Introduction
Cancer is a widely accepted risk factor for venous
thromboembolism (VTE) and, to a lesser extent, arterial thrombosis.
This risk is attributed to factors such as the expression of
prothrombotic factors by tumors, tumor compression of vessels,
inflammatory host response to malignancies, immobility, surgery,
indwelling central venous catheters and certain antitumor therapies
(1). In cancer patients undergoing
surgery, VTE is the most common cause of mortality in the first 30
postoperative days (2). VTE is
also a common complication of surgery for gynecologic cancer and
gynecologic malignancies are classified as the highest risk group
(3,4). It has been reported that 90% of
pulmonary thromboembolism (PTE) resulted from deep venous
thromboses (DVT). Kearon (5)
reported that, in a large number of cases, VTE occurred between the
intraoperative period and the first 3 postoperative days and that
thromboemboli in many of these patients resolved spontaneously (50%
resolved within 72 h). The risk of progression of postoperative VTE
appears to be greater if the initial thrombosis is large and if
there are continuing risk factors for thrombosis. The risk of
symptomatic VTE is generally the highest within the first 2 weeks
after surgery.
D-dimer, a marker of the hypercoagulable state, is a
stable end product of fibrin degradation. D-dimer levels increase
because of fibrin formation and fibrinolysis. Plasma D-dimer
measurement has been widely used in the screening for VTE. The time
course of changes in plasma D-dimer levels following surgery has
not been defined, although a recent study clarified the time course
associated with changes in plasma D-dimer levels after surgery in
patients with gynecologic cancer (6). The European Society for Medical
Oncology (ESMO) Clinical Practice Guidelines recommends the use of
low-molecular-weight heparin (LMWH) [e.g., enoxaparin (ENO) 4,000
units], unfractionated heparin (UFH) 5,000 units, 3 times daily, or
fondaparinux (FPX) 2.5 mg in patients undergoing major cancer
surgery (7). ENO and FPX were
approved by the Japanese Ministry of Health, Labor and Welfare for
use in the prevention of VTE in 2008 and 2009, respectively, and
have since been routinely used postoperatively for VTE prophylaxis.
No studies have evaluated the efficacy of ENO or FPX in terms of
postoperative D-dimer levels in patients with gynecologic cancer.
The purpose of the present study was to evaluate the effects of ENO
and FPX on postoperative plasma D-dimer levels in patients with
gynecologic cancer. Furthermore, pre-, intra- and postoperative
parameters, including use of ENO or FPX usage, were investigated as
possible risk factors for postoperative VTE or PTE.
Materials and methods
Study population
For this study, 434 patients with invasive
gynecologic cancer, who underwent surgery at Okayama University
Hospital (Okayama, Japan) between August, 2005 and August, 2011
were recruited. Written informed consent was obtained from all
patients. The median age of the patients was 55 years (range, 16–85
years). Patients with a range of gynecologic cancers were enrolled,
including 118 ovarian, 204 endometrial, 146 cervical and 3 vulvar
cancers. Patients with preoperative VTE were excluded from the
study. This study was approved by the ethics committee of Okayama
University Hospital.
Measurement of D-dimer levels and
detection of VTE
The plasma D-dimer level was measured prior to
surgery and on postoperative days 0, 1, 3, 5, 7, 10, 14 and 21, and
a final level was measured after day 28. The D-dimer level was
measured with a latex photometric immunoassay system using LPIA-Ace
D-D dimer II (Mitsubishi Chemical Medience Corporation, Tokyo,
Japan) as the reagent. Inter-assay variability (coefficient of
variation) was <10%. Preoperative plasma D-dimer levels were
used as baseline and the postoperative cut-off values were set at
10–15 μg/ml, in accordance with previous studies (6). Following surgery, primary screening
for VTE was performed using meticulous evaluation of the clinical
signs and elevation of the plasma D-dimer level. The clinical signs
of VTE evaluated in this study were leg swelling, tenderness along
the distribution of deep veins, acute cardiovascular dysfunction,
dyspnea, chest pain and loss of consciousness. Patients whose
plasma D-dimer concentration exceeded the pre-set cut-off values
(10–15 μg/ml) and patients who showed clinical signs of VTE
underwent CT scanning of the chest, abdomen and lower extremities
by using MDCT (Toshiba).
Demographic data
Preoperative autologous blood collection was
performed to minimize homologous blood transfusion in selected
cases of radical hysterectomy and/or para-aortic lymphadenectomy.
Erythropoiesis-stimulating agents (ESAs) were preferentially used
to correct anemia associated with the collection of autologous
blood. Preoperative autologous blood was obtained from 227 patients
and ESAs were administered to 180 of these patients. The patients
received general anesthesia. Calf-length external sequential
compression devices (SCD) were placed on both legs at the start of
surgery in all the patients; the use of these devices continued
postoperatively. In all, 270 patients underwent chemical
anticoagulation: 107 patients received UFH (10,000 U/day) following
surgery, 51 patients received FPX (2.5 mg/day) and 112 patients
received ENO (4,000 units) after the surgery. UFH, ENO and FPX were
continued for a median of 5 days (range, 3–23 days), 10 days
(range, 1–15 days) and 10 days (range, 3–20 days), respectively.
