Introduction
The standard treatment for patients with
International Federation of Gynecology and Obstetrics (FIGO) stage
IB-II cervical cancer is radical hysterectomy and/or radiotherapy
(RT), including concurrent chemoradiotherapy (CCRT). In Japan, the
majority of gynecologic oncologists select radical hysterectomy for
patients with stage IB-II cervical cancer (1). By contrast, the National
Comprehensive Cancer Network (NCCN) clinical practice guidelines
recommend radical hysterectomy for patients with IA2, IB1 and IIA1
and CCRT for patients with stage IB2, IIA2 and IIB cervical cancer
(2).
A previous study by the Gynecologic Oncology Group
(GOG) demonstrated that adjuvant pelvic RT following radical
hysterectomy reduced the number of recurrences in stage IB patients
with intermediate risk factors (3). In addition, another GOG study
(GOG109/SWOG 8797/RTOG91-12) suggested that RT with concurrent
cisplatin-containing chemotherapy (CT) was more effective for stage
IA2-IIA patients with pelvic lymph node involvement, parametrial
extension or compromised surgical margin compared to RT alone
following radical hysterectomy (4). Therefore, the NCCN clinical practice
guidelines recommend adjuvant RT including CCRT for cervical cancer
patients with pathological risk factors following radical
hysterectomy (2).
Since the incidence of adenocarcinoma (AC) of the
uterine cervix has increased from ∼12 to 24% of cervical cancer
cases over the past 24 years (5),
effective therapeutic strategies for AC are required. Whether the
prognosis of patients with cervical cancer is dependent on
histological type remains controversial (6–11). A
previous GOG study of 813 patients with stage IB cervical cancer,
645 of whom had squamous cell carcinoma (SCC) and 168 AC, including
adenosquamous cell carcinoma (ASCC), demonstrated that there were
no statistically significant differences regarding the
recurrence-free interval among histological types (6). The NCCN clinical practice guidelines
also suggest that AC may be effectively treated in a similar manner
to SCC (2). In a previous study,
we reported that stage II patients with AC had a significantly
worse prognosis compared to those with SCC, although the survival
of stage IB patients did not differ between AC and SCC (12). Additionally, findings of previous
studies suggested that the radiosensitivity of AC may be lower
compared to that of SCC (13–15).
Consequently, adjuvant RT, which is recommended as the standard
adjuvant treatment for high- or intermediate-risk patients with
cervical cancer, may be of limited value for patients with AC
following radical hysterectomy. This retrospective study was
conducted to evaluate the efficacy of adjuvant RT for patients with
AC compared to those with SCC following radical hysterectomy.
Patients and methods
Patient data
A total of 820 patients with FIGO stage IB-IIB
cervical cancer, who underwent type III radical hysterectomy at 10
institutes (Hyogo Cancer Center, Kagoshima City Hospital, National
Hospital Organization Shikoku Cancer Center, National Hospital
Organization Kure Medical Center, Fukushima Medical University,
Yamagata University, Tohoku University School of Medicine, Hirosaki
University School of Medicine, Iwate Medical University and Tottori
University Hospital) between 1997 and 2003, were enrolled in this
study. Data were collected from patient medical records. The
retrospective study protocol was approved by the Institutional
Review Board of each institution. There were 540 SCC and 280 AC
patients. The indications for adjuvant treatment were as follows:
pelvic lymph node involvement, parametrial extension, deep stromal
invasion, vessel permeation and a compromised surgical margin;
however, the indications for adjuvant treatment were not identical
among the 10 institutes. The chemotherapeutic regimens and number
of cycles were also decided on by each institution, although the
majority of adjuvant CT was platinum-based combination CT.
