Introduction
Thromboembolism is a known vascular toxicity
associated with tumor chemotherapy. The combination of pemetrexed
and carboplatin has exhibited significant antitumor activity, with
mild manageable toxicity in patients with advanced non-small-cell
lung cancer (NSCLC) (1), whereas
cerebral arterial embolism has not been recognized as a side effect
associated with this regimen. This is the case report of an unusual
case of NSCLC, in which the patient suffered a left middle cerebral
arterial embolism following administration of pemetrexed and
carboplatin.
Case report
A 62-year-old non-smoking woman was diagnosed with
stage IV adenocarcinoma of the lung in October, 2011. The patient
had no previous medical history of arrhythmia, ischemic heart
disease, diabetes mellitus or stroke and did not receive any daily
medications. Mutation analysis of lung cancer specimens obtained
prior to the administration of first-line chemotherapy revealed the
presence of an L858R point mutation of the epidermal growth factor
receptor gene. The patient was administered gefitinib (250 mg
daily) as a first-line therapy; however, progressive multiple brain
metastases were detected after 4 months of therapy. Following
whole-brain radiation therapy (WBRT) at a total dose of 30 Gy, the
patient resumed gefitinib treatment. Five months after WBRT,
positron emission tomographic and computed tomographic (CT) imaging
revealed disease progression in the liver and bone. An
echocardiogram prior to administration of second-line chemotherapy
revealed no thrombus in the heart, no atrioventricular septal
defects, no patent foramen ovale and no valvular vegetations. The
combination of pemetrexed and carboplatin was then administered as
second-line treatment.
On the day of the completion of the first regime
cycle, the patient was readmitted to the emergency department with
complaints of sudden-onset right hemiplegia and agitation.
Laboratory testing revealed grade 1 anemia (hemoglobin, 11.3 g/dl),
grade 1 thrombocytopenia (116,000/μl), a slight elevation of the
prothrombin time/international normalized ratio to 1.18 (normal
range, 0.90–1.10), a substantial increase in D-dimer levels to 19.2
μg/ml (normal range, 0–0.9 μg/ml) and fibrin degradation product
(FDP) levels of 47.8 μg/ml (normal range, 0.1–4.99 μg/ml). A
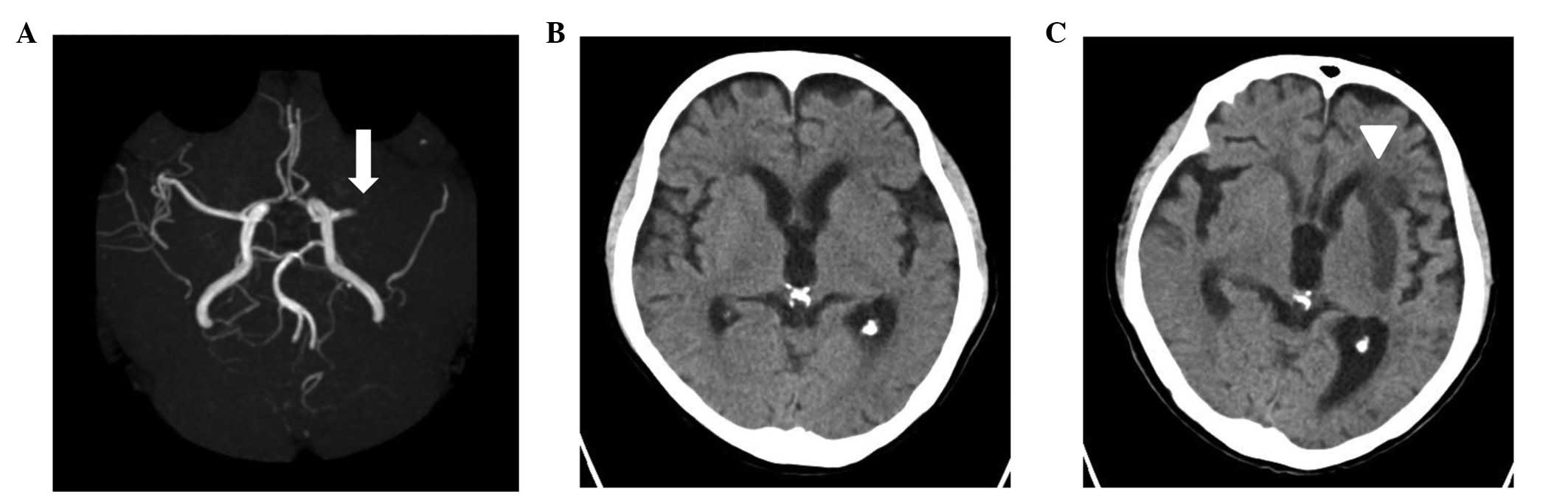
non-contrast brain CT revealed no new abnormalities (Fig. 1B); however, brain magnetic
resonance imaging and magnetic resonance angiography revealed an
occlusion of the left middle cerebral artery (MCA) (Fig. 1A). Following consultation with a
neurologist and considering the substantial risk of cerebral
hemorrhage caused by thrombolytic agents, the patient was not
administered thrombolytic therapy. Six days after the stroke, a
cranial CT scan revealed the presence of an infarction in the
supply area of the MCA (Fig. 1C).