UFH was used at the surgeon’s discretion prior to the regulatory
approval of the other agents (e.g., prior to 2008) and ENO and FPX
were routinely used subsequent to 2009. Age, history, body-mass
index, hematologic data, the results of biochemical tests, type of
surgery, intraoperative findings and postoperative information were
obtained from the medical records of the patients.
Statistical analysis
The Mann-Whitney U test was used to compare plasma
D-dimer levels or postoperative day of VTE detection in the groups.
The Chi-square test, Mann-Whitney U test and logistic regression
analysis were used to investigate the relationship between various
variables and the occurrence of VTE or PTE. P<0.05 was
considered to indicate a statistically significant difference.
Results
Postoperative VTE and PTE
When the groups of patients were combined, VTE was
detected in 31 (7.1%) patients on postoperative days 1–21 (median,
day 7). The median postoperative day of VTE detection was
significantly later in the case of patients treated with ENO or FPX
(median, day 11) compared to patients who did not receive ENO or
FPX (median, day 5.5) (P=0.028). The incidence of VTE in patients
with ovarian, endometrial and cervical cancer was 7.4, 7.7 and
5.6%, respectively (Table I). PTE
was found in 14 patients (3.2%). The incidence of PTE in patients
with ovarian, endometrial and cervical cancer was 3.2, 3.1 and
3.5%, respectively (Table I). PTE
was clinically symptomatic (dyspnea or chest pain) in 3 patients,
but not fatal in any patient. A substantial number of thrombi and
PTEs were treated using anticoagulation therapy with parenteral UFH
followed by oral warfarin. Insertion of an inferior vena cava
filter was required in 5 cases.
 | Table I.Incidence of postoperative VTE and PTE
in patients with gynecologic cancer. |
Table I.
Incidence of postoperative VTE and PTE
in patients with gynecologic cancer.
| Cancer type | VTE, n (%) | PTE, n (%) |
|---|
| Ovarian (n=95) | 7 (7.4) | 3 (3.2) |
