Introduction
The surgical strategy for gallbladder cancer (GBC)
depends on the extent of the disease, particularly the T stage from
the tumor, node, metastasis (TNM) classification (1). Identification of useful prognostic
markers exerting a strong prognostic impact for each T stage would
be beneficial in the development of rational therapeutic strategies
for each T stage of GBC. Useful histological markers are well-known
and easily evaluated in ordinary pathological examinations. This
study therefore focused on CD8+ tumor-infiltrating
lymphocytes (TIL), Ki-67 labeling index (LI), p53 nuclear
expression and mitotic count (MC), all of which have been well
investigated in other solid cancers as candidate prognostic markers
in GBC.
CD8+ TIL have been considered as
manifestations of host immune reactions against cancer cells and
strong prognostic impact of CD8+ TIL has been found in a
wide variety of solid cancer tissues (2–10). A
gene on chromosome 10 encodes a nuclear protein of 345–395 kDa that
is recognized by the antibody of the Ki-67 antigen. Ki-67 protein
is expressed during the active phases of the cell cycle (G1, S, G2,
and mitosis), but is absent from resting cells (G0). Ki-67 LI is
thus considered a marker for cell proliferation and the prognostic
impact of Ki-67 LI has been reported in various solid cancer
tissues (11–14). p53 is a well-known tumor suppressor
protein that is encoded by the TP53 gene, located on the short arm
of chromosome 17. Mutations of the TP53 gene lead to loss of
production of the normal p53 protein and synthesis of a mutated
protein with an increased half-life which tends to accumulate in
the nucleus and can be detected by immunohistochemical staining
(15). The prognostic role of p53
nuclear expression as assessed by immunohistochemistry has been
reported in various types of solid cancer (16–19).
MC is widely recognized as an indicator of tumor malignancy and the
prognostic impact of MC and classification or grading by MC status
have been reported for various types of tumor (20–24).
The purpose of this study was to assess the
prognostic impact of CD8+ TIL, Ki-67 LI, p53 nuclear
expression and MC in GBC, according to T stage.
Materials and methods
Patients and staging
A total of 101 GBC patients underwent surgical
treatment for the primary lesion at the Saga University Hospital
between January, 1989 and December, 2011. Of these, 11 patients
showing non-invasive intramucosal cancer and 4 patients for whom no
tissue samples were preserved were excluded from the study. As a
result, a final total of 86 patients with invasive GBC were
enrolled in this study. Informed consent for the use of resected
tissue was obtained from the patients, and the study protocol was
approved by the Ethics Committee of the Faculty of Medicine at the
Saga University. Clinical and histopathological staging were based
on the TNM Classification of Malignant Tumors established by the
International Union Against Cancer (7th edition, 2009) (1).
Immunohistochemical staining and
evaluation of MC
Sections cut from formalin-fixed paraffin-embedded
tissue blocks were used. The primary antibodies used were CD8
(dilution 1:50, clone C8/144B; DakoCytomation, Glostrup, Denmark),
p53 (prediluted, clone DO-7; Nichirei Biosciences, Tokyo, Japan)
and Ki-67 (dilution 1:30, clone MIB-1; DakoCytomation). The slides
were heated in ethylenediaminetetraacetic acid (EDTA) (pH 9.0) in a
microwave oven for antigen retrieval. The EnVision™+ system
(DakoCytomation) was used as the secondary antibody. Slides were
visualized by diaminobenzidine tetrahydrochloride (DAB 4HCl) and
nuclei were counterstained with hematoxylin. An Autostainer
Plus® automatic stainer (DakoCytomation) was used for
staining. Ki-67 LI was determined using the ratio of positive
nuclear staining of Ki-67 and classified as ≤10% (low group) or
>10% (high group). Assessment of p53 was also determined by
positive nuclear staining and classified as ≤30% (low group) or
>30% (high group). The cut-off value of p53 and Ki-67 LI in
previous studies varied greatly. The cut-off value of p53 and Ki-67
was determined to divide the cohort into two comparable groups
effectively. CD8+ lymphocytes within the cancer cell
nest were regarded as CD8+ TIL, according to a previous
report (2). CD8+ TIL
were counted in the three fields showing the most abundant
distribution of CD8+ TIL using a ×10 objective lens. The
number of CD8+ TIL was then determined as the mean count
for these three fields. CD8+ TIL was analyzed separately
on the tumor surface and the invasion front and was categorized as
≤10 (low group) and >10 (high group). Mitoses were counted in 10
high-power fields (HPF; magnification, ×400) on slides stained
using hematoxylin and eosin (H&E) and categorized as ≤10/10 HPF
or >10/10 HPF. Assessments of immunohistochemical staining and
mitoses were performed and confirmed by consensus decision by two
pathologists (M.M. and K.K. or Y.T. and K.K.).
