Introduction
Acute myeloid leukemia (AML) is a heterogeneous
clonal disorder characterized by autonomous proliferation and
impaired differentiation of hematopoietic progenitor cells
(1,2). It is the most common malignant
myeloid disorder in adults. Cytogenetic aberrations and molecular
genetic alterations provide significant prognostic information for
determining the response to chemotherapy and survival outcome
(3,4). An increasing number of genetic
abnormalities revealed in AML have also contributed to our
understanding of the mechanisms and process of leukemogenesis,
which leads to an improvement of risk-stratification, and the
development of individualized therapies and response assessment
(5).
Among these genetic alterations, a potential
prognostic genetic marker is the nucleophosmin 1 (NPM1) gene, which
is important in many tumor-associated chromosomal translocations
(6). NPM1 is an ubiquitously
expressed phosphoprotein and continuously shuttles between the
cytoplasm and nucleus (7,8). Several functions for this protein
have been described, including the binding of p53 (9), the initiation of centrosome
duplication (10), and ribosomal
protein assembly and transport (11). More recently, NPM1 exon 12
mutations have been reported to be involved in leukemogenesis, and
detected in ~35% of AML cases (12,13).
However, the prognostic implications of NPM1 mutations are less
clear and are notably variable among different institutions
(13–18). A study conducted by Konoplev et
al including 252 AML patients suggested NPM1 mutations did not
impact overall survival (OS) and event-free survival (EFS)
(17). However, findings of
previous studes have indicated that the NPM1 mutation has a
favorable effect on the outcome for AML (13–16,19–22).
For this reason, we performed an updated meta-analysis of 9
published studies in order to investigate the prognostic
significance of NPM1 mutations for AML.
Materials and methods
Selection of studies
Studies were eligible for inclusion in the
meta-analysis if they were: i) original articles written in English
and published up to January 2013; ii) dealt only with untreated AML
patients; iii) offered survival information based on the NPM1
