Introduction
Cervical cancer develops from the cells that
constitute the cervical epithelium and involves an abnormal
increase in the number of immature cells in this region. Cervical
cancer is the second most commonly diagnosed malignancy among
females worldwide according to the International Federation of
Gynecology and Obstetrics (FIGO) (1). Despite the generally favorable
prognosis of early cervical cancer patients, approximately
one-third of these patients eventually succumb to the disease.
The optimal treatment selection for cervical cancer
is multidisciplinary and is based on several factors, including
patient age, histological subtype, tumor grade, stage and lymph
node status, depth of stromal invasion and lymphovascular space
invasion (LVSI). The immunoresponse was also identified as an
important prognostic factor for survival in cervical cancer
(2). B7 family members and their
receptors are crucial in the regulation of antigen-specific immune
responses (3).
The modulation and suppression of antitumor immune
response is a characteristic that enables tumor cells to escape
immune surveillance. Members of the B7 family are involved in this
process, since the level of activation of the antitumor immune
response depends on the balance between co-stimulatory and
co-inhibitory signals. Certain molecules, which are often
overexpressed in tumors, have been associated with the pathogenesis
and progression of malignancies, as well as their immunological and
non-immunological functions. The B7 homologs play a key role in the
maintenance of self-tolerance and regulation of innate and adaptive
immunity in tumor-bearing hosts (4).
B7-H4 (also known as B7× and B7S1) is the most
recently identified immunoregulatory member of the B7 family
(3,5,6).
B7-H4 has been implicated in the inhibition of T-cell-mediated
immunity and the downregulation of the T-cell response through the
inhibition of T-cell proliferation, cytokine production and cell
cycle progression (3,7,8).
Although the expression of B7-H4 is characteristic in lymphoid
cells, B7-H4 mRNA is highly expressed in human breast and ovarian
cancers, but not in the majority of normal tissues (9–11).
Aberrant B7-H4 expression was also demonstrated in the cancer cells
of patients with various malignancies, such as melanoma, ovarian,
gastric and non-small-cell lung cancer, renal cell carcinoma,
esophageal and endometrial cancer, and its expression was found to
be correlated with the progression of the disease (7,8,11–18).
Cervical cancer is difficult to detect early and it may also occur
at a young age. While early detection improves the cure rate, the
stage at diagnosis remains one of the most significant prognostic
factors. Therefore, it is crucial to identify novel biomarkers for
early detection and new targets for the treatment of cervical
cancer. B7-H4 is reportedly overexpressed in early-stage ovarian
cancer and is independent of CA125, suggesting that B7-H4 may be a
novel biomarker (19).
B7-H4 was shown to be a prognostic maker in various
tumors, although the correlation between B7-H4 expression and the
prognosis of cervical cancer has not been fully elucidated. In this
study, we measured the B7-H4 expression intensity in cervical
cancer tissues using an immunohistochemical method and the
association of B7-H4 expression with clinicopathological parameters
and the intensity of expression was analyzed. B7-H4 may serve as a
novel prognostic predictor for human cervical cancer and is also a
potential target for therapeutic intervention.
Materials and methods
Tissue samples
Tissues were obtained from 102 patients who
underwent surgery at the Nagoya University Hospital. The age of the
patients ranged between 23 and 80 years, with a median age of 50
years. Patients who underwent pre-operative treatment, such as
radiotherapy and/or chemotherapy, were excluded. All tissue samples
were fixed in 10% formalin, embedded in paraffin and routinely
stained with hematoxylin and eosin for histological
examination.
Immunohistochemical B7-H4 staining and
evaluation
Formalin-fixed, paraffin-embedded tissue blocks were
cut into 4-mm sections and mounted on charged glass slides,
deparaffinized and rehydrated in a graded series of ethanol.
