Introduction
The intraductal papillary neoplasm of the bile duct
(IPNB) is a novel disease concept that was recently classified as a
biliary cystic tumor by the revised World Health Organization
classification released in 2010 (1). Recently, an additional type of IPNB,
the branch duct type, was described and relevant cases have been
reported (2–5). Since IPNB is usually treated
surgically at the time of diagnosis, the natural history of the
tumor is largely unknown. This report describes a rare case of
branch duct type IPNB, in which the patient initially declined
surgery and was followed up with a tentative diagnosis of
mucus-producing hepatic tumor for 11 years, after which time she
finally consented to surgery, enabling a definitive diagnosis.
Case report
A 70-year-old female patient developed obstructive
jaundice and cholangitis in 2000, originally attributed to a
mucus-producing hepatic tumor, and was treated at a local hospital.
Surgery was advised due to the repeated episodes; however, the
patient refused and continued to receive medical treatment. In May,
2011, the patient developed jaundice and fever and was treated with
antibiotics. The symptoms did not resolve and she was admitted to
the Tokyo Rosai Hospital. The patient’s medical history included
surgery for appendicitis at the age of 50 years and cholecystectomy
for cholecystitis due to gallstones at the age of 62 years. There
was no significant family history. In addition, the patient had
developed allergic shock induced by iodinated contrast medium
during the contrast-enhanced computed tomography (CT) in 2000;
therefore, no iodinated contrast medium was used in 2011.
On admission, the patient exhibited a clear
sensorium, with a blood pressure of 123/73 mmHg, body temperature
of 37.5°C and pulse rate of 60 beats/min. The palpebral conjunctiva
was not anemic, whereas the bulbar conjunctiva was colored
yellow.
Breathing and heart sounds were clear. The abdomen
was flat and soft, with tenderness localized to the right upper
quadrant, without rebound tenderness or muscular rigidity. Blood
testing on admission indicated mild anemia, with a hemoglobin level
of 9.9 g/dl, predominantly direct hyperbilirubinemia and jaundice,
with a total bilirubin level of 4.6 mg/dl and a direct bilirubin
level of 3.8 mg/dl, and increased inflammatory reaction, with a
C-reactive protein level of 14.7 mg/dl. All tumor markers were
normal (Table I).
 | Table I.Blood laboratory findings on
admission. |
Table I.
