Introduction
The concept of the sentinel lymph node (SLN) refers
to the first lymph node to receive lymphatic drainage from the
primary tumor. Sentinel lymph node biopsy (SLNB) is used to
determine the local and regional lymph node status of solid tumors.
It was first described by Cabanas (1) for the management of penile cancer and
has been widely used as an effective regional nodal staging
procedure after a 1992 landmark study on melanoma patients
(2). It is currently used in
several solid tumors, including breast cancer and melanoma
(3,4). The application and validity of SLNB
in cervical cancer should be practiced with caution, since the
lymphatic drainage of the cervix is significantly more complicated,
due to its midline position. Although a number of feasibility
studies for SLNB in cervical cancer have been conducted, SLNB does
not appear to be suitable for clinical application, due to the wide
range of reported detection rates, from 55.5 (5) to 100% (6). A high detection rate may render SLBN
in cervical cancer feasible in clinical practice, which may
decrease the complications, such as prolonged operation time, blood
loss, lymphocyst and lymphedema, experienced by patients undergoing
lymph node dissection (7). The
pelvic nodal involvement rate in early-stage cervical cancer cases
eligible for surgery was reported to be 0–4.8% in stage IA, 17% in
stage IB and 12–27% in stage IIA disease (8,9),
suggesting that lymph node dissection may not be beneficial in
>90% of stage IA cases. Therefore, a reliable SLBN is
crucial.
There is currently no clear assessment of SLNB
diagnostic performance. Therefore, a meta-analysis of the published
studies was conducted, with the aim to provide a comprehensive and
up-to-date overview of the feasibility and diagnostic value of SLNB
in cervical cancer.
Materials and methods
Search strategy
A comprehensive, systematic search for published
studies was performed using the search terms ‘cervical cancer’,
‘sentinel lymph node’, ‘sensitivity’ and ‘negative predictive
value’ in the PubMed database, with a time cutoff of September,
2012. The selected articles were limited to the English language.
Reviews, comments, letters, conference abstracts and case reports
were excluded from this analysis. Publications with a sample size
of <10 were also excluded, since they were considered as case
reports (10). The SLNB appears to
be a better testing method compared to positron emission
tomography, magnetic resonance imaging and computed tomography
(11). Furthermore, surgical
resection is the preferred therapeutic method for early cervical
cancer. Therefore, we analyzed the studies in which sentinel lymph
nodes (SLNs) were detected by the blue dye technique and/or by the
use of a radiotracer intraoperatively.
Data extraction
Two independent investigators carefully extracted
data from the selected articles using predefined tables, including
first author, year of publication, sample size, route of surgery,
detection method, type of pathological assessment and diagnostic
results (detection rate, mean SLN number, bilateral detection rate,
sensitivity and negative predictive value).
Statistical analysis
The detection rate, sensitivity and negative
predictive values were pooled with the random effects model of
DerSimonian and Laird (13), using
MetaAnalyst Beta 3.13 software (Tufts Medical Center, Boston, MA,
USA) (12). The potential
heterogeneity among the studies was assessed using the Q-statistic
and P<0.1 was considered to indicate a statistically significant
difference. If heterogeneity was present, a subgroup analysis was
used for further assessment.