Statistical analysis
Statistical analysis was performed using the JMP
software version 8 (SAS Institute, Cary, NC, USA). Statistical
analysis to compare the two groups was performed using the
Student’s t-test, the χ2 test and Fisher’s exact test,
as appropriate. The survival analyses were performed as
disease-specific survival, determined from the time of surgery to
the time of cancer-related death or the most recent follow-up. The
Cox proportional hazards model was applied for univariate and
multivariate analyses. Postoperative survival curves were
calculated using the Kaplan-Meier method. Differences in survival
curves were compared using the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics,
status of CD8+ TIL, Ki-67 LI, p53 expression and MC and
survival analysis for overall T stage
Patients comprised 27 men and 59 women, with a mean
age of 68.8 years (range, 45–87 years) at the time of surgery. Nine
patients (10.5%) were classified as T1b, 31 (36.0%) as T2, 40
(46.5%) as T3 and 6 (7.0%) as T4. Of the 86 patients, 68 (79.0%)
were classified as the surface CD8+ TIL-high group and
18 (21.0%) as the surface CD8+ TIL-low group. Sixty-two
patients (72.1%) were classified as the invasive site
CD8+ TIL-high group and 24 (27.9%) as the invasive site
CD8+ TIL-low group. Sixty patients (69.8%) were
classified as the MC-low group and 26 (30.2%) as the MC-high group.
Forty-two patients (48.8%) were classified as the Ki-67 LI-low
group and 44 (51.2%) as the Ki-67 LI-high group. Fifty-seven
patients (66.3%) patients were classified as the p53 expression-low
group and 29 (33.7%) as the p53 expression high group. Uni- and
multivariate analyses for disease-specific survival in the 86 GBC
patients are shown in Table I. In
the univariate analysis by Cox’s proportional hazards model,
factors significantly correlated with survival were T, N and MC
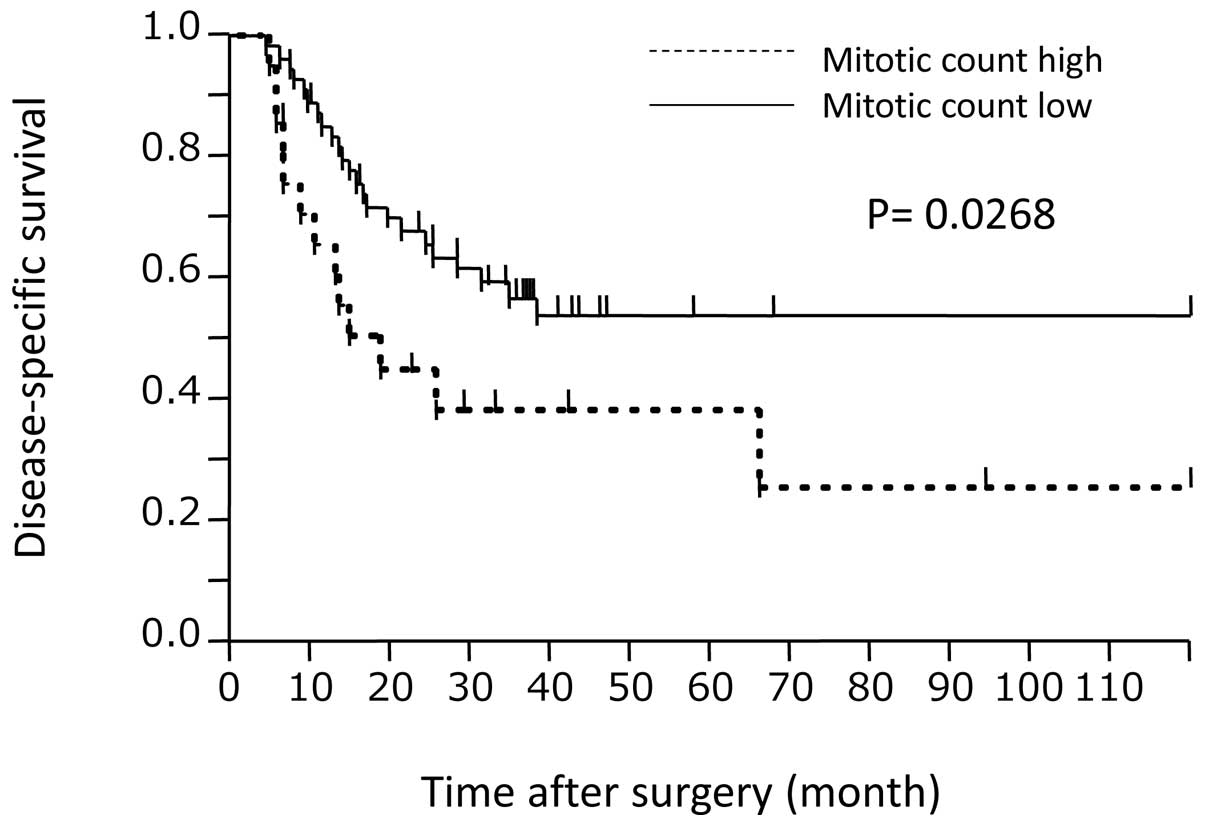
(P<0.0001, P<0.0001 and P=0.0383, respectively). The survival