Statistical analysis
Patient survival distribution was calculated using
the Kaplan-Meier method. The significance of the survival
distribution in each group was assessed by the log-rank test. The
Chi-square test was used to compare any associations between
prognostic factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient data
A total of 354 patients, 141 of whom had AC and 213
SCC, underwent radical hysterectomy alone (Table I). The remaining 466 patients, of
whom 139 had AC and 327 SCC, received adjuvant treatment following
radical hysterectomy. The distribution of patients with SCC
receiving adjuvant treatment was significantly higher compared to
those with AC (60.6 vs. 49.6%, respectively; P= 0.0028). Out of the
139 AC patients, 69 received RT alone or CCRT with weekly cisplatin
(CDDP), 54 received CT alone and 16 received concomitant RT and CT.
Out of the 327 SCC patients receiving adjuvant treatment, 258
received RT alone or CCRT with weekly CDDP, 36 received CT alone
and 33 received concomitant RT and CT.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Histological type
|
|---|
| AC (n=280) | SCC (n=540) |
|---|
| Age, years [mean
(range)] | 46.2 (18–84) | 49.0 (19–84) |
| FIGO stage | | |
| IB1 | 184 | 258 |
| IB2 | 39 | 67 |
| IIA | 11 | 83 |
| IIB | 46 | 132 |
| Adjuvant
treatment | | |
| Yes | 139 | 327 |
| RT or CCRT | 69 | 258 |
| CT | 54 | 36 |
| RT + CT | 16 | 33 |
| No | 141 | 213 |
The 5-year overall survival (OS) rate for patients
with AC and SCC was 87.4 and 83.4%, respectively (P=0.2509). The
5-year OS for stage IB1 was 92.0% in AC, 94.7% in SCC and for stage
IB2 survival was 75.5% in AC and 74.2% in SCC. Patients with AC
exhibited a significantly worse outcome compared to those with SCC
in stage II (stage IIA: 54.5 vs. 87.4%, respectively and stage IIB:
63.3 vs. 78.8%, respectively; P<0.05).
Survival and lymph node involvement
Patients with SCC exhibited significantly higher
lymph node involvement compared to those with AC in stage IB1
(Table II). By contrast, lymph
node involvement did not differ between AC and SCC in stages IB2
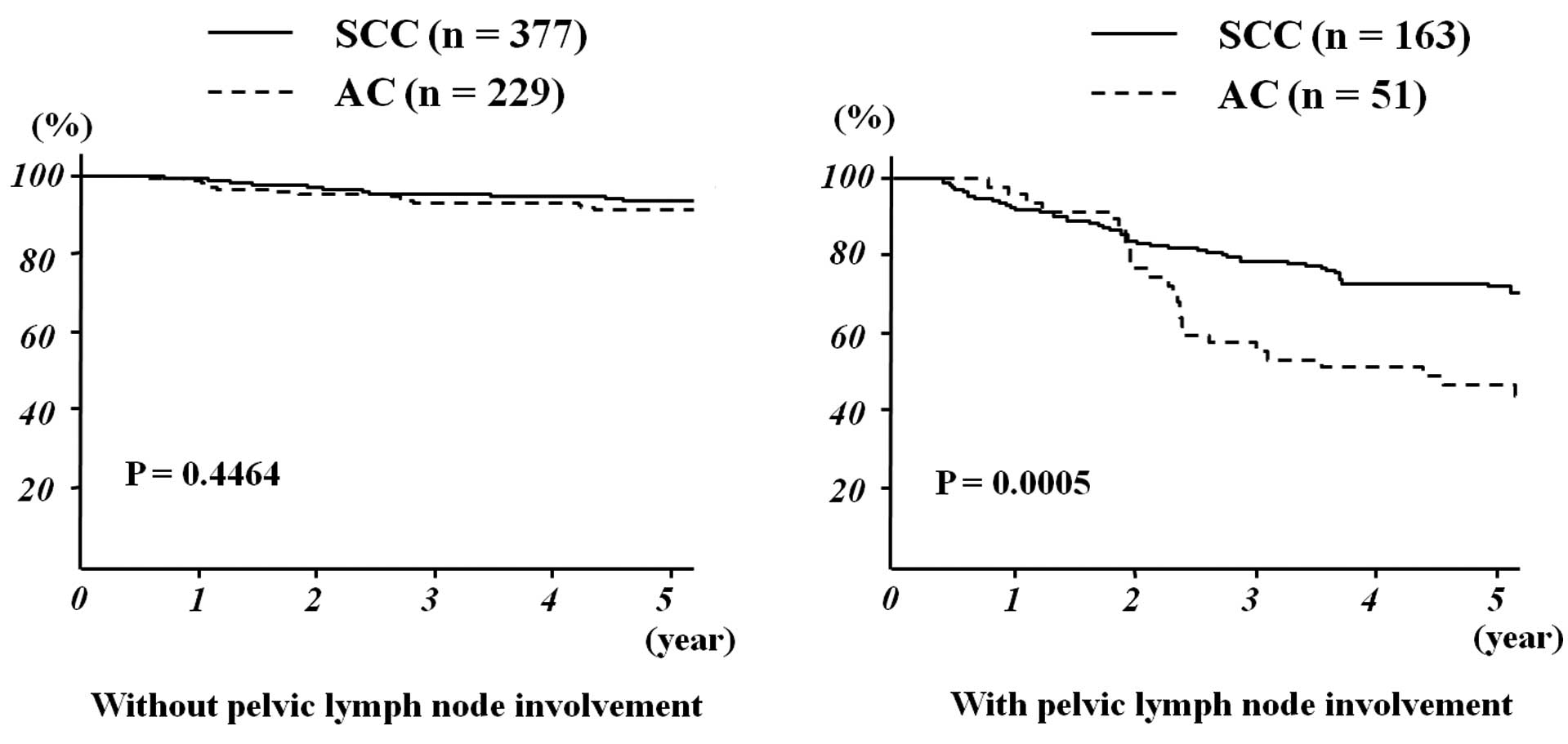
and II. Among patients with lymph node involvement, those with AC
exhibited a significantly worse outcome compared to those with SCC
(5-year OS: 46.4% vs. 72.3%, respectively; P=0.0005). However, the
histological type did not significantly affect the outcome of
patients without lymph node involvement (5-year OS: AC, 91.2% and
SCC, 93.9%; P= 0.4464) (Fig.
1).
 | Table II.Incidence of pelvic lymph node
