These reactions are complex and are
important for understanding how metastasis works. It has been
previously suggested that different types of liver cells cooperate
with tumour cells and this cooperative relationship has a dual
nature. On one hand, this relationship may aid with the invasion
and metastasis of tumour cells, facilitating the spread of tumours
within the body, whilst on the other hand it also has the potential
to activate the body's immune defence mechanisms, helping the body
recognize and effectively kill tumour cells (Fig. 2) (48).
Hepatocytes account for ~60% of the cellular
composition of the liver and participate in several metabolic
activities, such as glycogen synthesis and decomposition, fat and
protein metabolism and the biosynthesis of hormones (49). These cells are distinguished by
their high mitochondrial content and extensive endoplasmic
reticulum, which facilitate the synthesis of coagulation factors,
albumin and other serum proteins that are crucial for the metabolic
and detoxification functions of the liver (49). In response to hepatic injury,
hepatocytes can undergo rapid proliferation, contributing to the
regenerative capacity of the liver (50). However, elucidating the precise
mechanisms underlying liver regeneration remains an active area of
research. Specifically, whether this process is driven primarily by
stem or progenitor cells or through the replication of existing
hepatocytes is still under investigation (51). With respect to their interaction
with neoplastic cells, it has been reported that hepatocytes can
express integrins capable of binding to malignant cells via
osteopontin. They may also form desmosomes with tumour cells,
facilitating cell aggregation within the liver (52). In addition, fibrinogen like 1,
which is secreted by hepatocytes, can help cancer cells survive by
preventing attacks from T cells. This interaction between
hepatocytes and cancer cells is currently under investigation,
though it is suggested that liver cells serve a significant role in
cancer progression (53).
LSECs serve important roles in both immune and
physiological actions, such as filtering, carrying out endocytic
processes and antigen presentation (54). As selective filters, LSECs
facilitate the passage of various molecules, such as plasma
proteins, pharmaceuticals and viruses <200 nm into the Disse
space while effectively preventing cellular entry (55). Kupffer cells, which are found in
the liver and make up a small portion of liver cells, are critical
for certain processes, such as removing infectious agents,
processing cholesterol and assisting the immune system (56). Kupffer cells originate from the
yolk sac early in development and travel to the liver where they
renew and help maintain the immune balance, cellular homeostasis
and metabolic balance of the liver (57). When mature, Kupffer cells express
several types of surface receptors, such as scavenger receptors,
Toll-like receptors and nucleotide-binding and oligomerization
domain-like receptors, which help identify and eliminate harmful
pathogens or dying cells (58).
When they are activated, Kupffer cells can produce chemicals such
as cytokines and chemokines, including TNF-β and IL-1, which
attract other immune cells to sites of infection (58). When tumour cells enter the liver,
they first meet LSECs and Kupffer cells in the sinusoids and can
often be killed quickly because of physical stress or mechanical
problems, such as shear forces caused by blood flow in the liver
sinus environment, mechanical stresses caused by the pressure from
surrounding cells and occurrences of tumour cells getting stuck in
the liver sinus, which can cause cell death. Moreover, Kupffer
cells can resist tumour metastasis by killing tumour cells through
phagocytosis using receptors such as Dectin-2 (59). Like LSECs, Kupffer cells also
produce inflammatory chemicals, such as TNF-α, nitric oxide and
reactive oxygen species (ROS), which contribute to cancer cell
apoptosis. NK cells from the liver also help to kill cancer cells,
releasing perforin or granzyme or activating the Fas/FasL pathway
to cause cancer cell death (60).
After cancer cells die, IL-1, IL-6, IL-8 and C-C motif chemokine
ligand (CCL) 5 are released, facilitating the mobilization and
activation of additional immune cells to eliminate additional
cancer cells (61). However, this
process can create a local immune response that increases the
expression of certain adhesion receptors on LSECs, such as
E-selectin, vascular cell adhesion molecule 1 and
intercellular-adhesion molecule 1, which mediate cancer cell
adhesion and transmigration into the Disse space (62). Notably, LSECs can increase EMT,
which helps tumour cells move and invade more easily, allowing them
to escape immune detection (62,63). While Kupffer cells initially
display antitumour properties, they can also have adverse effects,
including the release of growth factors, such as hepatocyte growth
factor, and angiogenic factors, such as VEGF, which can help cancer
cells migrate (61). Kupffer
cells also attract other immune cells that help cancer spread, such
as macrophages and myeloid-derived suppressor cells (MDSCs),
fostering an immune-tolerant microenvironment (64). Therefore, LSECs not only serve an
antitumour role but also serve an immunosuppressive role and
ultimately impair the antitumour response of the immune system
(65).
Typically, the liver harbours a substantial
population of NK cells, which serve crucial roles in combating
infections and eliminating malignancies (66). When NK cells interact with cancer
cells, they can release chemicals such as perforin and granzymes,
which cause the target cells to break apart and undergo programmed
cell death (67). NK cells can
also destroy tumour cells directly via other methods, such as the
use of TNF-related apoptosis-inducing ligands to induce tumour cell
death (68). Their role in tumour
immunosurveillance encompasses the induction of inflammatory
responses via the secretion of various cytokines and chemokines
(69). For example,
granulocyte-macrophage colony-stimulating factor secreted by NK
cells can stimulate the bone marrow to produce more granulocytes
and macrophages. These immune cells are recruited to the tumour
site to participate in the inflammatory response and the killing of
tumour cells. Additionally, TNF-α secreted by NK cells is also an
important factor in inducing the inflammatory response. In liver
cancer, TNF-α can also promote the infiltration of inflammatory
cells together with IFN-γ (69).
IL-8 secreted by NK cells can attract inflammatory cells, such as
neutrophils, to migrate to the tumour site and alter the tumour
microenvironment to be unfavourable for the growth of tumour cells
(68). NK cells are also key in
preventing the spread of tumours as they can attack cancer cells
that have already spread to other parts of the body (70), and a high concentration of these
cells often correlates with improved overall survival in patients
with CRLM (71). However, the
glycolytic conditions prevalent in CRLM create an environment rich
in lactic acid, which leads to NK cell apoptosis via the reduction
of intracellular pH levels (72).
Moreover, MDSCs can also reduce the power of NK cells by releasing
nitric oxide, which disrupts functions mediated by Fc receptors,
including antibody-dependent cellular cytotoxicity and cytokine
production (73). A previous
study utilizing a murine model of CRLM has demonstrated that
liver-resident NK (LrNK) cells exhibit increased expression of
retinoid-related orphan nuclear receptor alpha (RORα), a regulator
that is essential for LrNK cell maintenance but does not affect
conventional NK cells (74). The
targeted deletion of RORα in these models exacerbates CRLM,
highlighting the necessity of RORα for LrNK cell-mediated
antitumour immunity. Additionally, treatment with the RORα agonist
SR1078 has been shown to slow CRLM progression (74). In clinical contexts, the number of
liver NK cells is often reduced in patients with CRLM due to the
negative effects of lactate produced by the tumour cells (72). Furthermore, NK cells are capable
of promoting a proinflammatory metastatic environment in CRC,
leading to increased levels of IFN-γ, IL-2, IL-12p70 and IFN-α
within the CRC microenvironment while simultaneously decreasing
IL-6 levels. This finding indicates their effective antitumour
activity (75). Overall, NK cells
exhibit significant antitumour effects in patients with CRLM and
are closely associated with patient prognosis, positioning them as
promising specific therapeutic targets for this condition.
Macrophages function as APCs with multiple functions
that are essential in mediating immune responses against tumours
(76). They present exogenous
antigens to T cells through the major histocompatibility complex
(MHC)-I and MHC-II pathways, utilizing costimulatory and inhibitory
signals, along with cytokines, to modulate T cell activation
(76). Macrophages that are
recruited to tumour sites are referred to as tumour-associated
macrophages (TAMs), which serve a comprehensive regulatory role in
metastasis (77). TAMs contribute
to an immunosuppressive tumour environment through the upregulation
of inhibitory receptors such as programmed death-ligand (PD-L) 1
and PD-L2 (78). TAMs also
produce cytokines such as IL-10 and TGF-β. These molecules activate
regulatory T cells, which further reduce the ability of the immune
system to fight tumours (79).
Moreover, TAMs secrete remodelling factors that alter the
extracellular matrix (ECM) and enzymes that breakdown ECM
components, aiding in the dispersal of colorectal tumour cells
(80).
Novel analytical techniques, such as single-cell
analysis and spatiotemporal transcriptomics, have significantly
enhanced our understanding of the specific TAM subsets that drive
CRC metastasis (81). A previous
study compared tumour cells from the colon with their metastatic
counterparts found in the liver and reported the presence of a type
of TAM that expresses secreted phosphoprotein 1 (SPP1). This type
of TAM is associated with more aggressive cancers and poorer
outcomes for patients (82,83). SPP1+ TAMs interact with
fibroblasts that express fibroblast activation protein (FAP). This
interaction likely hinders lymphocyte infiltration and is
associated with decreased patient survival rates. These findings
underscore the complex interactions between stromal and immune
components within the tumour microenvironment (84,85). In alignment with these
observations, another study identified a group of M2-like TAMs
positive for mannose receptor c-type 1 and CCL18 with elevated SPP1
expression within CRLM. These macrophages have different metabolic
profiles and are also immunosuppressive. This suggests that TAMs
can have complex characteristics that contribute to cancer
progression (86). These findings
indicate that targeting the interactions between FAP+
fibroblasts and SPP1+ TAMs could be a potential strategy
for improving CRC treatment outcomes. However, this approach needs
further research to fully explore its effectiveness (87).
HSCs constitute ~15% of the nonparenchymal cell
population in the liver and serve a crucial role in mediating the
liver's response to injury (88).
Typically, they are not active; rather, they rest within the Disse
space. However, upon liver damage, activated hepatic stellate cells
expressing smooth muscle actin are activated and differentiate into
a myofibroblast-like phenotype and begin to produce ECM. The ECM is
mostly made up of collagen types I and IV, which are key parts of
the fibrotic processes that occur in the liver (88). In addition to producing ECM, HSCs
also produce various growth factors, such as TGF-β, and molecules
that aid in the production of blood vessels, such as VEGF and
angiopoietin-1. HSCs also release cytokines and chemokines such as
CCL2 and CCL21 (89,90), which help attract immune cells to
injured areas of the liver. HSCs also produce matrix
metalloproteinases (MMPs), including MMP-2, MMP-9 and MMP-13, which
are involved in remodelling the ECM (89,90). Angiogenesis is significantly
enhanced by the synergistic actions of VEGF, angiopoietin-1, ECM
components and MMPs. Together, these factors facilitate the
migration of cancer cells (20,91). Activated HSCs secrete CCL20, which
further activates the CCL20/C-C motif chemokine receptor
6/ERK1/2/ETS transcription factor ELK1/microRNA-181a-5p positive
feedback loop. This pathway affects how the tumour microenvironment
functions and helps form a niche to which cancer cells can be
transferred (28). While HSCs can
engage in antigen presentation to T cells and potentially initiate
an adaptive immune response, they more commonly promote immune
tolerance, particularly through the PD-L1/programmed cell death
protein 1 (PD-1) pathway and by inducing regulatory T cells
(92,93). Additionally, they influence the
transformation of monocytes into MDSCs, a process that depends on
CD44, further augmenting the immunosuppressive microenvironment.