| Endometrial
(n=194) | 15 (7.7) | 6 (3.1) |
| Cervical (n=142) | 8 (5.6) | 5 (3.5) |
| Vulvar (n=3) | 1 (33.3) | 0 (0.0) |
Time course of changes in postoperative
plasma D-dimer levels
The plasma D-dimer value gradually increased
postoperatively, peaked on postoperative days 7–10 and then
decreased. The D-dimer value significantly differed between
VTE-positive and -negative patients preoperatively (P=0.047) and on
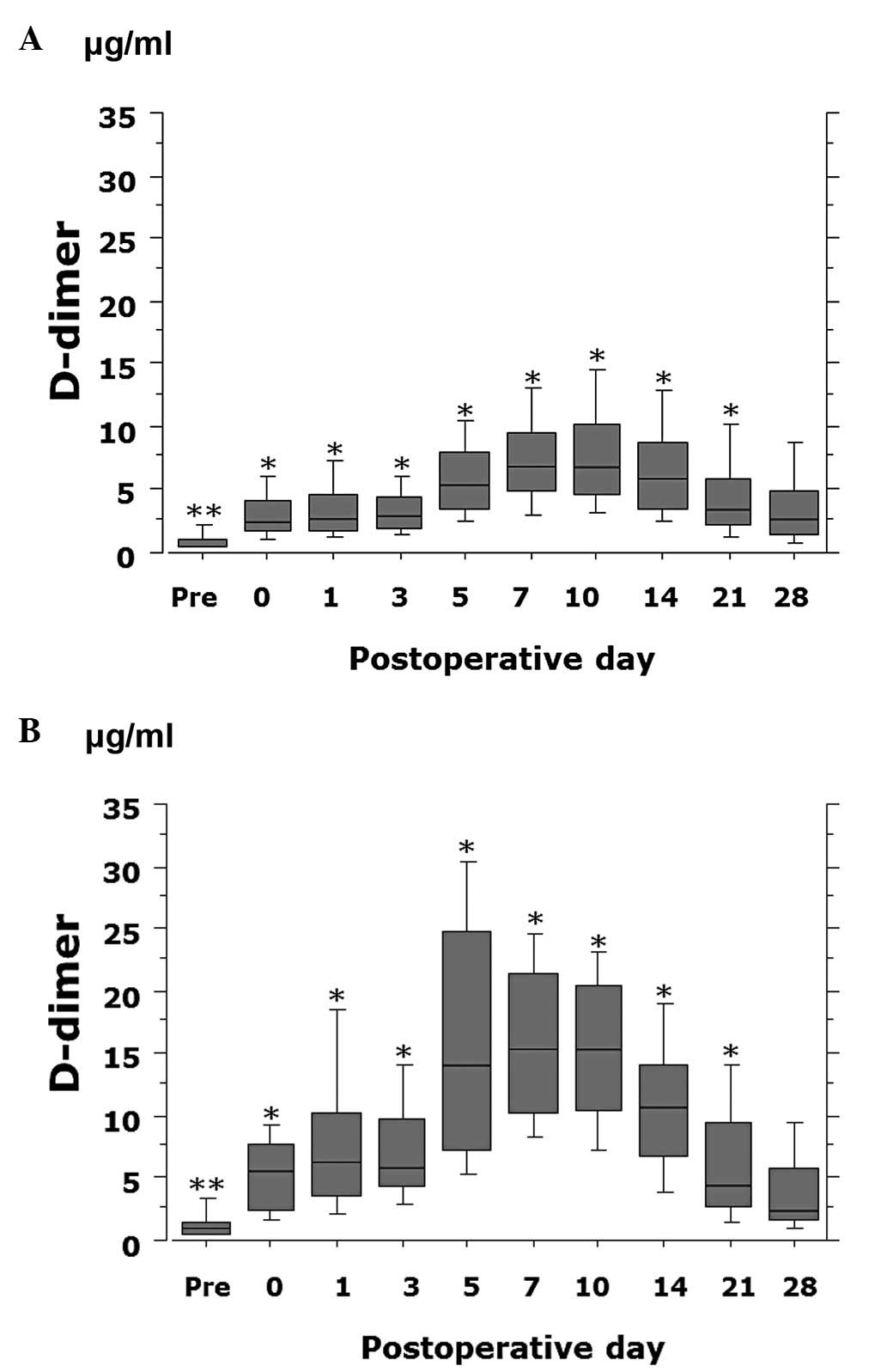
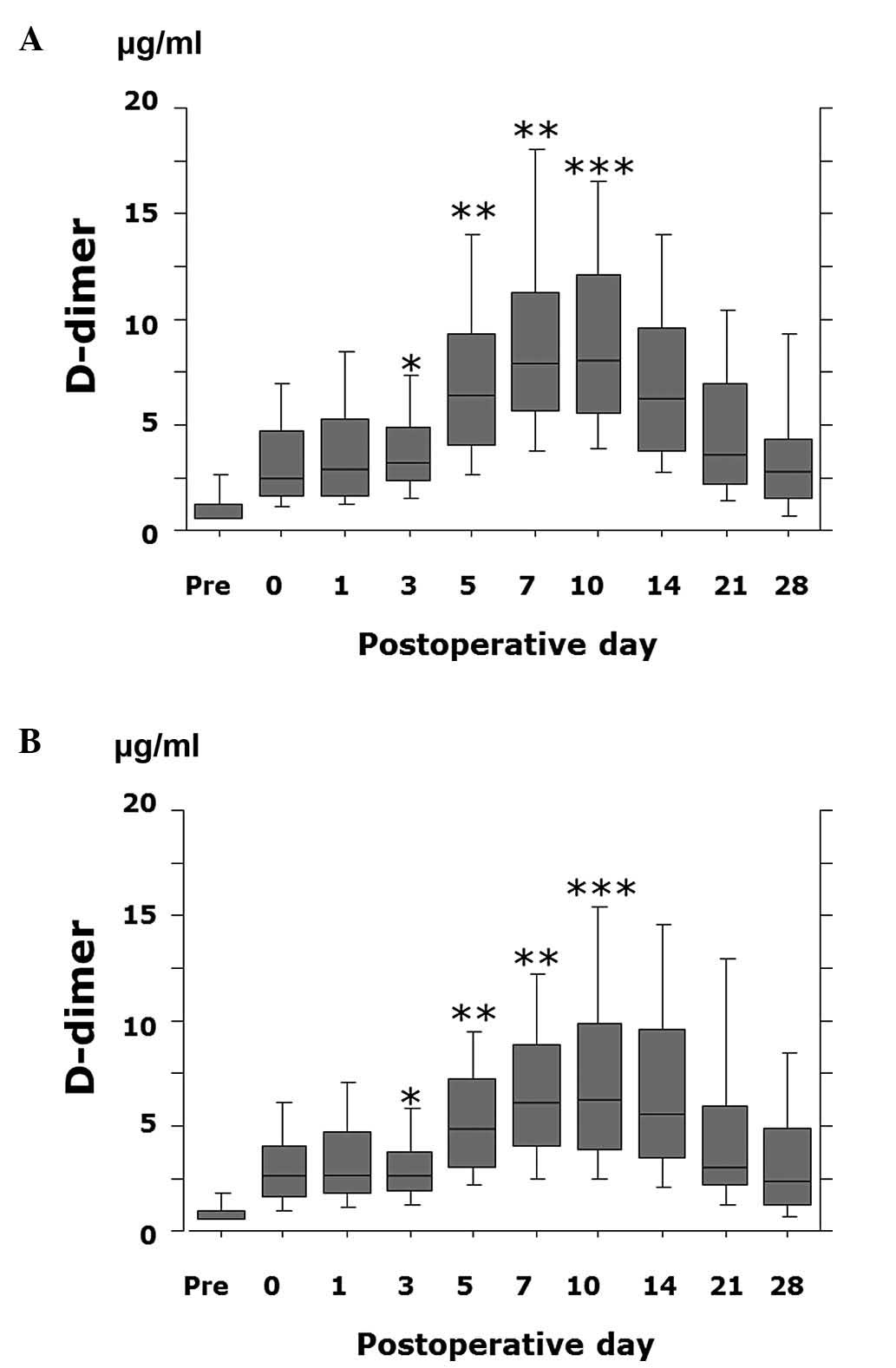
postoperative day 0–21 (P<0.0001) (Fig. 1). The D-dimer value was
significantly lower on postoperative day 3 (P=0.0004), days 5–7
(P<0.0001) and day 10 (P=0.0009) in patients receiving ENO or
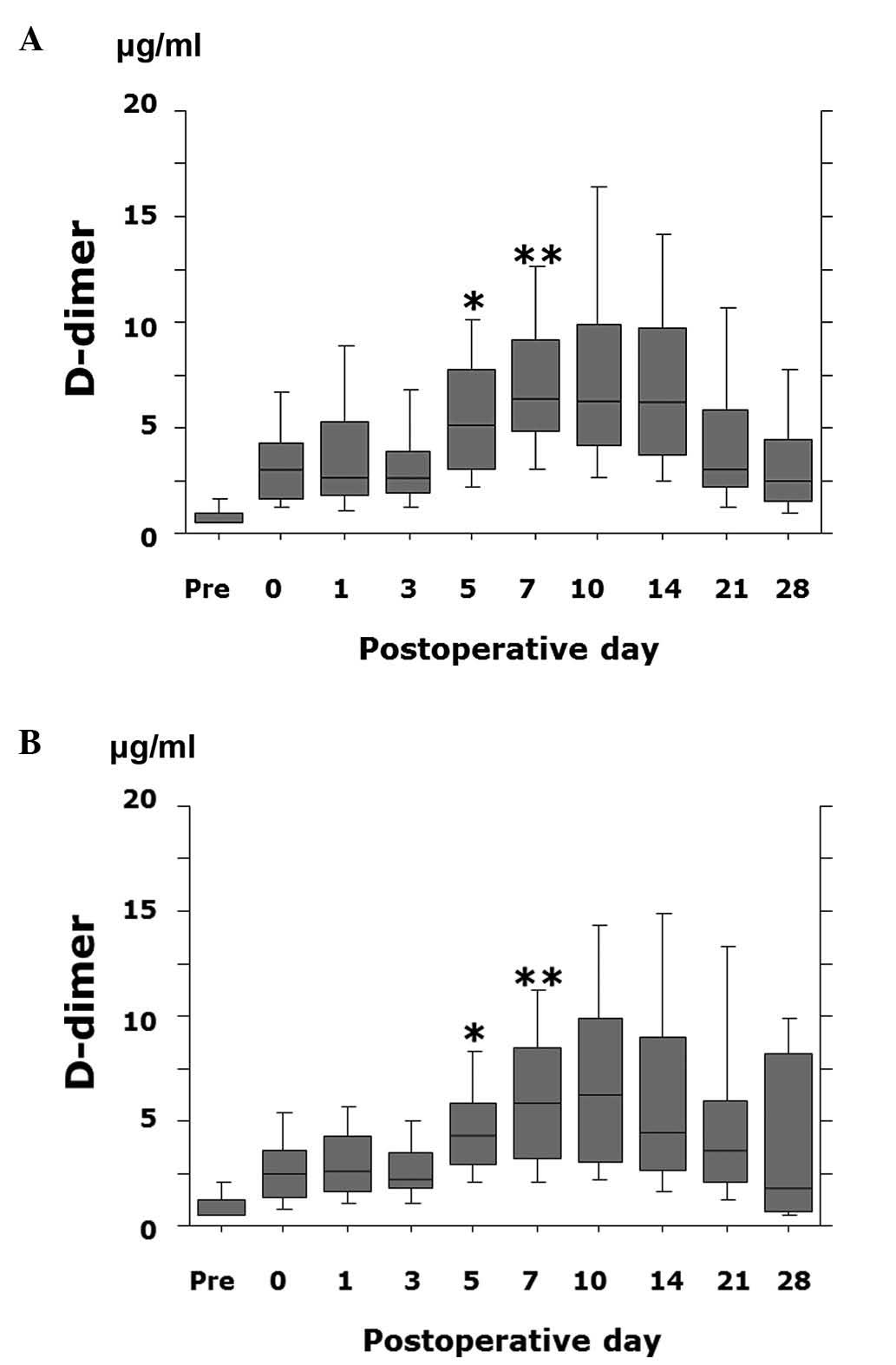
FPX compared to patients not treated with ENO or FPX (Fig. 2). In addition, the D-dimer value
was significantly lower on postoperative day 5 (P=0.028) and day 7
(P=0.049) in patients receiving FPX compared to patients treated
with ENO (Fig. 3).
