Introduction
Angiosarcoma (AS) is an aggressive, malignant
endothelial cell tumor of vascular or lymphatic origin (1). The presentation and clinical behavior
of AS may vary, depending on its location. Therefore, ASs are
considered as several closely related tumors rather than a single
entity and may be divided into several groups. Cutaneous AS (CAS)
is a rare aggressive tumor of the scalp and face of elderly
patients, which spreads widely, recurring locally with early
metastasis (2). Cases of
thrombocytopenia during tumor progression and metastasis related to
intratumor sequestration have been rarely reported (3–8). The
present study describes a case of CAS of the buttock complicated by
severe thrombocytopenia and reviews the literature regarding the
clinical behavior of this entity. The study was conducted following
a clinical research review by our Ethics Committee. The patient was
informed that data from the case would be submitted for publication
and provided the required consent.
Case report
A 56-year-old female sought medical assistance due
to a 2-month history of dark reddish eruptions and pain in her left
buttock. There was no reported history of disease or trauma. Three
discrete eruptions without edema were identified on the skin. The
surface was smooth with minor inflammation and the borders were
poorly defined. The lesions were tender to palpation. The
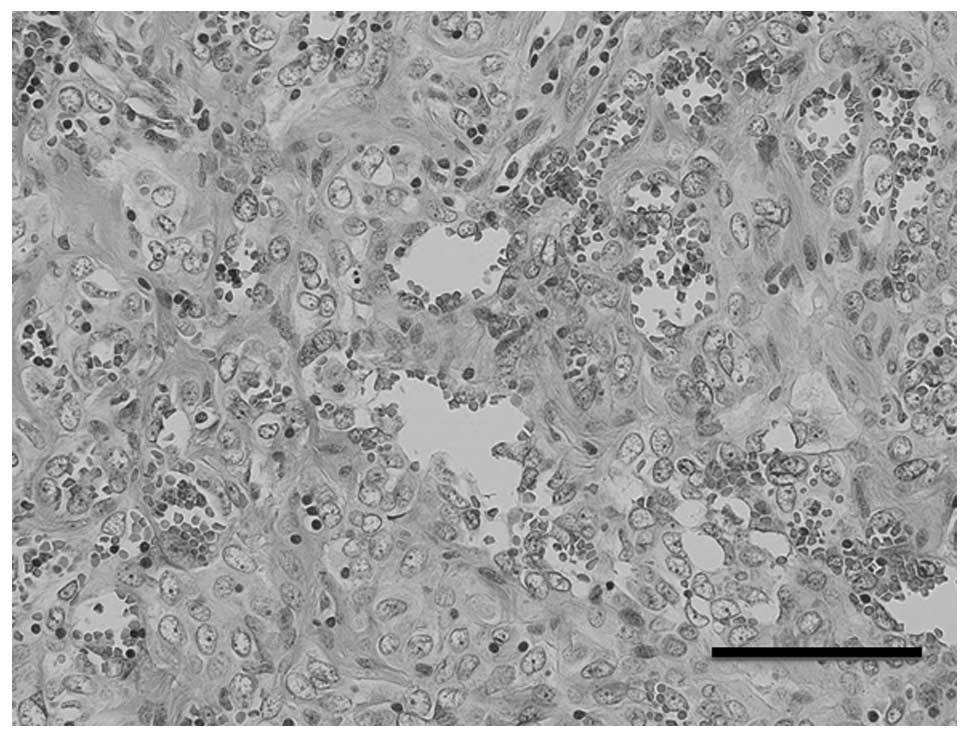
radiological and laboratory findings were normal. A biopsy was
performed and sheets of highly atypical, mitotically active,
epithelioid endothelial cells were observed under a light
microscope, with formation of irregular sinusoidal vascular
channels (Fig. 1). The lesion was
diagnosed as epithelioid hemangioendothelioma based on the
histopathological findings.
Wide surgical resection was performed; however, the
patient experienced seven local recurrences over a period of seven
years. Whenever the tumor recurred, it was surgically resected.
