Introduction
In women, lung cancer is the most common cause of
cancer-related mortality and the second most commonly occurring
cancer worldwide (1). Lung cancer
is directly associated with a delayed presentation. Signs and
symptoms are rarely present until the malignancy is advanced and
often unresectable. Patients with stage IA (T1N0M0) disease have a
61–75% five-year survival rate subsequent to surgical resection
(2). Most of these patients have
no symptoms and their lung lesions are initially detected as
solitary pulmonary nodules (SPNs) in computed tomography (CT)
screening.
An SPN is defined as a focal round or oval area
(diameter <3 cm) of increased radiographic opacity in the lung
(3). In the United States,
>150,000 patients visit their physicians annually with symptoms
generating a diagnostic dilemma of an SPN. CT screening has
increased the detection rate of SPNs with its detection rate
increasing even further, due to incidental findings of lung nodules
on chest CT (2).
In the past 30 years, substantial advances have been
made in the outcome of patients with early-stage breast cancer,
resulting from early detection, the identification of prognostic
factors and the improvements in surgical techniques, such as radio-
and systemic therapy (4). These
improvements in breast cancer outcomes have increased the
curability or survival period of several patients, thereby
increasing their risk of developing a second malignancy.
Pulmonary metastases are common in patients with
breast cancer. Most physicians traditionally consider an SPN
occurring in a patient with breast cancer to represent a metastasis
from the breast (5). However, a
differential diagnosis using radiological features is often
difficult (5,6). Thus, an SPN occurring in a patient
with documented past or present breast cancer presents a diagnostic
challenge.
The nature of SPNs in patients with breast cancer
and the effectiveness of surgery in their treatment were
retrospectively evaluated.
Materials and methods
Patient population and surgical
procedure
In total, 30 SPN specimens were obtained from women
(mean age, 58 years; range, 41–80 years), who underwent surgical
resection at the Gunma University Hospital between January, 2002
and July, 2011. Institutional approval and written informed consent
from the patients were obtained prior to surgery. The patients had
previously undergone curative surgery for breast cancer. The
patient characteristics are shown in Table I. Patients with metastases in other
organs were excluded. Pre-operative evaluation included chest
X-rays, total body CT scans, fiberoptic bronchoscopy, isotopic bone
scanning, cardiopulmonary function tests and total body fluorine-18
deoxyglucose positron emission tomography (FDG-PET). Peripheral
lesions were submitted pre-operatively to CT-guided needle
aspiration biopsy (CT-NAB), where the lesion diameter and location
were appropriate. No patient had a centrally located SPN and no
transbronchial needle aspiration or other bronchoscopic sampling
procedure was required during pre-operative bronchoscopy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | N | % |
|---|
| No. of patients | 30 | |
| Age (years) | | |
| Mean | 58 | |
| Range | 41–80 | |
| Nodule diameter
(mm) | | |
| Mean | 12 | |
| Range | 6–22 | |
| Nodule type | | |
| Solid | 14 | 47 |
| Mixed GGO | 14 | 47 |
| Pure GGO | 2 | 6 |
| Coincidence | | |
| Yes | 7 | 23 |
| No | 23 | 77 |
| DFI (months) | | |
| Mean | 68 | |
| Range | 6–229 | |
| Smoking status | | |
| Smoker | 3 | 10 |
| Non-smoker | 27 | 90 |
| Stage of breast
cancer | | |
| pT | | |
| pT1 | 10 | 33 |
| pT2 | 17 | 57 |
| pT3 | 3 | 10 |
| pN | | |
| pN0 | 15 | 50 |
| pN1 | 14 | 47 |
| pN2 | 1 | 3 |
| Laterality between
breast cancer and SPN | | |
| Ipsilateral | 15 | 50 |
| Contralateral | 15 | 50 |
| Past history of
malignancy | | |
| Breast cancer
only | 27 | 90 |
| Breast cancer and
other types | | |
| Uterine
cancer | 2 | 7 |
| Colon cancer | 1 | 3 |
Wedge resection or segmentectomy by video-assisted
thoracoscopic surgery (VATS) was planned to confirm the diagnosis
of peripherally-located SPNs. Frozen sections with hematoxylin and
eosin (H&E) staining were evaluated intraoperatively and the
operation was terminated when a nodule was definitively diagnosed
as metastatic lung cancer or a benign condition. In cases of
nodules diagnosed as primary lung cancer or suspected as malignant,
although not identified as either primary or metastatic lung cancer
at intraoperative diagnosis, the VATS was converted to an open
procedure including anatomical resection (lobular dissection or
segmentectomy) and hilar and mediastinal lymph node
dissections.
