Introduction
Primary ovarian carcinoma is often chemosensitive,
particularly the serous papillary adenocarcinoma type. Therefore,
patients with advanced ovarian carcinoma often undergo aggressive
surgical cytoreduction, followed by combination antitumor
chemotherapy. Peritoneal tuberculosis is rare and may present with
symptoms similar to those of advanced ovarian carcinoma.
The treatment of advanced ovarian carcinoma
coexistent with peritoneal tuberculosis requires the administration
of chemotherapy effective against both diseases. However, the
immunosuppression induced by the antitumor chemotherapy may
exacerbate the tuberculosis and the antituberculous chemotherapy
may limit the administration of anticancer chemotherapy due to its
adverse effects. There are few available case reports of patients
treated with antituberculous and antitumor chemotherapy for
Müllerian carcinoma (1–3).
The plasma concentration of paclitaxel was measured
at various time intervals after the first combined administration
of antituberculous and antitumor chemotherapy and further treatment
was planned based on these data. To the best of our knowledge, this
is the first case report of a patient with primary advanced ovarian
carcinoma coexistent with peritoneal tuberculosis.
Materials and methods
Methods
Staging was performed based on the criteria of the
International Federation of Gynecology and Obstetrics (FIGO). After
pathological examination had confirmed the diagnosis of ovarian
serous papillary adenocarcinoma coexistent with peritoneal
tuberculosis, informed consent was obtained from the patient and
her family prior to treatment. Blood (6 ml) was collected for
analysis at 8 time points. The blood samples were centrifuged
(1,600 × g for 10 min) to separate the plasma, which was stored at
−80°C until assayed. The paclitaxel concentrations were measured by
