Introduction
Members of the aldehyde dehydrogenase (ALDH) family
are NAD(P)-dependent enzymes involved in catalyzing the oxidation
of various endogenous and exogenous aldehydes to corresponding
carboxylic acids (1) and are
classified into 11 families and 4 subfamilies (2). ALDH1 is an isoform that is highly
expressed in the stem cells of various lineages including
hematopoietic tissues, neural tissues and mammary gland, and has
been shown to play an important functional role in stem cells
(3).
In normal tissue, strong ALDH1-positive cells are
present in the putative epithelial stem/progenitor cell zones
located in the breast, colon and stomach (4). Moreover, a high percentage of
ALDH1-positive tumor cells are found in various types of cancer,
such as ovarian, colon, lung, pancreatic and liver (4). However, analyses of ALDH1 expression
in basal cell carcinoma (BCC), actinic keratosis (AK) and Bowen’s
disease (BD) of the skin as well as normal skin tissue have not yet
been performed. In this study, we assessed ALDH1 expression in BCC,
AK and BD by immunohistochemistry and compared the findings with
that of normal skin.
Materials and methods
Case selection
Twenty-five formalin-fixed and paraffin-embedded
tissue specimens each from consecutive operative BCC, AK and BD
cases were selected from the archives of our Diagnostic Pathology
Division. The 25 cases of BCC included 16 males and 9 females with
an average age of 70.2 years (range, 41–92 years). The 25 AK cases
comprised 14 males and 8 females with an average age of 76.6 years
(range, 46–95 years). These cases included patients with 2 or 3
lesions. The 25 cases of BD included 12 males and 13 females with
an average age of 76.7 years (range, 63–89 years). This study was
approved by the ethics committee of Shiga University of Medical
Science (Shiga, Japan). All patients gave their consent to
participate in this study.
The cases were reviewed by diagnostic pathologists
to confirm the diagnosis of BCC, AK and BD, and subclassification
of BCC was performed according to the World Health Organization
Classification of Tumours. Pathology and Genetics of Skin Tumours
(5).
ALDH1 expression was also examined in 10 normal skin
specimens from the scalp and face.
Immunohistochemistry
Immunohistochemical stainings were performed using
an autostainer (Benchmark XT System; Ventana Medical Systems,
Tucson, AZ, USA) according to the manufacturer’s instructions. The
primary antibody used was a mouse monoclonal antibody against human
ALDH1 (clone 44/ALDH; BD Transduction Laboratories, Franklin Lakes,
NJ, USA), as previously described (4,6,7).
Evaluation of immunoreactivity
The expression pattern of ALDH1 was evaluated
semiquantatively as a percentage of positively stained tumor cells,
as described in a previous study (8) and scored as: 0 (<5% of positive
tumor cells), 1+ (5–9%), 2+ (10–50%) and 3+ (>51%).
Results
Normal skin
ALDH1 expression was observed in the suprabasal
cells of the follicular infundibulum, mature sebocytes and the
inner cells of the outer root sheath (Fig. 1). No ALDH1-positive cells were
found in the epidermis, basal cells of the follicular infundibulum
or outer root sheath, with the exception of the inner cells, bulge,
inner root sheath, hair matrix and supramatrix (Fig. 1).