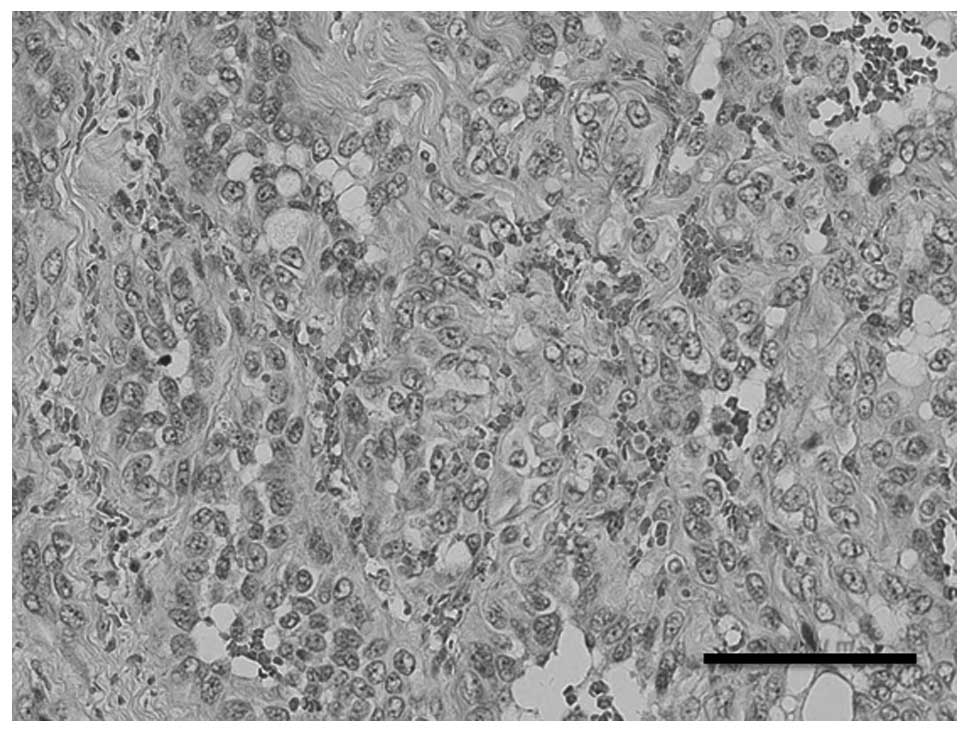
After the third resection the tumor was histopathologically
re-evaluated and the diagnosis was changed to AS (Fig. 2). Immunohistochemically, tumor
cells were positive for CD31 and negative for CD34. The specimen
was submitted for cytogenetic analysis. Six years after the onset,
the tumor had significantly enlarged. Following the seventh
surgery, radiotherapy (RT, 60 Gy) and intensive multidrug
chemotherapy [MAID (doxorubicin 30 mg/m2, ifosfamide 1.5
g/m2 and dacarbazine 300 mg/m2) × 3 days]
were administered (9). However,
one year after the RT the patient developed local recurrence,
inguinal lymph node metastasis and multiple lung metastases.
Lymphedema also developed due to femoral lymphatic vessel
obstruction. Nine years after the initial operation, lymph node
dissection and free-skin grafting were performed during the ninth
resection procedure. However, the tumor subsequently recurred
around the grafted skin and grew progressively, with secondary
necrosis and hemorrhage. The hemorrhage persisted for 2 months and
the patient developed severe anemia (5.2 g/dl) and thrombocytopenia
(52,000/μl). Despite supportive care with hemorrhage control and
transfusion of platelets and erythrocytes, anemia and
thrombocytopenia continued to progress rapidly. The patient
eventually developed disseminated intravascular coagulation and
succumbed to the disease 6 weeks after the ninth surgery (9 years
and 6 months after the initial consultation).
Cytogenetic analysis was performed on G-band by
trypsin banding using surgical specimens. Standard culture and
harvesting procedures were used, as previously described (10). The karyotypes were expressed in
accordance with the International System for Human Cytogenetic
Nomenclature (11). Twenty cells
were analyzed and the composite karyotype was
46,XX,t(12;20)(p13;p11.2)[3]/47,X,add(X)(q13),del(6)(q?),add(12)(p13),−21,+2mar[2]/45,XX,der(1)add(1)(p36.3)del(1)(q41),−20[1]/46,XX[13]
(Fig. 3).