a local laboratory (SRL Co., Ltd., Tokyo, Japan), using the
high-performance liquid chromatography-ultraviolet method (4).
Case report
A 47-year-old gravida 1, para 1 patient underwent
exploratory surgery for ovarian carcinoma by a gynecological
surgeon at another hospital, which revealed severe adhesions around
a primary large right ovarian tumor and severe carcinomatous
peritonitis. A metastatic tumor was identified on the left ovary
and left salpingo-oophorectomy was performed. Based on these
findings, the patient was diagnosed with serous papillary
adenocarcinoma of the ovary, clinical stage IIIc and was referred
to our hospital. After obtaining informed consent, the patient
underwent three courses of neoadjuvant chemotherapy with tri-weekly
paclitaxel and carboplatin [TC; paclitaxel 175 mg/m2
body surface area, carboplatin area under the concentration/time
curve (AUC)=6]. Subsequently, the patient underwent total abdominal
hysterectomy, right salpingo-oophorectomy, omentectomy and pelvic
and para-aortic lymphadenectomy. There were numerous residual
metastases (<2mm) on the peritoneum with mild fibrous adhesions
around the right adnexa and one 22-mm tumor on the peritoneum over
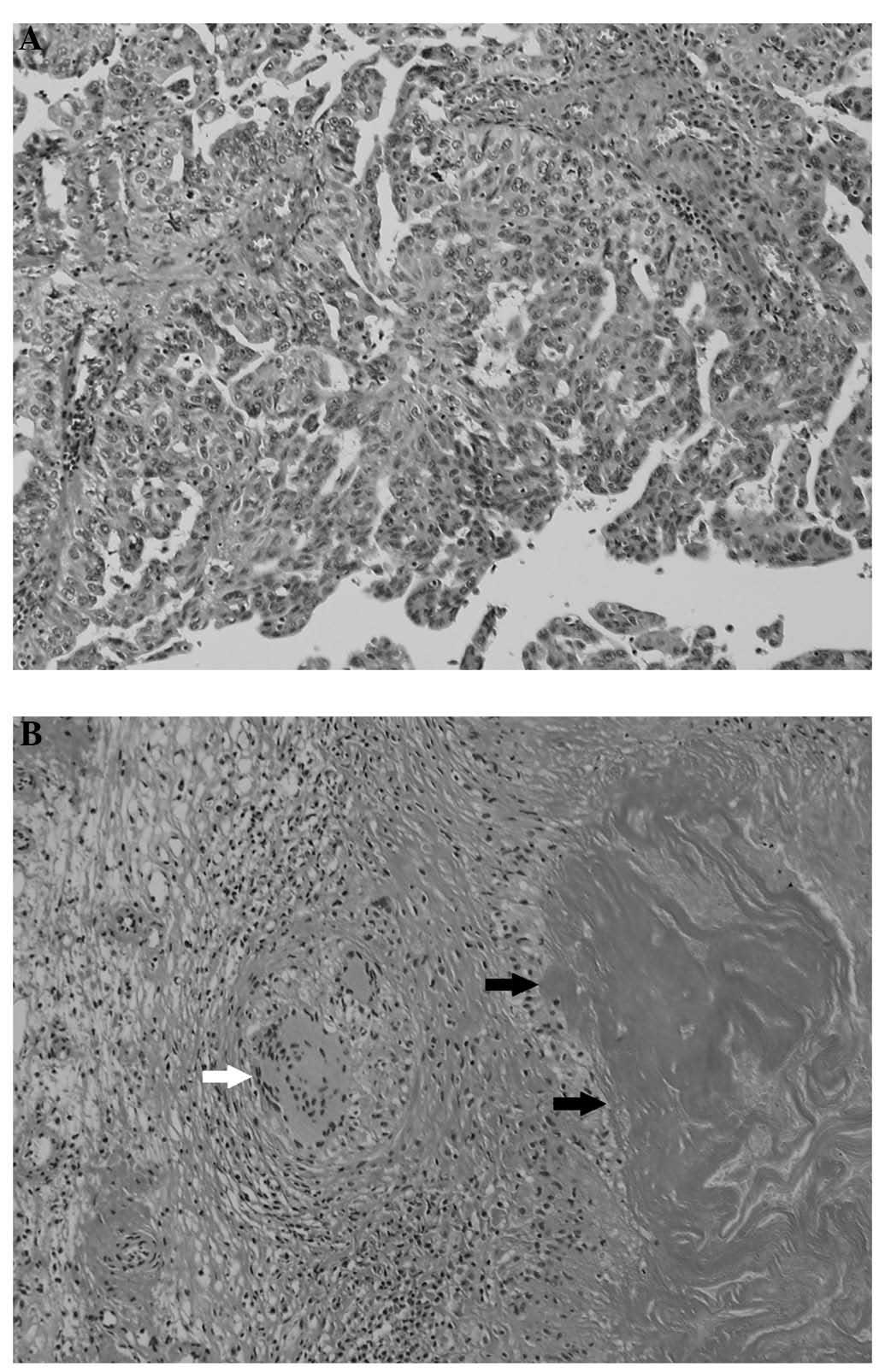
the ileum with mild fibrous adhesions. The microscopic examination
of the tumor revealed Langhans-type giant cells, caseous necrosis
and lymphocytic infiltration (Fig.
1). The Ziehl-Neelsen staining was negative. The culture and
polymerase chain reaction examination of the sputum did not reveal
tuberculosis bacteria and the interferon-γ release assay
(QuantiFERON-TB Gold; Cellestis Ltd, Victoria, Australia) was
negative. No evidence of tuberculosis of the lungs or other organs
was detected on computed tomography and the diagnosis was confirmed
as ovarian serous papillary adenocarcinoma coexistent with
peritoneal tuberculosis.