Blood laboratory findings on
admission.
| Markers | Values |
|---|
| Biochemistry | |
| CRP | 14.7 mg/dl |
| Na | 139 mEq/l |
| K | 3.3 mEq/l |
| Cl | 99 mEq/l |
| TP | 6.3 g/dl |
| Alb | 3.0 g/dl |
| T-Bil | 4.6 mg/dl |
| D-Bil | 3.8 mg/dl |
| AST | 60 IU/l |
| ALT | 67 IU/l |
| LDH | 171 IU/l |
| ALP | 565 IU/l |
| γ-GT | 205 IU/l |
| BUN | 11 mg/dl |
| Cr | 0.6 mg/dl |
| BS | 107 mg/dl |
| PT | 77% |
| PT-INR | 1.1 |
| APTT | 29.7 sec |
| Hematology | |
| WBC | 5,200 μl |
| RBC |
353×104/μl |
| Hgb | 9.9 g/dl |
| Hct | 29.8% |
| PLT |
34.3×104/μl |
| Serological
tests | |
| HCV-Ab | (−) |
| HBs-Ag | (−) |
| HBs-Ab | (−) |
| CEA | 1.8 ng/ml |
| CA19-9 | 2.0 U/ml |
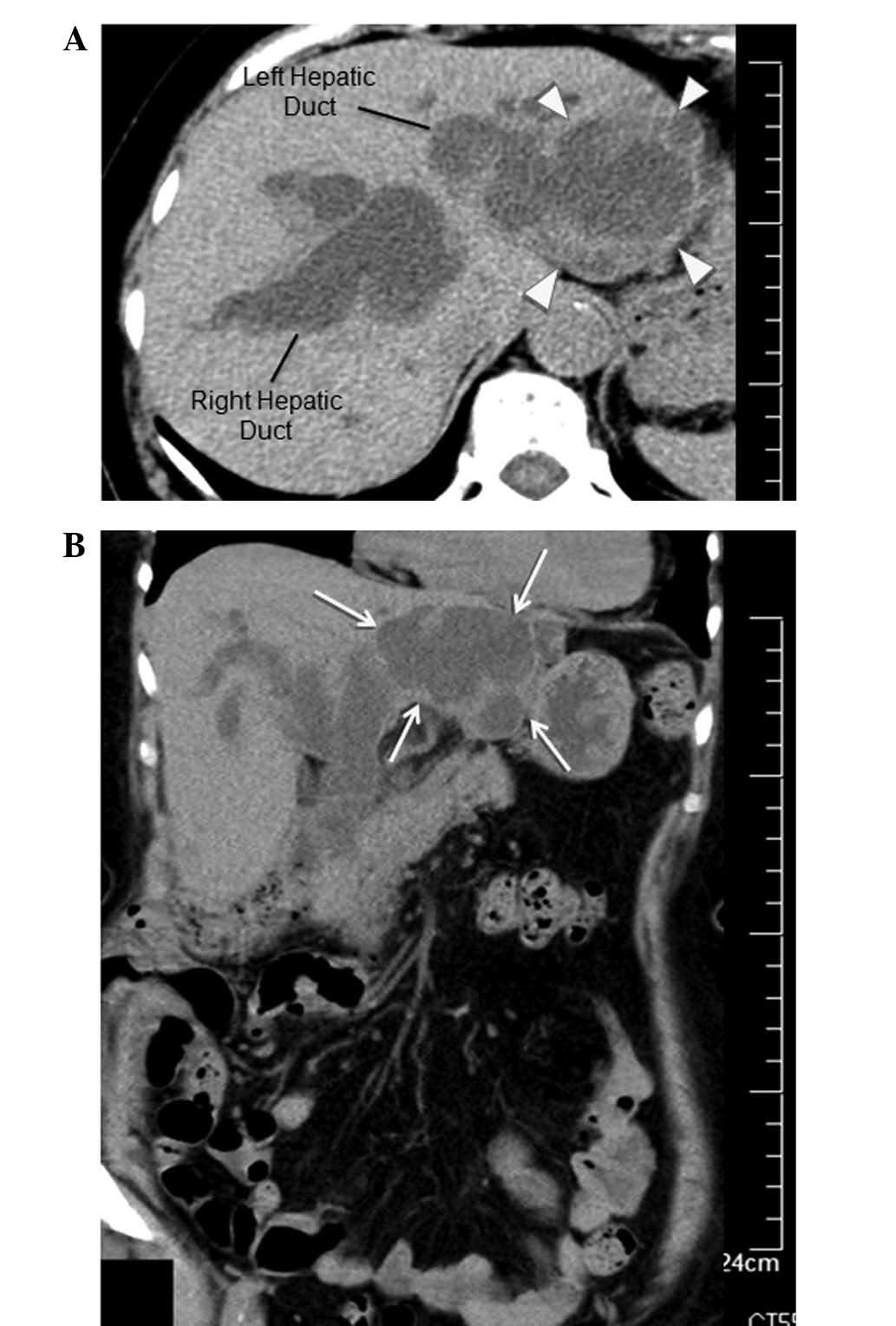
The non-contrast-enhanced CT scan performed on
hospital day 1 revealed a 50-mm cystic mass with an internal septum
in the left hepatic lobe and dilated intra- and extra-hepatic bile
ducts (Fig. 1). The
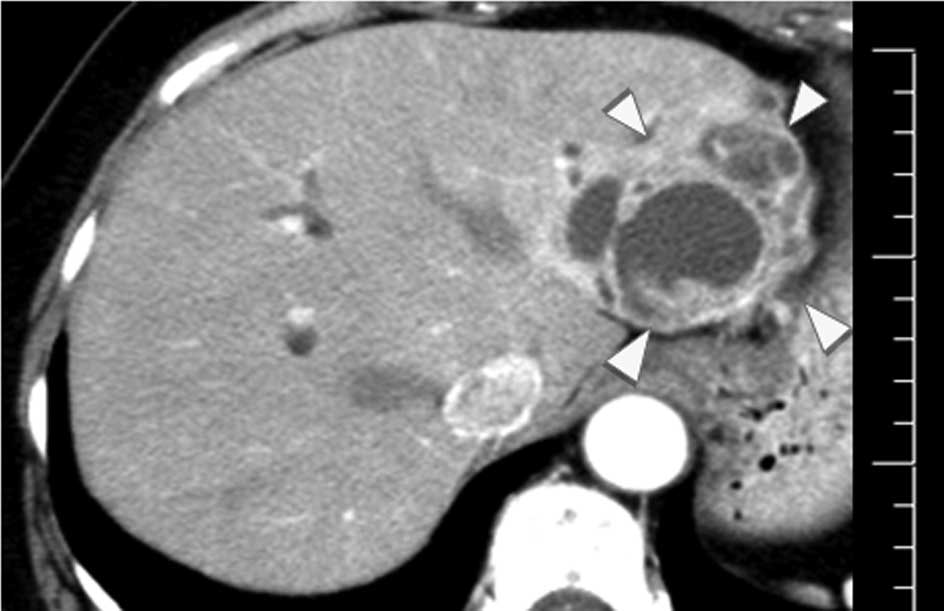
contrast-enhanced CT performed on initial presentation at another
hospital in 2000, had also revealed a 50-mm cystic mass with an
internal septum in the left hepatic lobe, suggesting that the mass
had remained unchanged in size between 2000 and 2011. However,
there was no dilation of the intra- and extra-hepatic bile ducts on