Together, these actions help create conditions where immune
responses are dampened, which is beneficial for the survival of
tumour cells in the liver (94).
B cells are a major component of the tumour
microenvironment and are generally associated with tertiary
lymphoid structures (TLSs) (114). TLSs are lymphoid-like aggregates
that form in nonlymphoid tissues (115). Their main function involves
facilitating the immune response in nonlymphoid tissues, especially
in states of malignancy (115).
It has been reported that in some CRLMs, the proportion of B cells
that produce antibodies is significantly increased and concentrated
around TLSs and these antibodies are associated with antitumour
immunity (116). However,
another study reported the immunosuppressive role of B cells within
the tumour microenvironment (117).
In conclusion, the activity of B cells in liver
metastases from colorectal carcinoma has many facets; however, B
cells contribute to tumour growth via immunosuppressive mechanisms
and are also active in TLSs in the production of antitumour
antibodies and participation in antitumour responses.
The establishment of a microenvironment prior to
metastasis in the liver is a consequence of interactions between
certain cells. Resident cells of the liver interact with CRC cells,
altering the hepatic microenvironment by accumulating inflammatory
mediators, remodelling the ECM and enhancing tumour-induced
immunosuppression. Consequently, these concurrent processes create
a supportive 'soil' that facilitates the establishment and
proliferation of CRC cells within the hepatic landscape.
CRLM is present in ~20% of patients with CRC, which
are liver metastases that are diagnosed either before or at the
time of CRC diagnosis (118).
Biomarker testing is routine for the correct diagnosis of
synchronous liver metastasis. Serum CEA is typically a first-line
test, whereas CA19-9 serves a subsidiary role in patients in whom
CEA levels are not informative (119). RAS mutation status is considered
a predictive biomarker that affects the effectiveness of anti-EGFR
therapies and therefore influences treatment options. Thus,
mutation testing of NRAS exons 2, 3 and 4 and KRAS exons 2, 3 and 4
is recommended for all patients with CRC (120). Furthermore, it is suggested that
patients with CRLM undergo BRAF mutation testing, as BRAF is a
prognostic biomarker (121,122).
The basic diagnostic workup for CRC patients should
combine serum tumour markers and pathology staging with imaging
studies, including routine liver ultrasound and abdominal
contrast-enhanced CT (123). In
patients with a high suspicion of CRLM based on ultrasound or CT
images that cannot be confirmed, further tests may include serum
alpha-fetoprotein, liver contrast-enhanced ultrasound and both
plain and enhanced MRI of the liver (124). MRI is generally recommended
preoperatively in cases where metastasis to the liver is
resectable, whereas PET-CT is not a routine standard modality.
However, in specific clinical situations where there is a need to
accurately assess the extent of disease beyond the liver, or to
distinguish between viable tumour and post-treatment changes,
PET-CT has its indications. Needle biopsies of liver metastases are
generally not required as biopsies may cause tumour cells to spread
along the needle track and may lead to bleeding, especially when
the tumour is located near major blood vessels. Meanwhile,
non-invasive imaging techniques such as CT, MRI and PET-CT can
provide thorough diagnostic information (125). Intraoperative exploration of the
liver should be a routine part of surgery for CRC to exclude
metastatic disease and any suspicious nodule detected at the time
of surgery may necessitate biopsy (125).
Regular follow-up examinations are necessary after
surgery to identify the occurrence of metachronous liver
metastases. CEA and CA19-9 levels must be measured every 3-6 months
for 2 years after surgical resection, with regular monitoring every
6 months for the following 3 years and yearly monitoring after 5
years (126). In cases of high
suspicion on ultrasound or CT, liver MRI should be considered and
abdominal/pelvic enhanced CT scan or liver MRI enhancement should
be performed every 3 months for 2 years after surgery, with liver
cell-specific contrast agent enhanced MRI, if deemed necessary.
Imaging, using a consistent modality, is recommended every 6-12
months for a total of 5 years (127). Routine PET-CT scans are not
indicated. Finally, electronic colonoscopy should be performed
within 1 year following surgery. In the case of abnormalities,
re-examination should be performed within 1 year. If no abnormality
is found, then re-examination at 3 years after surgery and every 5
years thereafter is recommended (128).
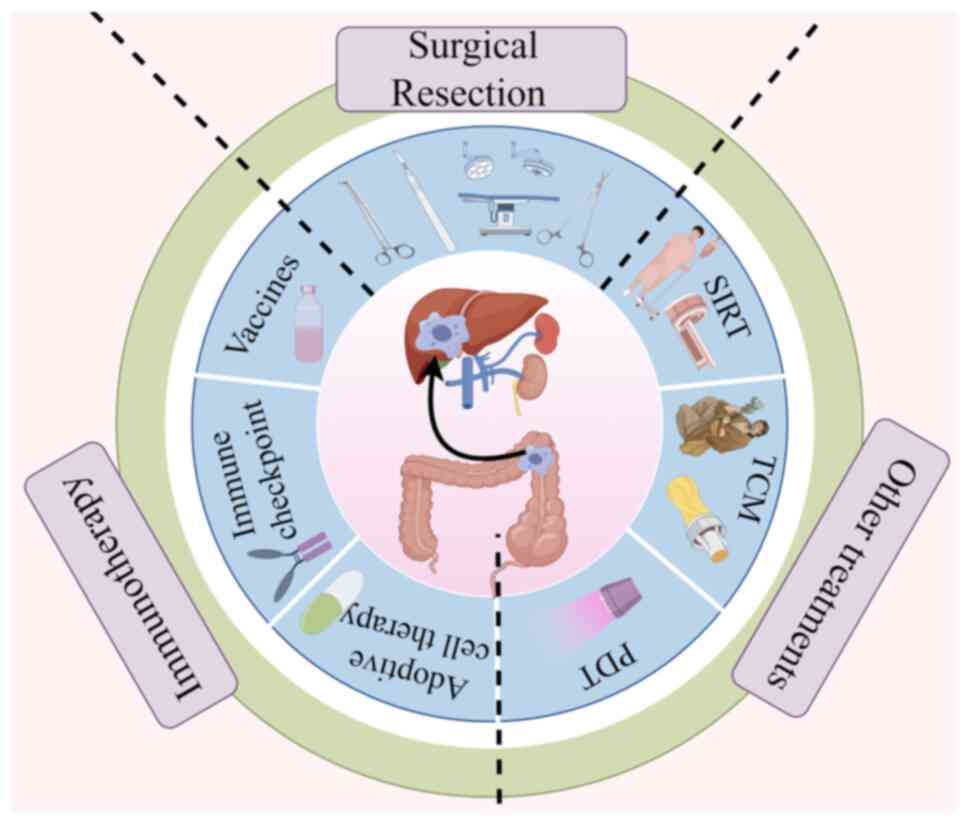
The clinical therapeutic approaches for liver
metastasis from CRC primarily involve surgical resection,
immunotherapy and other treatments (Fig. 3). The surgical removal of liver
metastatic foci is optimal for curing liver metastases and
achieving significant cure rates. Immunotherapy, particularly in
combination with other cancer therapies, may activate
antigen-specific T cells to overcome tumour immunosuppression.
Comprehensive therapeutic strategies are crucial for improving
outcomes in patients with liver metastasis from CRC.
Surgical removal of liver metastatic foci remains
the optimal approach for curing liver metastases in patients with
CRC (129). Therefore, it is
critical that eligible patients receive surgical treatment when
appropriate. For patients with liver metastases, resection can
achieve a 20% cure rate (130),
with a 5 year survival rate >50% (131). Additionally, for patients whose
dissemination is accompanied by lymph node infiltration and occult
micrometastasis who are suitable for complete surgical resection,
adjuvant radiotherapy, chemotherapy and targeted therapy are
required (16). However, ~70% of
patients are ineligible for surgical treatment due to widespread
disease, multiple tumours, significant systemic diseases or poor
liver reserve function (132).
Moreover, the best surgical strategy for patients
with liver metastases from CRC remains controversial. The METASYNC
trial is a published prospective controlled trial (133) in which the outcomes of patients
who underwent simultaneous tumour resection were compared with
those of patients who underwent staged surgical resection. A total
of 85 patients with liver metastasis of CRC were analysed. There
was no significant difference in the rate of postoperative
complications between the two groups. After 2 years, patients who
underwent simultaneous resection had improved overall survival (OS)
and disease-free survival (DFS) compared with patients who
underwent staged resection. However, OS improvement was not
statistically significant after 47 months, which may be due to
several limitations, including the small sample size of the trial
and the long time period over which the trial was conducted, which
could lead to the loss of patient follow-up data.
In brief, while surgical treatment of liver
metastases from CRC has limitations, the criteria for surgery are
not uniform and further investigations based on well-designed
prospective, multicentre studies are needed. Additionally, the
prognostic factors of liver metastasis of CRC mainly include the
size, differentiation and depth of invasion of the primary tumour,
the number, size and distribution of liver metastases, the age, sex
and physical condition of the patient and the levels of serological
markers such as CEA and CA19-9, as well as the mutation status of
genes such as KRAS and BRAF (134,135). For liver metastases of CRC,
surgical resection may be considered if there are a small number
and size of tumours and they have not metastasized. Among the
prognostic factors, the primary tumour and metastases directly
affect the feasibility and effect of surgery. For example, patients
with highly differentiated tumours have an improved prognosis and
experience increased surgical removal success rates (136).
The purpose of immunotherapy is to enhance the
intrinsic immunity and antitumour capabilities of T cells, target
immunosuppressive TAMs and amplify the antitumour actions of the
patient's own immune system (137). Immunotherapy encompasses immune
checkpoint inhibitors (ICIs), cancer vaccines and other biologics
such as chimeric antigen receptor (CAR) T cells. However, due to
the suppressive effect of the tumour immune microenvironment, the
efficacy of immunotherapy may not be ideal (138).
PD-1 and cytotoxic T lymphocyte-associated antigen-4
(CTLA-4) are receptors that inhibit T cell responses to ensure
peripheral tolerance, permitting the proliferation of tumour cells
rather than the elimination of tumour cells by the immune system.
