Introduction
Lung cancer is one of the leading causes of
mortality, with a curing rate of only ∼13% (1). Non-small cell lung cancers (NSCLC)
account for 80% of lung cancers. Approximately 75–80% of NSCLC
patients, when diagnosed, are already in the late stage of the
cancer. Surgery is the main treatment for NSCLC patients in
clinical stage I–IIIa. Following surgery, administration of
adjuvant chemotherapy (combination of two platinum drugs) may lead
to significant survival benefits (2), even though 30–60% of patients
receiving adjuvant chemotherapy experience tumor recurrence or
distant metastasis (3). Multidrug
resistance (MDR) constitutes a challenge with regard to effective
chemotherapeutic interventions. The occurrence of MDR, regardless
of whether it is congenital or acquired, is a serious challenge for
effective administration of NSCLC treatments.
p53 tumor suppressor gene is a multifunctional
protein that is involved in the regulation of cell cycles,
apoptosis, gene transcription, stress response and DNA repair.
Previous studies focused on the correlation between p53 protein
expression and cisplatin-based chemotherapy for late-stage NSCLC
patients. In their study, Tsao et al(4) observed that among 253 NSCLC cases,
positive p53 protein expression was more likely to be found in male
and squamous cell carcinoma patients. However, Bai et
al(5) observed that a high
expression of p53 protein is correlated with tumor invasion status
in hilar, pericardium, blood vessels and thrombosis.
Multidrug resistance proteins (MRP), including MRP1
and MRP2, are key members of the ATP-binding transporter
superfamily (ABC proteins). These proteins are involved in the
transmembrane transportation of prokaryotic and eukaryotic cells.
By regulating the pH in cytoplasm and organelles, MRP proteins may
decrease the amount of drugs at the functioning sites and reduce
the intracellular concentration of drugs, leading to drug
resistance. MRP expression may be associated with drug resistance
and prognosis of lung cancers (6).
However, the correlation between MRP expression and lung cancer
types, differentiations and clinical stages remains to be
clarified.
Overexpression of c-erbB2 protein expression may be
detected in a variety of malignancies, including NSCLC. c-erbB2
protein is a marker of endogenous MDR, which can be used as an
independent predictor (7). c-erbB2
protein overexpression is associated with mutations in chromosome
17q21 locus (8). Turken et
al(9) demonstrated that 35% of
NSCLC cases were associated with a high c-erbB2 protein expression.
A high c-erbB2 protein expression is frequently detected in lung
adenocarcinoma tissues in stage IIIb–IV (P=0.04). NSCLC patients
with a positive c-erbB2 protein expression are prone to recurrence
and metastasis following treatment. Therefore, a high c-erbB2
protein expression is an indicator of tumor progression (8,9), but
a high c-erbB2 protein expression is not associated with the
sensitivity of chemotherapy (9).
In this study, immunohistochemical methods were used
to investigate expression levels of three drug
resistance-associated proteins (p53, c-erbB2 and MRP) in NSCLC
tissues. The correlation between expression levels of p53, c-erbB2
and MRP and the clinicopathological characteristics of NSCLC, as
well as the prognostic significance of these protein expressions,
was investigated.
Materials and methods
Patients
In total, 152 NSCLC samples were confirmed by
surgery and pathological detection. Inclusion criteria were: i)
pathologically proven NSCLC cells; ii) primary lung cancer cases;
iii) no adjuvant radiotherapy or chemotherapy received prior to
surgery; iv) no distant metastasis found prior to surgery and v) no
serious heart and lung diseases or other combined diseases
diagnosed (Table I).
 | Table ICorrelation between expression of p53,
MRP and c-erB2 proteins and clinicopathological characteristics of
NSCLC patients. |
Table I
Correlation between expression of p53,
MRP and c-erB2 proteins and clinicopathological characteristics of
NSCLC patients.