status, including, NPM1 mutations and NPM1-wild-type (NPM1-wt), and
iv) provided survival information on response to induction therapy,
including, complete remission (CR), disease-free survival (DFS)
and/or OS. Studies were excluded if they focused exclusively on
acute promyelocytic leukemia. Multiple reports of a single study
were considered as one publication, and only the most recent
article was examined.
A computerized literature search of the PubMed,
Medline and EMBASE databases was conducted using the free text
search term AML AND nucleophosmin AND survival, with the
publication period limited to prior to January 2013, and the
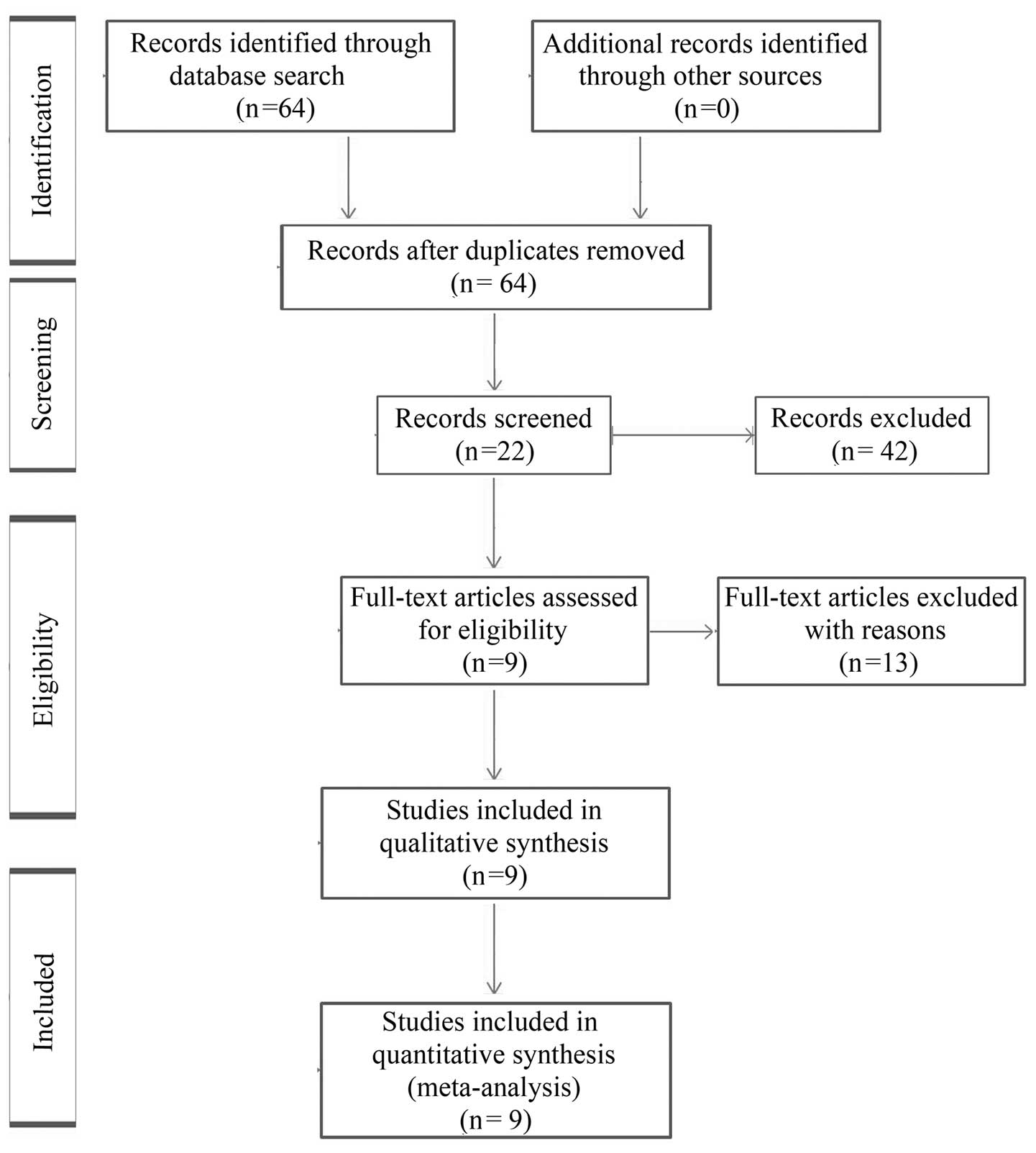
language to English. The initial search yielded a total of 64
articles, and the titles and abstracts of these papers were
reviewed, resulting in the exclusion of 42 articles, with 22
candidate articles. Of the 22 articles, 13 full-text articles were
excluded as survival information was unavailable. In total, 9
studies satisfied the eligibility criteria and were included in the
meta-analysis (Table I). The
reasons for excluding articles are shown in Fig. 1 (23–35).
 | Table IList of studies included in the
meta-analysis. |
Table I
List of studies included in the
meta-analysis.
| Author (Refs.) | Publication
year | Region | Subjects (n) | NPM1 mutation
(%) | Normal karyotype
(%) |
|---|
| Thiede et al
(14) | 2006 | Germany | 1485 | 27.47 | 79.41 |
| Döhner et al
(15) | 2005 | Germany and
USA | 300 | 48.33 | NR |
| Schnittger et
al (16) | 2005 | Germany and
Italy | 401 | 52.90 | 100.00 |
| Suzuki et al
(19) | 2005 | Japan | 190 | 30.53 | 63.79 |
| Verhaak et
al (13) | 2005 | The
Netherlands | 275 | 34.55 | 77.89 |
| Mullighan et
al (18) | 2007 | USA | 93 | 6.45 | 50.00 |
| Gale et al
(20) | 2008 | United Kingdom | 1217 | 41.33 | 67.59 |
| Becker et al
(21) | 2010 | Germany and
USA | 148 | 56.08 | NR |
| Boonthimat et
al (22) | 2008 | Thailand | 400 | 26.25 | 86.67 |
Data extraction and quality
assessment
In order to avoid bias in the data abstraction
process, the reviewers Y.F. Liu and P.C. He independently retrieved
the data from the articles and subsequently compared the results.