Results
Study selection and description
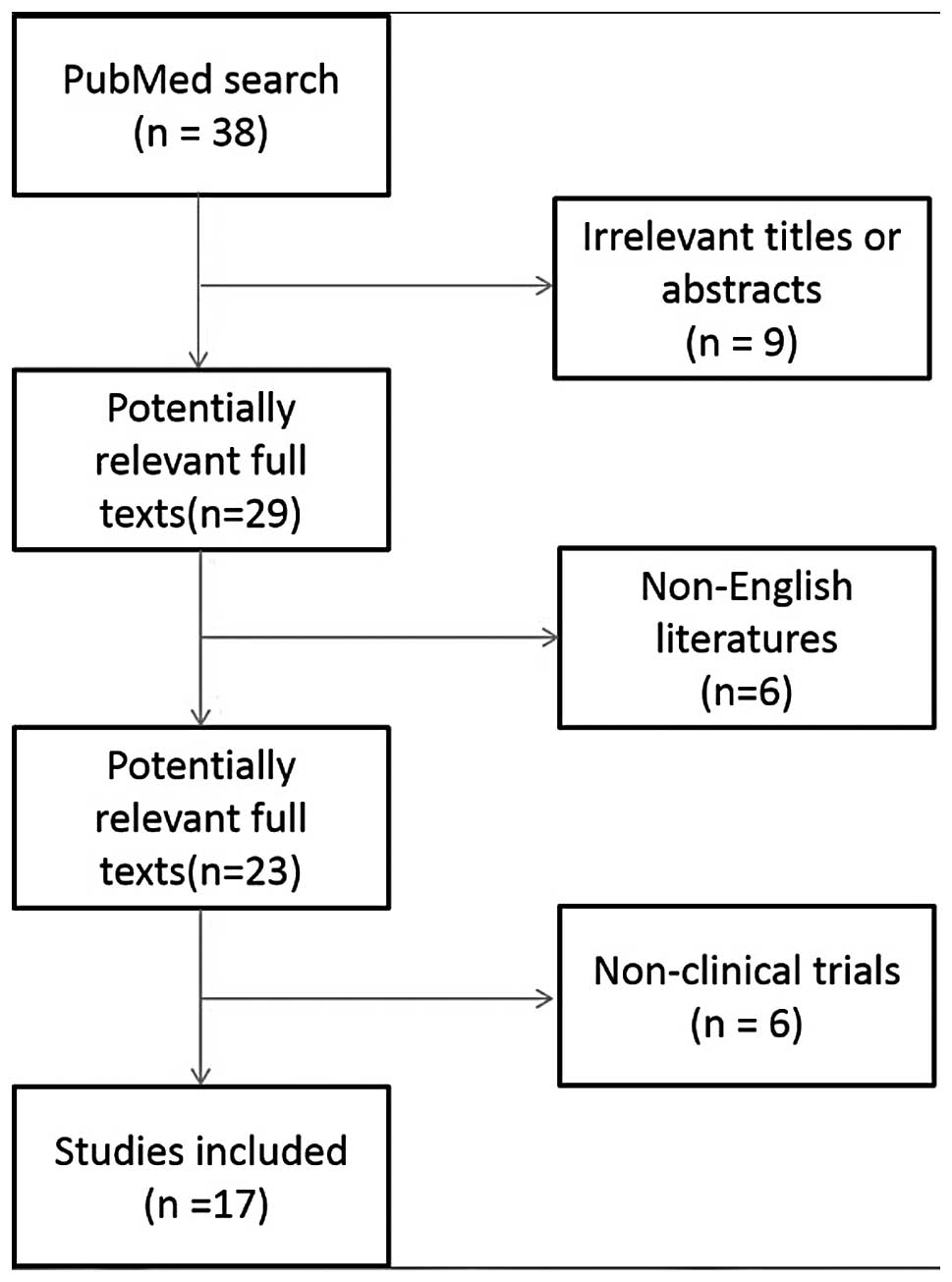
A total of 38 studies were identified with the
established search strategy. Studies that were clearly not
eligible, as indicated by the information provided in the abstract,
were excluded. For the remaining studies, the full text was read.
Finally, a total of 17 studies were included in the analysis,
involving a total of 1,112 patients (5,6,14–28).
The study selection process is summarized in Fig. 1. The median number of included
patients per study was 50 (range, 12–211). The detailed
characteristics of the 17 eligible studies are summarized in
Table I and the diagnostic
performance of SLNB is summarized in Table II.
 | Table I.Characteristics of the 17 studies
included in the meta-analysis. |
Table I.