Pathological examination and
immunohistochemical diagnosis
For a strict differential diagnosis of pulmonary
nodules, a pathological examination was performed by two or more
pathologists. For the histological diagnosis, a conventional
morphopathological examination using H&E staining was
performed. The pathological findings were evaluated and compared to
those of primary breast cancer. For the strict differential
diagnosis of pulmonary nodules, immunohistochemical examination was
performed using the standard avidin-biotin peroxidase complex
method, with monoclonal antibodies against estrogen receptor
(ER)-α, progesterone receptor (PR), gross cystic disease fluid
protein-15 (GCDFP-15), thyroid transcription factor-1 (TTF-1) and
surfactant apoprotein-A (SP-A) for the cases. The immunopositive
results for some ER, PR and GCDFP-15 support the specimen diagnosis
of metastasis from breast cancer, although certain lung cancers are
known to be immunopositive for these antibodies (6–8).
TTF-1 and SP-A are known to be specific markers for primary lung
cancer, especially adenocarcinoma (9,10).
ER and PR staining was analyzed based on the Allred system, where
intensity of staining is recorded on a 5-scale grade (0, no
staining; 1, 20%; 2, 40%; 3, 60%; 4, 80%; and 5, 100%) (11). For GCDFP-15, TTF-1 and SP-A
staining, tissue were positive if ≥10% of the tumor cells were
stained. The final diagnosis of SPNs was confirmed based on the
results of the immunohistochemical and morphopathological
examinations.
Data management
The in- and outpatient medical records were reviewed
for the patients. The follow-up of the post-operative clinical
course was conducted relying on outpatient medical records and
telephone or written inquiries. The patients were retrospectively
analyzed for age, diameter of SPN, disease-free interval (DFI),
pre-operative follow-up period, smoking status, stage of breast
cancer, laterality between breast cancer and SPN, past history of
malignancy, histological subtype of the SPN, relapse and follow-up.
The DFI was measured between the dates of initial treatment for
breast cancer and the first SPN detection. The pre-operative
follow-up period was measured between the dates of the first SPN
detection and the date of operation. Overall survival (OS) was
defined as the time period between the resection of the SPN and the
date of the last follow-up or decease. Disease-free survival (DFS)
was defined as the time period between the resection of the SPN and
the date of the first documented relapse or the last follow-up. The
available survival data were updated in December, 2011.
Statistical analysis
OS and DFS were estimated using the Kaplan-Meier
method. The Student’s t-test and the χ2 test were used
to compare percentages and the mean values, respectively. Seven
patients with a pulmonary nodule detected simultaneously with
breast cancer were excluded from the DFI analysis. The values were
considered to indicate a statistically significant difference at
P<0.05. The statistical analyses were performed using the SPSS
for Windows ver. 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient groups
A differential diagnosis using only radiological
features was difficult to make, since certain metastatic lung
cancers revealed mixed ground-glass opacity (GGO) on the CT scans
(Fig. 1 and Table II).
 | Table IIRadiological features of solitary
pulmonary nodules. |
Table II
Radiological features of solitary
pulmonary nodules.
| Pure GGO
| Mixed GGO
| Solid
|
|---|
| Diagnosis | N | % | N | % | N | % |
|---|
| Primary lung
cancer | 2 | 6 | 11 | 37 | 7 | 24 |
| Metastasis | | | | | | |
| Breast cancer | 0 | 0 | 3 | 10 | 4 | 13 |
| Colon cancer | 0 | 0 | 0 | 0 | 1 | 3 |
| Benign tumor | 0 | 0 | 0 | 0 | 2 | 7 |
| Total | 2 | 6 | 14 | 47 | 14 | 47 |
Of the 30 patients undergoing surgery during the
study period, three underwent pre-operative CT-NAB yielding a
diagnosis of primary lung adenocarcinoma. Twenty-six patients
underwent wedge resections, while one required segmentectomy using
VATS for the diagnosis of a deep intraparenchymal nodule.