involvement. |
Table II.
Incidence of pelvic lymph node
involvement.
| FIGO stage | Pelvic lymph node
involvement
| P-value |
|---|
| AC (%) | SCC (%) |
|---|
| IB1 | 9.8 (18/184) | 16.7 (43/258) | 0.0391 |
| IB2 | 23.9 (11/39) | 46.3 (31/67) | 0.0667 |
| IIA | 36.4 (4/11) | 34.9 (29/83) | 0.9259 |
| IIB | 39.1 (18/46) | 45.4 (60/132) | 0.4566 |
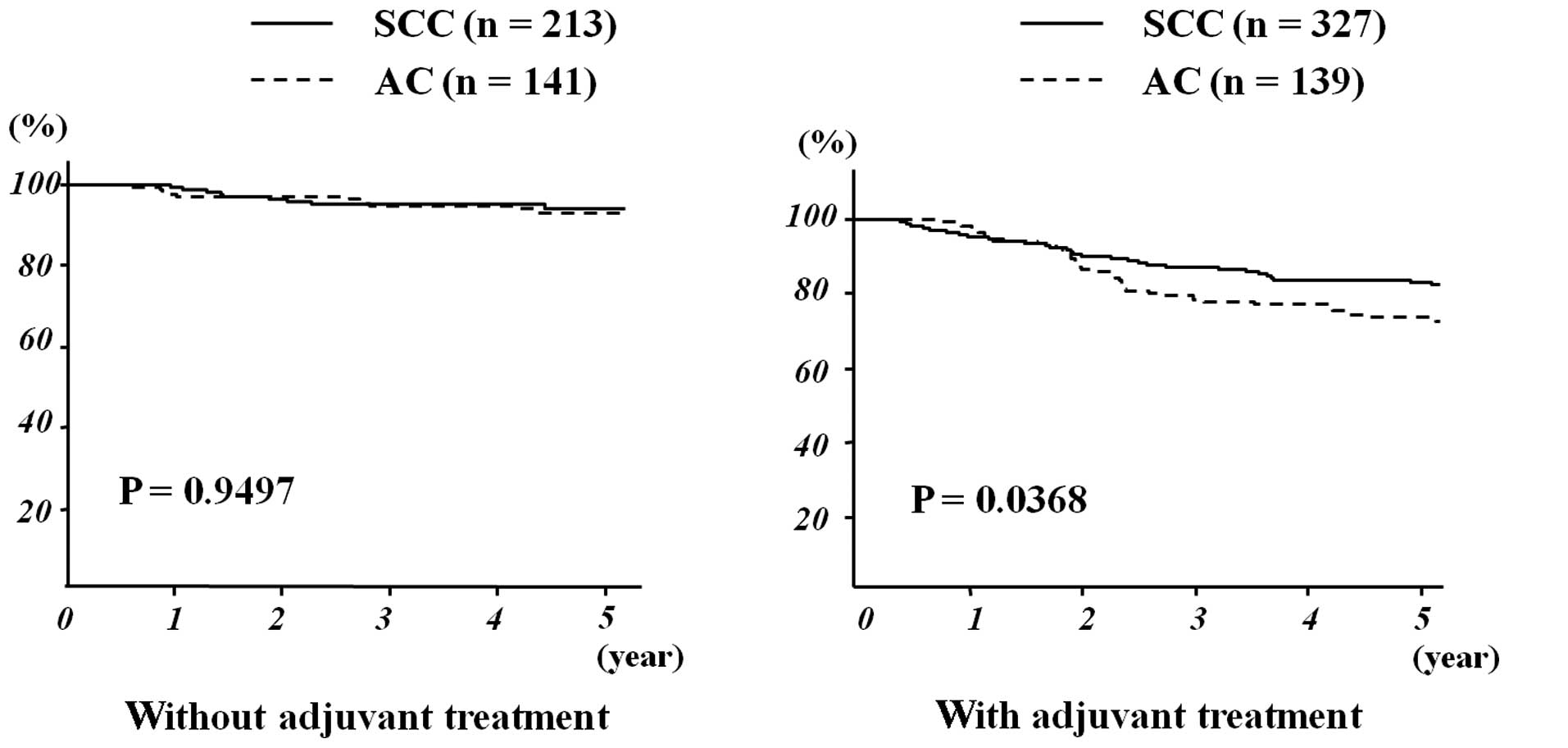
Among patients not receiving adjuvant treatment
following radical hysterectomy, patients with AC exhibited an
outcome similar to those with SCC (5-year OS: 93.1 vs. 94.0%,
respectively; P= 0.9497) (Fig. 2).
However, among patients who underwent adjuvant treatment, those
with AC had a significantly worse outcome compared to those with
SCC (5-year OS: 73.7 vs. 83.1%, respectively; P= 0.0368). Among
stage II patients undergoing adjuvant treatment, patients with AC
exhibited a significantly worse outcome compared to those with SCC
(5-year OS: stage IIA, 50.0 vs. 86.9%, respectively, P= 0.0032;
stage IIB, 61.1 vs. 75.5%, respectively, P= 0.037). However, the
histological type did not significantly affect the outcome of
patients with stage I disease (5-year OS: stage IB1: AC, 84.1 and
SCC, 91.2%, P= 0.3374; stage IB2: AC, 74.6 and SCC, 76.1%,
P=0.9127).