Characteristics of the 17 studies
included in the meta-analysis.
| First author | Year | Sample size | Route of surgery | Detection method | Pathological
assessment | Refs. |
|---|
| Malur | 2001 | 50 |
Laparoscopy/laparotomy | D+I | HE | (5) |
| Levenback | 2002 | 39 | Laparotomy | D+I | HE | (6) |
| Rhim | 2002 | 26 | Laparotomy | D+I | HE+IHC | (14) |
| Lambaudie | 2003 | 12 | Laparoscopy | D+I | HE+IHC | (15) |
| Plante | 2003 | 70 | Laparoscopy | D+I | HE+IHC | (16) |
| Niikura | 2004 | 20 | Laparotomy | D+I | HE+IHC | (17) |
| Roca | 2005 | 40 |
Laparoscopy/laparotomy | D+I | HE+IHC | (18) |
| Silva | 2005 | 56 | Laparotomy | Isotope | HE+IHC | (19) |
| Wydra | 2006 | 100 | Laparotomy | D+I | HE+IHC | (20) |
| Zhang | 2006 | 27 | ND | D+I | HE | (21) |
| Schwendinger | 2006 | 47 | Laparotomy | Dye | HE+IHC | (22) |
| Kara | 2008 | 32 | Laparotomy | Isotope | HE+IHC | (23) |
| Pazin | 2009 | 50 | ND | Dye | ND | (24) |
| Darlin | 2010 | 105 |
Laparoscopy/laparotomy | Isotope | HE+IHC | (25) |
| Ogawa | 2010 | 82 | ND | Isotope | HE | (26) |
| Lecuru | 2011 | 145 | Laparoscopy | D+I | HE+IHC | (27) |
| Roy | 2011 | 211 | Laparoscopy | D+I | HE+IHC | (28) |
 | Table II.Diagnostic performance of the 17
studies included in the meta-analysis. |
Table II.
Diagnostic performance of the 17
studies included in the meta-analysis.
| First author | Year | Sample size | Detection rate
(%) | Bilateral detection
rate (%) | Mean no. of SLNs
(%) | Sensitivity | Negative predictive
value (%) | Refs. |
|---|
| Malur | 2001 | 50 | 78 | NA | 2 | 83.3 | 97 | (5) |
| Levenback | 2002 | 39 | 100 | 72 | 4 | 87.5 | 97 | (6) |
| Rhim | 2002 | 26 | 100 | NA | 2 | 80 | 95.2 | (14) |
| Lambaudie | 2003 | 12 | 92 | 83 | 3.1 | 66.7 | 90 | (15) |
| Plante | 2003 | 70 | 87 | 60 | 1.9 | 100 | 100 | (16) |
| Niikura | 2004 | 20 | 90 | 75 | 2.3 | 100 | 100 | (17) |
| Roca | 2005 | 40 | 100 | NA | 2.5 | 100 | 100 | (18) |
| Silva | 2005 | 56 | 93 | 41 | 2.3 | 82.3 | 92.1 | (19) |
| Wydra | 2006 | 100 | 84 | 66 | 1.8 | 86.4 | 95.5 | (20) |
| Zhang | 2006 | 27 | 100 | 74 | 2.6 | 85.7 | 95.2 | (21) |
| Schwendinger | 2006 | 47 | 83 | NA | 2 | 90 | 97 | (22) |
| Kara | 2008 | 32 | 100 | 50 | 2.1 | 100 | 100 | (23) |
| Pazin | 2009 | 50 | 92 | 38 | 2.6 | 85 | 89.6 | (24) |
| Darlin | 2010 | 105 | 90 | 59 | 1 | 94 | 99 | (25) |
| Ogawa | 2010 | 82 | 88 | 64 | 2.2 | 100 | 100 | (26) |
| Lecuru | 2011 | 145 | 98 | 76 | 3 | 92 | 98.2 | (27) |
| Roy | 2011 | 211 | 99 | 86 | NA | 90.6 | 94.2a | (28) |
Analysis of the 17 studies
The diagnostic value of SLNB was affected by several
factors and significant heterogeneity was identified (Table I). Therefore, data were pooled
using a random effects model.
The pooled detection rate of SLN was 92.2% (95% CI:
88.3–94.8%; Fig. 2), whereas the
pooled sensitivity and negative predictive values were 88.8 and
95.0%, respectively (Fig. 3A and
B).