Definitive pathological diagnoses of SPN are listed in Table III, and immunohistochemical
features are shown in Table IV.
Three patients, who underwent only wedge resection by VATS due to
small nodules showing pure or mixed GGO, were diagnosed with
non-invasive primary lung cancer. In two cases, the absence of
malignant cells was demonstrated by intraoperative examination,
thus the operation was terminated (one patient received a wedge
resection and one segmentectomy). Intraoperative diagnoses of
malignant lesions were made in 22 patients by wedge resection,
while 12 patients were diagnosed with primary lung cancer, 11 of
whom had adenocarcinoma and one a carcinoid tumor. Ten of these 11
patients underwent lobectomy and lymph node dissection, whereas one
underwent segmentectomy and lymph node dissection. In one case,
dissemination was found intraoperatively and the operation was
terminated subsequent to the wedge resection and the dissection of
the dissemination. In five cases, intraoperative examination did
not distinguish between primary or metastatic adenocarcinoma. These
patients underwent lobectomy or segmentectomy and lymph node
dissection. Two were definitively diagnosed with primary lung
cancer and three with metastases of breast cancer. Five cases were
diagnosed as metastatic adenocarcinoma, and these operations were
terminated after wedge resection. Four of these patients had
metastases from breast and colon cancers. Eight patients diagnosed
with metastatic lung cancer started systemic treatment subsequent
to pulmonary resection.
 | Table IIIHistological diagnosis of solitary
pulmonary nodules in breast cancer patients. |
Table III
Histological diagnosis of solitary
pulmonary nodules in breast cancer patients.
| Diagnosis | No. of
patients | % |
|---|
| Malignancy | 28 | 93 |
| Primary lung
cancer | 20 | 67 |
| Histology | | |
|
Adenocarcinoma | 19 | 64 |
|
Carcinoid | 1 | 3 |
| Pathological
stage | | |
| IA
(T1aN0M0) | 15 | 75 |
| IIA
(T2aN1M0) | 1 | 5 |
| IIIA
(T1aN2M0) | 3 | 15 |
| IV
(T1aN0M1a) | 1 | 5 |
| Metastatic
carcinoma | 8 | 26 |
| From breast
cancer | 7 | 23 |
| From colon
cancer | 1 | 3 |
| Benign
condition | 2 | 7 |
| Histology | | |
| Epithelioid
cell granuloma | 1 | |
| Fibrosis and
elastosis | 1 | |
 | Table IVImmunohistochemical features of
solitary pulmonary nodules. |
Table IV
Immunohistochemical features of
solitary pulmonary nodules.
| | ER
| PR
| GCDFP-15
| TTF-1
| SPA
|
|---|
| Diagnosis | Total | N | % | N | % | N | % | N | % | N | % |
|---|
| Primary lung
cancer | 19 | 3 | 16 | 0 | 0 | 3 | 16 | 15 | 79 | 13 | 68 |
| Metastasis | | | | | | | | | | | |
| Breast
cancer | 7 | 4 | 57 | 2 | 29 | 5 | 71 | 0 | 0 | 2 | 29 |
| Colon cancer | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benign tumor | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Of the 20 patients with primary lung cancer, 15
(75%) had stage IA tumors (T1aN0M0), one a stage IIA tumor
(T2aN1M0), three stage IIIA tumors (T1aN2M0) and one a stage IV
tumor (T1aN0M1a), based on the International Association for the
Study of Lung Cancer (IASLC) criteria (7th edition) (Aurora, CO,
USA) (Table III). The median
follow-up period between the first SPN detection and the pulmonary
resection was 9.5 months, while the follow-up period did not exceed
six months in 12 of the 15 stage IA primary lung cancer
patients.
A significant difference was observed only in the
DFI between the primary lung cancer and metastasis from breast
cancer (Table V). The average DFI
in patients with primary lung cancer and metastatic breast cancer
was 94 (range, 6–229) months and 41 (range, 12–88) months,
respectively. DFI of the primary lung cancer group was markedly
longer compared to the metastatic breast cancer group (P=0.031).