Discussion
ASs are, collectively, one of the rarest types of
soft tissue tumors. They comprise <1% of all sarcomas. Among all
cases of AS, one third occur in the skin, one fourth in soft tissue
and the remainder in other sites. Since the clinical behavior of AS
varies depending on its location, ASs are divided into several
clinical groups. CAS without lymphedema is the most frequently
encountered type of AS. It usually develops after the age of 60
years and more commonly affects females. Due to the fact that AS
readily invades the surrounding tissues, complete resection is
difficult. The prognosis of CAS is poor, with a 5-year survival
rate of 10–34% (12–14).
Only 9 cases of CAS with thrombocytopenia, including
the present case, have been described in the English literature
thus far (3–8) (Table
I). The mean age at presentation was 72.7 years (range, 56–87
years) and it occurred more commonly among males. The tumors were
located in the scalp (5/9, 55.6%), face (2/9, 22.2%) and trunk
(2/9, 22.2%). The prognosis of CAS complicated by thrombocytopenia
is poor, even following treatment with combined chemotherapy and
RT. The average period from the initial diagnosis to the
development of thrombocytopenia was 4.1 months (range, 0–10 months)
and the average survival was 9.7 months (range, 3–36 months). Of
the 9 reported cases of CAS with thrombocytopenia (including the
present case), all the patients succumbed to the disease within 4
months after the onset of thrombocytopenia. Our patient survived
for 76 months subsequent to the initial consultation but succumbed
to the disease 2 months following tumor necrosis. These findings
suggest that local control of the tumor is critical.
 | Table IClinical characteristics of cutaneous
angiosarcoma with thrombocytopenia. |
Table I
Clinical characteristics of cutaneous
angiosarcoma with thrombocytopenia.
| Case | Age
(years)/gender | Location | Treatment | Durationa (months) | Outcome (months) | Authors (Refs.) |
|---|
| 1 | 66/F | Face | RE | 0 | DOD (3) | Arcomano et
al(3) |
| 2 | 59/M | Scalp | CT, RT | 7 | DOD (10) | Satoh et
al(4) |
| 3 | 79/M | Face | RT, CT | 3 | DOD (4) | |
| 4 | 79/M | Chest | CT | 2 | DOD (6) | |
| 5 | 69/M | Scalp | RE, RT, CT | 3 | DOD (4) | Salameh et
al(5) |
| 6 | 67/M | Scalp | CT, RT | 10 | DOD (36) | Imafuku et
al(6) |
| 7 | 76/M | Scalp | RT, CT | 7 | DOD (9) | Kluger et
al(7) |
| 8 | 87/M | Scalp | RT, CT | 1 | DOD (3) | Tan et
al(8) |
| 9 | 56/F | Buttock | RE, CT, RT | 72 | DOD (76) | Present case |
The present case describes the development of
thrombocytopenia in a patient with metastatic AS of the buttock.
The mechanism of thrombocytopenia is considered to be related to
the Kasabach-Merritt syndrome (KMS) (15). KMS has been used to describe
various tumors that broadly fit the initial description (16), such as thrombocytopenia associated
with giant hemangioendothelioma (5), intestinal angioma (17), hepatic epithelioid
hemangioendothelioma (18), occult
visceral hemangiomatosis (19) and
AS (20). KMS is defined as
consumption of platelets within tumors due to the accumulation of
blood in the tumor vasculature and platelet accretion to the
abnormal vascular endothelium (16,20).
In our case, tumor growth and recurrence led to KMS secondary to
hyperplasia of the abnormal blood vessels and continuous bleeding
due to tumor necrosis.
