Introduction
The standard postoperative chemotherapy for
epithelial ovarian cancer is currently a combination therapy
including platinum and taxanes (1). Although the treatment outcome of
epithelial ovarian cancer has improved, it remains unsatisfactory
in terms of long-term survival. A recent study demonstrated that
bevacizumab administered in combination with paclitaxel/carboplatin
(TC) prolongs survival and may be used as maintenance chemotherapy
(2). Furthermore, dose-dense
weekly TC was reported to be significantly superior to TC therapy
regarding progression-free survival (PFS) and overall survival
(3). The therapeutic efficacy of
intraperitoneal chemotherapy was also verified in a randomized
controlled study (4). A
combination of molecular-targeted agents or refined regimens has
improved the outcome of first-line treatment for epithelial ovarian
cancer.
Epithelial ovarian cancer is highly sensitive to
chemotherapy and ~75% of patients achieve a clinical complete
response (CCR) with first-line treatment. However, several patients
relapse, develop chronic disease and ultimately succumb to ovarian
cancer. The disease-free survival of optimal disease (advanced
cancer) was reported to be 18–24 months and that of suboptimal
disease 18 months (5).
Furthermore, a previous study assessing optimal and suboptimal
disease reported a disease-free survival of 16–17 months (5). The approximate prevalence of relapse
was 10% in low-risk groups, 20% in high-risk groups for early
cancer, 60–70% in optimal surgery groups and 80–85% in suboptimal
surgery groups for advanced cancer. Thus, ≥60% of patients with
ovarian cancer are candidates for second-line treatment (5) and determining the second-line
therapeutic options is vital for improving the outcome.
The treatment-free interval (TFI) following the
first-line treatment is currently recognized as the most
significant parameter for determining the optimal regimen for the
treatment of relapsed cancer. Increasing the TFI results in an
improved response to platinum (6).
Commonly, the treatment regimen is selected for platinum-sensitive
tumors with a TFI of ≥6 months and for platinum-resistant tumors
with a TFI of <6 months.
However, whether relapsed ovarian cancer with a TFI
of 6–12 months may be treated as platinum-sensitive has not been
determined. Furthermore, it has not been established whether tumors
may be considered drug-sensitive or -resistant according to TFI,
regardless of the differences in drug sensitivity according to
histological type. In the present study, the medical records of a
relatively large number of patients with relapsed stage III/IV
epithelial ovarian cancer were reviewed, the PFS was calculated
according to the TFI and the degree of platinum sensitivity was
retrospectively verified with the TFI. Furthermore, we investigated
the degree of platinum sensitivity with TFI according to
histological type.
Materials and methods
Study population and inclusion
criteria
The study population comprised 747 patients with
epithelial ovarian cancer who underwent treatment at seven
institutions participating in the Tohoku Gynecologic Cancer Unit
between January, 2003 and December, 2007; these were: Hirosaki
University Graduate School of Medicine (Hirosaki, Japan), Akita
University School of Medicine (Akita, Japan), Iwate Medical
University School of Medicine (Morioka, Japan), Tohoku University
School of Medicine (Sendai, Japan), Yamagata University School of
Medicine (Yamagata, Japan), Fukushima Medical University
(Fukushima, Japan) and the Miyagi Cancer Center (Natori, Japan). Of
the 747 patients, 405 were diagnosed with stage III/IV epithelial
ovarian cancer, including 156 patients with relapsed or recurrent
disease. Patients in whom a complete response (CR) was maintained,
those who had received neoadjuvant chemotherapy, incomplete
first-line chemotherapy or radiotherapy and those with an unknown
prognosis were excluded; finally, a total of 107 patients with
relapsed epithelial ovarian cancer after attaining a CCR with
first-line treatment were assessed. CCR was defined as the cases
which became negative for the tumor marker CA125 at the end of
first-line treatment, with no lesions detected on computed
tomography (CT) and positron emission tomography-CT. Informed
consent was obtained from the patients or their family members to
collect data, following approval by the Institutional Review Boards
of the involved institutions.
