Long non-coding RNAs (lncRNAs) are transcribed by
RNA polymerase II and range in length from 200 nucleotides to 100
kilobases without coding for proteins (1); ~95% of the human genome consists of
nc sequences that are transcribed into lncRNAs (2). These lncRNAs can be classified into
long intergenic nc (linc), enhancer, intronic and antisense lncRNAs
(3). Additionally, lncRNAs
modulate gene expression involved in multiple biological processes,
including cell apoptosis, proliferation and differentiation and
post-transcriptional, translational and epigenetic regulation
(4,5).
lncRNAs play important roles in regulating chromatin
structure and oncogene expression, thereby contributing to
tumorigenesis (6).
Mechanistically, lncRNAs directly recruit epigenetic and/or
epitranscriptomic regulators to control oncogene expression,
driving tumor development and progression (7). Rapid advancements in genome-wide
technologies are accelerating identification of novel lncRNAs and
their regulatory mechanisms in carcinogenesis. Notably, lncRNAs are
involved in epigenetic regulation by modifying chromatin structures
(primarily acetylation and methylation) through specific enzymes,
including DNA- and histone-modifying enzymes, and chromatin
organization-associated proteins (8). Furthermore, lncRNAs are essential
for modulating the three-dimensional (3D) genomic architecture
(9). CCCTC binding factor (CTCF),
regulated by lncRNAs, serves as a master regulator of mammalian
chromatin topologically associated domain (TAD) (10). CTCF controls oncogene expression
by mediating chromatin TAD architecture and enhancer-promoter
contacts within TADs (11). CTCF
contains RNA-binding regions (RBRs) within its zinc finger (ZF)
domains recognized by lncRNAs, which are key for CTCF
self-clustering and CTCF-mediated long-range chromatin interaction
(12,13). Deletion of RBRs notably disrupts
half of CTCF mediated chromatin loops to cause deregulation of gene
expression (12). lncRNAs
cooperate with CTCF to mediate genome topological regulation,
thereby leading to carcinogenesis (9). lncRNAs also regulate oncogene
expression and carcinogenesis by recruitment of epitranscriptomic
regulators, including RNA-modifying enzymes mediating RNA
N6-methyladenosine (m6A) modification and RNA
adenosine-to-inosine (A-to-I) editing (14). Thus, future studies may elucidate
the interlocking functions of lncRNAs with both epigenome and
epitranscriptome to develop novel cancer therapies and improve
prognostic strategies (15).
Understanding the epigenetic and epitranscriptomic
regulatory roles of lncRNAs in carcinogenesis is key for unraveling
the molecular mechanisms underlying cancer development and
progression. The present review aims to provide a comprehensive
overview of how lncRNAs influence gene expression in carcinogenesis
and their potential as diagnostic markers and therapeutic targets
in oncology.
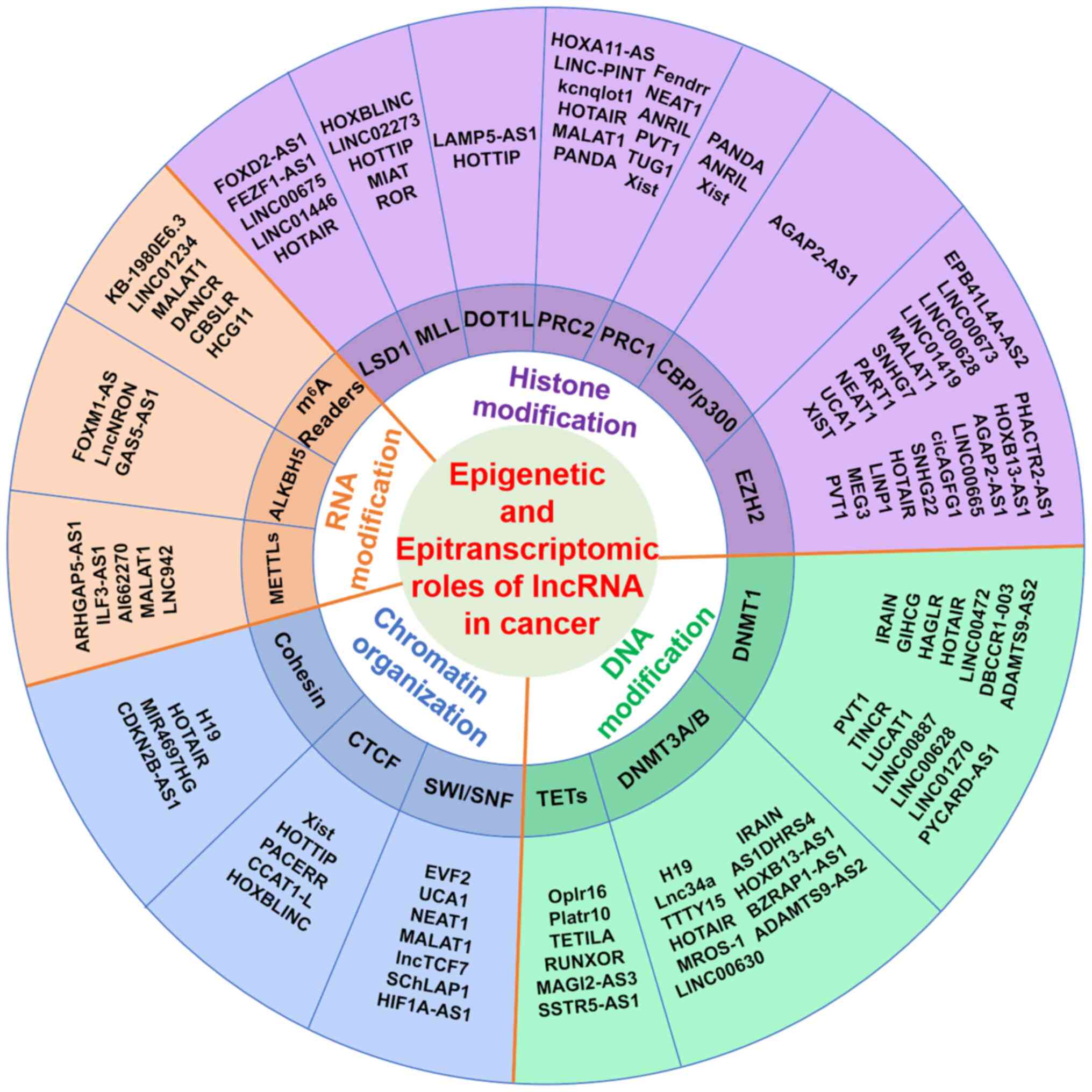
lncRNAs play key roles in mediating DNA
modification, histone modification and chromatin organization to
regulate oncogene expression in cancers. lncRNAs recruit DNA- and
histone-modifying enzymes and chromatin organization-associated
proteins at specific genomic loci to modulate gene expression in
carcinogenesis (5,7,16-18).
DNA methylation is a critical epigenetic process in
which methyl groups are added to cytosine residues to form
5-methylcytosine (5mC) modification in the DNA sequence,
particularly in promoter CpG islands (19). This modification serves a key role
in epigenetic regulation, specifically in controlling oncogene
expression and influencing tumor development and progression
(20). In cancer, there are five
types of DNA methyltransferases (DNMTs) involved in DNA 5mC
modification, namely DNMT1, DNMT2, DNMT3A, DNMT3B and DNMT3L
(21). Abnormal DNA methylation
patterns, such as hypermethylation of tumor suppressor genes or
hypomethylation of oncogenes, are associated with the onset and
progression of various types of cancer (19). For example, hypermethylation
reduces the expression of p16(INK4a) in melanoma (22), the BRCA1 gene is
hypermethylated in breast cancer (23), and the Myc gene is
hypomethylated in hepatocellular carcinoma (HCC) (24).
DNMT3s, specifically DNMT3A and DNMT3B, serve a
crucial role in initiating DNA methylation (25). These lncRNAs reprogram the DNA 5mC
methylation landscape, facilitate DNA self-assembly, and serve as
universal cancer biomarkers to promote carcinogenesis (20). Mechanistically, lncRNAs recruit
DNMT3A and DNMT3B to target oncogenes and influence methylation
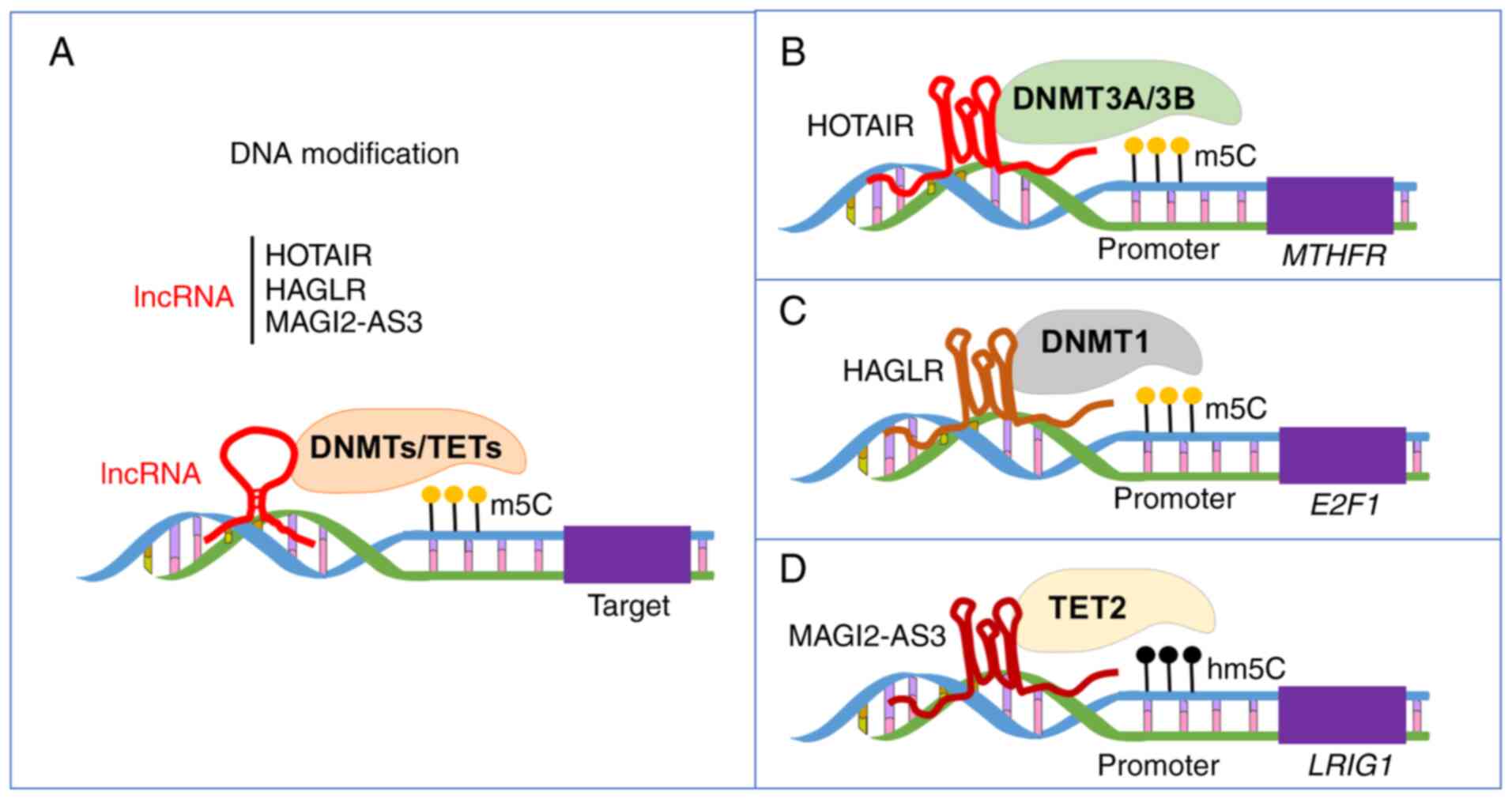
status of their promoters and regulatory regions (16) (Fig.