Subgroup analysis of three factors
The Q-statistic P-values of the heterogeneity test
were <0.1. We performed subgroup analyses according to the route
of surgery (laparoscopy or laparotomy), the detection method (dye,
isotope or a combination of the two), pathological assessment type
[hematoxylin-eosin staining (HE) or HE + immunohistochemistry
(IHC)]. The results of the subgroup analyses are presented in
Table III.
 | Table III.Meta-analysis results of the 17
studies. |
Table III.
Meta-analysis results of the 17
studies.
| Diagnostic
parameters | Detection rate %
(95% CI) | Sensitivity % (95%
CI) | Negative predictive
value% (95% CI) |
|---|
| Overall (17
studies) | 92.2
(88.3–94.8) | 88.8
(85.1–91.7) | 95.0
(92.8–96.6) |
| Laparoscopy (4
studies) | 96.1
(85.5–99.0) | 89.8
(79.5–95.2) | 96.2
(90.9–98.5) |
| Laparotomy (7
studies) | 90.2
(83.1–94.5) | 86.3
(81.4–90.1) | 95.3
(92.2–97.2) |
| Dye (2
studies) | 87.5
(75.3–94.1) | 87.2
(78.9–92.6) | 93.2
(79.9–97.9) |
| Isotope (4
studies) | 90.3
(86.0–93.4) | 94.4
(82.6–98.4) | 94.5
(85.6–98.1) |
| D+I (11
studies) | 94.3
(88.5–97.2) | 88.0
(83.2–91.6) | 95.7
(93.8–97.0) |
| HE (4 studies) | 89.4
(75.8–95.8) | 88.0
(77.3–94.1) | 93.7
(86.1–97.3) |
| HE+IHC (12
studies) | 93.1
(88.6–96.0) | 89.6
(85.0–92.9) | 95.7
(93.7–97.1) |
When considering the route of surgery, the pooled
SLN detection rate in the laparoscopy subgroup (4 studies) was
96.1%, compared to 90.2% in the laparotomy subgroup (7 studies).
The sensitivity and negative predictive values of the two subgroups
were 89.8 vs. 86.3% and 96.2 vs. 95.3%, respectively.
When considering the detection method, the pooled
SLN detection rate in dye subgroup (2 studies), the isotope
subgroup (4 studies) and the combination of the two subgroup (11
studies) was 87.5, 90.3 and 94.3%, respectively. The pooled
negative predictive value of the combination subgroup was higher
compared to that of the dye and isotope subgroups.
When considering the pathological assessment type,
the pooled detection rates in the HE+IHC subgroup (12 studies) was
93.1% and in the HE subgroup 89.4% (4 studies).
Discussion
In the present meta-analysis we pooled the detection
rate, sensitivity and negative predictive values with data
extracted from 17 studies. The overall SLN detection rate was
92.2%, which was satisfactory in the SLNB of cervical cancer,
whereas the high pooled sensitivity (88.8%) and negative predictive
values (95.0%) also indicated that this procedure is feasible.
However, the diagnostic parameters were affected by
several factors, such as the route of surgery, the detection
method, the pathological assessment type and other
predictable/unpredictable factors. Subgroup analyses were performed
for the three factors mentioned above.
First, in the subgroup analysis according to the
route of surgery, we observed that the detection rate of
laparoscopy was superior to that of laparotomy (96.1 vs. 90.2%).
Furthermore, laparoscopy exhibited a higher sensitivity compared to
laparotomy (89.8 vs. 86.3%), with wide visual fields and minimal
incisions. Therefore, the technologically improved laparoscopic
equipment is recommended for the surgical treatment of cervical
cancer.
Second, the SLN detection rate in cervical cancer
with the combination of dye and isotope (94.3%) was higher compared
to that of dye (87.5%) or isotope (90.3%) alone. In addition, the
pooled negative predictive value exhibited the same trend. Van de
Lande et al (29) also
reported that the combination of a radionuclide with a blue dye was
the optimal method of SLN detection in a systematic review that
mainly compared the three methods.
