Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer worldwide (1). The median survival time of patients
with unresectable tumors and untreated patients with less advanced
disease is <4 months and <1 year, respectively (2–6). The
total survival rate of HCC patients is 3–5% (7), due to the high rate of recurrence
following resection and the resistance to chemotherapy.
This type of cancer is particularly aggressive as a
result of its high degree of vascularization. Multiple angiogenic
and anti-angiogenic factors released by the tumor and host cells
are involved in this process (8).
The microvascular density of HCCs correlates with disease prognosis
and postoperative disease recurrence (9–12).
Angiogenesis, the formation of new blood vessels from preexisting
vasculature, is crucial in the development, growth and metastasis
of various neoplasms, including HCCs (13,14).
Although angiogenesis constitutes a promising avenue for the
identification of markers and novel therapeutic approaches, the
ramifications of the signaling pathways are complex and have not
yet been fully elucidated, particularly with respect to
vascularization.
This study aimed to identify angiogenic genes that
are deregulated by HCC and determine their potential as predictors
of postoperative survival. Liver tissue samples and nodules from
three groups of HCC patients were used to perform TaqMan gene array
analysis and to identify the most promising biomarker of HCC in
terms of patient characteristics, survival rates and tissue
histology.
Materials and methods
Paired analysis of angiogenic gene
expression in HCC nodules and non-HCC liver tissue
A preliminary experiment was conducted, using tissue
samples from 12 HCC patients to identify the affected
angiogenesis-related target genes to be investigated in this study.
All the patients were Japanese and they had undergone surgical HCC
resection between October, 2008 and October, 2009 at the Department
of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical
University, Japan. The majority of the patients were male, with
moderately differentiated HCC histology and negative for
intrahepatic metastases (IM), portal vein invasion (Vp) or venous
invasion (Vv). Half of the patients had liver cirrhosis or chronic
hepatitis resulting from viral infection (Table I). The patients provided written
informed consent according to the institutional regulations. This
study was approved by the Ethics Committee and Institutional Review
Board of the Tokyo Women’s Medical University.
 | Table I.Characteristics of the 12 HCC
patients who provided liver samples for the identification of
angiogenic genes deregulated by HCC. |
Table I.
Characteristics of the 12 HCC
patients who provided liver samples for the identification of
angiogenic genes deregulated by HCC.
|
Characteristics | Frequency | Percentage |
|---|
| Age (years) | | |
| Mean (range) | 12 (51–81) | - |
| Gender | | |
| Male | 10 | 83 |
| Female | 2 | 17 |
| Tumor size
(cm) | | |
| Mean (range) | 2.4 (1.5–4.2) | - |
| Histology | | |
| Well
differentiated | 1 | 8 |
| Moderately
differentiated | 11 | 92 |
| IM | | |
| Positive | 2 | 17 |
| Negative | 10 | 83 |
| Vp | | |
| Positive | 2 | 17 |
| Negative | 10 | 83 |
| Vv | | |
| Negative | 12 | 100 |
| Macroscopic
findings | | |
| SNIM | 2 | 17 |
| SN | 6 | 50 |
| SNEG | 4 | 33 |
| Child-Pugh
classification | | |
| A | 12 | 100 |
| Liver status | | |
| Cirrhosis | 6 | 50 |
| Chronic
hepatitis | 5 | 42 |
| Normal | 1 | 8 |
| Infection | | |
| HBV | 3 | 25 |
| HCV | 7 | 58 |
| HCV+HBV | 1 | 8 |
| Negative | 1 | 8 |
The tissue samples collected from primary HCC
nodules and non-HCC liver tissue of each patient were immediately
snap-frozen and stored at −80°C until further use. The samples were
then homogenized and total RNA was isolated using the
RNeasy® Mini kit (Qiagen, Valencia, CA, USA).
Subsequently, complementary DNA (cDNA) was synthesized using 2
μg of total RNA and High Capacity RNA-to-cDNA Master Mix
(Applied Biosystems Inc., Foster City, CA, USA) according to the
manufacturer’s protocol. We used the TaqMan® Array Gene
Expression 96-well Human Angiogenesis Plate (Applied Biosystems
Inc.) to determine the angiogenic gene profiles of the specimens in
each sample set. A total of 92 angiogenesis- or
lymphangiogenesis-associated gene assays and 4 control endogenous
gene assays were performed in each plate. The target genes
investigated in this study are listed in Table II. The gene expression level was
analyzed using a 7500 Real-Time PCR system (Applied Biosystems
Inc.). Polymerase chain reaction (PCR) using TaqMan®
Gene Expression Master Mix (Applied Biosystems Inc.) was performed
under the following conditions: 2 min at 50°C, 10 min at 95°C,
followed by 40 cycles of 30 sec at 95°C and 1 min at 60°C. Data
were analyzed using SDS software, version 1.4 (Applied Biosystems
Inc.) and gene expression levels were compared using the ΔΔCt
method (15). Significantly
upregulated or downregulated genes were screened using a cut-off
P-value of <0.01.
 | Table II.List of the angiogenic genes included
in the gene array platea. |
Table II.