All data were assessed for internal consistency and disagreements
were resolved by discussion. Characteristics abstracted from the
articles included the name of the first author, year of
publication, location of the study, number of subjects, mean or
median values of age and median white blood cell (WBC) counts, the
incidence of NPM1 mutations, percentage of cases with normal
karyotype, outcomes including hematologic CR rate, hazard ratio
(HR) and 95% confidence interval (CI) for DFS and OS according to
the NPM1 status based on multivariate analysis. When the data
required for the analysis could not be abstracted, attempts were
made to contact the investigators who conducted the studies.
The quality of evidence and the strength of
recommendations were evaluated by GRADE profiler (version 3.2)
(36). Any discrepancies in
quality assessments were resolved by consensus among the authors.
The overall quality of the data was graded as moderate.
Quantitative data synthesis
HR was used to assess the survival effect of NPM1
mutations compared with wild-type. The natural logarithm of a crude
HR and its variance within the study was calculated by using the
abstracted survival probabilities at each time point with the
methods proposed by Parmar et al (37), and those described previously
(38). HR was calculated to show
how many times higher the probability of survival failure was for
the patients with NPM1 mutations cmpared with those with wild-type,
as an HR less than unity suggests that NPM1 mutations yield a
better survival rate compared with wild-type.
The odds ratio (OR) was calculated to describe the
probability of CR following induction therapy based on NPM1
mutation status. An OR >1 indicates that patients with NPM1
mutations are associated with an improved CR rate compared with
those without the mutations.
A DerSimonian Laird random method was used to
calculate summary HR or OR and their 95% CI. Initially, the fixed
effect and random-effect models were used to calculate summary HRs,
but eventually the random-effect model was selected. Begg’s funnel
plots (39) and Egger’s test
(40) were used to detect possible
publication bias. The between-study variation (τ2) from
the Q statistic was also calculated (41).
Statistical analysis
Statistical analyses were performed using STATA 12
software (College Station, TX, USA). P<0.05 was considered to