Finally, when the pathological assessment with HE +
IHC was used to determine the lymph node status, higher detection
and sensitivity rates were achieved compared to the HE group (93.1
vs. 89.4% and 89.6 vs. 88.0%, respectively). IHC may accurately
determine the lymph node status, since it is able to detect
micrometastases, compared to HE alone (30). Therefore, under the appropriate
conditions, IHC is recommended in SLNB.
In this meta-analysis, the predetermined search
strategy described above was used for the selection of studies from
the available literature. Several studies on SLNB in cervical
cancer were identified; however, the data used by each study to
describe the performance characteristics were inconsistent, due to
the different objectives of the studies. Several studies reported
data that were not sufficient to calculate sensitivity or negative
predictive values, leading to the exclusion of those studies.
Furthermore, in view of the limited time and
capacity, newly published literature was not included in our
study.
In conclusion, we analyzed the performance
characteristics of SLNB in cervical cancer using data from 17
studies, including a total of 1,112 patients. Although SLNB appears
to exhibit a satisfactory diagnostic performance in the
meta-analysis, further studies are required to determine the true
performance of the clinical application of SLNB in cervical
cancer.
References
|
1.
|
Cabanas RM: An approach for the treatment
of penile carcinoma. Cancer. 39:456–466. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Morton DL, Wen DR, Wong JH, Economou JS,
Cagle LA, Storm FK, et al: Technical details of intraoperative
lymphatic mapping for early stage melanoma. Arch Surg. 127:392–399.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Morrow M, Rademaker AW, Bethke KP,
Talamonti MS, Dawes LG, Clauson J and Hansen N: Learning sentinel
node biopsy: results of a prospective randomized trial of two
techniques. Surgery. 126:714–720. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lyman GH, Giuliano AE, Somerfield MR, et
al: American Society of Clinical Oncology: American Society of
Clinical Oncology guideline recommendations for sentinel lymph node
biopsy in early-stage breast cancer. J Clin Oncol. 23:7703–7720.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Malur S, Krause N, Kohler C and Schneider
A: Sentinel lymph node detection in patients with cervical cancer.
Gynecol Oncol. 80:254–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Levenback C, Coleman RL, Burke TW, Lin WM,
Erdman W, Deavers M and Delpassand ES: Lymphatic mapping and
sentinel node identification in patients with cervix cancer
undergoing radical hysterectomy and pelvic lymphadenectomy. J Clin
Oncol. 20:688–693. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Achouri A, Huchon C, Bats AS, Bensaid C,
Nos C and Lecuru F: Complications of lymphadenectomy for
gynecologic cancer. Eur J Surg Oncol. 39:81–86. 2013. View Article : Google Scholar
|
|
8.
|
Ayhan A, Celik H, Dursun P, Gultekin M and
Yuce K: Prognostic and therapeutic importance of lymphadenectomy in
gynecological cancers. Eur J Gynaecol Oncol. 25:279–286.
2004.PubMed/NCBI
|
|
9.
|
Hauspy J, Beiner M, Harley I, Ehrlich L,
Rasty G and Covens A: Sentinel lymph node in vulvar cancer. Cancer.
110:1015–1023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kang S, Yoo HJ, Hwang JH, Lim MC, Seo SS
and Park SY: Sentinel lymph node biopsy in endometrial cancer:
meta-analysis of 26 studies. Gynecol Oncol. 123:522–527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Selman TJ, Mann C, Zamora J, Appleyard TL
and Khan K: Diagnostic accuracy of tests for lymph node status in
primary cervical cancer: a systematic review and meta-analysis.
CMAJ. 178:855–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wallace BC, Schmid CH, Lau J and
Trikalinos TA: Meta-Analyst: software for meta-analysis of binary,
continuous and diagnostic data. BMC Med Res Methodol. 9:802009.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Rhim CC, Park JS, Bae SN and Namkoong SE:
Sentinel node biopsy as an indicator for pelvic nodes dissection in
early stage cervical cancer. J Korean Med Sci. 17:507–511. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lambaudie E, Collinet P, Narducci F,
Sonoda Y, Papageorgiou T, Carpentier P, Leblanc E and Querleu D:
Laparoscopic identification of sentinel lymph nodes in early stage
cervical cancer: prospective study using a combination of patent
blue dye injection and technetium radiocolloid injection. Gynecol
Oncol. 89:84–87. 2003. View Article : Google Scholar
|
|
16.
