Introduction
Locally advanced breast cancer (LABC) is defined as
patients who present with the clinical stages of T4 N0-1M0 and T3N1
(stage IIIa), T0-3 N2M0 (stage IIIb), and T0-4 N3M0 (stage IIIc),
without distant metastasis. T and N staging were as per the 6th
edition of the American Joint Committee on Cancer, the TNM staging
classification and revision of the American Joint Committee on
Cancer staging system for breast cancer (1). LABC is characterized by large breast
tumors involving the skin or muscles of the chest wall and
extensive involvement of the local lymph nodes. Therefore treatment
involves combined modality including systemic chemotherapy, surgery
and radiotherapy (2,3).
Good local control and down-staging increase the
possibility for surgery. Intra-arterial infusion is suggested to
take advantage of the first pass effect of chemotherapeutics,
generating higher local drug concentrations at the tumor cell
membrane and therefore enhancing cellular drug uptake. Drug
exposure to the tumor starts at the time of drug uptake through the
cell membrane. Studies have focused on intra-arterial infusion
chemotherapy for LABC and results have demonstrated high local
control (4–9). However, the main complications of
intra-arterial infusion chemotherapy have yet to be reported. In
the present study, we report on the results and main complications
of intra-arterial infusion chemotherapy through the subclavian and
thoracic arteries for 53 LABC cases for the period December, 2006
to December, 2010. The results demonstrated the efficacy of good
local control for LABC and revealed serious complications with
intra-arterial infusion chemotherapy.
Materials and methods
Subjects
The patients with LABC were recruited from the
Department of Breast and Endocrine Surgery, The First Affiliated
Hospital of Chongqing Medical University, between December, 2006
and December, 2010. Clinical stage IIIa was identified in 10
patients, stage IIIb in 24 patients and stage IIIc in 19 patients.
The patients were female with a mean age of 47.7 years (range,
28–67 years). Histological examination confirmed carcinoma of the
breast (left side in 30 and right side in 23) by pathological
diagnosis via fine needle biopsy pre-operative intra-arterial
infusion chemotherapy. The findings of the chest X-ray, ventral
ultrasonography, radioisotope scan and head nuclear magnetic