indicate a statistically significant test result for summary HR or
OR.
Results
Study characteristics
As shown in Table
I, 9 studies including a total of 4,509 subjects (2,903 with
NPM1-wt, 1,606 with NPM1 mutations) were included in the
meta-analysis. Four studies originated from Europe (13,14,16,20),
two from Asia (19,22) and three from the USA, two of which
contained information pertaining to Germany (15,18,21).
The frequency of NPM1 mutations varied between 6.45 and 56.08% for
AML patients. NPM1 mutations were associated with a higher
frequency of FLT3-ITD mutations in 7 studies (Table II) (13–15,19–22).
The frequency of the normal karyotype was higher among NPM1 mutant
patients (Table I) (13,14,16,18–20,22).
No graphical or statistical evidence of publication bias for DFS or
OS was identified.
 | Table IIDiagnostic characteristics according
to the NPM1 status in the AML patients. |
Table II
Diagnostic characteristics according
to the NPM1 status in the AML patients.
| Author (Refs.) | NPM1 status | Subjects (n) | Age (years) | Median WBC count
(109/l) | FLT3-ITD mutation
(%) |
|---|
| Thiede et al
(14) | NPM1-wt | 1077 | 58.0
(15.0–87.0) | 0.3–465 |
13.74a |
| NPM1-mt | 408 | 60.0
(18.0–83.0) | 0.5–380 | 40.20 |
| Döhner et al
(5) | NPM1-wt | 155 | 16.0–60.0 | 0.41–369 |
24.52a |
| NPM1-mt | 145 | 18.0–60.0 | 0.2–345 | 40.69 |
| Schnittger et
al (16) | NPM1-wt | 189 | 58.1 | 10.0 (0.1–361) | NR |
| NPM1-mt | 212 | 55.8 | 38.7 (0.2–486) | NR |
| Suzuki et al
(19) | NPM1-wt | 141 | 47.0
(15.0–85.0) | 23.3
(0.9–337.6) |
11.35a |
| NPM1-mt | 49 | 58.0
(15.0–77.0) | 52.2 (1.0–372) | 55.10 |
| Verhaak et
al (13) | NPM1-wt | 180 | 39.7±13.3 | NA |
16.76a |
| NPM1-mt | 95 | 47.3±10.7 | NA | 49.47 |
| Mullighan et
al (18) | NPM1-wt | 87 | 9.7 | 38.0 | NR |
| NPM1-mt | 6 | 14.6 | 35.3 | 16.67 |
| Gale et al
(20) | NPM1-wt | 714 | 41.0 | 18.5 |
19.19a |
| NPM1-mt | 503 | 46.0 | 35.4 | 41.35 |
| Becker et al
(21) | NPM1-wt | 65 | 71.0
(60.0–83.0) | 7.0
(1.0–434.1) |
20.00a |
| NPM1-mt | 83 | 67.0
(60.0–81.0) | 26.2
(1.0–249.3) | 39.76 |
| Boonthimat et
al (22) | NPM1-wt | 295 | 40.0 | 25.4 |
20.68a |
| NPM1-mt | 105 | 51.0 | 47.0 | 43.81 |
Treatment outcomes
Table III shows
the CR rate and HR for DFS and OS among AML patients with NPM1
mutations compared with patients without the mutations in