However, in 2 of 7 patients with metastases from breast cancer, DFI
was >5 years. In 6 of the 20 patients with primary lung cancer,
DFI was <5 years, while in seven patients, pulmonary nodules
were detected during the pre-operative examination of breast
cancer.
 | Table VComparison of patient characteristics
between primary lung cancer and metastasis from breast cancer. |
Table V
Comparison of patient characteristics
between primary lung cancer and metastasis from breast cancer.
| Primary lung cancer
| Metastasis from
breast cancer
| |
|---|
| Patient
characteristics | N | Range | % | N | Range | % | P-value |
|---|
| No. of
patients | 20 | | | 7 | | | |
| Age (years) | 62 | 41–77 | | 54 | 41–58 | | 0.121 |
| Nodule diameter
(mm) | 13 | 7–22 | | 10 | 6–22 | | 0.237 |
| DFI (months) | 94 | 6–229 | | 41 | 12–88 | | 0.031 |
| Pre-operative
follow-up period (months) | 11 | 1–84 | | 5 | 1–12 | | 0.232 |
| Smoker | 1 | | 5 | 1 | | 14 | 0.459 |
| Ipsilaterality | 10 | | 50 | 4 | | 57 | 0.765 |
| Stage of breast
cancer | | | | | | | |
| T | | | | | | | |
| T1, 2 | 18 | | 90 | 6 | | 86 | 0.610 |
| T3 | 2 | | 10 | 1 | | 14 | |
| N | | | | | | | |
| N0 | 10 | | 50 | 2 | | 29 | 0.298 |
| N1, 2 | 10 | | 50 | 5 | | 71 | |
Survival and outcomes
Follow-up was complete for the patients in this
study. The median follow-up was 46 months (range, 5–115). The
five-year survival rate for lung metastasis from breast cancer
patients was 100% subsequent to pulmonary resection, although the
median follow-up was 52 months (Fig.
2). Of these 7 patients, five were event-free and two had
breast cancer relapse: one with lung metastasis and the other with
hilar lymph nodes. For the patient with lung metastasis relapse,
subsequent lung resection was performed, with no recurring
relapse.
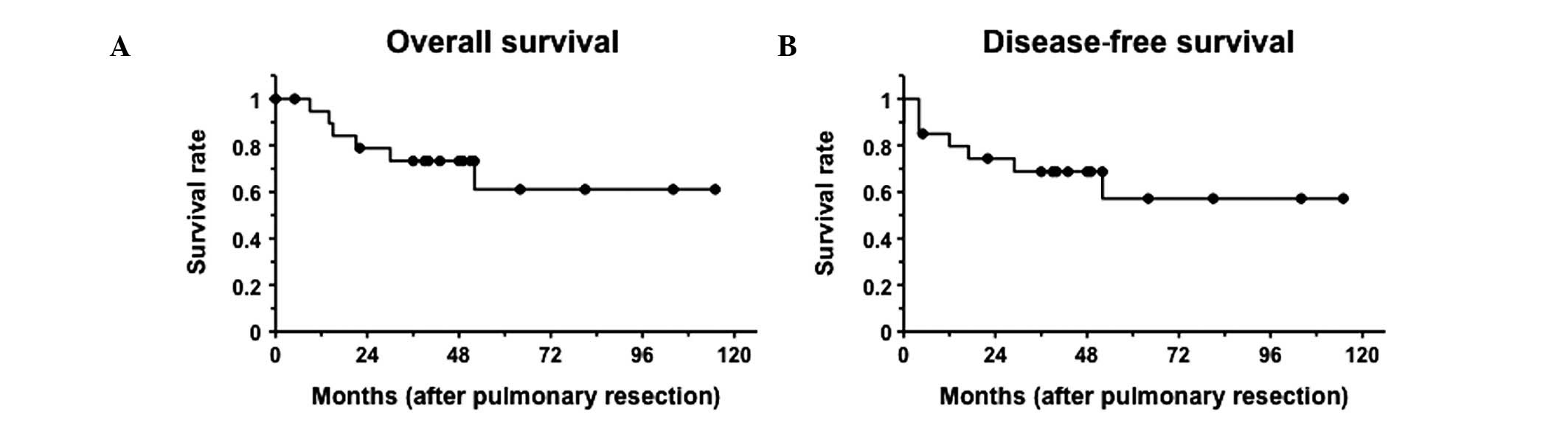
The five-year survival period for the 20 primary
lung cancer patients was 61.1% subsequent to pulmonary resection
(Fig. 3). Six patients diagnosed
with primary lung cancer succumbed to the disease during the
observation period, and four of lung cancer relapse (three had
stage IIIA tumors and one a stage IV tumor), one from breast cancer
relapse and one from cerebral apoplexy. The remaining 14 patients,
with stage I or IIA tumor, were event-free, with the exception of
one patient, who developed a new primary lung cancer.