| Clinicopathological
characteristics | No. | p53 | MRP | c-erbB2 |
|---|
|
|
|
|---|
| Positive (%) | χ2 | Positive (%) | χ2 | Positive (%) | χ2 |
|---|
| Gender | | | | | | | |
| Male | 127 | 73 (57.5) | 54 (42.5) | 55(43.3) | | | |
| Female | 25 | 9 (36.0) | 3.879a | 12 (48.0) | 0.255 | 12 (48.0) | 0.214 |
| Age (years) | | | | | | | |
| ≤59 | 69 | 32 (46.4) | 30(43.5) | 35 (50.7) | | | |
| >59 | 83 | 50 (60.2) | 2.915 | 36 (43.4) | 0.000 | 32 (38.6) | 1.237 |
| Pathological
types | | | | | | | |
| Squamous cell
carcinoma | 97 | 35 (36.1) | 32 (33.0) | 46 (47.4) | | | |
| Adenocarcinoma | 37 | 15 (40.5) | 25 (67.6) | 13 (35.1) | | | |
| Others | 18 | 5 (27.8) | 1.520 | 9 (50.0) | 7.545a | 8 (44.4) | 0.978 |
| Differentiation | | | | | | | |
| High and
medium | 99 | 43 (43.4) | 38 (38.4) | 51 (51.5) | | | |
| Low | 53 | 39 (73.6) | 12.63b | 28 (52.8) | 2.932 | 16 (30.2) | 2.548 |
| TNM staging | | | | | | | |
| I–II | 99 | 46 (46.5) | 49 (49.5) | 50 (50.5) | | | |
| III | 53 | 36 (67.9) | 6.399a | 17 (32.1) | 3.125 | 17 (32.1) | 3.087 |
| Lymph node
metastasis | | | | | | | |
| Yes | 78 | 51 (65.4) | 32 (41.0) | 29 (37.2) | | | |
| No | 74 | 31 (41.9) | 8.436b | 34 (45.9) | 0.374 | 38 (51.4) | 1.963 |
| Vascular tumor
thrombus | | | | | | | |
| Yes | 17 | 7 (41.2) | 5 (29.4) | 6 (35.3) | | | |
| No | 135 | 75 (55.6) | 1.257 | 61 (45.2) | 1.529 | 61 (45.2) | 1.102 |
| Margin | | | | | | | |
| Negative | 148 | 79 (53.4) | 65 (43.9) | 66 (44.6) | | | |
| Positive | 4 | 3 (75.0) | 0.733 | 1 (25.0) | 0.567 | 1 (25.0) | 0.379 |
Immunohistochemistry (IHC)
Tissue samples were fixed with formaldehyde
solution, embedded in paraffin, followed by regular (4 μm)
slicing. IHC was performed by the S-P method, as per the
manufacturer’s instructions, using p53, c-erbB2 and MRP mouse
anti-human monoclonal antibodies and the S-P immunohistochemistry
kit (Kit-9710; Maixin Biotechnology, Fujian, China). Samples were
treated under the same conditions, including staining and
3,3′-diaminobenzidine (DAB) coloring. Lung sections with known
protein expression were used as positive controls under the same
conditions. Phosphate-buffered saline (PBS) was used as the blank
control for primary antibodies. Normal mouse serum was used as the
negative control for primary antibodies. For each
immunohistochemical staining sample, 10 high-power views
(magnification, ×200) were randomly selected for microscopic
observation.
Criteria for p53 protein staining were (10): cells without nucleus-stained
particles were considered negative, while cells with
nucleus-stained particles were considered positive. For the tissue
samples: i) if <30% of the cells were positive, the tissues were
considered to be weak positive; ii) if 30–70% of the cells were
positive, the tissues were considered to be moderately positive and
iii) if >70% of the cells were positive, the tissues were
considered to be strongly positive. MRP and c-erbB2 protein
staining was analyzed based on the Hercep score (7,11),
as recommended by the Food and Drug Administration (FDA). Staining
scores were described as: 0–1, negative; 1–2, weakly positive and
2–3, strongly positive.
Statistical analysis
Data were analyzed using the SPSS 16.0 software.
Survival rate analyses were performed using the Kaplan-Meier
method. Sample rates were compared using the χ2 test.
Single-factor analyses were carried out using the log-rank test.
