Introduction
Malignant pleural mesothelioma (MPM) arises from the
mesothelial surface of the pleural cavity and is a locally invasive
tumor with poor prognosis (1,2). In
>70% of patients, the tumor is associated with exposure to
asbestos fibers following a long latent period of 20–50 years
(3). The incidence of mesothelioma
is rare in the general population; however, it is expected to
increase in the next 20 years in industrialized countries as a
result of past asbestos use (4,5).
MPM is refractory to the currently available
treatment options. The efficacy of surgical therapy has not been
precisely defined (6) and
radiotherapy may be palliative but does not prolong survival
(7). For the majority of patients
with MPM, systemic chemotherapy remains the standard of care
(8). Prior to 2003, the majority
of studies on chemotherapy for MPM were conducted using either
single agents or combination regimens in the setting of small phase
II trials. The results demonstrated <20% of tumor regression
with no significant effect on patient survival, which was 6–9
months (8,9). Since 2003, the combination of
cisplatin and pemetrexed (PTX) has been used as standard
chemotherapy for MPM (10). This
was based on a randomized phase III study in which PTX plus
cisplatin achieved a response rate of 41.3% and a median survival
of 12.1 months, compared to 16.7% response rate and 9.3-month
median survival achieved by cisplatin alone (10).
In this study, a non-platinum-based combination
therapy with two anti-metabolites (methotrexate and gemcitabine)
was devised. Methotrexate is an analogue of folic acid known to be
effective against breast cancer, lymphoblastic leukemia and
osteosarcoma (11,12). Gemcitabine is a pyrimidine
analogue, effective against a wide range of solid tumors, including
pancreatic carcinoma and non-small cell lung carcinoma (13). Methotrexate and gemcitabine have
been reported to exhibit single-agent activity in MPM (8,9);
however, the combined administration of these agents has not yet
been investigated.
In the present study, we evaluated the feasibility
and efficacy of methotrexate and gemcitabine combination therapy in
the treatment of MPM, through the analysis of toxicity, response
and survival data.
Patients and methods
Patients
Patients with histologically confirmed MPM who had
previously received 0–1 chemotherapy cycles, not including
gemcitabine and methotrexate, were considered eligible for this
single-center study. Tumor extension was classified according to
the tumor-node-metastasis (TNM) staging system developed by the
International Mesothelioma Interest Group (IMIG) (14). Patients were 18–75 years of age,
with an Eastern Cooperative Oncology Group (ECOG) performance
status of 0–2, had adequate bone marrow function (hemoglobin
concentration ≥10 g/dl, total leukocyte count
≥3.0×109/l, granulocyte count ≥1.5×109/l and
platelet count ≥100×109/l), adequate renal function
(serum creatinine level <1.5 mg/dl) and adequate hepatic
function (total bilirubin level ≤1.5 times the upper limit of
normal and serum alanine transferase and alkaline phosphatase
levels ≤3 times the upper limit of normal). Patients with a
concurrent malignancy of another type or symptoms and/or signs of
metastases in the central nervous system were excluded. Patients
with prior surgery were considered eligible. This study was
approved by the Institutional Review Board of Hyogo College of
Medicine and informed consent was obtained from each patient.
Treatment
Patients received a 30-min intravenous (i.v.)
infusion of 100 mg/m2 methotrexate and, 30 min later, a
30-min i.v. infusion of 800 mg/m2 gemcitabine. For
leucovorin rescue, calcium leucovorin (10 mg/m2, p.o. or
i.v.) was administered 4 times at 6-h intervals, initiated 24 h
after the administration of methotrexate. These treatments were
administered weekly, with 4 treatments constituting a cycle of
therapy. A maximum of 6 cycles were administered, unless therapy
was terminated due to tumor progression, patient death or wish of
treatment discontinuation, or in the presence of convincing
evidence that further treatment was not beneficial. Antiemetic and
symptomatic treatments were permitted. Analyses of blood cell count
and chemistry were performed weekly. Treatment was delayed in the
case of i) absolute neutrophil count <1.5×109/l
and/or platelet count <100×109/l; ii) any grade 3 or
4 non-hematological toxicity (except for nausea/vomiting) that did
not resolve to grade 1 or less. If these toxicities were not
resolved within the cycle, the dose was reduced to 75% of the
previous dose level for the next cycle.
