Introduction
Glomus tumors constitutes 1.6% of all soft tissue
tumors and are distinctive neoplasms that resemble the
Sucquet-Hoyer canal of the normal glomus body, located in the
subcutaneous tissue, which is responsible for the regulation of
temperature and blood pressure (1–3).
These tumors are usually solitary, deep blue to purple in color,
and accompanied by the classic triad of pain, cold sensitivity and
point tenderness (2). The glomus
tumor was originally considered to be a form of angiosarcoma until
the findings demonstrated by Masson (4) in 1924 (3). Masson compared the tumors with the
normal glomus body and suggested that the lesion represented
hyperplasia or overgrowth of this structure (3). The most common site for these tumors
is the distal extremities, particularly in the subungual digital
areas, although tumors have been identified in extradigital sites
including the bone, tongue, stomach, rectum, mesentery, lung,
mediastinum, sacrum, coccyx, and the head and neck (5,6). In
the present study, we report a case of extradigital glomus tumor of
elbow.
Case report
A 45-year-old male patient presented at Chosun
University Hospital (Chosun, China) with a painful nodular lesion
of the elbow. The patient presumed this lesion to be a puncture
wound caused by pricking his elbow on a tree two weeks previously.
Local examination revealed a violet-colored, mobile nodule. Mass
excision was performed following a clinical diagnosis of
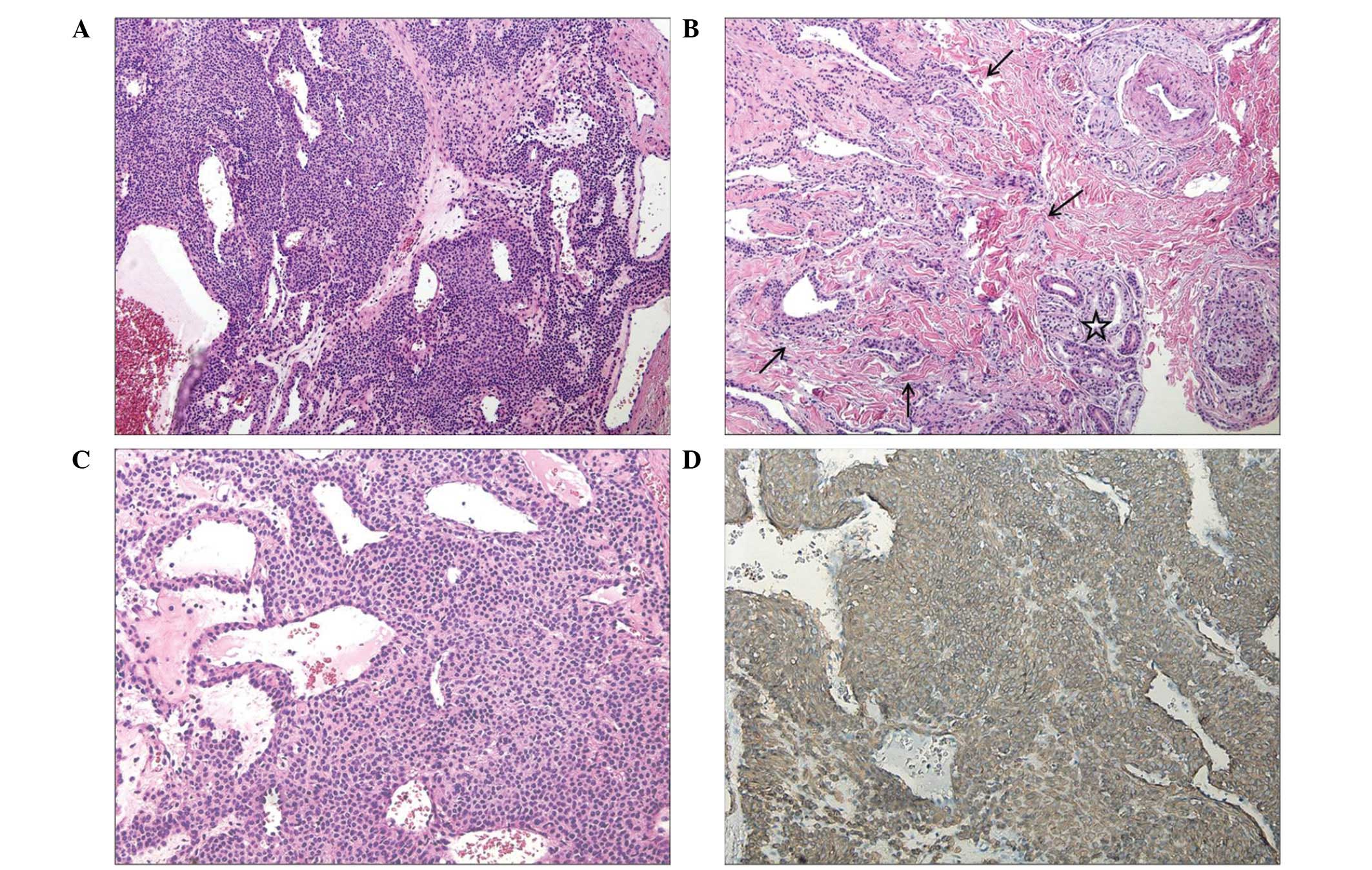
hemangioma. Histopathologically, the surgical specimen indicated a
subcutaneous, well-circumscribed nodule with focal infiltration
into the upper dermis in the subcutaneous region (Fig. 1B). The tumor was 0.9×0.7 cm and was
comprised of sheets and a nest of uniform and round cells,
interrupted by vessels of varying size (Fig. 1A). Certain areas had an organoid or
epitheliod growth pattern. High-power examination with a light
microscope at ×200 magnification (BX51, Olympus, Tokyo, Japan)
revealed that the glomus tumor cells exhibited punched-out and
hyperchromatic nuclei and pale cytoplasm (Fig. 1C). A dense fibrous pseudocapsule
surrounded the solid sheet of tumor cells. The tumor cells were
immunoreactive for smooth muscle actin (SMA) (Fig. 1D) and vimentin (VMT). The final
pathologic diagnosis was that of a glomus, solid-type tumor.
Discussion
The presence of glomus tumors in sites other than
the hand makes an early and accurate diagnosis difficult. However,
following the correct diagnosis, the treatment involves complete
surgical excision (7). Diagnosis
of a glomus tumor is primarily clinical as imaging techniques, such
as plain radiography, magnetic resonance imaging, ultrasonography