curve according to MC is shown in Fig.
1. The P-value calculated by log-rank testing was 0.0268.
Multivariate analysis of the significant variables in the
univariate analysis revealed only T and N as independent prognostic
factors (P=0.005 and P=0.0113, respectively).
 | Table IAnalysis of prognostic factors in 86
GBC patients. |
Table I
Analysis of prognostic factors in 86
GBC patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Characteristics | No. | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| T | | | <0.0001 | | 0.0050 |
| T1b, T2 | 40 | 1.00 | | 1.00 | |
| T3, T4 | 46 | 4.88
(2.35–11.17) | | 3.22 (1.41–8.00) | |
| N | | | <0.0001 | | 0.0113 |
| N0 | 41 | 1.00 | | 1.00 | |
| N1 | 45 | 6.39
(3.05–14.68) | | 3.21 (1.30–8.27) | |
| M | | | <0.0001 | | 0.2407 |
| M0 | 69 | 1.00 | | 1.00 | |
| M1 | 17 | 4.69
(2.30–9.30) | | 1.60
(0.73–3.53) | |
| Surface TIL | | | 0.7485 | | |
| >10 | 68 | 1.00 | | | |
| ≤10 | 18 | 1.13
(0.54–2.67) | | | |
| Invasion front
TIL | | | 0.4719 | | |
| >10 | 62 | 1.00 | | | |
| ≤10 | 24 | 1.31
(0.64–2.96) | | | |
| Mitotic count | | | 0.0383 | | 0.1096 |
| ≤10/10 HPF | 60 | 1.00 | | 1.00 | |
| >10/10
HPF | 26 | 2.13
(1.04–4.15) | | 1.85
(0.87–3.85) | |
| Ki-67 LI | | | 0.5191 | | |
| ≤10% | 42 | 1.00 | | | |
| >10% | 44 | 1.24
(0.64–2.46) | | | |
| p53 expression | | | 0.9139 | | |
| ≤30% | 57 | 1.00 | | | |
| >30% | 29 | 0.96
(0.48–1.87) | | | |
Analysis in T2 patients
Univariate and multivariate analyses for
disease-specific survival in T2 patients are shown in Table II. In the univariate analysis by
Cox’s proportional hazards model, the only factors significantly
correlated with survival were M and p53 expression (P=0.0311 and
P=0.0154, respectively). Notably, the p53 expression-high group
demonstrated significantly better outcomes compared with the low
group. Multivariate analysis using significant variables from
univariate analyses was conducted, but identified as independent
prognostic factors.
