Introduction
Hepatocellular carcinoma (HCC) is ranked as the
third most common cause of cancer-related mortality worldwide, with
>500,000 cases diagnosed annually (1). Epidemiologic evidence demonstrated
that HCC is frequently associated with hepatitis B virus (HBV)
infection in China (2). Hepatic
resection and transplantation, resulting in 5-year survival rates
of 30–70%, were shown to be beneficial for <20% of patients
(3). Therefore, local tumor
control is crucial for patients with unresectable HCC prior to
liver transplantation. Over the last decade, transarterial
chemoembolization (TACE), radiofrequency ablation, Yttrium-90
microsphere embolization and percutaneous ethanol injection have
been commonly used for improving local tumor control (4). However, these regimens exhibit
limitations regarding lesion size, location, number and
distribution.
The incidence of portal vein tumor thrombosis (PVTT)
is 30–40% in HCC patients. PVTT is a poor prognostic factor, which
may lead to wide dissemination of tumor cells throughout the liver,
severely compromising liver function (5). TACE has become one of the major
treatment options for patients with unresectable HCC, although its
efficacy remains controversial (6–7).
Alternative treatment options, such as intra-arterial chemotherapy
and immunotherapy, have not demonstrated survival benefits
(8–9). Therefore, an efficient way to improve
local control for HCC with PVTT is required.
The application of traditional radiotherapy in the
treatment of HCC is limited, due to radiation-induced liver disease
(RILD) and the low tolerance of the liver to radiotherapy (10). Recent advances in radiotherapy
techniques, including three-dimensional conformal radiotherapy
(3D-CRT) and image-guided radiotherapy, have enabled the delivery
of a higher radiation dose to the tumor rather than to the
surrounding normal tissues (11).
Stereotactic body radiotherapy (SBRT), a 3D-CRT irradiation
technique developed to optimize target dose delivery and normal
tissue sparing, is emerging as a viable treatment option for HCC
patients. Accumulating evidence demonstrated that SBRT has become a
treatment option for local tumor control of primary and secondary
malignancies of the liver. Promising responses to high-dose
radiotherapy to partial liver volume in patients with unresectable
HCC have been observed (12–14).
The combination of TACE with SBRT has been previously used in the
management of HCC patients with PVTT (15–16).
Despite its potential advantages, the combination of TACE with SBRT
to treat advanced HCC with PVTT is poorly documented, with only a
few available case reports (15).
In this study, we retrospectively investigated patients with
primary HCC and evaluated the response rate and toxicity of SBRT
alone and SBRT combined with TACE for advanced HCC with PVTT.
Materials and methods
Ethics statement
The patients completed a questionnaire and provided
written informed consent. The study was performed according to the
Helsinki Declaration and the samples were processed following
approval of the written consent statement by the Ethics Committee
of the Navy General Hospital.
Patients
Between February, 2004 and March 2008, a consecutive
case series of HCC patients with major vascular invasion (PVTT and
inferior vena cava tumor thrombus), treated by TACE and γ-SBRT at
the Department of Radiotherapy, Navy General Hospital, Beijing,
were retrospectively investigated. The eligibility criteria were as
follows: patient age of ≥18 years, definite diagnosis of primary
HCC with major vascular invasion (PVTT and inferior vena cava tumor
thrombus), class A or B liver function according to the Child-Pugh
classification and absence of previous radiotherapy to the liver.
In all the patients, the diagnosis of HCC was based on histological
confirmation or a characteristic tumor appearance on at least two
imaging studies [including dynamic computed tomography (CT) scans,
positron emission tomography-CT and dynamic contrast-enhanced
magnetic resonance imaging (MRI) scans] and the presence of risk
factors including HBV and HCV infection and cirrhosis. The presence
and extent of PVTT were assessed by multiphase dynamic CT scans
with a routine slice thicknesses of 5 mm, using the following
criteria: i) a low-attenuation intraluminal filling defect adjacent
to the primary tumor during the portal phase and ii) an enhanced
inner side of the filling defect during the arterial phase
(17).
Study design and procedures
The study protocol was approved by the Ethics
Committee of our hospital. A total of 120 patients were enrolled in
the study and SPSS software, version 12.0 (SPSS Inc., Chicago, IL,
USA) was used to randomly assign the observations into 3 equal
sized groups (seed=85,123), with each group including 40 patients.
Finally, 101 patients met the inclusion criteria. After picking out
the ineligible observations, the group assignment was as follows:
group A, 34 patients treated with γ-SBRT followed by TACE within
the next 2–3 weeks; group B, 37 patients treated with TACE followed
by γ-SBRT within the next 2–3 weeks; and group C, 30 patients
treated with γ-SBRT alone.
Treatment process
The Seldinger method was used to puncture and
cannulate the proper hepatic artery through the femoral artery.