1A). lncRNA ADAMTS9-AS2 recruits DNMT3s to the cadherin
3 promoter CpG islands, thereby decreasing proliferation, invasion
and migration of esophageal cancer cells (26). lncRNA HOTAIR regulates
MTHFR (methylenetetrahydrofolate reductase) gene expression
by recruiting DNMT3s to mediate DNA methylation at the MTHFR
gene promoter, conferring chemoresistance in esophageal cancer
(27) (Fig. 1B). Additionally, lnc34a
recruits DNMT3A and prohibitin 2 to silence microRNA
(miR)-34a expression, promoting colorectal cancer
proliferation (28). lncRNA
TTTY15 mediates DNMT3A to increase 5mC modification at
TBX4 promoter, leading to dysregulation of TBX4 gene
expression associated with non-small cell lung cancer (NSCLC) cell
proliferation and metastasis (29). lncRNA AS1DHRS4 enhances DNA
methylation at the DHRS4L2 (Dehydrogenase/reductase member 4
like 2) promoter region to suppress the DHRS4 gene
expression in carcinogenesis (30). lncRNA MROS-1 modulates
PRUNE2 (prune homolog 2 with BCH domain) expression to
enhance oral cancer migration by interacting with DNMT3A (31). lncRNA IRAIN inhibits
VEGFA expression to suppress renal carcinoma tumor growth by
recruitment of DNMT3A/B to the VEGFA promoter region
(32). Collectively, lncRNAs
serve a critical role in recruiting DNMT3s to mediate aberrant DNA
methylation patterns in cancer development and carcinogenesis
(Fig. 1B).
Notably, lncRNAs serve a critical role in
maintaining DNA 5mC methylation in cancer genome by recruiting
DNMT1 (Fig. 1C) (25). For example, lncRNA HAGLR
functions as a tumor suppressor by recruiting DNMT1 to the promoter
of E2F Transcription Factor 1) gene, inhibiting lung adenocarcinoma
cell proliferation (33)
(Fig. 1C). Depletion of lncRNA
LUCAT1 promotes the ubiquitination of DNMT1 and enhances
expression of UHRF1 (Ubiquitin Like with PHD and Ring Finger
Domains 1) gene in esophageal squamous cell carcinoma (34). Additionally, loss of CCDC26
(Coiled-Coil Domain Containing 26) results in genome-wide
hypomethylation, increasing double-stranded DNA breaks and inducing
hepatocellular carcinoma cell death (35). Similarly, lncRNA DBCCR1-003
inhibits DNA methylation at the DBCCR1 (Deleted in bladder
cancer chromosome region 1) promoter region by sequestering DNMT1,
decreasing bladder cancer cell proliferation (36). lncRNA H19 mediates DNA
methylation and NAT1 (N-acetyltransferase 1) gene
expression, contributing to breast cancer chemoresistance (37). Moreover, lncRNA PVT1
recruits DNMT1 to the miR-18b-5p DNA promoter, forming the
PVT1 (plasmacytoma variant translocation gene
1)/miR-18b-5p/HIF1A (hypoxia inducible factor 1
subunit alpha) regulation axis in gallbladder cancer (38), suggesting its potential
therapeutic role (38). Thus,
lncRNAs play critical roles in cooperation with DNMT1
methyltransferase to maintain DNA 5mC methylation and regulate
oncogene expression in the cancer genome (Fig. 1C).
Additionally, ten-eleven translocation (TET) family
proteins (TET1, TET2 and TET3) oxidize 5mC) to
5-hydroxymethylcytosine, activating DNA demethylation (39,40) (Fig.
1D). Previous studies have highlighted a subset of lncRNAs that
interact with TETs to regulate DNA demethylation in carcinogenesis
(41-43). For instance, lncRNA Oplr16
recruits TET2 to the OCT4 (Octamer-Binding Transcription
Factor 4) promoter, mediating promoter-enhancer loops regulation of
OCT4 expression in tumorigenesis (44). Similarly, lncRNA Platr10
interacts with TET1 to mediate DNA demethylation at the OCT4
promoter (45). Additionally,
lncRNA TETILA mediates TET2 subcellular localization by
binding to the double-stranded β-helix domain in acute myeloid
leukemia (AML) (42). lncRNA
MAGI2-AS3 recruits TET2 to the LRIG1 (leucine rich
repeats and immunoglobulin like domains 1) promoter, upregulating
LRIG1 expression and inhibiting leukemic stem cell (LSC)
proliferation (46) (Fig. 1D). lncRNA RUNXOR triggers
DNA demethylation and activates expression of RUNX1 gene to
suppress breast cancer proliferation by interacting with TETs
(47). In short, lncRNAs are key
for regulating the DNA demethylation in tumorigenesis by recruiting
TET family proteins (Fig.
1D).
Collectively, lncRNAs exert their influence by
interacting with DNA-modifying enzymes. Dysregulation of
DNA-modifying enzymes alters the epigenetic landscape of the
genome, driving cancer development and progression. Specifically,
lncRNAs promote DNA methylation or demethylation at promoters of
the tumor suppressor or activator genes by interacting with the
DNMTs or TETs, thereby inducing carcinogenesis (Fig. 1A-D; Table SI).
lncRNAs interact with histone-modifying complexes,
which catalyze reversible histone modification, regulating
chromatin accessibility, DNA replication and gene transcription
during the development and progression of cancer (48). The histone modifications are
catalyzed by histone-modifying complexes, including 'writer' and
'eraser' proteins. Writer proteins comprise histone
methyltransferases (HMTs) and histone acetyltransferases (HATs),
which deposit methyl and acetyl groups on the lysine-rich
amino-terminal tails of histone proteins, respectively. By
contrast, eraser proteins include histone demethylases and
deacetylases, which can remove the aforementioned groups. lncRNAs
directly interact with histone-modifying enzymes to modulate gene
expression associated with cell proliferation, cell cycle
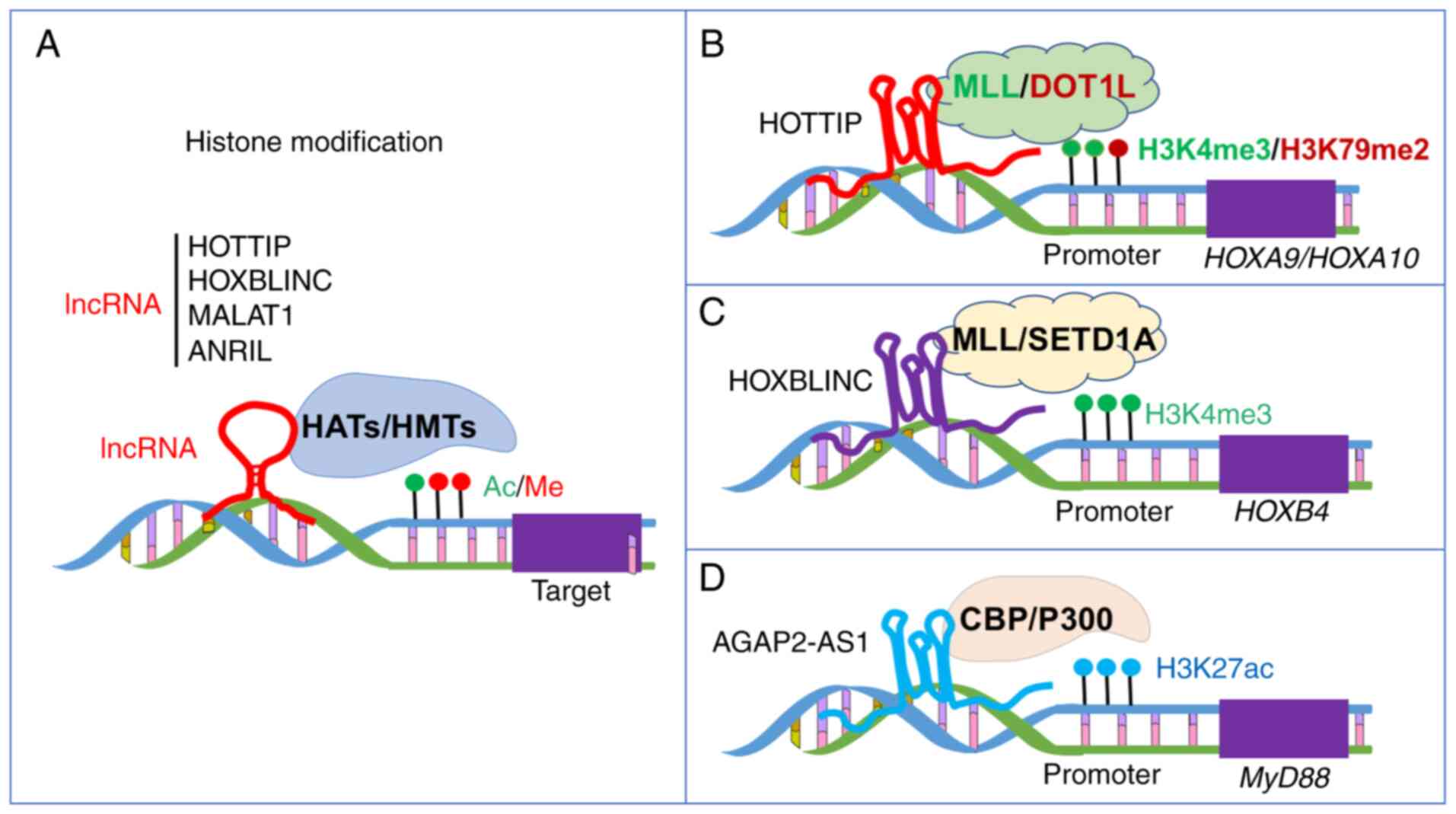
progression, apoptosis and metastasis (Fig. 2A).
lncRNAs are key players in regulating gene
expression and impacting various cellular processes. Specifically,
lncRNAs serve critical roles in silencing oncogene expression by
recruiting H3K9me or H3K27me-associated histone methyltransferase
complexes. For example, lncRNA PHACTR2-AS1 mediates H3K9me
of ribosomal DNA, leading to the suppression of rRNA transcription
and inhibiting cell proliferation and metastasis of breast cancer
by recruiting EZH2 (Enhancer of Zeste Homolog 2) (56,57). Similarly, the antisense ncRNA
Kcnq1ot1 interacts with polycomb repressive complex (PRC2)
components EZH2, SUZ12 (suppressor of zeste 12 homolog), and G9a to
silence the potassium voltage-gated channel subfamily Q member 1)
gene, contributing to tumorigenesis (58). Furthermore, lncRNA HOTAIR
lncRNA has been implicated in promoting tumor progression and
metastasis in various types of cancer, including breast and
pancreatic cancer, NSCLC and gastrointestinal stromal tumor
(59-62). Mechanistically, HOTAIR
recruits PRC2 and LSD1 (Lysine specific demethylase 1)/REST
corepressor 1) epigenetic complexes to increase H3K27me3 and
decrease H3K4me2 to downregulate p21 expression (63-66). Moreover, lncRNA FEZF1-AS1
specifically binds LSD1 to regulate the expression of CDKN1A
gene, contributing to pathogenesis of colorectal carcinoma, glioma
and gastric cancer (67). lncRNA
Air recruits G9a enzyme to mediate H3K9 methylation at the
Slc22a3 (Solute carrier family 22 member 3) gene promoter,
thereby repressing Slc22a3 expression in carcinogenesis
(51). lncRNA EPB41L4A-AS2
regulates RARRES1 (Retinoic acid receptor responder 1) and
MyD88 via H3K27me3 modification to suppress breast cancer
invasion and metastasis (68).