BCC
The 25 cases of BCC included 18 nodular types, 4
micronodular types and 3 superficial types. ALDH1 was expressed in
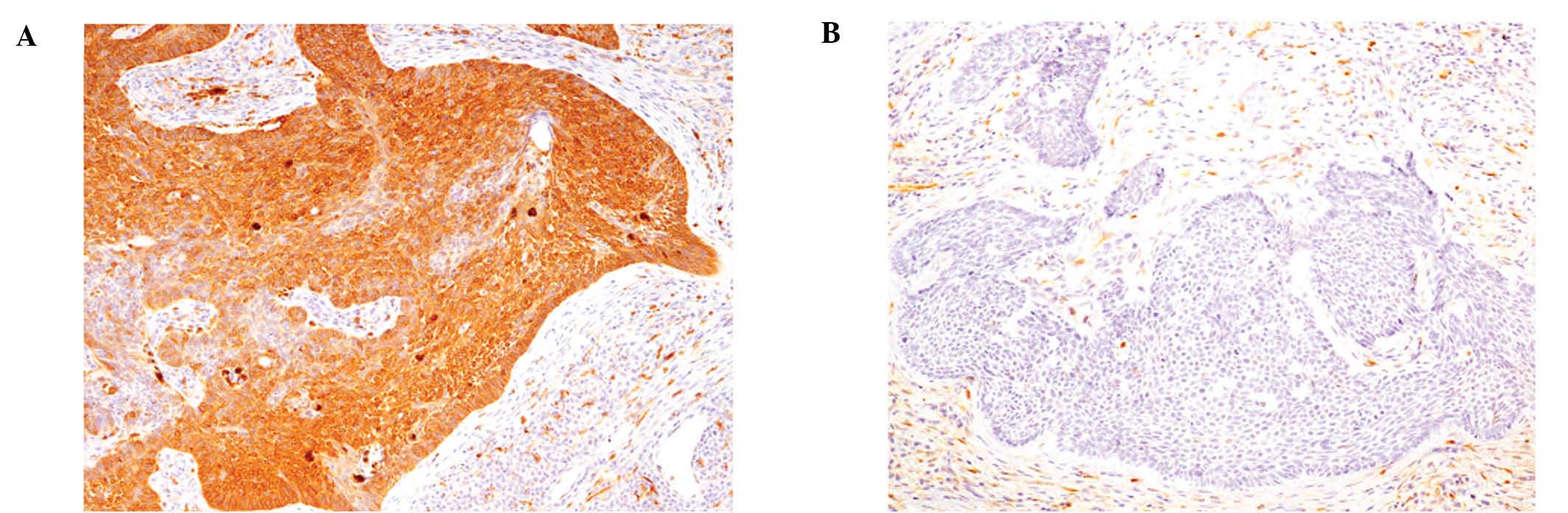
68% of BCC cases. However, only 2 cases showed diffuse positive
immunoreactivity (score 3+) (Fig.
2A) and 1 case was scored 2+, whereas 56% of BCC cases showed
only focal immunopositivity (score 1+) and 32% were negative (score
0) (Fig. 2B and Table I). Only nodular types of BCC had
scores of 2+ or 3+, while the micronodular cases had a score of 1+
and the superficial cases were scored 0 (Table I).
 | Table IALDH1 expression in basal cell
carcinoma, actinic keratosis and Bowen’s disease. |
Table I
ALDH1 expression in basal cell
carcinoma, actinic keratosis and Bowen’s disease.
| ALDH1 expression
|
|---|
| Carcinoma type | 0 | 1+ | 2+ | 3+ |
|---|
| Basal cell
carcinoma | 8/25 | 14/25 | 1/25 | 2/25 |
| Nodular type | 5/18 | 10/18 | 1/18 | 2/18 |
| Micronodular
type | 0/4 | 4/4 | 0/4 | 0/4 |
| Superficial type | 3/3 | 0/3 | 0/3 | 0/3 |
| Actinic
keratosis | 14/25 | 11/25 | 0/25 | 0/25 |
| Bowen’s disease | 2/25 | 5/25 | 2/25 | 16/25 |
AK
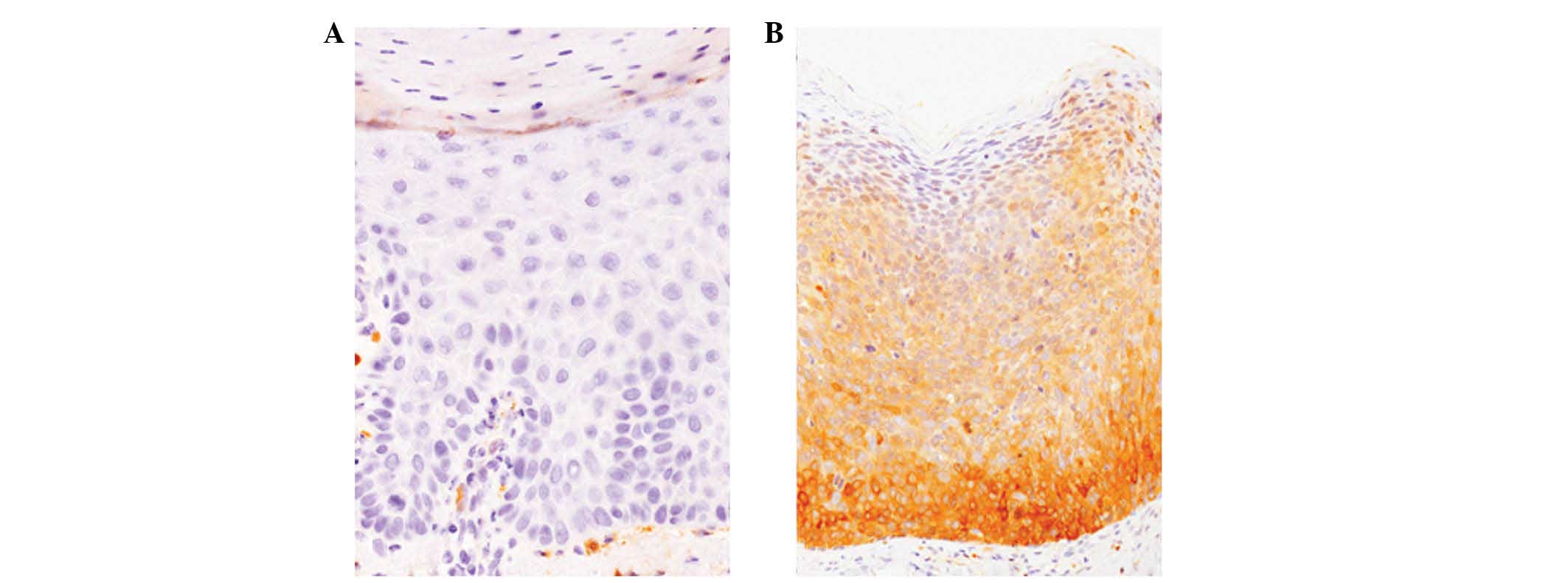
Most of the AK cases (56%) were negative for ALDH1
(score 0) (Fig. 3A) and the
remaining 44% of cases had a score of 1+. No diffusely
ALDH1-positive cases were observed (Table I).
BD
ALDH1 expression was observed in 92% of BD cases, of
which 64% showed diffuse positive immunoreactivity for ALDH1 (score
3+) (Fig. 3B). Only 8% of BD cases
were ALDH1-negative (Table I).
Discussion
ALDH1 has been proven to be useful for the
identification of normal stem cells of various organs.
ALDH1-positive tumor cells exhibit cancer stem cell properties and
are resistant to chemotherapy in certain types of cancer (3). For example, it has been demonstrated
that ALDH1-positive cells have stem or progenitor cell abilities in
normal breast and breast cancer cells (8). It has also been shown that the
presence of ALDH1-positive tumor cells in lymph node metastatic
lesions after neoadjuvant chemotherapy correlated with poor
prognosis and reduced survival in breast cancer patients (6). Moreover, a high expression of ALDH1