Multivariate analyses were performed using Cox’s regression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of p53, c-erbB2 and MRP
proteins in NSCLC cells
In this study, correlations between the survival
rates of 152 NSCLC patients and positive expression of p53, c-erbB2
and MRP proteins in NSCLC tissues were investigated. Positive
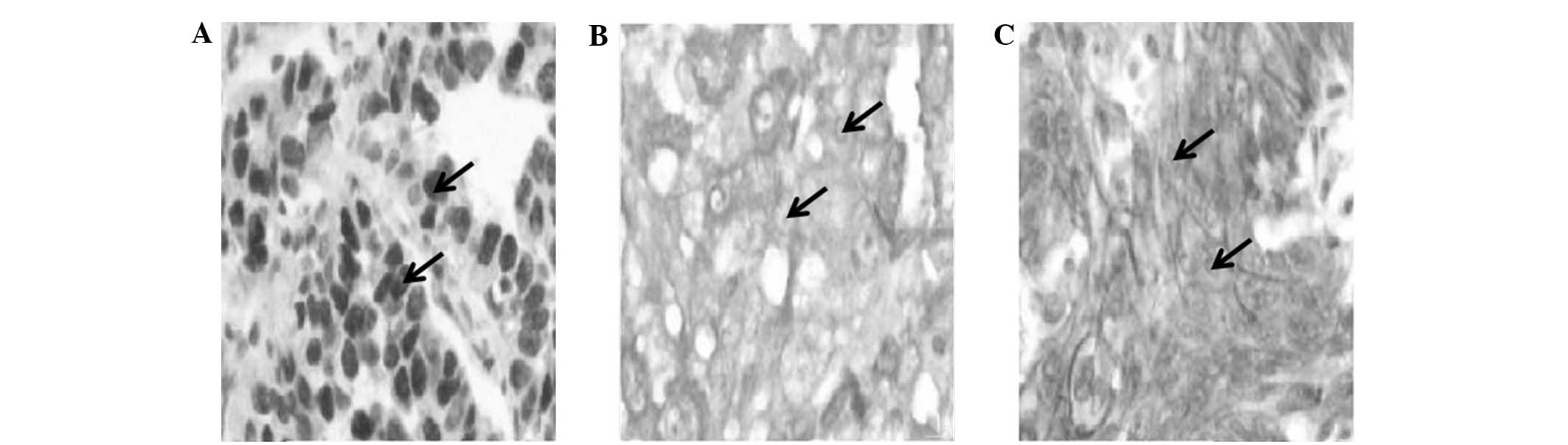
expression of p53, c-erbB2 and MRP was detected by IHC (Fig. 1). IHC staining results indicated
that of the 152 NSCLC cases, the positive expression of p53,
c-erbB2 and MRP proteins was 53.9 (82/152), 44.1 (67/152) and 43.4%
(66/152), respectively. Spearman’s rank correlation coefficient
revealed a correlation in the expression of the three proteins in
NSCLC tissues. The r-values between p53 and MRP, and those between
p53 and c-erbB-2 were 0.248 (P=0.019) and 0.335 (P=0.002),
respectively. The r-values between MRP and c-erbB-2 were 0.321
(P=0.005).
Correlations between p53, c-erbB2, MRP
expression and clinicopathological characteristics of NSCLC
p53 protein expression in NSCLC tissues was markedly
correlated with patient gender, cancer cell differentiation,
clinical stages of NSCLC and lymph node metastasis (P<0.05 and
P<0.01) (Table I). A positive
expression rate of MRP was markedly higher in lung adenocarcinomas
(67.6%) compared with lung squamous cell carcinoma (33.0%)
(P<0.05). However, c-erbB2 expression was not correlated with
the clinicopathological characteristics of NSCLC (P>0.05).
Correlations between expression of p53,
c-erbB2 and MRP proteins and the survival rates of NSCLC
patients
The combination of positive p53, c-erbB2 and MRP
expression indicated poor prognosis (Table II). One, 2- and 3-year survival
rates of patients with a positive expression of these three
proteins were markedly lower compared with those of patients with a
negative expression of the three proteins (P=0.02, 0.01 and 0.00,
respectively).
 | Table IICorrelation between expression of
p53, MRP and c-erbB2 in tumor tissues and survival rates of NSCLC
patients. |
Table II
Correlation between expression of
p53, MRP and c-erbB2 in tumor tissues and survival rates of NSCLC
patients.