lncRNA MALAT1 suppresses E-cadherin expression to
promote osteosarcoma (OS) metastasis by interacting with PRC2
complex component EZH2, embryonic ectoderm development gene) and
SUZ12 (69,70). lncRNA ANRIL interacts with
PRC1 and PRC2 complexes to suppress gene transcription, including
p15/CDKN2B, p16/CDKN2B and p14ARF gene clusters
(71,72). lncRNA PANDA interacts with
PRC1, PRC2 and the transcription factor NF-YA (Nuclear
transcription factor Y, alpha) to suppress senescence in cancer
cells (73). HOTAIR
interacts with PRC2 complex to silence its target gene expression
by increasing H3K27me3 enrichment in its target loci in breast
cancer cells (62,74). Critically, lncRNA could also
regulate histone modification via histone acetyltransferase in
cancer cells. lncRNA transcribed upstream of the CCND1 gene
recruits translocated in liposarcoma to the CCND1 promoter
region and suppresses CCND1 transcription by inhibiting the
histone acetyltransferase CBP (CREB Binding Protein)/p300 in
tumorigenesis (75). lncRNA
circAGFG1 recruits EZH2 to inhibit p53 gene
expression, regulating proliferation and cell cycle progression in
cervical cancer (76). lncRNA
LINC01419 interacts with EZH2 to mediate the histone
methylation at the reversion-inducing cysteine-rich protein with
kazal motifs) promoter, controlling hepatocellular carcinoma growth
and metastasis (77). lncRNA
lnc-ATB directly binds EZH2 to regulate cell proliferation,
invasion and migration in ovarian cancer (78). lncRNA LINP1 recruits EZH2
to the promoter regions of tumor suppressors KLF2 (KLF
transcription factor 2) and PRSS8 (Serine protease 8),
regulating cell apoptosis in cervical cancer (79). lncRNA XIST facilitates cell
proliferation, migration and invasion in neuroblastoma by
interacting with PRC2 complex to downregulate DKK1
(Dickkopf-1) gene expression (80). lncRNA UCA1 confers
tamoxifen resistance in breast cancer through regulation of the
EZH2/p21 axis and the PI3K/AKT signaling pathway (81). lncRNA AGAP2-AS1 mediates
the H3K27 acetylation at the promoter of the carcinogenic protein
MyD88 by binding with CBP, resulting in progression and
chemoresistance of breast cancer (82) (Fig.
2D; Table SI).
Collectively, lncRNAs not only serve important roles
in contributing to carcinogenesis by recruiting the H3K4me3 or
H3K79me2-related HMT complexes, MLL and DOT1L, but also interact
with chromatin PRC2 or PRC1 complex leading to suppressed gene
expression in carcinogenesis (Fig.
2A-D; Table SI). The
interaction between lncRNAs and histone-modifying enzymes
represents a novel and complex regulatory network in
carcinogenesis. Further research into the mechanisms underlying
these interactions may provide valuable insights into the molecular
mechanisms driving cancer development and potentially lead to the
identification of the novel drug targets for cancer
therapeutics.
Nucleosome formation involves DNA wrapping around
structural histone proteins, which are organized into chromatin.
Gene regulation is directly influenced chromatin organization,
which includes the accessible/active euchromatin and
condensed/suppressed heterochromatin (83). Chromatin and its regulatory
elements are widely distributed in cancer genomes. Previous
research has highlighted the importance of chromatin TAD structure
in interacting with structural/regulatory protein complexes and
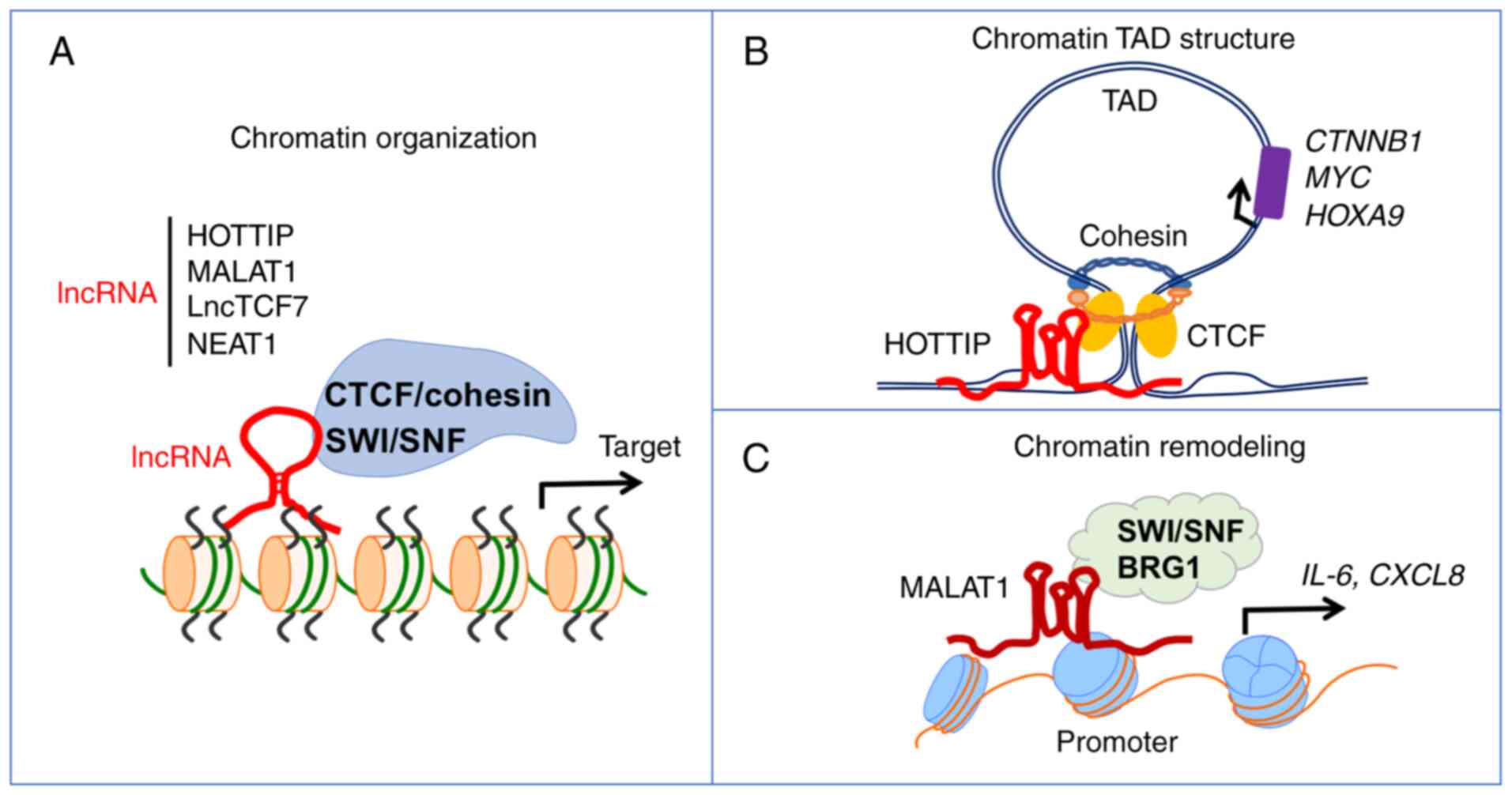
lncRNAs (84) (Fig. 3A). CTCF is a key regulator of
mammalian 3D genome organization (10). The CTCF/cohesin complex is
responsible for modulating chromatin TAD boundaries, as well as
enhancer-promoter long-range contacts within TADs to regulate gene
expression in cancer genomes (11,85). Additionally, the SWItch/Sucrose
Non-Fermentable (SWI/SNF) chromatin remodeling complex serves a
critical regulatory role in the development and progression of
various types of cancer (86).
Recent studies have underscored the key role of
lncRNAs in chromatin remodeling and gene transcription in
carcinogenesis (97-99). Tang et al (88) demonstrated that lncRNAs play a
crucial role in recruiting imitation SWI/SNF family proteins to
specific genomic regions, thereby activating the transcription of
target genes in carcinogenesis. lncRNA UCA1 interacts with
Brahma-related gene 1 (BRG1) of the chromatin SWI/SNF remodeling
complex to hinder its binding to p21 promoter locus,
promoting gallbladder cancer (100). Moreover, lncRNA MALAT1 is
essential for forming a complex with the chromatin remodeling
component BRG1, epigenetically promoting inflammation-related
hepatocellular carcinoma progression (101,102) (Fig. 3C). lncRNA nuclear enriched
transcript 1 (NEAT1) interacts with the subunit AT rich
interactive domain 1B of the BRM-associated factor)-type SWI/SNF
complex through the formation of paraspeckles (nuclear bodies) in
tumorigenesis (97). NEAT1
regulates nuclear paraspeckle assembly by the recruitment of the
subunit of SWI/SNF complex component BRG1 in colorectal cancer
cells (103,104). lncRNA LncTCF7 has been
shown to enhance the activation of the TCF7 transcriptional
promoter and the WNT signaling pathway by recruiting SWI/SNF
complex, thereby increasing stemness of cancer cells (105,106). lncRNA SCHLAP1 promotes
aggressive prostate cancer invasion and metastasis by interacting
with and antagonizing SWI/SNF complex (107,108).
In summary, lncRNAs interact with chromatin
organization regulators to regulate gene expression in cancer
cells. Mechanistically, lncRNAs such as MALAT1, LncTCF7 and
SCHLAP1 interact with chromatin remodeling complexes to
influence chromatin structure and accessibility. HOTTIP
recruits CTCF/cohesin to activate oncogenic TADs in AML, while
MALAT1 and LncTCF7 coordinate with SWI/SNF to promote
hepatocellular and colorectal cancer, respectively. Therefore,
lncRNAs serve key roles in modulating chromatin organization by
interacting with CTCF/cohesin complex or ISWI/SNF family proteins
to promote transcription of oncogenes in cancer development and
progression (Fig. 3A-D; Table SI).