Response and toxicity criteria
Chest imaging by computed tomography (CT) was
performed at baseline, following completion of every other
treatment cycle and every 8weeks following completion of therapy.
Objective response was evaluated and calculated using the modified
Response Evaluation Criteria in Solid Tumors (RESIST) criteria for
MPM (15). Treatment-related
toxicities were evaluated according to the National Cancer
Institute Common Toxicity Criteria version 3.0 (16).
Statistical analysis
Survival was calculated as the time period from
treatment initiation to death, using the Kaplan-Meier method
(17).
Results
Patient characteristics
The characteristics of the 21 eligible patients are
listed in Table I. There were 16
males and 5 females, with a median age of 63 years (range, 51–75
years). The histological pattern of MPM was epithelial in 17 cases,
sarcomatous in 3 cases and biphasic in 1 case. Nineteen patients
(90.4%) had stage III and IV disease according to the IMIG staging
system at the time of enrollment. Thirteen patients (61.9%) had an
ECOG performance status of 0 or 1.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | No. (%) |
|---|
| Gender | |
| Male | 16 (76.2) |
| Female | 5 (23.8) |
| Age, years | |
| Median | 63 |
| Range | 51–75 |
| Performance
status | |
| 0 | 1 (4.8) |
| 1 | 12 (57.1) |
| 2 | 8 (38.1) |
| IMIG stage | |
| Ib | 1 (4.8) |
| II | 1 (4.8) |
| III | 4 (19.0) |
| IV | 15 (71.4) |
| Histological
subtype | |
| Epithelial | 17 (81.0) |
| Sarcomatous | 3 (14.3) |
| Biphasic | 1 (4.7) |
| Previous
treatment | |
| None | 10 (47.6) |
| Surgery | 2 (9.5) |
| Chemotherapy | 9 (42.9) |
| Asbestos
exposure | |
| Yes | 13 (61.9) |
| No | 8 (38.1) |
Responses to treatment
A total of 88 cycles were administered to the 21
patients. Each patient received a median 4.2 cycles (range, 2–10
cycles). Response to chemotherapy is shown in Table II. No patients exhibited a complete
response. Eight patients (38.1%) exhibited a partial response.
According to the histological pattern, a PR was observed in 6 out
of the 17 patients with epithelial type and in 2 out of the 3
patients with sarcomatous type MPM. Out of the total 21 patients,
10 (47.6%) had stable disease and 3 (14.3%) had progressive disease
with no period of stabilization.
 | Table IIResponse to chemotherapy and
histologic subtype. |
Table II
Response to chemotherapy and
histologic subtype.
| | Histologic subtype
|
|---|
| Response | Overall no. (%) | Epithelial no.
(n) | Sarcomatous no.
(n) | Biphasic no. (n) |
|---|
| Complete
response | 0 | 0 | 0 | 0 |
| Partial response | 8 (38.1) | 6 | 2 | 0 |
| Stable disease | 10 (47.6) | 8 | 1 | 1 |
| Progressive
disease | 3 (14.3) | 3 | 0 | 0 |
Toxicity
The toxicity observed in each patient is shown in
Table III. There was no
treatment-related mortality. The most frequently observed
hematological side effects were neutropenia and thrombocytopenia.