List of the angiogenic genes included
in the gene array platea.
| Gene symbol | Assay ID |
|---|
| 18S | Hs99999901_s1 |
| GAPDH | Hs99999905_m1 |
| HPRT1 | Hs99999909_m1 |
| GUSB | Hs99999908_m1 |
| FGA | Hs00241027_m1 |
| PLG | Hs00264877_m1 |
| CXCL12 | Hs00171022_m1 |
| EDIL3 | Hs00174781_m1 |
| EPHB2 | Hs00362096_m1 |
| FGF1 | Hs00265254_m1 |
| FGF2 | Hs00266645_m1 |
| FGF4 | Hs00173564_m1 |
| PDGFB | Hs00234042_m1 |
| PTN | Hs00383235_m1 |
| PROK1 | Hs00260905_m1 |
| TGFA | Hs00608187_m1 |
| TGFB1 | Hs99999918_m1 |
| TNF | Hs00174128_m1 |
| TNFSF15 | Hs00270802_s1 |
| ITGA4 | Hs00168433_m1 |
| IFNB1 | Hs01077958_s1 |
| IFNG | Hs00174143_m1 |
| CXCL10 | Hs00171042_m1 |
| IL12A | Hs00168405_m1 |
| CD44 | Hs00153304_m1 |
| CDH5 | Hs00174344_m1 |
| CXCL2 | Hs00601975_m1 |
|
SERPINB5 | Hs00184728_m1 |
| FLT1 | Hs00176573_m1 |
| SEMA3F | Hs00188273_m1 |
| ANGPTL3 | Hs00205581_m1 |
| CEACAM1 | Hs00236077_m1 |
| HEY1 | Hs00232618_m1 |
| ITGAV | Hs00233808_m1 |
| PECAM1 | Hs00169777_m1 |
| LYVE-1 | Hs00272659_m1 |
| FOXC2 | Hs00270951_s1 |
| COL4A1 | Hs00266237_m1 |
| COL4A2 | Hs01098873_m1 |
| COL15A1 | Hs00266332_m1 |
| HSPG2 | Hs00194179_m1 |
| COL18A1 | Hs00181017_m1 |
| CSF3 | Hs99999083_m1 |
| GRN | Hs00963711_g1 |
| THBS2 | Hs01568063_m1 |
| LECT1 | Hs00993254_m1 |
| ANGPTL4 | Hs01101127_m1 |
| ITGB3 | Hs01001469_m1 |
|
SERPINC1 | Hs00166654_m1 |
| PRL | Hs00168730_m1 |
| MMP2 | Hs00234422_m1 |
| ANG,
RNASE4 | Hs02379000_s1 |
| ANGPT1 | Hs00181613_m1 |
| ANGPT2 | Hs00169867_m1 |
| FST | Hs00246256_m1 |
| HGF | Hs00300159_m1 |
| IL8 | Hs00174103_m1 |
| LEP | Hs00174877_m1 |
| MDK | Hs00171064_m1 |
| TYMP | Hs00157317_m1 |
| VEGFA | Hs00900054_m1 |
| VEGFB | Hs00173634_m1 |
| VEGFC | Hs00153458_m1 |
| CTGF | Hs00170014_m1 |
| FBLN5 | Hs00197064_m1 |
| THBS1 | Hs00962914_m1 |
|
SERPINF1 | Hs00171467_m1 |
| PF4 | Hs00427220_g1 |
| VASH1 | Hs00208609_m1 |
| ADAMTS1 | Hs00199608_m1 |
| ANGPTL1 | Hs00559786_m1 |
| AMOT | Hs00611096_m1 |
| TEK | Hs00176096_m1 |
| TIE1 | Hs00178500_m1 |
| TNMD | Hs00223332_m1 |
| TIMP2 | Hs00234278_m1 |
| TIMP3 | Hs00165949_m1 |
| ANGPTL2 | Hs00765775_m1 |
| KIT | Hs00174029_m1 |
| TNNI1 | Hs00913333_m1 |
| NRP2 | Hs00187290_m1 |
| KDR | Hs00176676_m1 |
| ENPP2 | Hs00196470_m1 |
| FIGF | Hs00189521_m1 |
| FN1 | Hs01549940_m1 |
| COL4A3 | Hs01022527_m1 |
| F2 | Hs01011995_g1 |
| BAI1 | Hs01105174_m1 |
| CHGA | Hs00900373_m1 |
| ANGPT4 | Hs00211115_m1 |
| PDGFRA | Hs00998026_m1 |
| PDGFRB | Hs00387364_m1 |
| FLT4 | Hs01047677_m1 |
| NRP1 | Hs00826128_m1 |
| S1PR1 | Hs01922614_s1 |
| PROX1 | Hs00896294_m1 |
Paired analysis of lymphatic vessel
endothelial hyaluronan receptor-1 (LYVE-1) expression in HCC
nodules and non-HCC liver tissue
Archived liver tissue samples (primary HCC tumors;
>95% HCC cells and non-HCC tissue from the same patient) from
HCC patients were tested for LYVE-1 expression. The 58
complete sets were obtained from Japanese patients who had
undergone surgical HCC resection between December, 1993 and May,
2007 at the Department of Surgery, Institute of Gastroenterology,
Tokyo Women’s Medical University, Japan. Similar to the 12-patient
group, the archived samples were collected primarily from males
with moderately differentiated HCC histology and cirrhosis or
chronic hepatitis resulting from viral infection (Table III). The patients provided written
informed consent in accordance with institutional regulations.
 | Table III.Characteristics of the 58 HCC
patients investigated for the histology of HCC nodules and non-HCC
liver tissue and survival curves. |
Table III.