and angiography, do not yield a specific image of the tumor as they
may only show the precise location and size of the tumor (8).
Beaton et al (9) suggested that the frequency of
extradigital cases varied from 11 to 65% and may be more common in
males than females. Lee et al demonstrated that extradigital
glomus tumors are more common in males, whereas digital tumors are
more frequent in females (2). The
most prominent sites of extradigital glomus tumors have been
reported to be the hands, followed by the feet and forearms
(10). Other atypical locations
where glomus tumors occurred and were excised include the lower lip
(11), mediastinum (12), shoulder (13) and upper back (13). Folpe et al (14) examined 52 atypical glomus tumors
located on thigh, calf and ankle, foot, buttock, trunk and abdomen,
arm, lung, stomach and L3 vertebra.
Symptoms of glomus tumors are typical and often out
of proportion to the size of the neoplasm. Paroxysms of pain
radiating away from the lesion are the most common complaint
(3). These epidoses may be
elicited by changes in temperature, particularly by exposure to the
cold, and tactile stimulation even to a minor degree (3). The mechanism of pain production
requires further elucidation, however, identification of nerve
fibers containing immunoreactive substance P (SP) in glomus tumors
suggests pain mediation through the release of this substance
(3). SP is a pain-related peptide
that acts as the main afferent pain transmitter in glomus tumors
(15,16). McKemy suggested that
thermosensitive afferents express ion channels of the transient
receptor potential (TRP) family, which respond at distinct
temperature thresholds, thus establishing a molecular basis for
thermosensation (17). TRPV1 is a
capsaicin receptor that acts through the release of SP.
Furthermore, SP and TRPV1 correlate closely, although the exact
association remains unclear (2).
Accurate diagnosis followed by complete excision is
regarded as curative for patients with solitary lesions, and
recurrence rates for solitary tumors have been found to range from
12 to 33% (18,19). It is rare for malignant glomus
tumors to occur. Refined criteria have been suggested to define
malignant lesions (14), including
deep location and a size of >2 cm, or atypical mitotic figures,
or moderate to high nuclear grade and ≥5 mitotic figures per 50
high-power field (14). Lesions
with marked nuclear atypia but no other malignant features are
termed symplastic. Glomus tumors of uncertain malignant potential
are defined as lesions that lack criteria for the diagnosis of
malignant or symptomatic glomus tumors but have high mitotic
activity and superficial location, large size only or deep location
only (14).