resonance did not provide evidence of distant metastasis. This
study was approved by the Ethics Committee of Chongqing Medical
University. All patients provided written consent.
Treatment
The patients were treated with angiography (Siemens
Coroskop Plus, Germany) under local anesthesia. According to the
Seldinger technique, a 6F catheter was inserted percutaneously into
the femoral artery. Subsequently, the catheter was guided to the
opening of the subclavian artery. An arteriogram of the subclavian,
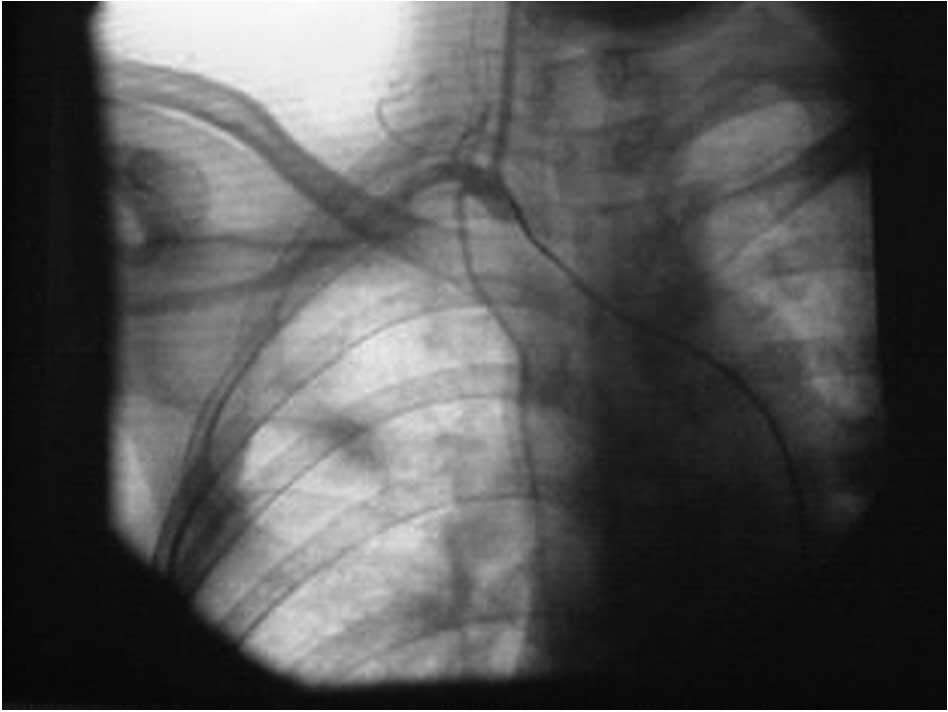
thoracic and vertebral arteries was obtained for the patients
(Fig. 1). For patients in stage
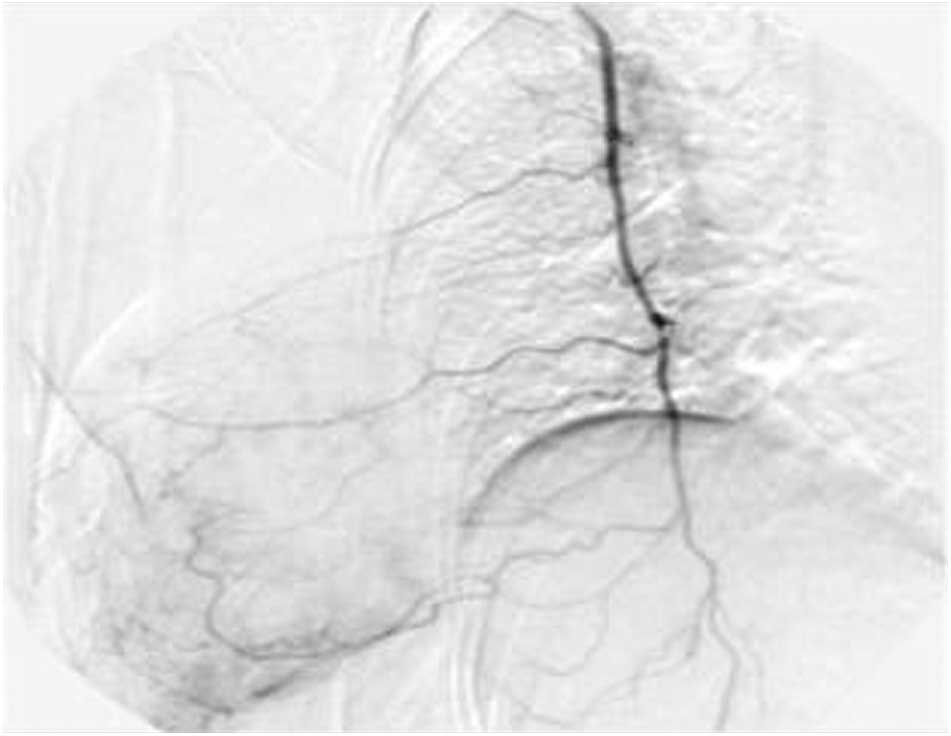
IIIa, the catheter was inserted into the thoracic artery (Fig. 2). For patients in stage IIIb and
IIIc, the catheter was guided to the opening of the subclavian and
lateral thoracic arteries (Fig.
1). Prior to drug administration, contrast agent (iohexol) was
injected to comfirm the location of the catheter again and the
ipsilateral upper arm was bundled with a tourniquet, at a pressure
of 260–280 mmHg.
Drug doses administered included taxotere (100
mg/m2), epidoxorubicin (100 mg/m2),
5-fluorouracil (1,000 mg) and/or cyclophosphamide (800
mg/m2). The tumor response, local lymph nodes and
occurrence of local or systemic complications determined the number
of cycles of chemotherapy for which there was an interval time of
21 days between two cycles.
Response criteria
Based on the WHO criteria the response was estimated
according to the clinical features following treatment and all the
patients were evaluated by computerised tomography scan
preoperation. Complete disappearance of all the lesions was
considered a complete response (CR); macroscopic reduction in size
by ≥50% was considered a partial response (PR); a reduction of
25–50% was designated as stable disease (SD); and the appearance of
any new lesions not previously identified or an estimated increase
of 25% in existent lesions was considered progressive disease.
Results
Of the 53 patients, 7 cases (13.2%) were CR; 41
cases (77.4%) were PR, with a rate of effectiveness of (CR + PR:
90.6%, 48/53); 5 cases (9.4%) were SD and no case was progressive.
The treatment results of LABC are provided in Table I.
 | Table I.Patient characteristics, treatment,
response and complications. |
Table I.