the initial CT scan (Fig. 2). On
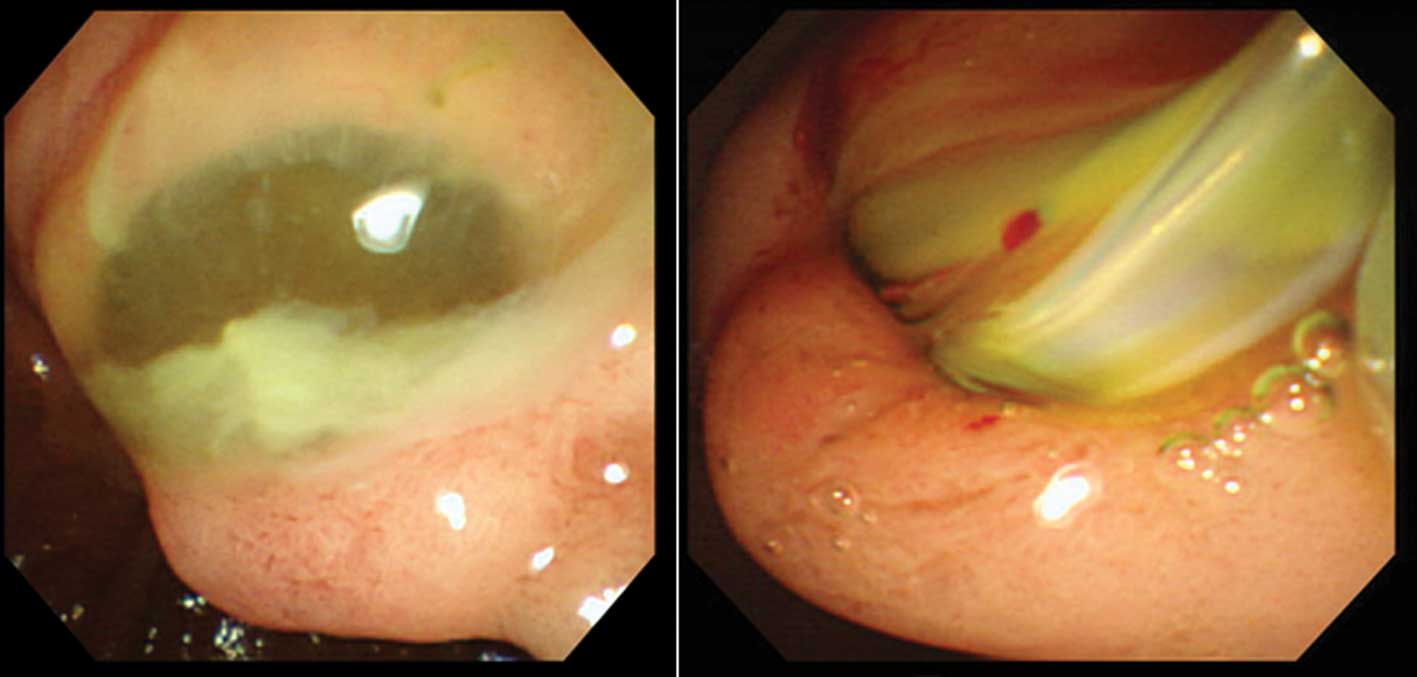
hospital day 2, endoscopic retrograde cholangiopancreatography
(ERCP) was performed and revealed an expanded papilla of Vater due
to the presence of a mucous plug. A retrieval balloon catheter
(Extractor Pro 15/18 mm, 6–7 Fr; Boston Scientific, Cork, Ireland)
was inserted into the upper bile duct, inflated to fit the diameter
of the bile duct and the mucous plug was removed. This procedure
resulted in drainage of copious amounts of mucus and infected bile
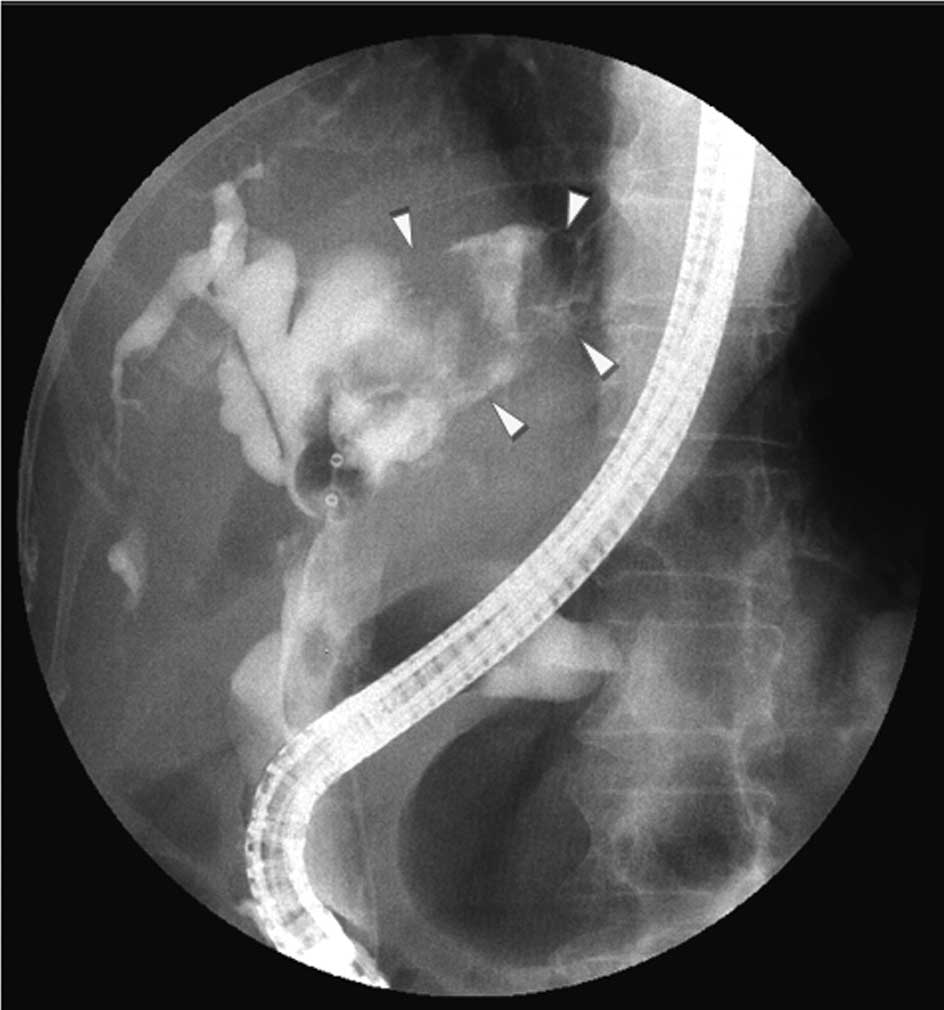
(Fig. 3). After the same procedure
was repeated 3–4 times, cholangiography was performed and revealed
that the cystic mass in the left lobe was communicating with the
bile duct, with a clear zone in the cystic cavity that appeared to
represent a large amount of mucus (Fig. 4). After informing the patient of
the increased risk of recurrent cholangitis due to increased mucus
production by the cystic mass in the left hepatic lobe, despite the
absence of change in size over 11 years, and the potentially
malignant nature of the cystic mass, the patient finally consented
to surgery. Left hepatic lobectomy was performed on hospital day
34.