Following placement of a catheter, a mixture comprising 3–10 ml of
iodized oil (Lipiodol; Laboratoires André Guerbet,
Aulnay-sous-Bois, Paris, France), 600–1,000 mg fluorouracil and
30–50 mg cisplatin was infused using a catheter placed directly
into the right or left hepatic artery, with embolization performed
using gelatin sponge cubes (Gelfoam; Upjohn, Kalamazoo, MI, USA).
The treatment was repeated at 6–8-week intervals until complete
disappearance of the viable intrahepatic tumor, provided that
hepatic function was preserved. TACE (median number of sessions, 2;
range, 1–4) was performed prior to or following SBRT in the
patients of group A or B, whereas patients in group C were treated
with SBRT alone. The interval between TACE and SBRT was at least 4
weeks.
SBRT was planned following identification of PVTT at
initial presentation or follow-up imaging and was initiated 2–3
weeks prior to or following TACE. Tumors imaged on the planning
triphasic CT and/or MRI enhancing large vessel thromboses were
included within the gross target volume [gross tumor volume (GTV)].
An 8-mm margin around the GTV within the liver and non-enhancing
thromboses was included within the clinical target volume. The
planning target volume (PTV) margins were individualized (minimum,
5 mm), as previously described (18). The PTV around the GTV was the
primary target (PTVPrimary), whereas the PTV around the
clinical target volume (PTVSecondary) was a secondary
target. Conformal planning was used, with 3–10 coplanar or
non-coplanar beams of 6–18 MV, with up to 3 segments within each
field.
The dose-volume histogram for the liver minus the
GTV (referred to as liver) was used to estimate the risk of RILD
and to allocate the dose to the PTVPrimary. The dose to
the PTVPrimary was allocated depending on the effective
volume of irradiated liver (Veff) (Appendix, online
only) and the uninvolved liver volume with a maximum dose of 60 Gy.
The target dose to PTVSecondary, containing possible
microscopic disease, was 24 Gy. SBRT was delivered in 6 fractions
distributed over 2 weeks, usually on alternate days. The maximum
permitted dose to 0.5 ml of the esophagus, stomach, duodenum or
bowel was 30 Gy. The maximum dose to the spinal cord was 27 Gy and
to the heart 40 Gy. Efforts were made to minimize the irradiation
of normal tissues.
Evaluation
Patients underwent abdominal CT scans 1 month
following the completion of SBRT, after which time the tumor
response was assessed at 2–3-month intervals. Tumor responses were
classified according to the modified World Health Organization
Response Evaluation Criteria (11)
as follows: complete response (CR), complete disappearance of the
irradiated tumor; partial response (PR), >50% reduction in tumor
volume; stable disease (SD), decrease of <50% or >25% in
tumor volume; and progressive disease (PD), >25% increase in
tumor volume.
The response of PVTT to γ-SBRT was evaluated by
serial CT scans performed 3 months after the completion of
radiotherapy. The product of the largest perpendicular diameter of
the tumor thrombus was calculated and compared to the initial value
and the PVTT response was defined as follows: CR, complete
disappearance of the PVTT; PR, ≥50% decrease in the diameter of the
thrombus; SD, <50% decrease or <25% increase in the diameter
of the thrombus; and PD, ≥25% increase in the diameter of the
thrombus. The objective response was estimated based on the
combined number of patients with CR and PR and the progression-free
rate of PVTT included patients with CR, PR and SD. The sum of CR
and PR was defined as the objective response rate.
Toxicity was graded using the National Cancer
Institute Common Toxicity Criteria for Adverse Events, version 3.0.
Any grade 4 or 5 hepatic or gastrointestinal toxicity or
thrombocytopenia occurring within 1 month of SBRT, or RILD
requiring treatment in the absence of disease progression within 3
months following SBRT was considered as dose-limiting toxicity
(17). For Child-Pugh liver
function determination, the international normalized ratio was
considered to be stable in patients requiring warfarin
treatment.
The European Organization for Research and Treatment
of Cancer QLQ-HCC18 questionnaire was used to assess abdominal
distension, jaundice and ascites as the life quality dimension of
patients.
Statistical analysis
The overall and progression-free survival rates of
PVTT were estimated from the date of detection of PVTT to the date
of death or last follow-up and to the date of PVTT progression,
respectively. The probability of cumulative survival was calculated
according to the Kaplan-Meier method. The univariate and
multivariate analyses were performed using a Cox proportional
hazards models. Variables with P<0.05 by univariate analysis
were selected for multivariate analysis. P<0.05 was considered
to indicate a statistically significant difference. All the
aforementioned analyses were performed using SPSS statistical
software, version 12.0 (SPPS Inc., Chicago, IL, USA).
Results
Patient characteristics
The characteristics of the patients and the tumors
prior to treatment are summarized in Table I. The majority of the subjects were
male (68.3%), with a median age of 53 years (range, 19–79 years).