Patient characteristics
The recorded patient characteristics and variables
included age, histological type of ovarian cancer, debulking
surgery, first-line treatment, response to first-line treatment,
time to relapse, site of relapse and second-line treatment
(Table I). With regard to
debulking surgery, the size of the residual tumor was graded as 0,
<1 and ≥1 cm for complete, optimal and suboptimal debulking,
respectively. A central pathological review was conducted to assess
the histological type.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | No. of patients |
|---|
| Age, years [median
(range)] | 56 (26–78) |
| Histological
type | |
| Serous | 101 |
| Endometrioid | 18 |
| Clear cell | 26 |
| Mucinous | 11 |
| First-line
regimen | |
| TC | 135 |
| DC | 10 |
| CPT-P | 6 |
| CAP | 5 |
| No. of first-line
chemotherapy cycles [median (range)] | 6 (1–13) |
| Debulking
surgery | |
| Complete | 31 |
| Optimal | 39 |
| Suboptimal | 86 |
| Response to
first-line chemotherapy | |
| Complete
response | 107 |
| Partial
response | 26 |
| Stable disease | 4 |
| Progressive
disease | 19 |
| CR according to
histological type [CR/non-CR (%)] | |
| Serous | 73/101 (72.3) |
| Endometrioid | 12/18 (66.7) |
| Clear cell | 16/26 (61.5) |
| Mucinous | 6/11 (54.5) |
| Recurrence sites
after CR | |
| Intraabdominal | 45 |
| Intrapelvic | 44 |
| Distant | 18 |
| Second-line
regimen | |
| Platinum-based | 70 |
|
Non-platinum-based | 37 |
The TFI was defined as the period from the
completion of the first-line treatment to the initiation of
second-line treatment after confirming disease relapse on imaging.
Increased CA125 levels alone were not considered to reflect
relapse. PFS was defined as the interval from the initiation of
second-line treatment for relapsed lesions to confirmed disease
progression.
Statistical analysis
The degree of platinum sensitivity was calculated by
comparing the PFS values. PFS was estimated using the Kaplan-Meier
method and compared using the log-rank test. Hazard ratios (HRs)
with 95% confidence intervals (CIs) were calculated with the Cox
proportional hazards regression model. P≤0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median age of the
patients was 56 years. With regard to histological type, serous
adenocarcinoma was diagnosed in 101 patients (65%) and clear cell
adenocarcinoma, which was reported to have a measurable incidence
in the USA and Europe (7), was
identified in 26 patients (17%). The median number of first-line
treatment cycles was 6 and the TC regimen was administered to 135
patients (87%), whereas docetaxel/carboplatin was selected for
patients with paclitaxel hypersensitivity or peripheral
neurotoxicity. As regards debulking surgery, complete or optimal
outcomes were achieved in 70 patients (45%). A CR following
administration of the first-line treatment was observed in 107
patients (69%). Although the prevalence of CR following
administration of the first-line regimen varied by histological
type from 72.3% in serous adenocarcinoma to 54.5% in mucinous
adenocarcinoma, there were no statistically significant differences
in the prevalence of CR among the four histological types. The
number of relapse sites was similar for abdominal and pelvic
cavities. The majority of distant metastases were located in the
lungs and in the mediastinal, supraclavicular or inguinal lymph
nodes. In patients with a relapse following a CR (70 patients in
the platinum and 37 in the non-platinum group), a second-line
regimen was initiated.