Antigen retrieval was performed in 1 mmol/l EDTA solution (Nacalai
Tesque Inc., Kyoto, Japan) (pH 8.0) at 98ºC for 15 min. Endogenous
peroxidase activity was blocked with 3.0% hydrogen peroxide (Wako,
Osaka, Japan) for 5 min at room temperature. Blocking was performed
in 10% normal goat serum (NGS; DakoCytomation, Glostrup, Denmark)
with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) in
Tris-buffered saline (TBS) with 0.025% Tween-20 (TBS-T, Kanto
Chemical Co., Inc., Tokyo, Japan) for 60 min. The sections were
incubated with anti-B7-H4 rabbit monoclonal antibody in blocking
buffer at 4ºC overnight (clone EP1165; Abcam, Cambridge, MA, USA).
After three washings, the sections were incubated with biotinylated
goat anti-rabbit horseradish peroxidase for 60 min at a 1:500
dilution, followed by incubation with avidin-biotin complex reagent
for 30 min at a 1:100 dilution (PK-4001; Vector Laboratories,
Burlingame, CA, USA) and finally visualized with
3,3′-diaminobenzidine (DAB peroxidase substrate kit, SK-4100,
Vector Laboratories) in Milli-Q purified water (Millipore,
Billerica, MA, USA). The slides were counterstained with Mayer’s
hematoxylin (Wako). The sections were dehydrated, cleared and
mounted. Negative controls were run on all sections with 3% NGS in
TBS-T, generated against unrelated antigens. The intensity of
immunostaining for B7-H4 was scored as 0 (negative), 1 (weak), 2
(medium) and 3 (strong).
Statistical analysis
The association between negative vs. positive B7-H4
expression and the clinicopathological parameters was evaluated
using χ2 tests. The various clinicopathological
parameters were evaluated using the Kaplan-Meier method by
univariate survival analysis. The comparison between the survival
curves was analyzed using the log-rank test for differences in
