Introduction
The prediction of the host response to an
administered therapy by means of a serum biomarker may offer a
useful and convenient prognostic or predictive factor in the
planning of cancer treatment. Follézou and Bizon (1) reported an increase in serum iron
levels following administration of various anticancer drugs,
including 5-FU, actinomycin D, adriamycin and cyclophosphamide.
Recently, we also reported a significant increase in serum iron
levels during therapy with leucovorin and fluorouracil plus
oxaliplatin (FOLFOX) or leucovorin and fluorouracil plus irinotecan
(FOLFIRI). Moreover, the levels of aspartate aminotransferase,
alanine aminotransferase and hemoglobin were unaffected and the
levels of transferrin and ferritin were only minimally altered
during chemotherapy, while a molecularly-targeted drug exerted no
effect on serum iron levels (2).
The aim of this study was to investigate the
correlation between serum iron levels and prognosis in advanced
colorectal cancer (CRC) patients treated with FOLFOX/FOLFIRI ±
molecularly-targeted drugs, in order to establish their potential
as a new biomarker.
Patients and methods
Patients
Seventy-two patients with unresectable advanced or
metastatic CRC were enrolled in this study. Treatments based on the
Japanese Society for Cancer of the Colon and Rectum guidelines were
administered to all the patients at our institution (3). Patients were treated with FOLFOX or
FOLFIRI therapy alone or in combination with molecularly-targeted
drugs (bevacizumab/cetuximab/panitumumab). All patients succumbed
to their disease between December, 2005 and February, 2012. No
patients received radiotherapy. Informed consent for the
measurement of serum iron levels was obtained from the patients.
Approval for this study was obtained from the Tobu Chiiki Hospital
Institutional Review Board (no. 12.09.10. no. 2).
Serum iron levels
Serum iron levels were measured as part of routine
blood analysis at our hospital laboratory prior to and 48 h after
chemotherapy, to determine whether an adverse reaction had
occurred. The normal range of serum iron levels was established as
60–210 μg/dl for men and 50–170 μg/dl for women. Changes in serum
iron levels during chemotherapy were assessed. Taking the median
rate of increase in serum iron levels as the cut-off value in each
therapy, the patients were divided into two cohorts: cohort I
(increase rate greater than the cut-off value in at least one
therapy) or cohort II (increase rate less than the cut-off value in
all therapies). Prognosis was prospectively evaluated and compared
between the two cohorts.
Statistical analysis
Patient characteristics were compared between the
two cohorts using the χ2 test (gender, number of
patients treated with molecularly-targeted drugs, Dukes’ stage,
histological type, primary tumor site and recurrence type) and the
t-test (age and frequency of chemotherapy). The median survival
time (MST) by cut-off value of serum iron levels was calculated by
the Kaplan-Meier method. The overall survival (OS) curves of the
two cohorts as determined by the cut-off value were compared by the
log-rank test. The Cox proportional hazards regression analysis was
used in the univariate and multivariate analyses of prognostic
factors for OS. P<0.05 was considered to indicate a