Discussion
The present study demonstrated that 93% of SPNs
found in patients with breast cancer were malignant. The majority
of pathological diagnosis of these SPNs was primary lung cancer
(67%) or pulmonary metastasis from breast cancer (23%). The early
resection of SPNs was demonstrated to possibly result in good
prognosis in patients with malignant tumors, regardless of their
diagnosis as primary lung cancer or metastasis.
A differential diagnosis between primary and
metastatic lung cancer using radiological features was difficult,
as seen in previous studies (5,6). No
visible differences were detected in the radiological features or
the dimension (Fig. 1; Table II). GGO is one of the
characteristic signs of primary lung adenocarcinoma, although cases
of metastatic lung tumor from the adenocarcinoma of the
gastrointestinal tract or malignant melanoma showing GGOs have also
been reported (12,13). In the present study, >40% (3/7)
of the metastases from breast cancer showed mixed GGO on CT.
Therefore, an SPN showing GGO on CT is not likely to indicate
pulmonary adenocarcinoma in patients with a history of breast
cancer. Furthermore, a differential diagnosis of focal GGO includes
inflammatory diseases, focal fibrosis and atypical adenomatous
hyperplasia (14). Several studies
have demonstrated that sometimes even a benign and malignant
distinction is difficult to make (5,6).
Therefore, a qualitative diagnosis is required for the definitive
diagnosis of SPNs in breast cancer patients.
Previous studies have reported that ∼50% of SPNs are
benign (2,15). However, when the population is
limited to patients, who have undergone surgery for breast cancer,
the malignancy rate of SPNs has been shown to be >80% (5,6,16).
Thus, it is necessary to monitor SPNs in patients with breast
cancer as potentially malignant, as in primary or metastatic lung
cancers. In the present study, 93% of SPNs found in patients with
breast cancer were malignant, while 67% were diagnosed as primary
lung cancer. The percentage of primary lung cancer was the highest
in previous studies (5,6), as in their case of Rena et
al(5), who reported that 82%
of the SPNs found in patients subsequent to mastectomy were
malignant, while 48% were diagnosed as primary lung cancer.
Consistent with the present findings, that study reported that the
major population of SPNs found in patients subsequent to
mastectomies was primary lung cancer, however, the frequency of
primary lung cancer in this study was higher, especially in
adenocarcinoma (19/20 patients with primary lung cancer). This
tendency may be associated with the increasing number of patients
with lung adenocarcinoma, especially among young Asian, non-smoking
women (17,18); all patients but three were
non-smoking Japanese women. Okasaka et al(6) reported that 100% (48/48) of their
cases were malignant, while 83% were diagnosed as breast cancer
metastases. In that study, specimens were obtained by CT-NAB or
transbronchial lung biopsy (TBLB) in 44 (91.7%), and by surgical
resection in 4 patients. Moreover, 32 patients were diagnosed by
conventional pathological examination, and immunohistochemical
staining and molecular marker examination were performed for the
remaining patients in a step-wise manner (6). By contrast, in this study, the entire
tumor was evaluated by surgical resection and immunohistochemical
staining performed in all cases. The discrepancy in the rate is
likely to be due to the different specimen collection method or
candidates for immunohistochemical staining.
Lung resection is a controversial treatment for
pulmonary metastasis from breast cancer (5,19–23).