ICIs can enhance the anticancer immune response by inhibiting the
expression of the suppressive receptor PD-L1 on the surface of T
cells and malignant cells, particularly CRC cells (139). The PD-1/PD-L1 inhibitors
commonly used in current mCRC clinical trials include nivolumab,
pembrolizumab, durvalumab, atezolizumab and avelumab (140-144). These medications have shown
significant effectiveness in treating microsatellite
instability-high (MSI-H)/mismatch repair deficient (dMMR) mCRC,
though they have demonstrated poor efficacy in the majority
(>90%) of patients with microsatellite stable (MSS)/metastatic
MMR-proficient (pMMR) mCRC (145). Specifically, the blockade of
PD-1 by pembrolizumab or nivolumab has resulted in frequent and
lasting remission for ~75% of patients with dMMR mCRC (146). Patients with mCRC with high
microsatellite instability or mismatch repair defects did not have
significantly improved overall survival rates compared with those
achieved with chemotherapy. Nevertheless, the median
progression-free survival (PFS) for those treated with
pembrolizumab was 16.5 months compared with 8.2 months for the
chemotherapy group (range, 6.1-10.2 months), indicating that the
PFS of the patients in the pembrolizumab group was at least twice
that of those receiving chemotherapy, with fewer treatment-related
adverse events (147). Increased
infiltration of CD8+ T cells and expression of immune
checkpoint molecules, including PD-L1 (137), are characteristics of dMMR CRC,
which may partially explain why PD-1/PD-L1 inhibitors have achieved
significant efficacy in patients with MSI-H or dMMR mCRC.
Ipilimumab, a CTLA-4 inhibitor antibody, can be used
in conjunction with PD-1/PD-L1 inhibitors for the clinical
treatment of MSI-H/dMMR mCRC. The combination of nivolumab with a
low dose of ipilimumab has provided sustained clinical benefits,
marked by an objective response rate (ORR) of 65% (complete
response rate of 13%) and a disease control rate of 81% after a
median follow-up of 50.9 months, with durable responses (148). While this therapy can be highly
effective for dMMR CRC, pMMR tumours exhibit no response.
Immunotherapy targeting the PD-1/PD-L1 axis is a
promising strategy for eliminating cancer. However, patients with
CRC with MSS do not respond to anti-PD-1/PD-L1 therapies. The
efficacy of immunotherapy is largely determined by the intensity of
the immune response and the limited effectiveness can be explained
by the immunosuppressive state of the tumour microenvironment
(149). Currently, various
combination therapies against PD-1/PD-L1 have enhanced the
regeneration of an immunogenic TME, however, their synergistic
effects and clinical efficacy for patients with MSS CRC remain
underexplored (150). For
patients with refractory CRC, there is a need to develop an
increased number of potential combination therapies to overcome the
ineffectiveness of anti-PD-1/PD-L1 treatments (151). Furthermore, patients with MSI-H
or dMMR status have a better response to immunotherapy and an
improved prognosis and are more suitable for immunotherapy
(152).
Cancer cells elicit an immune response due to the
expression of altered self-antigens; thus, cancer vaccines induce a
potent immune reaction against one or several tumour-specific
antigens (153,154). Currently, there are three main
types of cancer vaccines for CRC liver metastases: Molecular-based
vaccines, cancer cell vaccines and dendritic cell vaccines. Among
these, the most widely used approach is the use of dendritic cell
vaccines for treating CRC liver metastases. However, no product has
yet been approved for the development of cancer vaccines targeting
CRC. A previous study demonstrated that patients with CRC
metastases who received a dendritic cell vaccine and were
disease-free after metastasectomy and perioperative chemotherapy
had a survival rate that surpassed that of the contemporary
unvaccinated group, with a relapse-free median survival time of
25.7 months and a median OS of 44.1 months (155). A randomized phase II clinical
trial of dendritic cell vaccination following the resection of CRC
liver metastases showed that, compared with the observation group
(median DFS, 9.53 months), the vaccine group had fewer and later
disease recurrences (median DFS, 25.26 months) (156). Therefore, the combination of DC
vaccines with other cancer therapies such as chemotherapy,
radiotherapy and ICIs could activate antigen-specific effector T
cells and potentially overcome tumour immunosuppression.
Adoptive T cell therapy is an antitumour treatment
that involves the transfer of T cells from a donor to the patient
(157). The process involves
isolating T cells with antitumour activity, followed by their
extensive ex vivo expansion, activation and infusion into
patients for tumour treatment (158). In solid tumours, three main
types of adoptive T-cell therapies have been developed:
Tumour-infiltrating lymphocytes, CAR-engineered T cells and
high-affinity T cell receptor-engineered T cells (159). In CRC, CAR T cell therapy
remains a primary focus of research. In a mouse model of CRC liver
metastasis, the administration of anti-CEA CAR T cell therapy
significantly controlled tumour growth, inhibited tumour
proliferation and improved liver function (160). For patients with CEA-positive
mCRC, CAR T cell therapy stabilized the condition of 7 patients who
had progressive disease in previous treatments after receiving CAR
T cell therapy. Of these individuals, 2 patients maintained stable
disease for >30 weeks and a reduction in both tumour size and
number was observed in 2 patients through PET/CT and MRI analysis.
Additionally, after long-term observation, a significant reduction
in serum CEA levels was evident in ~60% of patients. Moreover, a
sustained presence of CAR T cells in the peripheral blood was noted
in patients treated with high doses of CAR T cell therapy (161). Another previous study
demonstrated the application of HER2-specific CAR T cells for the
treatment of mCRC. HER2 CAR T cells displayed potent specific
killing activity against CRC cells and their antitumour efficacy
was confirmed in both in vitro and mouse model studies
(162). Zhou et al
(163) reported that the use of
mesothelin-targeted CAR T cells for the treatment of mouse CRLMs
demonstrated good efficacy and safety. When a local administration
method was used, the treatment effectively reduced the tumour
burden and enhanced cytotoxic T cell infiltration without
significant toxicity. CAR T cell therapy, which targets specific
antigens such as HER2 and mesothelin, has shown potential in the
treatment of mCRC, with preclinical models demonstrating a reduced
tumour burden and increased survival rates. The use of localized
delivery methods, including portal vein administration, has
improved both the safety and effectiveness of treatment (164). Nevertheless, there are still
challenges to address, such as overcoming the immunosuppressive
tumour microenvironment and ensuring the longevity and
effectiveness of CAR-T cells within solid tumours. Ongoing research
and clinical trials aim to refine CAR T cell treatment strategies
for mCRC, with a focus on overcoming these obstacles to fully
exploit their therapeutic potential.
Among the most frequently used targeted therapies in
clinical practice, anti-VEGF and anti-EGFR treatments have resulted
in favourable outcomes in patients with mCRC. However, the optimal
first-line approach for a more precise and personalized therapeutic
strategy continues to be a subject of debate (167). The results of several large
clinical studies comparing the combination regimens of bevacizumab
and cetuximab with FOLFOX/FOLFIRI were fairly consistent, with no
significant difference in response rate or PFS between the two
groups and prolonged OS in the cetuximab group (168,169). Similarly, the PEAK trial, which
evaluated the combination of panitumumab and bevacizumab with
FOLFOX, reported comparable response rates and PFS rates, with a
slight OS advantage for panitumumab compared with bevacizumab with
FOLFOX (34.2 vs. 24.3 months) (170).
However, one-sided evaluation of the efficacy of
these two treatment methods on the basis of the results of the
above clinical trials still has a large bias. To clarify the
potential role of tumour heterogeneity in the effectiveness of
these targeted therapies, the latest research further explores the
mechanism of action of drugs and the impact of differences in the
mutation sites of genes.
Post hoc analyses of the FIRE-3 trial suggested
that cetuximab might be superior to anti-VEGF therapy in patients
with wild-type RAS, achieving higher objective response rates (72.0
vs. 56.1%) and faster tumour regression (68.2 vs. 49.1%) (171). Meta-analyses encompassing the
FIRE-3, CALGB and PEAK trials have reinforced the preference for
anti-EGFR over anti-VEGF in patients with CRC with wild-type RAS
(172).
Moreover, anatomical location appears to modulate
the efficacy of targeted therapies. Left-sided tumours,
characterized by higher EGFR expression when compared with
right-sided lesions, result from a variety of complex biological
and molecular mechanisms and tend to exhibit superior responses to
anti-EGFR agents, whereas right-sided tumours are more responsive
to anti-VEGF drugs (173,174).
The FIRE-3 trial corroborated these observations, with left-sided
tumours demonstrating a greater sensitivity to cetuximab compared
with bevacizumab (OS, 38.3 vs. 28.0 months) and right-sided tumours
showing the opposite pattern (OS, 8.3 vs. 23.0 months) (175). Similar trends were observed in
the CALGB study (174) and
panitumumab trial (176),
indicating that even in the absence of BRAF mutations, right-sided
cancers may derive minimal benefit from anti-EGFR therapy (176).
For second-line therapy, transitioning from a
bevacizumab maintenance regimen to cetuximab or panitumumab did not
yield significant improvements in patients with progressing RAS
wild-type CRC, as per findings from the independent phase II trial
(177).
Consistent with the findings of a number of studies
regarding mCRC, the use of anti-EGFR drugs in combination with
chemotherapy has the potential to be effective in patients with
surgically resectable liver metastases of CRC if the KRAS gene is
wild type (178-180). However, the EPOC trial showed
that incorporating cetuximab into perioperative chemotherapy did
not confer a survival advantage and was associated with a shorter
PFS (14.1 vs. 20.5 months) (179,181), highlighting the uncertainty
surrounding the role of cetuximab in this setting. Conversely, the
perioperative administration of bevacizumab in conjunction with
chemotherapy has been linked to prolonged survival in patients with
liver-limited mCRC, although recurrence remains a common outcome
(182-185).
The mutation status of KRAS and BRAF is a key
factor affecting the selection and effectiveness of targeted
therapy (186). For example,
patients with abnormal EGFR expression may be treated using a drug
that targets EGFR, such as cetuximab. Patients with mutations in
KRAS, NRAS and BRAF genes should be treated with other
corresponding targeted drugs. The status of gene mutation in
prognostic factors not only affects the selection of targeted
drugs, but also directly affects the therapeutic effect and
prognosis of patients (187).
Despite advancements in the targeted treatment landscape of CRC,
the requirement for more efficacious therapeutic modalities
persists. Future endeavours should focus on refining the
combination of targeted therapies with conventional chemotherapy
and using precision medicine paradigms to optimize patient outcomes
and prolong survival.
In addition to surgery and immunotherapy, a range
of other treatments exists, including selective internal radiation
therapy (SIRT) and Traditional Chinese Medicine (TCM). Furthermore,
new developments in cancer nanotechnology and photodynamic therapy
(PDT) may offer potential solutions for the diagnosis and treatment
of mCRC in the liver.
SIRT is a process in which microspheres filled with
the β-emitting radionuclide Yttrium-90 (Y-90) are embolized into
the arterial supply of the liver to enhance the management of liver
metastases (188). Currently,
the European Society for Medical Oncology consensus guidelines
consider SIRT as a potential treatment option only for patients who
are unresponsive to chemotherapy drugs (189). In a previous systematic review
and network meta-analysis, SIRT with Y-90 resin microspheres
demonstrated positive therapeutic effects for patients with
chemotherapy-resistant or chemotherapy-intolerant mCRC. The
analysis, which included 15 studies, showed that all active
treatments enhanced OS compared with best supportive care, with
SIRT having the longest OS, albeit without reaching statistical
significance. Specifically, compared with best supportive care,
SIRT has a hazard ratio for OS of 0.48 (95% CI, 0.27-0.87),
demonstrating its potential advantages in terms of safety and
efficacy (190). The unique
delivery mechanism of SIRT, which targets liver metastases while
minimally affecting healthy tissues, renders it a feasible choice
in the mCRC treatment landscape, warranting additional exploration
and consideration in clinical settings.