has been found to be associated with lymph node metastasis in oral
squamous cell carcinoma (9) and is
also associated with postoperatrive recurrence and poor prognosis
in esophageal squamous cell carcinoma (10).
However, the expression of ALDH1 in normal tissues
is not always restricted to stem cells. Deng et al (4) classified ALDH1 expression patterns in
normal tissues into three types: i) tissues with absent or limited
ALDH1 expression (breast and lung); ii) tissues with relatively
weak ALDH1 expression (stomach and colon); and iii) tissues with
extensive and high ALDH1 expression (liver and pancreas). Based on
these results, those authors concluded that ALDH1 can be an
effective and useful stem cell marker for tissues that usually do
not express ALDH1 at a high level, such as breast, stomach and
colon. However, ALDH1 should not be used in organs that usually
express a high level of ALDH1, such as liver and pancreas (3). To the best of our knowledge, the
present study is the first to clearly show that ALDH1 is expressed
in the suprabasal cells of the follicular infundibulum, inner cells
of the outer root sheath and sebocytes of normal skin tissue. These
distribution patterns do not correspond to those of stem cells in
the skin, which are thought to be located in the bulge (11). Therefore, ALDH1 is not a useful
stem cell marker for normal skin tissue and may have other
functional roles as it has been reported that ALDH1 is, not only a
putative stem cell marker, but may also have numerous functions,
such as in differentiation and self-renewal (3).
This study also clearly demonstrated that over half
of AK cases (56%) were negative or negligible for ALDH1. By
contrast, 64% of BD cases were diffusely positive for ALDH1. Basal
cells of the epidermis are important in the pathogenesis of AK, but
not in BD, in which the neoplastic cells have been reported to
originate from the pilar outer root sheath or acrotrichium
(12). Therefore, the differential
ALDH1 expression patterns suggest that in AK and BD, the neoplastic
squamous cells harbor distinct phenotypes and may reflect the
different origin of these two conditions.