In this study, the five-year survival period for solitary lung
metastasis from breast cancer patients was 100% after pulmonary
resection. The majority of breast oncologists opt for systemic
treatment, even in the case of a single lesion metastasis, given
that pulmonary metastasis is a systemic disease (5). The median survival period of
pulmonary metastasis patients subsequent to systemic treatment
remains approximately 24 months (19,20).
Several studies have suggested that surgical treatment for operable
lung metastases from breast cancer followed by systemic treatment
prolongs survival to a greater extent compared to standard systemic
treatment alone, in patients with <4 pulmonary metastases or
with a DFI >3 years (21–23).
The present study also suggests that pulmonary resection for
solitary lung metastasis from breast cancer is likely to contribute
to the improvement of prognosis, although a combined systemic
therapy might be required.
Furthermore, a substantial discordance between the
human epidermal growth factor receptor 2 (HER2) expression and
hormone receptors, such as ER and PR, in primary and recurrent
tumors in patients with recurrent breast cancer has been reported
(24,25). These reports showed a ∼40%
discordance in the hormone receptor status and 8–14% discordance in
HER2 status (24,25). Discordant cases have poor survival
rates, probably due to inappropriately targeted therapies. In the
present study, discordance in the PR status was also observed,
although in only one patient (14.3%). Therefore, tissue
confirmation is suggested for the appropriate treatment in patients
with clinically or radiologically suspected metastasis from breast
cancer. Moreover, the entire tumor needs to be examined to avoid
intratumoral heterogeneity generating discordance in a limited
number of specimens (25,26).
To provide an appropriate treatment, the diagnosis
of an SPN must be early and certain. Consistent with the findings
of this study, DFI for metastases from breast cancer has been
demonstrated to be significantly shorter compared to primary lung
cancer (5). However, 29% of
patients with metastases from breast cancer have DFI >5 years,
while 30% of primary lung cancer cases have DFI <5 years.
Although the DFI is available for assistance with differential
diagnosis, it is difficult to diagnose only by clinical data,
including the DFI criteria. Therefore, a pathological examination
is necessary to confirm the diagnosis. Step-wise examination using
biopsy specimens, initiating with CT-NAB or TBLB, for the
differential diagnosis of SPN (6,27)
has been previously made. However, limitations and complications
occur during these examinations. The examinations are limited due
to the tumor location and size, since when the nodules are small or
in an intricate location, it is difficult to extract an appropriate
specimen for pathological examination, including several
immunohistochemical stainings (2,28).
Another limitation of these examinations is their being only
diagnostic at best, rather than therapeutic, albeit being less
invasive compared to surgery (2).
Moreover, several complications, such as pneumothorax,
intrapulmonary hemorrhage, air embolism and pleural implantation,
associated with CT-NAB, have been reported (29). Being a minimally invasive surgical
procedure with high diagnostic sensitivity and specificity, VATS
has become a popular technique for nodule resection (30). Additionally, VATS may be converted
to therapeutic resection when a nodule is diagnosed
intraoperatively as primary lung cancer.
Evidently, the earlier the stage of primary lung
cancer, the better the prognosis. In primary lung cancer cases in
this study, 75% of the patients had a stage IA tumor at the time of
the surgery. The patients with stage IA tumor are still alive, with
the exception of two patients, deceased from other causes. The
five-year cancer-specific survival period for stage IA primary lung
cancer patients was 100% subsequent to pulmonary resection,
possibly due to a relatively short pre-operative follow-up period
leading to an earlier detection and more frequent opportunity for
chest CT scan compared to cancer-free women. Advanced-stage lung
cancer patients had a tendency for long follow-up or delay in the
detection of pulmonary involvement since the DFI occurred over a
long period of time and no periodic follow-up was carried out.
Therefore, early resection of SPN found in patients with breast
cancer is likely to also contribute to the improvement of prognosis
for primary lung cancer patients.