statistically significant difference. Data were expressed as the
means ± SD and were analyzed using SPSS for Windows version 15
(SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and serum iron
levels
The patient characteristics are shown in Table I. The typical pattern of change in
the serum iron levels prior to and following each chemotherapy
regimen is shown in Fig. 1. The
serum iron levels were transiently elevated following treatment,
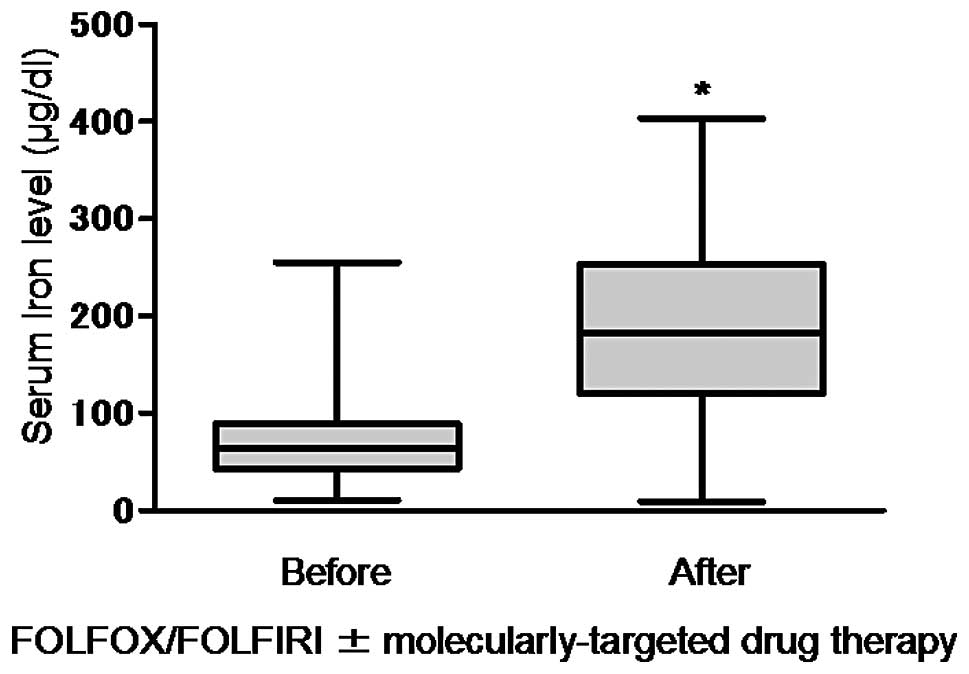
returning to baseline within 2 weeks. The serum iron level was
68.16±32.46 μg/dl prior to treatment, increasing significantly to
185.87±80.11 μg/dl following treatment (1,454 blood samples,
P<0.001, Fig. 2). The median
increase rate in the serum iron levels (cut-off value) is shown in
Table II. No significant bias in
patient characteristics was observed between cohorts I (n=44) and
II (n=28) (Table III).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | Value |
|---|
| No. of patients | 72 |
| Age in years [mean,
(range)] | 70.1 (45–84) |
| Gender
(male/female) | 41/31 |
| Histological
type |
| Adenocarcinoma |
|
Well-differentiated | 8 |
|
Moderately-differentiated | 50 |
|
Poorly-differentiated | 4 |
| Mucinous
carcinoma | 8 |
| Unknown | 2 |
| Primary cancer
site |
| Colon/rectum | 54/18 |
| Dukes’ stage
(A/B/C/D) | 1/6/35/30 |
| Recurrence type |
| Lymph node | 4 |
| Liver | 33 |
| Local
recurrence | 3 |
| Bone | 2 |
| Mediastinum | 1 |
| Lung | 12 |
| Unresectable | 7 |
| Peritoneum | 9 |
| Ovary | 1 |
| Molecularly-targeted
drug (+/−) | 29/43 |
| Frequency of
chemotherapy (range) | 21.5 (1–73) |
 | Table IIMedian increase rate in serum iron
levels (cut-off values). |
Table II
Median increase rate in serum iron
levels (cut-off values).
| Parameters | FOLFOX4 (n=96) | mFOLFOX6 (n=4) | FOLFIRI (n=69) |
|---|
| Alone | 214.2% (n=65) | 577.3% (n=2) | 344.3% (n=41) |
| Molecularly-targeted
drug | 190.2% (n=31) | 501.6% (n=2) | 395.3% (n=28) |
 | Table IIIPatient characteristics. |
Table III
Patient characteristics.
| Variables | Cohort I | Cohort II | P-value |
|---|
| No. of
patients | 44 | 28 | |
| Age in years [mean,
(range)] | 71.8 (53–84) | 67.5 (45–81) | 0.062 |
| Gender
(male/female) | 27/17 | 14/14 | 0.464 |
| Histological
type | | | 0.181 |
|
Adenocarcinoma |
|
Well-differentiated | 4 | 4 | |
|
Moderately-differentiated | 30 | 20 | |
|
Poorly-differentiated | 3 | 1 | |
| Mucinous
carcinoma | 7 | 1 | |
| Unknown | 0 | 2 | |
| Primary cancer
site | | | |
| Colon/rectum | 34/10 | 20/8 | 0.463 |
| Dukes’ stage
(A/B/C/D) | 1/3/24/16 | 0/3/11/14 | 0.470 |
| Recurrence
type | | | 0.096 |
| Lymph node | 3 | 1 | |
| Liver | 19 | 14 | |
| Local
recurrence | 3 | 0 | |
| Bone | 0 | 2 | |
| Mediastinum | 0 | 1 | |
| Lung | 10 | 2 | |
| Unresectable | 2 | 5 | |
| Peritoneum | 6 | 3 | |
| Ovary | 1 | 0 | |
|
Molecularly-targeted drug (+/−) | 21/23 | 8/20 | 0.141 |
| Frequency of
chemotherapy (range) | 23.6 (1–73) | 18.3 (1–41) | 0.113 |
Prognosis
The MST in cohorts I (n=44) and II (n=28) was 430
and 377 days, respectively. The MST was significantly higher in
cohort I (P=0.0382) (Fig. 3). The
results of univariate analysis are shown in Table IV. A significant difference was
observed in the serum iron levels. Multivariate analysis identified
a small increase in the serum iron levels as an independent risk
factor for OS (Table V).