TFI in relapsed patients following
CR
The median TFI in relapsed patients was 11.5 months
(Table II). Significant
differences in the TFI by histological type were observed between
cases with serous adenocarcinoma and those with clear cell
adenocarcinoma (HR=0.53; 95% CI: 0.30–0.95; P<0.02) (Table II).
 | Table IITreatment-free interval (TFI) of
patients who relapsed after achieving a complete response. |
Table II
Treatment-free interval (TFI) of
patients who relapsed after achieving a complete response.
| Histological
type | No. of patients | Median TFIa |
|---|
| All types | 107 | 11.5 |
| Serous | 73 | 11.5 |
| Endometrioid | 12 | 16.0 |
| Clear cell | 16 | 8.0b |
| Mucinous | 6 | 11.0 |
PFS following second-line treatment
A total of 20, 37 and 50 patients relapsed after a
CR at <6, 6–12 and ≥12 months, respectively, and the median PFS
following administration of a second-line regimen was 3.0, 5.5 and
13.0 months, respectively (Table
III). There were no statistically significant differences in
the distribution of the interval from the end of first-line
treatment to relapse according to the histological type (Table IV). There were no significant
differences in the median PFS (3.0 months) between the platinum-
and non-platinum-based treatment groups who relapsed within 6
months after CR, when the PFS was compared based on the observation
period for all histological types (Fig. 1A). In patients who relapsed between
6 and 12 months, the median PFS was 8.0 and 3.0 months in the
platinum- and non-platinum based groups, respectively, with PFS
being significantly longer in the platinum group (HR=0.35; 95% CI:
0.18–0.69; P<0.002) (Fig. 1B).
In patients who relapsed after 12 months, the median PFS was 14.0
and 11.5 months in the platinum- and non-platinum-based groups,
respectively, with the PFS being significantly longer in the