Characteristics of the 58 HCC
patients investigated for the histology of HCC nodules and non-HCC
liver tissue and survival curves.
|
Characteristics | Frequency | Percentage |
|---|
| Age (years) | | |
| Mean (range) | 63 (39–81) | - |
| Gender | | |
| Male | 45 | 77 |
| Female | 13 | 23 |
| Histology | | |
| Well
differentiated | 7 | 12 |
| Moderately
differentiated | 44 | 73 |
| Poorly
differentiated | 9 | 15 |
| Child-Pugh
classification | | |
| A | 53 | 88 |
| B | 7 | 12 |
| Liver status | | |
| Cirrhosis | 23 | 38 |
| Chronic
hepatitis | 35 | 58 |
| Normal | 2 | 3 |
| Viral
infection | | |
| HBV | 17 | 28 |
| HCV | 30 | 50 |
| Negative | 13 | 22 |
The formalin-fixed paraffin-embedded (FFPE) samples
were preserved using the general protocol of the Institute of
Pathology, Tokyo Women’s Medical University, Japan. Each FFPE
specimen was cut into 10-μm sections, deparaffinized in
xylene and rehydrated in graded ethanols. The tissues were
dissected and total RNA was isolated using the RNeasy®
FFPE kit (Qiagen). Subsequently, cDNA was synthesized using
High-Capacity cDNA Reverse Transcription kits (Applied Biosystems
Inc.) with 1 μg of total RNA, according to the
manufacturer’s protocol. The expression of LYVE-1 and β-2
microglobulin (B2M), which was used as endogenous control,
were measured using a StepOne™ Real-Time PCR system (Applied
Biosystems Inc.). The TaqMan® primers/probe for
LYVE-1 (Assay ID: Hs00272659_m1) and B2M (Assay ID:
Hs99999907_m1) were purchased from TaqMan® Gene
Expression Assays (Applied Biosystems Inc.). PCR was performed
using TaqMan® Fast Master Mix under the following
conditions: 20 sec at 95°C, followed by 40 cycles of 1 sec at 95°C
and 20 sec at 60°C. Data were analyzed using StepOne™ software,
version 2.1 and the gene expression level was quantified by the
ΔΔCt method.
Histological analysis of the nodules
All the HCC specimens, including the fresh specimens
from the 12 patients, were histologically evaluated according to
the general rules for the clinical and pathological study of
primary liver cancer (16). The
clinicopathological parameters of the specimens, including tumor
diameter, liver status, IM, Vp, Vv and histopathological
classification were obtained.
Correlations between LYVE-1 expression,
HCC differentiation and patient survival
We analyzed archived HCC samples from 103 HCC
patients. Those archived samples had been primarily collected from
males with moderately differentiated HCCs and cirrhosis or chronic
hepatitis resulting from viral infection (Table IV). The patients were Japanese and
had undergone surgical HCC resection between December, 1993 and
May, 2007 at the Department of Surgery, Institute of
Gastroenterology, Tokyo Women’s Medical University, Japan. The
patients provided written informed consent in accordance with
institutional regulations.
 | Table IV.Characteristics of the 103 HCC
patients investigated for survival curves. |
Table IV.
Characteristics of the 103 HCC
patients investigated for survival curves.
|
Characteristics | Frequency | Percentage |
|---|
| Age (years) | | |
| Mean (range) | 63 (39–81) | - |
| Gender | | |
| Male | 78 | 76 |
| Female | 25 | 24 |
| Histology | | |
| Well
differentiated | 19 | 18 |
| Moderately
differentiated | 67 | 65 |
| Poorly
differentiated | 17 | 16 |
| Child-Pugh
classification | | |
| A | 90 | 87 |
| B | 12 | 12 |
| C | 1 | 1 |
| Liver status | | |
| Cirrhosis | 44 | 43 |
| Hepatitis | 56 | 54 |
| Normal | 3 | 3% |
| Viral
infection | | |
| HBV | 24 | 24 |
| HCV | 49 | 47 |
| HCV+HBV | 1 | 1 |
| Negative | 29 | 28 |
| IM | | |
| Positive | 17 | 16 |
| Negative | 86 | 84 |
| Vp | | |
| Positive | 19 | 18 |
| Negative | 84 | 82 |
| Vv | | |
| Positive | 6 | 6 |
| Negative | 97 | 94 |
| Macroscopic
findings | | |
| SNIM | 25 | 24 |
| SN | 30 | 29 |
| SNEG | 38 | 38 |
| Conflict
multinodular type | 4 | 4 |
| Massive type | 6 | 6 |
| Tumor size
(cm) | | |
| Mean (range) | 4.2 (0.8–17) | - |
Statistical analysis
We used Wilcoxon signed-rank tests to compare gene
expression levels between HCC nodules and non-HCC liver tissue. The
correlation between LYVE-1 expression levels in HCC nodules
and the degree of nodule differentiation was assessed using
Steel-Dwass tests. Disease-free survival (DFS) and overall survival
(OS) were calculated by the Kaplan-Meier method and differences in
survival curves were analyzed using log-rank tests. The follow-up
time was defined as the time from the date of surgery to the date
of death or the last known follow-up. The correlation of
LYVE-1 expression to the clinicopathological parameters was
evaluated using Fisher’s exact probability tests or Chi-square
tests. Independent prognostic factors were analyzed using the Cox
proportional hazards regression model. P<0.05 was considered to
indicate a statistically significant difference. All tests were
two-sided. We used JMP® software, version 9.0.1 (SAS
Institute Inc., Cary, NC, USA) to compute all the statistics.