RNA modifications, including methylation and RNA
editing, are key for various cellular processes in cancer cells,
including RNA stability, structure and metabolism, localization and
translation (109). lncRNAs play
key roles in carcinogenesis by recruiting RNA modification
complexes at the specific gene loci. lncRNAs directly interact with
RNA modification complexes, such as RNA methyltransferases and
editing enzymes, to modulate RNA modification and gene expression
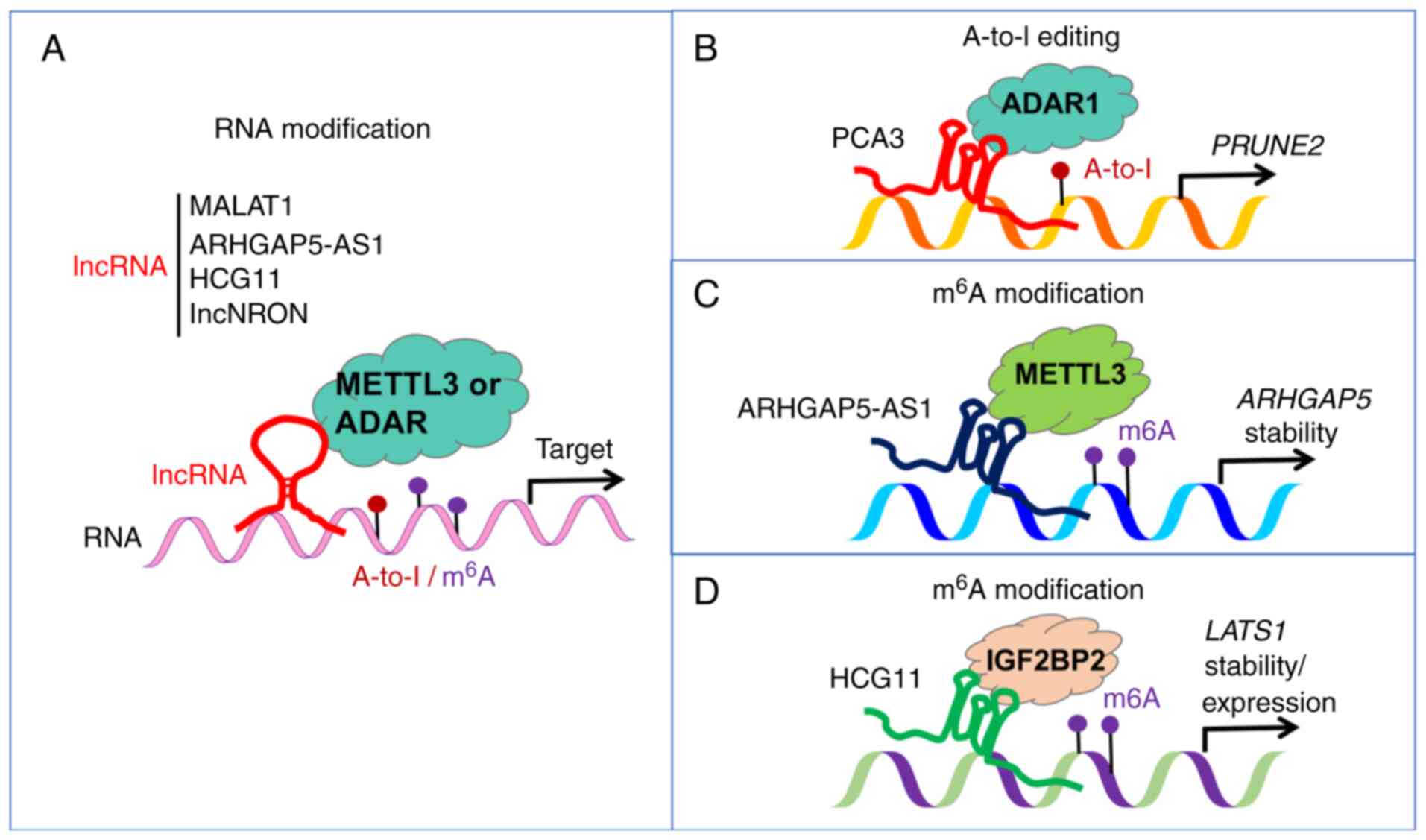
in cancer genomes (Fig. 4A)
(110-116). By serving as molecular guides or
scaffolds, lncRNAs directly recruit RNA modifiers to target RNAs,
thereby influencing RNA modification patterns and driving cancer
progression.
In summary, studies have highlighted the role of
lncRNAs in mediating epitranscriptomic modifications, which are
reversible chemical modifications on RNA molecules that impact
oncogene expression and carcinogenesis (15,131,132). The interplay between lncRNAs and
epitranscriptomic modification regulators in carcinogenesis
underscores the complexity of regulatory networks that control
oncogene expression in cancer (15). Some specific lncRNAs, such as
MALAT1, ARHGAP5-AS1 and lncNRON, have been implicated
in carcinogenesis by recruiting METTL3/METTL14 or ALKBH5 enzymes.
Additionally, lncRNAs such as HCG11, lncRNA-CBSLR and
KB-1980E6.3 regulate oncogene expression by recruitment of
m6A readers. lncRNAs play a direct role in recruiting
RNA modification complexes, including RNA methyltransferases and
RNA editing complexes, to regulate RNA modifications and abnormal
oncogene expression, ultimately contributing to carcinogenesis
(Figs. 1-4; Table SI). Collectively, these findings
underscore the diverse and complex regulatory roles that lncRNAs
serve in mediating m6A modification and gene expression
in various types of cancer, highlighting their potential as
therapeutic targets for cancer treatment.
lncRNAs are key regulators of cancer biology,
operating through both epigenetic and epitranscriptomic mechanisms
(17,133,134). As epigenetic regulators, lncRNAs
interact with chromatin-modifying complexes (such as polycomb
repressors and DNA methyltransferases) to silence tumor suppressor
genes or activate oncogenes (131,135). In their epitranscriptomic
capacity, lncRNAs orchestrate RNA modification (such as
m6A methylation and A-to-I editing) to stabilize
oncogenic transcripts or enhance their translational efficiency
(132). These dual regulatory
roles establish lncRNAs as master regulators of cancer hallmarks,
including proliferation, metastasis and therapy resistance. Beyond
their mechanistic roles, lncRNAs are as critical diagnostic and
prognostic biomarkers in carcinogenesis. Their tissue- and
cancer-specific expression profiles, combined with their ability to
regulate key oncogenic pathways (such as those controlling
proliferation, apoptosis and metastasis), position them as
promising tools for early cancer detection, risk stratification and
personalized therapeutic strategies (136).
Advancements in transcriptome sequencing data
availability may facilitate the discovery of lncRNA biomarkers. HOX
family genes, such as HOTAIR, HOXBLINC and
HOTTIP, are frequently upregulated in cancer and closely
linked to carcinogenesis (18,59,61,92). Utilizing HOTAIR measurement
for risk stratification of patients undergoing surgery may enhance
precision medicine strategies for aggressive esophageal cancer
(27). Mechanistically,
HOTAIR influences the expression of the MTHFR gene by
recruiting DNMT3s to the MTHFR gene promoter, resulting in
esophageal cancer chemoresistance (27) (Fig.
1B). The function of HOTAIR lncRNA may be context- or
cell-type-specific, but it still serves as a valuable clinical
prognostic indicator. In patients with metastatic AML, high levels
of HOTTIP expression are associated with shorter overall
survival and increased responsiveness to WNT inhibitor ICG-001
treatment compared with those with low levels (18). Mechanistically, HOTTIP
regulates HOXA9 oncogene expression and the WNT/β-Catenin
signaling pathway, influencing AML development and progression
(18) (Fig. 2B). In addition, HOTTIP
coordinates with CTCF in mediating R-loops and TAD formation at
crucial hematopoietic/leukemogenic loci to regulate expression of
leukemia-related genes in AML leukemogenesis (91) (Fig.
3B). HOXBLINC expression is associated with poor
prognosis in AML based on The Cancer Genome Atlas datasets
(50) (Fig. 2C). HOXBLINC shows the
highest levels of elevated expression in patients with AML with
disease progression compared with patients without progression,
suggesting its potential as a reliable biomarker with cancer- or
tissue-specific expression profiles in AML.
Aberrant expression of lncRNAs and their
involvement in cellular processes establish them as promising
therapeutic targets for cancer. Studies have highlighted the
importance of elucidating lncRNA-mediated mechanisms in cancer
development and metastasis (59,77,125,137). For example, inhibitors targeting
LINC01212 have efficacy in melanoma treatment (138). lncMyoD has been
identified as a functional regulator of IMP1 (IGF2 mRNA-binding
protein (IMP)1 and IMP2, highlighting its therapeutic potential in
sarcoma (139). Additionally,
lncRNA-6585 and its associated antibody are under
investigation for cervical cancer diagnosis and therapy (138). Emerging strategies, such as
nanomaterials-based technologies, represent cutting-edge approaches
for targeting lncRNAs in cancer therapy (140). These innovations, including
nanoparticle delivery systems, enhance specificity and efficacy of
RNA-based therapies (140). A
recent study systematically summarized advances in targeting the
lncRNA-Wnt axis with flavonoids for colorectal cancer (CRC)
therapy, underscoring the potential of flavonoid-based strategies
to exploit epigenetic mechanisms for CRC prevention and treatment
(141).
lncRNAs are increasingly leveraged in nucleic
acid-based therapeutics in cancer, including CRISPR/Cas9 (Clustered
regularly interspaced short palindromic repeats associated protein
9) sgRNA design, small interfering RNA (siRNA) and antisense
oligonucleotides (ASOs) (142).
For example, CRISPR/Cas9 sgRNA-mediated silencing of UCA1,
NEAT1 or MALAT1 inhibits cancer cell metastasis
(70,143). MALAT1 serves as a
druggable lncRNA for precision anti-cancer strategies, while
microRNAs and lncRNAs represent promising targets in drug
development (70,144). Furthermore, siRNA and ASOs
enable precise gene silencing, making them key tools for research
and clinical applications (143,144). For example, siRNA targeting
DDX11 antisense RNA 1) has been developed for liver cancer therapy
(145). In a luminal mouse
mammary tumor virus-PyMT mouse mammary carcinoma model, promoter
depletion or ASO-mediated knockdown of MALAT1 notably
decreases lung cancer metastasis (120).
Collectively, lncRNAs are associated with cancer
development and progression, underscoring their potential as
prognostic and predictive biomarkers. Their cancer- or
tissue-specific expression profiles across malignancies highlight
their clinical relevance. The integration of lncRNAs into clinical
oncology signifies a new era of precision medicine. Their dual use
as diagnostic/prognostic markers and therapeutic targets may
transform cancer management by enabling earlier detection,
personalized treatment and dynamic monitoring. As research
advances, lncRNAs are poised to transition from experimental
discoveries to key assets in treating cancer, offering now avenues
for improving patient outcomes.
The aberrant expression of lncRNAs is associated
with poor prognosis of patients with cancer. Understanding the
mechanisms by which lncRNAs drive cancer progression is key for
developing effective therapy (146). lncRNAs possess structural
versatility, enabling them to recruit epigenetic and
epitranscriptomic regulators, including PRC1, PRC2, LSD1, MLL,
DNMTs, CTCF, cohesin, METTL3 and ALKBH5. By interacting with these
regulators, lncRNAs modulate the epigenome and amplify oncogenic
pathways in carcinogenesis (147,148). Therefore, future studies may
elucidate the interlocking functions of lncRNAs with epigenetic or
epitranscriptomic regulators to develop new cancer therapies and
earlier prognosis strategies (46).
Dysregulation of lncRNA-mediated DNA modification
is implicated in tumor initiation, progression and metastasis
(27). Understanding crosstalk
between lncRNAs and DNA modification in cancer is crucial for
investigating underlying molecular mechanisms of tumorigenesis.
Aberrant DNA 5mC methylation patterns, such as hypermethylation of
tumor suppressor genes and hypomethylation of oncogenes, are
implicated in cancer development and progression. lncRNAs recruit
DNMTs complex to the specific genomic loci, leading to changes in
DNA methylation patterns and oncogene expression during
carcinogenesis (26,27). For example, lncRNA HOTAIR
recruits DNMT3A/3B complex to the promoter regions of tumor
suppressor gene MTHFR, promoting cancer cell proliferation
and metastasis (27). Moreover,
HOTAIR epigenetically suppresses the miR-122
expression via DNMTs-mediated DNA methylation, contributing to
hepatocarcinogenesis (149).
Additionally, lncRNAs, such as HAGLR, CCDC26 and
TTTY15, have been implicated in recruiting DNMTs to
influence the DNA methylation of their targets in cancer cells
(33-35). Overall, recruitment of DNMTs by
lncRNAs represents the epigenetic mechanism by which lncRNAs
regulate oncogene expression in carcinogenesis (Fig. 1A-D). Although numerous lncRNAs can
influence the DNA methylation of their targets leading to
carcinogenesis, it is unknown which regulators are recruited by
lncRNA in cancer. Further studies should explore the epigenetic
mechanisms of lncRNA-mediated DNA modification in cancer
genome.
The recruitment of histone modification complexes
by lncRNAs serves a crucial role in the regulation of gene
expression patterns associated with cancer development and
progression. lncRNAs recruit HAT complexes to specific genomic
loci, resulting in changes in histone acetylation patterns during
carcinogenesis (59,60). This recruitment of histone
modifying regulators by lncRNAs regulates changes in gene
expression patterns associated with cancer cell proliferation,
invasion, metastasis, apoptosis, cell cycle progression and drug
resistance (Fig. 5). For example,
lncRNA HOTAIR interacts with the HAT complex PRC2 to
increase H3K27me3 at oncogene promoters and subsequently silence
their expression (59-61) (Fig.