Findings of this study, demonstrated that 88% of BCC
cases showed no or only focal-positive immunoreactivity for ALDH1.
The possible origin of BCC cells is thought to be the outer root
sheath of hair follicles, particularly basal cells (13). ALDH1 expression was observed in the
inner cells, but not in the basal cells of the outer root sheath of
normal hair follicles. Thus, a low ALDH1 expression in this disease
may reflect the possibility that BCC originates from the basal
cells of the outer root sheath.
In addition, a recent study demonstrated that high
ALDH1-expressing breast cancer cells preferentially survive both
chemotherapy and radiation compared with low ALDH1-expressing
cancer cells and a specific ALDH inhibitor
(diethylaminobenzalaldehyde) may result in significant
sensitization to therapy in the former cells (14). Our results have shown that 92% of
BD cases showed positive immunoreactivity for ALDH1 (of which 64%
showed diffuse expression). Therefore, current administration of an
ALDH1 inhibitor might be a candidate treatment for BD.
References
|
1.
|
Sladek NE: Human aldehyde dehydrogenases:
potential pathological, pharmacological, and toxicological impact.
J Biochem Mol Toxicol. 17:7–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Black WJ, Stagos D, Marchitti SA, et al:
Human aldehyde dehydrogenase genes: alternatively spliced
transcriptional variants and their suggested nomenclature.
Pharmacogenet Genomics. 19:893–902. 2009. View Article : Google Scholar
|
|
3.
|
Ma I and Allan AL: The role of human
aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell
Rev and Rep. 7:292–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Deng S, Yang X, Lassus H, et al: Distinct
expression levels and patterns of stem cell marker, aldehyde
dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS
One. 5:e102772011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kossard S, Epstein EH Jr, Cerio R, Yu LL
and Weedon D: Basal cell carcinoma. World Health Organization
Classification of Tumours. Pathology and Genetics of Skin Tumours.
LeBoit PE, Burg G, Weedon D and Sarasain A: IARC Press; Lyon: pp.
13–19. 2006
|
|
6.
|
Sakakibara M, Fujimori T, Miyoshi T, et
al: Aldehyde dehydrogenase 1-positive cells in axillary lymph node
metastases after chemotherapy as a prognostic factor in patients
with lymph node-positive breast cancer. Cancer. 118:3899–3910.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Isfoss BL, Holmqvist B, Alm P and Olsson
H: Distribution of aldehyde dehydrogenase 1-positive stem cells in
benign mammary tissue from women with and without breast cancer.
Histopathology. 60:617–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ginestier C, Hur MH, Charafe-Jauffret E,
et al: ALDH1 is a marker of normal and malignant human mammary stem
cells and a predictor of poor clinical outcome. Cell Stem Cell.
1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Michifuri Y, Hirohashi Y, Torigoe T, et
al: High expression of ALDH1 and SOX2 diffuse staining pattern of
oral squamous cell carcinomas correlates to lymph node metastasis.
Pathol Int. 62:684–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Minato T, Yamamoto Y, Seike J, et al:
Aldehyde dehydrogenase 1 expression is associated with poor
prognosis in patients with esophageal squamous cell carcinoma. Ann
Surg Oncol. July 31–2012.(Epub ahead of print).
|
|
11.
|
Goldstein J and Horsley V: Home sweet
home: skin stem cell niches. Cell Mol Life Sci. 69:2573–2582. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Saglam O, Salama M, Meier F, et al:
Immunohistochemical staining of palisading basal cells in Bowen’s
disease and basal involvement in actinic keratosis: contrasting
staining patterns suggest different cells of origin. Am J
Dermatopathol. 30:123–126. 2008.PubMed/NCBI
|
|
13.
|
Ishida M, Kushima R and Okabe H:
Immunohistochemical demonstration of D2-40 in basal cell carcinomas
of the skin. J Cutan Pathol. 35:926–930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Croker AK and Allan AL: Inhibition of
aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and
radiation resistance of stem-like ALDHhiCD44+ human breast cancer
cells. Breast Cancer Res Treat. 133:75–87. 2012.PubMed/NCBI
|