Grade 3–4 hematologic toxicities were observed in 3 patients
(14.3%), which, however, were manageable and did not result in
life-threatening complications. Six patients (28.6%) experienced
grade 1–2 gastrointestinal toxicities (nausea, vomiting and
anorexia) and 7 patients (33.3%) developed grade 1–2 liver
dysfunction. Two patients developed interstitial pneumonitis (grade
2) and were administered glucocorticosteroid therapy.
 | Table IIIChemotherapy-related toxicity in
eligible patients. |
Table III
Chemotherapy-related toxicity in
eligible patients.
| Toxicity | Grade 1 no. (%) | Grade 2 no. (%) | Grade 3 no. (%) | Grade 4 no. (%) |
|---|
| Hematologic | 5 | 2 (9.5) | 2 (9.5) | 1 (4.8) |
| Gastrointestinal | 5 (23.8) | 1 (4.8) | 0 (0) | 0 (0) |
| Hepatobiliary | 4 (19.0) | 3 (14.3) | 0 (0) | 0 (0) |
| Pulmonary | 0 (0) | 2 (9.5) | 0 (0) | 0 (0) |
Survival
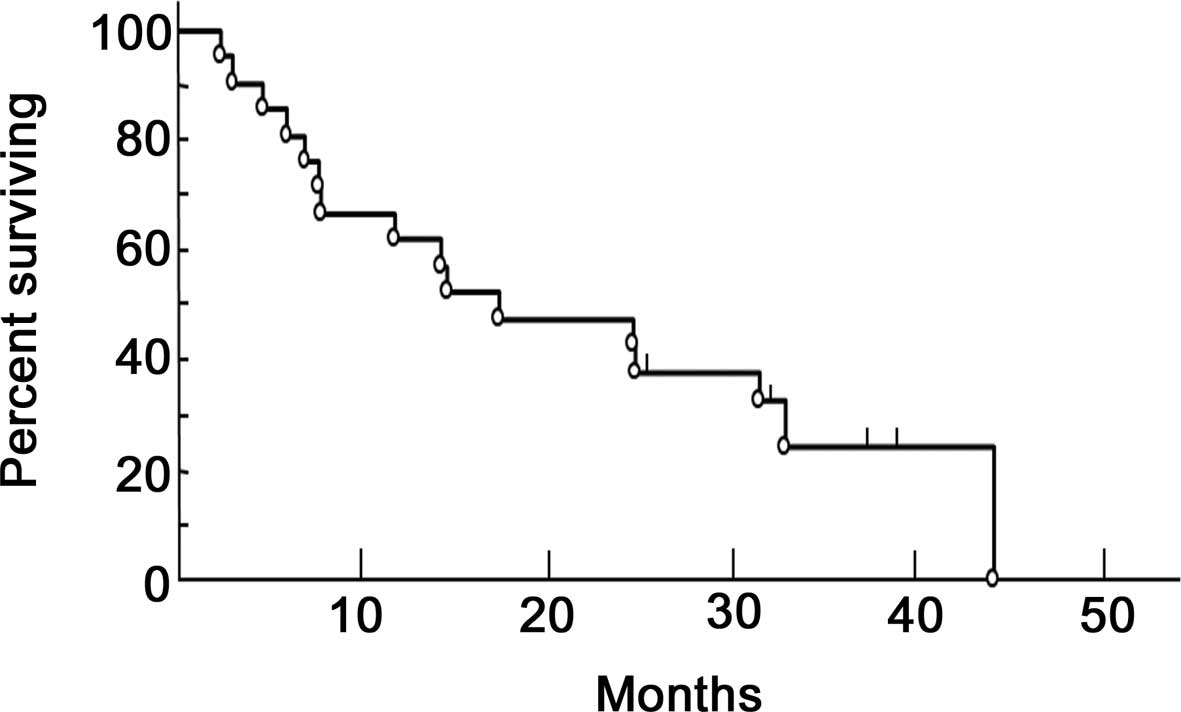
The median overall survival was 19.4 months (range,
2–41 months), with a 1- and 2-year survival rates of 61.9 and
38.1%, respectively (Fig. 1). As
regards the histological pattern, the median survival was 19.6
months for the epithelial, 22.6 months for the sarcomatous and 7.1
months for the biphasic type of MPM.