Treatment
The standard antituberculous therapy used in Japan
was administered, with isoniazid (300 mg/day), rifampicin (600
mg/day) and ethambutol (1.5 g/day). The patient completed 6 months
of ethambutol and 12 months of isoniazid and rifampicin
administration, according to the guidelines. After confirming the
safety of the antituberculous drugs for 2 weeks, four courses of
paclitaxel (70 mg/m2) and carboplatin (AUC=2) were
administered on days 1, 8 and 15 every 4 weeks (weekly TC regimen)
for ovarian carcinoma. The patient developed an elevation of
aspartate aminotransferase and alanine aminotransferase levels and
thrombocytopenia. The rifampicin administration was discontinued on
days 1, 8, and 15 and the paclitaxel and carboplatin doses were
reduced by 20% from the third course of antitumor chemotherapy
onwards.
Follow-up
After the completion of four courses of antitumor
chemotherapy, the patient was regularly followed up at our
hospital. Imaging examination findings and carbohydrate antigen 125
levels remained within normal limits. The patient has remained
alive and recurrence-free for 5 years.
Results
Plasma concentrations of paclitaxel
The antitumor chemotherapy was initiated after we
confirmed the safety of the antituberculous chemotherapy for 2
weeks. In the morning of day 1 of the first course of weekly TC,
the patient received antituberculous drugs and 2 h later paclitaxel
was administered over 1 h, followed by carboplatin over 1 h. For
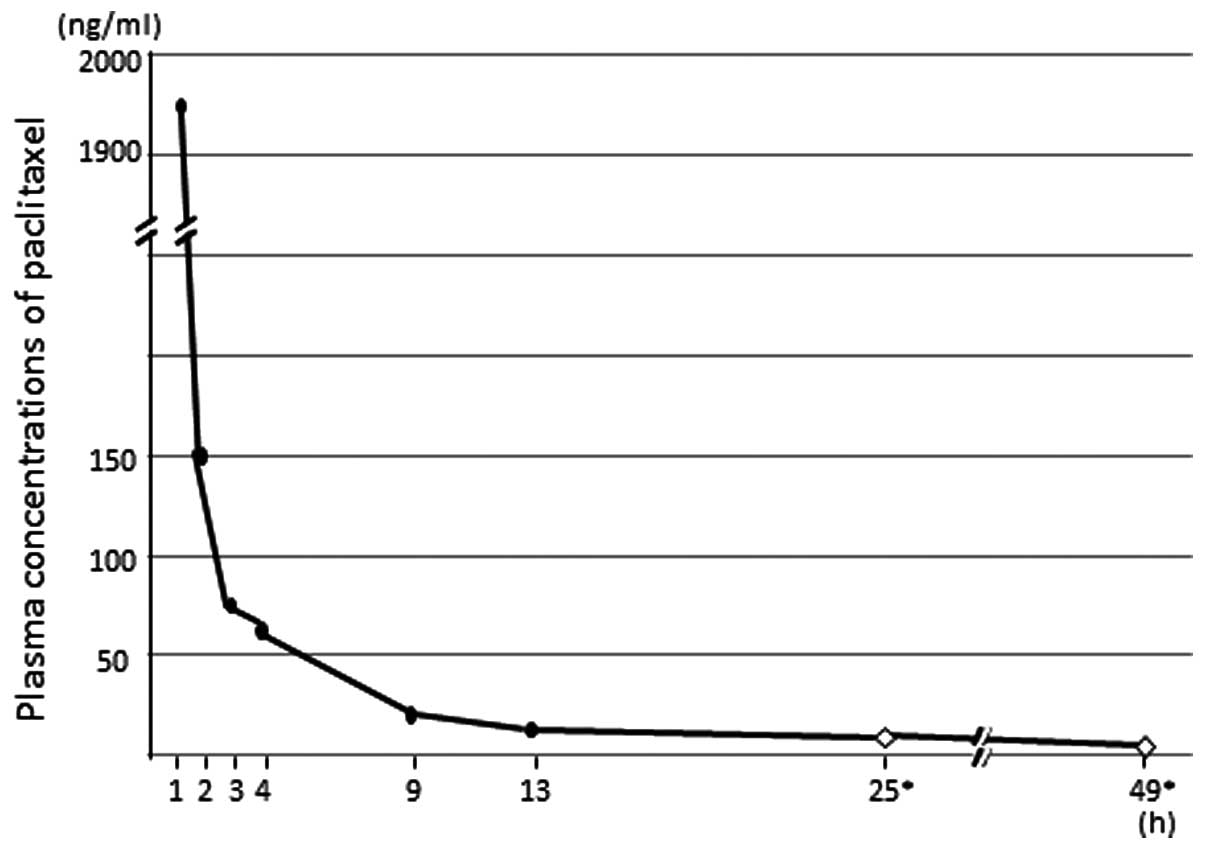
pharmacokinetic analysis of paclitaxel, blood samples were
collected at 1, 2, 3, 4, 9, 13, 25 and 49 h after the initiation of
paclitaxel administration. The plasma concentrations of paclitaxel
after the first course of antitumor chemotherapy are illustrated in
Fig. 2.
