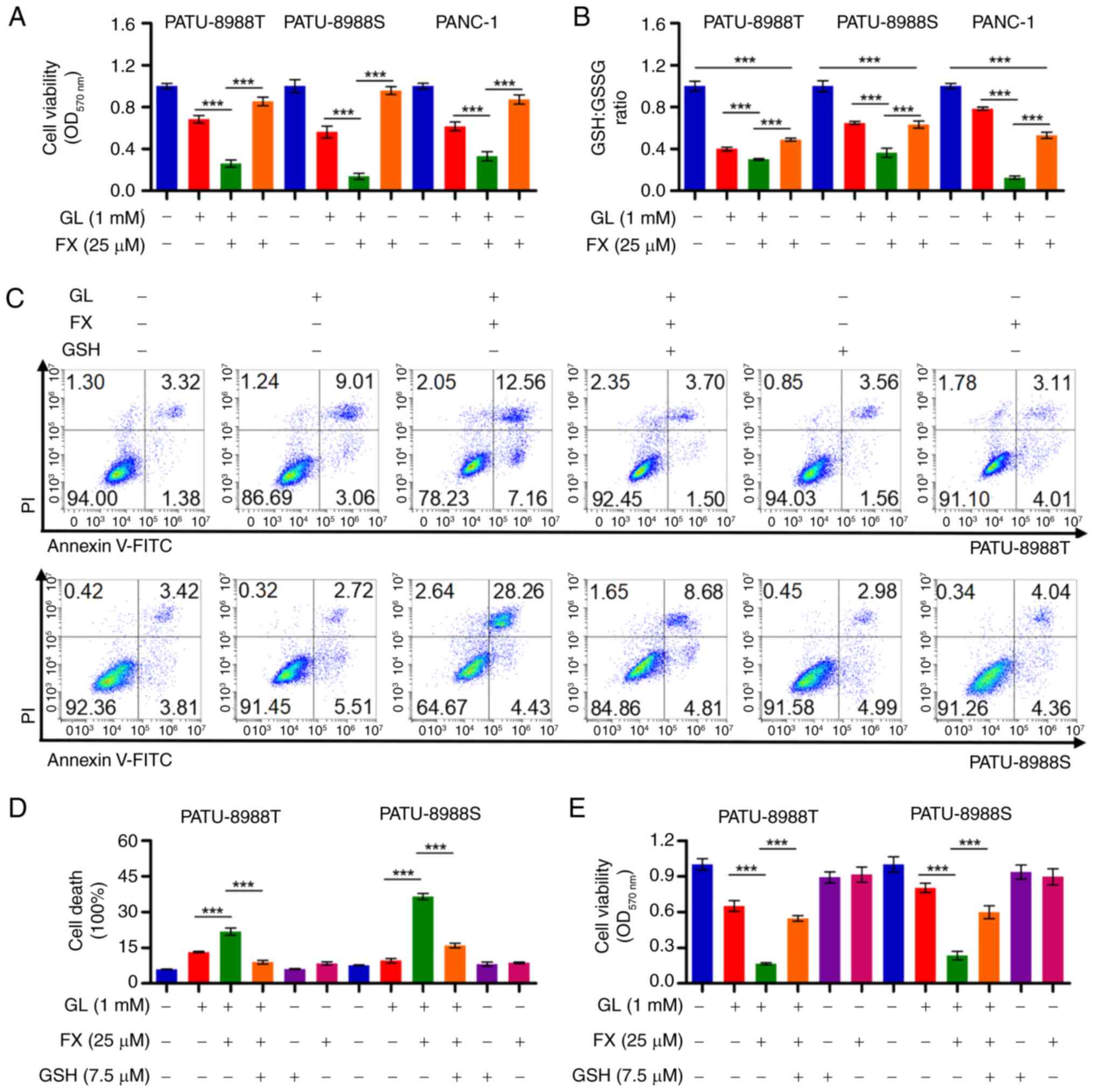
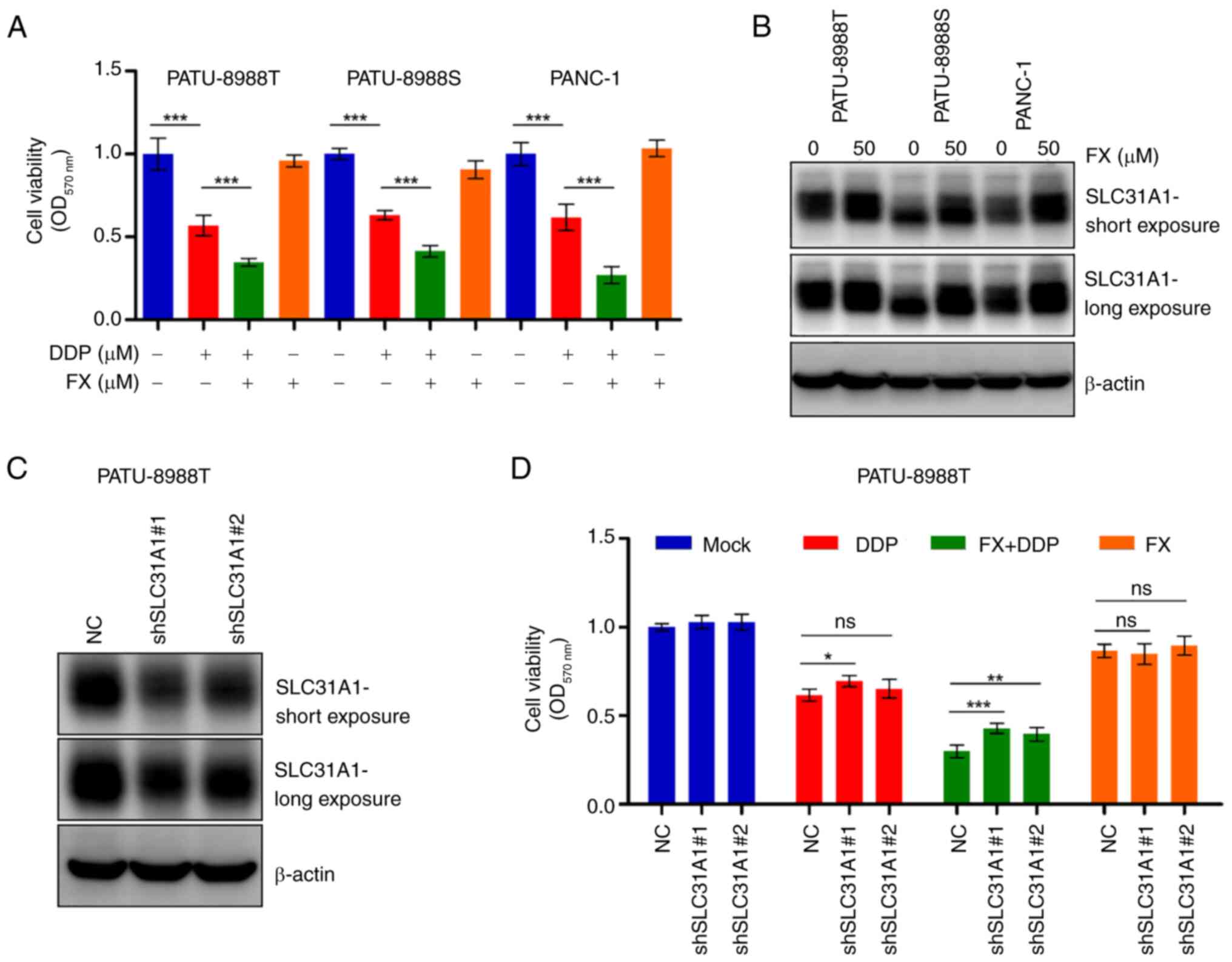
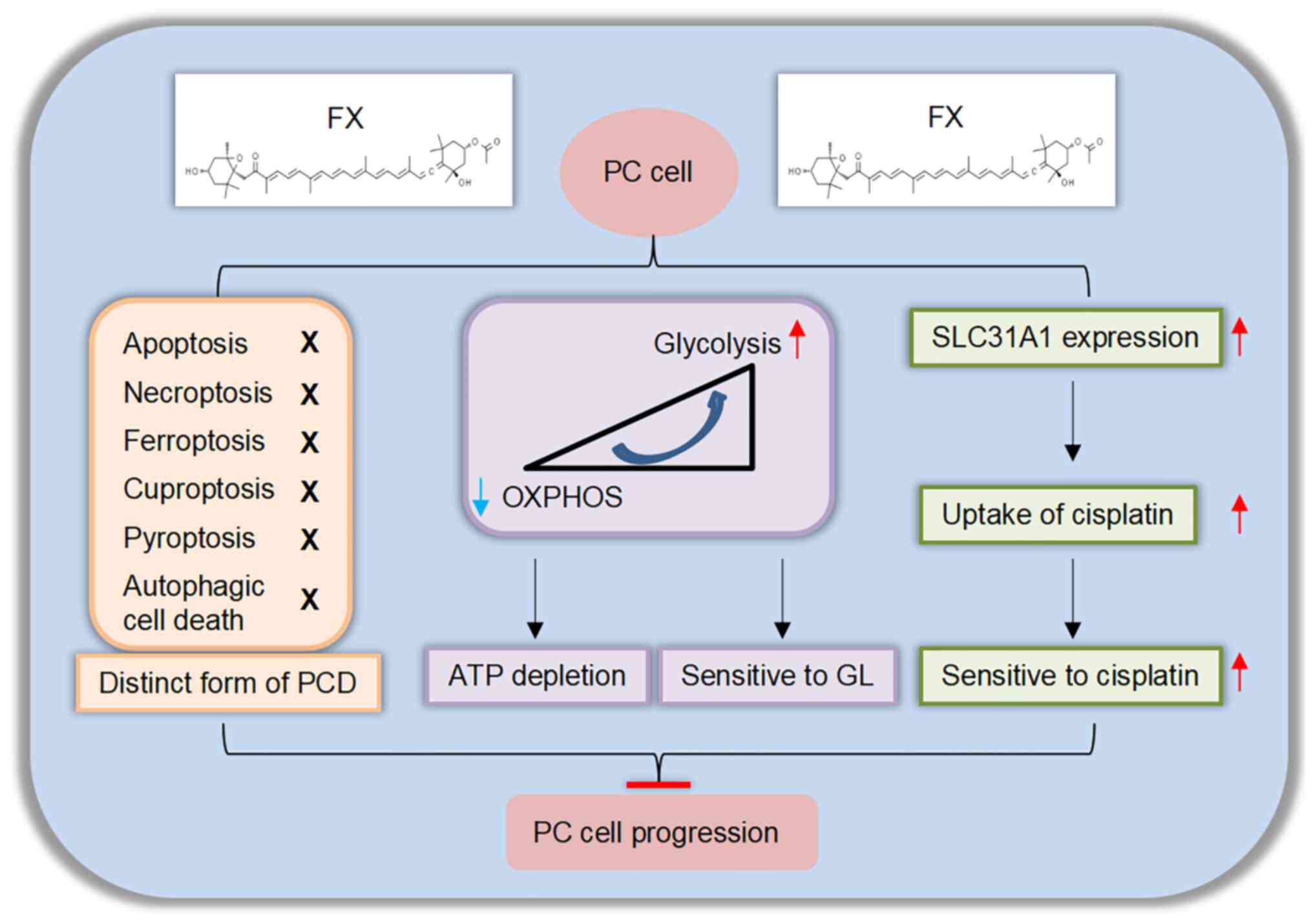
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain T and Dudeja V: The war against
pancreatic cancer in 2020-advances on all fronts. Nat Rev
Gastroenterol Hepatol. 18:99–100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Din NAS, Mohd Alayudin S, Sofian-Seng NS,
Rahman HA, Mohd Razali NS, Lim SJ and Wan Mustapha WA: Brown algae
as functional food source of fucoxanthin: A Review. Foods.