Invasion of the main portal vein was observed in all the patients
and of the unilateral first-order branch of the portal vein in 34
patients (33.7%). Radiotherapy was initiated in all the patients
2–3 weeks prior to or following TACE. The median radiotherapy dose
was 40.2 Gy (range, 21–60 Gy). Since different doses per fraction
were used, the biologically effective dose and equivalent dose in
2-Gy fractions, as the a/b ratio of 10, were also calculated. A
small proportion of the patients (16.7%) received radiotherapy with
a target volume that included the whole HCC and PVTT.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Total | Group A (n) | Group B (n) | Group C (n) | P-value |
|---|
| Gender | | | | | 0.892 |
| Male | 69 | 23 | 25 | 21 | |
| Female | 32 | 11 | 12 | 9 | |
| Age | | | | | 0.232 |
| Range | 19–79 | 19–69 | 25–70 | 20–79 | |
| Median age | 53 | 50 | 53 | 55 | |
| Child-Pugh grade | | | | | 0.743 |
| Grade A | 67 | 23 | 25 | 19 | |
| Grade B | 34 | 11 | 12 | 11 | |
| α-fetoprotein | | | | | 0.174 |
| >500
μg/l | 36 | 15 | 12 | 9 | |
| 20–500
μg/l | 39 | 9 | 16 | 14 | |
| <20
μg/l | 26 | 10 | 9 | 7 | |
| Tumor stage | | | | | 0.708 |
| IIb | 67 | 22 | 23 | 22 | |
| IIIa | 34 | 11 | 13 | 10 | |
| Maximum lesion
diameter | | | | | 0.981 |
| <5 cm | 32 | 12 | 11 | 9 | |
| ≥5 cm<10 cm | 49 | 17 | 18 | 14 | |
| ≥10 cm | 20 | 5 | 7 | 8 | |
| No. of lesions | | | | | 0.793 |
| 1 | 57 | 19 | 21 | 17 | |
| 2 | 35 | 12 | 12 | 11 | |
| ≥3 | 9 | 3 | 4 | 2 | |
| Location of
embolism | | | | | 0.886 |
| Trunk | 34 | 11 | 13 | 10 | |
| Branches | 67 | 23 | 24 | 20 | |
| Clinical
manifestations | | | | | 1.000 |
| Abdominal
distension | 53 | 17 | 20 | 16 | |
| Jaundice | 25 | 9 | 10 | 6 | |