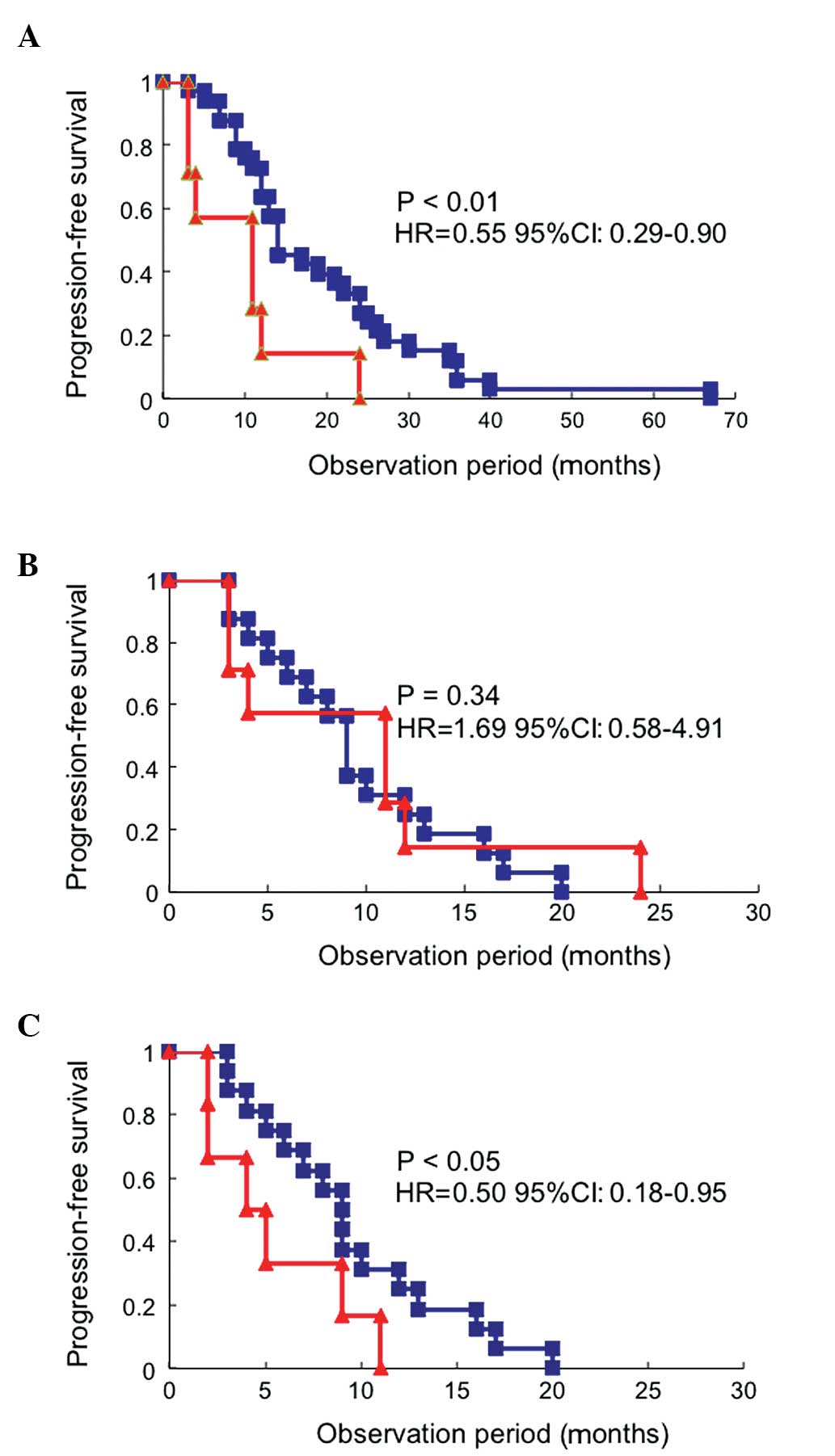
platinum group (HR=0.79; 95% CI: 0.50–0.97; P=0.02) (Fig. 1C). Therefore, the platinum group
alone was further investigated. Patients who relapsed after 12
months were further classified every 6 months (Fig. 2). There were significant
differences in PFS between patients who relapsed within 6 months
and those who relapsed between 6 and 12 months (HR=0.45; 95% CI:
0.16–0.88; P=0.01), as well as between those who relapsed between 6
and 12 months and those who relapsed between 12 and 18 months
(HR=0.41; 95% CI: 0.19–0.91; P=0.03; Fig. 2). However, there were no
significant differences in the PFS between patients who relapsed
between 12 and 18 months and those who relapsed between 18 and 24
months, or between those who relapsed between 18 and 24 months and
those who relapsed after 24 months (Fig. 2). The patients who relapsed were
divided into those who relapsed between 12 and 24 months and those
who relapsed after 24 months and the PFS was compared between the
groups: no significant differences in PFS were observed between the
two groups (HR=1.03; 95% CI: 0.61–1.72; P=0.12).
 | Table IIIProgression-free survival (PFS)
according to the interval from the end of first-line treatment to
relapse. |
Table III
Progression-free survival (PFS)
according to the interval from the end of first-line treatment to
relapse.
| Interval from the end
of first-line treatment to relapse (months) |
|---|
|
|
|---|
| Variables | <6 | 6–12 | >12 |
|---|
| No. of patients | 20 | 37 | 50 |
| Median PFS
(months) | 3.0 | 5.5 | 13.0 |
| Second-line
treatment |
| Platinum-based | 6 | 23 | 41 |
|
Non-platinum-based | 14 | 14 | 9 |
 | Table IVInterval from the end of first-line
treatment to relapse according to histological type. |
Table IV
Interval from the end of first-line
treatment to relapse according to histological type.