Risk factors for postoperative VTE and
PTE
Results of univariate analysis indicated that the
D-dimer value on postoperative day 3, non-O blood group, the use of
ESAs, length of surgery, the extent of lymph node dissection, age
and the preoperative D-dimer value were significant risk factors
for postoperative VTE (Tables II
and III). The use of ENO or FPX
was not associated with the incidence of VTE. Logistic regression
multivariate analysis revealed that the D-dimer value on
postoperative day 3, the use of ESAs, advanced age and non-O blood
group were independent risk factors for predicting the occurrence
of postoperative VTE (Table IV).
The D-dimer value on postoperative day 3, the use of ESAs and not
receiving treatment with ENO or FPX were significant risk factors
for postoperative PTE (Tables V
and VI). Logistic regression
multivariate analysis revealed that the D-dimer value on
postoperative day 3 and the use of ESAs were independent risk
factors for predicting the occurrence of postoperative PTE
(Table VII).
 | Table II.Univariate analysis of preoperative
predictors of VTE. |
Table II.
Univariate analysis of preoperative
predictors of VTE.
| Variables | VTE (−) (n=403) | VTE (+) (n=31) | P-value |
|---|
| Age (years) | | | 0.023 |
| Median (range) | 55 (23–85) | 58 (32–75) | |
| BMI
(kg/m2) | | | 0.827 |
| Median (range) | 22.9 (13.5–46.5) | 22.3 (15.0–32.0) | |
| Comorbidity | 103 (25.6) | 12 (38.7) | 0.110 |
| Smoking | 38 (9.4) | 0 (0.0) | 0.074 |
| Blood group | | | 0.004 |
| O | 105 (26.1) | 1 (3.3) | |
| Non-O | 298 (73.9) | 30 (96.7) | |
| Diagnosis group | | | 0.296 |
| Ovarian | 88 (21.8) | 7 (22.6) | |
| Endometrial | 179 (44.4) | 15 (48.4) | |
| Cervical | 134 (33.3) | 8 (25.8) | |
| Vulvar | 2 (0.5) | 1 (3.2) | |
| Usage of ESAs | 160 (39.7) | 20 (64.5) | 0.007 |
| Pre-operative D-dimer
(μg/ml) | | | 0.030 |
| Median (range) | 0.5 (0.5–24.1) | 0.9 (0.5–6.2) | |
| White blood cell
(/mm3) | | | 0.067 |
| Median (range) | 6360
(2090–18300) | 5800 (3700–9890) | |
| Hemoglobin
(g/dl) | | | 0.813 |
| Median (range) | 12.8 (2.9–15.7) | 12.9 (9.0–15.9) | |
| Platelet
(/mm3) | | | 0.199 |
| Median (range) | 27.8 (3.4–97.1) | 26.2 (13.6–43.0) | |
| CRP (mg/dl) | | | 0.971 |
| Median (range) | 0.06 (0.0–14.0) | 0.06 (0.0–7.5) | |
| PT-INR | | | 0.123 |
| Median (range) | 0.93 (0.79–1.44) | 0.91 (0.84–1.10) | |
| APTT | | | 0.890 |
| Median (range) | 30.5 (21.2–53.2) | 30.5 (20.0–43.3) | |
 | Table III.Univariate analysis of
intra-/postoperative predictors of VTE. |
Table III.
Univariate analysis of
intra-/postoperative predictors of VTE.
| Variables | VTE (−) (n=403) | VTE (+) (n=31) | P-value |
|---|
| Type of
hysterectomy | | | 0.4180 |
| Not removed | 37 (9.2) | 1 (3.2) | |
| SH | 206 (51.1) | 15 (48.4) | |
| RH | 160 (39.7) | 15 (48.4) | |
| Extent of
lymphadenectomy | | | 0.0220 |
| None | 48 (11.9) | 1 (3.2) | |
| Pelvic | 248 (61.5) | 15 (48.4) | |
|
Pelvic-paraaortic | 107 (26.6) | 15 (48.4) | |
| Operation time
(min) | | | 0.2290 |
| Median (range) | 300 (140–455) | 275 (40–470) | |
| Blood loss (ml) | | | 0.3490 |
| Median (range) | 460 (0–4790) | 530 (50–7000) | |
| Blood
transfusion | 340 (84.4) | 25 (80.6) | 0.1300 |
| Usage of
anticoagulant | | | 0.1610 |
| None/UFH | 248 (61.5) | 23 (74.2) | |
| ENO/FPX | 155 (38.5) | 8 (25.8) | |
| D-dimer
(μg/ml) (day 3) | | | |
| Median (range) | 2.8 (0.5–24.2) | 5.8 (1.6–21.5) | <0.0001 |
 | Table IV.Stepwise multivariate logistic
regression analysis for predictors of VTE. |
Table IV.