individual studies. The summary OR for CR in the NPM1 mutant group
was 2.23 (95% CI: 1.86–2.67, P<0.001) (Fig. 2). The summary HR for DFS of
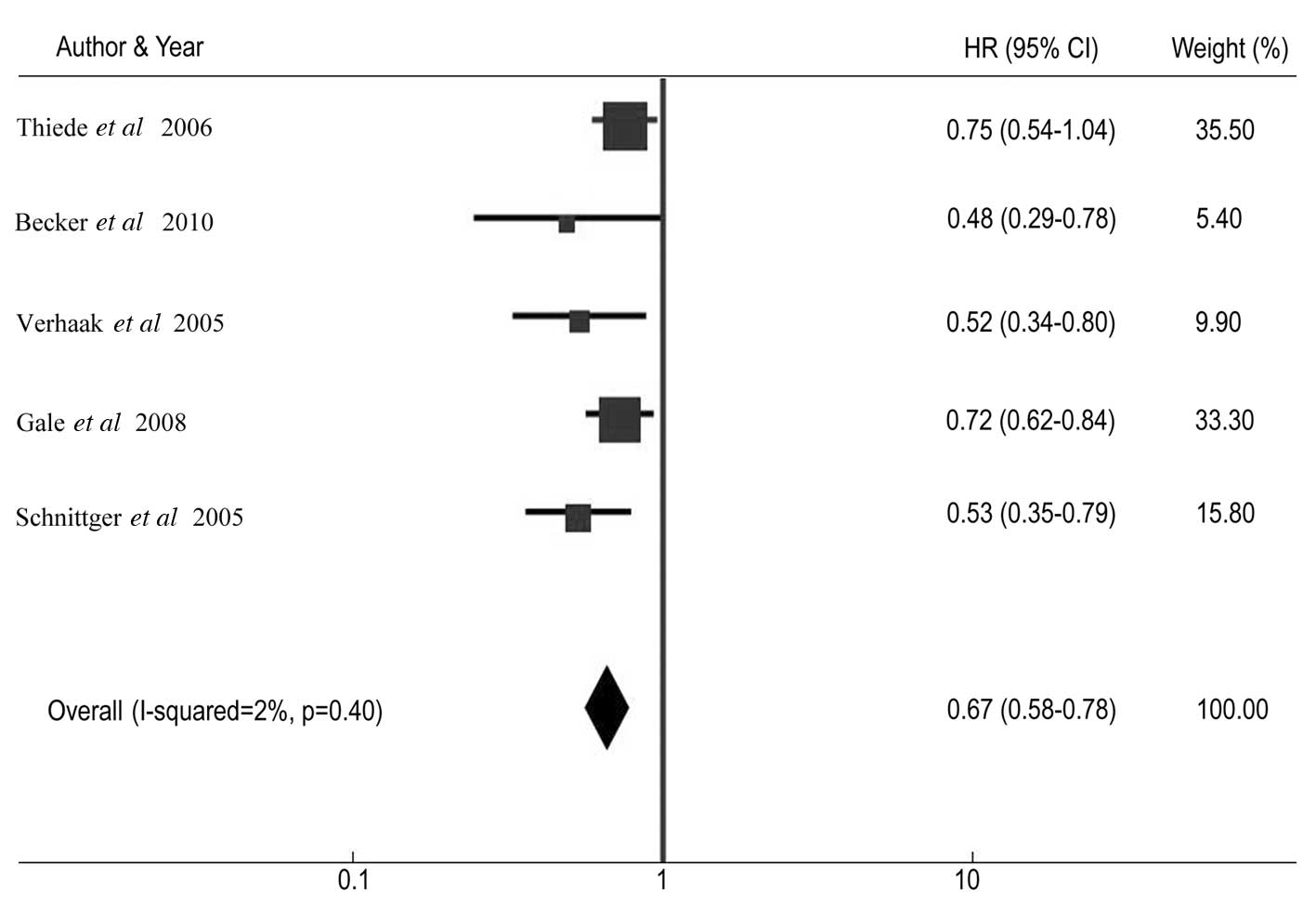
NPM1-mt/NPM1-wt was 0.67 (95% CI: 0.58–0.78; P<0.001) (Fig. 3), and the overall HR for OS of
NPM1-mt/NPM1-wt was 0.63 (95% CI: 0.54–0.74; P<0.001) (Fig. 4). The test for heterogeneity, which
evaluates the variation in study outcomes between studies in a
meta-analysis, showed no significant heterogeneity among studies
included in the DFS analysis (Q=4.08, df=4, P=0.40,
τ2=2) and OS analysis (Q=5.52, d=4, P=0.24,
τ2=28).
 | Table IIINPM1 mutations and outcomes in acute
myeloid leukemia. |
Table III
NPM1 mutations and outcomes in acute
myeloid leukemia.
| Author (Refs.) | NPM1 status | Subjects (n) | CR (%) | HR for OS | 95% CI for OS | HR for DFS | 95% CI for DFS |
|---|
| Thiede et al
(14) | NPM1-wt | 1077 |
41.60a | 1.00 | Reference | 1.00 | Reference |
| NPM1-mt | 408 | 58.60 | 0.73 | 0.58–0.91 | 0.75 | 0.54–1.04 |
| Döhner et al
(15) | NPM1-wt | 155 |
68.50a | 1.00 | Reference | NR | NR |
| NPM1-mt | 145 | 86.00 | 0.44 | 0.30–0.66 | NR | NR |
| Schnittger et
al (16) | NPM1-wt | 189 |
54.70a | NR | NR | 1.00 | Reference |
| NPM1-mt | 212 | 70.50 | NR | NR | 0.53 | 0.35–0.79 |
| Suzuki et al
(19) | NPM1-wt | 141 |
68.80a | NR | NR | NR | NR |
| NPM1-mt | 49 | 85.70 | NR | NR | NR | NR |
| Verhaak et
al (13) | NPM1-wt | 180 | NA | 1.00 | Reference | 1.00 | Reference |
| NPM1-mt | 95 | NA | 0.49 | 0.33–0.72 | 0.52 | 0.34–0.80 |
| Mullighan et
al (18) | NPM1-wt | 87 | 85.06 | NA | NA | NA | NA |
| NPM1-mt | 6 | 100.00 | NA | NA | NA | NA |
| Gale et al
(20) | NPM1-wt | 714 | NA | 1.00 | Reference | 1.00 | Reference |
| NPM1-mt | 503 | NA | 0.67 | 0.58–0.77 | 0.72 | 0.62–0.84 |
| Becker et al
(21) | NPM1-wt | 65 |
47.69a | 1.00 | Reference | 1.00 | Reference |
| NPM1-mt | 83 | 84.34 | 0.43 | 0.30–0.63 | 0.48 | 0.29–0.78 |
Furthermore, we performed a sensitivity test during
the meta-analysis. Exclusion of any single study did not affect the
overall results.
Discussion
Previous studies have investigated the prognostic
significance of NPM1 mutation status in AML patients. Certain
studies demonstrated the positive prognostic effect of NPM1
mutations (13–16,19–22),
whereas in other studies no clinical outcome difference between
patients with and without NPM1 mutations (17,18).