In China, TCM has been widely used as an adjunct
therapy for cancers, including mCRC (191). TCM, which serves as a supportive
therapy, can diminish the adverse effects of cancer treatments and
may increase the effectiveness of chemotherapy (192,193). A previous study integrating
Huangci granules, a TCM, with chemotherapy and either cetuximab or
bevacizumab for the treatment of mCRC. These results indicated
that, compared with the placebo group, the treatment group
experienced significantly prolonged PSF, improved quality of life
and fewer related grade 3 or 4 adverse events (194). A cohort study by Zhang et
al (195) compared the
results of 110 patients treated with a combination of Chinese and
Western medicine with those of 225 patients treated exclusively
with Western medicine. These findings indicated that combined
therapy notably increased the OS and PFS in women, patients with
right-sided colon lesions and those receiving first-line treatment.
Studies regarding the correlation between Chinese herbal medicine
(CHM) and stage II and III CRC have shown that long-term use of CHM
(>18 months) can significantly reduce the postoperative
recurrence and metastasis of CRC (196). At present, the study of TCM in
the treatment of CRC has focused largely on the molecular level.
The lack of robust evidence-based studies and standardization of
herbal products are major barriers to the globalization of TCM
(197). With the advent of new
technologies, such as whole-genome sequencing, genome editing and
quantitative proteomics analysis, a deeper and more accurate
understanding of the molecular targets and signalling pathways
involved in the antimetastatic effects of CRC can be gained. With
further research in this area, TCM interventions for CRC can be
combined with other treatment methods being developed, leading to
safer, evidence-based use of these herbs.
Photodynamic therapy (PDT) is an emerging minimally
invasive treatment method with promising outcomes for CRC
treatment. PDT involves the administration of a photosensitizer
that accumulates selectively within cancer cells. When exposed to
light at the appropriate wavelength, PDT can destroy cancer cells
by generating ROS (16,198). In a phase I study, 24 patients
with mCRC to the liver were treated with PDT with the
photosensitizer
5,10,15,20-tetrakis(m-hydroxyphenyl)bacteriochlorin, which caused
tumour necrosis in all treated lesions 1 month after PDT (199). The treatment of 18 patients with
CRC with PDT using Hematoporphyrin resulted in a significantly
longer OS in the PDT group compared with the non-PDT group, with a
44.4% ORR and 88.9% disease control rate 2 months after PDT
(200). Currently, the primary
challenges of PDT are the limited depth of penetration by the
activating light source, inadequate immunogenicity induction and
complexity of the tumour microenvironment, all of which restrict
the effectiveness of PDT. These challenges have been addressed
through the use of nanotechnology. The combined use of
meta-tetrahydroxy-phenylchlorin in PDT with hepatitis B virus
core-like particles for treating mice with CRC effectively
controlled tumour growth and significantly improved the survival
rates of the treated mice (201). Liang et al (202) used perfluorocarbon@porphyrin
nanoparticles to assist PDT in treating mice with CRC, which
resulted in an enhanced therapeutic effect of PDT and
downregulation of COX-2 expression, eliminating tumour hypoxia and
directly inhibiting hypoxia-driven CRLM. In addition to
conventional treatment methods, the development of a variety of new
interdisciplinary methods are required for the successful treatment
of patients with CRC.
The management of CRLMs remains a significant
challenge in oncology due to the intricate interactions between
tumour cells and the hepatic microenvironment. Early detection and
a comprehensive understanding of the molecular mechanisms driving
CRLM are crucial for improving patient prognosis. Surgical
resection offers the best chance for a cure, though many patients
are ineligible due to advanced disease or poor liver function.
Chemotherapy and targeted therapies have shown promise, yet
resistance and side effects are common. Immunotherapy, particularly
in patients with dMMR CRC, has emerged as a promising treatment
modality, but its efficacy in the majority of patients with CRC
remains limited. The use of SIRTs and TCMs offers alternative
approaches, highlighting the potential benefits of integrating
these approaches with conventional treatments. Continued research
is imperative to refine therapeutic strategies, overcome treatment
resistance and develop personalized medicine for patients with
CRLM. The future of CRLM management lies in the combination of
novel therapies with a deeper understanding of the tumour
microenvironment, ultimately aiming to increase the survival and
quality of life of patients.
Not applicable.
MW and ZJ designed the study. YL and HY wrote the
manuscript. SD, CW and WZ produced the figures using Figdraw
(https://www.figdraw.com/). ZZ read the
literature search. All authors read and approved the final version
of the manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was funded by the National Natural Science Foundation
of China (grant nos. 82103645 and 82260596), the Natural Science
Foundation of Jiangxi Province (grant no. 20242BAB25506), the China
Postdoctoral Science Foundation (grant no. 2023M741523) and the
Science and Technology Program of Jiangxi Provincial Health and
Family Planning Commission (grant no. 202410246).
|
1
|
Singh S, Gomez HJ, Thakkar S, Singh SP and
Parihar AS: Overcoming acquired drug resistance to cancer therapies
through targeted STAT3 inhibition. Int J Mol Sci. 24:47222023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshino T, Portnoy DC, Obermannová R,
Bodoky G, Prausová J, Garcia-Carbonero R, Ciuleanu T,
García-Alfonso P, Cohn AL, Van Cutsem E, et al: Biomarker analysis
beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and
second-line ramucirumab efficacy in patients with metastatic
colorectal carcinoma from RAISE-a global phase III study. Ann
Oncol. 30:124–131. 2019. View Article : Google Scholar
|
|
3
|
Stewart CL, Warner S, Ito K, Raoof M, Wu
GX, Kessler J, Kim JY and Fong Y: Cytoreduction for colorectal
metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When
does it palliate, prolong survival, and potentially cure? Curr
Probl Surg. 55:330–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norén A, Sandström P, Gunnarsdottir K,
Ardnor B, Isaksson B, Lindell G and Rizell M: Identification of
inequalities in the selection of liver surgery for colorectal liver
metastases in sweden. Scand J Surg. 107:294–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margonis GA, Sergentanis TN,
Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K,
Psaltopoulou T, Wang J, Buettner S, Papalois ΑE, et al: Impact of
surgical margin width on recurrence and overall survival following
R0 hepatic resection of colorectal metastases: A systematic review
and Meta-analysis. Ann Surg. 267:1047–1055. 2018. View Article : Google Scholar
|
|
6
|
Qin S, Liu GJ, Huang M, Huang J, Luo Y,
Wen Y, Wang Y and Chen L: The local efficacy and influencing
factors of ultrasound-guided percutaneous microwave ablation in
colorectal liver metastases: A review of a 4-year experience at a
single center. Int J Hyperthermia. 36:36–43. 2019. View Article : Google Scholar
|
|
7
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
8
|
Peña C, García JM, Silva J, García V,
Rodríguez R, Alonso I, Millán I, Salas C, de Herreros AG, Muñoz A
and Bonilla F: E-cadherin and vitamin D receptor regulation by
SNAIL and ZEB1 in colon cancer: Clinicopathological correlations.
Hum Mol Genet. 14:3361–3370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He X, Chen Z, Jia M and Zhao X:
Downregulated E-cadherin expression indicates worse prognosis in
Asian patients with colorectal cancer: Evidence from meta-analysis.
PLoS One. 8:e708582013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh BY, Hong HK, Lee WY and Cho YB: Animal
models of colorectal cancer with liver metastasis. Cancer Lett.
387:114–120. 2017. View Article : Google Scholar
|
|
12
|
Helling TS and Martin M: Cause of death
from liver metastases in colorectal cancer. Ann Surg Oncol.
21:501–506. 2014. View Article : Google Scholar
|
|
13
|
Tsilimigras DI, Hyer JM, Bagante F,
Guglielmi A, Ruzzenente A, Alexandrescu S, Poultsides G, Sasaki K,
Aucejo F and Pawlik TM: Resection of colorectal liver metastasis:
Prognostic impact of tumor burden vs KRAS mutational status. J Am
Coll Surg. 232:590–598. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsilimigras DI, Brodt P, Clavien PA,
Muschel RJ, D'Angelica MI, Endo I, Parks RW, Doyle M, de Santibañes
E and Pawlik TM: Liver metastases. Nat Rev Dis Primers. 7:272021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsilimigras DI, Ntanasis-Stathopoulos I,
Bagante F, Moris D, Cloyd J, Spartalis E and Pawlik TM: Clinical
significance and prognostic relevance of KRAS, BRAF, PI3K and TP53
genetic mutation analysis for resectable and unresectable
colorectal liver metastases: A systematic review of the current
evidence. Surg Oncol. 27:280–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou H, Liu Z, Wang Y, Wen X, Amador EH,
Yuan L, Ran X, Xiong L, Ran Y, Chen W and Wen Y: Colorectal liver
metastasis: Molecular mechanism and interventional therapy. Signal
Transduct Target Ther. 7:702022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuzhalin AE and Yu D: Brain metastasis
organotropism. Cold Spring Harb Perspect Med. 10:a0372422020.
View Article : Google Scholar
|
|
18
|
Mielgo A and Schmid MC: Liver tropism in
cancer: The hepatic metastatic niche. Cold Spring Harb Perspect
Med. 10:a0372592020. View Article : Google Scholar
|
|
19
|
Yoon H, Sabate Del Rio J, Cho SW and Park
TE: Recent advances in micro-physiological systems for
investigating tumor metastasis and organotropism. Lab Chip.
24:1351–1366. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milette S, Sicklick JK, Lowy AM and Brodt
P: Molecular pathways: Targeting the microenvironment of liver
metastases. Clin Cancer Res. 23:6390–6399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elsayes KM, Shaaban AM, Rothan SM, Javadi
S, Madrazo BL, Castillo RP, Casillas VJ and Menias CO: A
comprehensive approach to hepatic vascular disease. Radiographics.
37:813–836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Huang S, Lu X, Huang Y and Chi P:
Incidence of and risk factors for gastroepiploic lymph node
involvement in patients with cancer of the transverse colon
including the hepatic flexure. World J Surg. 45:1514–1525. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poisson J, Lemoinne S, Boulanger C, Durand
F, Moreau R, Valla D and Rautou PE: Liver sinusoidal endothelial
cells: Physiology and role in liver diseases. J Hepatol.
66:212–227. 2017. View Article : Google Scholar
|
|
24
|
Zheng M and Tian Z: Liver-mediated
adaptive immune tolerance. Front Immunol. 10:25252019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heymann F, Peusquens J, Ludwig-Portugall
I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N,
Ochando JC, Randolph GJ, et al: Liver inflammation abrogates
immunological tolerance induced by Kupffer cells. Hepatology.