Only a few cases of AS have been cytogenetically
investigated (21–29). The cytogenetic findings of AS are
occasionally complex and limited. However, certain cytogenetic
changes reported in tumors from different locations revealed
similarities (Table II). The most
common changes were gain of 5pter-p11, 8p12-qter and 20pter-q12,
losses of 4p, 7p15-pter and Y and abnormalities in 22q (1). In our case, t(12;20)(p13;p11.2)
including a site of 20pter-q12 was observed.
 | Table IIChromosomal changes in
angiosarcoma. |
Table II
Chromosomal changes in
angiosarcoma.
| Case | Age
(years)/gender | Location | Karyotype (no. of
cells) | Authors (Refs.) |
|---|
| 1 | 52/F | Soft tissue |
41,XY,−3,−6,−6,+der(7)t(6;7)(p21.1;p22),−17,−17,−20[1]/45,XX,−1,+2,−22[1]/45,XX,−6[1]/45,XX,−10[1]/45,
XX,−13[1]/45,XX,−14[1]/45,XX,−16[1]/45,XX,−20[1]/45,XX,−22[1]/45,X,−X[1]/46,X,+del(1)(q21),
t(6:8)
(q22–23;q24),−X[1]/46,XXt(1;12)(p34.1–34.3;q13)[1]/46,XX,t(5;?)(p13;?)[1]/46,X,t(5;19)(q21–22;p13.3),
−X,+mar[1]/46,XX,t(10;17)(q11.2;p11.2)[1]/47,XX,t(3;21)(p21;p13),del(7)(q21),+19[1]/47,XX,
+3,t(X;10)(q28;q11.2)[1]/48,XX,+2,+7[1]/46,XX[13] | Kindblom et
al(21) |
| 2 | 34/F | Ovary |
48,XX,t(1;3)(q11;p11),+3,+12 | Fletcher et
al(22) |
| 3 | 61/F | Breast |
47,X,der(X)t(X;9)(q11;q12),t(1;3)(p13;q29),t(1;17)(p13;p13),−2,−2,del(4)(q25q31),
+der(4)del(4)(p14)t(2;4)(p13;q35),del(7)(p11),add(8)(q13),+12,der(15)t(5;15)(p11;p11),
+der(20)t(6;20)(q13;q23),der(22)t(6;22)(q15;q13) | Gil-Benso et
al(23) |
| 4 | NA | Soft tissue |
46,XX,add(1)(p36),t(1;3)(q32;q21),del(2)(p21),del(7)(p15),add(12)(q13),add(17)(q24) | Van den Berg et
al 24) |
| 5 | 67/M | Kidney |
46,XY,inv(7)(p15q11)[2]/46–47,X,−Y,del(1)(q25),add(4)(q25),add(5)(q31),+mar1[cp15]/46,XY[3] | Cerilli et
al(25) |
| 6 | 41/M | Soft tissue |
39–43,XY,der(1;11)(q10;q10),−4,t(5;15)(q11;q24),+der(8)t(8;?17)(p12;q11)add(8)(q24),
−9,−11,−13,−17,−17,−18,add(18)(p11),+del(20)(q12–13),+der(?)t(?;10)(?;q22)[cp9]/46,XY[2] | Schuborg et
al(26) |
| 7 | 69/F | Soft tissue |
45–47,XX,add(8)(q22),+add(22)(q12),inc[cp9] | |
| 8 | 75/M | Soft tissue |
46,XY,der(7)del(7)(p15)del(7)(q11q22)[3]/47,XY,der(7),+mar[2]/45,X,
−Y[13]/47,XY,+8[7]/48,XY,+X,+8[6] | |
| 9a | 71/F | Soft tissue |
73–79,XX,−X,add(1)(q11),−3,+5,t(5;14)(q13;p13),+6,+7,+8,+9,+9,
(primary)
der(9;10)(q10;q10)x2,+11,−13,+20,−21,−22,inc[cp3]/46,XX[11] | |
| 9b | 71/F | Soft tissue
(recurrence) |
77–79,XXX,add(1),−3,i(5)(q10),t(5;14),+6,+6,+7,der(9;10)x2,
+11,add(15)(p11),+16,+20,inc[2]/46,XX[p] | Wong et
al(27) |
| 10 | 34/F | Nasopharynx |
43,XX,?dup(3)(q21q29),del(4)(p11p16),−8,der(9;17)(q10;q10),−13,add(14)(q32),
−16,add(16)(q22),der(18)t(1;18)(q21;q21),−19,−20,add(22)(q13),+r,+mar1,+mar2[4]/43,
idem,
−14,+20[3]/33–43,idem,−3,−4,−5,−6,−7,−10,−11,−16,−18,−20[cp10]/61–84,XXX,?dup(3),+?dup(3),
del(4),+del(4),+5,+7,−8,der(9;17),?i(9)(q10),+10,+11,+12,+14,+15,−16,add(16)x2,−17,add(18),
add(19)(p13),−20,add(22),+add(22),+rx2,+mar1×2,+mar2×2[cp6]/46,XX[3] | |
| 11 | 29/M | Heart |
55,XY,+der(1;17)(q10;q10),+2,+7,+8,+8,+19,+20,+21,+22 | Zu et