|
Plante M, Renaud MC, Tetu B, Harel F and
Roy M: Laparoscopic sentinel node mapping in early-stage cervical
cancer. Gynecol Oncol. 91:494–503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Niikura H, Okamura C, Akahira J, Takano T,
Ito K, Okamura K and Yaegashi N: Sentinel lymph node detection in
early cervical cancer with combination 99mTc phytate and
patent blue. Gynecol Oncol. 94:528–552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Roca I, Caresia AP, Gil-Moreno A, et al:
Usefulness of sentinel lymph node detection in early stages of
cervical cancer. Eur J Nucl Med Mol Imaging. 32:1210–1216. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Silva LB, Silva-Filho AL, Traiman P, et
al: Sentinel node detection in cervical cancer with
99mTc-phytate. Gynecol Oncol. 97:588–595. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wydra D, Sawicki S, Wojtylak S, Bandurski
T and Emerich J: Sentinel node identification in cervical cancer
patients undergoing transperitoneal radical hysterectomy: a study
of 100 cases. Int J Gynecol Cancer. 16:649–654. 2006. View Article : Google Scholar
|
|
21.
|
Zhang WJ, Zheng R, Wu LY, Li XG, Li B and
Chen SZ: Clinical application of sentinel lymph node detection to
early stage cervical cancer. Chin J Cancer. 25:224–228. 2006.(In
Chinese).
|
|
22.
|
Schwendinger V, Muller-Holzner E, Zeimet
AG and Marth C: Sentinel node detection with the blue dye technique
in early cervical cancer. Eur J Gynaecol Oncol. 27:359–362.
2006.PubMed/NCBI
|
|
23.
|
Kara PP, Ayhan A, Caner B, Gultekin M,
Ugur O, Bozkurt MF and Usubutun A: Sentinel lymph node detection in
early stage cervical cancer: a prospective study comparing
preoperative lymphoscintigraphy, intraoperative gamma probe, and
blue dye. Ann Nucl Med. 22:487–494. 2008. View Article : Google Scholar
|
|
24.
|
Pazin V, Dragojević S, Miković Z, et al:
The value of sentinel lymphadenectomy in radical operative
treatment of cervical cancer. Mil Med Pharm Rev. 66:539–543.
2009.PubMed/NCBI
|
|
25.
|
Darlin L, Persson J, Bossmar T, Lindahl B,
Kannisto P, Måsbäck A and Borgfeldt C: The sentinel node concept in
early cervical cancer performs well in tumors smaller than 2 cm.
Gynecol Oncol. 117:266–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ogawa S, Kobayashi H, Amada S, et al:
Sentinel node detection with 99mTc phytate alone is
satisfactory for cervical cancer patients undergoing radical
hysterectomy and pelvic lymphadenectomy. Int J Clin Oncol.
15:52–58. 2010.
|
|
27.
|
Lécuru F, Mathevet P, Querleu D, et al:
Bilateral negative sentinel nodes accurately predict absence of
lymph node metastasis in early cervical cancer: results of the
SENTICOL study. J Clin Oncol. 29:1686–1691. 2011.
|
|
28.
|
Roy M, Bouchard-Fortier G, Popa I,
Grégoire J, Renaud MC, Têtu B and Plante M: Value of sentinel node
mapping in cancer of the cervix. Gynecol Oncol. 122:269–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Van de Lande J, Torrenga B, Raijmakers PG,
Hoekstra OS, van Baal MW, Brölmann HA and Verheijen RH: Sentinel
lymph node detection in early stage uterine cervix carcinoma: a
systematic review. Gynecol Oncol. 106:604–613. 2007.PubMed/NCBI
|
|
30.
|
Van Trappen PO and Pepper MS: Lymphatic
dissemination of tumour cells and the formation of micrometastases.
Lancet Oncol. 3:44–52. 2002.PubMed/NCBI
|