Patient characteristics, treatment,
response and complications.
| No. | Age (years) | Stage | Location | Cycles | Drugs | Response | Complication |
|---|
| 1 | 32 | IIIb | Left | 2 | EPI | PR | No |
| 2 | 28 | IIIb | Right | 1 | EPI+CTX+5-FU | SD | Inappetence |
| 3 | 53 | IIIb | Right | 1 | EPI+CTX+5-FU | PR | No |
| 4 | 67 | IIIb | Left | 1 | EPI | PR | Pain of upper
extremity |
| 5 | 65 | IIIb | Left | 3 | EPI+CTX+5-FU | CR | Inappetence |
| 6 | 52 | IIIb | Right | 1 | EPI+5-FU | PR | No |
| 7 | 51 | IIIa | Left | 1 | EPI+CTX+5-FU | PR | No |
| 8 | 47 | IIIa | Right | 1 | EPI+CTX+5-FU | PR | No |
| 9 | 28 | IIIa | Left | 2 | T | PR | Hematological
toxicity over grade 3 |
| 10 | 32 | IIIb | Left | 4 | T | CR | No |
| 11 | 57 | IIIa | Right | 1 | EPI+CTX+5-FU | SD | Pain of upper
extremity |
| 12 | 67 | IIIc | Left | 4 | T | CR | Hematological
toxicity over grade 3 |
| 13 | 31 | IIIa | Left | 1 | EPI+CTX+5-FU | PR | No |
| 14 | 34 | IIIc | Right | 1 | EPI+CTX+5-FU | PR | No |
| 15 | 51 | IIIa | Left | 1 | T | PR | No |
| 16 | 66 | IIIc | Right | 1 | T | PR | Pain of upper
extremity |
| 17 | 56 | IIIc | Right | 2 | T | PR | Necrosis of local
skin |
| 18 | 41 | IIIa | Left | 1 | T | PR | No |
| 19 | 46 | IIIc | Right | 2 | EPI+CTX+5-FU | PR | No |
| 20 | 39 | IIIc | Left | 2 | T | PR | No |
| 21 | 41 | IIIb | Left | 2 | EPI+CTX+5-FU | PR | No |
| 22 | 45 | IIIa | Left | 1 | EPI+CTX+5-FU | PR | Pain of upper
extremity |
| 23 | 50 | IIIb | Right | 1 | T | PR | Pain of upper
extremity; nausea |
| 24 | 50 | IIIb | Left | 2 | EPI+CTX+5-FU | PR | No |
| 25 | 50 | IIIc | Left | 1 | EPI+CTX+T | PR | No |
| 26 | 49 | IIIb | Left | 2 | EPI+CTX+T | CR | Pain of upper
extremity |
| 27 | 48 | IIIb | Left | 2 | EPI+CTX+T | PR | Diarrhea |
| 28 | 43 | IIIb | Right | 1 | EPI+CTX+5-FU | PR | No |
| 29 | 36 | IIIc | Left | 1 | EPI+CTX+5-FU | PR | Nausea |
| 30 | 36 | IIIb | Right | 2 | EPI+CTX+T | PR | Hematological
toxicity over grade 3 |
| 31 | 54 | IIIb | Left | 1 | EPI+CTX+T | PR | No |
| 32 | 60 | IIIc | Right | 2 | EPI+CTX+T | CR | Pain of upper
extremity |
| 33 | 66 | IIIc | Right | 1 | EPI+CTX+T | PR | Diarrhea |
| 34 | 53 | IIIc | Left | 1 | EPI+CTX+T | PR | No |
| 35 | 42 | IIIc | Right | 1 | EPI+CTX+T | PR | No |
| 36 | 42 | IIIb | Right | 1 | EPI+CTX+T | PR | Stomachache |
| 37 | 42 | IIIa | Right | 2 | EPI+CTX+T | PR | No |
| 38 | 47 | IIIb | Left | 2 | EPI+CTX+5-FU | SD | No |
| 39 | 45 | IIIb | Right | 3 | EPI+CTX+T | CR | Ipsilateral upper
extremity atrophy |
| 40 | 29 | IIIb | Right | 2 | EPI+CTX+T | PR | Hematological
toxicity over grade 3 |
| 41 | 53 | IIIc | Left | 2 | EPI+CTX+T | PR | Nausea |
| 42 | 29 | IIIb | Right | 2 | EPI+CTX+T | PR | No |
| 43 | 60 | IIIc | Left | 1 | EPI+CTX+T | PR | No |
| 44 | 61 | IIIc | Left | 3 | EPI+CTX+T | PR | Hematological
toxicity over grade 3 |
| 45 | 60 | IIIc | Left | 1 | EPI+CTX+5-FU | SD | No |
| 46 | 52 | IIIb | Left | 2 | EPI+CTX+5-FU | PR | Pain of neck and
headache |
| 47 | 47 | IIIb | Left | 1 | EPI+CTX+5-FU | SD | No |
| 48 | 61 | IIIc | Left | 2 | EPI+CTX+T | PR | No |
| 49 | 62 | IIIc | Right | 1 | T | PR | No |
| 50 | 54 | IIIb | Right | 1 | EPI+CTX+T | PR | No |
| 51 | 42 | IIIb | Right | 2 | EPI+CTX+T | PR | Hematological
toxicity over grade 3 |
| 52 | 55 | IIIa | Left | 2 | EPI+CTX+T | CR | Vomiting |
| 53 | 60 | IIIb | Left | 1 | EPI+CTX+T | PR | Ipsilateral upper
extremity atrophy |
Main complications
Complications occurred mainly in the local areas.