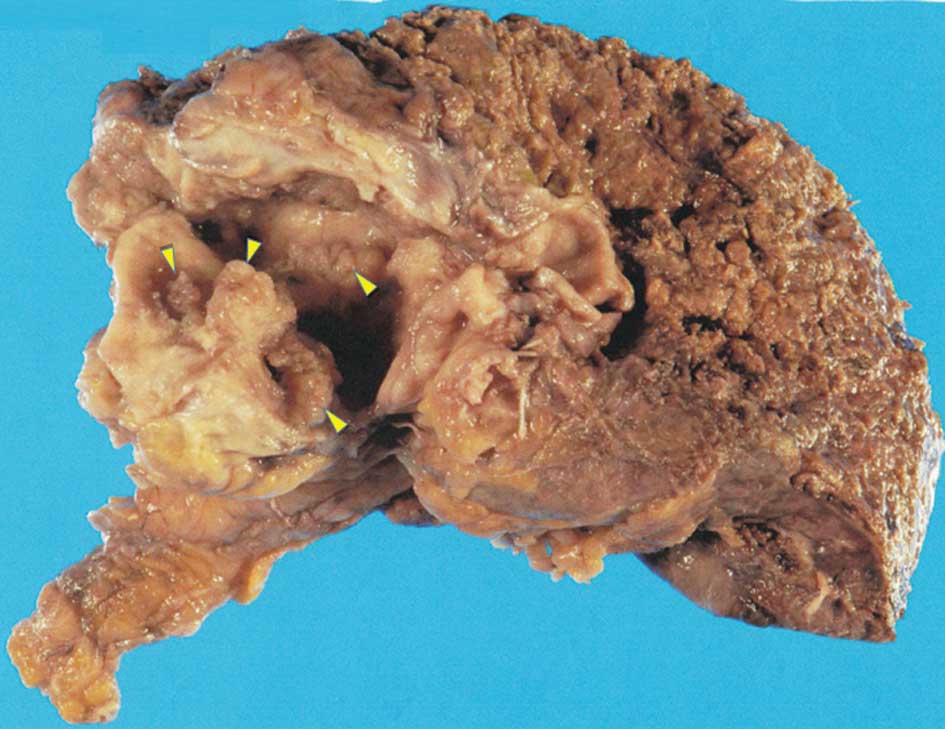
Macroscopically, the tumor was 50×60 mm in size, the
intrahepatic segmental B3 bile duct was markedly dilated and
multiple papillary projections were observed in the bile duct lumen
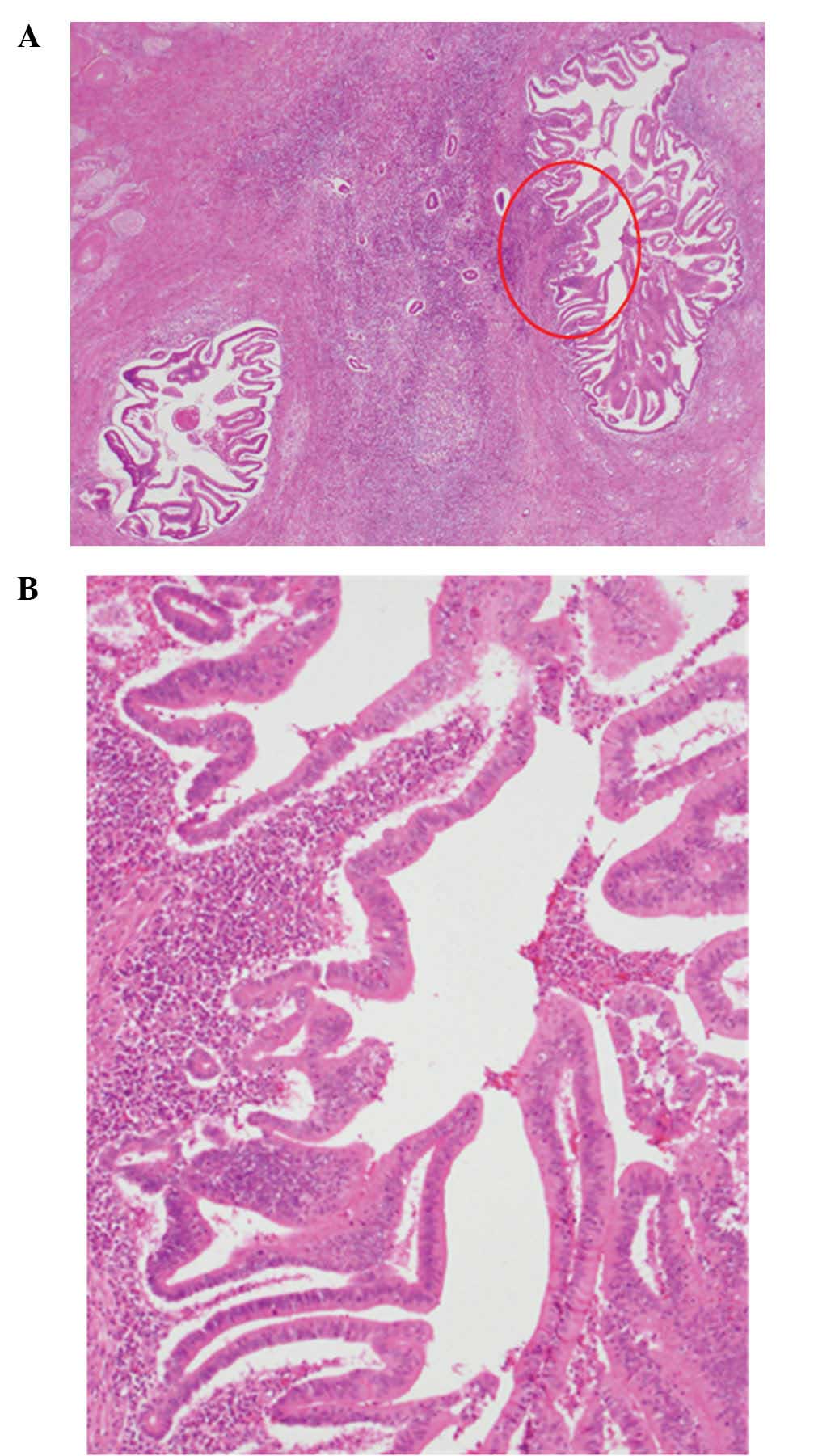
(Fig. 5). On microscopic
examination, the intrahepatic bile ducts were markedly dilated,
with papillary projections in the lumen. The tumor cells exhibited
marked nuclear atypia and pseudostratification and the tumor was
diagnosed as a low-grade intraductal papillary neoplasm of the bile
duct. No ovarian-like stroma was identified (Fig. 6).
Discussion
IPNB is defined as a bile duct epithelial tumor with
papillary proliferation in the bile duct lumen and has been
classified, along with biliary mucinous cystic neoplasm, under the
category of biliary cystic tumors in the revised World Health
Organization classification released in 2010 (1). IPNB is considered a precancerous
lesion or an intraepithelial neoplasm of the biliary tract and
divided, according to tumor cell grade, into intraductal papillary
neoplasm with low-, intermediate- or high-grade intraepithelial
neoplasia (1). The disease concept
of IPNB was first proposed by Chen et al (6) and Nakanuma et al (7). IPNB is often classified into gastric,
intestinal, pancreatobiliary or oncocytic subtypes, similar to
intraductal papillary mucinous neoplasm (IPMN) (1). Ohtsuka et al (8) reported that the pancreatobiliary is
the most common type of non-mucus-producing IPNB, whereas the
intestinal type is the most common type of mucus-producing IPNB.
That study also reported that the former type has a higher grade of
malignancy compared to the latter type and that the degree of
malignant transformation and tumor extension vary depending on the
status of mucus production. Kim et al (9) also reported that mucus-producing
IPNBs were more frequently encountered in patients with the gastric
or intestinal subtypes compared to in those with the oncocystic or
pancreatobiliar subtypes. The frequency of invasive cancer was also
significantly higher in the cases with the pancreatobiliary
compared to those with the gastric subtype. Accordingly, patients
with pancreatobiliary IPNB exhibited significantly poorer prognosis
compared to those with gastric or intestinal IPNB. The 5-year
survival rate was the highest among patients with the gastric
subtype (83.9%), whereas for the intestinal and pancreatobiliary
subtypes it was 75.4 and 46.8%, respectively.