| Histology | No. of
patients | Interval from the
end of first-line treatment to relapse (months) |
|---|
|
|---|
| <6 | 6–12 | >12 |
|---|
| Serous | 73 | 10 (14%) | 27 (37%) | 36 (49%) |
| Endometrioid | 12 | 3 (25%) | 3 (25%) | 6 (50%) |
| Clear cell | 16 | 5 (31%) | 6 (38%) | 5 (31%) |
| Mucinous | 6 | 2 (33%) | 1 (17%) | 3 (50%) |
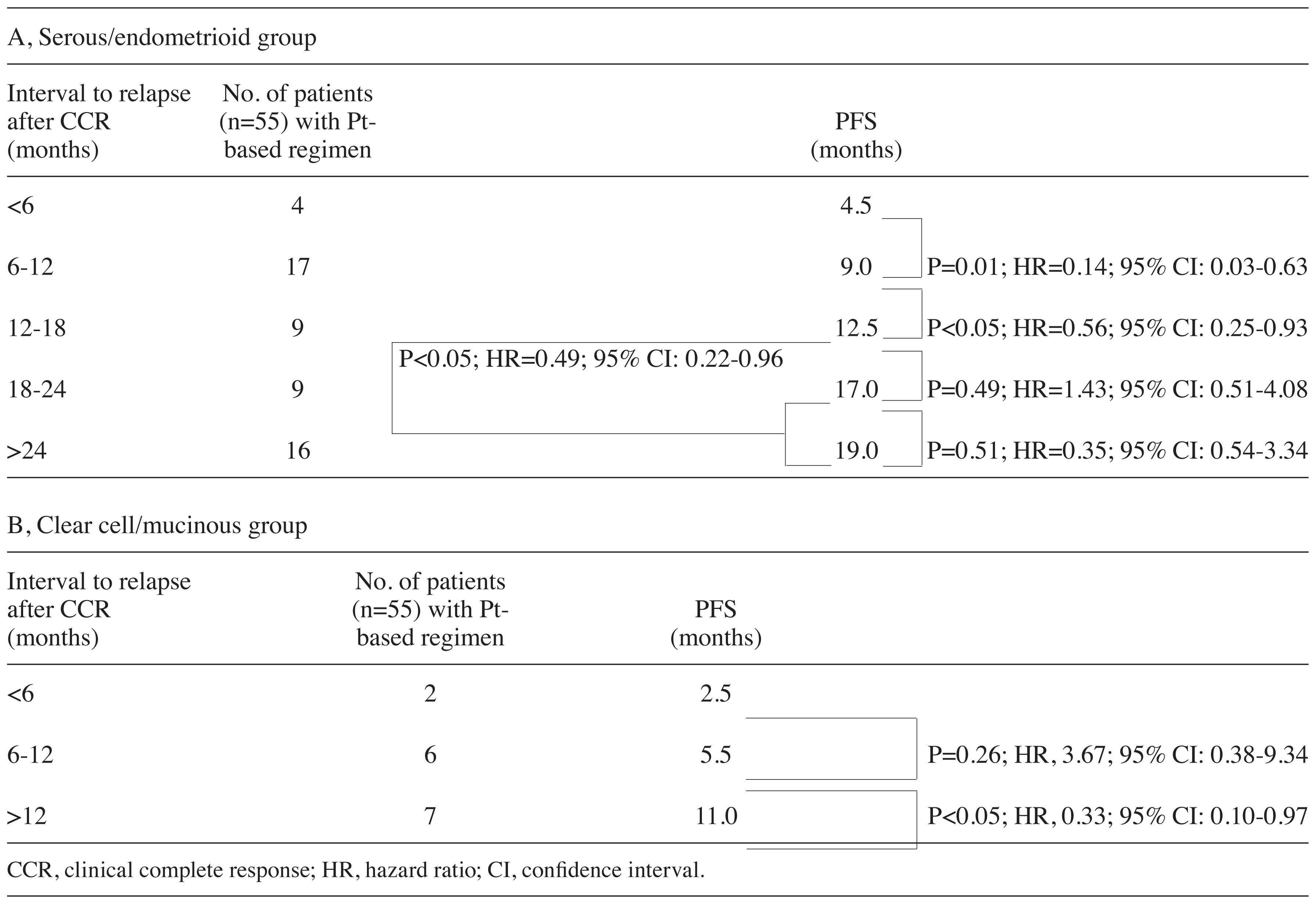
PFS by histological type following
second-line treatment
The differences in PFS by histological type were
investigated. The PFS in the serous/endometrioid group treated with
platinum after relapse was 4.5, 9.0, 12.5, 17.0 and 19.0 months in
patients who relapsed at <6, 6–12, 12–18, 18–24 and ≥24 months,
respectively (Table V). There were
significant differences in the PFS between patients who relapsed
within 6 months and those who relapsed between 6 and 12 months
(HR=0.14; 95% CI: 0.03–0.63; P=0.01), as well as between those who
relapsed between 6 and 12 months and those who relapsed between 12
and 18 months (HR=0.56; 95% CI: 0.25–0.93; P<0.05) (Table V). Although there were no
significant differences in the PFS between those who relapsed
between 12 and 18 and those who relapsed between 18 and 24 months,
or between those who relapsed between 18 and 24 and those who
relapsed after 24 months, there were significant differences in the
PFS between patients who relapsed between 12 and 18 and those who
relapsed after 18 months (HR=0.49; 95% CI: 0.22–0.96; P<0.05)
(Table V). Furthermore, in the
clear cell/mucinous adenocarcinoma group treated with platinum as a
second-line regimen, there were no significant differences in the
PFS between patients who relapsed within 6 months and those who
relapsed between 6 and 12 months, while there were significant
differences in the PFS between patients who relapsed between 6 and
12 months and those who relapsed after 12 months (HR=0.33; 95% CI:
0.10–0.97; P<0.05) (Table V).