Pain of the ipsilateral upper extremity was noted in 7 cases. One
case experienced neck pain and headache; this patient recovered 2
weeks later without any special treatment. Two cases had
ipsilateral upper extremity atrophy and disability and did not
recover within a time period of 6 months, following drug
administration from the opening of the subclavian and lateral
thoracic arteries. One case had necrosis of local skin, but
recovered with conservative treatment. The systemic toxicity was
mild and did not affect the quality of life of patients (Table I).
Other complications
Of the 53 patients, no complications related to the
angiographic technique were observed. Hematological toxicity over
grade 3 such as fever and without bleeding was observed in 6 cases
(11.3%), while 9 patients had gastrointestinal symptoms including
nausea, vomiting, diarrhea and stomachache. Cardiovascular toxicity
was not observed (Table I).
Discussion
At present, a combination of systemic therapy with
locoregional treatment (surgery and/or radiotherapy) constitutes
the standard of care in LABC patients since improving locoregional
control is associated with improved survival (10). In patients with stage III breast
cancer treated with induction chemotherapy followed by surgery,
radiotherapy or combination therapy, the risk of locoregional
recurrence is at a range of 20% (9). The use of induction systemic therapy
results in tumor downstaging and in selected LABC patients even
allows for breast conserving surgery (11–14).
Intra-arterial infusion chemotherapy is an effective
and safe treatment for the local tumor control of LABC (7). In our data, which also demonstrated
the good local control of LABC with intra-arterial infusion
chemotherapy, the CR + PR was 90.6%, which was higher than that
reported by Shimamoto et al (8), who noted that the local response rate
was 77.3% (at least more than two regimens) and Pacetti et
al (7) who noted that the
response rate was 80%. Factors such as the drugs used and
administration of chemotheraputic cycles likely affected the
results of those authors. However, in the present study, treatment
involved different methods of drug administration. For patients in
stage IIIa, large breast tumors and the skin or muscles of the
chest wall were usually involved, thus local control tumors were
primary tumors. We inserted a catheter into the thoracic artery and
administered the majority of the drugs into the chest area, as
there would be more effective local control and downstaging. For
patients in stage IIIb and IIIc, the local lymph nodes were usually
extensively involved. Control of the regional lymph nodes was
considered crucial, therefore, the catheter was guided to the
opening of the subclavian and lateral thoracic arteries, allowing
more drugs to be administered in the subclavian, superclavian and
axilla regions These methods contributed to improving the response
rate of local lesions and increased the possibility of surgery.
However, results of the follow-up revealed that overall and
disease-free survival had not improved.
Few studies have reported on local complications
following intra-arterial infusion chemotherapy for LABC. In the
present study, severe complications were observed during treatment.
Two patients in stage IIIb had ipsilateral upper extremity atrophy
leading to disability, and these patients did not recover within a
6-month time period. Pain of the ipsilateral upper extremity was
noted in 7 cases that recovered two weeks later without any special
treatment. The reasons for these complications included the
loosening of the tourniquet during drug administration, which
caused the drugs to flow into the ipsilateral upper extremity,
and/or rapid drug administration. One patient experienced neck pain
and headache, but recovered without any special treatment. The
reason for the symptoms involved drugs flowing into the vertebral
artery.
Previous studies have reported toxicity with
systemic chemotherapy and hematological toxicity over grade 3 in
4–65% of patients (15,16). Of the 53 patients included in the
present study, no complications associated with the angiographic
technique were observed. Hematological toxicity over grade 3 such
as fever and without bleeding was observed in 6 cases (11.3%). The
patients were treated with human granulocyte colony-stimulating
factor (human GCSF) and recovered. Nine patients had
gastrointestinal symptoms including nausea, vomiting, diarrhea and
stomachache. The patients were administered timely symptomatic
treatment and recovered. Cardiovascular toxicity was not
observed.