2A-B). Additionally, HOTAIR and Air interact with
PRC2 complex or G9a to regulate histone acetylation or methylation
at specific gene loci, promoting tumorigenesis (51,59-61). Furthermore, oncogenic lncRNAs
HOTTIP, HOXBLINC, MIAT, LAMP5-AS1,
ANRIL, CircAGFG1, PANDA and LINP1,
directly interact with MLL, DOT1L, SETD1A and EZH2, influencing
cancer cell proliferation, cell cycle progression and apoptosis
(Fig. 5). lncRNA AGAP2-AS1
directly binds CBP/P300 to mediate the H3K27 acetylation at the
promoter of the carcinogenic protein MyD88, leading to
chemoresistance in breast cancer (82) (Figs.
2D and 5). While the role of
lncRNA-mediated histone modification in carcinogenesis is
well-established (18,50,51,53,55,69), further research is needed to
understand how lncRNAs recruit histone modification complexes. The
present review provides an overview of the current understanding of
how lncRNAs mediate the recruitment of histone modification
complexes in carcinogenesis, emphasizing the potential of targeting
lncRNA-histone modifier interactions as a therapeutic approach for
cancer treatment (Fig. 5).
RNA modifications, such as m6A methylation and
A-to-I editing, influence mRNA stability, translation and splicing.
Dysregulation of m6A methylation is implicated in various cancers.
lncRNAs recruit the METTL3/METTL14 complex to mediate m6A
methylation, regulating target gene expression and driving tumor
growth and metastasis (150,151). lncRNA MALAT1 recruits the
METTL3/METTL14 complex to promote methylation and subsequent
translation at specific mRNAs, enhancing NSCLC cell proliferation
and invasion (112). lncRNAs
recruit m6A readers and eraser to mediate oncogene
expression or inactivate tumor suppressive genes in carcinogenesis,
such as IGF2BP1, IGF2BP2, YTHDC1, YTHDF2 and ALKBH5 (116,125,126,128,129). Additionally, ADAR1-dependent
A-to-I editing serves a critical role in the progression and
metastasis of cancers (117,118). lncRNAs PCA3 and LINC00944
recruit ADAR to regulate the expression of the tumor-related genes
in carcinogenesis (110,119) (Fig. 4A-D). Overall, the recruitment of
the RNA A-to-I editing enzyme, METTL3/METTL14 complex and
m6A or m6A readers by lncRNAs represents a
novel epitranscriptomic mechanism by which lncRNAs modulate
oncogene expression driving carcinogenesis.
In addition, lncRNAs serve essential roles in 3D
chromatin organization by recruiting, bridging, and guiding the
CTCF/cohesin complex to specific genomic regions. Depletion of RBRs
disrupts CTCF-mediated DNA recognition and binding and chromatin
loops in cancer genome (12).
Thus, lncRNAs coordinate with CTCF, contributing to the genome
topological regulation via the RNA-dependent mechanism. lncRNA
HOTTIP coordinates with CTCF to mediate TAD formation at key
hematopoietic/leukemogenic loci for AML development and progression
(18) (Fig. 3B). lncRNA HOTTIP also
cooperates with CTCF/cohesin-mediated TAD structure in LSC
regulation and AML leukemogenesis according to its function in
specific leukemic genome topology (91) (Fig.
3B). These findings suggest that lncRNAs contribute to genome
topological regulation via RNA-dependent mechanisms. Further
research is needed to explore the roles of architectural RNAs and
regulatory lncRNAs in CTCF/cohesin-mediated chromatin organization
across numerous types of cancer.
lncRNAs serve physiological and pathological roles
in numerous aspects of genome function and biological processes,
such as cell development, differentiation, proliferation, invasion
and migration. Unlike protein-coding genes, lncRNAs lack
well-defined domains, making their regulatory mechanisms diverse
and complex (152). Notably,
lncRNAs >300 bp contain multiple functional domains that
interact with various factors to coordinate activity in both time
and space (153). For example,
HOTTIP cooperates with WDR5/MLL/DOT1L, a large family of
RNA-binding proteins involved in cellular processes such as
alternative splicing, mRNA stability and transcriptional regulation
(18). Furthermore, HOTTIP
can bind to the RNA binding domains of CTCF/cohesin, leading to
aberrant induction of oncogene expression and the WNT pathway in
leukemogenesis (91). lncRNA
HOTTIP mediates histone modification and chromatin
organization to regulate HOXA oncogene expression and WNT
signaling pathways by recruiting modifiers, including
WDR5/MLL/DOT1L and CTCF/cohesin. The 3,343 bp lncRNA HOTTIP
contains different functional domains that can recruit different
DNA or RNA modifying regulators. Experimental frameworks for
studying the cis- and trans-acting functions of this lncRNA have
been detailed in previous research (18,91). Similarly, upregulation of
MALAT1 lncRNA in various types of cancer, along with its
pleiotropic roles in gene regulation, has made it a target for
therapeutic interventions in cancer (70). lncRNA MALAT1 suppresses
E-cadherin expression, promoting OS metastasis by
recruitment of the PRC2 member EZH2 (69). lncRNA MALAT1 also plays a
key role in chromatin remodeling to promote inflammation-associated
hepatocellular carcinoma progression by interaction with BRG1
(101,102). Additionally, MALAT1
regulates the expression of miR-26b by recruiting METTL3,
leading to the invasive and metastatic behavior of breast cancer
via the MALAT1/miR-26b/HMGA2 axis (120,121). These examples illustrate how
lncRNAs with multiple functional domains interact with diverse
modifiers to regulate cancer progression. While experimental
studies have provided insight into lncRNA functions, further
research is needed to clarify their regulatory mechanisms across
numerous types of cancer.
Previous studies have challenged traditional views
of lncRNA function, highlighting their key roles in cancer
development (154,155). Advances in RNA-associated
technologies, such as ChIRP-seq (chromatin isolation by RNA
purification sequencing), ChIRP-Mass Spectrometry), and iDRiP
(identification of direct RNA interacting proteins), have enabled
identification of lncRNAs that interact with DNA-modifying enzymes,
histone modifiers, RNA-modifying complexes and chromatin-organizing
proteins (156-158). Moreover, a deep understanding of
lncRNA-driven epigenetic and epitranscriptomic regulation through
next-generation sequencing technology strengthen its association
with carcinogenesis (84). The
regulation of lncRNAs in human may lead to the discovery of
promising targets for cancer therapeutics. This has spurred the
rapid growth of epigenetic drug discovery, with drug-targeting
epigenetic enzymes being tested in the clinic for the treatment of
various types of human cancer (159-161). lncRNAs provide a platform for
identifying epigenetic targets, enabling the development of
epi-drugs to counteract aberrant epigenetic enzymes. lncRNA
expression shows high specificity, as they are expressed at
different developmental stages and in a cancer type-specific manner
(162,163). Disrupting expression of a
specific lncRNA associated with epigenetic regulation can lead to
the upregulation of its target (6).
Understanding of how lncRNAs mediate epigenetic and
epitranscritomic regulators to control the cancer biological
processes, such as invasion-metastasis, and influence the tumor
microenvironment is steadily advancing (Fig. 5; Table SI). Critically, lncRNAs form
relatively stable secondary and higher structures to facilitate
cellular organization and gene regulation, including DNA
replication, RNA transcription, protein translation and cell and
cell differentiation (164,165). The complex structural features
make lncRNAs potential players in epigenetic regulation in various
types of cancer (96). Multiple
pieces of evidence suggest that structural features of lncRNAs are
essential for understanding their function and roles in cancer
development and progression (7,15,29,33,69). In conclusion, the present review
highlighted the role of lncRNA in mediating epigenetic and
epitranscriptomic regulation to control oncogene expression.
Directly recruiting DNA modifying complex, histone modifying
regulators, chromatin organization associated regulators, and RNA
modifying complex plays a role in carcinogenesis. Understanding
lncRNA functions and structures is key for developing targeted
cancer therapy.
Not applicable.
HL conceived the study and wrote and revised the
manuscript. CD, HQ, RF and HY wrote the manuscript. AS and HL
wrote, reviewed and edited the manuscript. CD, HQ, RF and HL
constructed figures. HL supervised the study. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
No applicable.
The present study was supported by National Natural Science
Foundation of China (grant nos. 82270193 and 82470156) and Zhejiang
Provincial Natural Science Foundation of China (grant no.
YXD24H0801).
|
1
|
Ransohoff JD, Wei Y and Khavari PA: The
functions and unique features of long intergenic non-coding RNA.
Nat Rev Mol Cell Biol. 19:143–157. 2018. View Article : Google Scholar :
|
|
2
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang D, Garcia-Bassets I, Benner C, Li W,
Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, et al:
Reprogramming transcription by distinct classes of enhancers
functionally defined by eRNA. Nature. 474:390–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Sun W, Qin Z, Guo S, Kang Y, Zeng
S and Yu L: LncRNA regulation: New frontiers in epigenetic
solutions to drug chemoresistance. Biochem Pharmacol.
189:1142282021. View Article : Google Scholar
|
|
5
|
Morlando M and Fatica A: Alteration of
epigenetic regulation by long noncoding RNAs in cancer. Int J Mol
Sci. 19:5702018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khorkova O, Hsiao J and Wahlestedt C:
Basic biology and therapeutic implications of lncRNA. Adv Drug
Deliv Rev. 87:15–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beckedorff FC, Amaral MS,
Deocesano-Pereira C and Verjovski-Almeida S: Long non-coding RNAs
and their implications in cancer epigenetics. Biosci Rep.
33:e000612013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Chan YT, Tan HY, Li S, Wang N and
Feng Y: Epigenetic regulation in human cancer: The potential role
of epi-drug in cancer therapy. Mol Cancer. 19:792020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tachiwana H, Yamamoto T and Saitoh N: Gene
regulation by non-coding RNAs in the 3D genome architecture. Curr
Opin Genet Dev. 61:69–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phillips JE and Corces VG: CTCF: Master
weaver of the genome. Cell. 137:1194–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang C, Rao S, Crispino JD and
Ntziachristos P: Determinants and role of chromatin organization in
acute leukemia. Leukemia. 34:2561–2575. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansen AS, Hsieh TS, Cattoglio C, Pustova
I, Saldaña-Meyer R, Reinberg D, Darzacq X and Tjian R: Distinct
classes of chromatin loops revealed by deletion of an RNA-binding
region in CTCF. Mol Cell. 76:395–411.e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saldana-Meyer R, Rodriguez-Hernaez J,
Escobar T, Nishana M, Jácome-López K, Nora EP, Bruneau BG, Tsirigos
A, Furlan-Magaril M, Skok J and Reinberg D: RNA interactions are
essential for CTCF-mediated genome organization. Mol Cell.
76:412–422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui L, Ma R, Cai J, Guo C, Chen Z, Yao L,
Wang Y, Fan R, Wang X and Shi Y: RNA modifications: Importance in
immune cell biology and related diseases. Signal Transduct Target
Ther. 7:3342022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dinescu S, Ignat S, Lazar AD, Constantin
C, Neagu M and Costache M: Epitranscriptomic signatures in lncRNAs
and their possible roles in cancer. Genes (Basel). 10:522019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Z, Xu F, Teschendorff AE, Zhao Y, Yao
L, Li J and He Y: Insights into the role of long non-coding RNAs in
DNA methylation mediated transcriptional regulation. Front Mol
Biosci. 9:10674062022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Statello L, Guo CJ, Chen LL and Huarte M:
Gene regulation by long non-coding RNAs and its biological
functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar
|
|
18
|
Luo H, Zhu G, Xu J, Lai Q, Yan B, Guo Y,
Fung TK, Zeisig BB, Cui Y, Zha J, et al: HOTTIP lncRNA promotes
hematopoietic stem cell Self-renewal leading to AML-like disease in
mice. Cancer Cell. 36:645–659.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greenberg MVC and Bourc'his D: The diverse
roles of DNA methylation in mammalian development and disease. Nat
Rev Mol Cell Biol. 20:590–607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao SG, Chen WS, Li H, Foye A, Zhang M,
Sjöström M, Aggarwal R, Playdle D, Liao A, Alumkal JJ, et al: The
DNA methylation landscape of advanced prostate cancer. Nat Genet.