The aim of the present meta-analysis was to clarify the prognostic
significance of NPM1 mutation status in AML patients. Meta-analysis
is a useful statistical method for integrating results from
independent studies for a specified outcome. Combining the relevant
studies increases statistical power, thus the effects that may be
missed by individual studies may be detected (42). The present meta-analysis
demonstrated the effects of NPM1 mutations with the summary OR of
2.23 (95% CI: 1.86–2.67) for CR, HR of 0.67 (95% CI: 0.58–0.78) for
DFS, and HR of 0.63 (95% CI: 0.54–0.74) for OS. Furthermore, the
present study indicated that NPM1 mutations were associated with a
higher frequency of normal karyotype and FLT3-ITD mutations.
Notably, FLT3-ITD mutations have been shown to be
the most important abnormality in AML patients and correlated with
marked poor outcome (high percentage of bone marrow blast cells,
increased risk of relapse from CR, and reduced survival) (43,44).
However, our study indicated that patients with FLT3-ITD and NPM1
mutations have an improved CR (14,15,19,20),
DFS (14,20) and OS (14,20)
compared with those who only have the FLT3-ITD mutation, although
this result was inferior to only NPM1 mutation cases.
The present study has several limitations. Firstly,
the analyses were based on observational studies rather than
prospective controlled studies or randomized trials. Secondly, we
used abstracted data, while an individual patient data-based
meta-analysis would have provided a more robust estimate of the
association. The results reported should therefore be interpreted
carefully by clinical physicians. Thirdly, as is often the case
with meta-analysis, a substantial effect of heterogeneity should be
considered. Although median WBC counts at the time of diagnosis
were not identified as sources of heterogeneity, we cannot rule out
the potential effect of other factors, such as differences in
treatment and distinct cytogenetic categories, which were not
examined in our analysis. Publication bias, although not directly
detected in the present study, may also have had an impact on the
accuracy of our study (42,45).
Although these limitations should be considered, the
results of our meta-analysis demonstrated that NPM1 mutations have
a favorable effect on the outcome for AML. Thus, distinguishing AML
with NPM1 mutations from AML without mutations and justifying the
risk-adapted therapeutic strategy for AML based on the NPM1
status.
A large number of patients should be prospectively
studied in order for definitive conclusions to be reached. In
addition to the presence or absence of NPM1 mutations, several
factors relevant to NPM1 have been suggested to have a prognostic
value, including the expression levels of NPM1 transcripts,
mutant/wild-type allelic ratios for NPM1, and certain types of NPM1
exon mutations. These factors should be investigated in order to
arrive at a more accurate estimation of the prognosis for AML.
Acknowledgements
The authors would like to thank Dr Di Wu and Dr
Jieying Xi for their technological assistance.
References
|
1
|
Estey E and Döhner H: Acute myeloid
leukemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar
|
|
2
|
Rubnitz JE, Gibson B and Smith FO: Acute
myeloid leukemia. Hematol Oncol Clin North Am. 24:35–63. 2010.
View Article : Google Scholar
|
|
3
|
Harrison CJ, Hills RK, Moorman AV, et al:
Cytogenetics of childhood acute myeloid leukemia: United Kingdom
Medical Research Council Treatment trials AML 10 and 12. J Clin
Oncol. 28:2674–2681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grimwade D: The clinical significance of
cytogenetic abnormalities in acute myeloid leukaemia. Best Pract
Res Clin Haematol. 14:497–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Döhner H, Estey EH, Amadori S, et al:
Diagnosis and management of acute myeloid leukemia in adults:
recommendations from an international expert panel, on behalf of
the European LeukemiaNet. Blood. 115:453–474. 2010.PubMed/NCBI
|
|
6
|
Naoe T, Suzuki T, Kiyoi H and Urano T:
Nucleophosmin: a versatile molecule associated with hematological
malignancies. Cancer Sci. 97:963–969. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borer RA, Lehner CF, Eppenberger HM and
Nigg EA: Major nucleolar proteins shuttle between nucleus and
cytoplasm. Cell. 56:379–390. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan WY, Liu QR, Borjigin J, et al:
Characterization of the cDNA encoding human nucleophosmin and
studies of its role in normal and abnormal growth. Biochemistry.