62:279–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang D, Wang X, Si M, Yang J, Sun S, Wu H,
Cui S, Qu X and Yu X: Exosome-encapsulated miRNAs contribute to
CXCL12/CXCR4-induced liver metastasis of colorectal cancer by
enhancing M2 polarization of macrophages. Cancer Lett. 474:36–52.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng W, Huang W, Chen J, Qiao C, Liu D, Ji
X, Xie M, Zhang T, Wang Y, Sun M, et al: CXCL12-mediated HOXB5
overexpression facilitates Colorectal Cancer metastasis through
transactivating CXCR4 and ITGB3. Theranostics. 11:2612–2633. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao S, Mi Y, Zheng B, Wei P, Gu Y, Zhang
Z, Xu Y, Cai S, Li X and Li D: Highly-metastatic colorectal cancer
cell released miR-181a-5p-rich extracellular vesicles promote liver
metastasis by activating hepatic stellate cells and remodelling the
tumour microenvironment. J Extracell Vesicles. 11:e121862022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of Epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ang HL, Mohan CD, Shanmugam MK, Leong HC,
Makvandi P, Rangappa KS, Bishayee A, Kumar AP and Sethi G:
Mechanism of epithelial-mesenchymal transition in cancer and its
regulation by natural compounds. Med Res Rev. 43:1141–1200. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jayachandran J, Srinivasan H and Mani KP:
Molecular mechanism involved in epithelial to mesenchymal
transition. Arch Biochem Biophys. 710:1089842021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho ES, Kang HE, Kim NH and Yook JI:
Therapeutic implications of cancer epithelial-mesenchymal
transition (EMT). Arch Pharm Res. 42:14–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang N, Ng AS, Cai S, Li Q, Yang L and
Kerr D: Novel therapeutic strategies: Targeting
epithelial-mesenchymal transition in colorectal cancer. Lancet
Oncol. 22:e358–e368. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kulus M, Farzaneh M, Bryja A, Zehtabi M,
Azizidoost S, Abouali Gale Dari M, Golcar-Narenji A, Ziemak H,
Chwarzyński M, Piotrowska-Kempisty H, et al: Phenotypic transitions
the processes involved in regulation of growth and proangiogenic
properties of stem cells, cancer stem cells and circulating tumor
cells. Stem Cell Rev Rep. 20:967–979. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akhurst RJ: From shape-shifting embryonic
cells to oncology: The fascinating history of epithelial
mesenchymal transition. Semin Cancer Biol. 96:100–114. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou S, Xu H, Duan Y, Tang Q, Huang H and
Bi F: Survival mechanisms of circulating tumor cells and their
implications for cancer treatment. Cancer Metastasis Rev.
43:941–957. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie Q, Liu S, Zhang S, Liao L, Xiao Z,
Wang S and Zhang P: Research progress on the multi-omics and
survival status of circulating tumor cells. Clin Exp Med.
24:492024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gkountela S, Castro-Giner F, Szczerba BM,
Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C,
Stirnimann CU, et al: Circulating tumor cell clustering shapes DNA
methylation to enable metastasis seeding. Cell. 176:98–112.e14.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rozenberg JM, Buzdin AA, Mohammad T,
Rakitina OA, Didych DA, Pleshkan VV and Alekseenko IV: Molecules
promoting circulating clusters of cancer cells suggest novel
therapeutic targets for treatment of metastatic cancers. Front
Immunol. 14:10999212023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hou JM, Krebs M, Ward T, Sloane R, Priest
L, Hughes A, Clack G, Ranson M, Blackhall F and Dive C: Circulating
tumor cells as a window on metastasis biology in lung cancer. Am J
Pathol. 178:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Francescangeli F, Magri V, De Angelis ML,
De Renzi G, Gandini O, Zeuner A, Gazzaniga P and Nicolazzo C:
Sequential isolation and characterization of single CTCs and large
CTC clusters in metastatic colorectal cancer patients. Cancers
(Basel). 13:63622021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leblanc R and Peyruchaud O: Metastasis:
New functional implications of platelets and megakaryocytes. Blood.
128:24–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Plantureux L, Mège D, Crescence L,
Carminita E, Robert S, Cointe S, Brouilly N, Ezzedine W,
Dignat-George F, Dubois C and Panicot-Dubois L: The Interaction of
platelets with colorectal cancer cells inhibits tumor growth but
promotes metastasis. Cancer Res. 80:291–303. 2020. View Article : Google Scholar
|
|
44
|
Szczerba BM, Castro-Giner F, Vetter M,
Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R,
Singer J, et al: Neutrophils escort circulating tumour cells to
enable cell cycle progression. Nature. 566:553–557. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Duda DG, Duyverman AM, Kohno M, Snuderl M,
Steller EJ, Fukumura D and Jain RK: Malignant cells facilitate lung
metastasis by bringing their own soil. Proc Natl Acad Sci USA.
107:21677–21682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ormseth B, Onuma A, Zhang H and Tsung A:
The hepatic Pre-metastatic niche. Cancers (Basel). 14:37312022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Trefts E, Gannon M and Wasserman DH: The
liver. Curr Biol. 27:R1147–R1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li X, Ramadori P, Pfister D, Seehawer M,
Zender L and Heikenwalder M: The immunological and metabolic
landscape in primary and metastatic liver cancer. Nat Rev Cancer.
21:541–557. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gong J, Tu W, Liu J and Tian D:
Hepatocytes: A key role in liver inflammation. Front Immunol.
13:10837802022. View Article : Google Scholar
|
|
50
|
Carvalho-Gontijo R, Han C, Zhang L, Zhang
V, Hosseini M, Mekeel K, Schnabl B, Loomba R, Karin M, Brenner DA
and Kisseleva T: Metabolic injury of hepatocytes promotes
progression of NAFLD and AALD. Semin Liver Dis. 42:233–249. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rigual MDM, Sanchez Sanchez P and Djouder
N: Is liver regeneration key in hepatocellular carcinoma
development? Trends Cancer. 9:140–157. 2023. View Article : Google Scholar
|
|
52
|
Amilca-Seba K, Sabbah M, Larsen AK and
Denis JA: Osteopontin as a regulator of colorectal cancer
progression and its clinical applications. Cancers (Basel).
13:37932021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li JJ, Wang JH, Tian T, Liu J, Zheng YQ,
Mo HY, Sheng H, Chen YX, Wu QN, Han Y, et al: The liver
microenvironment orchestrates FGL1-mediated immune escape and
progression of metastatic colorectal cancer. Nat Commun.
14:66902023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pandey E, Nour AS and Harris EN: Prominent
receptors of liver sinusoidal endothelial cells in liver
homeostasis and disease. Front Physiol. 11:8732020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Szafranska K, Kruse LD, Holte CF, McCourt
P and Zapotoczny B: The wHole story about fenestrations in LSEC.
Front Physiol. 12:7355732021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu GX, Wei S, Yu C, Zhao SQ, Yang WJ, Feng
YH, Pan C, Yang KX and Ma Y: Activation of Kupffer cells in NAFLD
and NASH: Mechanisms and therapeutic interventions. Front Cell Dev
Biol. 11:11995192023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li W, Chang N and Li L: Heterogeneity and
function of kupffer cells in liver injury. Front Immunol.
13:9408672022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kubes P and Jenne C: Immune responses in
the liver. Annu Rev Immunol. 36:247–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Matsumura H, Kondo T, Ogawa K, Tamura T,
Fukunaga K, Murata S and Ohkohchi N: Kupffer cells decrease
metastasis of colon cancer cells to the liver in the early stage.
Int J Oncol. 45:2303–2310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Williamson T, Sultanpuram N and Sendi H:
The role of liver microenvironment in hepatic metastasis. Clin
Transl Med. 8:212019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Keirsse J, Van Damme H, Geeraerts X,
Beschin A, Raes G and Van Ginderachter JA: The role of hepatic
macrophages in liver metastasis. Cell Immunol. 330:202–215. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang M and Zhang C: The role of liver
sinusoidal endothelial cells in cancer liver metastasis. Am J
Cancer Res. 11:1845–1860. 2021.PubMed/NCBI
|
|
63
|
Ou J, Peng Y, Deng J, Miao H, Zhou J, Zha
L, Zhou R, Yu L, Shi H and Liang H: Endothelial cell-derived
fibronectin extra domain A promotes colorectal cancer metastasis
via inducing epithelial-mesenchymal transition. Carcinogenesis.
35:1661–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ciner AT, Jones K, Muschel RJ and Brodt P:
The unique immune microenvironment of liver metastases: Challenges
and opportunities. Semin Cancer Biol. 71:143–156. 2021. View Article : Google Scholar
|
|
65
|
Yu X, Chen L, Liu J, Dai B, Xu G, Shen G,
Luo Q and Zhang Z: Immune modulation of liver sinusoidal
endothelial cells by melittin nanoparticles suppresses liver
metastasis. Nat Commun. 10:5742019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Crispe IN: The liver as a lymphoid organ.
Annu Rev Immunol. 27:147–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Prager I, Liesche C, van Ooijen H, Urlaub
D, Verron Q, Sandström N, Fasbender F, Claus M, Eils R, Beaudouin
J, et al: NK cells switch from granzyme B to death
receptor-mediated cytotoxicity during serial killing. J Exp Med.
216:2113–2127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shimasaki N, Jain A and Campana D: NK
cells for cancer immunotherapy. Nat Rev Drug Discov. 19:200–218.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wiltrout RH: Regulation and antimetastatic
functions of liver-associated natural killer cells. Immunol Rev.
174:63–76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lopez-Soto A, Gonzalez S, Smyth MJ and
Galluzzi L: Control of Metastasis by NK Cells. Cancer Cell.
32:135–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Donadon M, Hudspeth K, Cimino M, Di
Tommaso L, Preti M, Tentorio P, Roncalli M, Mavilio D and Torzilli
G: Increased infiltration of natural killer and T cells in
colorectal liver metastases improves patient overall survival. J
Gastrointest Surg. 21:1226–1236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Harmon C, Robinson MW, Hand F, Almuaili D,
Mentor K, Houlihan DD, Hoti E, Lynch L, Geoghegan J and O'Farrelly
C: Lactate-mediated acidification of tumor microenvironment induces
apoptosis of Liver-resident NK cells in colorectal liver
metastasis. Cancer Immunol Res. 7:335–346. 2019. View Article : Google Scholar
|
|
73
|
Stiff A, Trikha P, Mundy-Bosse B,
McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S,
Abood D, et al: Nitric oxide production by Myeloid-derived
suppressor cells plays a role in impairing Fc Receptor-mediated
natural killer cell function. Clin Cancer Res. 24:1891–1904. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Song J, Song H, Wei H, Sun R, Tian Z and
Peng H: Requirement of RORα for maintenance and antitumor immunity
of liver-resident natural killer cells/ILC1s. Hepatology.
75:1181–1193. 2022. View Article : Google Scholar
|
|
75
|
Toffoli EC, van Vliet AA, Verheul HWM, van
der Vliet HJ, Tuynman J, Spanholtz J and de Gruijl TD: Allogeneic
NK cells induce monocyte-to-dendritic cell conversion, control
tumor growth, and trigger a pro-inflammatory shift in
patient-derived cultures of primary and metastatic colorectal
cancer. J Immunother Cancer. 11:e0075542023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lin Y, Xu J and Lan H: Tumor-associated
macrophages in tumor metastasis: Biological roles and clinical
therapeutic applications. J Hematol Oncol. 12:762019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang H, Wang X, Zhang X and Xu W: The
promising role of tumor-associated macrophages in the treatment of
cancer. Drug Resist Updat. 73:1010412024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hou S, Zhao Y, Chen J, Lin Y and Qi X:
Tumor-associated macrophages in colorectal cancer metastasis:
Molecular insights and translational perspectives. J Transl Med.