Discussion
MPM is notoriously refractory to the majority of
treatments and the standard first-line treatment is currently
cisplatin and PTX chemotherapy (10). In the present study, we evaluated
the feasibility of a non-platimum regimen for MPM, involving the
sequential administration of the anti-metabolites, methotrexate and
gemcitabine.
Methotrexate, an antifolate, has long been used as
an anticancer agent and exerts its action through the inhibition of
dihydrofolate reductase (DHFR) (12). High-dose methotrexate (1500
mg/m2) has been reported to be effective in the
treatment of MPM, with a response rate of 37% (18). However, high-dose methotrexate was
associated with severe toxicity and this method of treatment has
been abandoned. The efficacy of low- or medium-dose methotrexate
has not been assessed in MPM. In the treatment of gastric cancer
(19) and head and neck cancer
(20), weekly administration of
medium-dose methotrexate (100–200 mg/m2) combined with
sequential administration of 5-fluorouracil (5-FU) (600
mg/m2) has been reported to be effective and of low
toxicity. In this study, a moderate dose of methotrexate (100
mg/m2) was administered weekly in combination with
gemcitabine.
PTX is a newly developed antifolate that targets
multiple enzymes involved in DNA synthesis and folate metabolism.
Single use of PTX has been reported to be moderately effective
against MPM (21). Following
combination therapy with 1,250 mg/m2 gemcitabine
administered on days 1 and 8 and 500 mg/m2 PTX
administered on day 8 or 1, chemotherapy-naïve MPM patients
exhibited a response rate of 17–26%, with a median survival of 8–10
months (22). Hematologic
toxicities included grade 3–4 neutropenia (60%) and febrile
neutropenia (10%). These results indicated that the combination of
PTX and gemcitabine was moderately effective in MPM patients but
was associated with a notably high incidence of neutropenia
(22).
In this study on the methotrexate and gemcitabine
doublet regimen, 3 patients (14.3%) exhibited grade 3–4 hematologic
toxicity, with no sepsis or hemorrhage. There was no observed grade
3–4 non-hematological toxicity. Two patients developed interstitial
pneumonitits (grade 2) which responded well to steroid therapy.
Thus, the tolerability and toxicity profiles were considered
acceptable.
The response rate with the methotrexate and
gemcitabine combination chemotherapy was 38.1%, which is within the
range of 20–50% observed with other ‘active’ agents for MPM
(8,10). Median survival was 19.4 months.
Antifolates may be one of the key agents for MPM, since the
majority of mesothelioma cells of all histological MPM subtypes
express high-affinity α folate receptor (23). In our combination regimen, we
observed that methotrexate, an old-type antifolate, exhibited
desirable efficacy. Methotrexate has also been reported to be more
efficient compared to PTX, a newly developed antifolate, against
osteosarcoma cells (24),
indicating that methotrexate possesses a therapeutic potential.
In the present study, 10 out of the 21 patients were
chemotherapy-naïve and their response rate to this regimen was
similar to the overall response rate described above. This suggests
that methotrexate plus gemcitabine may be beneficial as the
first-line treatment for MPM. Eleven patients who had been
previously treated also exhibited a response rate similar to the
overall response rate. Although the optimal regimen constituting
the second-line chemotherapy remains to be determined, results of
the present study suggest that methotrexate plus gemcitabine may
also be beneficial as a second-line treatment.
In conclusion, the present study demonstrated that
the methotrexate and gemcitabine combination therapy is feasible,
with a more favorable toxicity profile and efficient in the
treatment of MPM. Further clinical evaluation is required, with
prospective trials including a larger cohort of patients.
References
|
1.