11:22352022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Méresse S, Fodil M, Fleury F and Chénais
B: Fucoxanthin, a Marine-derived carotenoid from brown seaweeds and
microalgae: A promising bioactive compound for cancer therapy. Int
J Mol Sci. 21:92732020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rengarajan T, Rajendran P, Nandakumar N,
Balasubramanian MP and Nishigaki I: Cancer preventive efficacy of
marine carotenoid fucoxanthin: Cell cycle arrest and apoptosis.
Nutrients. 5:4978–4989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou LL, Gao C, Chen L, Hu GQ and Xie SQ:
Essential role of autophagy in fucoxanthin-induced cytotoxicity to
human epithelial cervical cancer HeLa cells. Acta Pharmacol Sin.
34:1403–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Cheng J, Min Z, Yin T, Zhang R,
Zhang W, Hu L, Cui Z, Gao C, Xu S, et al: Effects of fucoxanthin on
autophagy and apoptosis in SGC-7901 cells and the mechanism. J Cell
Biochem. 119:7274–7284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long Y, Cao X, Zhao R, Gong S, Jin L and
Feng C: Fucoxanthin treatment inhibits nasopharyngeal carcinoma
cell proliferation through induction of autophagy mechanism.
Environ Toxicol. 35:1082–1090. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murase W, Kamakura Y, Kawakami S, Yasuda
A, Wagatsuma M, Kubota A, Kojima H, Ohta T, Takahashi M, Mutoh M,
et al: Fucoxanthin prevents pancreatic tumorigenesis in C57BL/6J
mice that received allogenic and orthotopic transplants of cancer
cells. Int J Mol Sci. 22:136202021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Terasaki M, Takahashi S, Nishimura R,
Kubota A, Kojima H, Ohta T, Hamada J, Kuramitsu Y, Maeda H, Mutoh
M, et al: A marine carotenoid of fucoxanthinol accelerates the
growth of human pancreatic cancer PANC-1 cells. Nut Cancer.
74:357–371. 2020. View Article : Google Scholar
|
|
11
|
Wang YP, Zhou W, Wang J, Huang X, Zuo Y,
Wang TS, Gao X, Xu YY, Zou SW, Liu YB, et al: Arginine methylation
of MDH1 by CARM1 inhibits glutamine metabolism and suppresses
pancreatic cancer. Mol Cell. 64:673–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashton TM, McKenna WG, Kunz-Schughart LA
and Higgins GS: Oxidative phosphorylation as an emerging target in
cancer therapy. Clin Cancer Res. 24:2482–2490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Molina JR, Sun Y, Protopopova M, Gera S,
Bandi M, Bristow C, McAfoos T, Morlacchi P, Ackroyd J, Agip AA, et
al: An inhibitor of oxidative phosphorylation exploits cancer
vulnerability. Nat Med. 24:1036–1046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Passaniti A, Kim MS, Polster BM and
Shapiro P: Targeting mitochondrial metabolism for metastatic cancer
therapy. Mol Carcinog. 61:827–838. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu Y, Ricciardiello F, Yang G, Qiu J,
Huang H, Xiao J, Cao Z, Zhao F, Liu Y, Luo W, et al: The role of
mitochondria in the chemoresistance of pancreatic cancer cells.
Cells. 10:4972021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gentric G, Mieulet V and Mechta-Grigoriou
F: Heterogeneity in cancer metabolism: New concepts in an old
field. Antioxid Redox Signal. 26:462–485. 2017. View Article : Google Scholar :
|
|
19
|
Tong Y, Guo D, Lin SH, Liang J, Yang D, Ma
C, Shao F, Li M, Yu Q, Jiang Y, et al: SUCLA2-coupled regulation of
GLS succinylation and activity counteracts oxidative stress in
tumor cells. Mol Cell. 81:2303–2316.e8. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jacque N, Ronchetti AM, Larrue C, Meunier
G, Birsen R, Willems L, Saland E, Decroocq J, Maciel TT, Lambert M,
et al: Targeting glutaminolysis has antileukemic activity in acute
myeloid leukemia and synergizes with BCL-2 inhibition. Blood.