Results
Identification of angiogenic genes
deregulated by HCC
The gene array analysis of liver tissue samples
collected from the initial 12-patient group identified 14 genes
differentially expressed in HCC and non-HCC tissues (Table V). Among these, the genes encoding
collagen type XVα1, IVα1 and IVα2, as well as two growth
factor-related genes [EGF-like repeats and discoidin I-like domains
3 (EDIL3) and platelet-derived growth factor β polypeptide (PDGFB)]
were upregulated by HCC. HCC was also associated with upregulation
of the gene encoding neurite growth-promoting factor 2 (midkine,
MDK), which is involved in embryonic development and inflammation.
By contrast, HCC caused downregulation of genes encoding
inflammatory chemokines (CXCL2 and CXCL12) and genes associated
with vessel growth, namely neuropilin 2 (NRP2) and
LYVE-1 (Table V).
 | Table V.Differentially expressed genes in HCC
and non-HCC tissues. |
Table V.
Differentially expressed genes in HCC
and non-HCC tissues.
| A, Genes
upregulated in primary HCC nodules compared to non-HCC liver
tissue. |
|
| No. | Gene name | Description | P-value |
|
| 1 | COL15A1 | Collagen, type
XVα1 | 0.0020 |
| 2 | COL4A1 | Collagen, type
IVα1 | 0.0010 |
| 3 | COL4A2 | Collagen, type
IVα2 | 0.0034 |
| 4 | EDIL3 | EGF-like repeats
and discoidin I-like domains 3 | 0.0098 |
| 5 | MDK | Midkine | 0.0005 |
| 6 | PDGFB | Platelet-derived
growth factor β polypeptide | 0.0010 |
|
| B, Genes
downregulated in primary HCC nodules compared to non-HCC liver
tissue. |
|
| No. | Gene name | Description | P-value |
|
| 1 | ANGPTL1 | Angiopoietin-like
1 | 0.0010 |
| 2 | CXCL12 | Chemokine (C-X-C
motif) ligand 12 | 0.0024 |
| 3 | CXCL2 | Chemokine (C-X-C
motif) ligand 2 | 0.0010 |
| 4 | HGF | Hepatocyte growth
factor | 0.0049 |
| 5 | LYVE-1 | Lymphatic vessel
endothelial hyaluronan receptor-1 | 0.0010 |
| 6 | NRP2 | Neuropilin 2 | 0.0068 |
| 7 | PDGFRA | Platelet-derived
growth factor receptor α polypeptide | 0.0005 |
| 8 | PLG | Plasminogen | 0.0034 |
Interpatient variability in LYVE-1
downregulation by HCC
The effect of HCC on LYVE-1 expression was
verified using a larger cohort of 58 patients. LYVE-1
expression was significantly lower in HCC nodules compared to the
corresponding non-HCC liver tissue (P<0.0001). Paired analysis
of HCC nodule and non-HCC liver tissue samples from each patient
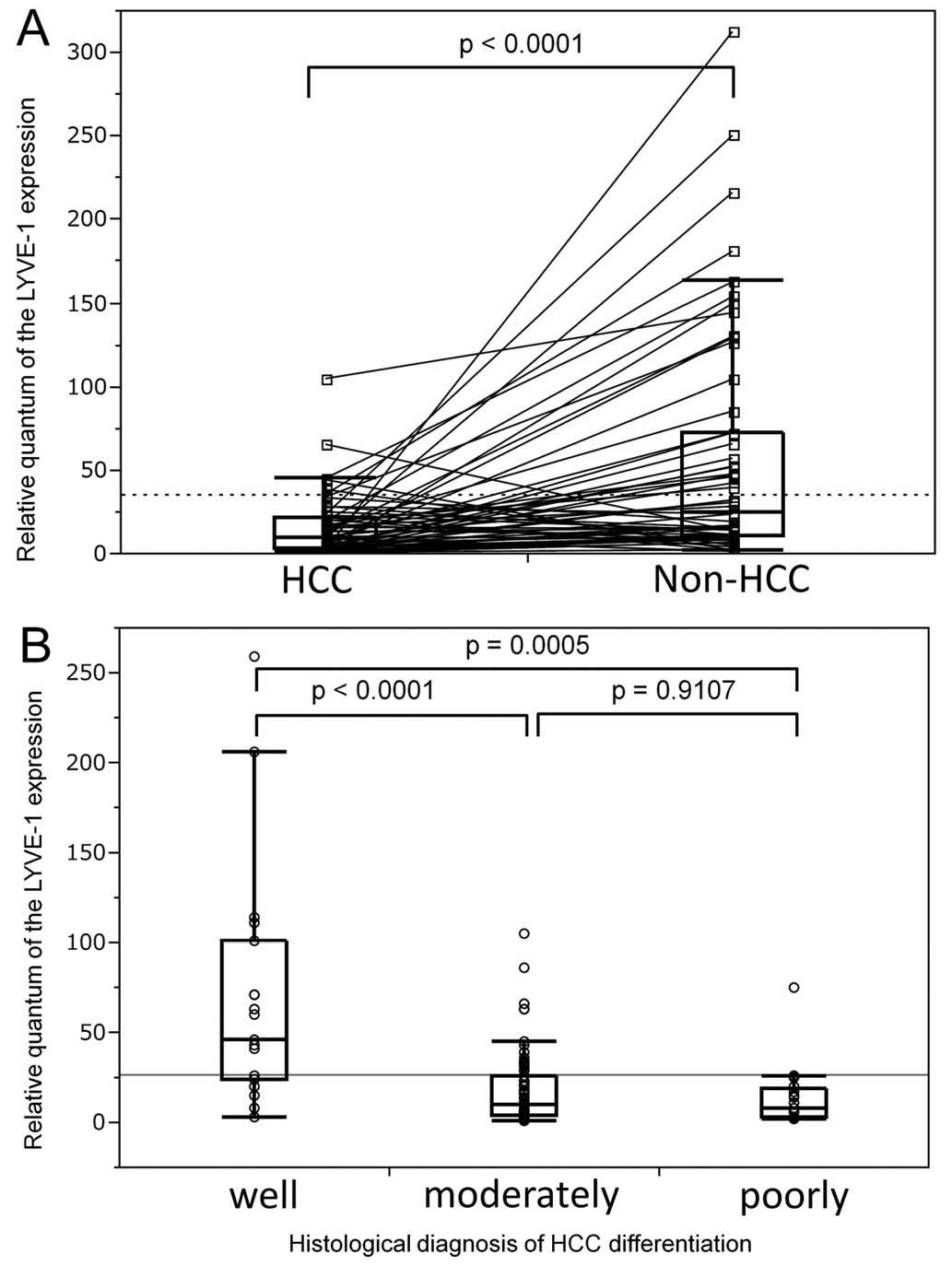
revealed a large variability in LYVE-1 expression between
the patients (Fig. 1A).