overall patient survival. The overall survival (OS) was defined as
the time between the date of surgery and last date of follow-up or
date of death from cervical cancer. The disease-free survival (DFS)
was defined as the time interval between the date of surgery and
date of recurrence or that of the last follow-up. The prognostic
significance of B7-H4 expression according to other pathological
variables was assessed using the multivariate Cox’s proportional
hazards analysis. Statistical analyses were conducted using free R
software and P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunohistochemical detection of B7-H4 in
cervical cancer tissues
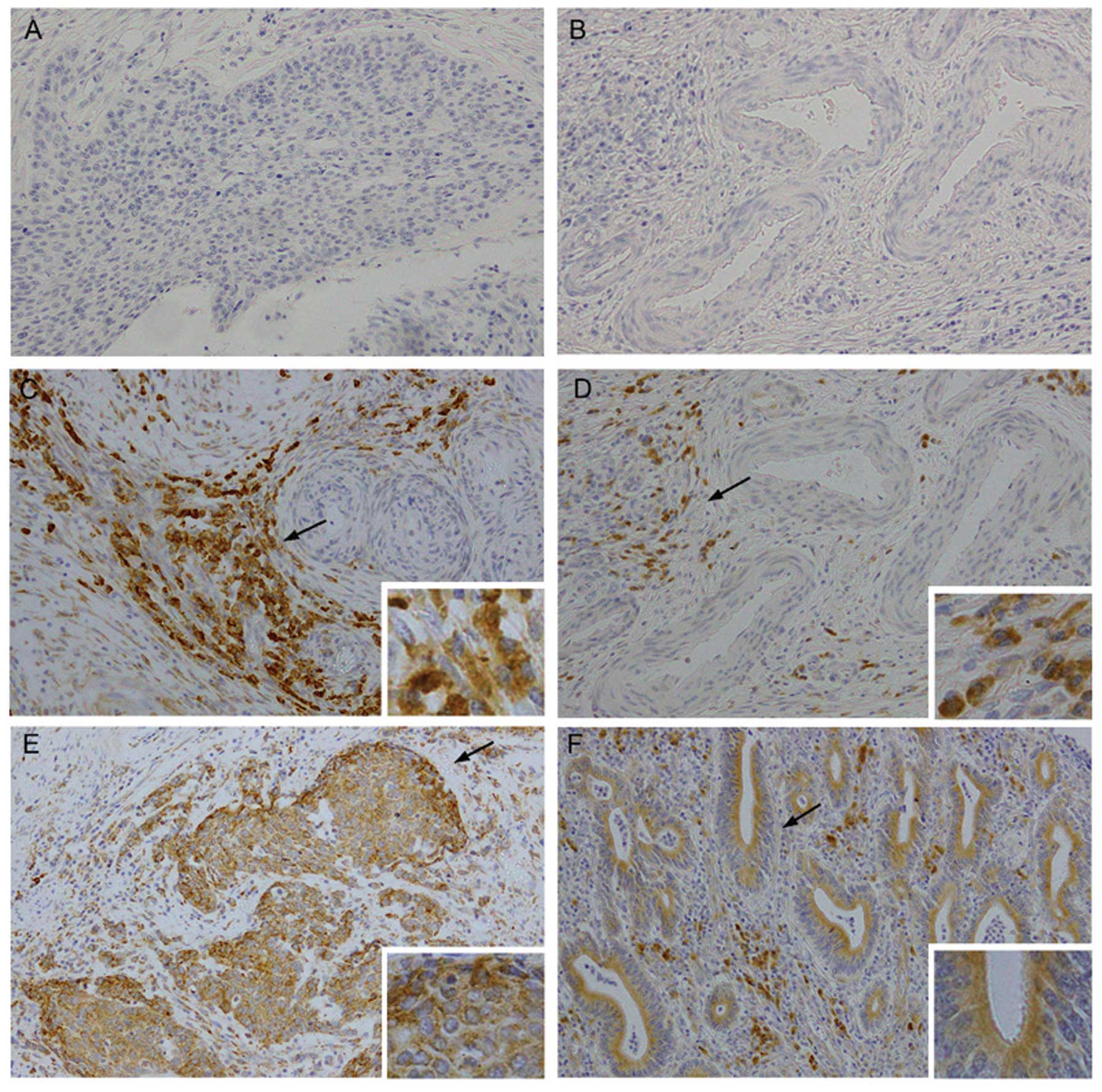
A total of 102 cervical cancer specimens were
investigated in this study and 71 (69.6%) were found to be positive
for B7-H4 immunoexpression, of which 23 were strongly positive.
B7-H4 was detected on the cell membrane as well as in the cytoplasm
of cervical cancer cells (Fig. 1).
In several cases, the expression of B7-H4 in the tumor stroma was
found to be higher compared to that in tumor cells (Fig. 1C and D). There were also cases in
which cancer involving vascular spaces exhibited strong B7-H4
staining (Fig. 1C and D). The
strongly positive cases were included in positive cases for the
statistical analysis. The association between B7-H4 expression and
clinicopathological variables is shown in Table I. There was no significant
correlation between the expression of B7-H4 and patient age, FIGO
stage, histological type, LVSI, or lymph node status.
Representative images of B7-H4 staining are shown in Fig. 1.
 | Table IAssociation between B7-H4 expression
and clinicopathological parameters. |
Table I
Association between B7-H4 expression
and clinicopathological parameters.