In conclusion, this study has shown that SPNs found
in patients with breast cancer have a high probability of
malignancy, especially primary lung adenocarcinoma. Furthermore,
the early resection of SPN led to good prognosis in patients
diagnosed with both primary and metastatic lung cancers. Thus,
early pathological diagnosis by surgical resection was suggested to
be conducted for the early diagnosis and the appropriate treatment
of SPNs in patients with breast cancer.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2.
|
Tan BB, Flaherty KR, Kazerooni EA and
Iannettoni MD: The solitary pulmonary nodule. Chest. 123:89–96.
2003.
|
|
3.
|
Jeong YJ, Yi CA and Lee KS: Solitary
pulmonary nodules: detection, characterization, and guidance for
further diagnostic workup and treatment. Am J Roentgenol.
188:57–68. 2007.
|
|
4.
|
Lorigan P, Califano R, Finn CF, Howell A
and Thatcher N: Lung cancer after treatment for breast cancer.
Lancet Oncol. 11:1184–1192. 2010.
|
|
5.
|
Rena O, Papalia E, Ruffini E, Filosso PL,
Oliaro A, Maggi G and Casadio C: The role of surgery in the
management of solitary pulmonary nodule in breast cancer patients.
Eur J Surg Oncol. 33:546–550. 2007.
|
|
6.
|
Okasaka T, Usami N, Mitsudomi T, Yatabe Y,
Matsuo K and Yokoi K: Stepwise examination for differential
diagnosis of primary lung cancer and breast cancer relapse
presenting as a solitary pulmonary nodule in patients after
mastectomy. J Surg Oncol. 98:510–514. 2008.
|
|
7.
|
Mazoujian G, Pinkus GS, Davis S and
Haagensen DE Jr: Immunohistochemistry of a gross cystic disease
fluid protein (GCDFP-15) of the breast. A marker of apocrine
epithelium and breast carcinomas with apocrine features. Am J
Pathol. 110:105–112. 1983.
|
|
8.
|
Yang M and Nonaka D: A study of
immunohistochemical differential expression in pulmonary and
mammary carcinomas. Mod Pathol. 23:654–661. 2010.
|
|
9.
|
Holzinger A, Dingle S, Bejarano PA, Miller
MA, Weaver TE, DiLauro R and Whitsett JA: Monoclonal antibody to
thyroid transcription factor-1: production, characterization, and
usefulness in tumor diagnosis. Hybridoma. 15:49–53. 1996.
|
|
10.
|
Nicholson AG, McCormick CJ, Shimosato Y,
Butcher DN and Sheppard MN: The value of PE-10, a monoclonal
antibody against pulmonary surfactant, in distinguishing primary
and metastatic lung tumours. Histopathology. 27:57–60. 1995.
|
|
11.
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168. 1998.
|
|
12.
|
John HW and Benedek B: CT halo sign in
pulmonary metastases from mucinous adenocarcinoma of the pancreas.
South Med J. 94:448–449. 2001.
|
|
13.
|
Okita R, Yamashita M, Nakata M, Teramoto
N, Bessho A and Mogami H: Multiple ground-grass opacity in
metastasis of malignant melanoma diagnosed by lung biopsy. Ann
Thorac Surg. 79:e1–e2. 2005.
|
|
14.
|
Yanagitani N, Kaira K, Ishizuka T, Aoki H,
Utsugi M, Shimizu Y, Sugano N, Endou K, Hisada T and Mori M:
Multiple lung metastases presenting as ground-glass opacities in a
pulmonary adenocarcinoma: a case report. Cases J. 2:69102009.
|
|
15.
|
Swanson SJ, Jaklitsch MT, Mentzer SJ,
Bueno R, Lukanich JM and Sugarbaker DJ: Management of solitary
pulmonary nodule: role of thoracoscopy in diagnosis and therapy.
Chest. 116(Suppl 6): S523–S524. 1999.
|
|
16.
|
Casey JJ, Stempel BG, Scanlon EF and Fry
WA: The solitary pulmonary nodule in the patient with breast
cancer. Surgery. 4:801–805. 1984.
|
|
17.
|
Charloux A, Quoix E, Wolkove N, Small D,
Pauli G and Kreisman H: The increasing incidence of lung
adenocarcinoma: reality or artifact? A review of the epidemiology
of lung adenocarcinoma. Int J Epidemiol. 26:14–23. 1997.
|
|
18.
|
Lam B, Lam WK, Lam CL, Ooi GC, Ho JCM,
Wong MP and Tsang KW: Adenocarcinoma of the lung in Chinese
patients: a revisit and some perspectives from the literature.