Correlation between LYVE-1 downregulation
and HCC nodule differentiation
Since the only parameter affected by LYVE-1
expression was the histology of the nodules, this association was
further investigated by analysis of HCC nodule samples. The
possible contribution of disease severity to interpatient
variability in LYVE-1 expression was assessed using a large
number of patients for whom nodule histology reports and archived
tissue samples were available for correlation analysis. The loss of
nodule differentiation was associated with a decrease in
LYVE-1 expression, which would occur early in the evolution
of the disease (P=0.0006). The LYVE-1 expression level was
decreased >5-fold between the first two stages (P<0.0001) and
remained comparable in poorly differentiated HCC nodules (P=0.91).
These data support an association between LYVE-1 expression
and HCC progression (Fig. 1B).
Correlation between LYVE-1 expression and
patient survival
The detrimental effect of LYVE-1
downregulation on the survival of HCC patients was confirmed in the
cohort of the 103 HCCs based on a similar analysis of HCC nodules.
Based on a median observation frequency of 2,752 days, this group
was characterized by a 5-year DFS rate of 34.1% and a 5-year OS
rate of 66.6% and was used to assess the effect of LYVE-1
expression on survival by dividing the patients into groups with
high expression (>7-fold relative to the lowest value) and low
expression (<7-fold relative to the lowest value) in HCC
nodules. Fig. 2A shows that DFS
was not significantly affected by the LYVE-1 expression
level in HCC nodules. By contrast, the OS curve decayed less
rapidly for the high-expression group compared to that for the
low-expression group, resulting in 5-year OS rates of 81 and 45%,
respectively (P=0.004; Fig. 2B).
In fact, all the patients with low LYVE-1 expression reached
the 45% OS plateau phase within 4 years after surgery. Accordingly,
these data were confirmed by univariate Cox regression analyses for
DFS [hazard ratio (HR)=1.394; 95% confidence interval (CI):
0.864–2.203; P=0.1694] and OS (HR=2.458; 95% CI: 1.298–4.625;
P=0.0063). Multivariate Cox regression analyses identified
LYVE-1 expression as a significant independent prognostic
parameter of OS (HR=3.067; 95% CI: 1.507–6.273; P=0.0021) (Tables VI and VII).
 | Table VI.Uni- and multivariate Cox regression
analyses for disease-free survival (DFS) in HCC. |
Table VI.
Uni- and multivariate Cox regression
analyses for disease-free survival (DFS) in HCC.
| A, Univariate
analysis of DFS among the 103 HCC patients. |
|
| Variables | Univariate analysis
|
| HR | 95% CI | P-value |
|
| Age ≥65 years | 1.230 | 0.784–1.942 | 0.3682 |
| Female gender | 0.962 | 0.557–1.588 | 0.8847 |
| Histopathological
grade | | | |
| Poor | 1.995 | 1.065–3.488 | 0.0321a |
| Moderate | 1.235 | 0.778–2.004 | 0.3744 |
| Child-Pugh
classification B or C | 0.871 | 0.384–1.714 | 0.7083 |
| Cirrhosis | 0.898 | 0.564–1.409 | 0.6418 |
| Viral
infection-positive | 0.900 | 0.553–1.523 | 0.6860 |
| IM-positive | 16.345 | 7.297–37.151 | <0.0001b |
| Vp-positive | 3.868 | 2.059–6.857 | <0.0001b |
| Vv-positive | 3.999 | 1.355–9.525 | 0.0153a |
| Macroscopic
findings | | | |
| SNEG or massive
or conflict multinodular type | 3.504 | 2.164–5.709 | <0.0001b |
| Tumor size ≥3
cm | 2.608 | 1.623–4.187 | <0.0001b |
| Low LYVE-1
in HCC | 1.394 | 0.864–2.203 | 0.1694 |
|
| B, Multivariate
analysis of DFS among the 103 HCC patients. |
|
| Variables | Multivariate
analysis
|
| HR | 95% CI | P-value |
|
| Poor
histopathological grade | 1.043 | 0.522–1.985 | 0.9003 |
| IM-positive | 8.902 | 3.687–21.910 | <0.0001b |
| Vp-positive | 1.450 | 0.673–2.951 | 0.3309 |
| Vv-positive | 1.880 | 0.567–5.256 | 0.2809 |
| Macroscopic
findings | | | |
| SNEG or massive or
conflict multinodular type | 2.192 | 0.995–4.764 | 0.0516 |
| Tumor size ≥3
cm | 1.071 | 0.517–2.197 | 0.8531 |
 | Table VII.Univariate and multivariate Cox
regression analyses for overall survival (OS) in HCC. |
Table VII.