| | B7-H4 | |
|---|
| |
| |
|---|
| Parameters | n | Negative (%) | Positive (%) | P-value |
|---|
| Total | 102 | 31 (30.4) | 71 (69.6) | - |
| Age (years) |
| <50 | 53 | 15 (28.3) | 38 (71.7) | 0.793 |
| ≥51 | 49 | 16 (32.7) | 33 (67.3) | |
| FIGO stage |
| I–IIA | 73 | 24 (32.9) | 49 (67.1) | 0.531 |
| IIB–IV | 29 | 7 (24.1) | 22 (75.9) | |
| Histological
type |
| Squamous | 83 | 27 (32.5) | 56 (67.5) | 0.481 |
| Adenocarcinoma | 19 | 4 (21.1) | 15 (78.9) | |
| LVSI |
| Negative | 54 | 14 (25.9) | 40 (74.1) | 0.410 |
| Positive | 48 | 17 (35.4) | 31 (64.6) | |
| Lymph node |
| Negative | 74 | 22 (29.7) | 52 (70.3) | 0.996 |
| Positive | 28 | 9 (32.1) | 19 (67.9) | |
Association of B7-H4 expression with the
survival of cervical cancer patients
The median follow-up for all patients was 68 months
(range, 4.8–169 months). Of the 102 patients, 20 (19.6%) had
developed a relapse of cervical cancer at the time of the last
follow-up and 16 (15.6%) eventually succumbed to the disease. The
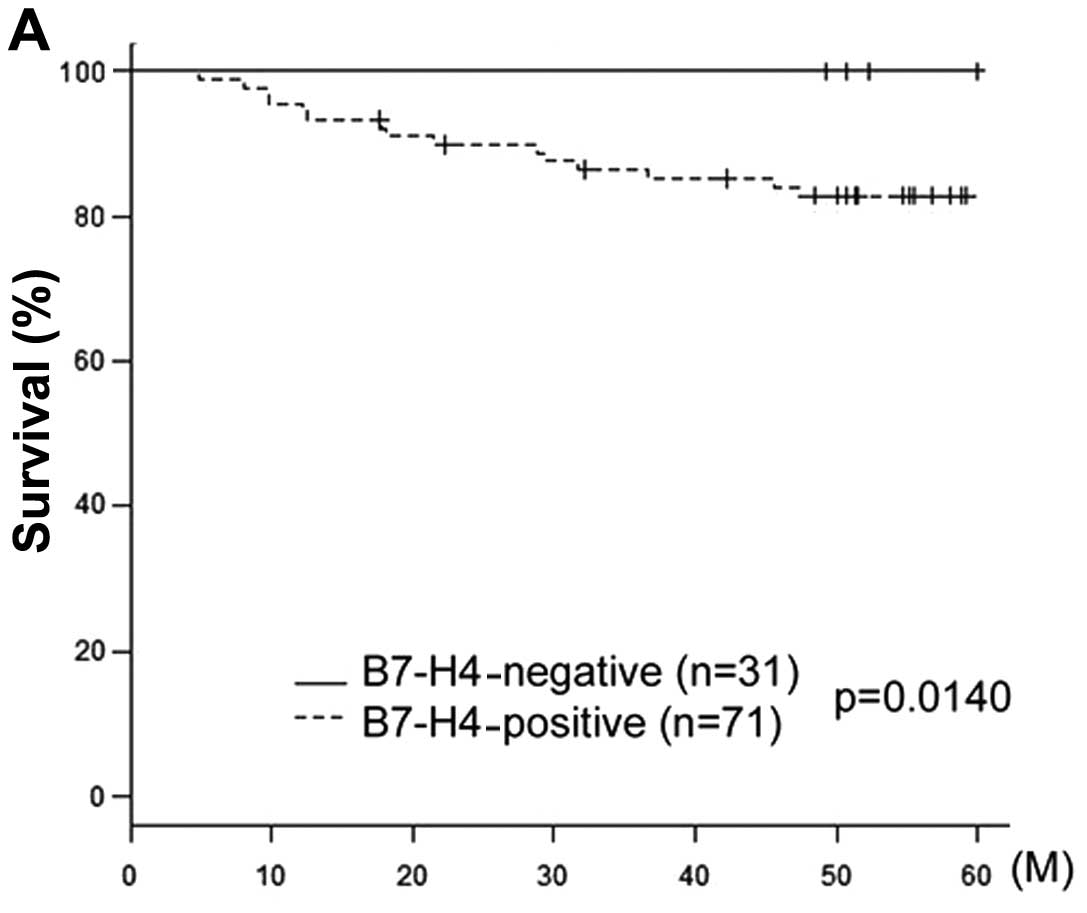
5-year OS rate for patients who were negative (n=31) and positive
(n=71) for B7-H4 expression were 94.7 and 78.4%, respectively
(Table II). Fig. 2 shows the OS and DFS curves with
respect to B7-H4 expression. The OS of B7-H4-positive patients was
significantly lower compared with that of B7-H4-negative patients
(P=0.014). The DFS in B7-H4-positive patients exhibited a tendency
to be associated with positive B7-H4 expression, although this
association was not significant (P=0.0813).
 | Table IIUnivariate analysis of various
clinicopathological parameters in relation to survival of patiens
with cervical cancer. |
Table II
Univariate analysis of various
clinicopathological parameters in relation to survival of patiens
with cervical cancer.