Recurrence in patients undergoing
adjuvant radiotherapy
In patients undergoing adjuvant treatment, there was
no significant difference in the outcome between different
treatments (5-year OS: CT, 79.4%; RT, 70.4%; RT + CT, 68.8%; P=
0.4522). In patients receiving adjuvant RT including CCRT, the
5-year OS was 70.4% in patients with AC and 81.7% in those with SCC
(P=0.0858). Among patients receiving adjuvant RT, those with AC
recurred more frequently compared to those with SCC (Table III). Furthermore, patients with AC
recurred more frequently in the pelvic cavity, including the
vaginal stump and/or pelvis, compared to those with SCC. By
contrast, the histological type did not affect the incidence of
recurrence in the paraaortic lymph nodes and/or distant
recurrence.
 | Table III.Recurrence in patients receiving
adjuvant radiotherapy. |
Table III.
Recurrence in patients receiving
adjuvant radiotherapy.
| Pathological risk
factor | AC (%) | SCC (%) | P-value |
|---|
| Recurrence outside RT
field | 15.9 (11/69) | 14.3 (37/258) | 0.7385 |
| Recurrence within RT
field | 24.6 (17/69) | 10.5 (27/258) | 0.0022 |
| Total recurrence | 40.6 (28/69) | 24.8 (64/258) | 0.0096 |
Discussion
Radiosensitivity is one of the important prognostic
factors in the treatment of uterine cervical cancer; however, the
number of clinical studies that have focused on patients with AC is
limited. Eifel et al (16)
reported that among 1,767 patients with stage I cervical cancer
undergoing initial RT, those with AC had a worse prognosis compared
to those with SCC, due to the higher incidence of distant
metastasis in AC patients, although there were no significant
differences in the rate of recurrence in the pelvic cavity between
AC and SCC patients. By contrast, there was no significant
difference in the incidence of distant recurrence between AC and
SCC patients. According to a previous study, RT was less effective
compared to surgery in patients with AC (13). Recently, Niibe et al
(14) reported that the 5-year OS
for stage IIIB patients with AC treated with high-dose
intracavitary brachytherapy combined with external beam
radiotherapy was 20.2%. The 5-year OS for stage IIIB patients with
SCC has been reported to be 47.2–55.2% in Japan (17–19).
These findings suggested that the radiosensitivity of AC was lower
compared to that of SCC. In our series, among the patients
undergoing adjuvant RT including CCRT, those with AC recurred
significantly more frequently, particularly in the pelvic cavity,
compared to those with SCC. Our data also indicated lower
sensitivity to adjuvant RT in patients with AC. Consequently, RT
including CCRT may not be the optimal adjuvant treatment for
high-risk patients with AC following radical hysterectomy.
Pelvic lymph node involvement is an independent
prognostic factor in patients with cervical cancer; however, it has
not been elucidated whether patients with AC had a higher incidence
of pelvic lymph node involvement compared to those with SCC.
According to a previous study, the incidence of lymph node
involvement in patients with SCC was significantly higher compared
to those with AC and ASCC in stage IB (12.6 vs. 9.5%, respectively;
P=0.0466) (8). By contrast, other
studies suggested that there were no significant differences in the
incidence of lymph node involvement between patients with AC and
SCC (20,21). To the best of our knowledge, this
is the first study to elucidate the incidence of pelvic lymph node
involvement in stage IB1, IB2, IIA and IIB uterine cervical cancer
patients. Furthermore, there was no significant difference in the
incidence of lymph node involvement between patients with AC and
SCC in stage IB2, IIA and IIB, although patients with SCC exhibited
a significantly higher lymph node involvement compared to those
with AC in stage IB1.
Among patients with pelvic lymph node involvement,
those with AC had a significantly worse outcome compared to those
with SCC. These results suggested that the poorer outcome of AC
patients may be due to ineffective adjuvant treatment administered
to patients with AC compared to those with SCC, rather than a
higher incidence of lymph node involvement in AC compared to SCC.