| Ascites | 25 | 8 | 9 | 8 | |
Overall and PVTT response rates
Of the 101 patients assessed for tumor response at 6
months after diagnosis of PVTT, 29 patients (28.7%) achieved a CR,
59 (58.4%) achieved a PR and 7 patients (8.3%) had SD, yielding an
objective response rate of 87.1% and a progression-free rate of
95.4%. As shown in Table II, the
response rates of the patients in groups A (88.2%, 30/34) and B
(89.2%, 33/37) were higher compared to those in group C (83.3%,
25/30), although the difference was not statistically significant.
Of the 101 patients assessed for response of PVTT, 18 patients
(17.8%) achieved a CR, 53 (52.5%) achieved a PR, 15 (14.9%) had SD
and 15 patients (14.8%) had PD at 2–3 months after the completion
of SBRT. The objective response rate and the progression-free rate
of PVTT was 70.3 and 85.1%, respectively (Table II). The response rates of the
patients in groups A (73.5%, 25/34) and B (70.3%, 26/37) were
higher compared to those in group C (66.7%, 20/30), although the
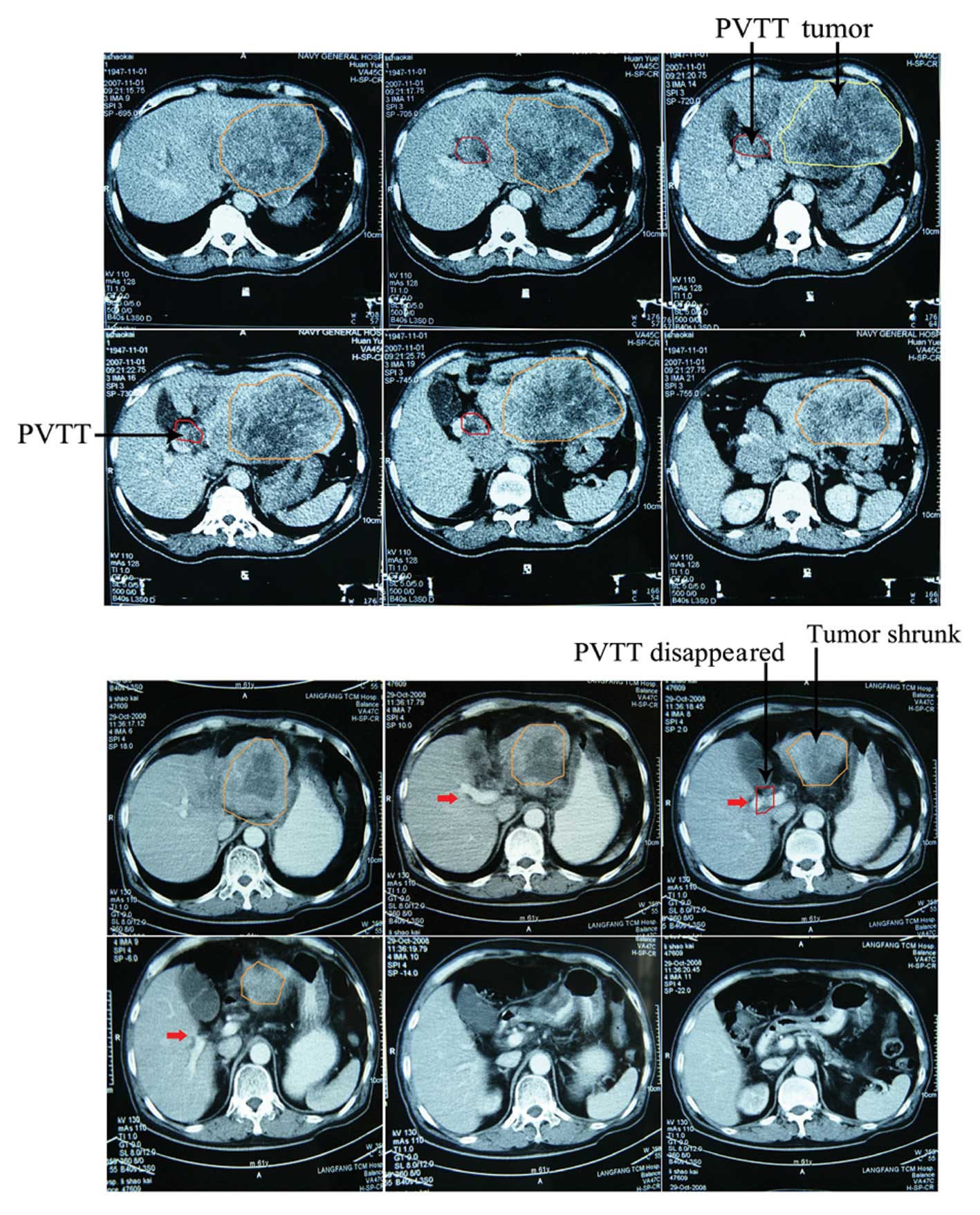
difference was not statistically significant. An example of
follow-up CT images of patients who achieved a CR at 12 months
after treatment with combined TACE and radiotherapy is presented in
Fig. 1.
 | Table II.Tumor and portal vein tumor thrombus
(PVTT) response rates. |
Table II.
Tumor and portal vein tumor thrombus
(PVTT) response rates.
| Cases | CR | PR | SD | PD | RR (CR+PR) | P-value |
|---|
| Tumor response
(n) | | | | | | NS |
| Group A (34) | 9 | 21 | 2 | 2 | 88.2 % (30/34) | |
| Group B (37) | 11 | 22 | 2 | 2 | 89.2 % (33/37) | |
| Group C (30) | 9 | 16 | 3 | 2 | 83.3% (25/30) | |
| Total (101) | 29 | 59 | 7 | 6 | 87.1% (88/101) | |
| PVTT response
(n) | | | | | | NS |