Imaging modalities, such as ERCP and CT, do not
provide clear contrast-enhanced images of bile ducts or
differentiation between clear zones representing mucus and tumors
in patients with high mucus-producing IPNBs. Tsuyuguchi et
al (10) reported that
accurate diagnosis of the size and localization of tumors by
imaging modalities, such as cholangiography, is not feasible in
such cases. Thus, it is difficult to determine the exact size of
IPNBs, although in the present case the tumor size, as assessed by
CT, remained almost unchanged over a period of 11 years. However,
the prominent dilation of intra- and extra-hepatic bile ducts, as
compared to that observed on CT scans performed 11 years earlier,
indicated increased mucus production by the tumor. The surgically
resected tumor was histologically diagnosed as intestinal IPNB and
was considered, according to Ohtsuka et al (8), to be of the high mucus-producing
subtype. The tumor was histologically classified as low-grade,
suggesting a relatively slow rate of disease progression.
Another possible reason for the slow progression is
that the tumor was branch duct type IPNB. IPNB continues to draw
attention due to its morphological and phenotypical resemblance to
the main duct type of IPMN (1,6,11).
The branch duct type IPMN has also been reported in the pancreas.
This lesion forms at the bifurcation of the main pancreatic duct,
where branched pancreatic ducts become cystically dilated and
assume a botryoidal shape, exhibiting excessive mucus production
(12,13). Recently, several case reports of
branch duct type IPNB were reported (2–5). In
all of these cases, cystic or papillary lesions were identified in
the peribiliary glands. The peribiliary glands are accessory
glandular tissues located around the extrahepatic and major
intrahepatic bile ducts (6,14,15).
These accessory glands are distributed in the connective tissue
surrounding the bile ducts or in the bile duct wall and are
connected to the bile duct lumen through a specific duct. A recent
study indicated that various lesions may occur in the peribiliary
glands (16). Lim et al
(2) reviewed imaging findings of
cystic IPNBs and pathological findings of resected specimens and
reported that certain types of cystic IPNB, particularly those with
against bile ducts, originate from the peribiliary glands and are
considered as the counterpart of branch duct type IPMN. In a review
of 12 cases of IPNB (2), 10 cases
were carcinoma in situ and 2 cases were mucinous
adenocarcinoma with partial invasion. Those findings suggested that
IPNB originating from peribiliary glands may also undergo gradual
transformation from adenoma to malignant tumor and eventually to
invasive cancer.
Nakanishi et al (17) reported 2 cases of IPNB in which a
multilocular cyst was formed in the sparse connective tissue
surrounding the bile duct wall, with the cystic cavity
communicating with the bile duct. The authors of that study
suggested that, among the epithelial, luminal and glandular
structures communicating with the bile duct, the peribiliary gland
is the most likely candidate as the origin of this type of
epithelial tumor. It was also suggested that the multilocular
structure of the tumor may be the result of the extension of tumor
cells arising from a single acinus to an adjoining acinus through a
specific duct and that the cystic morphology may be due to the
expansion of the inner cavity of the gland by mucus secreted from a
papillary tumor arising from the peribiliary gland. The authors of
that study also hypothesized that a tumor arising in a peribiliary
gland extends into the epithelium through a dilated specific duct
and further extends through the duct opening into the bile duct
lumen, forming a papillary tumor protruding into the lumen, while
continuously replacing the biliary epithelium.
In the present case, the mass included surrounding
peribiliary glands and the histological images revealed no typical
papillary growth in the peribiliary glands. However, imaging
studies revealed a cystic mass, a characteristic finding of branch
duct type IPNB, in the left hepatic lobe. Another important finding
was that the size of the tumor remained almost unchanged for 11
years. If branch duct type IPNB is the counterpart of branch duct
type IPMN, the progression of the lesion should occur as slowly as
branch duct type IPMN. Thus, this finding also supports the
diagnosis of branch duct type IPNB in the present case. Considering
the limited number of available case reports on branch duct type
IPNB and the fact that IPNB is usually treated surgically at the
time of diagnosis, the present case, with its long-term follow-up
of 11 years, provides valuable insight into the natural history of
this type of tumor.