al(28) |
| 12 | 79/F | Bone |
46,XX,t(1;14)(p21;q24)[16]/46,XX[3] | Dunlap et
al(29) |
| 13 | 56/F | Cutaneous |
46,XX,t(12;20)(p13;p11.2)[3]/47,X,add(X)(q13),del(6)(q?),add(12)(p13),−21,
+2mar[2]/45,XX,der(1)add(1)(p36.3)del(1)(q41),−20[2]/46,XX[13] | Present case |
Treatment of CAS is challenging and an optimal
treatment protocol has not yet been determined. The treatment
usually consists of surgical excision with wide safety margins and
postoperative radiation (2,30),
although radiation monotherapy appears to be insufficient. In
addition, the role of chemotherapy remains undetermined and
definitive application of adjuvant modalities has not yet been
established (2,30). Additional comprehensive studies on
the treatment of CAS are required.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Scientific Research (C) 24592227 (KAKENHI).
References
|
1
|
Weiss SW and Goldblum JR: Angiosarcoma.
Enzinger and Weiss's Soft Tissue Tumors. Weiss SW and Goldblum JR:
Mosby; St. Louis: pp. 703–720. 2007
|
|
2
|
Mendenhall WM, Mendenhall CM, Werning JW,
Reith JD and Mendenhall NP: Cutaneous angiosarcoma. Am J Clin
Oncol. 29:524–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arcomano MA, Shulkin BL, Petry NA and Wahl
RL: Metastatic angiosarcoma with thrombocytopenia and intratumoral
indium-111-platelet deposition. J Nucl Med. 32:2278–2280.
1991.PubMed/NCBI
|
|
4
|
Satoh T, Takahashi Y, Yokozeki H, Katayama
I and Nishioka K: Cutaneous angiosarcoma with thrombocytopenia. J
Am Acad Dermatol. 40:872–876. 1991. View Article : Google Scholar
|
|
5
|
Salameh F, Henig I, Bar-Shalom R and Maza
I: Metastatic angiosarcoma of the scalp causing Kasabach-Merritt
syndrome. Am J Med Sci. 333:293–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imafuku S, Hosokawa C, Moroi Y and Furue
M: Kasabach-Merritt syndrome associated with angiosarcoma of the
scalp successfully treated with chemoradiotherapy. Acta Derm
Venereol. 88:193–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kluger N, Girard C, Boissier E, Sibille L,
Mariano-Goulart D and Guillot B: Metastatic cutaneous angiosarcoma
complicated with severe thrombocytopenia. Eur J Dermatol.
20:662–663. 2010.PubMed/NCBI
|
|
8
|
Tan SM, Tay YK, Liu TT and Mancer K:
Cutaneous angiosarcoma associated with the Kasabach-Merritt
syndrome. Ann Acad Med Singapore. 39:941–942. 2010.PubMed/NCBI
|
|
9
|
Elias A, Ryan L, Sulkes A, Collins J,
Aisner J and Antman KH: Response to mesna, doxorubicin, ifosfamide,
and dacarbazine in 108 patients with metastatic or unresectable
sarcoma and no prior chemotherapy. J Clin Oncol. 7:1208–1216.
1989.PubMed/NCBI
|
|
10
|
Yasuda T, Nishio J, Sumegi J, et al:
Aberrations of 6q13 mapped to the COL12A1 locus in chondromyxoid
fibroma. Mod Pathol. 22:1499–1506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaffer LG and Tommerup N: An
International System for Human Cytogenetic Nomenclature. S Karger;
Basel: 2005
|
|
12
|
Holden CA, Spittle MF and Jones EW:
Angiosarcoma of the face and scalp, prognosis and treatment.