| Variables | No. | NSCLC survival
rates (146 cases)
|
|---|
| 1-year (%) | P-value | 2-year (%) | P-value | 3-year (%) | P-value |
|---|
| MRP | | | | | | | |
| Negative | 82 | 82.3 | 0.35 | 75.8 | 0.04 | 54.7 | 0.04 |
| Positive | 64 | 77.6 | 60.2 | 35.6 | | | |
| c-erbB2 | | | | | | | |
| Negative | 81 | 87.4 | 0.07 | 73.3 | 0.10 | 50.4 | 0.03 |
| Positive | 65 | 79.2 | 65.4 | 36.1 | | | |
| p53 | | | | | | | |
| Negative | 67 | 86.5 | 0.14 | 71.6 | 0.11 | 59.8 | 0.09 |
| Positive | 79 | 81.3 | 67.2 | 54.3 | | | |
| p53, MRP,
c-erbB2 | | | | | | | |
| All negative | 28 | 92.1 | 0.02 | 78.5 | 0.01 | 63.4 | 0.00 |
| p53, MRP,
c-erbB2 | | | | | | | |
| All positive | 23 | 72.6 | 54.8 | 32.2 | | | |
| p53 and MRP | | | | | | | |
| All negative | 16 | 88.3 | 0.08 | 76.5 | 0.03 | 61.8 | 0.02 |
| p53 and MRP | | | | | | | |
| All positive | 18 | 75.2 | 57.1 | 34.2 | | | |
| p53 or MRP | | | | | | | |
| Single
positive | 35 | 83.5 | 69.3 | 51.2 | | | |
| p53 and
c-erbB2 | | | | | | | |
| All Negative | 12 | 90.2 | 0.13 | 74.8 | 0.07 | 61.7 | 0.02 |
| p53 and
c-erbB2 | | | | | | | |
| All positive | 15 | 76.4 | 60.9 | 35.3 | | | |
| p53 or c-erbB2 | | | | | | | |
| Single
positive | 39 | 84.3 | 71.4 | 56.5 | | | |
| MRP and
c-erbB2 | | | | | | | |
| All negative | 23 | 88.6 | 0.08 | 76.2 | 0.03 | 58.7 | 0.01 |
| MRP and
c-erbB2 | | | | | | | |
| All positive | 11 | 74.2 | 58.1 | 32.6 | | | |
| MRP and
c-erbB2 | | | | | | | |
| Single
positive | 28 | 82.5 | 67.3 | 43.4 | | | |
Patients with a positive expression of two of the
three proteins had lower 1-, 2- and 3-year survival rates compared
with those of patients with a positive expression of one or a
negative expression of two proteins (P=0.02 and P=0.01,
respectively).
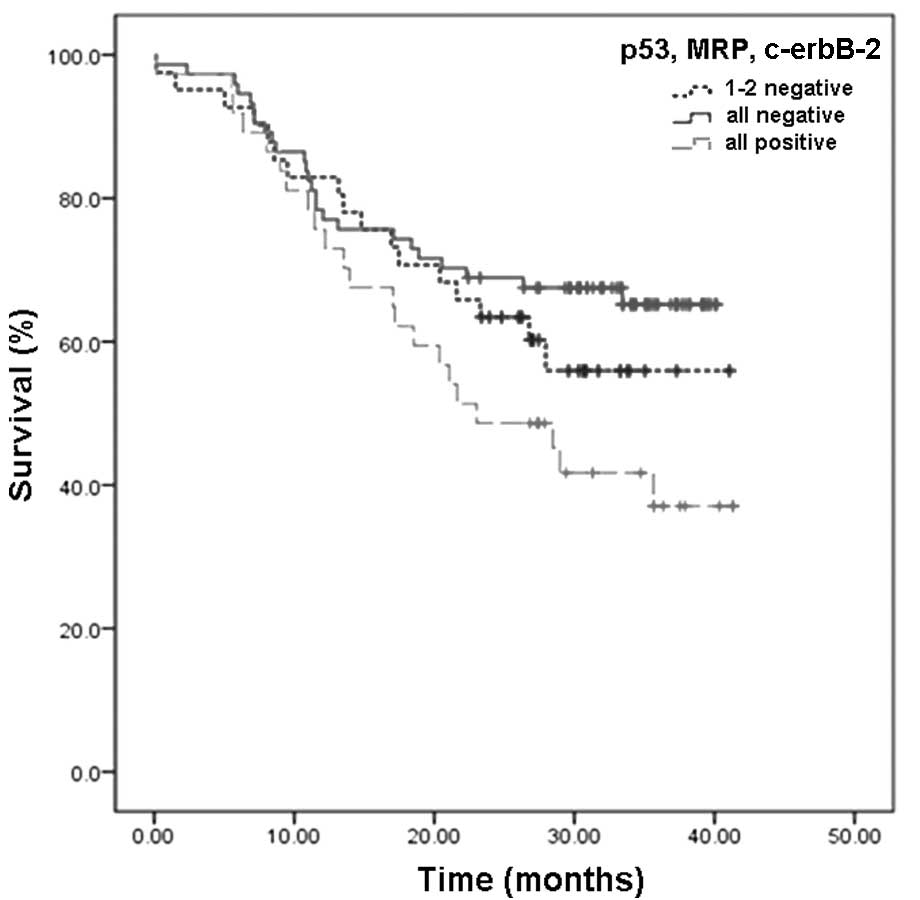
Correlations between expression of p53,
c-erbB2 and MRP proteins and the cumulative survival rates of NSCLC
patients receiving surgery