Abbreviations:
|
IPNB
|
intraductal papillary neoplasm of the
bile duct
|
|
CT
|
computed tomography
|
|
ERCP
|
endoscopic retrograde
cholangiopancreatography
|
|
IPMN
|
intraductal papillary mucinous
neoplasm
|
References
|
1.
|
Nakanuma Y, Curabo MP, Franceschi S, et
al: Intrahepatic cholangiocarcinoma. WHO Classification of Tumours
of the Digestive System. Bosman FT, Carnerio F, Hruban RH and
Theise ND: 4th edition. IARC Press; Lyon: pp. 217–224. 2010
|
|
2.
|
Lim JH, Zen Y, Jang KT, Kim YK and
Nakanuma Y: Cyst-forming intraductal papillary neoplasm of the bile
ducts: description of imaging and pathologic aspects. AJR Am J
Roentgenol. 197:1111–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nakanishi Y, Nakanuma Y, Ohara M, et al:
Intraductal papillary neoplasm arising from peribiliary glands
connecting with the inferior branch of the bile duct of the
anterior segment of the liver. Pathol Int. 61:773–777. 2011.
View Article : Google Scholar
|
|
4.
|
Nakanishi Y, Zen Y, Hirano S, et al:
Intraductal oncocytic papillary neoplasm of the bile duct: the
first case of peribiliary gland origin. J Hepatobiliary Pancreat
Surg. 16:869–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zen Y, Amarapurkar AD and Portmann BC:
Intraductal tubulopapillary neoplasm of the bile duct: potential
origin from peribiliary cysts. Hum Pathol. 43:440–445. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chen TC, Nakanuma Y, Zen Y, et al:
Intraductal papillary neoplasia of the liver associated with
hepatolithiasis. Hepatology. 34:651–658. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nakanuma Y, Sasaki M, Ishikawa A, Tsui W,
Chen TC and Huang SF: Biliary papillary neoplasm of the liver.
Histol Histopathol. 17:851–861. 2002.PubMed/NCBI
|
|
8.
|
Ohtsuka M, Kimura F, Shimizu H, et al:
Similarities and differences between intraductal papillary tumors
of the bile duct with and without macroscopically visible mucin
secretion. Am J Surg Pathol. 35:512–521. 2011. View Article : Google Scholar
|
|
9.
|
Kim KM, Lee JK, Shin JU, et al:
Clinicopathologic features of intraductal papillary neoplasm of the
bile duct according to histologic subtype. Am J Gastroenterol.
107:118–125. 2012. View Article : Google Scholar
|
|
10.
|
Tsuyuguchi T, Sakai Y, Sugiyama H, et al:
Endoscopic diagnosis of intraductal papillary mucinous neoplasm of
the bile duct. J Hepatobiliary Pancreat Sci. 17:230–235. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nakanuma Y: A novel approach to biliary
tract pathology based on similarities to pancreatic counterparts:
is the biliary tract an incomplete pancreas? Pathol Int.
60:419–429. 2010. View Article : Google Scholar
|
|
12.
|
Adsay NV, Fukushima N, Furukawa T, et al:
Intraductal neoplasms of the pancreas. WHO Classification of
Tumours of the Digestive System. Bosman FT, Carnerio F, Hruban RH
and Theise ND: 4th edition. IARC Press; Lyon: pp. 304–313. 2010
|
|
13.
|
Ban S, Naitoh Y, Mino-Kenudson M, et al:
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its
histopathologic difference between 2 major types. Am J Surg Pathol.
30:1561–1569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nakanuma Y, Hoso M, Sanzen T, et al:
Microstructure and development of the normal and pathologic bilary
tract in humans, including blood supply. Microsc Res Tech.
38:552–570. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nakanuma Y, Zen Y and Portman BC: Diseases
of the bile ducts. MacSween’s Pathology of the Liver. Burt AD,
Portman BC and Ferrell LD: 6th edition. Churchill Livingstone;
Edinburg: pp. 491–562. 2011
|
|
16.
|
Cardinale V, Wang Y, Carpino G, et al:
Multipotent stem/progenitor cells in human biliary tree give rise
to hepatocytes, cholangiocytes and pancreatic islets. Hepatology.
54:2159–2172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nakanishi Y, Ohara M, Nakanuma Y, et al:
Intraductal papillary neoplasm of bile duct arising from the
peribiliary gland and its malignant progression. Kan Tan Sui.
65:495–502. 2012.(In Japanese).
|