 | Table IIAnalysis of prognostic factors in T2
GBC patients. |
Table II
Analysis of prognostic factors in T2
GBC patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Characteristic | No. | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| N | | | 0.0504 | | |
| N0 | 21 | 1.00 | | | |
| N1 | 10 | 3.80
(0.99–15.50) | | | |
| M | | | 0.0311 | | 0.1337 |
| M0 | 28 | 1.00 | | 1.00 | |
| M1 | 3 | 5.79
(1.20–22.5) | | 2.32
(0.46–10.11) | |
| Surface TIL | | | 0.5297 | | |
| >10 | 5 | 1.00 | | | |
| ≤10 | 26 | 1.86
(0.45–34.78) | | | |
| Invasion front
TIL | | | 0.4101 | | |
| >10 | 11 | 1.00 | | | |
| ≤10 | 20 | 1.87
(0.64–12.56) | | | |
| Mitotic counts | | | 0.8558 | | |
| ≤10/10 HPF | 24 | 1.00 | | | |
| >10/10
HPF | 7 | 0.87
(0.13–3.59) | | | |
| Ki-67 LI | | | 0.9994 | | |
| ≤10% | 11 | 1.00 | | | |
| >10% | 20 | 1.00
(0.26–4.76) | | | |
| p53 expression | | | 0.0154 | | 0.0623 |
| ≤30% | 18 | 1.00 | | 1.00 | |
| >30% | 13 | 0.13
(0.0070–0.71) | | 0.17
(0.0091–1.08) | |
Analysis in T3 patients
Univariate and multivariate analyses with
disease-specific survival in T3 patients are shown in Table III. In the univariate analysis
using Cox’s proportional hazards model, factors significantly
correlated with survival were N, M and MC (P=0.017, P=0.0476 and
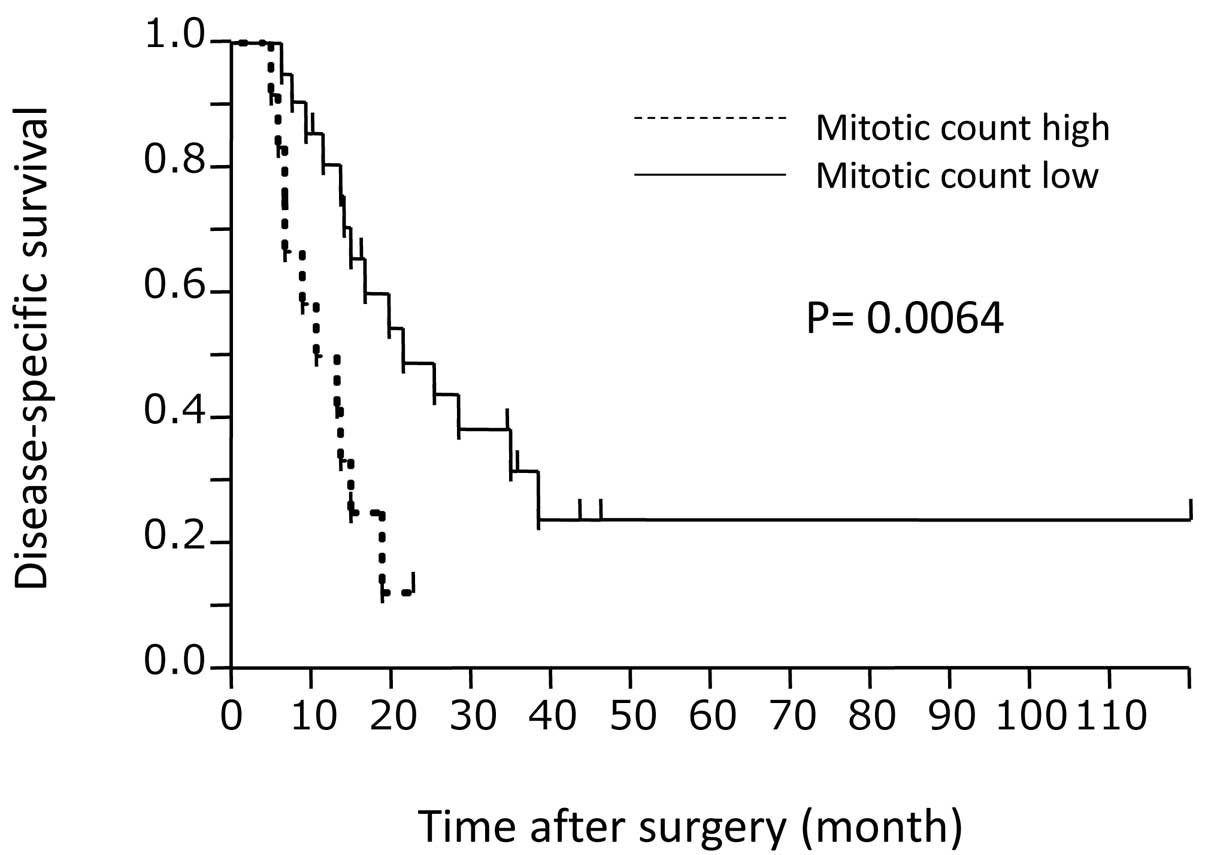
P=0.0113, respectively). The survival curve according to MC is
shown in Fig. 2. The P-value
calculated by the log-rank test was 0.0064. Multivariate analysis
using significant variables from the univariate analysis was
conducted, identifying only MC as an independent prognostic factor
(P=0.0419).