Correlations between expression of p53, c-erbB2 and
MRP proteins and the cumulative survival rates of NSCLC patients
receiving surgery were also investigated. The cumulative 1-, 2- and
3-year survival rates of patients with a positive expression of
p53, c-erbB2, and MRP proteins were markedly lower compared with
those of patients with a negative expression of the three proteins
(P<0.05) (Table III). Patients
with a negative expression of the three proteins had the highest
survival rates. Patients with a positive expression of one or two
proteins had mediate survival rates, while patients with a positive
expression of all three proteins had the lowest survival rates.
Survival rates in the subgroups were statistically significant
(Fig. 2, P<0.05).
 | Table IIICorrelation between expression of
p53, MRP and c-erbB2 in tumor tissues and survival rates of NSCLC
patients undergoing surgery only or surgery plus chemotherapy. |
Table III
Correlation between expression of
p53, MRP and c-erbB2 in tumor tissues and survival rates of NSCLC
patients undergoing surgery only or surgery plus chemotherapy.
| Variables | Surgery | Surgery and
chemotherapy after surgery |
|---|
|
|
|---|
| 1-year survival
(%) | 2-year survival
(%) | 3-year survival
(%) | P-value | 1-year survival
(%) | 2-year survival
(%) | 3-year survival
(%) | P-value |
|---|
| p53 | | | | | | | | |
| Positive | 79.5 | 59.0 | 43.6 | 0.03 | 82.5 | 70.0 | 62.5 | 0.82 |
| Negative | 84.8 | 69.7 | 58.7 | 94.1 | 73.5 | 61.8 | | |
| MRP | | | | | | | | |
| Positive | 76.4 | 61.3 | 34.9 | 0.04 | 78.2 | 58.3 | 36.9 | 0.03 |
| Negative | 79.7 | 74.5 | 49.6 | 84.6 | 76.7 | 59.4 | | |
| c-erbB2 | | | | | | | | |
| Positive | 74.2 | 62.2 | 32.7 | 0.04 | 83.6 | 70.7 | 38.7 | 0.01 |
| Negative | 80.5 | 72.4 | 48.9 | 94.3 | 74.1 | 52.9 | | |
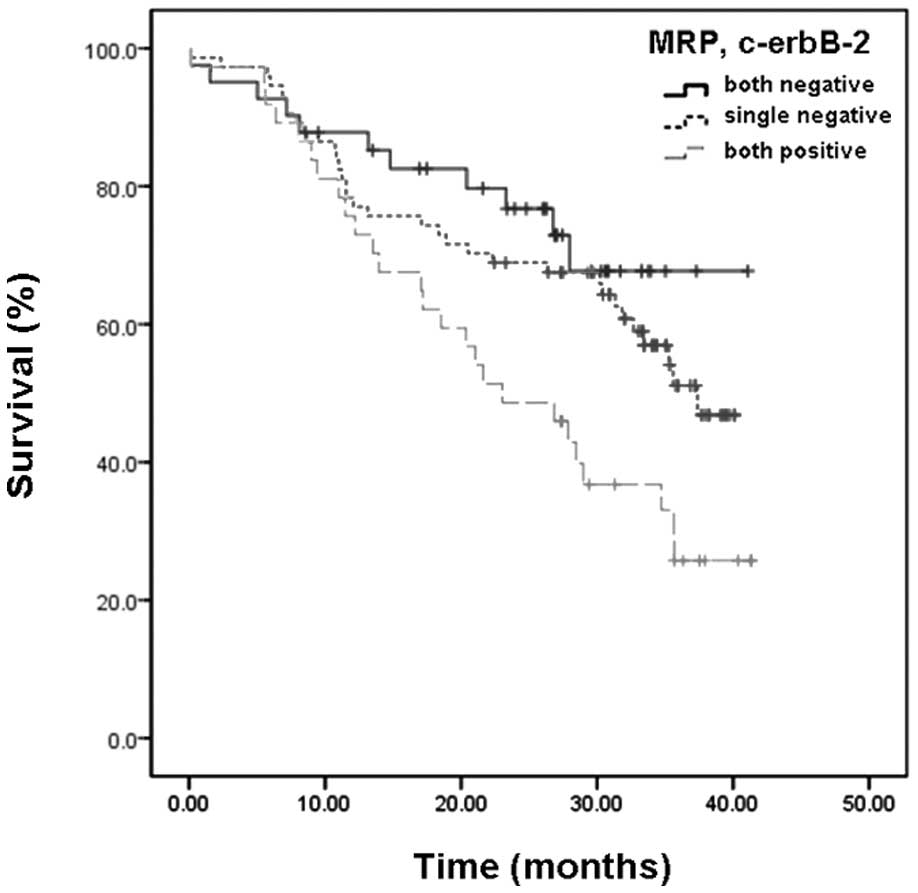
Correlations between expression of p53,
c-erbB2 and MRP proteins and the cumulative survival rates of NSCLC