No further chemotherapy was administered due to the deterioration
in the performance status of the patient associated with right
hemiplegia and the patient succumbed to progressive cancer 3 months
after the MCA embolism.
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Discussion
In the present case, the patient suffered an MCA
embolism on the day of completion of the first cycle of
chemotherapy with pemetrexed and carboplatin. The mechanisms
underlying the cerebrovascular event secondary to antineoplastic
agent administration are likely multifactorial, including tumor
microemboli, radiation-induced vasculopathy and
thromboembolism.
The suggested mechanism of systemic tumor
embolization from lung cancer is invasion of the pulmonary veins,
with or without invasion of the left atrium (2). Rarely, a tumor may invade the venous
circulation and spread to the left heart through a patent foramen
ovale, leading to systemic tumor embolization. The risk of tumor
embolization in the present case appeared to be low, since a chest
CT scan had revealed no evidence of pulmonary vein or left atrial
invasion by lung cancer.
Cumulative radiation-induced vascular damage in
terms of endothelial lesions may be another factor. An occlusive
vasculopathy with accelerated atherosclerosis may affect cranial
vessels within the irradiated field. Pathological studies
demonstrated that radiotherapy produces a sequence of arterial
changes characterized by initial endothelial cell damage,
thickening of the intimal layer caused by smooth muscle cell
proliferation, cell degeneration and hyaline transformation.
Intracranial occlusive vasculopathies associated with whole-brain
irradiation, gamma knife or other focused cranial radiation were
also reported (3,4).
Another possible explanation in the present case is
that thromboembolism may have accounted for the cerebrovascular
event. Platelet activation, alteration of the clotting cascade,
including hyperfibrinolysis, and disturbance of the
prostacyclin-thromboxane homeostasis, contribute towards an
increased risk of thrombosis by 4- to 6-fold in cancer patients.
compared to that in the general population (5). However, cerebral arterial embolism
has not been recognized as a side effect associated with this
regimen. Increased D-dimer and FDP levels may indicate the presence
of an abnormal clot. However, it was difficult to determine the
cause of the cerebral arterial embolism in the present case, since
an autopsy was not performed.
In conclusion, pemetrexed plus carboplatin is
routinely used for the treatment of advanced NSCLC. The present
case highlights the potential risk for the development of embolism
following pemetrexed-based chemotherapy. Future studies should be
conducted to elucidate the mechanisms through which these drugs may
eventually cause neurovascular adverse events.
References
|
1
|
Gronberg BH, Bremnes RM, Flotten O, et al:
Phase III study by the Norwegian lung cancer study group:
pemetrexed plus carboplatin compared with gemcitabine plus
carboplatin as first-line chemotherapy in advanced non-small-cell
lung cancer. J Clin Oncol. 27:3217–3224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Thenayan E and Maghfoor I:
Complications of malignancy: case 1. Systemic tumor embolism from
lung cancer at presentation. J Clin Oncol. 22:372–323. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernstein M, Lumley M, Davidson G,
Laperriere N and Leung P: Intracranial arterial occlusion
associated with high-activity iodine-125 brachytherapy for
glioblastoma. J Neurooncol. 17:253–260. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omura M, Aida N, Sekido K, Kakehi M and
Matsubara S: Large intracranial vessel occlusive vasculopathy after
radiation therapy in children: clinical features and usefulness of
magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 38:241–9.
1997. View Article : Google Scholar
|
|
5
|
Blom JW, Doggen CJ, Osanto S and Rosendaal
FR: Malignancies, prothrombotic mutations, and the risk of venous
thrombosis. JAMA. 293:715–722. 2005. View Article : Google Scholar : PubMed/NCBI
|