| Group A (34) | 7 | 18 | 4 | 5 | 73.5% (25/34) | |
| Group B (37) | 6 | 20 | 6 | 5 | 70.3% (26/37) | |
| Group C (30) | 5 | 15 | 5 | 5 | 66.7% (20/30) | |
| Total (101) | 18 | 53 | 15 | 15 | 70.3% (71/101) | |
Survival analysis and predictors of
survival
The median follow-up period was 15 months (range,
6–42 months). The median survival time was 17, 15 and 12 months in
groups A, B and C, respectively. The 1- and 2-year local control
rates were 55.9% (19/34) and 29.4% (10/34), 48.6% (18/37) and 24.3%
(9/37) and 43.3% (12/30) and 20.0% (6/30), respectively, in groups
A, B and C (Table III). The 1- and
2-year survival rates were 58.8 and 29.4%, 54.1 and 27.0% and 50.0
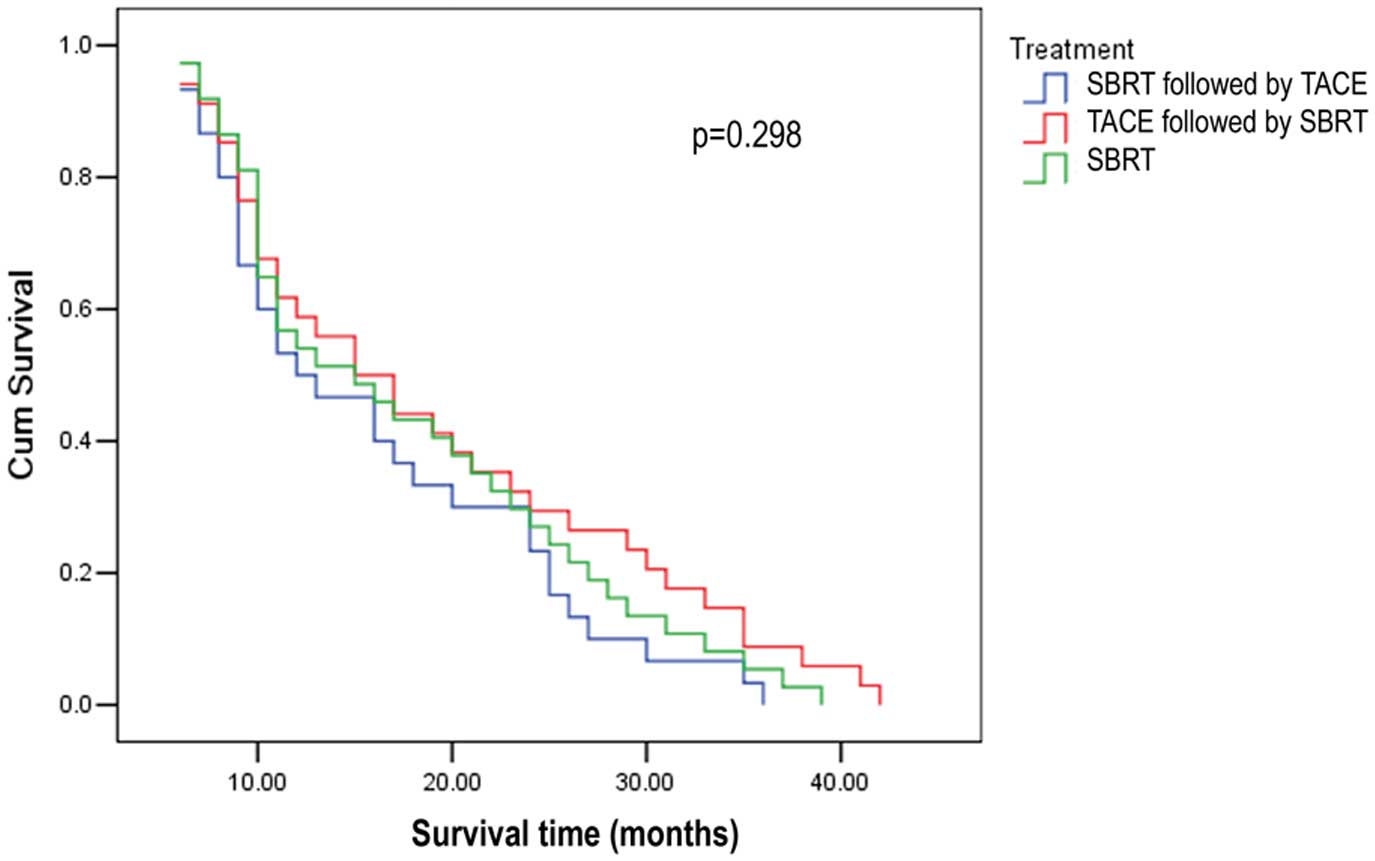
and 23.3%, respectively, in groups A, B and C. Based on the
log-rank (Mantel-Cox) test, the median survival time and the
survival rate of groups A and B were higher compared to those of
group C, although the difference was not statistically significant
(P=0.298). There was no significant difference between groups A and
B. The survival curves of the three groups are presented in
Fig. 2.
 | Table III.Median survival time, local control
rate and survival rate. |
Table III.
Median survival time, local control
rate and survival rate.
| Median survival
time (months) | Local control
rate | Survival rate |
|---|
|
|
|---|
| 1-year | 2-year | 1-year | 2-year |
|---|
| Group A | 15 | 55.9% (19/34) | 29.4% (10/34) | 58.8% | 29.4% |
| Group B | 15 | 48.6% (18/37) | 24.3% (9/37) | 54.1% | 27.0% |
| Group C | 12 | 43.3% (12/30) | 20.0% (6/30) | 50.0% | 23.3% |
Improvement of survival quality
Abdominal distension, jaundice and ascites are the
major complications affecting the quality of life of HCC patients.