Furthermore, in the present study, the outcome of patients with
stage I did not differ between AC and SCC; however, patients with
AC exhibited a significantly worse outcome compared to those with
SCC in stage II. Moreover, among patients undergoing adjuvant
treatment following radical hysterectomy, the outcome of patients
with AC was significantly worse compared to those with SCC.
Consequently, patients with AC may have a poorer outcome compared
to those with SCC when carcinoma invades beyond the uterine cervix,
including stage II disease and pelvic lymph node involvement.
Certain studies suggested the significance of
adjuvant CT for high- or intermediate-risk patients with cervical
cancer. Takeshima et al (22) reported that adjuvant combination
CT, containing bleomycin, vincristine, mitomycin and cisplatin,
achieved a 5-year disease-free survival rate of 93.3% in 30
intermediate-risk patients and of 85.7% in 35 high-risk patients.
Hosaka et al (23) reported
that out of the 27 patients without multiple lymph node involvement
who underwent adjuvant CT, only one patient recurred. In our
series, adjuvant CT achieved the same outcome as adjuvant RT or
concomitant RT and CT in patients with AC. These findings suggested
the potential role of adjuvant CT for cervical cancer patients,
particularly those with AC.
In conclusion, RT may not suffice as an adjuvant
treatment for pathological risk patients with cervical AC following
radical hysterectomy.
Acknowledgements
The authors are indebted to Satoshi
Yamaguchi (Hyogo Cancer Center), Toshiaki Nakamura (Kagoshima City
Hospital), Takashi Matsumoto (National Hospital Organization
Shikoku Cancer Center), Hiroshi Nishiyama (Fukushima Medical
University School of Medicine), Kenji Nakahara (Yamagata
University), Tadahiro Shoji (Iwate Medical University), Tadao
Takano (Tohoku University School of Medicine), and Nobuo Yaegashi
(Tohoku University School of Medicine) for their support.
References
|
1.
|
The annual report of the Japanese Society
of Obstetrics and Gynecology 2010. Acta Obstetricia Et Gynecologica
Japonica. 64:1029–1141. 2012.
|
|
2.
|
NCCN Clinical Practice Guidelines in
Oncology-cervical cancer v.1. 2011. National Comprehensive Cancer
Network (http://www.nccn.org/professionalsphysician_gls/f_guidelines.asp).
Accessed November 9, 2010.
|
|
3.
|
Sedlis A, Bundy BN, Rotman MZ, Lentz SS,
Muderspach LI and Zaino RJ: A randomized trial of pelvic radiation
therapy versus no further therapy in selected patients with stage
IB carcinoma of the cervix after radical hysterectomy and pelvic
lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol.
73:177–183. 1999. View Article : Google Scholar
|
|
4.
|
Peters WA III, Liu PY, Barrett RJ II, et
al: Concurrent chemotherapy and pelvic radiation therapy compared
with pelvic radiation therapy alone as adjuvant therapy after
radical surgery in high-risk early-stage cancer of the cervix. J
Clin Oncol. 18:1606–1613. 2000.
|
|
5.
|
Smith HO, Tiffany MF, Qualls CR and Key
CR: The rising incidence of adenocarcinoma relative to squamous
cell carcinoma of the United States - a 24-year population-based
study. Gynecol Oncol. 78:97–105. 2000.
|
|
6.
|
Look KY, Brunetto VL, Clarke-Pearson DL,
et al: An analysis of cell type in patients with surgically staged
stage IB carcinoma of the cervix: a Gynecologic Oncology Group
Study. Gynecol Oncol. 63:304–311. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Park JY, Kim DY, Kim JH, Kim YM, Kim YT
and Nam JH: Outcome after radical hysterectomy in patients with
early-stage adenocarcinoma of uterine cervix. Br J Cancer.