Stepwise multivariate logistic
regression analysis for predictors of VTE.
| Variables | Comparison | Multivariate
|
|---|
| Odds ratio | P-value | 95% CI |
|---|
| Age | Old:Young | 1.060 | 0.010 | 1.014–1.109 |
| Blood type | Non-O: O | 12.801 | 0.017 | 1.584–103.463 |
| Use of ESAs | (+):(−) | 4.183 | 0.003 | 1.610–10.970 |
| Preoperative
D-dimer | High: Low | 1.067 | 0.529 | 0.871–1.307 |
| Extent of
lymphadenectomy | Pelvic: None | 8.912 | 0.185 | 0.571–138.985 |
| Pelvic +
paraaortic: None | 10.496 | 0.099 | 0.643–171.427 |
| Operation time | Long: Short | 1.003 | 0.333 | 0.997–1.008 |
| Postoperative
D-dimer (day 3) | High: Low | 1.300 | <0.001 | 1.162–1.454 |
 | Table V.Univariate analysis of preoperative
predictors of PTE. |
Table V.
Univariate analysis of preoperative
predictors of PTE.
| Variables | PTE (−)
(n=420) | PTE (+) (n=14) | P-value |
|---|
| Age (years) | | | 0.412 |
| Median
(range) | 55 (16–85) | 58 (32–75) | |
| BMI
(kg/m2) | | | 0.866 |
| Median
(range) | 22.9
(13.5–46.5) | 22.3
(15.0–32.0) | |
| Comorbidity | 111 (26.4) | 4 (28.6) | 0.858 |
| Smoking | 38 (9.0) | 0 (0.0) | 0.239 |
| Blood group | | | 0.126 |
| O | 105 (25.0) | 1 (7.1) | |
| Non-O | 315 (75.0) | 13 (92.9) | |
| Diagnosis
group | | | 0.845 |
| Ovarian | 92 (21.8) | 3 (21.4) | |
| Endometrial | 189 (44.4) | 5 (35.7) | |
| Cervical | 136 (33.3) | 6 (42.9) | |
| Vulvar | 3 (0.5) | 0 (0.0) | |
| Use of ESAs | 169 (40.2) | 11 (78.6) | 0.004 |
| Pre-operative
D-dimer (μg/ml) | | | 0.105 |
| Median
(range) | 0.5 (0.5–24.1) | 0.9 (0.5–6.2) | |
| White blood cell
(/mm3) | | | 0.844 |
| Median
(range) | 6360
(2090–18300) | 5800
(3700–9890) | |
| Hemoglobin
(g/dl) | | | 0.427 |
| Median
(range) | 12.8
(2.9–15.7) | 12.9
(9.0–15.9) | |
| Platelet
(/mm3) | | | 0.674 |
| Median
(range) | 27.8
(3.4–97.1) | 26.2
(13.6–43.0) | |
| CRP (mg/dl) | | | 0.994 |
| Median
(range) | 0.06
(0.0–14.0) | 0.06 (0.0–7.5) | |
| PT-INR | | | 0.328 |
| Median
(range) | 0.93
(0.79–1.44) | 0.91
(0.84–1.10) | |
| APTT | | | 0.200 |
| Median
(range) | 30.5
(21.2–55.2) | 30.5
(20.0–43.3) | |
 | Table VI.Univariate analysis of
intra-/post-operative predictors of PTE. |
Table VI.
Univariate analysis of
intra-/post-operative predictors of PTE.
| Variables | PTE (−)
(n=420) | PTE (+) (n=14) | P-value |
|---|
| Type of
hysterectomy | | | 0.412 |
| Not removed | 40 (9.5) | 0 (0.0) | |
| SH | 215 (51.2) | 7 (50.0) | |
| RH | 165 (39.3) | 7 (50.0) | |
| Extent of
lymphadenectomy | | | 0.249 |
| None | 48 (11.9) | 1 (3.2) | |
| Pelvic | 248 (61.5) | 15 (48.4) | |
|
Pelvic-paraaortic | 107 (26.6) | 15 (48.4) | |
| Operation time
(min) | | | 0.098 |
| Median
(range) | 300 (140–455) | 275 (40–470) | |
| Blood loss
(ml) | | | 0.239 |
| Median
(range) | 460 (0–4790) | 530 (50–7000) | |
| Blood
transfusion | 354 (84.3) | 11 (78.6) | 0.581 |
| Use of
anticoagulant | | | 0.018 |
| None/UFH | 258 (61.6) | 13 (92.9) | |
| ENO/FPX | 162 (38.4) | 1 (7.1) | |
| D-dimer
(μg/ml) (Day 3) | | | |
| Median
(range) | 2.8 (0.5–24.2) | 5.8 (1.6–21.5) | <0.0001 |
 | Table VII.Stepwise multivariate logistic
regression analysis for predictors of PTE. |
Table VII.