Univariate and multivariate Cox
regression analyses for overall survival (OS) in HCC.
| A, Univariate
analysis of OS among the 103 HCC patients. |
|
| Variables | Univariate analysis
|
| HR | 95% CI | P-value |
|
| Age ≥65 years | 1.067 | 0.576–2.006 | 0.8381 |
| Female gender | 1.470 | 0.748–2.762 | 0.255 |
| Histopathological
grade | | | |
| Poor | 4.449 | 2.251–8.422 | <0.0001b |
| Moderate | 0.777 | 0.419–1.462 | 0.4274 |
| Child-Pugh
classification B or C | 2.285 | 0.977–4.737 | 0.0559 |
| Cirrhosis | 1.985 | 1.072–3.760 | 0.0292a |
| Viral
infection-positive | 0.854 | 0.437–1.794 | 0.6620 |
| IM-positive | 7.273 | 3.241–15.483 | <0.0001b |
| Vp-positive | 8.539 | 4.004–17.853 | <0.0001b |
| Vv-positive | 1.624 | 0.718–2.716 | 0.2010 |
| Macroscopic
findings | | | |
| SNEG or massive or
conflict multinodular type | 4.138 | 2.163–8.211 | <0.0001b |
| Tumor size ≥3
cm | 3.439 | 1.736–7.015 | 0.0004b |
| Low LYVE-1
in HCC | 2.458 | 1.298–4.625 | 0.0063b |
|
| B, Multivariate
analysis of OS among the 103 HCC patients. |
|
| Variables | Multivariate
analysis
|
| HR | 95% CI | P-value |
|
| Poor
histopathological grade | 1.523 | 0.638–3.536 | 0.3374 |
| Cirrhosis | 2.533 | 1.177–5.517 | 0.0175a |
| IM-positive | 3.993 | 1.386–11.846 | 0.0103a |
| Vp-positive | 2.676 | 0.9159–7.396 | 0.0711 |
| Macroscopic
findings | | | |
| SNEG or massive or
conflict multinodular type | 2.317 | 0.857–6.067 | 0.0964 |
| Tumor size ≥3
cm | 1.083 | 0.420–2.831 | 0.8693 |
| Low LYVE-1
in HCC | 3.067 | 1.507–6.273 | 0.0021b |
Specificity of factors affected by LYVE-1
expression in HCC patients
Analyses were performed to determine whether other
aspects of the disease were associated with the downregulation of
LYVE-1 expression. The patients were re-examined by
comparing the low- and high-expression groups with respect to the
general characteristics and the histology of the HCC nodules
(Table VIII). The expression of
LYVE-1 did not appear to exert any effect on basic
characteristics, such as age, gender ratio, liver status or viral
infection and IM, Vp and Vv in neither one of the two groups. With
respect to tissue histology, the HCC nodules were significantly
less differentiated in the low-expression group (P<0.0064;
Table VIII). These data suggest
that LYVE-1 downregulation may be a marker of nodule
dedifferentiation in HCC tissues.
 | Table VIII.Association between LYVE-1
expression in HCC liver nodules and clinicopathological
characteristics of the 103 patients. |
Table VIII.
Association between LYVE-1
expression in HCC liver nodules and clinicopathological
characteristics of the 103 patients.
| Clinicopathological
characteristics (n) | LYVE-1
expression
| P-value |
|---|
| High (n=65) | Low (n=38) |
|---|
| Age (years) | | | 0.1012 |
| <65 | 34 | 13 | |
| ≥65 | 31 | 25 | |
| Gender | | | 0.6387 |
| Male | 48 | 30 | |
| Female | 17 | 8 | |
| Histology | | | 0.0064a |
| Well
differentiated | 18 | 1 | |
| Moderately
differentiated | 38 | 29 | |
| Poorly
differentiated | 9 | 8 | |
| Child-Pugh
classification | | | 1.0000 |
| A | 57 | 33 | |
| B or C | 8 | 5 | |
| Liver status | | | 0.1001 |
| Cirrhosis | 32 | 12 | |
| Other | 33 | 26 | |
| Viral
infection | | | 1.0000 |
| Positive | 47 | 27 | |
| Negative | 18 | 11 | |
| IM | | | 0.5884 |
| Positive | 12 | 5 | |
| Negative | 53 | 33 | |
| Vp | | | 0.6085 |
| Positive | 11 | 8 | |
| Negative | 54 | 30 | |
| Vv | | | 1.0000 |
| Positive | 4 | 2 | |
| Negative | 61 | 36 | |
| Macroscopic
findings | | | 0.2208 |
| SNIM or SN | 38 | 17 | |
| Other | 27 | 21 | |
| Tumor size
(cm) | | | 0.2188 |
| <3 | 41 | 19 | |
| ≥3 | 24 | 19 | |
Discussion
The field of cancer research has benefited
significantly from genetic and functional analyses of oncogenes and
tumor suppressor genes (17).