 | Table IVUnivariate analysis of overall
survival. |
Table IV
Univariate analysis of overall
survival.
| Variables | No. of
patients | Hazard ratio | 95% CI | P-value |
|---|
| Age (years) | | 0.964 | 0.574–1.620 | 0.891 |
| <75 | 51 | | | |
| 75≤ | 21 | | | |
| Gender | | 0.622 | 0.378–1.022 | 0.061 |
| Male | 42 | | | |
| Female | 30 | | | |
| Histological
type | | 0.657 | 0.364–1.188 | 0.164 |
|
Differentiated | 58 | | | |
|
Undifferentiated/unknown | 14 | | | |
| Primary site | | 0.720 | 0.417–1.242 | 0.238 |
| Colon | 54 | | | |
| Rectum | 18 | | | |
| Dukes’ stage | | 1.236 | 0.764–1.999 | 0.388 |
| A/B/C | 42 | | | |
| D | 30 | | | |
| Recurrence
type | | 1.767 | 0.894–3.494 | 0.102 |
| Local
recurrence/Unresectable | 10 | | | |
| Metastasis | 62 | | | |
| Serum iron
level | | 1.686 | 1.023–2.779 | 0.040 |
| Cohort I | 44 | | | |
| Cohort II | 28 | | | |
 | Table VMultivariate analysis of overall
survival. |
Table V
Multivariate analysis of overall
survival.
| Variables | No. of
patients | Hazard ratio | 95% CI | P-value |
|---|
| Age (years) | | 1.216 | 0.664–2.229 | 0.526 |
| <75 | 51 | | | |
| 75≤ | 21 | | | |
| Gender | | 0.561 | 0.297–1.061 | 0.075 |
| Male | 42 | | | |
| Female | 30 | | | |
| Histological
type | | 0.589 | 0.285–1.219 | 0.154 |
|
Differentiated | 58 | | | |
|
Undifferentiated/unknown | 14 | | | |
| Primary site | | 0.731 | 0.389–1.375 | 0.331 |
| Colon | 54 | | | |
| Rectum | 18 | | | |
| Dukes’ stage | | 1.333 | 0.789–2.250 | 0.283 |
| A/B/C | 42 | | | |
| D | 30 | | | |
| Recurrence
type | | 2.096 | 0.960–4.575 | 0.063 |
| Local
recurrence/unresectable | 10 | | | |
| Metastasis | 62 | | | |
| Serum iron
level | | 1.961 | 1.143–3.365 | 0.015 |
| Cohort I | 44 | | | |
| Cohort II | 28 | | | |
Discussion
CRC is the third most common type of cancer
worldwide and the fourth most common cause of cancer-related
mortality (4). The OS rate in
advanced CRC patients has increased over the past decade as a
result of advances in chemotherapy. An increase in serum iron
levels with the administration of various anticancer drugs was
first reported several decades ago. We recently reported a
significant increase in serum iron levels during FOLFOX or FOLFIRI
therapy (2). In the present study,
serum iron levels were investigated as a potential new biomarker of
prognosis in chemotherapy.
Biomarkers play an important role in cancer
diagnosis, prognosis, treatment and monitoring. Several biomarkers
have been investigated with the development of new molecular
biological techniques and advances in cancer biology. Preoperative
increases in the serum levels of carcinoembryonic antigen,
C-reactive protein (CRP), pro-inflammatory cytokine interleukin-6
(IL-6) and other markers have been reported to provide prognostic
information (5–16). Prognostic factors for human CRC
have been the focus of extensive investigation (17–19).
Serum biomarkers have attracted attention as they offer a minimally
invasive and convenient tool for determining prognosis. Serum iron
levels are determined during the course of routine blood analysis.
Therefore, they are a potential easy-to-use biomarker for
chemotherapy in advanced CRC patients.