In patients who relapsed after 12 months, the PFS in the clear
cell/mucinous adenocarcinoma group was significantly shorter
compared to that in the serous/endometrioid adenocarcinoma group
(HR=0.55; 95% CI: 0.29–0.90; P<0.01; Fig. 3A), but similar to that of patients
who relapsed between 6 and 12 months in the serous/endometrioid
adenocarcinoma group (HR=1.69; 95% CI: 0.58–4.91; P=0.34; Fig. 3B). Furthermore, in patients who
relapsed between 6 and 12 months, the PFS of the clear
cell/mucinous adenocarcinoma group was significantly shorter
compared to that in the serous/endometrioid adenocarcinoma group
(HR=0.50; 95% CI: 0.18–0.95; P<0.05; Fig. 3C).
 | Table VProgression-free survival (PFS) in
patients with serous or endometrioid carcinoma and in those with
clear cell or mucinous carcinoma treated with a platinum (Pt)-based
regimen according to interval from the end of first-line treatment
to relapse. |
Table V
Progression-free survival (PFS) in
patients with serous or endometrioid carcinoma and in those with
clear cell or mucinous carcinoma treated with a platinum (Pt)-based
regimen according to interval from the end of first-line treatment
to relapse.
Discussion
The aim of first-line treatment is to cure, whereas
the primary goal of second-line treatment is palliation.
Accordingly, less toxic and more convenient regimens should be
selected, focusing on the balance between toxicity and
effectiveness. Quality of life, including improving symptoms and
preventing the onset of new symptoms is also preferentially
maintained. Therefore, it is crucial to appropriately determine how
the sensitivity or resistance to platinum is defined by TFI when
selecting a regimen and TFI should also be individualized according
to histological type.
Although the TFI of patients who relapsed within 6
months was suggested to indicate platinum sensitivity in this
study, the sensitivity to platinum was similar between patients who
relapsed between 12 and 24 months and those who relapsed after 24
months for all histological types. However, these results were
inconsistent with previous findings reporting a significantly
higher response rate in patients with a TFI of ≥24 months (6,8). In
this study, the overall proportion of clear cell and mucinous
adenocarcinomas, which are relatively refractory to treatment,
accounted for ~20% of the cases of ovarian cancer and platinum
sensitivity was evaluated according to histological type. In
patients with serous/endometrioid adenocarcinoma, the PFS following
platinum administration exhibited a significant increase in a
stepwise manner depending on the TFI (Table V). These findings suggested a novel
distribution of platinum sensitivity classification. The patients
who relapsed between 6 and 12 months following the completion of
first-line treatment (TFI of 6–12 months) may be classified as
‘intermediately sensitive’, those with a TFI of 12–18 months as
‘highly sensitive’ and those with a TFI of ≥18 months as ‘extremely
highly sensitive’, whereas the patients with a TFI of <6 months
were considered as platinum-resistant (Fig. 4A).
A significant difference in the PFS was only
detected between patients with a TFI of 6–12 and those with a TFI
of ≥12 months in the clear cell/mucinous adenocarcinoma group.
There were no significant differences in the PFS between patients
with a TFI of <6 months and those with a TFI of 6–12 months.