In conclusion, we reported the case of an
extradigital glomus tumor arising in the subcutaneous tissue of the
elbow. Unusual tumor sites and differing clinical symptoms
occasionally interfere with the diagnosis and treatment of patients
with extradigital tumors. Therefore, it is important to include the
glomus tumor in the differential diagnosis of patients with
extradigital painful or asymptomatic lesions that are purple in
color.
References
|
1
|
Rathi KR, Jena J, Dash BM, Mitra D,
Patnaik PK and Basu AR: Extradigital glomus tumor as a cause of
chronic perianal pain. Indian J Pathol Microbiol. 52:414–416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee DW, Yang JH, Chang S, Won CH, Lee MW,
Choi JH and Moon KC: Clinical and pathological characteristics of
extradigital and digital glomus tumors: a retrospective comparative
study. J Eur Acad Dermatol Venereol. 25:1392–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weiss SW and Goldblum JR: Perivascular
tumors. Enzinger and Weiss’s Soft tissue tumors. 5th edition.
Mosby; Maryland Heights, MO: pp. 751–756. 2007
|
|
4
|
Masson P: Le glomus neuromyoarterial des
regions tactile et ses tumors. Lyon Chir. 21:2571924.
|
|
5
|
Kale SS, Rao VK and Bentz ML: Glomus tumor
of the index finger. J Craniofac Surg. 17:801–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enzinger FM and Weiss SW: Perivascular
tumors. Enzinger and Weiss’s Soft tissue tumors. 4th edition.
Mosby; Maryland Heights, MO: pp. 985–1035. 2001
|
|
7
|
Tomak Y, Dabak N and Ozcan H: Extradigital
glomus tumor of the triceps tendon as a cause of elbow pain: a case
report. J Shoulder Elbow Surg. 12:401–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
González-Llanos F, López-Barea F, Isla A,
Fernández-Prieto A, Zubillaga A and Alvarez F: Periosteal glomus
tumor of the femur; a case report. Clin Orthop Relat Res.
380:199–203. 2000.
|
|
9
|
Beaton LI and Davis L: Glomus tumor:
report of three cases and analysis of 271 recorded cases. Q Bull
Northwest Univ Med School. 15:245–273. 1941.
|
|
10
|
Calonje E: Vascular tumors: tumors and
tumor-like conditions of blood vessels and lymphatics. Lever’s
Histopathology of the Skin. 10th edition. Lippincott Williams and
Wilkins; Philadelphia, PA: pp. 1047–1049. 2009
|
|
11
|
Lanza A, Moscariello A, Villani R and
Colella G: Glomus tumor of the lower lip. A case report. Minerva
Stomatol. 54:687–690. 2005.PubMed/NCBI
|
|
12
|
Gaertner EM, Steinberg DM, Huber M,
Hayashi T, Tsuda N, Askin FB, et al: Pulmonary and mediastinal
glomus tumors - Report of five cases including a pulmonary
glomangiosarcoma: a clinicopathologic study with literature review.
Am J Surg Pathol. 24:1105–1114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takei TR and Nalebuff EA: Extradigital
glomus tumour. J Hand Surg Br. 20:409–412. 1995. View Article : Google Scholar
|
|
14
|
Folpe AL, Fanburg-Smith JC, Miettinen M
and Weiss SW: Atypical and malignant glomus tumors: analysis of 52
cases, with a proposal for the reclassification of glomus tumors.
Am J Surg Pathol. 25:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishimoto S, Nagatani H, Miyashita A and
Kobayashi K: Immunohistochemical demonstration of substance
P-containing nerve fibres in glomus tumors. Br J Dermatol.
113:213–218. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu JS, Huang ZJ, Zhou JF, Lin M, Fang MR,
Pang ZJ and Yan HD: Expression and significance of substance P,
neurofilament-H in glomus tumors with chronic pain. Zhonghua Wai Ke
Za Zhi. 41:935–939. 2003.(In Chinese).
|
|
17
|
McKemy DD: How cold is it? TRPM8 and TRPA1
in the molecular logic of cold sensation. Mole Pain. 1:162005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rettig AC and Strickland JW: Glomus tumor
of the digits. J Hand Surg Am. 2:261–5. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strahan J and Bailie HW: Glomus tumour. A
review of 15 clinical cases. Br J Surg. 59:91–3. 1972. View Article : Google Scholar
|