In this study, we evaluated the quality of life of patients at 3
months after γ-SBRT treatment. As shown in Table IV, abdominal discomfort and
distension were alleviated in 69.1% (38/55) of the patients. Among
the patients treated with TACE prior to γ-SBRT or TACE following
γ-SBRT, 72.2% (13/18) and 71.4% (15/21), respectively, were
relieved from abdominal discomfort and distension, which was higher
compared to those treated with γ-SBRT alone, who exhibited a 62.5%
(10/16) relief rate. There was no significant difference in the
resolution of jaundice among the different treatment groups. Among
patients in group A, the jaundice and the icteric discoloration of
the skin resolved in all 9 cases and the icterus index was
decreased in 6 of the 9 patients. Of the 8 patients with a small or
medium amount of ascitic fluid, the ascites resolved in 1 patient
and was reduced in 4 patients. In group B, the jaundice and the
icteric skin discoloration resolved in all 10 cases and the icterus
index was decreased in 6 out of the 10 patients. The ascites
resolved in 1 patient, was reduced in 4 cases and was aggravated in
3 cases, with a remission rate of 55.6% (5/9). In group C, the
jaundice and the icteric skin discoloration resolved in all 6 cases
and the icterus index was decreased in 3 patients. The ascites
resolved in 1 patient and was reduced in 4 cases, with a remission
rate of 62.5% (5/8).
 | Table IV.Improvement of life quality following
radiotherapy. |
Table IV.
Improvement of life quality following
radiotherapy.
| Relief of abdominal
discomfort and distension | Jaundice
resolution | Ascites
release |
|---|
| Group A | 72.2% (13/18) | 66.6% (6/9) | 62.5% (5/8) |
| Group B | 71.4% (15/21) | 60.0% (6/10) | 55.6% (5/9) |
| Group C | 62.5% (10/16) | 50% (3/6) | 62.5% (5/8) |
Adverse effects
During the treatment, fatigue of various degrees,
loss of appetite, nausea and other symptoms were observed in 13, 15
and 11 cases in groups A, B and C, respectively. There were no
significant differences among the three groups. The incidence of
grade I-II acute bone marrow suppression among the patients of
group A (14/34, 41.2%) and group B (17/37, 45.9%) was higher
compared to that in group C (9/30, 29.6%), although the difference
was not statistically significant and they were all restored to
normal following treatment. During the period between 1 and 3
months after treatment, exacerbation of liver function was observed
in 32.4% (11/34), 40.5% (15/37) and 30.0% (9/30) of the patients in
group A, B and C, respectively. The exacerbation rate of liver
function in group B was higher compared to that in group A, with no
statistically significant difference among the three groups. In
detail, 8 cases deteriorated from grade A to B, 1 from A to C and 2
from B to C in group A: 10 cases deteriorated from grade A to B, 3
from A to C and 2 from grade B to C in group B; and 7 cases
deteriorated from grade A to B and 2 from B to C in group C. Fever
developed in 22 cases, with a body temperature of ~38°C). No other
serious complications were reported during the follow-up period
(Table V).
 | Table V.Adverse effects and exacerbation of
liver function during 3 months after treatment. |
Table V.
Adverse effects and exacerbation of
liver function during 3 months after treatment.
| Adverse
effects | Anorexia and
nausea | Acute bone marrow
suppression (grade I+II) | Exacerbation grade
of liver function
|
|---|
| Grade A to B
(case) | Grade A to C
(case) | Grade B to C
(case) | Exacerbation
rate |
|---|
| Group A | 13 | 14 | 8 | 1 | 2 | 32.4% (11/34) |
| Group B | 15 | 17 | 10 | 3 | 2 | 40.5% (15/37) |
| Group C | 11 | 9 | 7 | - | 2 | 30.0% (9/30) |
Discussion
The advantages of γ-SBRT in the treatment of HCC may
be attributed to its ability to increase the radiation dose
delivered to the tumor target while decreasing the irradiation of
the surrounding normal tissues, which may result in improved local
treatment and normal tissue protection (19–20).
To the best of our knowledge, there is no available study on the
sequence of γ-SBRT and TACE in patients with HCC with PVTT.
Therefore, we compared the clinical efficacy, toxicity and adverse
effects among the patients of three groups who received γ-SBRT
followed by TACE, TACE followed by γ-SBRT, or γ-SBRT alone. The
results demonstrated that the efficiency, control and survival
rates in the two combined-modality groups were higher compared to
those in the group who received γ-SBRT alone, whereas the liver
function compromise in the patients who received TACE followed by
γ-SBRT was more severe compared to that of the patients who were
treated with γ-SBRT alone. These results demonstrated that TACE may
result in liver function damage in HCC patients with PVTT, which
should be considered during treatment planning. Therefore, if TACE
is required first, due to the state of the disease, any changes in
liver function should be monitored and the appropriate treatment
planned accordingly.