However, the PFS of patients who relapsed after 12 months was
significantly shorter in the clear cell/mucinous adenocarcinoma
group compared to that in the serous/endometrioid adenocarcinoma
group and was similar to the PFS of patients who relapsed between 6
and 12 months in the serous/endometrioid adenocarcinoma group.
Furthermore, the PFS of patients who relapsed between 6 and 12
months was significantly shorter in the clear cell/mucinous
adenocarcinoma group compared to that in the serous/endometrioid
adenocarcinoma group. Therefore, it may be rational to classify
patients with clear cell/mucinous adenocarcinoma who relapsed
within 12 months as ‘platinum-resistant’ and those who relapsed
after 12 months as ‘intermediately sensitive’ (Fig. 4B).
In the 1990s, several studies focused on the
identification and differentiation of platinum-sensitive from
platinum-resistant relapse (6,8,9).
Harries and Gore (10) suggested
that sensitivity and resistance to platinum were separated by a PFS
of 6 months. Furthermore, previous studies defined sensitivity and
resistance to platinum according to the clinical response, such as
the frequency of CR for agents administered to relapsed patients
(6,8,9).
However, in the present study, drug sensitivity was evaluated by
the interval to disease progression (observation period), as well
as the PFS of relapsed patients under platinum- and
non-platinum-based treatment. It is widely accepted that the
patients with a longer time interval between the completion of
first-line treatment and the initiation of second-line treatment
exhibit a higher response rate to the second-line regimen. In order
to avoid ineffective treatment with resistant regimens, the length
of the observation period should be considered on an individual
basis. The results of the present study suggested that a TFI of
<6 and ≥6 months after the completion of first-line treatment
may be appropriate for patients with serous/endometrioid
adenocarcinoma to determine sensitivity or resistance to platinum
as second-line treatment, whereas a TFI of 6 months is too short to
apply to patients with clear cell/mucinous adenocarcinoma.
Thus far, an observation period of ≥6 months
following the end of first-line treatment has been defined as
platinum-sensitive and phase III studies for patients with
platinum-sensitive recurrent ovarian cancer have been designed.
Although the incidence of relapsed patients with a TFI of ≥12
months was reported to be 60–70% in several randomized controlled
studies (11–14), the proportion of patients who
relapsed between 6 and 12 months was 30–40%, including those with
platinum-resistant clear cell/mucinous adenocarcinoma. Furthermore,
in the present study, although the TFI was ≥12 months in the
serous/endometrioid adenocarcinoma group, there were significant
differences in platinum sensitivity between patients who relapsed
between 12 and 18 months and those who relapsed after 18 months.
Thus, future clinical studies on the selection of chemotherapy for
recurrent ovarian cancer must consider the histological type and
platinum sensitivity with TFI.
With regard to taxane sensitivity in recurrent
ovarian cancer, it was demonstrated that the number of intervention
therapies following relapse, rather than the taxane-free interval,
is associated with taxane sensitivity (15). Although there were no differences
in the effects of taxanes on recurrent ovarian cancer, regardless
of whether the interval between the first and subsequent use of
taxanes was ≤12 or ≥24 months, additional intervention therapies
resulted in a decreased response to taxanes (15). Similarly, it was previously
reported that the taxane-free interval does not affect sensitivity
to taxanes (16). Therefore, drug
sensitivity to taxanes and platinum must be considered
separately.
Novel biological therapies, including
anti-angiogenic agents, signaling inhibitors, anti-CA125 antibody
and dendritic cell immunotherapy, have been developed for the
treatment of recurrent ovarian cancer. Bevacizumab was reported to
prolong survival in subjects with recurrent ovarian cancer
(17). Molecular-targeted agents
are expected to exert additive effects and platinum sensitivity is
considered to play a critical role in improving prognosis.
Furthermore, maintenance therapy with biological agents may
eventually alter the pattern of recurrence and novel
characterizations of platinum sensitivity/resistance may emerge.