102:1692–1698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shingleton HM, Bell MC, Fremgen A, et al:
Is there really a difference in survival of women with squamous
cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma of
the cervix? Cancer. 76:1948–1955. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kasamatsu T, Onda T, Sawada M, et al:
Radical hysterectomy for FIGO stage I-IIB adenocarcinoma of the
uterine cervix. Br J Cancer. 100:1400–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chen RJ, Lin YH, Chen CA, Huang SC, Chow
SN and Hsieh CY: Influence of histologic type and age on survival
rates for invasive cervical carcinoma in Taiwan. Gynecol Oncol.
73:184–190. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shimada M, Kigawa J, Nishimura R, et al:
Ovarian metastasis in carcinoma of the uterine cervix. Gynecol
Oncol. 101:234–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Irie T, Kigawa J, Minagawa Y, et al:
Prognosis and clinicopathological characteristics of Ib-IIb
adenocarcinoma of the uterine cervix in patients who have had
radical hysterectomy. Eur J Sur Oncol. 26:464–467. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Landoni F, Maneo A, Colombo A, et al:
Randomised study of radical surgery versus radiotherapy for stage
Ib-IIa cervical cancer. Lancet. 350:535–540. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Niibe Y, Kenjo M, Onishi H, et al:
High-dose-rate intracavitary brachytherapy combined with external
beam radiotherapy for stage IIIb adenocarcinoma of the uterine
cervix in Japan: a multi-institutional study of Japanese Society of
Therapeutic Radiology and Oncology 2006–2007 (study of JASTRO
2006–2007). Jpn J Clin Oncol. 40:795–799. 2010.PubMed/NCBI
|
|
15.
|
Baalbergen A, Ewing-Graham PC, Hop WC,
Struijk P and Helmerhorst TJ: Prognostic factors in adenocarcinoma
of the uterine cervix. Gynecol Oncol. 92:262–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Eifel PJ, Burke TW, Morris M and Smith TL:
Adenocarcinoma as an independent risk factor for disease recurrence
in patients with stage IB cervical carcinoma. Gynecol Oncol.
59:38–44. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Toita T, Sakumoto K, Higashi M, et al:
Therapeutic value of neoadjuvant intra-arterial chemotherapy
(cisplatin) and irradiation for locally advanced uterine cervical
cancer. Gynecol Oncol. 65:421–424. 1997. View Article : Google Scholar
|
|
18.
|
Hareyama M, Sakata K, Oouchi A, et al:
High-dose-rate versus low-dose-rate intracavitary therapy for
carcinoma of the uterine cervix: a randomized trial. Cancer.
94:117–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nakano T, Kato S, Ohno T, et al: Long-term
results of high-dose rate intracavitary brachytherapy for squamous
cell carcinoma of the uterine cervix. Cancer. 103:92–101. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nakanishi T, Ishikawa H, Suzuki Y, Inoue
T, Nakamura S and Kuzuya K: A comparison of prognoses of pathologic
stage Ib adenocarcinoma and squamous cell carcinoma of the uterine
cervix. Gynecol Oncol. 79:289–293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sakuragi N, Satoh C, Takeda N, et al:
Incidence and distribution pattern of pelvic and paraaortic lymph
node metastases in patients with stages Ib, IIa, and IIb cervical
carcinoma treated with radical hysterectomy. Cancer. 85:1547–1554.
1999. View Article : Google Scholar
|
|
22.
|
Takeshima N, Umayahara K, Fujiwara K, et
al: Treatment results of adjuvant chemotherapy after radical
hysterectomy for intermediate- and high-risk stage IB-IIA cervical
cancer. Gynecol Oncol. 103:618–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hosaka M, Watari H, Takeda M, et al:
Treatment of cervical cancer with adjuvant chemotherapy versus
adjuvant radiotherapy after radical hysterectomy and systematic
lymphadenectomy. J Obstet Gynaecol Res. 34:552–556. 2008.
View Article : Google Scholar
|