28:1033–1039. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colombo E, Marine JC, Danovi D, Falini B
and Pelicci PG: Nucleophosmin regulates the stability and
transcriptional activity of p53. Nat Cell Biol. 4:529–533. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okuda M, Horn HF, Tarapore P, et al:
Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome
duplication. Cell. 103:127–140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lindstrom MS: NPM1/B23: A multifunctional
chaperone in ribosome biogenesis and chromatin remodeling. Biochem
Res Int. 2011:1952092011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Falini B, Mecucci C, Tiacci E, et al:
Cytoplasmic nucleophosmin in acute myelogenous leukemia with a
normal karyotype. N Engl J Med. 352:254–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verhaak RG, Goudswaard CS, van Putten W,
et al: Mutations in nucleophosmin (NPM1) in acute myeloid leukemia
(AML): association with other gene abnormalities and previously
established gene expression signatures and their favorable
prognostic significance. Blood. 106:3747–3754. 2005. View Article : Google Scholar
|
|
14
|
Thiede C, Koch S, Creutzig E, et al:
Prevalence and prognostic impact of NPM1 mutations in 1485 adult
patients with acute myeloid leukemia (AML). Blood. 107:4011–4020.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Döhner K, Schlenk RF, Habdank M, et al:
Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger
adults with acute myeloid leukemia and normal cytogenetics:
interaction with other gene mutations. Blood. 106:3740–3746.
2005.PubMed/NCBI
|
|
16
|
Schnittger S, Schoch C, Kern W, et al:
Nucleophosmin gene mutations are predictors of favorable prognosis
in acute myelogenous leukemia with a normal karyotype. Blood.
106:3733–3739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Konoplev S, Huang X, Drabkin HA, et al:
Cytoplasmic localization of nucleophosmin in bone marrow blasts of
acute myeloid leukemia patients is not completely concordant with
NPM1 mutation and is not predictive of prognosis. Cancer.
115:4737–4744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mullighan CG, Kennedy A, Zhou X, et al:
Pediatric acute myeloid leukemia with NPM1 mutations is
characterized by a gene expression profile with dysregulated HOX
gene expression distinct from MLL-rearranged leukemias. Leukemia.
21:2000–2009. 2007. View Article : Google Scholar
|
|
19
|
Suzuki T, Kiyoi H, Ozeki K, et al:
Clinical characteristics and prognostic implications of NPM1
mutations in acute myeloid leukemia. Blood. 106:2854–2861. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gale RE, Green C, Allen C, et al: The
impact of FLT3 internal tandem duplication mutant level, number,
size, and interaction with NPM1 mutations in a large cohort of
young adult patients with acute myeloid leukemia. Blood.
111:2776–2784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Becker H, Marcucci G, Maharry K, et al:
Favorable prognostic impact of NPM1 mutations in older patients
with cytogenetically normal de novo acute myeloid leukemia and
associated gene- and microRNA-expression signatures: a Cancer and
Leukemia Group B study. J Clin Oncol. 28:596–604. 2010. View Article : Google Scholar
|
|
22
|
Boonthimat C, Thongnoppakhun W and
Auewarakul CU: Nucleophosmin mutation in Southeast Asian acute
myeloid leukemia: eight novel variants, FLT3 coexistence and
prognostic impact of NPM1/FLT3 mutations. Haematologica.
93:1565–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schnittger S, Haferlach C, Ulke M,
Alpermann T, Kern W and Haferlach T: IDH1 mutations are detected in
6.6% of 1414 AML patients and are associated with intermediate risk
karyotype and unfavorable prognosis in adults younger than 60 years
and unmutated NPM1 status. Blood. 116:5486–5496. 2010.
|
|
24
|
Yan L, Chen S, Liang J, et al: Analysis of
NPM1 gene mutations in Chinese adults with acute myeloid leukemia.