Iron is essential to all human cells, playing an
important role in numerous biological processes, such as electron
and oxygen transport and DNA synthesis (20,21).
However, excess iron poses a threat to cells and tissues due to its
ability to catalyze the generation of reactive radicals (22). Therefore, serum iron levels are
strictly regulated in the human body (23), mainly by the peptide hepcidin,
which is produced in the liver (24–26).
Hepcidin is a key regulator of the metabolism of iron, controlling
absorption and recycling (27,28).
Hepcidin decreases intestinal iron absorption and increases its
retention in reticuloendothelial cells (26). The target of hepcidin action is the
iron exporter ferroportin, which is mainly present in the
basolateral membranes of enterocytes and the cell membranes of
macrophages and hepatocytes (29).
Hepcidin is increased by iron loading and IL-6 and decreased by
anemia or hypoxia (27,30–33).
The majority of the iron required by the bone marrow for
erythropoiesis is provided by recycling iron from senescent red
blood cells via macrophages.
In this study, serum iron levels were transiently
elevated following chemotherapy, returning to baseline within 2
weeks. A number of factors may have contributed to this phenomenon.
Erythropoiesis, which consumes the largest amount of iron in the
body, exerting a profound effect on its distribution and
metabolism, is suppressed by chemotherapy. The subsequent reduction
in iron consumption for hemoglobin synthesis may have caused this
transient increase in serum iron levels. Vokurka et
al(34) observed an increase
in the expression of hepcidin associated with the
irradiation-induced suppression of erythropoiesis in mice.
Continuous iron absorption in the gut and its release from
macrophages is highly undesirable in situations where
erythropoiesis is suppressed. Moreover, the increase in the
expression of hepcidin was observed even in the presence of severe
anemia due to inhibition of hematopoiesis by irradiation. Hemolysis
and anemia decrease hepcidin expression only when erythropoiesis is
functional. However, if erythropoiesis is arrested, even severe
anemia does not lead to a decrease in hepcidin expression, which is
significantly increased. Hepcidin expression during chemotherapy
was not measured in the present study. However, if such an increase
in the expression of hepcidin was triggered by chemotherapy, the
underlying mechanism may be similar to that induced by
irradiation.
There were several limitations to this study. First,
the patient sample was limited. A larger patient sample may improve
data quality. Second, although serum iron levels appear to be a
biomarker for OS, the correlation between the increase in serum
iron levels and prognosis has not been fully elucidated, nor has
that between increases in CRP or IL-6 and prognosis. In addition to
cancer cells, chemotherapy suppresses erythropoiesis. If an
increase in serum iron levels is the result of suppression of
erythropoiesis, this may also indicate suppression of cancer cell
proliferation. Third, neither hepcidin as a key regulator of iron
metabolism nor IL-6 as a main inducer of hepcidin expression were
investigated in the present study. A study on a larger patient
population is currently being planned to investigate the
association of systemic iron metabolism with the clinical outcome
of chemotherapy.
In conclusion, no significant difference was
observed in the frequency of chemotherapy or the number of patients
treated with molecularly-targeted drugs between the two cohorts.
Cohort I exhibited a statistically significant improvement in
prognosis. Furthermore, the multivariate analysis revealed that the
change in serum iron levels was an independent predictive variable.
These results suggest that serum iron levels may be a useful and
convenient biomarker for OS in CRC patients.
Acknowledgements
The authors would like to thank Associate Professor
Jeremy Williams, Tokyo Dental College, for his assistance with the
English translation of this manuscript.
References
|
1
|
Follézou JY and Bizon M: Cancer
chemotherapy induces a transient increase of serum-iron level.
Neoplasma. 33:225–231. 1986.PubMed/NCBI
|
|
2
|
Mashiko S, Nagaoka I, Kitajima M, et al:
Evaluation of serum iron levels during FOLFOX4 and FOLFIRI
therapies. Exp Ther Med. 1:507–511. 2010.PubMed/NCBI
|
|
3
|
Watanabe T, Itabashi M, Shimada Y, et al:
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weitz J, Koch M, Debus J, et al:
Colorectal cancer. Lancet. 365:153–165. 2005. View Article : Google Scholar
|
|
5
|
Proctor MJ, Talwar D, Balmar SM, et al:
The relationship between the presence and site of cancer, an
inflammation-based prognostic score and biochemical parameters.