 | Table IIIAnalysis of prognostic factors in T3
GBC patients. |
Table III
Analysis of prognostic factors in T3
GBC patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
|
Characteristics | n | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| N | | | 0.0170 | | 0.1209 |
| N0 | 10 | 1.00 | | 1.00 | |
| N1 | 30 | 3.07
(1.21–9.42) | | 2.31
(0.81–7.52) | |
| M | | | 0.0476 | | 0.3942 |
| M0 | 28 | 1.00 | | 1.00 | |
| M1 | 12 | 2.54
(1.01–6.22) | | 1.53
(0.57–4.09) | |
| Surface TIL | | | 0.3747 | | |
| >10 | 7 | 1.00 | | | |
| ≤10 | 33 | 1.59
(0.60–5.49) | | | |
| Invasion front
TIL | | | 0.7769 | | |
| >10 | 10 | 1.00 | | | |
| ≤10 | 30 | 1.14
(0.47–3.18) | | | |
| Mitotic counts | | | 0.0113 | | 0.0419 |
| ≤10/10 HPF | 23 | 1.00 | | 1.00 | |
| >10/10
HPF | 17 | 3.30
(1.32–8.28) | | 2.66
(1.04–6.83) | |
| Ki-67 LI | | | 0.1816 | | |
| ≤10% | 22 | 1.00 | | | |
| >10% | 18 | 1.75
(0.77–4.03) | | | |
| p53 expression | | | 0.263 | | |
| ≤30% | 26 | 1.00 | | | |
| >30% | 14 | 1.59
(0.70–3.62) | | | |
Discussion
The prognosis following surgery for GBC is markedly
different according to the results for the T stage, as are the
therapeutic strategies (25,26).
Thus, studies for GBC according to T stage better reflect actual
prognosis after surgery and provide more useful information for
clinical treatment compared with studies on overall GBC. Generally,
survival of patients with T1 lesions is particularly good and
simple cholecystectomy with or without lymphadenectomy is thus
widely accepted as sufficient for T1 lesions (27). By contrast, survival of patients
with T4 lesions is extremely poor and chemotherapy or palliative
therapy is typically appropriate, except in rare cases where en
bloc resection of multiple organs is applicable. This study
therefore focused on patients with T2 and T3 tumors.
This study investigated correlations between
survival after surgery and the status of CD8+ TIL, Ki-67
LI, p53 expression and MC as potential prognostic markers for GBC.
However, results for these candidates were insufficient for use as
markers, with the exception of the results for MC. Concerning
CD8+ TIL, only one study that investigated the
prognostic impact of CD8+ TIL, reporting that
CD8+ TIL correlated with prolonged survival in
univariate analysis was available (28). However, no prognostic impacts of
surface or invasion front CD8+ TIL were observed in our
cohort. Therefore, we consider the prognostic impact of
CD8+ TIL in GBC to be controversial and suggest that
further investigation is required. Several previous studies have
reported no prognostic impact of p53 overexpression in GBC
(29–33), whereas reports of poor prognosis
with p53 overexpression are also available in the literature
(34–36). Of note, the present study showed an
association between p53 overexpression and favorable prognosis in
T2 GBC. Taken together, the correlation between p53 and prognosis
in GBC remains controversial, although gain of abnormalities in p53
protein is generally considered an early event in the progress of
carcinogenesis and is likely to be the usual route for GBC
development (37–40). A previous study reported that
patients with GBC showing high Ki-67 exhibited worse postoperative
prognosis compared with those showing low Ki-67 (41). Several previous studies reported
that Ki-67 LI of cancer cells did not correlate with patient
survival, supporting our results (30,32,33),
which is in agreement with findings of the present study. The
possible reason for Ki-67 not correlating with survival, despite
the MC significant correlation of MC with survival, is that Ki-67
LI involves the G1, S and G2 phases of the cell cycle, while MC
only involves the mitotic phase and might therefore reflect the
rapidity of cell proliferation more sensitively compared with Ki-67
LI.