patients receiving surgery and chemotherapy
In patients who received surgery plus chemotherapy
following surgery, and had a positive expression of MRP and c-erbB2
protein, the cumulative 1-, 2- and 3-year survival rates were
markedly lower compared with those of patients with a negative
expression of these proteins (Table
III, P<0.05). However, the survival rate differences of
patients with a positive or negative p53 expression were not
statistically significant (Table
III, P=0.82). Cumulative survival rates of patients with a
negative expression of MRP and c-erbB2 were higher compared with
those of patients with a positive expression of the two proteins.
Additionally, patients with a positive expression of either MRP or
c-erbB2 had cumulative survival rates between them. The results of
the comparisons were statistically significant (Fig. 3, P=0.01).
Cox multi-element analyses
Age, gender, pathological types, cancer cell
differentiation and other clinical or pathological characteristics,
as well as expression patterns of p53, c-erbB2 and MRP proteins
were analyzed in NSCLC patients, using the multi-element Cox model.
Cancer cell differentiation and c-erbB2 expression were identified
as two independent predictors of the prognosis of NSCLC patients
suitable for surgery (95% CI, P=0.000 and 0.029).
Discussion
MDR is the key reason for the poor efficacy of
chemotheraphy in NSCLC. Several factors are involved in MDR
development. The MRP phenotypes of various types of lung cancers
are different. The mechanisms of NSCLC MDR remain to be clarified,
but are possibly associated with changes in the expression levels
of a variety of proteins resulting in the process of tumor
formation. p53, MRP and c-erbB2 are three MDR-regulating proteins.
In this study, we observed that the three proteins are correlated
with the efficacy of surgery and chemotherapy in NSCLC
patients.