22:622024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Dou L, Shi X, He X and Gao Y: Macrophage
phenotype and function in liver disorder. Front Immunol.
10:31122019. View Article : Google Scholar
|
|
80
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang J, Zhu N, Su X, Gao Y and Yang R:
Novel tumor-associated macrophage populations and subpopulations by
single cell RNA sequencing. Front Immunol. 14:12647742023.
View Article : Google Scholar
|
|
82
|
Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu
K, Hu X, Bu Z, Peng J, Ren X and Zhang Z: Immune phenotypic linkage
between colorectal cancer and liver metastasis. Cancer Cell.
40:424–437.e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matusiak M, Hickey JW, van IDGP, Lu G,
Kidziński L, Zhu S, Colburg DRC, Luca B, Phillips DJ, Brubaker SW,
et al: Spatially segregated macrophage populations predict distinct
outcomes in colon cancer. Cancer Discov. 14:1418–1439. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xu C, Sun L, Jiang C, Zhou H, Gu L, Liu Y
and Xu Q: SPP1, analyzed by bioinformatics methods, promotes the
metastasis in colorectal cancer by activating EMT pathway. Biomed
Pharmacother. 91:1167–1177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sathe A, Mason K, Grimes SM, Zhou Z, Lau
BT, Bai X, Su A, Tan X, Lee H, Suarez CJ, et al: Colorectal cancer
metastases in the liver establish immunosuppressive spatial
networking between tumor-sssociated SPP1+ macrophages and
fibroblasts. Clin Cancer Res. 29:244–260. 2023. View Article : Google Scholar
|
|
86
|
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D,
Cheng Y, Huang S, Liu Y, Jiang S, et al: Spatiotemporal immune
landscape of colorectal cancer liver metastasis at single-cell
level. Cancer Discov. 12:134–153. 2022. View Article : Google Scholar
|
|
87
|
Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z,
Ding X, Bao R, Hong L, Jia W, et al: Single-cell and spatial
analysis reveal interaction of FAP+ fibroblasts and
SPP1+ macrophages in colorectal cancer. Nat Commun.
13:17422022. View Article : Google Scholar
|
|
88
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nielsen SR, Quaranta V, Linford A, Emeagi
P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, et al:
Macrophage-secreted granulin supports pancreatic cancer metastasis
by inducing liver fibrosis. Nat Cell Biol. 18:549–560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kisseleva T and Brenner D: Molecular and
cellular mechanisms of liver fibrosis and its regression. Nat Rev
Gastroenterol Hepatol. 18:151–166. 2021. View Article : Google Scholar
|
|
91
|
Carloni V, Luong TV and Rombouts K:
Hepatic stellate cells and extracellular matrix in hepatocellular
carcinoma: More complicated than ever. Liver Int. 34:834–843. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ali E, Trailin A, Ambrozkiewicz F, Liska V
and Hemminki K: Activated hepatic stellate cells in hepatocellular
carcinoma: Their role as a potential target for future therapies.
Int J Mol Sci. 23:152922022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu H, Qiu F, Wang Y, Zeng Q, Liu C, Chen
Y, Liang CL, Zhang Q, Han L, Dai Z, et al: CD8+CD122+PD-1+ tregs
synergize with costimulatory blockade of CD40/CD154, but Not
B7/CD28, to prolong murine allograft survival. Front Immunol.
10:3062019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hsieh CC, Hung CH, Chiang M, Tsai YC and
He JT: Hepatic stellate cells enhance liver cancer progression by
inducing myeloid-derived suppressor cells through Interleukin-6
signaling. Int J Mol Sci. 20:50792019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Assudani DP, Horton RB, Mathieu MG,
McArdle SE and Rees RC: The role of CD4+ T cell help in cancer
immunity and the formulation of novel cancer vaccines. Cancer
Immunol Immunother. 56:70–80. 2007. View Article : Google Scholar
|
|
96
|
Lee HG, Cho MJ and Choi JM: Bystander CD4+
T cells: Crossroads between innate and adaptive immunity. Exp Mol
Med. 52:1255–1263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chalmin F, Mignot G, Bruchard M, Chevriaux
A, Végran F, Hichami A, Ladoire S, Derangère V, Vincent J, Masson
D, et al: Stat3 and Gfi-1 transcription factors control Th17 cell
immunosuppressive activity via the regulation of ectonucleotidase
expression. Immunity. 36:362–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kroemer M, Turco C, Spehner L, Viot J,
Idirène I, Bouard A, Renaude E, Deschamps M, Godet Y, Adotévi O, et
al: Investigation of the prognostic value of CD4 T cell subsets
expanded from tumor-infiltrating lymphocytes of colorectal cancer
liver metastases. J Immunother Cancer. 8:e0014782020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tosolini M, Kirilovsky A, Mlecnik B,
Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH,
Pagès F and Galon J: Clinical impact of different classes of
infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in
patients with colorectal cancer. Cancer Res. 71:1263–1271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Farhood B, Najafi M and Mortezaee K: CD8+
cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell
Physiol. 234:8509–8521. 2019. View Article : Google Scholar
|
|
101
|
St Paul M and Ohashi PS: The roles of
CD8+ T cell subsets in antitumor immunity. Trends Cell
Biol. 30:695–704. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Khan O, Giles JR, McDonald S, Manne S,
Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et
al: TOX transcriptionally and epigenetically programs
CD8+ T cell exhaustion. Nature. 571:211–218. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Oshi M, Asaoka M, Tokumaru Y, Yan L,
Matsuyama R, Ishikawa T, Endo I and Takabe K: CD8 T cell score as a
prognostic biomarker for triple negative breast cancer. Int J Mol
Sci. 21:69682020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Voskoboinik I, Whisstock JC and Trapani
JA: Perforin and granzymes: Function, dysfunction and human
pathology. Nat Rev Immunol. 15:388–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Masuda K, Kornberg A, Miller J, Lin S,
Suek N, Botella T, Secener KA, Bacarella AM, Cheng L, Ingham M, et
al: Multiplexed single-cell analysis reveals prognostic and
nonprognostic T cell types in human colorectal cancer. JCI Insight.
7:e1546462022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Doherty DG: Immunity, tolerance and
autoimmunity in the liver: A comprehensive review. J Autoimmun.
66:60–75. 2016. View Article : Google Scholar
|
|
107
|
Blank CU, Haining WN, Held W, Hogan PG,
Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al:
Defining 'T cell exhaustion'. Nat Rev Immunol. 19:665–674. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Freitas-Lopes MA, Mafra K, David BA,
Carvalho-Gontijo R and Menezes GB: Differential location and
distribution of hepatic immune cells. Cells. 6:482017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li H and Xia N: The multifaceted roles of
B lymphocytes in metabolic dysfunction-associated steatotic liver
disease. Front Immunol. 15:14473912024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Xu Y, Wei Z, Feng M, Zhu D, Mei S, Wu Z,
Feng Q, Chang W, Ji M, Liu C, et al: Tumor-infiltrated activated B
cells suppress liver metastasis of colorectal cancers. Cell Rep.
40:1112952022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang Y, Jia J, Wang F, Fang Y, Yang Y,
Zhou Q, Yuan W, Gu X, Hu J and Yang S: Pre-metastatic niche:
formation, characteristics and therapeutic implication. Signal
Transduct Target Ther. 9:2362024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Taher TE, Bystrom J, Ong VH, Isenberg DA,
Renaudineau Y, Abraham DJ and Mageed RA: Intracellular B lymphocyte
signalling and the regulation of humoral immunity and autoimmunity.
Clin Rev Allergy Immunol. 53:237–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chandnani N, Gupta I, Mandal A and Sarkar
K: Participation of B cell in immunotherapy of cancer. Pathol Res
Pract. 255:1551692024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sautes-Fridman C, Petitprez F, Calderaro J
and Fridman WH: Tertiary lymphoid structures in the era of cancer
immunotherapy. Nat Rev Cancer. 19:307–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fridman WH, Meylan M, Petitprez F, Sun CM,
Italiano A and Sautes-Fridman C: B cells and tertiary lymphoid
structures as determinants of tumour immune contexture and clinical
outcome. Nat Rev Clin Oncol. 19:441–457. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang Y, Liu G, Zeng Q, Wu W, Lei K, Zhang
C, Tang M, Zhang Y, Xiang X, Tan L, et al: CCL19-producing
fibroblasts promote tertiary lymphoid structure formation enhancing
anti-tumor IgG response in colorectal cancer liver metastasis.
Cancer Cell. 42:1370–1385.e9. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang Z, Lu Z, Lin S, Xia J, Zhong Z, Xie
Z, Xing Y, Qie J, Jiao M, Li Y, et al:
Leucine-tRNA-synthase-2-expressing B cells contribute to colorectal
cancer immunoevasion. Immunity. 55:1067–1081.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
de Ridder J, de Wilt JH, Simmer F,
Overbeek L, Lemmens V and Nagtegaal I: Incidence and origin of
histologically confirmed liver metastases: An explorative
case-study of 23,154 patients. Oncotarget. 7:55368–55376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sala RJ, Ery J, Cuesta-Peredo D, Muedra V
and Rodilla V: Complete blood count alterations prior to the
diagnosis of colorectal cancer may help in the detection of
synchronous liver metastases. J Clin Med. 12:65402023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhao D, Wang L, Chen Z, Zhang L and Xu L:
KRAS is a prognostic biomarker associated with diagnosis and
treatment in multiple cancers. Front Genet. 13:10249202022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Johnson D, Chee CE, Wong W, Lam RCT, Tan
IBH and Ma BBY: Current advances in targeted therapy for metastatic
colorectal cancer-Clinical translation and future directions.
Cancer Treat Rev. 125:1027002024. View Article : Google Scholar
|
|
122
|
Sinicrope FA, Shi Q, Allegra CJ, Smyrk TC,
Thibodeau SN, Goldberg RM, Meyers JP, Pogue-Geile KL, Yothers G,
Sargent DJ and Alberts SR: Association of DNA mismatch repair and
mutations in BRAF and KRAS with survival after recurrence in Stage
III colon cancers: A secondary analysis of 2 randomized clinical
trials. JAMA Oncol. 3:472–480. 2017. View Article : Google Scholar :
|
|
123
|
Kuipers EJ and Grobbee EJ: Personalised
screening for colorectal cancer, ready for take-off. Gut.