| | Overall survival | Disease-free
survival |
|---|
| |
|
|
|---|
| Parameters | n | 5-year survival
(%) | P-value | 5-year survival
(%) | P-value |
|---|
| Age (years) |
| <50 | 53 | 90.6 | 0.0386 | 88.4 | 0.0685 |
| ≥51 | 49 | 79.0 | | 74.6 | |
| FIGO stage |
| I–IIA | 73 | 90.3 | 0.0077 | 90.3 | 0.0001 |
| IIB–IV | 29 | 71.5 | | 59.1 | |
| Histological
type |
| Squarmous | 83 | 85.4 | 0.6410 | 82.6 | 0.4700 |
|
Adenocarcinoma | 19 | 83.0 | | 78.3 | |
| LVSI |
| Negative | 54 | 90.6 | 0.0387 | 88.7 | 0.0679 |
| Positive | 48 | 79.1 | | 74.2 | |
| Lymph node
status |
| Negative | 74 | 90.4 | 0.0009 | 88.9 | 0.0009 |
| Positive | 28 | 70.4 | | 62.9 | |
| B7-H4 |
| Negative | 31 | 94.7 | 0.0140 | 90.2 | 0.0813 |
| Positive | 71 | 78.4 | | 78.2 | |
In the univariate analysis, age, FIGO stage,
positive LVSI and lymph node metastasis and positive expression of
B7-H4 were found to be significant predictors of poor OS.
Furthermore, FIGO stage and lymph node metastasis, which are
prognostic factors of DFS, were significantly poorer in
B7-H4-positive cases. Other factors, including age and LVSI,
exhibited a tendency to be associated with DFS, although this
association was not significant (Table II). Notably, with regard to OS and
DFS, FIGO stage and positive lymph node metastasis were found to be
more significant (P<0.01 and <0.001, respectively).
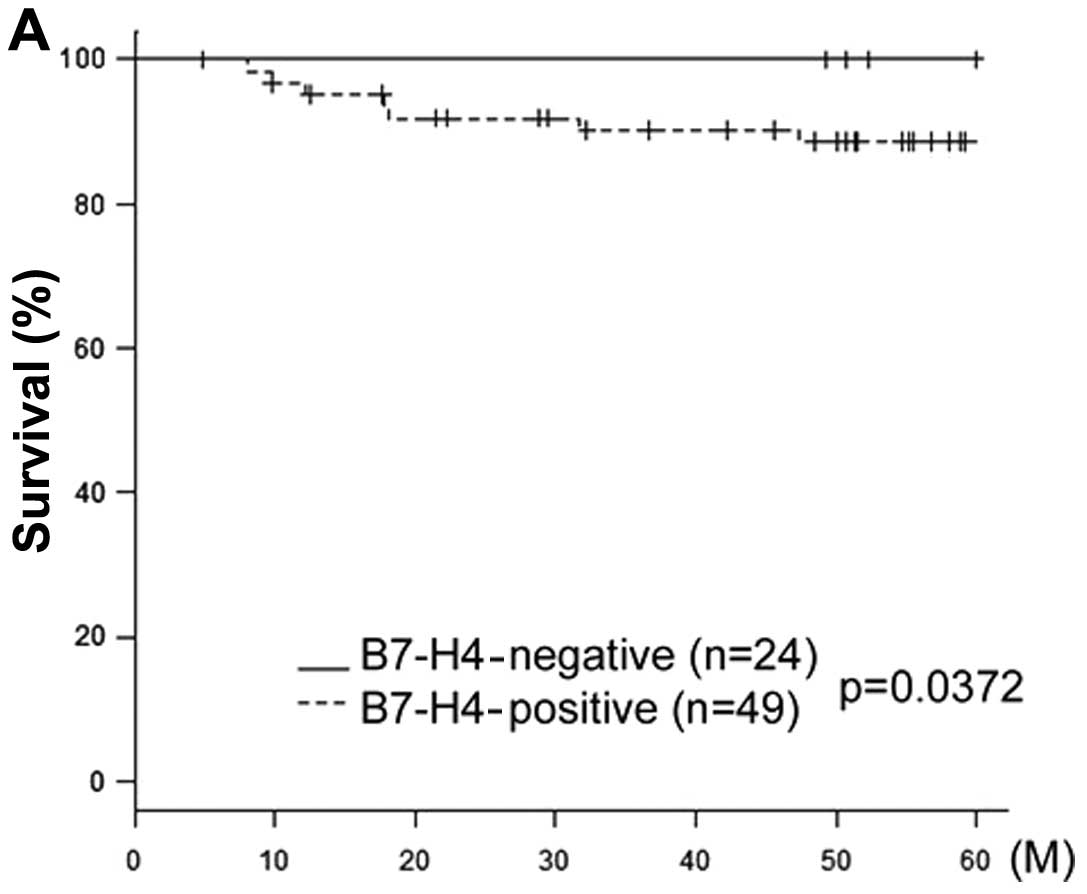
The cases were subdivided further into two groups
according to their FIGO stage (early stage I–IIA and advanced stage
IIB–IV). In the early-stage group, B7-H4 expression was
significantly associated with prognosis (P=0.0372) (Fig. 3A). However, in the advanced-stage
group, B7-H4 expression was not significantly associated with
prognosis (P=0.207) (Fig. 3B).