TACE has been frequently used in patients with
unresectable HCC. Izaki et al (21) applied TACE to treat patients with
HCC combined with PVTT and the results demonstrated a median
survival of 9.7 months, with accumulated 1-, 2- and 3-year survival
rates of 26.7, 13.3 and 13.3%, respectively. Those results
demonstrated that TACE is effective and safe for the treatment of
PVTT. The effectiveness of TACE in PVTT may be attributed to the
fact that PVTT receives its blood supply from the portal vein as
well as the hepatic artery. A previous study by Sawrie et al
(20) demonstrated that HCC was
supplied by the hepatic artery in 24.4% patients, by the portal
vein in 17.8% and by both in 57.8% patients. However, the long-term
effects of the administration of TACE alone are not satisfactory,
particularly in HCC patients with first-order branch or main portal
vein invasion.
Radiotherapy has been shown to be effective to a
certain extent on HCC with PVTT. Li et al (22) applied TACE followed by proton
radiotherapy on 46 patients with HCC and PVTT and observed the
effectiveness of the treatment. The experimental data suggested
that TACE combined with proton radiotherapy was a novel, safe and
effective treatment method. Seong et al (23) investigated the combination of CRT
with TACE for HCC and reported a local control rate of 66%. Ren
et al (24) also reported
that the thrombus remission rate following treatment by SBRT in
patients with HCC and PVTT was 62.8%. Therefore, if the patients
with PVTT are first treated by γ-SBRT to reduce the size of PVTT
and improve the blood supply of the portal vein, the addition of
TACE may enhance the therapeutic effect and decrease the incidence
of complications. Dang et al (25) used γ-SBRT to treat portal vein
thrombosis and reported a CR of 21.1% and a PR of 26.3% in the
γ-SBRT group, proving the effectiveness of γ-SBRT in the treatment
of PVTT. Our results demonstrated that the changes in liver
function in the group treated with γ-SBRT followed by TACE were
similar to those in the group treated with γ-SBRT alone. This may
be explained by the hypothesis that liver tumor cells exhibit an
early response, whereas normal liver tissue and vessels exhibit a
late response. This difference in the response to radiation may
result in the early response occurring during irradiation or within
the first few days or weeks after the treatment, whereas late
response may occur after several months or years. Therefore, the
first 2 weeks following γ-SBRT may be the time period of liver
tumor cell response to the radiation, while the changes in vessels
have not yet occurred and TACE performed during this period may not
affect the therapeutic efficacy of the drugs.
Approximately 25–30% of the blood supply of normal
liver tissue comes from the hepatic artery, which plays a role in
cancer embolization for tumor control and shrinking in patients
with partial obstruction of the portal vein, with drugs cycling in
the hepatic artery. The theoretical evidence of the treatment
option of TACE followed by γ-SBRT in patients with PVTT may be
based on this physiological phenomenon. Performing γ-SBRT after
TACE may be advantageous, as the reduction of the target volume by
TACE may allow increase of radiation dose delivery to the target
and/or decrease of the radiation damage of normal liver tissue. The
administration of γ-SBRT after TACE may contribute to killing or
inhibiting any residual tumor cells after TACE. The fact that prior
administration of TACE affects liver function is reflected by the
fact that the compromise of liver function in patients with PVTT
who were treated with TACE followed by γ-SBRT was significantly
different from that in patients treated by γ-SBRT alone.
Unlike linear accelertor-based SBRT that uses
multiple fixed beams, γ-SBRT employs highly focused revolving
radiation beams. These focused beams lead to the delivery of highly
conformal doses to the targets, while greatly sparing normal
tissues. As demonstrated in this study, γ-SBRT combined with TACE
sequentially is an effective treatment option for HCC with PVTT. In
particular, the remission effects of PVTT are comparable between
the two combined-modality groups and the γ-SBRT-alone group,
indicating that the remission may be the result of γ-SBRT.
In conclusion, the γ-knife-based SBRT combined with
TACE is a relatively effective local treatment for patients with
primary HCC and PVTT. Although there were no major radiation-
related complications associated with these treatments, there were
a few adverse reactions that were manageable with symptomatic
treatment. The bone marrow suppression was not significantly
different between the γ-SBRT alone and the γ-SBRT combined with
TACE groups. Compared to γ-SBRT alone and γ-SBRT followed by TACE,
TACE followed by γ-SBRT exerted a more prominent negative effect on
liver function. If TACE followed by γ-SBRT is used, the relevant
indicators of liver function should be closely monitored and, if
required, appropriate treatment should be administered
immediately.
References
|
1.
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yang HI, Lu SN, Liaw YF, et al: Hepatitis
B e antigen and the risk of hepatocellular carcinoma. N Engl J Med.
347:168–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
4.
|
Llovet JM: Updated treatment approach to
hepatocellular carcinoma. J Gastroenterol. 40:225–235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cabibbo G, Enea M, Attanasio M, Bruix J,
Craxi A and Camma C: A meta-analysis of survival rates of untreated
patients in randomized clinical trials of hepatocellular carcinoma.