Initial results of the Glasgow Inflammation Outcome Study. Br J
Cancer. 103:870–876. 2010. View Article : Google Scholar
|
|
6
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:257–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roxburgh CS and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Romero P, Palucka AK and
Marincola FM: Tumour immunity: effector response to tumour and role
of the microenvironment. Lancet. 371:771–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McDonald B, Spicer J, Giannais B, et al:
Systemic inflammation increases cancer cell adhesion to hepatic
sinusoids by neutrophil mediated mechanisms. Int J Cancer.
125:1298–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colotta F, Allavena P, Sica A, et al:
Cancer-related inflammation, the seventh hallmark of cancer: links
to genetic instability. Carcinogenesis. 30:1073–1081. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marsik C, Kazemi-Shirazi L, Schickbauer T,
et al: C-reactive protein and all-cause mortality in a large
hospital-based cohort. Clin Chem. 54:343–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldwasser P and Feldman J: Association of
serum albumin and mortality risk. J Clin Epidemiol. 50:693–703.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsushima H, Ito N, Tamura S, et al:
Circulating transforming growth factor beta 1 as a predictor of
liver metastasis after resection in colorectal cancer. Clin Cancer
Res. 7:1258–1262. 2001.PubMed/NCBI
|
|
18
|
Toiyama Y, Fujikawa H, Kawamura M, et al:
Evaluation of CXCL10 as a novel serum marker for predicting liver
metastasis and prognosis in colorectal cancer. Int J Oncol.
40:560–566. 2012.PubMed/NCBI
|
|
19
|
Sharma R, Zucknick M, London R, et al:
Systemic inflammatory response predicts prognosis in patients with
advanced-stage colorectal cancer. Clin Colorectal Cancer.
7:331–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponka P: Cellular iron metabolism. Kidney
Int Suppl. 69:S2–S11. 1999. View Article : Google Scholar
|
|
21
|
Aisen P, Enns C and Wessling-Resnick M:
Chemistry and biology of eukaryotic iron metabolism. Int J Biochem
Cell Biol. 33:940–959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Papanikolaou G and Pantopoulos K: Iron
metabolism and toxicity. Toxicol Appl Pharmacol. 202:199–211. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knutson M and Wessling-Resnick M: Iron
metabolism in the reticuloendothelial system. Crit Rev Biochem Mol
Biol. 38:61–88. 2003. View Article : Google Scholar
|
|
24
|
Krause A, Neitz S, Magert HJ, et al:
LEAP-1, a novel highly disulfide-bonded human peptide, exhibits
antimicrobial activity. FEBS Lett. 480:147–150. 2000.PubMed/NCBI
|
|
25
|
Park CH, Valore EV, Waring AJ, et al:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kemna EH, Tjalsma H, Willems HL and
Swinkels DW: Hepcidin: from discovery to differential diagnosis.
Haematologica. 93:90–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pigeon C, Ilyin G, Courselaud B, et al: A
new mouse liver-specific gene, encoding a protein homologous to
human antimicrobial peptide hepcidin, is overexpressed during iron
overload. J Biol Chem. 276:7811–7819. 2001. View Article : Google Scholar
|
|
28
|
Nicolas G, Bennoun M, Devaux I, et al:
Lack of hepcidin gene expression and severe tissue iron overload in
upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad
Sci USA. 98:8780–8785. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nemeth E, Tuttle MS, Powelson J, et al:
Hepcidin regulates cellular iron efflux by binding to ferroportin
and inducing its internalization. Science. 306:2090–2093. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nicolas G, Chauvet C, Viatte L, et al: The
gene encoding the iron regulatory peptide hepcidin is regulated by
anemia, hypoxia, and inflammation. J Clin Invest. 110:1037–1044.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nemeth E, Valore EV, Territo M, et al:
Hepcidin, a putative mediator of anemia of inflammation, is a type
II acute-phase protein. Blood. 101:2461–2463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemeth E, Rivera S, Gabayan V, et al: IL-6
mediates hypoferremia of inflammation by inducing the synthesis of
the iron regulatory hormone hepcidin. J Clin Invest. 113:1271–1276.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pietrangelo A, Dierssen U, Valli L, et al:
STAT3 is required for IL-6-gp130-dependent activation of hepcidin
in vivo. Gastroenterology. 132:294–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vokurka M, Krijt J, Sulc K and Necas E:
Hepcidin mRNA levels in mouse liver respond to inhibition of
erythropoiesis. Physiol Res. 55:667–674. 2006.PubMed/NCBI
|