Postgrad Med J. 77:708–712. 2001.
|
|
19.
|
Greenberg PA, Hortobagyi GN, Smith TL,
Ziegler LD, Frye DK and Buzdar AU: Long-term follow-up of patients
with complete remission following combination chemotherapy for
metastatic breast cancer. J Clin Oncol. 14:2197–2205. 1996.
|
|
20.
|
Diaz-Canton EA, Valero V, Rahman Z,
Rodriguez-Monge E, Frye D, Smith T, Buzdar AU and Hortobagyi GN:
Clinical course of breast cancer patients with metastases confined
to the lungs treated with chemotherapy. The University of Texas
M.D. Anderson Cancer Center experience and review of the
literature. Ann Oncol. 9:413–418. 1998.
|
|
21.
|
Yhim HY, Han SW, Oh DY, Han W, Im SA, Kim
TY, Kim YT, Noh DY, Chie EK, Ha SW, et al: Prognostic factors for
recurrent breast cancer patients with an isolated, limited number
of lung metastases and implications for pulmonary metastasectomy.
Cancer. 116:2890–2901. 2010.
|
|
22.
|
Chen F, Fujinaga T, Sato K, Sonobe M,
Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, Hirata T, et al:
Clinical features of surgical resection for pulmonary metastasis
from breast cancer. Eur J Surg Oncol. 35:393–397. 2009.
|
|
23.
|
Yoshimoto M, Tada K, Nishimura S, Makita
M, Iwase T, Kasumi F, Okumura S, Sato Y and Nakagawa K: Favourable
long-term results after surgical removal of lung metastases of
breast cancer. Breast Cancer Res Treat. 110:485–491. 2008.
|
|
24.
|
Simmons C, Miller N, Geddie W, Gianfelice
D, Oldfield M, Dranitsaris G and Clemons MJ: Does confirmatory
tumor biopsy alter the management of breast cancer patients with
distant metastases? Ann Oncol. 20:1499–1504. 2009.
|
|
25.
|
Liedtke C, Broglio K, Moulder S, Hsu L,
Kau SW, Symmans WF, Albarracin C, Meric-Bernstam F, Woodward W,
Theriault RL, et al: Prognostic impact of discordance between
triple-receptor measurements in primary and recurrent breast
cancer. Ann Oncol. 20:1953–1958. 2009.
|
|
26.
|
Gong Y, Symmans WF, Krishnamurthy S, et
al: Optimal fixation conditions for immunohistochemical analysis of
estrogen receptor in cytologic specimens of breast carcinoma.
Cancer. 102:34–40. 2004.
|
|
27.
|
Gould MK, Fletcher J, Iannettoni MD, Lynch
WR, Midthun DE, Naidich DP and Ost DE: Evaluation of patients with
pulmonary nodules: when is it lung cancer?: ACCP evidence-based
clinical practice guidelines (2nd edition). Chest. 132(Suppl 3):
S108–S130. 2007.
|
|
28.
|
Li H, Boiselle PM, Shepard JO,
Trotman-Dickenson B and McLoud TC: Diagnostic accuracy and safety
of CT-guided percutaneous needle aspiration biopsy of the lung:
comparison of small and large pulmonary nodules. AJR Am J
Roentgenol. 167:105–109. 1996.
|
|
29.
|
Inoue M, Honda O, Tomiyama N, Minami M,
Sawabata N, Kadota Y, Shintani Y, Ohno Y and Okumura M: Risk of
pleural recurrence after computed tomographic-guided percutaneous
needle biopsy in stage I lung cancer patients. Ann Thorac Surg.
91:1066–1072. 2011.
|
|
30.
|
McCormack PM, Bains MS, Begg CB, Burt ME,
Downey RJ, Panicek DM, Rusch VW, Zakowski M and Ginsberg RJ: Role
of video-assisted thoracic surgery in the treatment of pulmonary
metastases: results of a prospective trial. Ann Thorac Surg.
62:213–217. 1996.
|