126:1346–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong SM, Xiao C, Finley LW, Lahusen T,
Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, et al:
SIRT4 has tumor-suppressive activity and regulates the cellular
metabolic response to DNA damage by inhibiting mitochondrial
glutamine metabolism. Cancer Cell. 23:450–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tardito S, Oudin A, Ahmed SU, Fack F,
Keunen O, Zheng L, Miletic H, Sakariassen PØ, Weinstock A, Wagner
A, et al: Glutamine synthetase activity fuels nucleotide
biosynthesis and supports growth of glutamine-restricted
glioblastoma. Nat Cell Biol. 17:1556–1568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu W, Zhang C, Wu R, Sun Y, Levine A and
Feng Z: Glutaminase 2, a novel p53 target gene regulating energy
metabolism and antioxidant function. Proc Natl Acad Sci USA.
107:7455–7460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daemen A, Peterson D, Sahu N, McCord R, Du
X, Liu B, Kowanetz K, Hong R, Moffat J, Gao M, et al: Metabolite
profiling stratifies pancreatic ductal adenocarcinomas into
subtypes with distinct sensitivities to metabolic inhibitors. Proc
Natl Acad Sci USA. 112:E4410–E4417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Yu Z, Fu H, Guo Y, Hu P and Shi J:
Dual inhibitions on Glucose/glutamine metabolisms for nontoxic
pancreatic cancer therapy. ACS Appl Mater Interfaces.
14:21836–21847. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bae M, Kim MB and Lee JY: Fucoxanthin
attenuates the reprogramming of energy metabolism during the
activation of hepatic stellate cells. Nutrients. 14:19022022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye Z, Zhuo Q, Hu Q, Xu X, Mengqi Liu,
Zhang Z, Xu W, Liu W, Fan G, Qin Y, et al: FBW7-NRA41-SCD1 axis
synchronously regulates apoptosis and ferroptosis in pancreatic
cancer cells. Redox Biol. 38:1018072021. View Article : Google Scholar
|
|
28
|
Ma N, Shangguan F, Zhou H, Huang H, Lei J,
An J, Jin G, Zhuang W, Zhou S, Wu S, et al:
6-methoxydihydroavicine, the alkaloid extracted from Macleaya
cordata (Willd.) R. Br. (Papaveraceae), triggers
RIPK1/Caspase-dependent cell death in pancreatic cancer cells
through the disruption of oxaloacetic acid metabolism and
accumulation of reactive oxygen species. Phytomedicine.
102:1541642022. View Article : Google Scholar
|
|
29
|
Carvalho TMA, Audero MM, Greco MR, Ardone
M, Maggi T, Mallamaci R, Rolando B, Arpicco S, Ruffinatti FA, Pla
AF, et al: Tumor microenvironment modulates invadopodia activity of
Non-Selected and Acid-Selected pancreatic cancer cells and its
sensitivity to gemcitabine and C18-Gemcitabine. Cells. 13:7302024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Z, Zhou R, Zhao Y, Pan Y, Liang H,
Zhang JS, Tai S, Jin L and Teng CB: Blockage of SLC31A1-dependent
copper absorption increases pancreatic cancer cell autophagy to
resist cell death. Cell Prolif. 52:e125682019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng R, Ke N, Wang Z, Mou Y, Xiang B,
Zhang Z, Ji X, Zou J, Wang D, Yin Z, et al: Copper deprivation
enhances the chemosensitivity of pancreatic cancer to rapamycin by
mTORC1/2 inhibition. Chem Biol Interact. 382:1105462023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiu HW, Lin SW, Lin LC, Hsu YH, Lin YF,
Ho SY, Wu YH and Wang YJ: Synergistic antitumor effects of
radiation and proteasome inhibitor treatment in pancreatic cancer
through the induction of autophagy and the downregulation of TRAF6.