Stepwise multivariate logistic
regression analysis for predictors of PTE.
| Variables | Comparison | Multivariate
|
|---|
| Odds ratio | P-value | 95% CI |
|---|
| Use of ESAs | (+): (−) | 4.129 | 0.0350 | 1.108–15.389 |
| Use of
anticoagulants | ENO/FPX:
None/UFH | 0.190 | 0.1170 | 0.024–1.517 |
| Postoperative
D-dimer (day 3) | High: Low | 1.222 | 0.0002 | 1.102–1.356 |
Discussion
The present study measured D-dimer values
longitudinally in patients in whom sub-clinical VTE prior to
surgery was ruled out. These results showed that plasma D-dimer
values were significantly higher in VTE-positive patients compared
to VTE-negative patients preoperatively and on postoperative days
0–21, but not on day 28.
The changes in postoperative D-dimer levels
demonstrated the same pattern in patients treated with or without
UFH, although 5,000 units of UFH twice daily was commonly used for
a median of 5 days in cases requiring a more aggressive surgical
procedure (data not shown). Findings of a previous study have shown
that treatment with 5,000 units of UFH, 3 times daily,
significantly decreased the incidence of DVT in gynecologic cancer
patients, whereas this was not the case with treatment twice daily
(3). Recently, ENO and FPX were
approved for use as prophylaxis in Japanese patients undergoing
abdominal or pelvic surgery. Following approval, prophylaxis with
ENO or FPX for a median of 10 days has been routinely used at this
institution, (as well as other institutions). To the best of our
knowledge this is the first study to demonstrate a significantly
lower D-dimer value on postoperative days 3–10 in patients
receiving chemoprophylaxis with ENO or FPX compared to patients
receiving prophylaxis with SCDs or SCDs + UFH. These results
suggest that the decrease in the D-dimer level is a result of
fibrin formation and fibrinolysis due to administration of ENO or
FPX. Furthermore, this study demonstrated that the D-dimer value
was significantly lower in patients receiving FPX compared to those
treated with ENO on postoperative days 5 and 7. Therefore, 2.5 mg
of FPX may have stronger anticoagulant properties than 4,000 units
of ENO. Specifically, a subgroup analysis in the PEGAS study shed
some light on the efficacy of FPX relative to LMWH for
post-surgical thromboprophylaxis in patients with cancer (8).
The result of our previous study (9) demonstrated that the incidence of
preoperative VTE was significantly higher in patients with ovarian
cancer than in those with other types of gynecologic cancer. Unlike
preoperative VTE, ovarian cancer was not significantly associated
with postoperative VTE. In the present study population, VTE was
found in 7.9% of the patients after the surgery. Owing to the high
risk of postoperative VTE, this study also attempted to identify a
number of risk factors that may be useful in identifying
gynecologic cancer patients who are at even higher risk of
developing postoperative VTE. In contrast to results of the
previous study, results of the present study showed that a number
of factors were associated with VTE in the overall study
population. Age was an independent risk factor in the current study
population, as well as a high plasma D-dimer level on postoperative
day 3, the use of ESAs and non-O blood group, which have been
previously reported (6). These
results demonstrate that the use of ENO or FPX significantly
decreased the incidence of PTE, although therapy with these agents
did not reduce the incidence of VTE. The lack of statistical
significance detected for VTE is likely due to insufficient power
to show a difference in VTE rates. This study also showed that a
high plasma D-dimer level on postoperative day 3 and the use of
ESAs were independent risk factors for PTE.
The use of ESAs was demonstrated to be an important
risk factor for postoperative VTE and PTE. ESAs are biosynthetic
forms of erythropoietin, with similar biochemical structure and
biologic effects to those of erythropoietin (10). In this study, preoperative
autologous blood collection was undertaken to minimize homologous
blood transfusion in cases of radical hysterectomy and/or
para-aortic lymphadenectomy and ESAs were preferentially used to
correct anemia associated with the collection of autologous blood.
Analysis of the safety profile of ESAs for patients prior to their
going spinal surgery or open radical retropubic prostatectomy
revealed that the use of this hormone does not increase the risk of
thromboembolic events (11,12).
An open-level, randomized, parallel-group study was conducted to
confirm the safety and efficacy of epoetin α (PROCRIT) administered
perioperatively vs. the standard of care in blood conservation in
subjects undergoing major elective spinal surgery, available online
at http://clinicaltrial.gov/show/NCT00211146. This
observation led to the FDA issuing black box warnings for ESAs,
stating that ‘Perisurgery: Due to increased risk of DVT, DVT
prophylaxis is recommended’. The ESMO clinical practice guideline
states that the use of ESAs should be carefully reconsidered in the
case of patients at a high risk of thromboembolic events, such as
those undergoing surgery (13).
These results clearly show that the use of ESAs is an independent
risk factor for the development of VTE and PTE after surgery in
patients with gynecologic cancer. Additional research on the safety
of ESAs is required to confirm these results.