52:778–789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robertson KD: DNA methylation,
methyltransferases, and cancer. Oncogene. 20:3139–3155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Velden PA, Metzelaar-Blok JA,
Bergman W, Monique H, Hurks H, Frants RR, Gruis NA and Jager MJ:
Promoter hypermethylation: A common cause of reduced p16(INK4a)
expression in uveal melanoma. Cancer Res. 61:5303–5306.
2001.PubMed/NCBI
|
|
23
|
Saif I, Bouziyane A, Benhessou M, Karroumi
ME and Ennaji MM: Detection of hypermethylation BRCA1/2 gene
promoter in breast tumours among Moroccan women. Mol Biol Rep.
48:7147–7152. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang
Y, Jia L, Li S; Cancer Genome Atlas Research Network; Xie W and
Yang D: lncRNA epigenetic landscape analysis identifies EPIC1 as an
oncogenic lncRNA that interacts with MYC and promotes Cell-cycle
progression in cancer. Cancer Cell. 33:706–720.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lyko F: The DNA methyltransferase family:
A versatile toolkit for epigenetic regulation. Nat Rev Genet.
19:81–92. 2018. View Article : Google Scholar
|
|
26
|
Liu D, Wu K, Yang Y, Zhu D, Zhang C and
Zhao S: Long noncoding RNA ADAMTS9-AS2 suppresses the progression
of esophageal cancer by mediating CDH3 promoter methylation. Mol
Carcinog. 59:32–44. 2020. View Article : Google Scholar
|
|
27
|
Zhang S, Zheng F, Zhang L, Huang Z, Huang
X, Pan Z, Chen S, Xu C, Jiang Y, Gu S, et al: LncRNA
HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil
sensitivity in esophageal cancer cells. J Exp Clin Cancer Res.
39:1312020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Bu P, Ai Y, Srinivasan T, Chen HJ,
Xiang K, Lipkin SM and Shen X: A long non-coding RNA targets
microRNA miR-34a to regulate colon cancer stem cell asymmetric
division. Elife. 5:e146202016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai IL, Chang YS, Chan WL Lee YT, Yen JC,
Yang CA, Hung SY and Chang JG: Male-specific long noncoding RNA
TTTY15 inhibits Non-small cell lung cancer proliferation and
metastasis via TBX4. Int J Mol Sci. 20:34732019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q, Su Z, Xu X, Liu G, Song X, Wang R,
Sui X, Liu T, Chang X, Huang D, et al: AS1DHRS4, a head-to-head
natural antisense transcript, silences the DHRS4 gene cluster in
cis and trans. Proc Natl Acad Sci USA. 109:14110–14115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su SC, Yeh CM, Lin CW, Hsieh YH, Chuang
CY, Tang CH, Lee YC and Yang SF: A novel melatonin-regulated lncRNA
suppresses TPA-induced oral cancer cell motility through
replenishing PRUNE2 expression. J Pineal Res. 71:e127602021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Luo Q, Li Z, Wang Y, Zhu C, Li T and
Li X: Long Non-coding RNA IRAIN inhibits VEGFA expression via
enhancing Its DNA methylation leading to tumor suppression in renal
carcinoma. Front Oncol. 10:10822020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo X, Chen Z, Zhao L, Cheng D, Song W and
Zhang X: Long non-coding RNA-HAGLR suppressed tumor growth of lung
adenocarcinoma through epigenetically silencing E2F1. Exp Cell Res.
382:1114612019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon JH, You BH, Park CH, Kim YJ, Nam JW
and Lee SK: The long noncoding RNA LUCAT1 promotes tumorigenesis by
controlling ubiquitination and stability of DNA methyltransferase 1
in esophageal squamous cell carcinoma. Cancer Lett. 417:47–57.
2018. View Article : Google Scholar
|
|
35
|
Jones R, Wijesinghe S, Wilson C, Halsall
J, Liloglou T and Kanhere A: A long intergenic non-coding RNA
regulates nuclear localization of DNA methyl transferase-1.
iScience. 24:1022732021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu X, Lou Y, Tang J, Teng Y, Zhang Z, Yin
Y, Zhuo H and Tan Z: The long non-coding RNA Linc-GALH promotes
hepatocellular carcinoma metastasis via epigenetically regulating
Gankyrin. Cell Death Dis. 10:862019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vennin C, Spruyt N, Robin YM, Chassat T,
Le Bourhis X and Adriaenssens E: The long non-coding RNA 91H
increases aggressive phenotype of breast cancer cells and
up-regulates H19/IGF2 expression through epigenetic modifications.
Cancer Lett. 385:198–206. 2017. View Article : Google Scholar
|
|
38
|
Jin L, Cai Q, Wang S, Wang S, Wang J and
Quan Z: Long noncoding RNA PVT1 promoted gallbladder cancer
proliferation by epigenetically suppressing miR-18b-5p via DNA
methylation. Cell Death Dis. 11:8712020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu X and Zhang Y: TET-mediated active DNA
demethylation: Mechanism, function and beyond. Nat Rev Genet.
18:517–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rasmussen KD and Helin K: Role of TET
enzymes in DNA methylation, development, and cancer. Genes Dev.
30:733–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al-Imam MJ, Hussein UA, Sead FF, Faqri
AMA, Mekkey SM, Khazel AJ and Almashhadani HA: The interactions
between DNA methylation machinery and long non-coding RNAs in tumor
progression and drug resistance. DNA Repair (Amst). 128:1035262023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Ren M, Zeng T, Wang W, Wang X, Hu
M, Su S, Sun K, Wang C, Liu J, et al: TET2-interacting long
noncoding RNA promotes active DNA demethylation of the MMP-9
promoter in diabetic wound healing. Cell Death Dis. 10:8132019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roessner A, Franke S, Schreier J, Ullmann
S, Karras F and Jechorek D: Genetics and epigenetics in
conventional chondrosarcoma with focus on non-coding RNAs. Pathol
Res Pract. 239:1541722022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X, Du J, Yu J, Guo R, Feng Y, Qiao L,
Xu Z, Yang F, Zhong G, Liu F, et al: LncRNA NKILA regulates
endothelium inflammation by controlling a NF-κB/KLF4 positive
feedback loop. J Mol Cell Cardiol. 126:60–69. 2019. View Article : Google Scholar
|
|
45
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-kappaB
interacting long noncoding RNA blocks IkappaB phosphorylation and
suppresses breast cancer metastasis. Cancer Cell. 27:370–381. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen L, Fan X, Zhu J, Chen X, Liu Y and
Zhou H: LncRNA MAGI2-AS3 inhibits the self-renewal of leukaemic
stem cells by promoting TET2-dependent DNA demethylation of the
LRIG1 promoter in acute myeloid leukaemia. RNA Biol. 17:784–793.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nie Y, Zhou L, Wang H, Chen N, Jia L, Wang
C, Wang Y, Chen J, Wen X, Niu C, et al: Profiling the epigenetic
interplay of lncRNA RUNXOR and oncogenic RUNX1 in breast cancer
cells by gene in situ cis-activation. Am J Cancer Res. 9:1635–1649.
2019.PubMed/NCBI
|
|
48
|
Elsheikh SE, Green AR, Rakha EA, Powe DG,
Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, et
al: Global histone modifications in breast cancer correlate with
tumor phenotypes, prognostic factors, and patient outcome. Cancer
Res. 69:3802–3809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deng C, Li Y, Zhou L, Cho J, Patel B,
Terada N, Li Y, Bungert J, Qiu Y, Huang S, et al: HoxBlinc RNA
recruits Set1/MLL complexes to activate hox gene expression
patterns and mesoderm lineage development. Cell Rep. 14:103–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nagano T, Mitchell JA, Sanz LA, Pauler FM,
Ferguson-Smith AC, Feil R and Fraser P: The Air noncoding RNA
epigenetically silences transcription by targeting G9a to
chromatin. Science. 322:1717–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xiu B, Chi Y, Liu L, Chi W, Zhang Q, Chen
J, Guo R, Si J, Li L, Xue J, et al: LINC02273 drives breast cancer
metastasis by epigenetically increasing AGR2 transcription. Mol
Cancer. 18:1872019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu A, Hong F, Li D, Jin Y, Kon L, Xu Z, He
H and Xie Q: Long non-coding RNA ROR recruits histone
transmethylase MLL1 to up-regulate TIMP3 expression and promote
breast cancer progression. J Transl Med. 19:952021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai IL, Yang CA, Lin PC, Chan WL, Lee YT,
Yen JC, Chang YS and Chang JG: Long noncoding RNA MIAT promotes
non-small cell lung cancer proliferation and metastasis through
MMP9 activation. Oncotarget. 8:98148–98162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang WT, Chen TQ, Zeng ZC, Pan Q, Huang W,
Han C, Fang K, Sun LY, Yang QQ, Wang D, et al: The lncRNA LAMP5-AS1
drives leukemia cell stemness by directly modulating DOT1L
methyltransferase activity in MLL leukemia. J Hematol Oncol.
13:782020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chu W, Zhang X, Qi L, Fu Y, Wang P, Zhao
W, Du J, Zhang J, Zhan J, Wang Y, et al: The
EZH2-PHACTR2-AS1-Ribosome axis induces genomic instability and
promotes growth and metastasis in breast cancer. Cancer Res.
80:2737–2750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wanowska E, Samorowska K and Szczesniak
MW: Emerging roles of long Noncoding RNAs in breast cancer
epigenetics and epitranscriptomics. Front Cell Dev Biol.
10:9223512022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pandey RR, Mondal T, Mohammad F, Enroth S,
Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D and Kanduri C:
Antisense Noncoding RNA mediates lineage-specific transcriptional
silencing through Chromatin-Level regulation. Mol Cell. 32:232–246.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar
|
|
61
|
Niinuma T, Suzuki H, Nojima M, Nosho K,
Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki
Y, et al: Upregulation of miR-196a and HOTAIR drive malignant
character in gastrointestinal stromal tumors. Cancer Res.
72:1126–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long Noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kumar S, Gonzalez EA, Rameshwar P and
Etchegaray JP: Non-Coding RNAs as mediators of epigenetic changes
in malignancies. Cancers (Basel). 12:36572020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W,
De W, Wang Z and Wang R: The long noncoding RNA HOTAIR contributes
to cisplatin resistance of human lung adenocarcinoma cells via
downregualtion of p21(WAF1/CIP1) expression. PLoS One.
8:e772932013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu YW, Xia R, Lu K, Xie M, Yang F, Sun M,
De W, Wang C and Ji G: LincRNAFEZF1-AS1 represses p21 expression to
promote gastric cancer proliferation through LSD1-Mediated H3K4me2
demethylation. Mol Cancer. 16:392017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pang B, Wang Q, Ning S, Wu J, Zhang X,
Chen Y and Xu S: Landscape of tumor suppressor long noncoding RNAs
in breast cancer. J Exp Clin Cancer Res. 38:792019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huo Y, Li Q, Wang X, Jiao X, Zheng J, Li Z
and Pan X: MALAT1 predicts poor survival in osteosarcoma patients
and promotes cell metastasis through associating with EZH2.