In this study, IHC was used to detect p53 expression
in 152 NSCLC cases. The findings show that the positive rate of p53
expression in NSCLC cases was 53.9%. p53 protein expression was
correlated with NSCLC patient gender, cytological grades, clinical
stages, lymph node metastasis and other clinical characteristics
(P<0.05), but did not correlate with pathological types. In
their study, Rusch et al(10) found that NSCLC patients with a
positive expression of p53 protein are less sensitive to cisplatin
compared with patients with a negative expression of p53 protein, a
negative correlation. Sengupta et al(12) reported that overexpression of p53
protein may affect the efficacy of chemotherapy, leading to poor
prognosis of NSCLC. However, Huang et al(13) observed that the chemotherapeutic
efficacy of late-stage NSCLC patients with a negative expression of
p53 protein was less than that of patients with a positive p53
expression, a positive correlation. However, this positive
correlation is not adequate to be statistically significant. Our
studies showed that 1-, 2- and 3-year survival rates of patients
with a positive expression of p53 protein were not statistically
different from those of patients with a negative p53 protein
expression (P=0.82), indicating that the post-surgery
chemotherapeutic efficacy did not correlate with p53 protein
expression. These inconsistencies may be due to the two-way
regulation of chemotherapy sensitivity by p53 as reported
previously (14). In their study,
Lee et al(15) reported
that a positive expression of p53 protein is a good indicator of
early prognosis in resectable NSCLC cases. However, Kwiatkowski
et al(16) and Ebina et
al(17) demonstrated that p53
protein overexpression predicts a poor prognosis for NSCLC patients
in early stages. In this study, the 1-, 2- and 3-year survival
rates of patients, who only underwent surgery and had a positive
expression of p53, were lower compared to those of patients with a
negative expression of p53. This difference is statistically
significant (P=0.03). Although a positive p53 expression is
negatively correlated with the prognosis of NSCLC, the Cox
multi-element analyses indicated that p53 is not an independent
predictor of the prognosis of NSCLC patients.
Filipits et al(18) found that NSCLC patients suitable
for surgery had a positive MRP1 expression rate of 47% and a
positive MRP2 expression of 40%. The overall survival rates of
NSCLC patients with a positive MRP2 expression were markedly
shorter compared with those of patients with a negative MRP2
expression (P=0.007), suggesting that MRP2 protein expression may
be an indicator of poor prognosis of NSCLC patients suitable for
surgery. However, MRP1 expression did not correlate with survival
period. MRP1 and MRP2 are not significant for predicting the
efficacy of platinum-containing chemotherapy. Our results indicate
that in NSCLC tumor tissues, MRP protein had a positive expression
rate of 43.4% (66/152), which is markedly correlated with
pathological types and lymph node metastasis (P<0.05). The
positive MRP protein expression rate in lung adenocarcinoma was
67.6% (25/37), markedly higher compared with that of squamous cell
carcinoma (33.0%, 32/97), which was markedly different (P<0.05).
The 1-, 2- and 3-year survival rates of patients with a positive
MRP expression were markedly lower compared with those of patients
with a negative MRP expression, suggesting poor prognosis of NSCLC
patients with a positive MRP expression. However, the Cox
multi-element analyses suggest that MRP is not an independent
predictor of the prognosis of NSCLC patients.
Our findings suggest that the 1-, 2- and 3-year
survival rates of patients, who received surgery and chemotherapy,
and had positive c-erbB2 protein expression, were markedly lower
compared with those of patients with a negative c-erbB2 expression
(P=0.01), suggesting that NSCLC patients with a positive c-erbB2
expression may be resistant to platinum-containing chemotherapy.
Overexpression of c-erbB2 protein in NSCLC tissues may be a
prognostic indicator of tumor progression. Similarly, the Cox
multi-element analyses suggest that c-erbB2 is an independent
predictor of the prognosis of NSCLC patients.
Findings of this study have shown that a positive
expression of the three proteins in NSCLC patients indicates a poor
prognosis, as the 1-, 2- and 3-year survival rates were 72.6, 54.8
and 32.2%, respectively, which were markedly lower compared with
those of patients with a negative expression of the three proteins
(92.1, 78.5 and 63.4%, P=0.02, 0.01 and 0.00). The 3-year survival
rates of patients with a positive expression of two proteins were
lower compared with those of patients with a positive expression of
one protein or a negative expression of the two proteins (P=0.02
and P=0.01). Utilization of p53, c-erbB2 and MRP as a
three-indicator combination or of MRP and c-erbB2 as a
two-indicator combination, are useful for the prognostic evaluation
of the surgical and chemotherapeutic efficacies of NSCLC
patients.