Similarly, all the cases were stratified according
to histological subtype into squamous cell carcinoma (SCC) and
adenocarcinoma (Fig. 3C and D).
The expression of B7-H4 was a significant prognostic factor in SCC,
but not in adenocarcinoma (SCC, P=0.0341 and adenocarcinoma,
P=0.205). However, the squamous cell carcinoma subtype was a
significant poor prognostic factor.
Multivariate analysis of prognostic
variables in cervical cancer patients
As shown in Table
III, in the multivariate OS analysis, age, FIGO stage,
histological type, presence of LVSI, lymph node status and B7-H4
expression were included in the Cox proportional hazards analysis.
The relative risk (RR) for positive B7-H4 expression was 10.34 and
the 95% confidence interval (CI) was 1.345–79.478; P=0.025.
Similarly, the multivariate analysis of DFS revealed that only the
FIGO stage was a significant independent prognostic factor
(RR=3.47; 95% CI: 1.263–9.551; P=0.016). However, age, histological
type, LVSI, and lymph node status were not significantly
associated. With regard to DFS, there was a difference between
positive and negative expression of B7-H4, but it was not
considered to be a significant independent prognostic factor, due
to the narrow margin (RR=3.19; 95% CI: 0.903–11.247; P=0.072).
 | Table IIIMultivariate analysis of several
clinicopathological parameters in relation to survival of patiens
with cervical cancer. |
Table III
Multivariate analysis of several
clinicopathological parameters in relation to survival of patiens
with cervical cancer.
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|---|
| Parameters | Relative risk (95%
CI) | P-value | Relative risk (95%
CI) | P-value |
|---|
| Age (years) |
| <50 | 1 | 0.098 | 1 | 0.220 |
| ≥51 | 2.54
(0.841–7.659) | | 1.82
(0.694–4.783) | |
| FIGO stage |
| I–IIA | 1 | 0.240 | 1 | 0.016 |
| IIB–IV | 1.86
(0.658–5.268) | | 3.47
(1.263–9.551) | |
| Histological
type |
| Squarmous | 1 | 0.800 | 1 | 0.470 |
|
Adenocarcinoma | 0.86
(0.261–2.805) | | 1.49
(0.503–4.434) | |
| LVSI |
| Negative | 1 | 0.220 | 1 | 0.230 |
| Positive | 2.27
(0.615–8.405) | | 2.02
(0.640–6.360) | |
| Lymph node
status |
| Negative | 1 | 0.200 | 1 | 0.300 |
| Positive | 2.32
(0.642–8.349) | | 1.83
(0.578–5.787) | |
| B7-H4 |
| Negative | 1 | 0.025 | 1 | 0.072 |
| Positive | 10.34
(1.345–79.478) | | 3.19
(0.903–11.247) | |
Discussion
In the present study of 102 cervical cancer patients
with a long-term follow-up, our results demonstrated that B7-H4 was
highly and aberrantly expressed (71/102, 69.6%) and the B7-H4
expression level was not associated with any of the
clinicopathological parameters. B7-H4 immunoexpression was
evaluated based on stain intensity and area analysis. However,
there was no significant association between B7-H4 expression and
OS excluding intensity analysis. High B7-H4 expression was observed
in a predominantly cytoplasmic and circumferential membranous
distribution pattern. There was also limited immmunoreactivity for
B7-H4 in the tumor stroma and perivascular area. Similar results
were previously reported (20). In
addition to tumor cells, tumor-infiltrating macrophages (8,15,21)
and the endothelial cells of small blood vessels in the cancer
microenvironment were also found to constitutively express B7-H4.