69:403–404. 2020. View Article : Google Scholar
|
|
124
|
Bipat S, van Leeuwen MS, Ijzermans JN,
Comans EF, Planting AS, Bossuyt PM, Greve JW and Stoker J:
Evidence-base guideline on management of colorectal liver
metastases in the Netherlands. Neth J Med. 65:5–14. 2007.PubMed/NCBI
|
|
125
|
Bonney GK, Chew CA, Lodge P, Hubbard J,
Halazun KJ, Trunecka P, Muiesan P, Mirza DF, Isaac J, Laing RW, et
al: Liver transplantation for non-resectable colorectal liver
metastases: The International Hepato-Pancreato-Biliary Association
consensus guidelines. Lancet Gastroenterol Hepatol. 6:933–946.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr;
ASCO: ASCO 2006 update of recommendations for the use of tumor
markers in gastrointestinal cancer. J Clin Oncol. 24:5313–5327.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Desch CE, Benson AB III, Somerfield MR,
Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS
and Petrelli NJ; American Society of Clinical Oncology: Colorectal
cancer surveillance: 2005 update of an American Society of Clinical
Oncology practice guideline. J Clin Oncol. 23:8512–8519. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chinese College of Surgeons; Section of
Gastrointestinal Surgery, Branch of Surgery, Chinese Medical
Association; Section of Colorectal Surgery, Branch of Surgery,
Chinese Medical Association; Colorectal Cancer Professional
Committee, Chinese Anti-Cancer Association; Colorectal Cancer
Professional Committee, Chinese Medical Doctor Association;
Colorectal Cancer Expert Committee, Chinese Society of Clinical
Oncology; Chinese Society of Colon & Rectal Surgeons, Chinese
College of Surgeons, Chinese Medical Doctor Association; Metastasis
Research Committee, Anorectal Branch of Chinese Medical Doctor
Association; Section of Colorectal Oncology, Oncology Branch,
Chinese Medical Association; Branch of Metastatic Tumor Therapy,
China International Exchange and Promotive Association for Medical
and Health Care; Branch of Colorectal Disease, China International
Exchange and Promotive Association for Medical and Health Care:
China guideline for diagnosis and comprehensive treatment of
colorectal liver metastases (version 2023). Zhonghua Wei Chang Wai
Ke Za Zhi. 26:1–15. 2023.In Chinese.
|
|
129
|
Adam R, de Gramont A, Figueras J, Kokudo
N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L,
Sobrero A, et al: Managing synchronous liver metastases from
colorectal cancer: A multid isciplinary international consensus.
Cancer Treat Rev. 41:729–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Creasy JM, Sadot E, Koerkamp BG, Chou JF,
Gonen M, Kemeny NE, Balachandran VP, Kingham TP, DeMatteo RP, Allen
PJ, et al: Actual 10-year survival after hepatic resection of
colorectal liver metastases: What factors preclude cure? Surgery.
163:1238–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sa Cunha A, Laurent C, Rault A, Couderc P,
Rullier E and Saric J: A second liver resection due to recurrent
colorectal liver metastases. Arch Surg. 142:1144–1149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Prior TW, Leach ME and Finanger EL: Spinal
Muscular Atrophy. GeneReviews(®). Adam MP, Feldman J,
Mirzaa GM, Pagon RA, Wallace SE and Amemiya A: University of
Washington; Seattle: Copyright © 1993-2025, University of
Washington, Seattle. GeneReviews is a registered trademark of the
University of Washington, Seattle. All rights reserved., Seattle
(WA). 1993
|
|
133
|
Boudjema K, Locher C, Sabbagh C,
Ortega-Deballon P, Heyd B, Bachellier P, Métairie S, Paye F,
Bourlier P, Adam R, et al: Simultaneous versus delayed resection
for initially resectable synchronous colorectal cancer liver
metastases: A prospective, open-label, randomized, controlled
trial. Ann Surg. 273:49–56. 2021. View Article : Google Scholar
|
|
134
|
Ge Y, Lei S, Cai B, Gao X, Wang G, Wang L
and Wang Z: Incidence and prognosis of pulmonary metastasis in
colorectal cancer: A population-based study. Int J Colorectal Dis.
35:223–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Huo X, Zhang L and Li T: Analysis of the
association of the expression of KiSS-1 in colorectal cancer
tissues with the pathology and prognosis. Oncol Lett. 15:3056–3060.
2018.PubMed/NCBI
|
|
136
|
Jin LJ, Chen WB, Zhang XY, Bai J, Zhao HC
and Wang ZY: Analysis of factors potentially predicting prognosis
of colorectal cancer. World J Gastrointest Oncol. 11:1206–1217.
2019. View Article : Google Scholar
|
|
137
|
Ganesh K, Stadler ZK, Cercek A, Mendelsohn
RB, Shia J, Segal NH and Diaz LA Jr: Immunotherapy in colorectal
cancer: Rationale, challenges and potentia. Nat Rev Gastroenterol
Hepatol. 16:361–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. PubMed/NCBI
|
|
139
|
Yuan L, Tan Z, Huang J, Chen F, Hambly BD,
Bao S and Tao K: Exploring the clinical significance of IL-38
correlation with PD-1, CTLA-4, and FOXP3 in colorectal cancer
draining lymph nodes. Front Immunol. 15:13845482024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kim RD, Kovari BP, Martinez M, Xie H,
Sahin IH, Mehta R, Strosberg J, Imanirad I, Ghayouri M, Kim YC, et
al: A phase I/Ib study of regorafenib and nivolumab in mismatch
repair proficient advanced refractory colorectal cancer. Eur J
Cancer. 169:93–102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Saberzadeh-Ardestani B, Jones JC, Hubbard
JM, McWilliams RR, Halfdanarson TR, Shi Q, Sonbol MB, Ticku J, Jin
Z and Sinicrope FA: Association between survival and metastatic
site in mismatch repair–deficient metastatic colorectal cancer
treated with first-line pembrolizumab. JAMA Netw Open. 6:e230400.
2023. View Article : Google Scholar
|
|
142
|
Chen EX, Loree JM, Titmuss E, Jonker DJ,
Kennecke HF, Berry S, Couture F, Ahmad CE, Goffin JR, Kavan P, et
al: Liver metastases and immune checkpoint inhibitor efficacy in
patients with refractory metastatic colorectal cancer: A secondary
analysis of a randomized clinical trial. JAMA Netw Open.
6:e2346094. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Hecht JR, Raman SS, Chan A, Kalinsky K,
Baurain JF, Jimenez MM, Garcia MM, Berger MD, Lauer UM, Khattak A,
et al: Phase Ib study of talimogene laherparepvec in combination
with atezolizumab in patients with triple negative breast cancer
and colorectal cancer with liver metastases. ESMO Open.
8:1008842023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Taïeb J, Bouche O, André T, Le Malicot K,
Laurent-Puig P, Bez J, Toullec C, Borg C, Randrian V, Evesque L, et
al: Avelumab vs standard second-line chemotherapy in patients with
metastatic colorectal cancer and microsatellite instability: A
randomized clinical trial. JAMA Oncol. 9:1356–1363. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang R, Lian J, Wang X, Pang X, Xu B, Tang
S, Shao J and Lu H: Survival rate of colorectal cancer in China: A
systematic review and meta-analysis. Front Oncol. 13:10331542023.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Saberzadeh-Ardestani B, Jones JC, Hubbard
JM, McWilliams RR, Halfdanarson TR, Shi Q, Sonbol MB, Ticku J, Jin
Z and Sinicrope FA: Association between survival and metastatic
site in mismatch Repair-deficient metastatic colorectal cancer
treated with First-line pembrolizumab. JAMA Netw Open.
6:e2304002023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Diaz LA, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
André T, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Nivolumab plus low-dose ipilimumab in previously treated
patients with microsatellite instability-high/mismatch
repair-deficient metastatic colorectal cancer: 4-year follow-up
from CheckMate 142. Ann Oncol. 33:1052–1060. 2022. View Article : Google Scholar
|
|
149
|
Del Paggio JC: Immunotherapy: Cancer
immunotherapy and the value of cure. Nat Rev Clin Oncol.
15:268–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kong X, Li Q, Wang D, Wang M, Yang F and
Meng J: Mechanism of Qizhen decoction-mediated maturation of DC
cells to activate the IL-12/JAK2/STAT4 pathway to sensitise PD-1
inhibitors in the treatment of colorectal cancer. J Ethnopharmacol.
320:1173992024. View Article : Google Scholar
|
|
151
|
Chen L, Jiang X, Li Y, Zhang Q, Li Q,
Zhang X, Zhang M, Yu Q and Gao D: How to overcome tumor resistance
to anti-PD-1/PD-L1 therapy by immunotherapy modifying the tumor
microenvironment in MSS CRC. Clin Immunol. 237:1089622022.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Peng X, Xu Z, Guo Y and Zhu Y:
Necroptosis-related genes associated with immune activity and
prognosis of colorectal cancer. Front Genet. 13:9092452022.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Fan T, Zhang M, Yang J, Zhu Z, Cao W and
Dong C: Therapeutic cancer vaccines: Advancements, challenges, and
prospects. Signal Transduct Target Ther. 8:4502023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Sarvizadeh M, Ghasemi F, Tavakoli F, Sadat
Khatami S, Razi E, Sharifi H, Biouki NM and Taghizadeh M: Vaccines
for colorectal cancer: An update. J Cell Biochem. 120:8815–8828.
2019. View Article : Google Scholar
|
|
155
|
Morse MA, Niedzwiecki D, Marshall JL,
Garrett C, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ, Dessureault S,
Hobeika AC, et al: A randomized phase II study of immunization with
dendritic cells modified with poxvectors encoding CEA and MUC1
compared with the same poxvectors plus GM-CSF for resected
metastatic colorectal cancer. Ann Surg. 258:879–886. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Rodriguez J, Castañón E, Perez-Gracia JL,
Rodriguez I, Viudez A, Alfaro C, Oñate C, Perez G, Rotellar F,
Inogés S, et al: A randomized phase II clinical trial of dendritic
cell vaccination following complete resection of colon cancer liver
metastasis. J Immunother Cancer. 6:962018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Busch DH, Fräßle SP, Sommermeyer D,
Buchholz VR and Riddell SR: Role of memory T cell subsets for
adoptive immunotherapy. Semin Immunol. 28:28–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Chan JD, Lai J, Slaney CY, Kallies A,
Beavis PA and Darcy PK: Cellular networks controlling T cell
persistence in adoptive cell therapy. Nat Rev Immunol. 21:769–784.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Dougé A, El Ghazzi N, Lemal R and Rouzaire
P: Adoptive T cell therapy in solid tumors: State-of-the Art,
current challenges, and upcoming improvements. Mol Cancer Ther.