Owing to the suppression of the D-dimer value until
postoperative day 10 in patients undergoing treatment with ENO or
FPX, the median postoperative day of VTE detection was day 11 in
the present study. This observation suggests that
thromboprophylaxis reduces the incidence of VTE and delays the
occurrence of VTE. In the Enoxacan II study, a double-blinded,
multicenter, clinical trial of patients undergoing open abdominal
or pelvic cancer surgery, the incidence of VTE significantly
decreased from 12 to 4.8% when patients received inpatient
thromboprophylaxis for 27–31 days (14). The ESMO clinical practice
guidelines recommend that cancer patients undergoing elective major
abdominal or pelvic surgery should receive in-hospital and
post-discharge prophylaxis with LMWH for up to 1 month after
surgery (7). The present study
suggests that extended-duration prophylaxis may be considered for
patients with gynecologic cancer and other risk factors such as
advanced age, a high plasma D-dimer level on postoperative day 3,
the use of ESAs and non-O blood group. However, a systematic review
concluded that extended prophylaxis reduced asymptomatic VTE
without decreasing the risk of death at 3 months and that the
evidence for extended-duration regimens was limited and of poor
quality (15). More studies are
required to determine an optimal, cost-effective chemoprophylaxis
regimen.
The current findings demonstrate that the
postoperative D-dimer value was significantly lower in patients
with gynecologic cancer receiving ENO or FPX prophylaxis.
Furthermore, through multivariate analysis, the use of ESAs and the
presence of a high plasma D-dimer level on postoperative day 3 were
shown to be independent risk factors for postoperative VTE and
PTE.
References
|
1.
|
Lin A, Ryu J, Harvey D, Sieracki B,
Scudder S and Wun T: Low-dose warfarin does not decrease the rate
of thrombosis in patients with cervix and vulvo-vaginal cancer
treated with chemotherapy, radiation, and erythropoietin. Gynecol
Oncol. 102:98–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bradley CT, Brasel KJ, Miller JJ and
Pappas SG: Cost-effectiveness of prolonged thromboprophylaxis after
cancer surgery. Ann Surg Oncol. 17:31–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Einstein MH, Pritts EA and Hartenbach EM:
Venous thromboembolism prevention in gynecologic cancer surgery: a
sysytematic review. Gynecol Oncol. 105:813–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
NCCN Clinical practice guidelines in
oncology. Venous thromboembolic disease, version 1. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Accessed: April 6, 2013.
|
|
5.
|
Kearon C: Duration of venous
thromboembolism prophylaxis after surgery. Chest. 124:S386–S392.
2003. View Article : Google Scholar
|
|
6.
|
Kodama J, Seki N, Masahiro S, et al:
D-dimer level as a risk factor for postoperative venous
thromboembolism in Japanese women with gynecologic cancer. Ann
Oncol. 21:1651–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mandala M, Falanga A and Rolia F: Venous
thromboembolism in cancer patients: ESMO Clinical Practice
Guidelines for the management. Ann Oncol. 21:v274–v276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Agnelli G, Bergqvist D, Cohen AT, Gallus
AS and Gent M: Randomized clinical trial of postoperative
fondaparinux versus perioperative dalteparin for prevention of
venous thromboembolism in high-risk abdominal surgery. Br J Surg.
92:1212–1220. 2005. View
Article : Google Scholar
|
|
9.
|
Kodama J, Seki N, Fukushima C, et al:
Elevated preoperative plasma D-dimer levels and the incidence of
venous thromboembolism in Japanese females with gynecological
cancer. Oncol Lett. 5:299–304. 2013.PubMed/NCBI
|
|
10.
|
Egrie JC, Strickland TW, Lane J, et al:
Characterization and biological effects of recombinant human
erythropoietin. Immunobiology. 172:213–224. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Colomina MJ, Bagó J, Pellisé F, Godet C
and Villanueva C: Preoperative erythropoietin in spine surgery. Eur
Spine J. 13:S40–S49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lepor H, Lipkin M and Slova D: The
preoperative use of erythropoietin stimulating proteins prior to
radical prostatectomy is not associated with increased
cardiovascular or thromboembolic morbidity or mortality. Urology.
75:1424–1430. 2010. View Article : Google Scholar
|
|
13.
|
Schrijvers D, De Samblanx H and Rolia F:
Eruthropoiesis-stimulating agents in the treatment of anaemia in
cancer patients: ESMO Clinical Practice Guidelines for use. Ann
Oncol. 21:v244v2472010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bergqvist D, Agnelli G, Cohen AT, et al:
Duration of prophylaxis against venous thromboembolism with
enoxaparin after surgery for cancer. N Engl J Med. 346:975–980.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Akl EA, Terrenato I, Barba M, Sperati F,
Muti P and Schünemann HJ: Extended perioperative thromboprophylaxis
in patients with cancer. A systematic review. Thromb Haemost.
100:1176–1180. 2008.PubMed/NCBI
|