B7-H4 was also found to be highly expressed on tumor-associated
macrophages in the ascites of ovarian cancer patients and may
contribute to tumor progression (15).
In our study, high tumor B7-H4 expression was
associated with a poorer OS in the uni- and multivariate analysis
of all the factors affecting survival. The correlation between
B7-H4 expression and its clinical significance in patients with
malignancies was previously described (7,10,11,15–17,20,22,23).
Despite the unique patient populations, the differences in the
antibodies used, the variations in the definitions of endpoints and
the different pathologists evaluating the specimens, we
independently obtained markedly similar results. Those studies
suggested that aberrantly expressed B7-H4 was associated with
adverse pathological characteristics and an increased risk of a
poor prognosis or death from this disease. Our results demonstrated
that cervical cancer patients with B7-H4-positive tumors exhibited
a significantly worse OS compared to that of B7-H4-negative
patients.
B7-H4 staining intensity was found to be associated
with cancer spread along with clinical cancer recurrence and
subsequent death. The invasion of lymphatic or blood vessels by
carcinomatous cells is considered to be a critical step in the
formation of lymphatic or distant metastasis. The involvement of
lymphatic vessels is clearly an indicator of an unfavorable
prognosis, even in early-stage cervical cancer. Tumor B7-H4
expression was also associated with lymphocytic infiltration, which
predicts a poor clinical outcome for patients with cervical cancer
(7). Furthermore, we observed that
B7-H4 was associated with a significantly increased mortality risk
in cervical cancer patients with pathologically localized cervical
cancer tumors in the univariate analysis. Although FIGO stage, LVSI
and lymph node metastasis were not identified as significant
independent prognostic factors, they tended to be associated with
poor prognosis. B7-H4 expression in cervical cancer patients is an
independent prognostic predictor of the clinical outcome.
The role of B7-H4 has not been clearly determined
and functional analyses are currently difficult to perform. B7-H4
was demonstrated to be a negative regulator of T-cell responses and
to render tumors cells refractory to apoptosis (9,13).
Regarding the underlying mechanism, one possible explanation is
that B7-H4 expression inhibits T-cell proliferation, decreases
cytokine secretion and prevents apoptosis (8,13).
Two studies demonstrated that the overexpression of B7-H4 in human
ovarian cancer cell lines promoted tumor formation in severe
combined immunodeficiency mice, whereas short interfering
RNA-mediated knockdown of B7-H4 mRNA and protein expression in a
breast cancer cell line enhanced intracellular caspase activity,
leading to the acceleration of tumor cell apoptosis (9,24).
Furthermore, B7-H4 was shown to affect the immune response to and
the outcome of lethal pulmonary infection (25) and induce donor-specific tolerance
following transplantation (26,27).
Therefore, B7-H4 may also be used as an immunotherapeutic target
against malignancies.
In conclusion, in this study, we presented evidence
supporting the role of B7-H4 as an independent prognostic predictor
of clinical outcome in cervical cancer patients. Further functional
experiments may elucidate the molecular mechanism underlying the
effect of B7-H4 on the prognosis of cervical cancer. Furthermore,
B7-H4 may be a novel target for molecular-targeted therapy against
cervical cancer.
Acknowledgements
The authors would like to thank Dr Luo and Ms.