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nakano T: Current therapies for malignant
pleural mesothelioma. Environ Health Prev Med. 13:75–83. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wagner JC, Slegg CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in North western
Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
4.
|
Kaufman AJ and Pass HI: Current concepts
in malignant pleural mesothelioma. Expert Rev Anticancer Ther.
8:293–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yang H, Testa JR and Carbone M:
Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr
Treat Options Oncol. 9:147–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Maziak DE, Gagliardi A, Haynes AE, Mackay
JA and Evans WK: Surgical management of malignant pleural
mesothelioma: a systematic review and evidence summary. Lung
Cancer. 48:157–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ung YC, Yu E, Falkson C, Haynes AE,
Stys-Norman D and Evans WK: The role of radiation therapy in
malignant pleural mesothelioma: a systematic review. Radiother
Oncol. 80:13–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Steele JPC and Klabatsa A: Chemotherapy
options and new advances in malignant pleural mesothelioma. Ann
Oncol. 16:345–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fennell DA, Gaudino G, O’Byrne KJ, Mutti L
and van Meerbeeck J: Advances in the systemic therapy of malignant
pleural mesothelioma. Nat Clin Pract Oncol. 5:136–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Huennekens FM: The methotrexate story: a
paradigm for development of cancer chemotherapeutic agents. Adv
Enzyme Regul. 34:397–419. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
McGuire JJ: Anticancer antifolates:
current status and future directions. Curr Pharm Des. 9:2593–2613.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lund B, Kristjansen P and Hansen H:
Clinical and preclinical activity of 2′,2′-difluorodesoxycitidine
(Gemcitabine). Cancer Treat Rev. 19:45–55. 1993.
|
|
14.
|
Rusch VW: A proposed new international TNM
staging system for malignant pleural mesothelioma. From the
International Mesothelioma Interest Group Chest. 108:1122–1128.
1995.PubMed/NCBI
|
|
15.
|
Byrne MJ and Nowak AK: Modified RECIST
criteria for assessment of response in malignant pleural
mesothelioma. Ann Oncol. 15:257–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
National Cancer Institute Common Toxicity
Criteria for Adverse Events version 3.0. http://ctep.cancer.gov/reporting/ctc.html
Accessed August 9, 2003.
|
|
17.
|
Kaplan M and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
18.
|
Solheim OP, Saeter G, Finnanger AM and
Stenwig AE: High-dose methotrexate in the treatment of malignant
mesothelioma of the pleura: a phase II study. Br J Cancer.
65:956–960. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Imazawa M, Kojima T, Boku N, et al:
Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in
improving oral intake in patients with advanced gastric cancer with
severe peritoneal dissemination. Gastric Cancer. 12:153–157. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ringborg U, Ewert G, Kinnman J, Lundquist
PG and Strander H: Methotrexate and 5-fluorouracil in head and neck
cancer. Semin Oncol 10. (Suppl 2): 20–22. 1983.
|
|
21.
|
Scagliotti GV, Shin DM, Kindler HL, et al:
Phase II study of pemetrexed with and without folic acid and
vitamin B12 as front-line therapy in malignant pleural
mesothelioma. J Clin Oncol. 21:1556–1561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Jänne PA, Simon GR, Langer CJ, et al:
Phase II trial of pemetrexed and gemcitabine in chemotherapy-naive
malignant pleural mesothelioma. J Clin Oncol. 26:1465–1471.
2008.PubMed/NCBI
|
|
23.
|
Bueno R, Appasani K, Mercer H, Lester S
and Sugarbaker D: The alpha folate receptor is highly activated in
malignant pleural mesothelioma. J Thorac Cardiovasc Surg.
121:225–233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bodmer N, Walters DK and Fuchs B:
Pemetrexed, a multitargeted antifolate drug, demonstrates lower
efficacy in comparison to methotrexate against osteosarcoma cell
lines. Pediatr Blood Cancer. 50:905–908. 2008. View Article : Google Scholar : PubMed/NCBI
|