To the best of our knowledge, no previous studies
have indicated the prognostic impact of MC in GBC and MC has not
been applied in the histopathological diagnosis or grading of GBC.
The degree of MC had a strong impact on survival in the present
study. However, MC did not correlate with survival in patients with
T2 tumor. This finding suggests that T2 GBC could be controlled by
rational surgery involving regional lymphadenectomy and liver and
bile duct resections (42) even in
cases showing rapid growth with high MC tumor. By contrast, MC was
identified as an independent prognostic factor in patients with T3
tumor. This suggests that tumors with rapid growth directly affect
survival after surgery and that controlling rapidly growing tumors
is difficult using a surgical approach alone. MC is easily
evaluated by simple H&E staining. Additional investigation into
MC in T3 GBC might contribute to information regarding prognosis
after surgery as well as indications or selection of procedures for
surgical treatment and/or adjuvant chemotherapy.
Acknowledgements
The authors would like to thank Mr.
Fumihiro Mutoh for his valuable contributions to the
immunohistochemical stainings. There is no grant support for this
study.
References
|
1.
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumors. 7th edition. John Wiley
& Sons, Inc; Hoboken, NJ: 2009
|
|
2.
|
Naito Y, Saito K, Shiiba K, Ohuchi A,
Saigenji K, Nagura H and Ohtani H: CD8+T cells
infiltrated within cancer cell nests as a prognostic factor in
human colorectal cancer. Cancer Res. 58:3491–3494. 1998.PubMed/NCBI
|
|
3.
|
Guidoboni M, Gafà R, Viel A, Doglioni C,
Russo A, Santini A, Del Tin L, Macrì E, Lanza G, Boiocchi M and
Dolcetti R: Microsatellite instability and high content of
activated cytotoxic lymphocytes identify colon cancer patients with
a favorable prognosis. Am J Pathol. 159:297–304. 2001. View Article : Google Scholar
|
|
4.
|
Wakabayashi O, Yamazaki K, Oizumi S,
Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H and Nishimura M:
CD4+T cells in cancer stroma, not CD8+T cells
in cancer cell nests, are associated with favorable prognosis in
human non-small cell lung cancers. Cancer Sci. 94:1003–1009.
2003.
|
|
5.
|
Prall F, Dührkop T, Weirich V, Ostwald C,
Lenz P, Nizze H and Barten M: Prognostic role of
CD8+tumor-infiltrating lymphocytes in stage III
colorectal cancer with and without microsatellite instability. Hum
Pathol. 35:808–816. 2004.
|
|
6.
|
Fukunaga A, Miyamoto M, Cho Y, Murakami S,
Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y,
Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S and Katoh H:
CD8+tumor-infiltrating lymphocytes together with
CD4+tumor-infiltrating lymphocytes and dendritic cells
improve the prognosis of patients with pancreatic adenocarcinoma.
Pancreas. 28:e26–e31. 2004.
|
|
7.
|
Zlobec I, Minoo P, Baumhoer D, Baker K,
Terracciano L, Jass JR and Lugli A: Multimarker phenotype predicts
adverse survival in patients with lymph node-negative colorectal
cancer. Cancer. 112:495–502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Leffers N, Gooden MJ, de Jong RA,
Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG,
Daemen T and Nijman HW: Prognostic significance of
tumor-infiltrating T-lymphocytes in primary and metastatic lesions
of advanced stage ovarian cancer. Cancer Immunol Immunother.
58:449–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
de Jong RA, Leffers N, Boezen HM, ten Hoor
KA, van der Zee AG, Hollema H and Nijman HW: Presence of
tumor-infiltrating lymphocytes is an independent prognostic factor
in type I and II endometrial cancer. Gynecol Oncol. 114:105–110.