Int J Hematol. 86:143–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haferlach C, Mecucci C, Schnittger S, et
al: AML with mutated NPM1 carrying a normal or aberrant karyotype
show overlapping biologic, pathologic, immunophenotypic, and
prognostic features. Blood. 114:3024–3032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Rassidakis GZ, Li J, et al: High
frequency of NPM1 gene mutations in acute myeloid leukemia with
prominent nuclear invaginations (‘cuplike’ nuclei). Blood.
108:1783–1784. 2006.PubMed/NCBI
|
|
27
|
Paschka P, Schlenk RF, Gaidzik VI, et al:
IDH1 and IDH2 mutations are frequent genetic alterations in acute
myeloid leukemia and confer adverse prognosis in cytogenetically
normal acute myeloid leukemia with NPM1 mutation without FLT3
internal tandem duplication. J Clin Oncol. 28:3636–3643. 2010.
View Article : Google Scholar
|
|
28
|
Koh Y, Park J, Bae EK, et al: Non-A type
nucleophosmin 1 gene mutation predicts poor clinical outcome in de
novo adult acute myeloid leukemia: differential clinical importance
of NPM1 mutation according to subtype. Int J Hematol. 90:1–5. 2009.
View Article : Google Scholar
|
|
29
|
Palmisano M, Grafone T, Ottaviani E,
Testoni N, Baccarani M and Martinelli G: NPM1 mutations are more
stable than FLT3 mutations during the course of disease in patients
with acute myeloid leukemia. Haematologica. 92:1268–1269. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Zhang M, Yang L and Xiao Z: NPM1
mutations in myelodysplastic syndromes and acute myeloid leukemia
with normal karyotype. Leuk Res. 31:109–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wertheim G and Bagg A: Nucleophosmin
(NPM1) mutations in acute myeloid leukemia: an ongoing
(cytoplasmic) tale of dueling mutations and duality of molecular
genetic testing methodologies. J Mol Diagn. 10:198–202. 2008.
View Article : Google Scholar
|
|
32
|
Green CL, Koo KK, Hills RK, Burnett AK,
Linch DC and Gale RE: Prognostic significance of CEBPA mutations in
a large cohort of younger adult patients with acute myeloid
leukemia: impact of double CEBPA mutations and the interaction with
FLT3 and NPM1 mutations. J Clin Oncol. 28:2739–2747. 2010.
View Article : Google Scholar
|
|
33
|
Noguera NI, Ammatuna E, Zangrilli D, et
al: Simultaneous detection of NPM1 and FLT3-ITD mutations by
capillary electrophoresis in acute myeloid leukemia. Leukemia.
19:1479–1482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burnett AK, Hills RK, Green C, et al: The
impact on outcome of the addition of all-trans retinoic acid to
intensive chemotherapy in younger patients with nonacute
promyelocytic acute myeloid leukemia: overall results and results
in genotypic subgroups defined by mutations in NPM1, FLT3, and
CEBPA. Blood. 115:948–956. 2010.
|
|
35
|
Falini B: Therapy-related acute myeloid
leukaemia with mutated NPM1: treatment induced or de novo in
origin? Leukemia. 22:891–892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guyatt GH, Oxman AD, Vist GE, et al:
GRADE: an emerging consensus on rating quality of evidence and
strength of recommendations. BMJ. 336:924–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Meta-analysis of randomized clinical
trials comparing Cisplatin to Carboplatin in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 22:3852–3859. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Egger M, Davey SG, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yanada M, Matsuo K, Suzuki T, Kiyoi H and
Naoe T: Prognostic significance of FLT3 internal tandem duplication
and tyrosine kinase domain mutations for acute myeloid leukemia: a
meta-analysis. Leukemia. 19:1345–1349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Small D: FLT3 mutations: biology and
treatment. Hematol Am Soc Hematol Educ Program. 178–184. 2006.
View Article : Google Scholar
|
|
44
|
Kottaridis PD, Gale RE and Linch DC: Flt3
mutations and leukaemia. Br J Haematol. 122:523–538. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Feng JH, Guo XP, Chen YY, Wang ZJ, Cheng
YP and Tang YM: Prognostic significance of IDH1 mutations in acute
myeloid leukemia: a meta-analysis. Am J Blood Res. 2:254–264.
2012.PubMed/NCBI
|