Hepatology. 51:1274–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kim JH, Yoon HK, Kim SY, et al:
Transcatheter arterial chemoembolization vs. chemoinfusion for
unresectable hepatocellular carcinoma in patients with major portal
vein thrombosis. Aliment Pharmacol Ther. 29:1291–1298. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kothary N, Weintraub JL, Susman J and
Rundback JH: Transarterial chemoembolization for primary
hepatocellular carcinoma in patients at high risk. J Vasc Interv
Radiol. 18:1517–1527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chung YH, Song IH, Song BC, et al:
Combined therapy consisting of intraarterial cisplatin infusion and
systemic interferon-alpha for hepatocellular carcinoma patients
with major portal vein thrombosis or distant metastasis. Cancer.
88:1986–1991. 2000. View Article : Google Scholar
|
|
9.
|
Obi S, Yoshida H, Toune R, et al:
Combination therapy of intraarterial 5-fluorouracil and systemic
interferon-alpha for advanced hepatocellular carcinoma with portal
venous invasion. Cancer. 106:1990–1997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Emami B, Lyman J, Brown A, et al:
Tolerance of normal tissue to therapeutic irradiation. Int J Radiat
Oncol Biol Phys. 21:109–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Krishnan S, Dawson LA, Seong J, et al:
Radiotherapy for hepatocellular carcinoma: an overview. Ann Surg
Oncol. 15:1015–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Price TR, Perkins SM, Sandrasegaran K, et
al: Evaluation of response after stereotactic body radiotherapy for
hepatocellular carcinoma. Cancer. 118:3191–3198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Facciuto ME, Singh MK, Rochon C, et al:
Stereotactic body radiation therapy in hepatocellular carcinoma and
cirrhosis: evaluation of radiological and pathological response. J
Surg Oncol. 105:692–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tse RV, Hawkins M, Lockwood G, et al:
Phase I study of individualized stereotactic body radiotherapy for
hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J
Clin Oncol. 26:657–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Choi BO, Choi IB, Jang HS, et al:
Stereotactic body radiation therapy with or without transarterial
chemoembolization for patients with primary hepatocellular
carcinoma: preliminary analysis. BMC Cancer. 8:3512008. View Article : Google Scholar
|
|
16.
|
Zhao M, Wang JP, Li W, et al: Comparison
of safety and efficacy for transcatheter arterial chemoembolization
alone and plus radiofrequency ablation in the treatment of single
branch portal vein tumor thrombus of hepatocellular carcinoma and
their prognosis factors. Zhonghua Yi Xue Za Zhi. 91:1167–1172.
2011.(In Chinese).
|
|
17.
|
Dawson LA, Eccles C and Craig T:
Individualized image guided iso-NTCP based liver cancer SBRT. Acta
Oncol. 45:856–864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lawrence TS, Robertson JM, Anscher MS,
Jirtle RL, Ensminger WD and Fajardo LF: Hepatic toxicity resulting
from cancer treatment. Int J Radiat Oncol Biol Phys. 31:1237–1248.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lo SS, Dawson LA, Kim EY, et al:
Stereotactic body radiation therapy for hepatocellular carcinoma.
Discov Med. 9:404–410. 2010.PubMed/NCBI
|
|
20.
|
Sawrie SM, Fiveash JB and Caudell JJ:
Stereotactic body radiation therapy for liver metastases and
primary hepatocellular carcinoma: normal tissue tolerances and
toxicity. Cancer Control. 17:111–119. 2010.PubMed/NCBI
|
|
21.
|
Izaki K, Sugimoto K, Sugimura K and Hirota
S: Transcatheter arterial embolization for advanced tumor thrombus
with marked arterioportal or arteriovenous shunt complicating
hepatocellular carcinoma. Radiat Med. 22:155–162. 2004.
|
|
22.
|
Li Q, Zeng CJ and Wang Y: Interventional
chemoembolization combined with proton radiotherapy for the
treatment of hepatocellular carcinoma accompanied with portal
cancerous thrombus. J Intervent Radiol. 18:278–280. 2009.
|
|
23.
|
Seong J, Park HC, Han KH, et al: Clinical
results of 3-dimensional conformal radiotherapy combined with
transarterial chemoembolization for hepatocellular carcinoma in the
cirrhotic patients. Hepatol Res. 27:30–35. 2003. View Article : Google Scholar
|
|
24.
|
Ren B, Song JB, Wang XD, et al:
Stereotactic radiotherapy for hepatocellular carcinoma with portal
tumor thrombi. Chin J Hepatol. 14:308–309. 2006.(In Chinese).
|
|
25.
|
Dang YZ, Zhang XC, Lu WL, et al: Body
gamma knife and high intensity focused ultrasound (HIFU) for the
treatment of portal vein tumor thrombosis of hepatocellular
carcinoma. J Mod Oncol. 18:114–117. 2010.
|