Discussion
It has not been determined whether a regimen of
isoniazid and ethambutol affects the pharmacokinetics of
paclitaxel. The plasma concentration of paclitaxel was measured,
since rifampicin induces cytochrome P450 3A4 and may therefore
accelerate the metabolism of paclitaxel, resulting in a lower
concentration and effect of paclitaxel and increasing the risk of
hepatic dysfunction.
The plasma concentration of paclitaxel was highest 1
h after the initiation of its administration and decreased to
<10 ng/ml after 25 h. A previous study reported that the mean
26-h concentration after a dose of 100 mg/m2 was 0.04
μmol/l (∼34.16 ng/ml) (5)
and in the 9 patients for whom 26-h concentration data were
available, the plasma concentration was >0.01 μmol/l
(∼8.54 ng/ml), which is the minimum concentration required for its
antineoplastic effect (6). We
considered that rifampicin may enhance the metabolism of paclitaxel
and observed that the concentration of paclitaxel decreased below
its effective blood level soon after administration. Therefore, the
administration of rifampicin was discontinued on days 1, 8 and 15.
However, the plasma concentration of paclitaxel was not measured
after the first course of antitumor chemotherapy.
In conclusion, although rifampicin may enhance the
metabolism of paclitaxel, we suggest that it may be possible to
administer concurrent antituberculous and antitumor chemotherapy
under close observation.
References
|
1.
|
Chen CH, Huang CY and Chow SN: Early-stage
ovarian carcinoma combined with pulmonary tuberculosis mimicking
advanced ovarian cancer: a case report. Int J Gynecol Cancer.
14:1007–1011. 2004. View Article : Google Scholar
|
|
2.
|
Tuon FF, Miyaji KT, de Vidal PM, da Silva
LF, Kono A and Franca FO: Simultaneous occurrence of pulmonary
tuberculosis and carcinomatous lymphangitis. Rev Soc Bras Med Trop.
40:76–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ingec M, Erdogan F, Kumtepe Y, Isaoglu U,
Gundogdu C and Kadanali S: Management of bilateral fallopian tube
carcinoma coexistent with tuberculous salpingitis. J Obstet
Gynaecol Res. 31:65–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Longnecker SM, Donehower RC, Cates AE, et
al: High-performance liquid chromatographic assay for taxol in
human plasma and urine and pharmacokinetics in a phase I trial.
Cancer Treat Rep. 71:53–59. 1987.PubMed/NCBI
|
|
5.
|
Seidman AD, Hudis CA, Albanell J, Tong W,
Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J
and Norton L: Dose-dense therapy with weekly 1-hour paclitaxel
infusions in the treatment of metastatic breast cancer. J Clin
Oncol. 16:3353–3361. 1998.PubMed/NCBI
|
|
6.
|
Jordan MA, Wendell K, Gardiner S, Derry
WB, Copp H and Wilson L: Mitotic block induced in HeLa cells by low
concentrations of paclitaxel (Taxol) results in abnormal mitotic
exit and apoptotic cell death. Cancer Res. 56:816–825.
1996.PubMed/NCBI
|