Cancer. 59:1046–1057. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mark RJ, Poen JC, Tran LM, Fu YS and
Juillard GF: Angiosarcoma. A report of 67 patients and a review of
the literature. Cancer. 77:2400–2406. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morgan MB, Swann M, Somach S, Eng W and
Smoller B: Cutaneous angiosarcoma: a case series with prognostic
correlation. J Am Acad Dermatol. 50:867–874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasabach H and Merritt K: Capillary
hemangioma with extensive purpura. Am J Dis Child. 59:1063–1070.
1940. View Article : Google Scholar
|
|
16
|
Hall GW: Kasabach-Merrit syndrome:
pathogenesis and management. Br J Haematol. 112:851–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhandari B, Gupta BM and Agarwal SS: Giant
haemangioendothelioma with purpura (Kasabach-Merrit syndrome). (A
case report). Indian Pediatr. 9:718–720. 1972.PubMed/NCBI
|
|
18
|
Eugene C, Fingerhut A, Quevauvilliers J,
et al: Microangiopathic anaemia with thrombocytopaenia. Cure
following excision of an angioma of the small intestine (author's
transl). Nouv Presse Med. 7:735–737. 1978.(In French).
|
|
19
|
Frider B, Bruno A, Selser J, Vanesa R,
Pascual P and Bistoletti R: Kasabach-Merrit syndrome and adult
hepatic epithelioid hemangioendothelioma an unusual association. J
Hepatol. 42:282–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schulz AS, Urban J, Gessler P, Behnisch W,
Kohne E and Heymer B: Anaemia, thrombocytopenia and coagulopathy
due to occult diffuse infantile haemangiomatosis of spleen and
pancreas. Eur J Pediatr. 158:379–383. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kindblom LG, Stenman G and Angervall L:
Morphological and cytogenetic studies of angiosarcoma in
Stewart-Treves syndrome. Virchows Arch A Pathol Anat Histopathol.
419:439–445. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fletcher JA, Kozakewich HP, Hoffer FA, et
al: Diagnostic relevance of clonal cytogenetic aberrations in
malignant soft-tissue tumors. N Engl J Med. 324:436–442. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gil-Benso R, Lopez-Gines C, Soriano P,
Almenar S, Vazquez C and Llombart-Bosch A: Cytogenetic study of
angiosarcoma of the breast. Genes Chromosomes Cancer. 10:210–212.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van den Berg E, Van Oven MW, de Jong B, et
al: Comparison of cytogenetic abnormalities and deoxyribonucleic
acid ploidy of benign, borderline malignant, and different grades
of malignant soft tissue tumors. Lab Invest. 70:307–313.
1994.PubMed/NCBI
|
|
25
|
Cerilli LA, Huffman HT and Anand A:
Primary renal angiosarcoma: a case report with immunohistochemical,
ultrastructural, and cytogenetic features and review of the
literature. Arch Pathol Lab Med. 122:929–935. 1998.PubMed/NCBI
|
|
26
|
Schuborg C, Mertens F, Rydholm A, et al:
Cytogenetic analysis of four angiosarcomas from deep and
superficial soft tissue. Cancer Genet Cytogenet. 1:52–56. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong KF, So CC, Wong N, Siu LL, Kwong YL
and Chan JK: Sinonasal angiosarcoma with marrow involvement at
presentation mimicking malignant lymphoma: cytogenetic analysis
using multiple techniques. Cancer Genet Cytogenet. 129:64–68. 2001.
View Article : Google Scholar
|
|
28
|
Zu Y, Perle MA, Yan Z, Liu J, Kumar A and
Waisman J: Chromosomal abnormalities and p53 gene mutation in a
cardiac angiosarcoma. Appl Immunohistochem Mol Morphol. 9:24–28.
2001.PubMed/NCBI
|
|
29
|
Dunlap JB, Magenis RE, Davis C, Himoe E
and Mansoor A: Cytogenetic analysis of a primary bone angiosarcoma.
Cancer Genet Cytogenet. 194:1–3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abraham JA, Hornicek FJ, Kaufman AM, et
al: Treatment and outcome of 82 patients with angiosarcoma. Ann
Surg Oncol. 14:1953–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|