23:272–284. 2024. View Article : Google Scholar
|
|
160
|
Chai LF, Hardaway JC, Heatherton KR,
O'Connell KP, LaPorte JP, Guha P, Lopes MC, Rabinowitz BA, Jaroch
D, Cox BF, et al: Antigen receptor T cells (CAR-T) effectively
control tumor growth in a colorectal liver metastasis model. J Surg
Res. 272:37–50. 2022. View Article : Google Scholar
|
|
161
|
Zhang C, Wang Z, Yang Z, Wang M, Li S, Li
Y, Zhang R, Xiong Z, Wei Z, Shen J, et al: Phase I escalating-Dose
trial of CAR-T therapy targeting CEA+ metastatic colorectal
cancers. Mol Ther. 25:1248–1258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Xu J, Meng Q, Sun H, Zhang X, Yun J, Li B,
Wu S, Li X, Yang H, Zhu H, et al: HER2-specific chimeric antigen
receptor-T cells for targeted therapy of metastatic colorectal
cancer. Cell Death Dis. 12:11092021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhou X, Yang M, Yu J, Tan J, Xu N, Zhou Y,
Zhang W, Ma J, Zhang Z, Friedlaender A, et al: Regional delivery of
mesothelin-targeted chimeric antigen receptor T-cell effectively
and safely targets colorectal cancer liver metastases in mice. J
Gastrointest Oncol. 15:312–329. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Martínez Bedoya D, Dutoit V and Migliorini
D: Allogeneic CAR T cells: An alternative to overcome challenges of
CAR T cell therapy in glioblastoma. Front Immunol. 12:6400822021.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Pabla B, Bissonnette M and Konda VJ: Colon
cancer and the epidermal growth factor receptor: Current treatment
paradigms, the importance of diet, and the role of chemoprevention.
World J Clin Oncol. 6:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Christodoulou C: Reply to the reply to the
letter to the editor on 'Scalp metastases and scalp cooling for
chemotherapy-induced alopecia prevention', by W. P. M. Breed
(doi:10.1093/annonc/mdj085). Ann Oncol. 17:7252006. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH,
Atkins JN, et al: Effect of First-line chemotherapy combined with
cetuximab or bevacizumab on overall survival in patients with KRAS
Wild-type advanced or metastatic colorectal cancer: A randomized
clinical trial. JAMA. 317:2392–2401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Stintzing S, Modest DP, Rossius L, Lerch
MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U,
Al-Batran SE, Heintges T, et al: FOLFIRI plus cetuximab versus
FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3):
A post-hoc analysis of tumour dynamics in the final RAS wild-type
subgroup of this randomised open-label phase 3 trial. Lancet Oncol.
17:1426–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Khattak MA, Martin H, Davidson A and
Phillips M: Role of first-line anti-epidermal growth factor
receptor therapy compared with anti-vascular endothelial growth
factor therapy in advanced colorectal cancer: A meta-analysis of
randomized clinical trials. Clin Colorectal Cancer. 14:81–90. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Creasy JM, Sadot E, Koerkamp BG, Chou JF,
Gonen M, Kemeny NE, Saltz LB, Balachandran VP, Peter Kingham T,
DeMatteo RP, et al: The impact of primary tumor location on
Long-term survival in patients undergoing hepatic resection for
metastatic colon cancer. Ann Surg Oncol. 25:431–438. 2018.
View Article : Google Scholar
|
|
174
|
Arnold D, Lueza B, Douillard JY, Peeters
M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP,
Tabernero J, et al: Prognostic and predictive value of primary
tumour side in patients with RAS wild-type metastatic colorectal
cancer treated with chemotherapy and EGFR directed antibodies in
six randomized trials. Ann Oncol. 28:1713–1729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS Wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. 3:194–201. 2017. View Article : Google Scholar
|
|
176
|
Boeckx N, Koukakis R, Op de Beeck K, Rolfo
C, Van Camp G, Siena S, Tabernero J, Douillard JY, André T and
Peeters M: Primary tumor sidedness has an impact on prognosis and
treatment outcome in metastatic colorectal cancer: Results from two
randomized first-line panitumumab studies. Ann Oncol. 28:1862–1868.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Hecht JR, Cohn A, Dakhil S, Saleh M,
Piperdi B, Cline-Burkhardt M, Tian Y and Go WY: SPIRITT: A
randomized, multicenter, Phase II study of panitumumab with FOLFIRI
and bevacizumab with FOLFIRI as Second-line treatment in patients
with unresectable wild type KRAS metastatic colorectal cancer. Clin
Colorectal Cancer. 14:72–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Folprecht G, Gruenberger T, Bechstein W,
Raab HR, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J,
Lang H, Trarbach T, et al: Survival of patients with initially
unresectable colorectal liver metastases treated with
FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary
concept (CELIM study). Ann Oncol. 25:1018–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai
SY, Ye QH, Yu Y, Xu B, Qin XY and Xu J: Randomized controlled trial
of cetuximab plus chemotherapy for patients with KRAS wild-type
unresectable colorectal liver-limited metastases. J Clin Oncol.
31:1931–1938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Carrato A, Abad A, Massuti B, Grávalos C,
Escudero P, Longo-Muñoz F, Manzano JL, Gómez A, Safont MJ, Gallego
J, et al: First-line panitumumab plus FOLFOX4 or FOLFIRI in
colorectal cancer with multiple or unresectable liver metastases: A
randomised, phase II trial (PLANET-TTD). Eur J Cancer. 81:191–202.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Primrose J, Falk S, Finch-Jones M, Valle
J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M,
Iveson T, et al: Systemic chemotherapy with or without cetuximab in
patients with resectable colorectal liver metastasis: The New EPOC
randomised controlled trial. Lancet Oncol. 15:601–611. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Bisschop C, van Dijk TH, Beukema JC,
Jansen RLH, Gelderblom H, de Jong KP, Rutten HJT, van de Velde CJH,
Wiggers T, Havenga K and Hospers GAP: Short-Course radiotherapy
followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin
and subsequent radical treatment in primary Stage IV rectal cancer:
Long-term results of a phase II study. Ann Surg Oncol.
24:2632–2638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Chen HH, Lin JK, Chen JB, Chuang CH, Liu
MC, Wang JY and Changchien CR: Neoadjuvant therapy of bevacizumab
in combination with oxaliplatin and capecitabine (XELOX) for
patients with metastatic colorectal cancer with unresectable liver
metastases: A phase II, open-label, single-arm, noncomparative
trial. Asia Pac J Clin Oncol. 14:61–68. 2018. View Article : Google Scholar
|
|
184
|
Pietrantonio F, Cotsoglou C, Fuca G, Lo
Vullo S, Nichetti F, Milione M, Coppa J, Vaiani M, Alessi A,
Prisciandaro M, et al: Perioperative Bevacizumab-based triplet
chemotherapy in patients with potentially resectable colorectal
cancer liver metastases. Clin Colorectal Cancer. 18:34–43.e6. 2019.
View Article : Google Scholar
|
|
185
|
Yasuno M, Uetake H, Ishiguro M, Mizunuma
N, Komori T, Miyata G, Shiomi A, Kagimura T and Sugihara K:
mFOLFOX6 plus bevacizumab to treat liver-only metastases of
colorectal cancer that are unsuitable for upfront resection
(TRICC0808): A multicenter phase II trial comprising the final
analysis for survival. Int J Clin Oncol. 24:516–525. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Takii Y, Maruyama S and Nogami H: Can the
prognosis of colorectal cancer be improved by surgery? World J
Gastrointest Surg. 8:574–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Zhou F and Ding J: Prognosis and factors
affecting colorectal cancer with ovarian metastasis. Updates Surg.
73:391–398. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ramdhani K, Smits MLJ, Lam MGEH and Braat
AJAT: Combining selective internal radiation therapy with
immunotherapy in treating hepatocellular carcinoma and hepatic
colorectal metastases: A systematic review. Cancer Biother
Radiopharm. 38:216–224. 2023.PubMed/NCBI
|
|
189
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. PubMed/NCBI
|
|
190
|
Azeredo-da-Silva ALF, de Jesus VHF,
Agirrezabal I, Brennan VK, Carion PL, Amoury N, Vetromilla BM,
Zanotto BS, Shergill S and Ziegelmann PK: Selective internal
radiation therapy using Y-90 resin microspheres for metastatic
colorectal cancer: An updated systematic review and network
Meta-analysis. Adv Ther. 41:1606–1620. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhu Y, Yu J, Zhang K, Feng Y, Guo K, Sun L
and Ruan S: Network pharmacology analysis to explore the
pharmacological mechanism of effective Chinese medicines in
treating metastatic colorectal cancer using Meta-analysis approach.
Am J Chin Med. 49:1839–1870. PubMed/NCBI
|
|
192
|
Lam W, Bussom S, Guan F, Jiang Z, Zhang W,
Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45ra592010. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Sun Q, He M, Zhang M, Zeng S, Chen L, Zhao
H, Yang H, Liu M, Ren S and Xu H: Traditional Chinese medicine and
colorectal cancer: Implications for drug discovery. Front
Pharmacol. 12:6850022021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Liu N, Wu C, Jia R, Cai G, Wang Y, Zhou L,
Ji Q, Sui H, Zeng P, Xiao H, et al: Traditional Chinese medicine
combined with chemotherapy and cetuximab or bevacizumab for
metastatic colorectal cancer: A randomized, double-blind,
Placebo-controlled clinical trial. Front Pharmacol. 11:4782020.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Zhang T, He WT, Zi MJ, Song G, Yi DH and
Yang YF: Cohort study on prognosis of patients with metastatic
colorectal cancer treated with integrated Chinese and Western
medicine. Chin J Integr Med. 24:573–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Tang M, Zhang W, Qin W, Zou C, Yan Y, He
B, Xu Y, Zhang Y, Liu J, Sun H and Yang Y: Association between Oral
Chinese herbal medicine and recurrence and metastasis in patients
with stages II and III colorectal cancer: A cohort study in China.
Evid Based Complement Alternat Med. 2022:85293952022. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Hsiao WL and Liu L: The role of
traditional Chinese herbal medicines in cancer therapy-from TCM
theory to mechanistic insights. Planta Med. 76:1118–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Rodrigues JA and Correia JH: Photodynamic
therapy for colorectal cancer: An update and a look to the future.
Int J Mol Sci. 24:122042023. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
van Duijnhoven FH, Rovers JP, Engelmann K,
Krajina Z, Purkiss SF, Zoetmulder FA, Vogl TJ and Terpstra OT:
Photodynamic therapy with 5,10,15, 20 -Tetrak is (m-Hydroxyphenyl)
bacteriochlorin for colorectal liver metastases is safe and
feasible: Results from a phase I study. Ann Surg Oncol. 12:808–816.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Gu B, Wang B, Li X, Feng Z, Ma C, Gao L,
Yu Y, Zhang J, Zheng P, Wang Y, et al: Photodynamic therapy
improves the clinical efficacy of advanced colorectal cancer and
recruits immune cells into the tumor immune microenvironment. Front
Immunol. 13:10504212022. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Hao Y, Gu Z, Yu Z, Schomann T, Sayedipour
S, Aguilar JC, Ten Dijke P and Cruz LJ: Photodynamic therapy in
combination with the hepatitis B core virus-like particles (HBc
VLPs) to prime anticancer immunity for colorectal cancer treatment.
Cancers (Basel). 14:27242022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Liang X, Chen M, Bhattarai P, Hameed S and
Dai Z: Perfluorocarbon@Porphyrin nanoparticles for tumor hypoxia
relief to enhance photodynamic therapy against liver metastasis of
colon cancer. ACS Nano. 14:13569–13583. 2020. View Article : Google Scholar : PubMed/NCBI
|