Oncotarget. 8:46993–47006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Amodio N, Raimondi L, Juli G, Stamato MA,
Caracciolo D, Tagliaferri P and Tassone P: MALAT1: A druggable long
non-coding RNA for targeted anti-cancer approaches. J Hematol
Oncol. 11:632018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chi JS, Li JZ, Jia JJ, Zhang T, Liu XM and
Yi L: Long non-coding RNA ANRIL in gene regulation and its duality
in atherosclerosis. J Huazhong Univ Sci Technolog Med Sci.
37:816–822. 2017.PubMed/NCBI
|
|
72
|
Meseure D, Vacher S, Alsibai KD, Nicolas
A, Chemlali W, Caly M, Lidereau R, Pasmant E, Callens C and Bieche
I: Expression of ANRIL-Polycomb Complexes-CDKN2A/B/ARF genes in
breast tumors: Identification of a Two-Gene (EZH2/CBX7) signature
with independent prognostic value. Mol Cancer Res. 14:623–633.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Puvvula PK, Desetty RD, Pineau P, Marchio
A, Moon A, Dejean A and Bischof O: Long noncoding RNA PANDA and
scaffold-attachment-factor SAFA control senescence entry and exit.
Nat Commun. 5:53232014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Portoso M, Ragazzini R, Brenčič Ž, Moiani
A, Michaud A, Vassilev I, Wassef M, Servant N, Sargueil B and
Margueron R: PRC2 is dispensable for HOTAIR-mediated
transcriptional repression. EMBO J. 36:981–994. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang X, Arai S, Song X, Reichart D, Du K,
Pascual G, Tempst P, Rosenfeld MG, Glass CK and Kurokawa R: Induced
ncRNAs allosterically modify RNA-binding proteins in cis to inhibit
transcription. Nature. 454:126–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang XH and Li J: CircAGFG1 aggravates the
progression of cervical cancer by downregulating p53. Eur Rev Med
Pharmacol Sci. 24:1704–1711. 2020.PubMed/NCBI
|
|
77
|
Zhang G, Chen X, Ma L, Ding R, Zhao L, Ma
F and Deng X: LINC01419 facilitates hepatocellular carcinoma growth
and metastasis through targeting EZH2-regulated RECK. Aging (Albany
NY). 12:11071–11084. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen XJ and An N: Long noncoding RNA ATB
promotes ovarian cancer tumorigenesis by mediating histone H3
lysine 27 trimethylation through binding to EZH2. J Cell Mol Med.
25:37–46. 2021. View Article : Google Scholar :
|
|
79
|
Wu L, Gong Y, Yan T and Zhang H: LINP1
promotes the progression of cervical cancer by scaffolding EZH2,
LSD1, and DNMT1 to inhibit the expression of KLF2 and PRSS8.
Biochem Cell Biol. 98:591–599. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang J, Li WY, Yang Y, Yan LZ, Zhang SY,
He J and Wang JX: LncRNA XIST facilitates cell growth, migration
and invasion via modulating H3 histone methylation of DKK1 in
neuroblastoma. Cell Cycle. 18:1882–1892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li Z, Yu D, Li H, Lv Y and Li S: Long
non-coding RNA UCA1 confers tamoxifen resistance in breast cancer
endocrinotherapy through regulation of the EZH2/p21 axis and the
PI3K/AKT signaling pathway. Int J Oncol. 54:1033–1042.
2019.PubMed/NCBI
|
|
82
|
Dong H, Wang W, Mo S, Chen R, Zou K, Han
J, Zhang F and Hu J: SP1-induced lncRNA AGAP2-AS1 expression
promotes chemoresistance of breast cancer by epigenetic regulation
of MyD88. J Exp Clin Cancer Res. 37:2022018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Loe AKH, Zhu L and Kim TH: Chromatin and
noncoding RNA-mediated mechanisms of gastric tumorigenesis. Exp Mol
Med. 55:22–31. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Qiu Y, Xu M and Huang S: Long noncoding
RNAs: Emerging regulators of normal and malignant hematopoiesis.
Blood. 138:2327–2336. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Qiu Y and Huang S: CTCF-mediated genome
organization and leukemogenesis. Leukemia. 34:2295–2304. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ribeiro-Silva C, Vermeulen W and Lans H:
SWI/SNF: Complex complexes in genome stability and cancer. DNA
Repair (Amst). 77:87–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bammidi LS and Gayen S: Multifaceted role
of CTCF in X-chromosome inactivation. Chromosoma. 133:217–231.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Martitz A and Schulz EG: Spatial
orchestration of the genome: Topological reorganisation during
X-chromosome inactivation. Curr Opin Genet Dev. 86:1021982024.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Engreitz JM, Pandya-Jones A, McDonel P,
Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander
ES, et al: The Xist lncRNA exploits three-dimensional genome
architecture to spread across the X chromosome. Science.
341:12379732013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kung JT, Kesner B, An JY, Ahn JY,
Cifuentes-Rojas C, Colognori D, Jeon Y, Szanto A, del Rosario BC,
Pinter SF, et al: Locus-specific targeting to the X chromosome
revealed by the RNA interactome of CTCF. Mol Cell. 57:361–375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luo H, Zhu G, Eshelman MA, Fung TK, Lai Q,
Wang F, Zeisig BB, Lesperance J, Ma X, Chen S, et al:
HOTTIP-dependent R-loop formation regulates CTCF boundary activity
and TAD integrity in leukemia. Mol Cell. 82:833–851 e811. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhu G, Luo H, Feng Y, Guryanova OA, Xu J,
Chen S, Lai Q, Sharma A, Xu B, Zhao Z, et al: HOXBLINC long
non-coding RNA activation promotes leukemogenesis in NPM1-mutant
acute myeloid leukemia. Nat Commun. 12:19562021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lai Q, Hamamoto K, Luo H, Zaroogian Z,
Zhou C, Lesperance J, Zha J, Qiu Y, Guryanova OA, Huang S and Xu B:
NPM1 mutation reprograms leukemic transcription network via
reshaping TAD topology. Leukemia. 37:1732–1736. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Venkatraman A, He XC, Thorvaldsen JL,
Sugimura R, Perry JM, Tao F, Zhao M, Christenson MK, Sanchez R, Yu
JY, et al: Maternal imprinting at the H19-Igf2 locus maintains
adult haematopoietic stem cell quiescence. Nature. 500:345–349.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hacisuleyman E, Goff LA, Trapnell C,
Williams A, Henao-Mejia J, Sun L, McClanahan P, Hendrickson DG,
Sauvageau M, Kelley DR, et al: Topological organization of
multichromosomal regions by the long intergenic noncoding RNA
Firre. Nat Struct Mol Biol. 21:198–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hanly DJ, Esteller M and Berdasco M:
Interplay between long non-coding RNAs and epigenetic machinery:
Emerging targets in cancer? Philos Trans R Soc Lond B Biol Sci.
373:2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Reddy D, Bhattacharya S, Levy M, Zhang Y,
Gogol M, Li H, Florens L and Workman JL: Paraspeckles interact with
SWI/SNF subunit ARID1B to regulate transcription and splicing. EMBO
Rep. 24:e553452023. View Article : Google Scholar :
|
|
98
|
Bhattacharya A, Wang K, Penailillo J, Chan
CN, Fushimi A, Yamashita N, Daimon T, Haratake N, Ozawa H,
Nakashoji A, et al: MUC1-C regulates NEAT1 lncRNA expression and
paraspeckle formation in cancer progression. Oncogene.
43:2199–2214. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee VH, Tsang RK, Lo AWI, Chan SY, Chung
JC, Tong CC, Leung TW and Kwong DL: SMARCB1 (INI-1)-Deficient
sinonasal carcinoma: A systematic review and pooled analysis of
treatment outcomes. Cancers (Basel). 14:32852022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang X, Gong Y, Jin B, Wu C, Yang J, Wang
L, Zhang Z and Mao Z: Long non-coding RNA urothelial carcinoma
associated 1 induces cell replication by inhibiting BRG1 in 5637
cells. Oncol Rep. 32:1281–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Huang M, Wang H, Hu X and Cao X: lncRNA
MALAT1 binds chromatin remodeling subunit BRG1 to epigenetically
promote inflammation-related hepatocellular carcinoma progression.
Oncoimmunology. 8:e15186282019. View Article : Google Scholar
|
|
102
|
Lino Cardenas CL, Kessinger CW, Cheng Y,
MacDonald C, MacGillivray T, Ghoshhajra B, Huleihel L, Nuri S, Yeri
AS, Jaffer FA, et al: An HDAC9-MALAT1-BRG1 complex mediates smooth
muscle dysfunction in thoracic aortic aneurysm. Nat Commun.
9:10092018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Neve B, Jonckheere N, Vincent A and Van
Seuningen I: Epigenetic regulation by lncRNAs: An overview focused
on UCA1 in colorectal cancer. Cancers (Basel). 10:10092018.
View Article : Google Scholar
|
|
104
|
Chiba H, Muramatsu M, Nomoto A and Kato H:
Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and
Drosophila brahma are transcriptional coactivators cooperating with
the estrogen receptor and the retinoic acid receptor. Nucleic Acids
Res. 22:1815–1820. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li Y, Li W, Hoffman AR, Cui J and Hu JF:
The Nucleus/Mitochondria-Shuttling LncRNAs function as new
epigenetic regulators of mitophagy in cancer. Front Cell Dev Biol.
9:6996212021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cimadamore A, Gasparrini S, Mazzucchelli
R, Doria A, Cheng L, Lopez-Beltran A, Santoni M, Scarpelli M and
Montironi R: Long Non-coding RNAs in prostate cancer with emphasis
on second chromosome locus associated with Prostate-1 expression.
Front Oncol. 7:3052017. View Article : Google Scholar
|
|
109
|
Boo SH and Kim YK: The emerging role of
RNA modifications in the regulation of mRNA stability. Exp Mol Med.
52:400–408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
de Santiago PR, Blanco A, Morales F,
Marcelain K, Harismendy O, Sjöberg Herrera M and Armisén R:
Immune-related IncRNA LINC00944 responds to variations in ADAR1
levels and it is associated with breast cancer prognosis. Life Sci.
268:1189562021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sun Y, Ge J, Shao F, Ren Z, Huang Z, Ding
Z, Dong L, Chen J, Zhang J and Zang Y: Long noncoding RNA AI662270
promotes kidney fibrosis through enhancing METTL3-mediated
m6A modification of CTGF mRNA. FASEB J. 37:e230712023.
View Article : Google Scholar
|
|
112
|
Cao Y, Di X, Cong S, Tian C, Wang Y, Jin
X, Zhao M, Zhou X, Li R and Wang K: RBM10 recruits METTL3 to induce
N6-methyladenosine-MALAT1-dependent modification, inhibiting the
invasion and migration of NSCLC. Life Sci. 315:1213592023.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fang Y, Wu X, Gu Y, Shi R, Yu T, Pan Y,
Zhang J, Jing X, Ma P and Shu Y: LINC00659 cooperated with ALKBH5
to accelerate gastric cancer progression by stabilising JAK1 mRNA
in an m6 A-YTHDF2-dependent manner. Clin Transl Med.
13:e12052023. View Article : Google Scholar
|
|
114
|
Salameh A, Lee AK, Cardo-Vila M, Nunes DN,
Efstathiou E, Staquicini FI, Dobroff AS, Marchiò S, Navone NM,
Hosoya H, et al: PRUNE2 is a human prostate cancer suppressor
regulated by the intronic long noncoding RNA PCA3. Proc Natl Acad
Sci USA. 112:8403–8408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhu L, Zhu Y, Han S, Chen M, Song P, Dai
D, Xu W, Jiang T, Feng L, Shin VY, et al: Impaired autophagic
degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in
gastric cancer. Cell Death Dis. 10:3832019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mao J, Qiu H and Guo L: LncRNA HCG11
mediated by METTL14 inhibits the growth of lung adenocarcinoma via
IGF2BP2/LATS1. Biochem Biophys Res Commun. 580:74–80. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Picardi E, D'Erchia AM, Gallo A, Montalvo
A and Pesole G: Uncovering RNA editing sites in long Non-Coding
RNAs. Front Bioeng Biotechnol. 2:642014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ma CP, Liu H, Yi-Feng Chang I, Wang WC,
Chen YT, Wu SM, Chen HW, Kuo YP, Shih CT, Li CY and Tan BC: ADAR1
promotes robust hypoxia signaling via distinct regulation of
multiple HIF-1alpha-inhibiting factors. EMBO Rep. 20:e471072019.