Although TFI is a continuous variable with a wide boundary, it is
crucial to determine a definitive criterion of the sensitivity and
resistance to platinum in types of ovarian cancer with a prevalence
of relapse of ≥60%, in order to enable the selection of the most
efficient second-line regimen and design high-quality clinical
studies.
References
|
1
|
Ozols RF, Bundy BN, Greer BE, et al;
Gynecologic Oncology Group. Phase III trial of carboplatin and
paclitaxel compared with cisplatin and paclitaxel in patients with
optimally resected stage III ovarian cancer: a Gynecologic Oncology
Group study. J Clin Oncol. 21:3194–3200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burger RA, Brady MF, Bookman MA, et al:
Incorporation of bevacizumab in the primary treatment of ovarian
cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katsumata N, Yasuda M, Takahashi F, et al:
Dose-dense paclitaxel once a week in combination with carboplatin
every 3 weeks for advanced ovarian cancer: a phase 3, open-label,
randomised controlled trial. Lancet. 374:1331–1338. 2009.
View Article : Google Scholar
|
|
4
|
Armstrong DK, Bundy B, Wenzel L, et al:
Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl
J Med. 354:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugiyama T: Second-line treatment using
novel chemotherapeutic and biologic agents. Cancer and
Chemotherapy. 36:730–735. 2009.(In Japanese).
|
|
6
|
Markman M, Reichman B, Hakes T, et al:
Responses to second-line cisplatin-based intraperitoneal therapy in
ovarian cancer: influence of a prior response to intravenous
cisplatin. J Clin Oncol. 9:1801–1805. 1991.PubMed/NCBI
|
|
7
|
Hoskins PJ, Le N, Gilks B, et al:
Low-stage ovarian clear cell carcinoma: population-based outcomes
in British Columbia, Canada, with evidence for a survival benefit
as a result of irradiation. J Clin Oncol. 30:1656–1662. 2012.
View Article : Google Scholar
|
|
8
|
Gore ME, Fryatt I, Wiltshaw E, et al:
Treatment of relapsed carcinoma of the ovary with cisplatin or
carboplatin following initial treatment with these compounds.
Gynecol Oncol. 36:207–211. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blackledge G, Lawton F, Redman C, et al:
Response of patients in phase II studies of chemotherapy in ovarian
cancer: implications for patient treatment and the design of phase
II trials. Br J Cancer. 59:650–653. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harries M and Gore M: Part II:
chemotherapy for epithelial ovarian cancer-treatment of recurrent
disease. Lancet Oncol. 3:537–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parmar MK, Ledermann JA, Colombo N, et al:
Paclitaxel plus platinum-based chemotherapy versus conventional
platinum-based chemotherapy in women with relapsed ovarian cancer:
the ICON4/AGO-OVAR-2.2 trial. Lancet. 361:2099–2106. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfisterer J, Plante M, Vergote I, et al:
Gemcitabine plus carboplatin compared with carboplatin in patients
with platinum-sensitive recurrent ovarian cancer: an intergroup
trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin
Oncol. 24:4699–4707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pujade-Lauraine E, Wagner U,
Aavall-Lundqvist E, et al: Pegylated liposomal doxorubicin and
carboplatin compared with paclitaxel and carboplatin for patients
with platinum-sensitive ovarian cancer in late relapse. J Clin
Oncol. 28:3323–3329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aghajanian C, Blank SV, Goff BA, et al:
OCEANS: a randomized, double-blind, placebo-controlled phase III
trial of chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar
|
|
15
|
McCourt C, Dessie S, Bradley AM, et al: Is
there a taxane-free interval that predicts response to taxanes as a
later-line treatment of recurrent ovarian or primary peritoneal
cancer? Int J Gynecol Cancer. 19:343–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rose PG, Blessing JA, Ball HG, et al: A
phase II study of docetaxel in paclitaxel-resistant ovarian and
peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol
Oncol. 88:130–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aghajanian C, Blank SV, Goff BA, et al:
OCEANS: a randomized, double-blind, placebo-controlled phase III
trial of chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar
|