Daimon for their laboratory support.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibata K, Kajiyama H, Ino K, et al: Twist
expression in patients with cervical cancer is associated with poor
disease outcome. Ann Oncol. 19:81–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sica GL, Choi IH, Zhu G, et al: B7-H4, a
molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fauci JM, Straughn JM Jr, Ferrone S and
Buchsbaum DJ: A review of B7-H3 and B7-H4 immune molecules and
their role in ovarian cancer. Gynecol Oncol. 127:420–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7×: a widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003.
|
|
7
|
Krambeck AE, Thompson RH, Dong H, et al:
B7-H4 expression in renal cell carcinoma and tumor vasculature:
associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kryczek I, Wei S, Zhu G, et al:
Relationship between B7-H4, regulatory T cells, and patient outcome
in human ovarian carcinoma. Cancer Res. 67:8900–8905. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salceda S, Tang T, Kmet M, et al: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tringler B, Zhuo S, Pilkington G, et al:
B7-H4 is highly expressed in ductal and lobular breast cancer. Clin
Cancer Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tringler B, Liu W, Corral L, et al: B7-H4
overexpression in ovarian tumors. Gynecol Oncol. 100:44–52. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen LJ, Sun J, Wu HY, et al: B7-H4
expression associates with cancer progression and predicts
patient’s survival in human esophageal squamous cell carcinoma.
Cancer Immunol Immunother. 60:1047–1055. 2011.PubMed/NCBI
|
|
13
|
Seliger B, Marincola FM, Ferrone S and
Abken H: The complex role of B7 molecules in tumor immunology.
Trends Mol Med. 14:550–559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quandt D, Fiedler E, Boettcher D, Marsch
WCh and Seliger B: B7-H4 expression in human melanoma: its
association with patients’ survival and antitumor immune response.
Clin Cancer Res. 17:3100–3111. 2011.PubMed/NCBI
|
|
15
|
Kryczek I, Zou L, Rodriguez P, et al:
B7-H4 expression identifies a novel suppressive macrophage
population in human ovarian carcinoma. J Exp Med. 203:871–881.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyatake T, Tringler B, Liu W, et al:
B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid
adenocarcinomas and inversely correlated with tumor T-cell
infiltration. Gynecol Oncol. 106:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Wang Y, Zhao J, et al: B7-H3 and
B7-H4 expression in non-small-cell lung cancer. Lung Cancer.
53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arigami T, Uenosono Y, Ishigami S,
Hagihara T, Haraguchi N and Natsugoe S: Clinical significance of
the B7-H4 coregulatory molecule as a novel prognostic marker in
gastric cancer. World J Surg. 35:2051–2057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simon I, Katsaros D, Rigault de la
Longrais I, et al: B7-H4 is over-expressed in early-stage ovarian
cancer and is independent of CA125 expression. Gynecol Oncol.
106:334–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zang X, Thompson RH, Al-Ahmadie HA, et al:
B7-H3 and B7× are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007.
|
|
21
|
Galazka K, Oplawski M, Windorbska W, et
al: The immunohistochemical analysis of antigens such as RCAS1 and
B7H4 in the cervical cancer nest and within the fibroblasts and
macrophages infiltrating the cancer microenvironment. Am J Reprod
Immunol. 68:85–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Awadallah NS, Shroyer KR, Langer DA, et
al: Detection of B7-H4 and p53 in pancreatic cancer: potential role
as a cytological diagnostic adjunct. Pancreas. 36:200–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He C, Qiao H, Jiang H and Sun X: The
inhibitory role of B7-H4 in antitumor immunity: association with
cancer progression and survival. Clin Dev Immunol.
2011:6958342011.PubMed/NCBI
|
|
24
|
Cheng L, Jiang J, Gao R, et al: B7-H4
expression promotes tumorigenesis in ovarian cancer. Int J Gynecol
Cancer. 19:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hofmeyer KA, Scandiuzzi L, Ghosh K,
Pirofski LA and Zang X: Tissue-expressed B7× affects the immune
response to and outcome of lethal pulmonary infection. J Immunol.
189:3054–3063. 2012.
|
|
26
|
McGrath MM and Najafian N: The role of
coinhibitory signaling pathways in transplantation and tolerance.
Front Immunol. 3:472012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Hao J, Metzger DL, et al: B7-H4
treatment of T cells inhibits ERK, JNK, p38, and AKT activation.
PLoS One. 7:e282322012. View Article : Google Scholar : PubMed/NCBI
|