Cancer Lett. 365:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dasgupta A, Arneson-Wissink PC, Schmitt
RE, Cho DS, Ducharme AM, Hogenson TL, Krueger EW, Bamlet WR, Zhang
L, Razidlo GL, et al: Anticachectic regulator analysis reveals
Perp-dependent antitumorigenic properties of 3-methyladenine in
pancreatic cancer. JCI Insight. 7:e1538422022. View Article : Google Scholar :
|
|
34
|
Qi J, Xing Y, Liu Y, Wang MM, Wei X, Sui
Z, Ding L, Zhang Y, Lu C, Fei YH, et al: MCOLN1/TRPML1 finely
controls oncogenic autophagy in cancer by mediating zinc influx.
Autophagy. 17:4401–4422. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song CF, Hu YH, Mang ZG, Ye Z, Chen HD,
Jing DS, Fan GX, Ji SR, Yu XJ, Xu XW, et al: Hernandezine induces
autophagic cell death in human pancreatic cancer cells via
activation of the ROS/AMPK signaling pathway. Acta Pharmacol Sin.
44:865–876. 2023. View Article : Google Scholar :
|
|
36
|
Liu J, Tang H, Chen F, Li C, Xie Y, Kang R
and Tang D: NFE2L2 and SLC25A39 drive cuproptosis resistance
through GSH metabolism. Sci Rep. 14:295792024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou J, Zheng Y, Huang Y, Tang D, Kang R
and Chen R: The versatile gasdermin family: Their function and
roles in diseases. Front Immunol. 12:7515332021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nurcahyanti ADR, Kusmita L and Wink M:
Bixin and fucoxanthin sensitize human lung cancer and cervical
cancer cell to cisplatin in vitro. BMC Res Notes. 14:4542021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu CL, Lim YP and Hu ML: Fucoxanthin
enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway
and downregulates DNA repair gene expression in human hepatoma
HepG2 cells. Mar Drugs. 11:50–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandra F, Tania TF and Nurcahyanti ADR:
Bixin and Fuxoxanthin alone and in combination with cisplatin
regulate ABCC1 and ABCC2 transcription in A549 lung cancer cells. J
Pharm Bioallied Sci. 15:15–20. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu G, Peng H, Tang M, Yang M, Wang J, Hu
Y, Li Z, Li J, Li Z and Song L: ZNF711 down-regulation promotes
CISPLATIN resistance in epithelial ovarian cancer via interacting
with JHDM2A and suppressing SLC31A1 expression. EBioMedicine.
71:1035582021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Terasaki M, Inoue T, Murase W, Kubota A,
Kojima H, Kojoma M, Ohta T, Maeda H, Miyashita K, Mutoh M, et al: A
fucoxanthinol induces apoptosis in a pancreatic intraepithelial
neoplasia cell. Cancer Genomics Proteomics. 18:133–146. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang JY, Ou-Yang F, Hou MF, Huang HW, Wang
HR, Li KT, Fayyaz S, Shu CW and Chang HW: Oxidative
stress-modulating drugs have preferential anticancer
effects-involving the regulation of apoptosis, DNA damage,
endoplasmic reticulum stress, autophagy, metabolism, and migration.
Semin Cancer Biol. 58:109–117. 2019. View Article : Google Scholar
|
|
44
|
Su Z, Yang Z, Xie L, DeWitt JP and Chen Y:
Cancer therapy in the necroptosis era. Cell Death Differ.
23:748–756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu D, Wang S, Yu G and Chen X: Cell death
mediated by the pyroptosis pathway with the aid of nanotechnology:
Prospects for cancer therapy. Angew Chem Int Ed Engl. 60:8018–8034.
2021. View Article : Google Scholar
|
|
47
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen L, Chen D, Li J, He L, Chen T, Song
D, Shan S, Wang J, Lu X and Lu B: Ciclopirox drives growth arrest
and autophagic cell death through STAT3 in gastric cancer cells.
Cell Death Dis. 13:10072022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang P, Chen G, Jin W, Mao K, Wan H and
He Y: Molecular mechanisms of parthanatos and its role in diverse
diseases. Int J Mol Sci. 23:72922022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou Y, Liu L, Tao S, Yao Y, Wang Y, Wei
Q, Shao A and Deng Y: Parthanatos and its associated components:
Promising therapeutic targets for cancer. Pharmacol Res.