2009.PubMed/NCBI
|
|
10.
|
Shah W, Yan X, Jing L, Zhou Y, Chen H and
Wang Y: A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes
and a high percentage of CD4(+) FOXP3(+) regulatory T cells are
significantly associated with clinical outcome in squamous cell
carcinoma of the cervix. Cell Mol Immunol. 8:59–66. 2011.PubMed/NCBI
|
|
11.
|
Seethala RR, Hunt JL, Baloch ZW, Livolsi
VA and Leon Barnes E: Adenoid cystic carcinoma with high-grade
transformation: a report of 11 cases and a review of the
literature. Am J Surg Pathol. 31:1683–1694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Leuverink EM, Brennan BA, Crook ML,
Doherty DA, Hammond IG, Ruba S and Stewart CJ: Prognostic value of
mitotic counts and Ki-67 immunoreactivity in adult-type granulosa
cell tumour of the ovary. J Clin Pathol. 61:914–919. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wrba F, Reiner A, Markis-Ritzinger E,
Holzner JH, Reiner G and Spona J: Prognostic significance of
immunohistochemical parameters in breast carcinomas. Pathol Res
Pract. 183:277–283. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bruner JM: Neuropathology of malignant
gliomas. Semin Oncol. 21:126–138. 1994.PubMed/NCBI
|
|
15.
|
Greenblatt MS, Grollman AP and Harris CC:
Deletions and insertions in the p53 tumor suppressor gene in human
cancers: confirmation of the DNA polymerase slippage/misalignment
model. Cancer Res. 56:2130–2136. 1996.PubMed/NCBI
|
|
16.
|
Bosari S, Viale G, Radaelli U, Bossi P,
Bonoldi E and Coggi G: p53 accumulation in ovarian carcinomas and
its prognostic implications. Hum Pathol. 24:1175–1179. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fontanini G, Vignati S, Lucchi M, Mussi A,
Calcinai A, Boldrini L, Chiné S, Silvestri V, Angeletti CA, Basolo
F and Bevilacqua G: Neoangiogenesis and p53 protein in lung cancer:
their prognostic role and their relation with vascular endothelial
growth factor (VEGF) expression. Br J Cancer. 75:1295–1301. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Assimakopoulos D, Kolettas E, Zagorianakou
N, Evangelou A, Skevas A and Agnantis NJ: Prognostic significance
of p53 in the cancer of the larynx. Anticancer Res. 20:3555–3564.
2000.PubMed/NCBI
|
|
19.
|
Pancione M, Forte N, Fucci A, Sabatino L,
Febbraro A, Di Blasi A, Daniele B, Parente D and Colantuoni V:
Prognostic role of beta-catenin and p53 expression in the
metastatic progression of sporadic colorectal cancer. Hum Pathol.
41:867–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sumithran E, Susil BJ and Looi LM: The
prognostic significance of grading in borderline mucinous tumors of
the ovary. Hum Pathol. 19:15–18. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. CW Elston and IO Ellis. Histopathology.
1991.19:403–410, Histopathology 41: 151–153, 2002.
|
|
22.
|
Van Eeden S, Quaedvlieg PF, Taal BG,
Offerhaus GJ, Lamers CB and Van Velthuysen ML: Classification of
low-grade neuroendocrine tumors of midgut and unknown origin. Hum
Pathol. 33:1126–1132. 2002.PubMed/NCBI
|
|
23.
|
Kadota K, Suzuki K, Colovos C, Sima CS,
Rusch VW, Travis WD and Adusumilli PS: A nuclear grading system is
a strong predictor of survival in epitheloid diffuse malignant
pleural mesothelioma. Mod Pathol. 25:260–271. 2012.PubMed/NCBI
|
|
24.
|
Storr SJ, Safuan S, Mitra A, Elliott F,
Walker C, Vasko MJ, Ho B, Cook M, Mohammed RA, Patel PM, Ellis IO,
Newton-Bishop JA and Martin SG: Objective assessment of blood and
lymphatic vessel invasion and association with macrophage
infiltration in cutaneous melanoma. Mod Pathol. 25:493–504. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Pilgrim C, Usatoff V and Evans PM: A
review of the surgical strategies for the management of gallbladder
carcinoma based on T stage and growth type of the tumour. Eur J
Surg Oncol. 35:903–907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mekeel KL and Hemming AW: Surgical
management of gall-bladder carcinoma: a review. J Gastrointest
Surg. 11:1188–1193. 2007. View Article : Google Scholar
|
|
27.