Among the 92 angiogenic genes investigated, 14 genes were shown to
be significantly deregulated in HCC. Some of these genes
(COL15A1, COL4A1, COL4A2, PDGFB,
MDK and EDIL3) were upregulated, whereas others
(ANGPTL1, CXCL12, CXCL2, NRP,
HGF, LYVE-1, PDGFRA and PLG) were
downregulated, suggesting that they may be involved in the
mechanism of carcinogenesis or tumor growth. Among these genes,
LYVE-1 was one of the most strongly downregulated genes in
HCC nodules, compared to adjacent non-HCC tissue. This gene is of
particular interest, as the triad of glypican-3,
LYVE-1 and survivin was previously demonstrated to
provide a reliable diagnosis of early HCC (18). The present study demonstrates the
potential of LYVE-1 deregulation as an independent biomarker
of postsurgical outcome in HCC patients.
In the present study, the clinicopathological
findings revealed a significant correlation between LYVE-1
expression and the histology of HCC nodules. From a dynamic
perspective, the gradual loss of differentiation may be associated
with LYVE-1 downregulation occurring early during this
process. LYVE-1 expression levels in poorly or moderately
differentiated nodules were comparable and were decreased by
>5-fold compared to the levels in well-differentiated nodules.
These data are consistent with those of a previous study,
demonstrating that LYVE-1 expression decreases progressively
in HCC nodules transitioning from a polyclonal cirrhotic to a
monoclonal cirrhotic phenotype (19). In addition, our study suggests that
LYVE-1 may be an early marker of HCC tumorigenesis.
The potential of LYVE-1 as a predictor of
postsurgical outcome in HCC patients was clearly demonstrated in
terms of the 5-year OS. Logistic regression analyses revealed that
low LYVE-1 expression in HCC nodules was significantly
predictive of shorter OS. Since the decrease in LYVE-1
expression occurs early during the nodule transformation phase,
these data suggested that close monitoring of LYVE-1
expression after surgery may considerably improve survival in HCC
patients.
Our understanding of the role of LYVE-1 in
tumorigenesis is evolving rapidly as the dogma is challenged by
thorough immunohistochemical examination (20). This marker of lymphatic endothelial
cells has been detected in the endothelial cells of the hepatic
blood sinusoids of healthy subjects and patients diagnosed with
liver cancer and cirrhosis. Notably, this protein is not detected
in angiogenic blood vessels of liver tumors and is weakly detected
in the microcirculation of regenerative hepatic nodules in
cirrhosis, despite the fact that both types of vessels are derived
from liver sinusoids. Furthermore, the lymphatics are restricted to
the margins of HCCs and the surrounding tissues. This distribution
is consistent with the LYVE-1 downregulation observed in the
highly vascularized HCC nodules compared to non-HCC tissues.
Accordingly, the restriction of LYVE-1 to the periphery of
the tumor may translate into progressive decrease, in relative
expression with an increase in tumor size, as supported by a
previous study demonstrating that LYVE-1 attenuation in the
sinusoidal endothelium was associated with hepatic disease
progression (21).
The most common cause of mortality in HCC patients
is tumor recurrence following surgery, which may be caused by small
metastatic lesions or metachronous multicentric lesions in the case
of liver inflammation or cirrhosis. Chronic aggressive hepatitis is
a significant risk factor of HCC recurrence following hepatectomy
(22). Notably, the expression of
LYVE-1 in the lymphatic endothelium is downregulated by the
pro-inflammatory cytokine tumor necrosis factor-α in vitro
and in vivo(23–25), suggesting that LYVE-1
expression may be suppressed by hepatitis. The fact that
inflammation is initiated early during the course of liver disease
is consistent with our hypothesis that LYVE-1 may be an
early marker of HCC tumorigenesis.
LYVE-1 is a member of the Link protein
superfamily and is similar to the leukocyte hyaluronan receptor
CD44, which is known to facilitate tumor cell invasion. Hyaluronan
is a key substrate for cell migration among tissues during
inflammation, wound healing and neoplasia (26). Recent studies suggested that the
ligands of LYVE-1 receptors may enhance tumor cell adhesion
to the vessel wall (27) and open
lymphatic intercellular junctions (28), allowing tumor cells to invade the
surrounding tissue (29).
Therefore, although the overall LYVE-1 expression is
decreased in HCC nodules, the strategic positioning of its receptor
at the periphery of the tumor may favor tumorigenesis and
metastasis through the facilitation of tumor cell passage in and
out of the tumor. This hypothesis is consistent with the recent
finding that LYVE-1 expression may be associated with
chemoresistance (30). Therefore,
the progressive loss of LYVE-1 expression during the
transformation of HCC nodules may correlate with the severity of
inflammation and tumor growth.
To the best of our knowledge, this study is the
first to demonstrate a direct correlation between LYVE-1
expression and tumor dedifferentiation, which strengthens the
hypothesis that LYVE-1 may be a potent independent marker
for the clinical prognosis of HCC.
Acknowledgements
We would like to thank Mrs. Mieko
Hirokawa, Mr. Kanta Ohsuga and Mrs. Saki Okamoto for their
technical support. This study was supported by Health and Labour
Sciences Research Grants from the Ministry of Health, Labour and
Welfare of Japan - development of early detection systems for liver
cancer using molecular markers and diagnostic imaging in research
on hepatitis.
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Okuda K, Ohtsuki T, Obata H, et al:
Natural history of hepatocellular carcinoma and prognosis in
relation to treatment. Study of 850 patients. Cancer. 56:918–928.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nagasue N, Yukaya H, Hamada T, Hirose S,
Kanashima R and Inokuchi K: The natural history of hepatocellular
carcinoma. A study of 100 untreated cases. Cancer. 54:1461–1465.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Calvet X, Bruix J, Gines P, et al:
Prognostic factors of hepatocellular carcinoma in the west: a
multivariate analysis in 206 patients. Hepatology. 12:753–760.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Attali P, Prod’Homme S, Pelletier G, et
al: Prognostic factors in patients with hepatocellular carcinoma.