In conclusion, intra-arterial infusion chemotherapy
is an effective treatment for local tumor control and tumor
downstaging of LABC, thereby increasing the possibility for
surgery. Low systemic toxicity and good patient compliance are also
beneficial. However, severe complications may occur during
treatment. Thus, controlling the pressure of the tourniquet and
velocity of drug administration are crucial for reducing local
complications.
References
|
1.
|
Singletary SE, Allred C, Ashley P, et al:
Revision of the American Joint Committee on Cancer staging system
for breast cancer. J Clin Oncol. 20:3628–3636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kaufmann M, von Minckwitz G, Bear HD, et
al: Recommendations from an international expert panel on the use
of neoadjuvant (primary) systemic treatment of operable breast
cancer: new perspectives 2006. Ann Oncol. 18:1927–1934. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Olson JE, Neuberg D, Pandya KJ, et al: The
role of radiotherapy in the management of operable locally advanced
breast carcinoma: results of a randomized trial by the Eastern
Cooperative Oncology Group. Cancer. 79:1138–1149. 1997. View Article : Google Scholar
|
|
4.
|
Murakami M, Kuroda Y, Nishimura S, et al:
Intraarterial infusion chemotherapy and radiotherapy with or
without surgery for patients with locally advanced or recurrent
breast cancer. Am J Clin Oncol. 24:185–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kitagawa K, Yamakado K, Nakatsuka A, et
al: Preoperative transcatheter arterial infusion chemotherapy for
locally advanced breast cancer (stageIIIb) for down-staging and
increase of respectability. Eur J Radiol. 43:31–36. 2002.
View Article : Google Scholar
|
|
6.
|
Fiorentini G, Tsetis D, Bernardeschi P, et
al: First-line intra-arterial chemotherapy (IAC) with epirubicin
and mitoxantrone in locally advanced breast cancer. Anticancer Res.
23:4339–4345. 2003.PubMed/NCBI
|
|
7.
|
Pacetti P, Mambrini A, Paolucci R, et al:
Intra-arterial chemotherapy: a safe treatment for elderly patients
with locally advanced breast cancer. In Vivo. 20:761–764.
2006.PubMed/NCBI
|
|
8.
|
Shimamoto H, Takizawa K, Ogawa Y, et al:
Clinical efficacy and value of redistributed subclavian arterial
infusion chemotherapy for locally advanced breast cancer. Jpn J
Radiol. 29:236–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shenkier T, Weir L, Levine M, et al:
Steering committee on clinical practice guidelines for the care and
treatment of breast cancer. Clinical practice guidelines for the
care and treatment of breast cancer: 15. Treatment for women with
stage III or locally advanced breast cancer. CMAJ. 170:983–994.
2004. View Article : Google Scholar
|
|
10.
|
Huang EH, Tucker SL, Strom EA, et al:
Predictors of locoregional recurrence in patients with locally
advanced breast cancer treated with neoadjuvant chemotherapy,
mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys.
62:351–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fisher B, Bryant J, Wolmark N, et al:
Effect of preoperative chemotherapy on the outcome of women with
operable breast cancer. J Clin Oncol. 16:2672–2685. 1998.PubMed/NCBI
|
|
12.
|
Wolmark N, Wang J, Mamounas E, et al:
Preoperative chemotherapy in patients with operable breast cancer:
nine-year results from National surgical adjuvant breast and bowel
project B-18. J Natl Cancer Inst Monogr. 30:96–102. 2001.PubMed/NCBI
|
|
13.
|
van der Hage JA, van de Velde CJ, Julien
JP, et al: Preoperative chemotherapy in primary operable breast
cancer: results from the European organization for Research and
Treatment of Cancer trial 10902. J Clin Oncol. 19:4224–4237.
2001.
|
|
14.
|
Mauriac L, MacGrogan G, Avril A, et al:
Neoadjuvant chemotherapy for operable breast carcinoma larger than
3 cm: a unicentre randomized trial with a 124-month medial
follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann
Oncol. 10:47–52. 1999.PubMed/NCBI
|
|
15.
|
Eniu A, Palmieri FM and Perez EA: Weekly
administration of docetaxel and paclitaxel in metastatic or
advanced breast cancer. Oncologist. 10:665–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Heller W, Mazhar D, Ward R, et al:
Neoadjuvant 5-fluorouracil epirubicin and cyclophosphamide
chemotherapy followed by docetaxel in refractory patients with
locally advanced breast cancer. Oncol Rep. 17:253–259. 2007.
|