View Article : Google Scholar
|
|
119
|
Salameh A, Lee AK, Cardó-Vila M, Nunes DN,
Efstathiou E, Staquicini FI, Dobroff AS, Marchiò S, Navone NM,
Hosoya H, et al: PRUNE2 is a human prostate cancer suppressor
regulated by the intronic long noncoding RNA. Proc Natl Acad Sci
USA. 112:8403–8408. 2015. View Article : Google Scholar
|
|
120
|
Arun G, Diermeier S, Akerman M, Chang KC,
Wilkinson JE, Hearn S, Kim Y, MacLeod AR, Krainer AR and Norton L:
Differentiation of mammary tumors and reduction in metastasis upon
Malat1 lncRNA loss. Genes Dev. 30:34–51. 2016. View Article : Google Scholar :
|
|
121
|
Zhao C, Ling X, Xia Y, Yan B and Guan Q:
The m6A methyltransferase METTL3 controls epithelial-mesenchymal
transition, migration and invasion of breast cancer through the
MALAT1/miR-26b/HMGA2 axis. Cancer Cell Int. 21:4412021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sun T, Wu Z, Wang X, Wang Y, Hu X, Qin W,
Lu S, Xu D, Wu Y, Chen Q, et al: LNC942 promoting METTL14-mediated
m6A methylation in breast cancer cell proliferation and
progression. Oncogene. 39:5358–5372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pu J, Xu Z, Huang Y, Nian J, Yang M, Fang
Q, Wei Q, Huang Z, Liu G, Wang J, et al:
N6-methyladenosine-modified FAM111A-DT promotes
hepatocellular carcinoma growth via epigenetically activating
FAM111A. Cancer Sci. 114:3649–3665. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhou L, Jiang J, Huang Z, Jin P, Peng L,
Luo M, Zhang Z, Chen Y, Xie N, Gao W, et al: Hypoxia-induced lncRNA
STEAP3-AS1 activates Wnt/β-catenin signaling to promote colorectal
cancer progression by preventing m6A-mediated degradation of STEAP3
mRNA. Mol Cancer. 21:1682022. View Article : Google Scholar
|
|
125
|
Wang X, Liu C, Zhang S, Yan H, Zhang L,
Jiang A, Liu Y, Feng Y, Li D, Guo Y, et al:
N6-methyladenosine modification of MALAT1 promotes
metastasis via reshaping nuclear speckles. Dev Cell. 56:702–715.e8.
2021. View Article : Google Scholar
|
|
126
|
Yang H, Hu Y, Weng M, Liu X, Wan P, Hu Y,
Ma M, Zhang Y, Xia H and Lv K: Hypoxia inducible lncRNA-CBSLR
modulates ferroptosis through m6A-YTHDF2-dependent modulation of
CBS in gastric cancer. J Adv Res. 37:91–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang S, Wang Y, Zhang Z, Zhu C, Wang C, Yu
F and Zhao E: Long Non-Coding RNA NRON promotes tumor proliferation
by regulating ALKBH5 and nanog in gastric cancer. J Cancer.
12:6861–6872. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S,
Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al: m6A
Demethylase ALKBH5 maintains tumorigenicity of glioblastoma
Stem-like cells by sustaining FOXM1 expression and cell
proliferation program. Cancer Cell. 31:591–606.e6. 2017. View Article : Google Scholar
|
|
129
|
Zou Z, Zhou S, Liang G, Tang Z, Li K, Tan
S, Zhang X and Zhu X: The pan-cancer analysis of the two types of
uterine cancer uncovered clinical and prognostic associations with
m6A RNA methylation regulators. Mol Omics. 17:438–453. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhu P, He F, Hou Y, Tu G, Li Q, Jin T,
Zeng H, Qin Y, Wan X, Qiao Y, et al: A novel hypoxic long noncoding
RNA KB-1980E6.3 maintains breast cancer stem cell stemness via
interacting with IGF2BP1 to facilitate c-Myc mRNA stability.
Oncogene. 40:1609–1627. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nadhan R, Isidoro C, Song YS and
Dhanasekaran DN: LncRNAs and the cancer epigenome: Mechanisms and
therapeutic potential. Cancer Lett. 605:2172972024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang L, Tang L, Min Q, Tian H, Li L, Zhao
Y, Wu X, Li M, Du F, Chen Y, et al: Emerging role of RNA
modification and long noncoding RNA interaction in cancer. Cancer
Gene Ther. 31:816–830. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kim SY, Na MJ, Yoon S, Shin E, Ha JW, Jeon
S and Nam SW: The roles and mechanisms of coding and noncoding RNA
variations in cancer. Exp Mol Med. 56:1909–1920. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar
|
|
135
|
Liu S, Jiao B, Zhao H, Liang X, Jin F, Liu
X and Hu JF: LncRNAs-circRNAs as rising epigenetic binary
superstars in regulating lipid metabolic reprogramming of cancers.
Adv Sci (Weinh). 11:e23035702024. View Article : Google Scholar
|
|
136
|
Mattick JS, Amaral PP, Carninci P,
Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME,
Fitzgerald KA, et al: Long non-coding RNAs: Definitions, functions,
challenges and recommendations. Nat Rev Mol Cell Biol. 24:430–447.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Liu SJ, Dang HX, Lim DA, Feng FY and Maher
CA: Long noncoding RNAs in cancer metastasis. Nat Rev Cancer.
21:446–460. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Qian Y, Shi L and Luo Z: Long Non-coding
RNAs in Cancer: Implications for diagnosis, prognosis, and therapy.
Front Med (Lausanne). 7:6123932020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Gong C, Li Z, Ramanujan K, Clay I, Zhang
Y, Lemire-Brachat S and Glass DJ: A long non-coding RNA, LncMyoD,
regulates skeletal muscle differentiation by blocking IMP2-mediated
mRNA translation. Dev Cell. 34:181–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Davodabadi F, Farasati Far B, Sargazi S,
Fatemeh Sajjadi S, Fathi-Karkan S, Mirinejad S, Ghotekar S, Sargazi
S and Rahman MM: Nanomaterials-based targeting of long Non-Coding
RNAs in cancer: A Cutting-edge review of current trends.
ChemMedChem. 19:e2023005282024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Han S, Cao Y, Guo T, Lin Q and Luo F:
Targeting lncRNA/Wnt axis by flavonoids: A promising therapeutic
approach for colorectal cancer. Phytother Res. 36:4024–4040. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Parashar D, Singh A, Gupta S, Sharma A,
Sharma MK, Roy KK, Chauhan SC and Kashyap VK: Emerging roles and
potential applications of Non-Coding RNAs in Cervical cancer. Genes
(Basel). 13:12542022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Goyal A, Myacheva K, Gross M, Klingenberg
M, Duran Arque B and Diederichs S: Challenges of CRISPR/Cas9
applications for long non-coding RNA genes. Nucleic Acids Res.
45:e122017.PubMed/NCBI
|
|
144
|
Cetinkaya M and Baran Y: MicroRNAs and
long non-coding RNAs as novel targets in Anti-cancer drug
development. Curr Pharm Biotechnol. 24:913–925. 2023. View Article : Google Scholar
|
|
145
|
Ozcan G, Ozpolat B, Coleman RL, Sood AK
and Lopez-Berestein G: Preclinical and clinical development of
siRNA-based therapeutics. Adv Drug Deliv Rev. 87:108–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wahlestedt C: Targeting long non-coding
RNA to therapeutically upregulate gene expression. Nat Rev Drug
Discov. 12:433–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Alegra-Torres JA, Baccarelli A and Bollati
V: Epigenetics and lifestyle. Epigenomics. 3:267–277. 2011.
View Article : Google Scholar
|
|
148
|
Basu AK: DNA Damage, mutagenesis and
cancer. Int J Mol Sci. 19:9702018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Cheng D, Deng J, Zhang B, He X, Meng Z, Li
G, Ye H, Zheng S, Wei L, Deng X, et al: LncRNA HOTAIR
epigenetically suppresses miR-122 expression in hepatocellular
carcinoma via DNA methylation. EBioMedicine. 36:159–170. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Vaasjo LO: LncRNAs and chromatin
modifications pattern m6A methylation at the untranslated regions
of mRNAs. Front Genet. 13:8667722022. View Article : Google Scholar :
|
|
151
|
Vaid R, Thombare K, Mendez A,
Burgos-Panadero R, Djos A, Jachimowicz D, Lundberg KI, Bartenhagen
C, Kumar N, Tümmler C, et al: METTL3 drives telomere targeting of
TERRA lncRNA through m6A-dependent R-loop formation: A therapeutic
target for ALT-positive neuroblastoma. Nucleic Acids Res.
52:2648–2671. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar
|
|
153
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Cho SW, Xu J, Sun R, Mumbach MR, Carter
AC, Chen YG, Yost KE, Kim J, He J, Nevins SA, et al: Promoter of
lncRNA Gene PVT1 is a Tumor-suppressor DNA boundary element. Cell.
173:1398–1412.e22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Arun G, Aggarwal D and Spector DL: MALAT1
Long Non-coding RNA: Functional implications. Noncoding RNA.
6:222020.PubMed/NCBI
|
|
156
|
Chen PB, Chen HV, Acharya D, Rando OJ and
Fazzio TG: R loops regulate promoter-proximal chromatin
architecture and cellular differentiation. Nat Struct Mol Biol.
22:999–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Chu C, Qu K, Zhong FL, Artandi SE and
Chang HY: Genomic maps of long Noncoding RNA occupancy reveal
principles of RNA-Chromatin interactions. Mol Cell. 44:667–678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Chu C and Chang HY: ChIRP-MS: RNA-directed
proteomic discovery. Methods Mol Biol. 1861:37–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
de Lera AR and Ganesan A: Epigenetic
polypharmacology: From combination therapy to multitargeted drugs.
Clin Epigenetics. 8:1052016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Delgado-Morales R, Agís-Balboa RC,
Esteller M and Berdasco M: Epigenetic mechanisms during ageing and
neurogenesis as novel therapeutic avenues in human brain disorders.
Clin Epigenetics. 9:2017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wu SC, Kallin EM and Zhang Y: Role of
H3K27 methylation in the regulation of lncRNA expression. Cell Res.
20:1109–1116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kornienko AE, Dotter CP, Guenzl PM,
Gisslinger H, Gisslinger B, Cleary C, Kralovics R, Pauler FM and
Barlow DP: Long non-coding RNAs display higher natural expression
variation than protein-coding genes in healthy humans. Genome Biol.
17:142016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Pang KC, Frith MC and Mattick JS: Rapid
evolution of noncoding RNAs: Lack of conservation does not mean
lack of function. Trends Genet. 22:1–5. 2006. View Article : Google Scholar
|
|
164
|
Bohmdorfer G and Wierzbicki AT: Control of
chromatin structure by long noncoding RNA. Trends Cell Biol.
25:623–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xing Z, Lin A, Li C, Liang K, Wang S, Liu
Y, Park PK, Qin L, Wei Y, Hawke DH, et al: lncRNA directs
cooperative epigenetic regulation downstream of chemokine signals.
Cell. 159:1110–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|