|
Wakai T, Shirai Y, Yokoyama N, Nagakura S,
Watanabe H and Hatakeyama K: Early gallbladder carcinoma does not
warrant radical resection. Br J Surg. 88:675–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Nakakubo Y, Miyamoto M, Cho Y, Hida Y,
Oshikiri T, Suzuoki M, Hiraoka K, Itoh T, Kondo S and Katoh H:
Clinical significance of immune cell infiltration within
gallbladder cancer. Br J Cancer. 89:1736–1742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ajiki T, Onoyama H, Yamamoto M, Asaka K,
Fujimori T, Maeda S and Saitoh Y: p53 protein expression and
prognosis in gallbladder carcinoma and premalignant lesions.
Hepatogastroenterology. 43:521–526. 1996.PubMed/NCBI
|
|
30.
|
Hidalgo Grau LA, Badia JM, Salvador CA,
Monsó TS, Canaleta JF, Nogués JM and Sala JS: Gallbladder
carcinoma: the role of p53 protein overexpression and Ki-67 antigen
expression as prognostic markers. HPB; Oxford: 6. pp. 174–180.
2004
|
|
31.
|
Kim YW, Huh SH, Park YK, Yoon TY, Lee SM
and Hong SH: Expression of the c-erb-B2 and p53 protein in
gallbladder carcinomas. Oncol Rep. 8:1127–1132. 2001.PubMed/NCBI
|
|
32.
|
Jarnagin WR, Klimstra DS, Hezel M, Gonen
M, Fong Y, Roggin K, Cymes K, DeMatteo RP, D’Angelica M, Blumgart
LH and Singh B: Differential cell cycle-regulatory protein
expression in biliary tract adenocarcinoma: correlation with
anatomic site, pathologic variables, and clinical outcome. J Clin
Oncol. 24:1152–1160. 2006. View Article : Google Scholar
|
|
33.
|
Kim WB, Han HJ, Lee HJ, Park SS, Song TJ,
Kim HK, et al: Expression and clinical significance of cell cycle
regulatory proteins in gallbladder and extrahepatic bile duct
cancer. Ann Surg Oncol. 16:23–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lee CS and Pirdas A: p53 protein
immunoreactivity in cancers of the gallbladder, extrahepatic bile
ducts and ampulla of Vater. Pathology. 27:117–120. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Chang HJ, Yoo BC, Kim SW, Lee BL and Kim
WH: Significance of PML and p53 protein as molecular prognostic
markers of gallbladder carcinomas. Pathol Oncol Res. 13:326–335.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Roa EI, Lantadilla HS, Ibacache SG and de
Aretxabala UX: p53 and p27 gene expression in subserosal
gallbladder carcinoma. Rev Med Chil. 137:1017–1022. 2009.(In
Spanish).
|
|
37.
|
Wistuba II, Gazdar AF, Roa I and
Albores-Saavedra J: p53 protein overexpression in gallbladder
carcinoma and its precursor lesions: an immunohistochemical study.
Hum Pathol. 27:360–365. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Kamel D, Pääkkö P, Nuorva K, Vähäkangas K
and Soini Y: p53 and c-erbB-2 protein expression in adenocarcinomas
and epithelial dysplasias of the gall bladder. J Pathol. 170:67–72.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Itoi T, Watanabe H, Yoshida M, Ajioka Y,
Nishikura K and Saito T: Correlation of p53 protein expression with
gene mutation in gall-bladder carcinomas. Pathol Int. 47:525–530.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Oohashi Y, Watanabe H, Ajioka Y and
Hatakeyama K: p53 immunostaining distinguishes malignant from
benign lesions of the gall-bladder. Pathol Int. 45:58–65. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Shrestha ML, Miyake H, Kikutsuji T and
Tashiro S: Prognostic significance of Ki-67 and p53 antigen
expression in carcinomas of bile duct and gallbladder. J Med
Invest. 45:95–102. 1998.PubMed/NCBI
|
|
42.
|
Kohya N and Miyazaki K: Hepatectomy of
segment 4a and 5 combined with extra-hepatic bile duct resection
for T2 and T3 gallbladder carcinoma. J Surg Oncol. 97:498–502.
2008. View Article : Google Scholar : PubMed/NCBI
|