Acknowledgements
This study was supported by the
Traditional Chinese Medicine project of the Zhejiang Province
(grant no. 008CA036).
References
|
1.
|
Bunn PA: Future directions in clinical
research for lung cancer. Chest. 106:399–407. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Arriagada R, Bergman B, Dunant A, et al:
Cisplatin-based adjuvant chemotherapy in patients with completely
resected non-small cell lung cancer. N Engl J Med. 350:351–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mountain CF: Revisions in the
international system for staging Lung Cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tsao MS, Aviel-Ronen S, Ding K, et al:
Prognostic and predictive importance of p53 and RAS for adjuvant
chemotherapy in non-small cell lung cancer. J Clin Oncol.
25:5240–5247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bai H, Zhang XY, Ji H, et al: Expression
and the clinical significance of p53 and nm23 in non-small cell
lung cancers. J Prac Oncol. 19:497–500. 2004.
|
|
6.
|
Ota E, Abe Y, Oshika Y, et al: Expression
of the multidrug resistance-associated protein (MRP) gene in
non-small cell lung cancer. Br J Cancer. 72:550–554. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bu H and Pang ZG: Research progress of
C-erbB-2 gene in breast cancers. Gene News. 1:122001.
|
|
8.
|
Kristiansen G, Yu Y, Petersen S, et al:
Overexpression of c-erbB2 protein correlates with disease-stage and
chromosomal gain at the c-erbB2 locus in non-small cell lung
cancer. Eur J Cancer. 37:1089–1095. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Turken O, Kunter E, Cermik H, et al:
Prevalence and prognostic value of c-erbB2 expression in non-small
cell lung cancer (NSCLC). Neoplasma. 50:257–261. 2003.PubMed/NCBI
|
|
10.
|
Rusch V, Klimstra D, Venkatraman E, et al:
Aberrant p53 expression predicts clinical resistance to
cisplatin-based chemo-therapy in locally advanced non-small cell
lung cancer. Cancer Res. 55:5038–5042. 1995.PubMed/NCBI
|
|
11.
|
Izquierdo MA, Shoemaker RH, Flens MJ, et
al: Overlapping phenotypes of multidrug resistance among panels of
human cancer cell lines. Int J Cancer. 65:230–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sengupta S and Harris CC: p53: traffic cop
at the crossroads of DNA repair and recombination. Nat Rev Mol Cell
Biol. 6:44–55. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Huang PY, Liang XM, Lin SX, et al:
Correlations analysis among expression of ERCC1, metallothionein
p53 and platinum resistance and prognosis in advanced non-small
cell lung cancer. Chin J Cancer. 23:845–850. 2004.PubMed/NCBI
|
|
14.
|
Zhang HT: p53 and chemotherapy
sensitivity. Foreign Medicine: Oncology. 28:344–347. 2001.
|
|
15.
|
Lee JS, Yoon A, Kalapurakal SK, et al:
Expression of p53 onco-protein in non-small cell lung cancer: a
favorable prognostic factor. J Clin Oncol. 13:1893–1903.
1995.PubMed/NCBI
|
|
16.
|
Kwiatkowski DJ, Harpole DH, Godleski J, et
al: Molecular pathologic substaging in 244 stage I non-small cell
lung cancer patients: clinical implications. J Clin Oncol.
16:2468–2477. 1998.PubMed/NCBI
|
|
17.
|
Ebina SM, Mulshine JL, Linnoila RI, et al:
Relationship of p53 overexpression and up-regulation of
proliferating cell nuclear antigen with the clinical course of
non-small cell lung cancer. Cancer Res. 54:2496–2503.
1994.PubMed/NCBI
|
|
18.
|
Filipits M, Haddad V, Schmid K, et al:
Multidrug resistance proteins do not predict benefit of adjuvant
chemotherapy in patients with completely resected non-small cell
lung cancer: International Adjuvant Lung Cancer Trial Biologic
Program. Clin Cancer Res. 13:3892–3898. 2007. View Article : Google Scholar
|