Attempts for the selection of patients with prolonged survival.
Cancer. 59:2108–2111. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Poon D, Anderson BO, Chen LT, et al:
Management of hepatocellular carcinoma in Asia: consensus statement
from the Asian Oncology Summit 2009. Lancet Oncol. 10:1111–1118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yamamoto A, Dhar DK, El-Assal ON, Igarashi
M, Tabara H and Nagasue N: Thymidine phosphorylase
(platelet-derived endothelial cell growth factor), microvessel
density and clinical outcome in hepatocellular carcinoma. J
Hepatol. 29:290–299. 1998. View Article : Google Scholar
|
|
10.
|
Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR
and Ma ZC: Microvessel density of hepatocellular carcinoma: its
relationship with prognosis. J Cancer Res Clin Oncol. 125:419–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
El-Assal ON, Yamanoi A, Soda Y, et al:
Clinical significance of microvessel density and vascular
endothelial growth factor expression in hepatocellular carcinoma
and surrounding liver: possible involvement of vascular endothelial
growth factor in the angiogenesis of cirrhotic liver. Hepatology.
27:1554–1562. 1998. View Article : Google Scholar
|
|
12.
|
Poon RT, Ng IO, Lau C, et al: Tumor
microvessel density as a predictor of recurrence after resection of
hepatocellular carcinoma: a prospective study. J Clin Oncol.
20:1775–1785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yang ZF and Poon RT: Vascular changes in
hepatocellular carcinoma. Anat Rec (Hoboken). 291:721–734. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
No authors listed:. The general rules for
the clinical and pathological study of primary liver cancer. Liver
Cancer Study Group of Japan. Jpn J Surg. 19:98–129. 1989.
View Article : Google Scholar
|
|
17.
|
Levine AJ and Puzio-Kuter AM: The control
of the metabolic switch in cancers by oncogenes and tumor
suppressor genes. Science. 330:1340–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Llovet JM, Chen Y, Wurmbach E, et al: A
molecular signature to discriminate dysplastic nodules from early
hepatocellular carcinoma in HCV cirrhosis. Gastroenterology.
131:1758–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Colombat M, Paradis V, Bieche I, et al:
Quantitative RT-PCR in cirrhotic nodules reveals gene expression
changes associated with liver carcinogenesis. J Pathol.
201:260–267. 2003. View Article : Google Scholar
|
|
20.
|
Mouta Carreira C, Nasser SM, di Tomaso E,
et al: LYVE-1 is not restricted to the lymph vessels: expression in
normal liver blood sinusoids and down-regulation in human liver
cancer and cirrhosis. Cancer Res. 61:8079–8084. 2001.PubMed/NCBI
|
|
21.
|
Arimoto J, Ikura Y, Suekane T, et al:
Expression of LYVE-1 in sinusoidal endothelium is reduced in
chronically inflamed human livers. J Gastroenterol. 45:317–325.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ko S, Nakajima Y, Kanehiro H, et al:
Significant influence of accompanying chronic hepatitis status on
recurrence of hepatocellular carcinoma after hepatectomy. Result of
multivariate analysis. Ann Surg. 224:591–595. 1996. View Article : Google Scholar
|
|
23.
|
Johnson LA, Prevo R, Clasper S and Jackson
DG: Inflammation-induced uptake and degradation of the lymphatic
endothelial hyaluronan receptor LYVE-1. J Biol Chem.
282:33671–33680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Katoh S, Miyagi T, Taniguchi H, et al:
Cutting edge: an inducible sialidase regulates the hyaluronic acid
binding ability of CD44-bearing human monocytes. J Immunol.
162:5058–5061. 1999.PubMed/NCBI
|
|
25.
|
Gee K, Kozlowski M and Kumar A: Tumor
necrosis factor-alpha induces functionally active
hyaluronan-adhesive CD44 by activating sialidase through p38
mitogen-activated protein kinase in lipopolysaccharide-stimulated
human monocytic cells. J Biol Chem. 278:37275–37287. 2003.
View Article : Google Scholar
|
|
26.
|
Prevo R, Banerji S, Ferguson D, Clasper S
and Jackson D: Mouse LYVE-1 is an endocytic receptor for hyaluronan
in lymphatic endothelium. J Biol Chem. 276:19420–19430. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Du Y, Liu Y, Wang Y, He Y, Yang C and Gao
F: LYVE-1 enhances the adhesion of HS-578T cells to COS-7 cells via
hyaluronan. Clin Invest Med. 34:E45–E54. 2011.PubMed/NCBI
|
|
28.
|
Hou WH, Liua IH, Huang SS and Huang JS:
CRSBP-1/LYVE-1 ligands stimulate contraction of the
CRSBP-1-associated ER network in lymphatic endothelial cells. FEBS
Lett. 586:1480–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ramani P, Dungwa JV and May MT: LYVE-1
upregulation and lymphatic invasion correlate with adverse
prognostic factors and lymph node metastasis in neuroblastoma.
Virchows Arch. 460:183–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Qin Z, Dai L, Bratoeva M, Slomiany MG,
Toole BP and Parsons C: Cooperative roles for emmprin and LYVE-1 in
the regulation of chemoresistance for primary effusion lymphoma.
Leukemia. 25:1598–1609. 2011. View Article : Google Scholar : PubMed/NCBI
|