163:1052992021. View Article : Google Scholar
|
|
52
|
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu
L, Chen Y and Han B: Regulated cell death (RCD) in cancer: Key
pathways and targeted therapies. Signal Transduct Target Ther.
7:2862022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fraile-Martinez O, García-Montero C,
Pekarek L, Saz JV, Álvarez-Mon MÁ, Barrena-Blázquez S,
García-Honduvilla N, Buján J, Asúnsolo Á, Coca S, et al: Decreased
survival in patients with pancreatic cancer may be associated with
an increase in histopathological expression of inflammasome marker
NLRP3. Histol Histopathol. 39:35–40. 2024.
|
|
54
|
Ortega MA, Jiménez-Álvarez L,
Fraile-Martinez O, Garcia-Montero C, León-Oliva DD, Toledo-Lobo
MDV, Palacios E, Granado P, Esteban A, Guijarro LG, et al: Elevated
tissue expression of RANKL and RANK is associated with poorer
survival rates in pancreatic cancer patients. Histol Histopathol.
39:1133–1140. 2024.PubMed/NCBI
|
|
55
|
Ying H, Kimmelman AC, Lyssiotis CA, Hua S,
Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff
JL, et al: Oncogenic Kras maintains pancreatic tumors through
regulation of anabolic glucose metabolism. Cell. 149:656–670. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Humpton TJ, Alagesan B, DeNicola GM, Lu D,
Yordanov GN, Leonhardt CS, Yao MA, Alagesan P, Zaatari MN, Park Y,
et al: Oncogenic KRAS induces NIX-mediated mitophagy to promote
pancreatic cancer. Cancer Discov. 9:1268–1287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dey P, Li J, Zhang J, Chaurasiya S, Strom
A, Wang H, Liao WT, Cavallaro F, Denz P, Bernard V, et al:
Oncogenic KRAS-driven metabolic reprogramming in pancreatic cancer
cells utilizes cytokines from the tumor microenvironment. Cancer
Discov. 10:608–625. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Teng R, Liu Z, Tang H, Zhang W, Chen Y, Xu
R, Chen L, Song J, Liu X and Deng H: HSP60 silencing promotes
Warburg-like phenotypes and switches the mitochondrial function
from ATP production to biosynthesis in ccRCC cells. Redox Biol.
24:1012182019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qin S, Li J, Bai Y, Wang Z, Chen Z, Xu R,
Xu J, Zhang H, Chen J, Yuan Y, et al: Nimotuzumab plus gemcitabine
for K-Ras Wild-type locally advanced or metastatic pancreatic
cancer. J Clin Oncol. 41:5163–5173. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gaglio D, Metallo CM, Gameiro PA, Hiller
K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G and
Chiaradonna F: Oncogenic K-Ras decouples glucose and glutamine
metabolism to support cancer cell growth. Mol Syst Biol. 7:5232011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Son J, Lyssiotis CA, Ying H, Wang X, Hua
S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et
al: Glutamine supports pancreatic cancer growth through a
KRAS-regulated metabolic pathway. Nature. 496:101–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jaaks P, Coker EA, Vis DJ, Edwards O,
Carpenter EF, Leto SM, Dwane L, Sassi F, Lightfoot H, Barthorpe S,
et al: Effective drug combinations in breast, colon and pancreatic
cancer cells. Nature. 603:166–173. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lu J, Wu XJ, Hassouna A, Wang KS, Li Y,
Feng T, Zhao Y, Jin MF, Zhang BH, Ying TL, et al:
Gemcitabine-fucoxanthin combination in human pancreatic cancer
cells. Biomed Rep. 19:462023. View Article : Google Scholar
|
|
64
|
Cheng C, Ding Q, Zhang Z, Wang S, Zhong B,
Huang X and Shao Z: PTBP1 modulates osteosarcoma chemoresistance to
cisplatin by regulating the expression of the copper transporter
SLC31A1. J Cell Mol Med. 24:5274–5289. 2020. View Article : Google Scholar : PubMed/NCBI
|